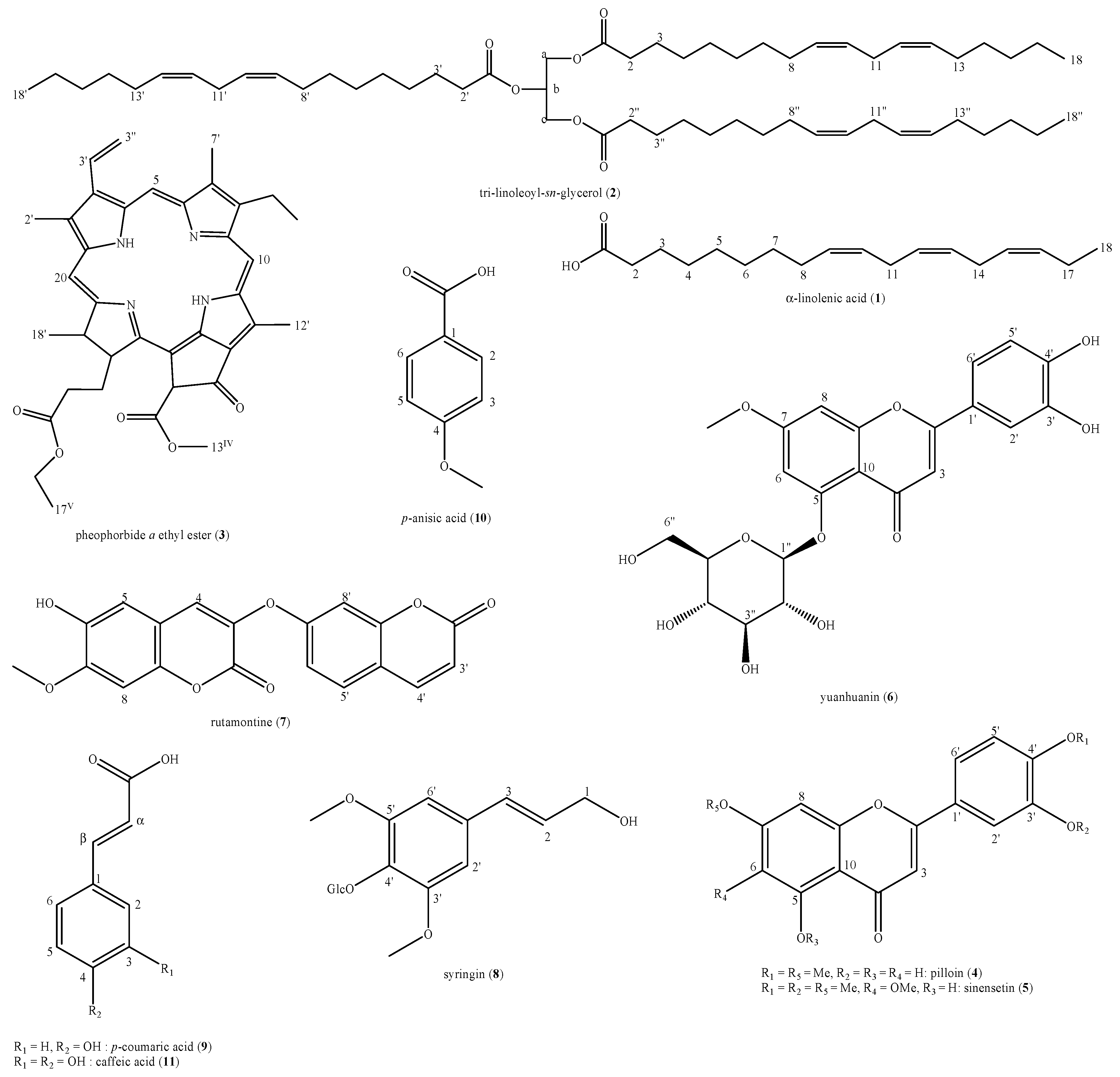
Abstract
In this paper, the first phytochemical analysis of the ethanolic extract of Daphne sericea Vahl flowering aerial parts collected in Italy and its biological activities were reported. Eleven compounds were identified i.e., α-linolenic acid (1), tri-linoleoyl-sn-glycerol (2), pheophorbide a ethyl ester (3), pilloin (4), sinensetin (5), yuanhuanin (6), rutamontine (7), syringin (8), p-coumaric acid (9), p-anisic acid (10) and caffeic acid (11). To the best of our knowledge, compounds (1-4, 7-8 and 10) were isolated from D. sericea for the first time during this work, whereas sinensetin (5) represents a newly identified component of the entire Thymelaeaceae family. The extract was found to possess radical scavenging against both DPPH• and 2,2′-azino-bis(3-thylbenzothiazoline-6-sulfonic acid (ABTS•+) radicals, with at least a 40-fold higher potency against the latter. Moreover, chelating abilities against both ferrous and ferric ions have been highlighted, thus suggesting a possible indirect antioxidant power of the extract. Although the precise bioactive compounds remain to be discovered, the polyphenolic constituents, including phenolic acids, tannins and flavonoids, seem to contribute to the antioxidant power of the phytocomplex. In addition, the extract produced cytotoxic effects in MDA-MB-231 and U87-MG cancer cell lines, especially at the concentration of 625 μg/mL and after 48–72 h. Further studies are required to clarify the contribution of the identified compounds in the bioactivities of the extract and to support possible future applications.
1. Introduction
Daphne sericea Vahl is an evergreen shrub belonging to the Thymelaeaceae family. Its name derives from the union of two terms: the Greek noun “Δάφνη” (Dáphne), a mythological nymph who turned into a laurel plant, therefore related to the similarity of its leaves to those of laurel, and the Latin adjective “sericeus, a, um” meaning silky because of those silky hairs that cover the buds and the corolla tube [1].
From the morphological point of view, this species is characterized by erect or decumbent branches and coriaceous leaves, oblong–obovate, almost glabrous in the superior face and with appressed hairs beneath. The flowers are very fragrant, pink, mostly collected in terminal heads, blooming between February and May. The fruit is a reddish–brown drupe [2,3] (Figure 1).

Figure 1.
Daphne sericea Vahl.
This species has a Central–Eastern Mediterranean distribution, comprising Crete, Greece, Lebanon, Syria, Turkey and Italy [4] where it can be only found in Tuscany, Latium, Abruzzo, Molise, Campania, Apulia and Sicily [5]. Its typical habitat is represented by rocky slopes, open pinewoods and garigues up to 1000 m a.s.l. [3].
In Turkey, leaves and flowers of D. sericea have been used as traditional remedies for the treatment of hemorrhoids [6]; furthermore, branches with leaves are exploited as dye sources [7]. Similarly, other Daphne species are utilized in many areas of the world, especially in Asia, Africa and Europe, to treat several ailments, such as bruises, stomachache, infections, rheumatisms, diarrhea, inflammations, cancer, sore throat, indolent ulcers, snakebites, laryngitis, malaria, fever and apoplexy [8].
Only a few studies about the phytochemistry and pharmacological properties of D. sericea are available in the literature. Tongur et al. [9] studied the phytochemical composition of the acetone and methanolic extracts of D. sericea collected in Turkey and highlighted the presence of several flavonoids and organic acids (i.e., quercetin, luteolin, kaempferol, apigenin, isorhamnetin, rhamnetin, chlorogenic acid, caffeic acid, syringic acid, p-coumaric acid, ferulic acid, and rosmarinic acid). Moreover, these extracts were found to possess radical scavenging properties against DPPH· and 2,2′-azino-bis(3-thylbenzothiazoline-6-sulfonic acid (ABTS·+) radicals, and against superoxide anion (O2−) [9]. Similarly, Ulubelen et al. [10] evaluated the phytochemical composition of a Soxhlet ethanolic extract from D. sericea collected in Turkey, reporting the presence of only flavonoids, including luteolin 7-methyl ether, isovitexin, apigenin and its 7-β-D-glucoside and two novel compounds, namely luteolin 7-methyl ether 5-β-D-glucoside and luteolin 7,3′-dimethyl ether 5-β-D glucoside. The aqueous layer remained after partitioning of the Soxhlet ethanolic extract with n-hexane, benzene and chloroform, mainly containing the coumarins daphnerotin and daphnin, sitosteryl 3-β-D-glucoside and several flavonoids (mainly luteolin 7-methyl ether, and lesser amounts of 5-glucoside of luteolin 7-methyl ether and 5-glucoside of luteolin 7,3′- dimethyl ether) exhibited promising antileukemic properties [10]. An antileukemic diterpenoid, namely mezerein, has been also identified in the hydroalcoholic extract of Daphne mezereum L. [11]. Lastly, 49 volatile compounds, mainly monoterpenes and fatty acids, representing 99.71 % of the total, were found in the essential oil of D. sericea flowers [12].
In the present study, a D. sericea specimen collected in Italy was studied for the first time for the phytochemical composition and the biological activities. Specifically, an ethanolic extract from the flowering aerial parts of D. sericea, has been prepared by maceration and characterized by an integrated phytochemical analysis. In addition, its antioxidant properties have been evaluated together with its cancer cell cytotoxicity for the first time.
