Cardiovascular “Patterns” of H2S and SSNO−-Mix Evaluated from 35 Rat Hemodynamic Parameters
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Ethical Approval
2.3. Animals, APW Measurement and Data Evaluation
3. Results
3.1. Effects of Na2S on Time-Dependent Changes of 35 HPs
3.2. Comparison of Transient Effects of Na2S with Published Data of GSNO on 35 HPs
3.3. Non-Hysteresis/Hysteresis Relationships between HPs to Systolic BP after Na2S Administration
3.4. Comparison of Non-Hysteresis/Hysteresis Relationships of Na2S with Published Relationships of GSNO
3.5. Effect of H2S on Distinct Fluctuation of Diastolic BP
3.6. Comparison of Na2S, GSNO and SSNO−-Mix Effects on Rat BP
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- O’Rourke, M.F.; Pauca, A.; Jiang, X.-J. Pulse wave analysis. Br. J. Clin. Pharmacol. 2001, 51, 507–522. [Google Scholar] [CrossRef]
- Žikić, D. A mathematical model of pressure and flow waveforms in the aortic root. Eur. Biophys. J. 2017, 46, 41–48. [Google Scholar] [CrossRef]
- Marais, L.; Pernot, M.; Khettab, H.; Tanter, M.; Messas, E.; Zidi, M.; Laurent, S.; Boutouyrie, P. Arterial Stiffness Assessment by Shear Wave Elastography and Ultrafast Pulse Wave Imaging: Comparison with Reference Techniques in Normotensives and Hypertensives. Ultrasound Med. Bio. 2019, 45, 758–772. [Google Scholar] [CrossRef]
- Misak, A.; Kurakova, L.; Berenyiova, A.; Tomasova, L.; Grman, M.; Cacanyiova, S.; Ondrias, K. Patterns and Direct/Indirect Signaling Pathways in Cardiovascular System in the Condition of Transient Increase of NO. Biomed. Res. Int. 2020, 2020, 6578213. [Google Scholar] [CrossRef]
- Tomasova, L.; Misak, A.; Kurakova, L.; Grman, M.; Ondrias, K. Characterization of Rat Cardiovascular System by Anacrotic/Dicrotic Notches in the Condition of Increase/Decrease of NO Bioavailability. Int. J. Mol. Sci. 2020, 21, 6685. [Google Scholar] [CrossRef] [PubMed]
- Kurakova, L.; Misak, A.; Tomasova, L.; Cacanyiova, S.; Berenyiova, A.; Ondriasova, E.; Balis, P.; Grman, M.; Ondrias, K. Mathematical relationships of patterns of 35 rat haemodynamic parameters for conditions of hypertension resulting from decreased nitric oxide bioavailability. Exp. Physiol. 2020, 105, 312–334. [Google Scholar] [CrossRef] [PubMed]