2. Material and Methods
2.1. Plant Material
The plant material consisting of 350 g of flowering aerial parts was collected in the San Venanzio gorges, Raiano municipality, Abruzzo region, Italy (geographical coordinates 13°79′84″ E, 42°10′77″ N) at an altitude of 450 m a.s.l. in the month of April 2018. The botanical identification was performed by two of us (M.D.C and G.C.) through the most used analytical floras [2,3] and comparing the collected material with those conserved in the principal herbariums of Central Italy. A portion of this collection was stored in our laboratory for further references and registered under the voucher name DS15042018.
2.2. Chemicals
The following solvents and reagents were used during this study: ethanol absolute for the extraction procedure and for the dissolution of the extract for the antioxidant activity assays; methanol and chloroform in mixture at different concentrations as eluting systems for the separation procedure on silica gel (60 Å, 70–230 mesh) columns and as mobile phases for the TLCs; 2N H2SO4 for the development of the TLCs; CDCl3, CD3OD and D2O as solvents for the identification of the metabolites by NMR spectroscopy; HPLC grade methanol for the identification of the metabolites by mass spectrometry (MS); MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) as an addition for the incubation of cells in the medium; DMSO for the dissolution of cells; Folin–Ciocalteu’s phenol reagent, tannic acid (Ph Eur purity), sodium carbonate (Na2CO3; 99.999% purity), aluminum chloride hexahydrate (AlCl3 × 6H2O; Ph Eur purity), 1,1-diphenyl-2-picryl-hydrazyl (DPPH·; 95% purity), 2,2′-azino-bis(3-thylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS·+; 98% purity), 2,2′-azo-bis(2-methylpropionamidine) dihydrochloride (AAPH; 97% purity), iron (II) sulfate heptahydrate (FeSO4 × 7H2O; 99% purity), iron (III) chloride (FeCl3 × 6H2O; 97% purity), ferrozine (97% purity), hydroxylamine hydrochloride (98% purity), trolox (97% purity) and quercetin (>95% purity) for the antioxidant activity assays.
All the solvents were RPE purity grade if not otherwise specified, and were purchased from Sigma Aldrich (San Louis, MI, USA) as well as all the deuterated solvents, 2N H2SO4, HPLC grade methanol and MTT, whereas silica gel, DMSO and all the reagents for the antioxidant activity assays were purchased from Merck (Darmstadt, Germany).
2.3. Extraction Procedure
The dried flowering aerial parts (350.2 g) were inserted into a flask and covered with a solution of 96% ethanol until complete immersion (about 300 mL). The plant material was macerated for at least 72 h so that metabolites could come into solution. This procedure was repeated three times in order to produce an exhaustive extraction. The ethanolic solutions, showing a greenish coloration, were gathered together and then filtered. All the ethanol was eliminated at reduced pressure at 50 °C until a water suspension was obtained. Throughout this part, pH was checked on litmus paper and this was about 8. This passage is necessary in order to verify that the pH is not too acid or basic (meaning in the range 5.5–8.5) because an extreme acidity or alkalinity might cause secondary reactions in the extract such as the hydrolysis of ester and glycosidic bonds [13]. The obtained suspension was then frozen and later lyophilized to preserve temperature-sensitive compounds eventually present. The obtained dried crude extract weighed 23.4 g and was a dark green color.
2.4. Isolation and Identification of the Metabolites
An aliquot of the dried crude extract (3.0 g) was subjected to a column chromatography procedure on silica gel as the stationary phase. The used amount of silica gel was 93.2 (ratio ~ 1:30 w/w) and the eluting system was a mixture of chloroform and methanol at different concentration ratios. The initial concentration ratio was 98:2 (v/v) (300 mL) but, during the chromatographic run, this was changed raising the polarity in order to allow the elution of the most polar compounds too. In particular, the concentration ratio passed to 95:5 (v/v) (300 mL), 9:1 (v/v) (400 mL), 8:2 (v/v) (500 mL), 7:3 (v/v) (300 mL) and then to 6:4 (v/v) (200 mL). From this chromatographic procedure, all the compounds were identified by means of exhaustive comparison of their spectroscopic data with those reported in the literature and with authentic standard compounds already available in our laboratory. In particular, the identified compounds were the following: tri-linoleoyl-sn-glycerol (2) and pheophorbide a ethyl ester (3) [14,15] in mixture in a ratio of 20:1 (w/w) from the assembly of fractions 6–11 for the total weight of 10.2 mg; α-linolenic acid (1) and pilloin (4) [14,16] in mixture in a ratio of 4:1 (w/w) from the assembly of fractions 21–30 for the total weight of 16.9 mg; pilloin (4) and sinensetin (5) [16,17] in mixture in a ratio of 5:1 (w/w) from the assembly of fractions 31–33 for the total weight of 5.4 mg; pilloin (4) and rutamontine (7) [16,18] in mixture in a ratio of 1.5:1 (w/w) from the assembly of fractions 34–35 for the total weight of 7.7 mg; p-coumaric acid (9) [19] in mixture with other unidentified compounds (ratio not calculable) from the assembly of fractions 115–127 for the total weight of 12.1 mg; syringin (8) [15] in mixture with ethoxy glucose and several saccharides (ratio not calculable) from the assembly of fractions 138–147 for the total weight of 6.8 mg; yuanhuanin (6) [20] and p-anisic acid (10) [21] in mixture in ratio 10:1 (w/w) from the assembly of fractions 161–179 for the total weight of 6.6 mg; caffeic acid (11) [22] in mixture with several saccharides (ratio not calculable) from the methanol column wash for the total weight of 33.8 mg. The identification of components in mixture was conducted by applying a well-established method developed by our research group [23,24,25,26,27,28].
2.5. NMR Analysis
NMR spectra were recorded on a Bruker Avance III instrument (Billerica, MA, USA). The potency of the instrument was 400 MHz. The chemical shifts were expressed in ppm from TMS (s, 0.00 ppm) as internal reference standard for the spectra recorded in CDCl3, whereas the internal solvent signal of CD2HOD (m5, δH 3.31 ppm; m7, δC 49.00 ppm) was the reference for the spectra recorded in CD3OD and the HDO signal (s, 4.79 ppm) was set as reference for the spectra recorded in D2O. All the experiments were conducted at 298 K.
The 2D NMR spectra were performed on the same Bruker Avance III 400 MHz instrument operating at 9.4 T and 298 K. The Heteronuclear Single Quantum Coherence (HSQC) experiments were acquired with a spectral width of 15 ppm for the proton nucleus and 250 ppm for the carbon nucleus. The average direct coupling (1JC-H) was set to 145 Hz. The recycle delay was 2.0 s and the data matrix set was 4 K × 256 points. The Heteronuclear Multiple Bond Correlation (HMBC) experiments were acquired with the same spectral width applied in the HSQC experiments and by using two different values of long-range coupling constants of 8 Hz with a recycle delay of 2.0 s and a data matrix of 4 K × 256 points. NMR spectra were analyzed by ACD NMR manager software ver. 12 (ACD/Labs, Toronto, ON, Canada).
2.6. MS Analysis
MS spectra were acquired with a triple quadrupole mass spectrometer PE-Sciex API-3000® (Perkin Elmer Sciex, Toronto, ON, Canada), equipped with an ESI source operating in positive ionization mode. The capillary voltage was 4500 V. High purity nitrogen was used as the curtain gas, while air was employed as the nebulizer and drying gas. The temperature to heat the drying gas was set at 100 °C. The flow rate of sample infusion was 20 μL/min. MS spectra were acquired with 50 acquisitions per sample. The full width at half maximum (FWHM) was set at m/z 0.7 ± 0.1 in each mass-resolving quadrupole to operate with a unit resolution. The mass spectrometer operated in Full Scan mode in a mass spectral range of 100–1000 m/z. MS data were acquired and elaborated by Analyst® 1.5 Software (AB Sciex, Framingham, MA, USA).
2.7. NMR and MS Data of the Isolated Compounds
α-linolenic acid (1): 1H NMR (400 MHz, CDCl3) δ: 5.42–5.32 (6H, m, overlapped olefinic protons), 2.82–2.75 (4H, m, H-11 and H-14), 2.36–2.30 (2H, m, H-2), 2.09–1.97 (4H, m, H-8 and H-17), 1.64–1.58 (2H, m, H-3), 1.25 (8H, br. s, H-4, H-5, H-6, H-7), 0.86 (3H, br. t, J = 7.2 Hz, 18-CH3).
ESI-MS: m/z 279.38 [M+H]+, m/z 301.41 [M+Na]+.
tri-linoleoyl-sn-glycerol (2): 1H NMR (400 MHz, CDCl3) δ: 5.42–5.36 (12H, m, overlapped olefinic protons), 5.34–5.31 (1H, partially overlapped, H-b), 4.17–4.09 (4H, m, H-a, H-c), 2.82–2.75 (6H, m, H-11, H-11′ and H-11”), 2.36–2.26 (6H, m, H-2, H-2′ and H-2”), 2.09–2.00 (12H, m, H-8, H-8′, H-8”, H-13, H-13′ and H-13”), 1.62–1.56 (6H, m, H-3, H-3′ and H-3”), 1.25 (52H, br. s, n-(CH2)), 0.89–0.85 (9H, m, 18-CH3, 18′-CH3, 18″-CH3).