- Wang, R. Physiological implications of hydrogen sulfide: A whiff exploration that blossomed. Physiol. Rev. 2012, 92, 791–896. [Google Scholar] [CrossRef] [PubMed]
- Drobná, M.; Misak, A.; Holland, T.; Kristek, F.; Grman, M.; Tomasova, L.; Berenyiova, A.; Cacanyiova, S.; Ondrias, K. Captopril partially decreases the effect of H2S on rat blood pressure and inhibits H2S-induced nitric oxide release from S-nitrosoglutathione. Physiol. Res. 2015, 64, 479–486. [Google Scholar] [CrossRef]
- Cacanyiova, S.; Berenyiova, A.; Balis, P.; Kristek, F.; Grman, M.; Ondrias, K.; Breza, J.; Breza, J., Jr. Nitroso-sulfide coupled signaling triggers specific vasoactive effects in the intrarenal arteries of patients with arterial hypertension. J. Physiol. Pharmacol. 2017, 68, 527–538. [Google Scholar]
- Zhao, W.; Zhang, J.; Lu, Y.; Wang, R. The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener. EMBO J. 2001, 20, 6008–6016. [Google Scholar] [CrossRef]
- Geng, B.; Yang, J.; Qi, Y.; Zhao, J.; Pang, Y.; Du, J.; Tang, C. H2S generated by heart in rat and its effects on cardiac function. Biochem. Biophys. Res. Commun. 2004, 313, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Szijártó, I.A.; Markó, L.; Filipovic, M.R.; Miljkovic, J.L.; Tabeling, C.; Tsvetkov, D.; Wang, N.; Rabelo, L.A.; Witzenrath, M.; Diedrich, A.; et al. Cystathionine γ-Lyase-Produced Hydrogen Sulfide Controls Endothelial NO Bioavailability and Blood Pressure. Hypertension 2018, 71, 1210–1217. [Google Scholar] [CrossRef]
- Yang, G.; Wu, L.; Jiang, B.; Yang, W.; Qi, J.; Cao, K.; Meng, Q.; Mustafa, A.K.; Mu, W.; Zhang, S.; et al. H2S as a physiologic vasorelaxant: Hypertension in mice with deletion of cystathionine gamma-lyase. Science 2008, 322, 587–590. [Google Scholar] [CrossRef]
- Cortese-Krott, M.M.; Kuhnle, G.G.; Dyson, A.; Fernandez, B.O.; Grman, M.; DuMond, J.F.; Barrow, M.P.; McLeod, G.; Nakagawa, H.; Ondrias, K.; et al. Key bioactive reaction products of the NO/H2S interaction are S/N-hybrid species, polysulfides, and nitroxyl. Proc. Natl. Acad. Sci. USA 2015, 112, 4651–4660. [Google Scholar] [CrossRef]
- Ali, M.Y.; Ping, C.Y.; Mok, Y.Y.; Ling, L.; Whiteman, M.; Bhatia, M.; Moore, P.K. Regulation of vascular nitric oxide in vitro and in vivo; a new role for endogenous hydrogen sulphide? Br. J. Pharmacol. 2006, 149, 625–634. [Google Scholar] [CrossRef]