ESI-MS: m/z 901.95 [M+Na]+.
pheophorbide a ethyl ester (3): 1H NMR (400 MHz, CDCl3) δ: 9.69 (1H, s, H-10), 9.54 (1H, s, H-5), 8.72 (1H, s, H-20), 8.01 (1H, dd, J =17.9, 11.5 Hz, H-3′), 6.22 (1H, dd, J = 11.5, 1.3 Hz, H-3”), 3.88 (3H, s, H-13IV), 3.72 (3H, s, H-12′), 3.43 (3H, s, H-2′), 3.27 (3H, s, H-7′), 1.87 (3H, s, H-18′), 1.12 (3H, t, J = 7.1 Hz, H-17V).
ESI-MS: m/z 643.68 [M+Na]+.
pilloin (4): 1H NMR (400 MHz, CDCl3) δ: 7.49 (1H, dd, J = 8.5, 2.0 Hz, H-6′), 7.33 (1H, d, J = 2.0 Hz, H-2′), 7.04 (1H, d, J = 8.5 Hz, H-5′), 6.58 (1H, s, H-3), 6.49 (1H, d, J = 2.2 Hz, H-8), 6.37 (1H, d, J = 2.2 Hz, H-6) 4.01 (3H, s, 4′-OMe), 3.89 (3H, s, 7-OMe).
ESI-MS: m/z 337.31 [M+Na]+, m/z 313.29 [M-H]−.
sinensetin (5): 1H NMR (400 MHz, CDCl3) δ: 7.57 (1H, dd, J = 8.4, 2.0 Hz, H-6′), 7.37 (1H, d, J = 2.0 Hz, H-2′), 7.02 (1H, d, J = 8.4 Hz, H-5′), 6.72 (1H, s, H-3), 6.62 (1H, s, H-8), 3.99 (3H, s, 4′-OMe), 3.98 (3H, s, 3′-OMe), 3.97 (3H, s, 6-OMe), 3.95 (3H, s, 7-OMe).
ESI-MS: m/z 395.38 [M+Na]+, m/z 371.40 [M-H]−.
yuanhuanin (6): 1H NMR (400 MHz, CD3OD) δ: 7.34 (1H, overlapped, H-6′), 7.33 (1H, overlapped, H-2′), 6.88 (1H, d, J = 9.0 Hz, H-5′), 6.86 (1H, d, J = 2.4 Hz, H-6), 6.82 (1H, d, J = 2.4 Hz, H-8), 4.86 (1H, d, J = 8.0 Hz, H-1″), 3.93 (1H, d, J = 12.0 Hz, Ha-6″), 3.91 (3H, s, 7-CH3O), 3.84 (1H, d, J = 12.0 Hz, Hb-6″), 3.70 (1H, m, H-3″*), 3.64 (1H, m, H-2″), 3.54 (1H, m, H-5″*), 3.44 (1H, m, H-4″).
13C-NMR (100 MHz, CD3OD) δ: 180.3 (C-4), 166.0 (C-7), 164.6 (C-2), 160.5 (C-9), 159.7 (C-5), 150.8 (C-4′), 147.0 (C-3′), 123.3 (C-1′), 120.2 (C-6′), 116.8 (C-5′), 114.1 (C-2′), 110.3 (C-10), 106.7 (C-3), 104.9 (C-1″), 104.1 (C-6), 97.4 (C-8), 77.5 (C-5″*), 76.7 (C-3″*), 71.7 (C-2″), 69.3 (C-4″), 62.6 (C-6″), 56.7 (O-Me).
* signals may be reversed.
ESI-MS: m/z 485.43 [M+Na]+, m/z 461.46 [M-H]−.
rutamontine (7): 1H NMR (400 MHz, CDCl3) δ: 7.68 (1H, d, J = 9.5 Hz, H-4′), 7.43 (1H, s, H-4), 7.41 (1H, d, J = 8.3 Hz, H-5′), 6.97(1H, br. d, J = 8.3 Hz, H-6′), 6.90 (1H, br. s, H-8′), 6.84 (1H, s, H-5), 6.35 (1H, d, J = 9.5 Hz, H-3′), 3.90 (3H, s, OMe).
ESI-MS: m/z 375.31 [M+Na]+, m/z 351.27 [M-H]−.
syringin (8): 1H NMR (400 MHz, CD3OD) δ: 6.73 (2H, s, H-2′, H-6′), 6.52 (1H, d, J = 15.9 Hz, H-3), 6.31 (1H, dt, J = 15.9, 5.6 Hz, H-2), 4.88 (1H, overlapped signal, H-1”), 4.22 (2H, d, J = 5.2 Hz, H-1), 3.84 (6H, s, 2xOMe), 3.75 (1H, dd, J = 12.1, 2.2 Hz, Ha-6”), 3.64 (1H, dd, J = 12.1, 5.1 Hz, Hb-6”), 3.57-3.40 (3H, m, H-3”, H-4”, H-5”), 3.30-3.26 (1H, m, H-2”).
ESI-MS: m/z 395.36 [M+Na]+, m/z 411.42 [M+K]+, m/z 767.58 [2M+Na]+.
p-coumaric acid (9): 1H NMR (400 MHz, CD3OD) δ: 7.59 (1H, d, J = 16.0 Hz, H-β), 7.44 (2H, d, J = 8.5 Hz, H-2 and H-6), 6.80 (2H, d, J = 8.5 Hz, H-3 and H-5), 6.28 (1H, d, J = 16.0 Hz, H-α).
ESI-MS: m/z 187.15 [M+Na]+, m/z 163.08 [M-H]−.
p-anisic acid (10): 1H NMR (400 MHz, CD3OD) δ: 7.86 (2H, d, J = 8.8 Hz, H-3, H-5), 6.93 (2H, d, J = 8.8 Hz, H-2, H-6), 3.88 (3H, s, 4-OCH3).
ESI-MS: m/z 175.27 [M+Na]+.
caffeic acid (11): 1H NMR (400 MHz, D2O) δ: 7.56 (1H, d, J = 15.9 Hz, H-β), 7.05 (1H, br. s, H-2), 6.92 (1H, br. d, J = 8.3 Hz, H-6), 6.71 (1H, d, J = 8.3 Hz, H-5), 6.29 (1H, d, J = 15.9 Hz, H-α).
ESI-MS: m/z 203.09 [M+Na]+, m/z 179.10 [M-H]−.
2.8. Antioxidant Activity
Different antioxidant activities, including radical scavenging, chelating and reducing ones, were assessed according to previous standardized spectrophotometric methods, using a microplate reader (Epoch Microplate Spectrophotometer, BioTeK® Instruments Inc., Winooski, VT, USA) [29]. Particularly, the radical scavenger activity towards the synthetic DPPH· (1,1-diphenyl-2-picryl-hydrazyl) and ABTS·+ (2,2′-azino-bis(3-thylbenzothiazoline-6-sulfonic acid) diammonium salt) radicals, and the ability of the extract to indirectly interfere with the reactive oxygen species (ROS)-generation through blocking the Fenton reaction by iron (both ferrous and ferric ions) chelating and reducing effects, were evaluated. The antioxidant properties were correlated with the polyphenol content of the extract. To this end, the total amounts of phenolics, tannins and flavonoids were measured and expressed by the microplate Folin–Ciocalteu and aluminum trichloride methods, described by Di Sotto et al. [30], and expressed as tannic acid (TAE) and quercetin equivalents (QE) per mg of extract, respectively.
To perform the antioxidant activity assays, D. sericea extract was dissolved in 100% EtOH (v/v) and assayed in the concentration range of 1–2000 µg/mL, using suitable dilution factors in order to achieve a concentration–response curve. Data obtained from at least two experiments and six technical replicates for each concentration were pooled for the statistical analysis. In each experiment, negative (vehicle) and positive controls (standard antioxidant agents) were included. Particularly, trolox (concentration range 0.1–100 µg/mL) and quercetin (concentration range 1–2000 µg/mL) were used as positive controls for the radical scavenger and reducing activity, and for the chelating activity, respectively. Moreover, suitable controls of the possible interference absorbance of the extract were included.
2.9. Cytotoxic Activity
HDF (human dermal fibroblast) cells, MDA-MB-231 (human breast cancer cells) and U-87 MG (human brain glioblastoma-astrocytoma cell lines) were obtained from ATCC, Manassas, VA, USA. HDF and MDA-MB-231 cells were grown in Dulbecco’s Modified Eagle’s Medium (DMEM) medium whereas U-87 MG was grown in DMEM/F12 medium with 10% fetal bovine serum (HyClone™ Fetal Bovine Serum (U.S.), Characterized) and 100 units/mL of penicillin/streptomycin at 37 °C in a humidified atmosphere in 5% CO2 incubator.
The HDF, MDA-MB-231 and U-87 MG cell lines were seeded into 96-well microtiter plates (Corning®, Saint Louis, MO, USA) at a density of 1.2*104, 1.0*104 and 1.5*104 cells/wells, respectively. After overnight incubation, cells were exposed to increased concentrations of the ethanolic extract of D. sericea Vahl (125, 625 and 1.25 mg/mL) in cell culture medium. After incubation times of 24, 48 and 72 h, medium was replaced by a new fresh one containing 0.5mg/mL of MTT. After 2 h, at 37°C in 5% CO2 cells were dissolved in DMSO. Absorbance was read at 570 nm by a spectrophotometer plate reader. The data were expressed as absorbance relative to untreated cells in the same experiment and standardized to 100%. All data points were performed in triplicate and in, at least, three independent experiments.