- Li, L.; Whiteman, M.; Guan, Y.Y.; Neo, K.L.; Cheng, Y.; Lee, S.W.; Zhao, Y.; Baskar, R.; Tan, C.H.; Moore, P.K. Characterization of a novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137): New insights into the biology of hydrogen sulfide. Circulation 2008, 117, 2351–2360. [Google Scholar] [CrossRef]
- Cacanyiova, S.; Krskova, K.; Zorad, S.; Frimmel, K.; Drobna, M.; Valaskova, Z.; Misak, A.; Golas, S.; Breza, J.; Breza, J., Jr.; et al. Arterial Hypertension and Plasma Glucose Modulate the Vasoactive Effects of Nitroso-Sulfide Coupled Signaling in Human Intrarenal Arteries. Molecules 2020, 25, 2886. [Google Scholar] [CrossRef] [PubMed]
- Cortese-Krott, M.M.; Fernandez, B.O.; Santos, J.L.; Mergia, E.; Grman, M.; Nagy, P.; Kelm, M.; Butler, A.; Feelisch, M. Nitrosopersulfide (SSNO−) accounts for sustained NO bioactivity of S-nitrosothiols following reaction with sulfide. Redox Bio. 2014, 2, 234–244. [Google Scholar] [CrossRef]
- Bogdándi, V.; Ditrói, T.; Bátai, I.Z.; Sándor, Z.; Minnion, M.; Vasas, A.; Galambos, K.; Buglyó, P.; Pintér, E.; Feelisch, M.; et al. Nitrosopersulfide (SSNO−) Is a Unique Cysteine Polysulfidating Agent with Reduction-Resistant Bioactivity. Antioxid. Redox Signal. 2020. [Google Scholar] [CrossRef]
- Grundy, D. Principles and standards for reporting animal experiments in The Journal of Physiology and Experimental Physiology. J. Physiol. 2015, 593, 2547–2549. [Google Scholar] [CrossRef]
- Lee, S.R.; Nilius, B.; Han, J. Gaseous Signaling Molecules in Cardiovascular Function: From Mechanisms to Clinical Translation. Rev. Physiol. Biochem. Pharmacol. 2018, 174, 81–156. [Google Scholar] [CrossRef]
- Bianco, C.L.; Akaike, T.; Ida, T.; Nagy, P.; Bogdandi, V.; Toscano, J.P.; Kumagai, Y.; Henderson, C.F.; Goddu, R.N.; Lin, J.; et al. The reaction of hydrogen sulfide with disulfides: Formation of a stable trisulfide and implications for biological systems. Br. J. Pharmacol. 2019, 176, 671–683. [Google Scholar] [CrossRef]
- Gheibi, S.; Jeddi, S.; Kashfi, K.; Ghasemi, A. Regulation of vascular tone homeostasis by NO and H2S: Implications in hypertension. Biochem. Pharmacol. 2018, 149, 42–59. [Google Scholar] [CrossRef]