2.10. Statistical Analysis
For the antioxidant activity studies, data are reported as the average and standard error of at least six replicates from two experiments. Statistical analysis was performed with GraphPadPrism™ software (GraphPad Software Inc., San Diego, CA, USA).
Differences among treatments were evaluated by one-way analysis of variance (one-way ANOVA), followed by Dunnett’s multiple comparison posttest. A p < 0.05 was considered as significant. The “Hill equation”: E = Emax/ 1 + (10 LogEC50/A)HillSlope, where E is the effect at a given concentration, Emax is the maximum activity, IC50 is the concentration producing a 50% inhibitory response, A is the agonist concentration, and Hill Slope is the slope of the agonist, was applied to obtain a concentration–response curve. The Pearson correlation coefficient was used to calculate the correlation between two variables, while statistical significance was determined by the two-tailed t-test.
For the cytotoxic activity studies, the normally distributed data were reported as the mean ± standard deviation and compared using analysis of variance (ANOVA). All experiments were quadruplicate; p < 0.05 was considered statistically significant.
3. Results and Discussion
3.1. Phytochemistry
The phytochemical analysis of the D. sericea flowering aerial parts collected in central Italy led to the identification of eleven compounds: α-linolenic acid (1), tri-linoleoyl-sn-glycerol (2), pheophorbide a ethyl ester (3), pilloin (4), sinensetin (5), yuanhuanin (6), rutamontine (7), syringin (8), p-coumaric acid (9), p-anisic acid (10) and caffeic acid (11) (Figure 2).
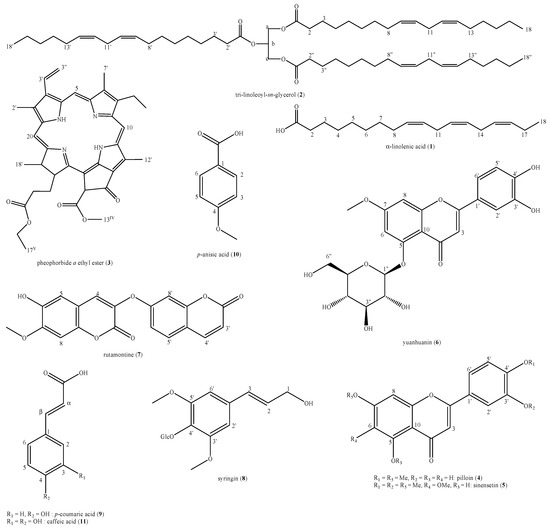
Figure 2.
Structures of the identified compounds in Daphne sericea Vahl flowering aerial parts.
These compounds belong to six different major classes of natural compounds i.e., fatty acids and derived triglycerides (1,2), tetrapyrrole derivative of chlorin family, also considered as degradation product of chlorophylls (3), flavonoids (4–6), coumarins (7), lignols (8) and natural organic acids (9–11).
3.2. Chemotaxonomy
To the best of our knowledge, compounds 1–4, 7–8 and 10 have been identified in D. sericea for the first time, during this work. In addition, sinensetin (5) represents a newly identified component of the entire Thymelaeaceae family. Indeed, this compound is more typical of another species i.e., Orthosiphon aristatus (Blume) Miq. (Lamiaecae family) [31,32,33,34]. This result is very important from the chemosystematic point of view since Lamiaceae and Thymelaceae are quite distant families. Therefore, it is possible that the biogenetic pathway leading to compound (5) evolved independently in Thymeleaceae and Lamiaceae. Obviously, further studies are necessary to confirm this hypothesis. On the contrary, p-coumaric acid (9) and caffeic acid (11) have been already isolated in this species [8]. Pheophorbide a ethyl ester (3), which may also be an artifact due to the extraction methodology adopted, has been already isolated from D. oleoides Schreb. [15]. As for pillion (4), it has been reported before only in D. aurantiaca Diels [35]. Yuanhuanin (6) was originally recognized in D. sericea [8] and then isolated also from D. koreana Nakai and D. pedunculata H.F.Zhou ex C.Y.Chang (syn. of D. esquirolii subsp. pedunculata (H.F.Zhou ex C.Yung Chang) Halda) [36]. Moreover, rutamontine (7) is quite a rare compound of Daphne species. In fact, its occurrence has been reported only in D. acutiloba Rehder [37] and D. oleoides Schreb. [38]. Syringin (8) has been identified in D. oleoides Schreb. [15,38] but also in many other Daphne species like D. arisanensis Hayata [39], D. kiusiana var. atrocaulis (Rehder) F.Maek. [40], D. tangutica Maxim. [41], D. mucronata subsp. linearifolia (Hart) Halda. [42] and D. feddei H.Lév. [43], thus resulting a highly represented metabolite within the genus. Lastly, p-anisic acid (10) has been previously isolated from Daphne species only from D. holosericea (Diels) Hamaya [44].
3.3. Biological Activities
3.3.1. Antioxidant Activity
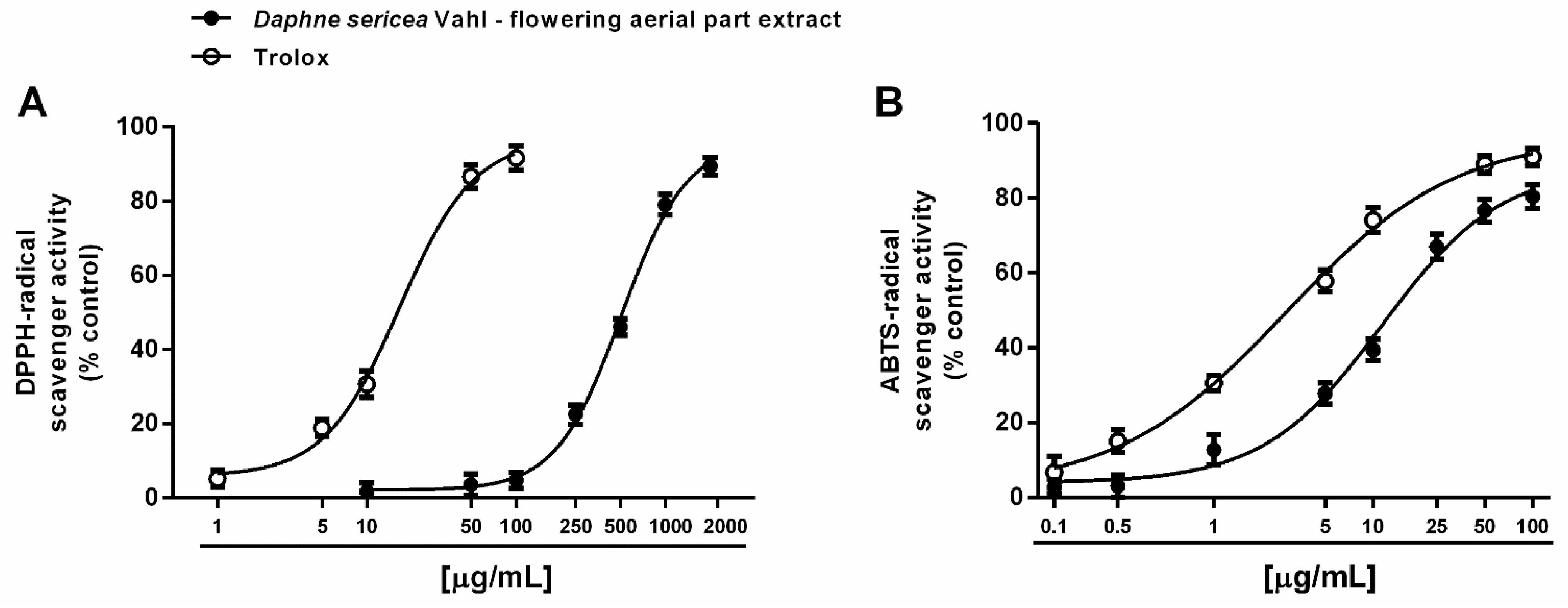
Both direct and indirect antioxidant properties of the ethanolic extract from the flowering aerial parts of D. sericea were evaluated. A direct antioxidant power has been assessed by measuring the ability of the extract to neutralize the synthetic DPPH· and ABTS·+ radicals. Under our experimental conditions, D. sericea flowering aerial parts extract exhibited scavenging properties towards both radicals, although with a different potency. Indeed, it inhibited DPPH·, starting from 250 μg/mL achieving a maximum effect at the concentration of 2000 μg/mL (Figure 3A), while the inhibition of ABTS·+ radical already appeared at 5 μg/mL and became complete at 100 μg/mL (Figure 3B).
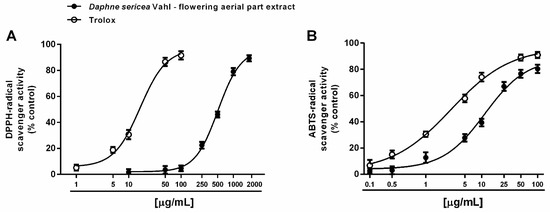
Figure 3.
Scavenger activity of the ethanolic extract obtained from the flowering aerial parts of Daphne sericea Vahl and the positive control Trolox against DPPH· (A) and ABTS·+ (B) radicals. Each data point represents the average and standard error of at least 6 replicates.