- Iring, A.; Jin, Y.J.; Albarrán-Juárez, J.; Siragusa, M.; Wang, S.; Dancs, P.T.; Nakayama, A.; Tonack, S.; Chen, M.; Künne, C.; et al. Shear stress-induced endothelial adrenomedullin signaling regulates vascular tone and blood pressure. J. Clin. Investig. 2019, 129, 2775–2791. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, G.C.; Herbert, K.E. Regulation of vascular function and blood pressure by circadian variation in redox signalling. Free Radic. Biol. Med. 2018, 119, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Vanhoutte, P.M.; Leung, S.W. Vascular nitric oxide: Beyond eNOS. J. Pharmacol. Sci. 2015, 129, 83–94. [Google Scholar] [CrossRef]
- Puzserova, A.; Bernatova, I. Blood pressure regulation in stress: Focus on nitric oxide-dependent mechanisms. Physiol. Res. 2016, 65, S309–s342. [Google Scholar] [CrossRef]
- Szabo, C. Hydrogen sulfide, an enhancer of vascular nitric oxide signaling: Mechanisms and implications. Am. J. Physiol. Cell Physiol. 2017, 312, C3–c15. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Wu, L.; Wang, R. Interaction of hydrogen sulfide with ion channels. Clin. Exp. Pharmacol. Physiol. 2010, 37, 753–763. [Google Scholar] [CrossRef]
- Cao, X.; Wu, Z.; Xiong, S.; Cao, L.; Sethi, G.; Bian, J.S. The role of hydrogen sulfide in cyclic nucleotide signaling. Biochem. Pharmacol. 2018, 149, 20–28. [Google Scholar] [CrossRef]
- Stubbert, D.; Prysyazhna, O.; Rudyk, O.; Scotcher, J.; Burgoyne, J.R.; Eaton, P. Protein kinase G Iα oxidation paradoxically underlies blood pressure lowering by the reductant hydrogen sulfide. Hypertension 2014, 64, 1344–1351. [Google Scholar] [CrossRef] [PubMed]
- Nier, B.A.; Harrington, L.S.; Carrier, M.J.; Weinberg, P.D. Evidence for a specific influence of the nitrergic pathway on the peripheral pulse waveform in rabbits. Exp. Physiol. 2008, 93, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wu, Y.M.; Li, Q.; Wang, X.; He, R.R. Electrophysiological effects of hydrogen sulfide on pacemaker cells in sinoatrial nodes of rabbits. Sheng Li Xue Bao 2008, 60, 175–180. [Google Scholar] [PubMed]