This different behavior was also confirmed by the IC50 values, which highlighted at least a 40-fold higher potency of the extract against ABTS·+ with respect to DPPH· (Table 1). The ABTS·+ radical scavenging activity was four-fold lower than that of the positive control Trolox, with a marked 30-fold lower potency against DPPH· (Table 1). The Pearson correlation analysis highlighted that these activities were not significantly correlated (Table 2).

Table 1.
The concentration producing a 50% inhibitory response (IC50) values of the ethanolic extract obtained from the flowering aerial parts of Daphne sericea Vahl and the positive controls in the antioxidant activity assays.

Table 2.
Pearson correlation coefficient among antioxidant activity assays for the flowering aerial parts extract from Daphne sericea Vahl.
Electron- or hydrogen-transfer mechanisms are required for scavenging both DPPH· and ABTS·+ radicals, although with a different specificity and kinetic profile [45]. Indeed, DPPH· is usually neutralized by small molecules, being sterically limited the access to the radical site to bulky compounds [45]. For instance, carotenoids have been reported to be not effective against DPPH· [46]. Conversely, ABTS·+ can react with both lipophilic and hydrophilic molecules, with poor selectivity for hydrogen-atom donors, thus being widely applied to characterize the radical scavenging power of different herbal extracts and vegetables. Considering the different behavior of DPPH· and ABTS·+ radicals and the specific ability of D. sericea extract against ABTS·+, the contribution of a complex pool of phytochemicals, including both lipophilic and hydrophilic, and either small or bulky structures, can be assumed; however, to confirm this hypothesis, further studies are required.
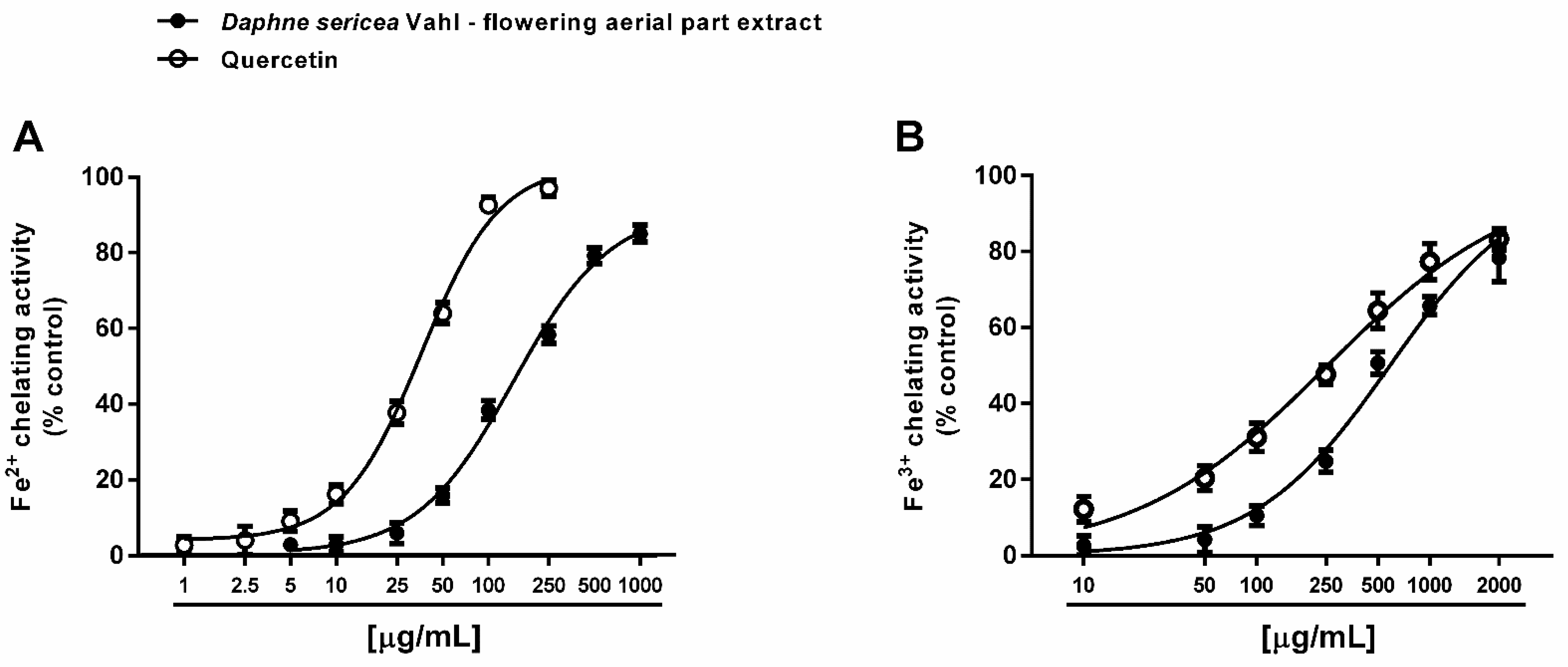
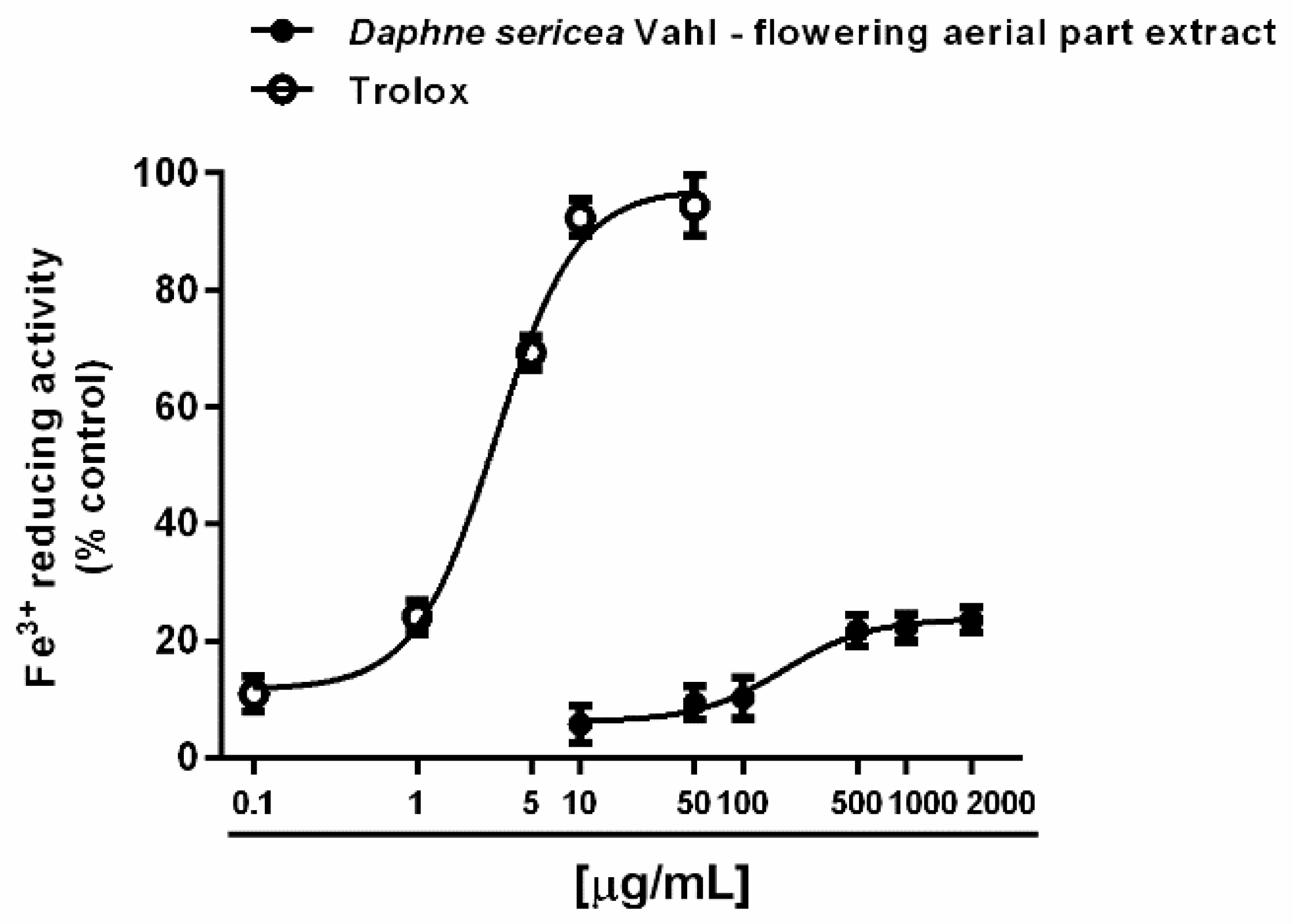
Under our experimental conditions, D. sericea flowering aerial parts extract was also able to interact with both ferrous and ferric ions. Particularly, it exhibited significant chelating properties starting from the concentration of 50 μg/mL (Figure 4A,B), although with a low reducing efficacy (Figure 5).
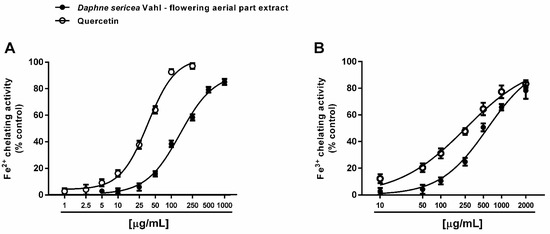
Figure 4.
Chelating activity of the ethanolic extract obtained from the flowering aerial parts of Daphne sericea Vahl and the positive control quercetin against ferrous (A) and ferric (B) ions. Each data point represents the average and standard error of at least 6 replicates.
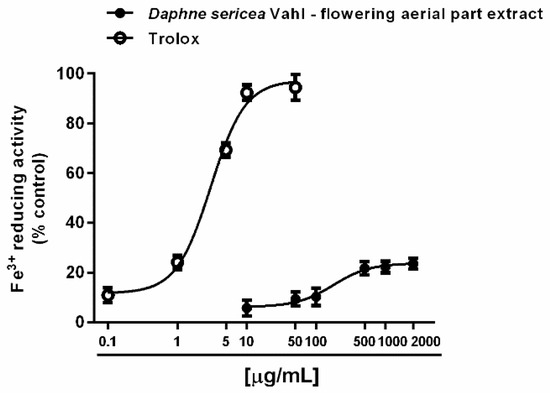
Figure 5.
Ferric ion reducing activity of the ethanolic extract obtained from the flowering aerial parts of Daphne sericea Vahl and the positive control trolox. Each data point represents the average and standard error of at least 6 replicates.
Comparing the IC50 values, the ferrous ion chelating ability of the extract was almost four-fold more potent than that of ferric ions, but significantly lower than those of the positive control quercetin. As estimated by the Pearson analysis, the ferrous and ferric ion chelating activities resulted significantly correlated each other and with DPPH· scavenging activity (at least p < 0.05) (Table 2).
Colorimetric determinations of polyphenols highlighted that the extract was characterized by a 3.4 polyphenols/tannins ratio, along with about 0.8% of total flavonoid compounds (Table 3).

Table 3.
Amounts of total phenolics and tannins, expressed as tannic acid equivalents (TAE), and flavonoids, expressed as quercetin equivalents (QE), in the ethanolic extract obtained from the flowering aerial parts of Daphne sericea Vahl. Data are reported as the average and standard error (SE) of at least six replicates from two experiments.
The antioxidant properties of D. sericea Vahl extracts have been scantily investigated, despite a higher interest for different Daphne species [47,48,49,50]. Recently, Tongur et al. [9] evaluated the antioxidant properties of the methanolic and acetone leaf extracts of D. sericea (ESM and ESA, respectively) in relation to its polyphenolic constituents, highlighting that total flavonoids and polyphenols (121.3 ± 19.7 and 602.4 ± 23.6 μg per mg of extract, expressed as rutin and gallic acid equivalents, respectively) were mainly recovered by methanolic extraction. This composition was found to be associated to interesting scavenging properties of extracts against both DPPH· and ABTS·+ radicals, with a higher potency of ESM. This evidence agrees with our findings, although our D. sericea ethanolic extract exhibited a more potent ABTS·+ scavenging activity (IC50 almost 14-fold lower than that of ESM), despite its eight-fold lower potency against DPPH·. Furthermore, Zengin et al. [51] characterized the antioxidant power of different extracts obtained from the leaves of the Turkish D. serica variety and highlighted that total flavonoids and polyphenols were mainly recovered by ethyl acetate and ethanol with respect to hexane and methanol; nevertheless, the methanolic extract exhibited higher potency as a DPPH· radical scavenger. This evidence suggests that the extraction method is crucial to recover bioactive compounds and can be responsible for the differences in the extract potency. Indeed, it is known that several factors, including the origin of plant material, harvesting time, extraction conditions (e.g., solvent, temperature, pH, extraction time) and methodology (e.g., classical maceration, Soxhlet extraction), and solvent evaporation, can affect the phytochemical composition of an extract and its bioactivities [52]. Further studies could clarify if modifying extraction conditions is a suitable strategy for better recovering the bioactive constituents of D. sericea flowering aerial parts, thus enhancing its antioxidant power.
Under our experimental conditions, D. sericea extract also exhibited iron chelating activities despite a weak ferric reducing power. Despite our results, [51] found that all the extracts from D. sericea, especially the methanolic and ethanolic ones, were able to reduce both ferric ions, although with a lower potency with respect to the standard antioxidants BHA (butylated hydroxyanisole)and BHT (butylated hydroxytoluene). Conversely, to the best of our knowledge, the chelating activity of D. sericea has been not previously published.
Iron is involved in metal-catalyzed oxidation of biological substrates and in the generation of reactive oxygen species (ROS) species through the Fenton cascade. The species can oxidate cell macromolecules, such as DNA, RNA, phospholipid biomembrane and proteins, thus leading to different chronic diseases, such as neurodegeneration, diabetes, cardiovascular and metabolic ailments, and cancer [53,54,55]. Therefore, an increasing interest has been devoted to the identification of novel antioxidant agents to be exploited in the prevention and treatment of such diseases, although clinical evidence of benefits and bioavailability remain important issues to be considered [55]. Electron donating compounds and chelating agents can affect Fenton reaction and lower ROS generation, thus acting as indirect antioxidant agents [56].
Several polyphenols and flavonoids, especially ellagic acid, rutin and catechin have been reported to possess a marked chelating power, which seems to be responsible for their antioxidant and cytoprotective properties [57].
Among the identified compounds in D. sericea flowering aerial parts extract, pilloin (4) has been previously reported to possess antioxidant properties likely due to the presence of a catechol ring in the structure [58]. Similarly, antioxidant activities have been reported for sinensetin (5) and syringin (8) although both compounds exhibited weak or null DPPH· scavenging [59,60,61]. Conversely, p-coumaric acid (9), p-anisic acid (10) and caffeic acid (11) are known to possess marked scavenging and chelating antioxidant properties [62,63,64]. These activities could be due to the presence of multiple functional groups, such as hydroxy or methoxy substituents with a carboxyl group as explained by Ivanova et al. [65]. Based on this evidence, the contribution of these compounds to the antioxidant activity of D. sericea extract can be hypothesized: this bioactivity could be a result of the subtle interactions of these phytochemicals, although the involvement of the entire phytocomplex cannot be excluded. Further studies could clarify the role of each bioactive constituent in the antioxidant properties of D. sericea flowering aerial parts extract.
3.3.2. Cytotoxic Activity
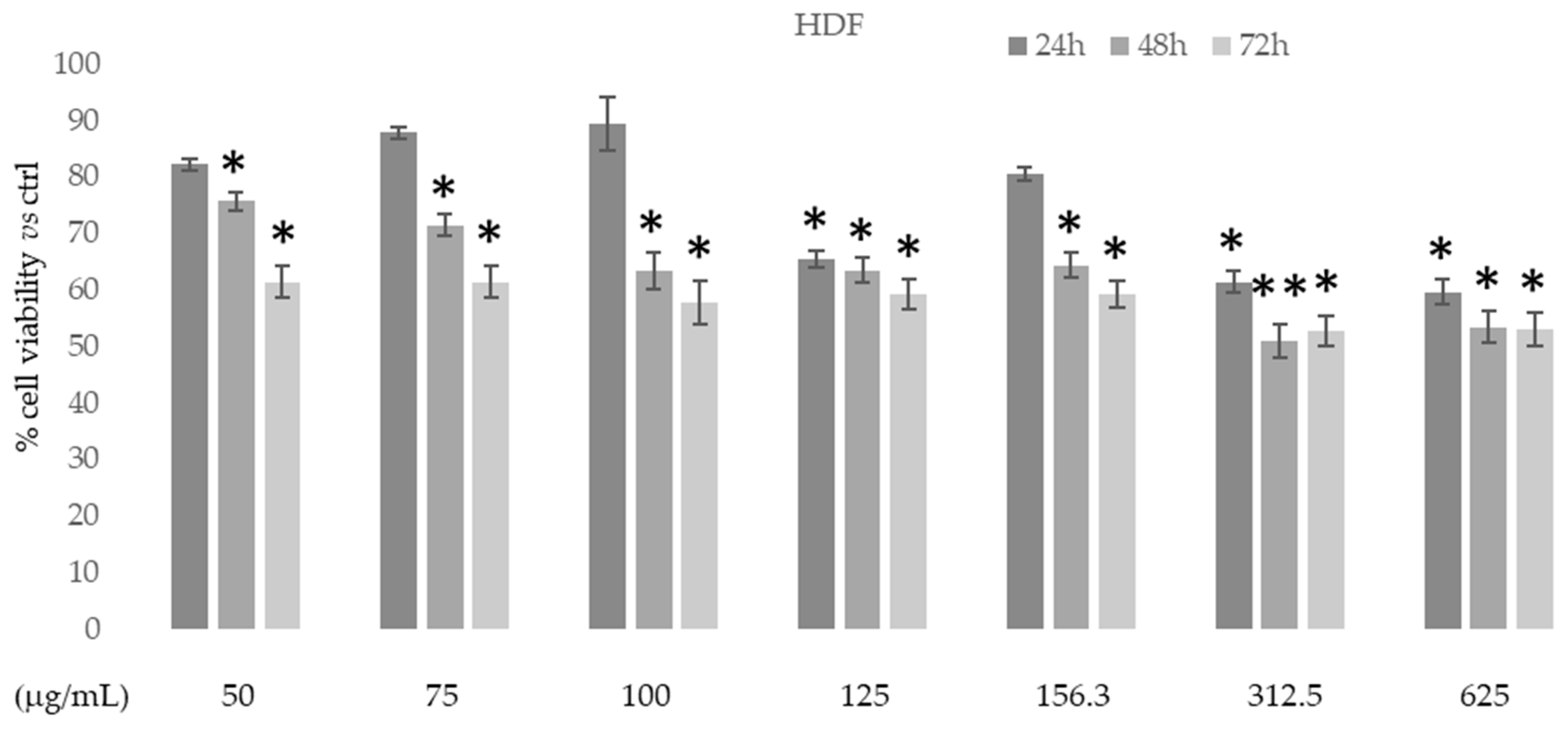
The in vitro cytotoxic activity of the ethanolic extract of D. sericea flowering aerial parts was tested on human fibroblast cell HDF (normal cells) using MTT colorimetric assay at different concentrations and times. Figure 6 shows that in this normal cell line the extract had no cytotoxic effects at all concentrations and times used.