- Yong, Q.C.; Hu, L.F.; Wang, S.; Huang, D.; Bian, J.S. Hydrogen sulfide interacts with nitric oxide in the heart: Possible involvement of nitroxyl. Cardiovasc. Res. 2010, 88, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Brunner, F.; Andrew, P.; Wölkart, G.; Zechner, R.; Mayer, B. Myocardial Contractile Function and Heart Rate in Mice With Myocyte-Specific Overexpression of Endothelial Nitric Oxide Synthase. Circulation 2001, 104, 3097–3102. [Google Scholar] [CrossRef] [PubMed]
- Layland, J.; Li, J.M.; Shah, A.M. Role of cyclic GMP-dependent protein kinase in the contractile response to exogenous nitric oxide in rat cardiac myocytes. J. Physiol. 2002, 540, 457–467. [Google Scholar] [CrossRef]
- Berenyiova, A.; Grman, M.; Mijuskovic, A.; Stasko, A.; Misak, A.; Nagy, P.; Ondriasova, E.; Cacanyiova, S.; Brezova, V.; Feelisch, M.; et al. The reaction products of sulfide and S-nitrosoglutathione are potent vasorelaxants. Nitric Oxide 2015, 46, 123–130. [Google Scholar] [CrossRef]
- Gladwin, M.T.; Crawford, J.H.; Patel, R.P. The biochemistry of nitric oxide, nitrite, and hemoglobin: Role in blood flow regulation. Free Radic. Biol. Med. 2004, 36, 707–717. [Google Scholar] [CrossRef]
- Farmer, P.J.; Sulc, F. Coordination chemistry of the HNO ligand with hemes and synthetic coordination complexes. J. Inorg. Biochem. 2005, 99, 166–184. [Google Scholar] [CrossRef]
- Vaughn, M.W.; Huang, K.T.; Kuo, L.; Liao, J.C. Erythrocytes possess an intrinsic barrier to nitric oxide consumption. J. Biol. Chem. 2000, 275, 2342–2348. [Google Scholar] [CrossRef] [PubMed]
- Deonikar, P.; Kavdia, M. Contribution of membrane permeability and unstirred layer diffusion to nitric oxide–red blood cell interaction. J. Theor. Bio. 2013, 317, 321–330. [Google Scholar] [CrossRef] [PubMed]
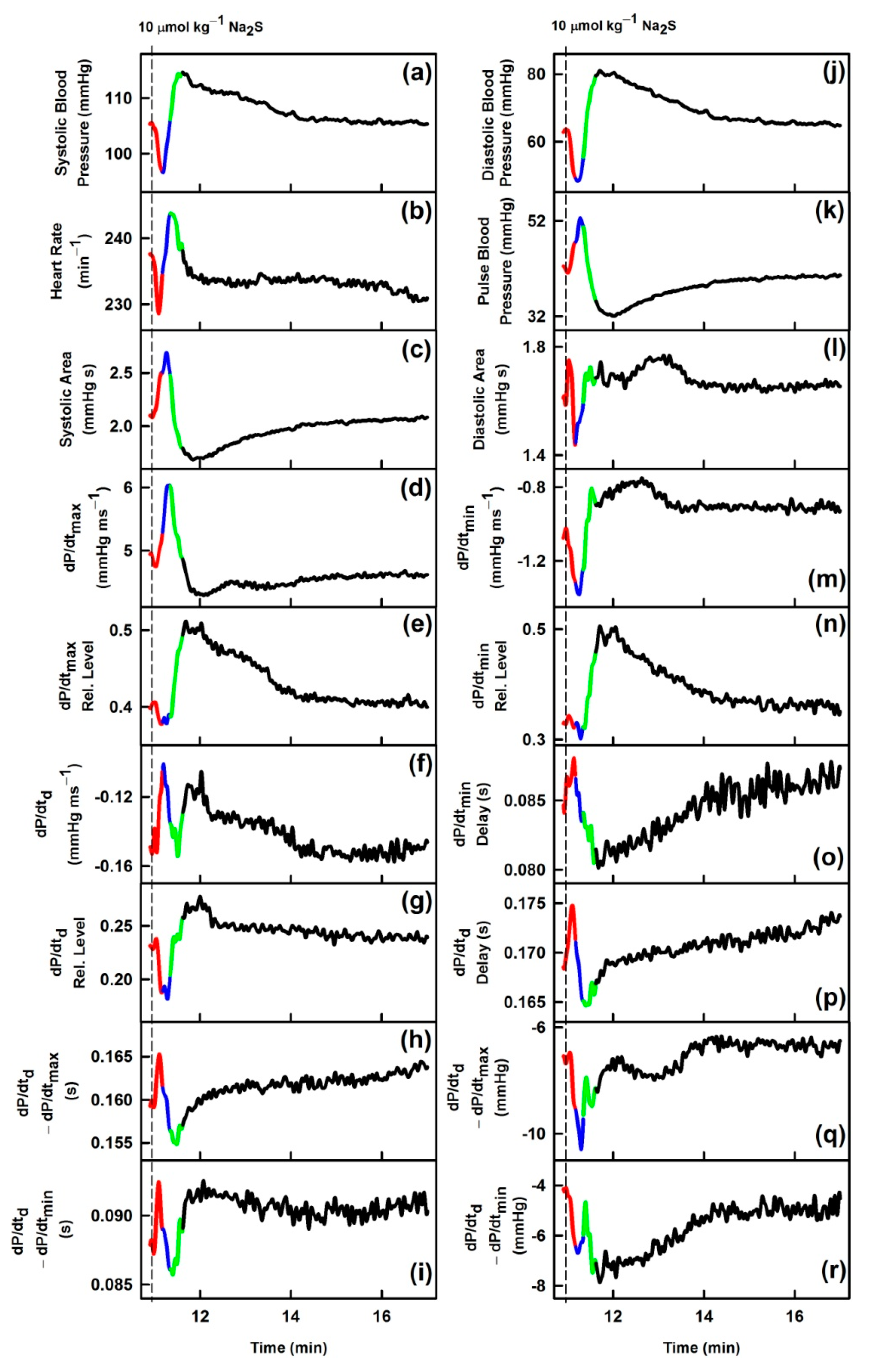

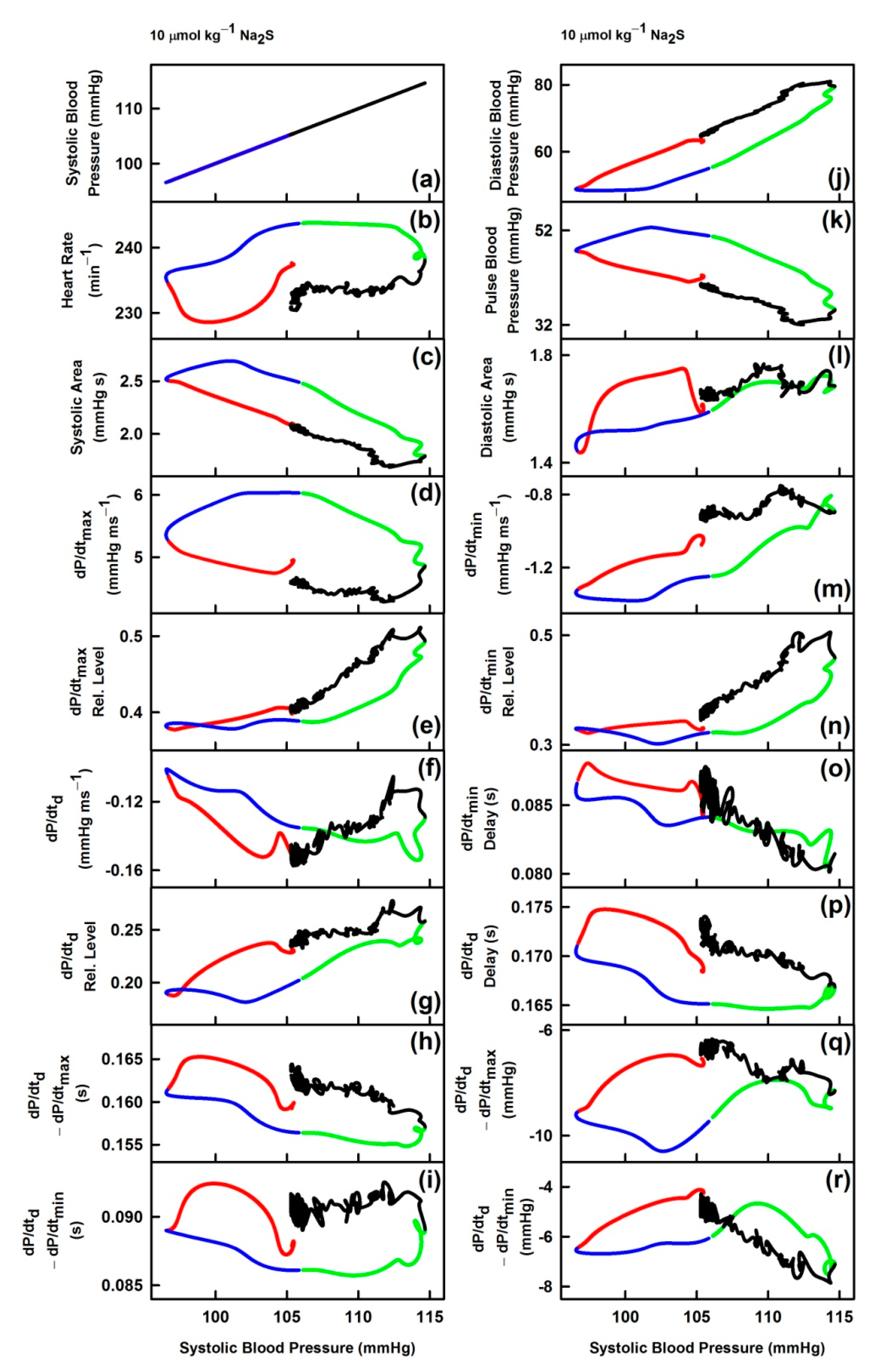






| Description | GSNO | Na2S | |
|---|---|---|---|
| (a) | Systolic blood pressure; in mmHg | ↓ | ↓ |
| (b) | Heart rate; in min−1 | ~ | ↓ |
| (c) | Systolic area; in mmHg s | ↑ | ↑ |
| (d) | dP/dtmax; in mmHg ms−1 | ↑ | ~ |
| (e) | dP/dtmax relative level | ↓ | ↓ |
| (f) | dP/dtd; in mmHg ms−1 | ↓↑ | ~ |
| (g) | dP/dtd relative level | ↓ | ↓ |
| (h) | dP/dtd − dP/dtmax; in s | ↑ | ↑ |
| (i) | dP/dtd − dP/dtmin; in s | ↑↓ | ↑ |
| (j) | Diastolic blood pressure; in mmHg | ↓ | ↓ |
| (k) | Pulse blood pressure; in mmHg | ↑ | ↑ |
| (l) | Diastolic area; in mmHg s | ↓ | ↑ |
| (m) | dP/dtmin; in mmHg ms−1 | ↓ | ↓ |
| (n) | dP/dtmin relative level | ↓ | ~ |
| (o) | dP/dtmin delay; in s | ↑ | ↑ |
| (p) | dP/dtd delay; in s | ↑ | ↑ |
| (q) | dP/dtd − dP/dtmax; in mmHg | ↓ | ↓ |
| (r) | dP/dtd − dP/dtmin; in mmHg | ↑ | ~ |
| (aa) | Systolic blood pressure; in mmHg | ↓ | ↓ |
| (bb) | Anacrotic notch; in mmHg | ↓ | ↓ |
| (cc) | Anacrotic notch relative level | ↑ | ↑ |
| (dd) | Anacrotic notch delay; in ms | ↑ | ↑ |
| (ee) | Anacrotic notch relative Delay | ↑ | ~ |
| (ff) | Dicrotic notch (DiN) − Anacrotic notch (AnN); in s | ↑ | ↑ |
| (gg) | (DiN − AnN)/dP/dtmin; in s/mmHg µs−1 | ↑ | ↑ |
| (hh) | (DiN − AnN)/dP/dtmax; in s/mmHg µs−1 | ↓ | ~ |
| (ii) | AnN − 1Max; in ms | ↑ | ↑ |
| (jj) | Augmentation index relative | ~ | ~ |
| (kk) | Dicrotic notch; in mmHg | ↓ | ↓ |
| (ll) | Dicrotic notch relative level | ↓ | ↓ |
| (mm) | Dicrotic notch delay; in ms | ↑ | ↑ |
| (nn) | Dicrotic notch relative delay | ↑ | ~ |
| (oo) | DiN − AnN; in mmHg | ↓ | ↓ |
| (pp) | (DiN − AnN)/dP/dtmin; in mmHg/mmHg ms−1 | ↑ | ↑ |
| (qq) | (DiN − AnN)/dP/dtmax; in mmHg/mmHg ms−1 | ↓ | ↓ |
| (rr) | AnN − 1Max; in mmHg | ~ | ~ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomasova, L.; Grman, M.; Misak, A.; Kurakova, L.; Ondriasova, E.; Ondrias, K. Cardiovascular “Patterns” of H2S and SSNO−-Mix Evaluated from 35 Rat Hemodynamic Parameters. Biomolecules 2021, 11, 293. https://doi.org/10.3390/biom11020293
Tomasova L, Grman M, Misak A, Kurakova L, Ondriasova E, Ondrias K. Cardiovascular “Patterns” of H2S and SSNO−-Mix Evaluated from 35 Rat Hemodynamic Parameters. Biomolecules. 2021; 11(2):293. https://doi.org/10.3390/biom11020293
Chicago/Turabian StyleTomasova, Lenka, Marian Grman, Anton Misak, Lucia Kurakova, Elena Ondriasova, and Karol Ondrias. 2021. "Cardiovascular “Patterns” of H2S and SSNO−-Mix Evaluated from 35 Rat Hemodynamic Parameters" Biomolecules 11, no. 2: 293. https://doi.org/10.3390/biom11020293
APA StyleTomasova, L., Grman, M., Misak, A., Kurakova, L., Ondriasova, E., & Ondrias, K. (2021). Cardiovascular “Patterns” of H2S and SSNO−-Mix Evaluated from 35 Rat Hemodynamic Parameters. Biomolecules, 11(2), 293. https://doi.org/10.3390/biom11020293