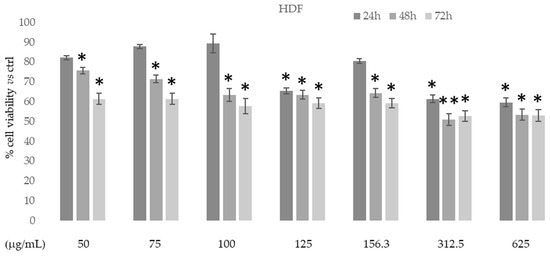
Figure 6.
Cytotoxic activity of Daphne sericea Vahl flowering aerial parts extract against human fibroblast cell HDF treated for 24, 48, and 72h. The percentage of cell viability (assayed by MTT test) was calculated considering the value of the control as 100%. Results are expressed as the mean value ± SD of quadruplicate determinations from 3 independent experiments. Asterisks indicate a significant reduction in cell viability in treated samples with respect to control cells (one-way ANOVA test, * p < 0.05, ** p < 0.01).
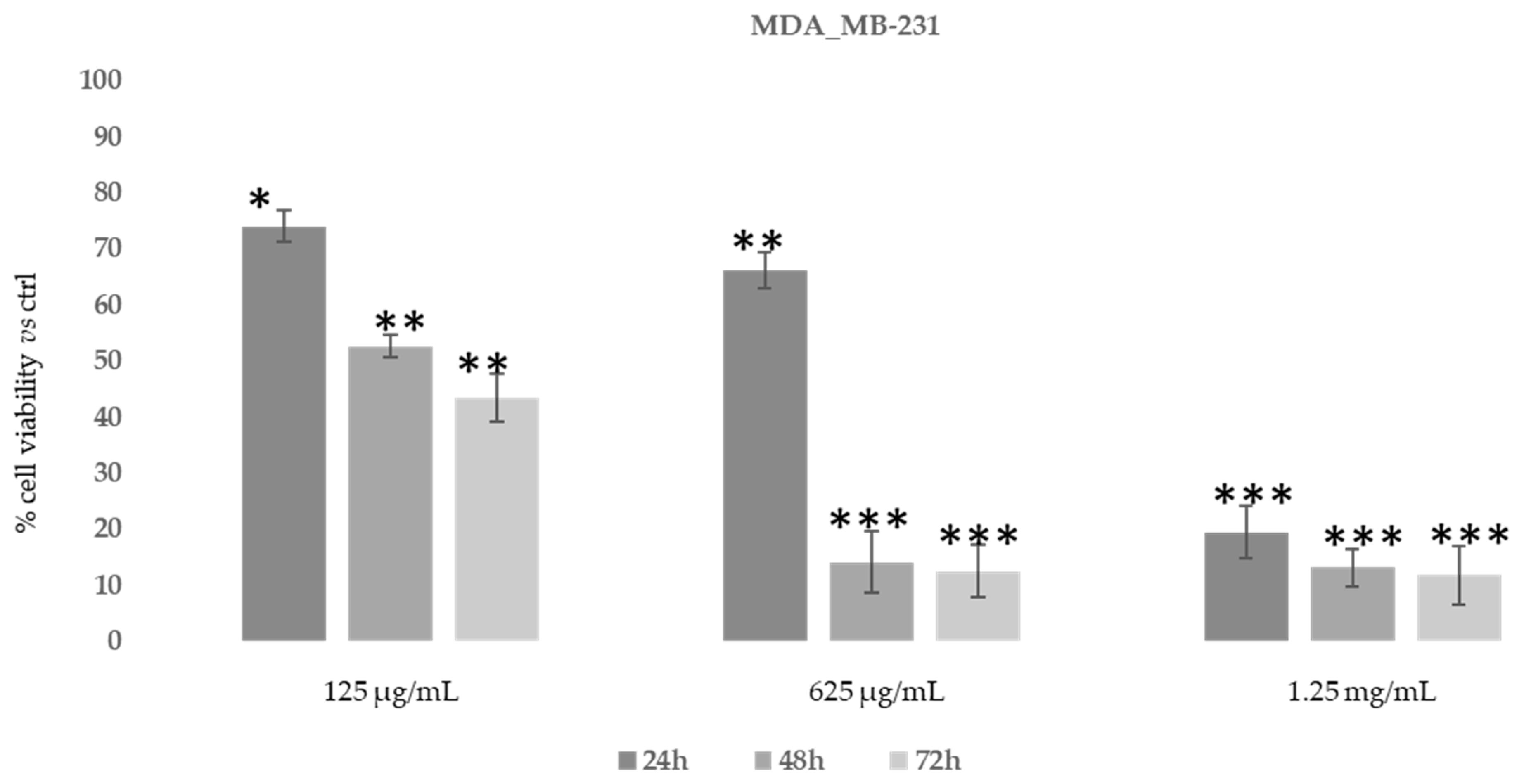
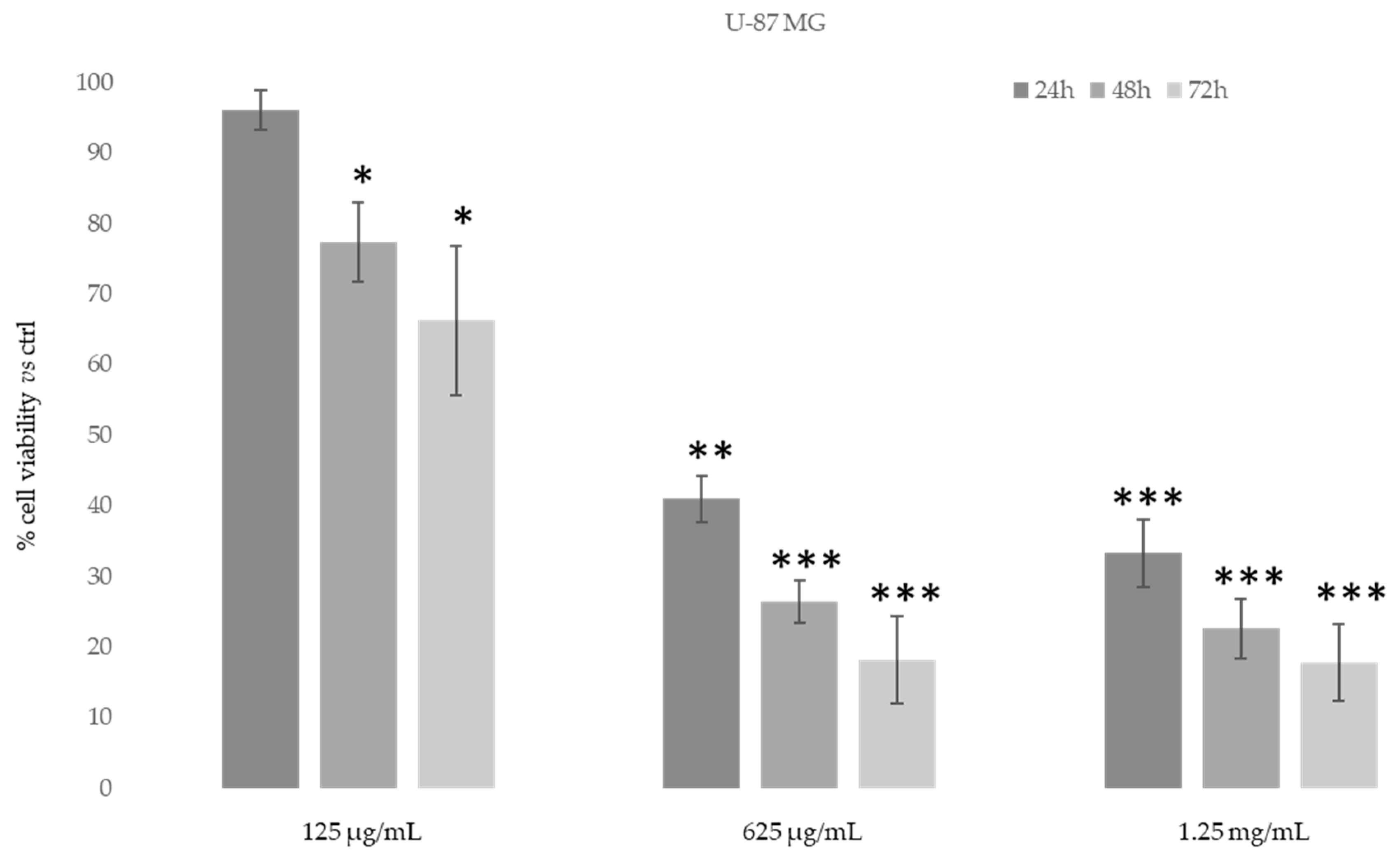
We also evaluated the inhibitory concentration to achieve 50% cell death (IC50) for D. sericea compound in two human cancer cell lines (MDA-MB-231 and U-87 MG) using MTT assay at the same concentrations of fibroblast cells and after 48h of treatment. The IC50 value after 48h of treatment with Daphne sericea Vahl found that the ethanolic extract was more effective in the MDA-MB-231 cell line (IC50 equal to 75 μg/mL) than in U-87 MG (IC50 equal to 150 μg/mL).
Then, in order to study the anti-proliferative properties of D. sericea compound we evaluated the in vitro cytotoxic activity in the same human cancer cell lines using MTT assay (Figure 7 and Figure 8).
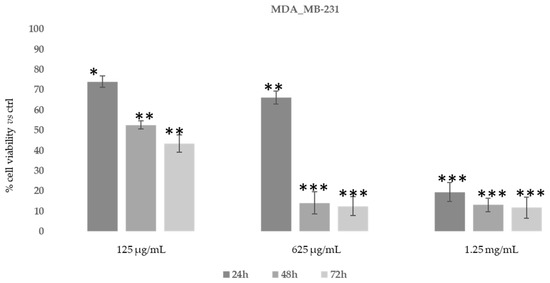
Figure 7.
Cytotoxic activity of Daphne sericea Vahl flowering aerial parts extract against the MDA-MB-231 cancer cell line treated for 24, 48 and 72 h. The percentage of cell viability (assayed by MTT test) was calculated considering the value of the control as 100%. Results are expressed as the mean value ± SD of quadruplicate determinations from 3 independent experiments. Asterisks indicate a significant reduction in cell viability in treated samples with respect of control cells (one-way ANOVA test, * p < 0.05, ** p < 0.01; *** p < 0.001).
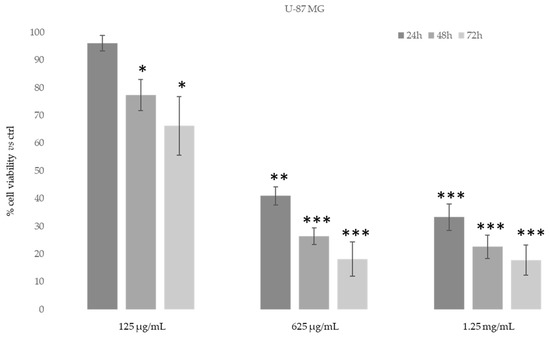
Figure 8.
Cytotoxic activity of Daphne sericea Vahl flowering aerial parts extract against the U-87 MG cancer cell line treated for 24, 48 and 72 h. The percentage of cell viability (assayed by MTT test) was calculated considering the value of the control as 100%. Results are expressed as the mean value ± SD of quadruplicate determinations from 3 independent experiments. Asterisks indicate a significant reduction in cell viability in treated samples with respect of control cells (one-way ANOVA test, * p < 0.05, ** p < 0.01; *** p < 0.001).
As shown in Figure 7, after 48–72 h, the ethanolic extract at the concentration of 625 μg/mL was able to induce an evident reduction in MDA-MB-231 cells’ proliferative capacity (85–90%). However, MDA-MB-231 cells also showed the same reduction after 24 h of treatment with 1.25 mg/mL of D. sericea extract. Instead, the U-87 MG cell line revealed to be less drastically susceptible to the anti-proliferative effect of D. sericea flowering aerial parts extract both at the concentrations of 625 μg/mL and 1.25 mg/mL after 24, 48 and 72 h of treatment (Figure 8).
Our results also revealed that the ethanolic extract of D. sericea flowering aerial parts, in a pathological condition such as cancer, showing important anti-proliferative activities against the two different human cancer cell lines studied. The cytotoxic effects observed in the D. sericea Vahl flowering aerial parts extract against the two studied cancer cell lines may also be explained by the total phytocomplex activity. Indeed, most of the identified compounds have already shown relevant antitumor properties against several cell lines [63,66,67,68,69,70,71,72,73]. Among these, sinensetin (5) seems likely to be the most responsible for the activity against MDA-MB-231 proliferation since it was observed to decrease the viability of MDA-MB-231 cells in a concentration and time dependent manner [74], whereas the poly-unsaturated fatty acids, p-anisic acid (10) and caffeic acid (11) seem to be the most effective compounds against U87-MG [63,66,75]. Then, these biological activities performed on the same extract showed good effects, in accordance with other previously published results, for what concerns the antioxidant tests and no toxic activities under normal conditions on a human normal fibroblast cell line. These results suggest a potential use of this natural product for integrative therapies thanks to its antioxidant properties. Moreover, the same compound at different concentrations has demonstrated good cytotoxic effects against the two studied cancer cell lines and it may be used in clinical practice as chemosensitizer to enhance the response to conventional therapy in future. Nevertheless, further pharmacological studies on the extract and on the single isolated compounds in this sense are necessary to verify this hypothesis, although the reported results are very promising.
4. Conclusions
The first phytochemical analysis of the ethanolic extract of Daphne sericea Vahl flowering aerial parts collected in Italy evidenced the presence of eleven compounds, belonging to six different classes of natural compounds. Among these, some were identified in the species and in the family for the first time, whereas the presence of the others confirms the correct botanical classification of the studied specimen as a member of the Daphne genus. The biological activities performed on the same extract showed good effects, in accordance with other previously published results, in terms of antioxidant activity and generally good cytotoxic effects against the two studied cancer cell lines. These findings suggest the possibility to list this species among the important medicinally and pharmacologically active Daphne species even if further studies are necessary to finally validate this hypothesis.
Author Contributions
Conceptualization, C.F., D.D.V., M.D.C., G.C., M.S., A.B.; methodology, C.F., A.V., F.S., P.T., M.F., A.D.S., A.S.; M.C.; formal analysis and investigation: C.F., A.V., D.D.V., F.S., P.T., M.F., M.D.C., G.C., A.D.S., A.S., M.C.; data analysis, C.F., A.V., P.T., M.F., A.D.S., A.S., M.C.; Writing—Review and editing, C.F., A.V., D.D.V., F.S., P.T., M.F., M.D.C., G.C., A.D.S., A.S., M.C., A.G., M.S., A.B.; supervision, A.G., M.S., A.B.; All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by Istituto Superiore di Sanità, ISS (Ministry of Health - ISS funding).
Institutional Review Board Statement
Not applicable since this study did not involve human or animals.
Informed Consent Statement
Not applicable since this study did not involve human.
Data Availability Statement
All the data are available in this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Vahl, M. Symbola Ebotanicae, Sive Plantarum, Tam Earum, Quas in Itinere, in Primis Orientali, Collegit Petrus Forskål, Quam Aliarum, Recen-tius Detectarum, Exactiores Descriptiones, nec non Observationes Circa Quasdam Plantas Dudum Cognitas; Pars prima; Nicolaus Möller & fil.: Copenhagen, Denmark, 1970; p. 28. [Google Scholar]
- Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. Flora Europaea; Cambridge University Press: Cambridge, UK, 1968; Volume 2. [Google Scholar]
- Pignatti, S.; Guarino, R.; La Rosa, M. Flora d’Italia, 2nd ed.; Edagricole—Edizioni Agricole di New Business Media: Milano, Italy, 2017; Volume 2. [Google Scholar]
- Pedrol, J. Thymelaeaceae. Euro+MedPlantbase—The Information Resource for Euro-Mediterranean Plant Diversity. Thymelaeaceae Juss. 2011. Available online: http://ww2.bgbm.org/euroPlusMed/PTaxonDetail.asp?UUID=0B0F7461-92DE-47A0-854C-5E9E595081C2 (accessed on 25 January 2021).
- Bartolucci, F.; Peruzzi, L.; Galasso, G.; Albano, A.; Alessandrini, A.; Ardenghi, N.M.G.; Astuti, G.; Bacchetta, G.; Ballelli, S.; Banfi, E.; et al. An updated checklist of the vascular flora native to Italy. Plant Biosyst. 2018, 152, 179–303. [Google Scholar] [CrossRef]
- Güzel, Y.; Güzelşemme, M.; Miski, M. Ethnobotany of medicinal plants used in Antakya: A multicultural district in Hatay province of Turkey. J. Ethnopharmacol. 2015, 174, 118–152. [Google Scholar] [CrossRef]
- Türkmen, N.; Kirici, S.; Özgüven, M.; İnan, M.; Kaya, D.A. An investigation of dye plants and their colourant substances in the eastern Mediterranean region of Turkey. Bot. J. Linn. Soc. 2004, 146, 71–77. [Google Scholar] [CrossRef]
- Moshiashvili, G.; Tabatadze, N.; Mshvildadze, V. The genus Daphne: A review of its traditional uses, phytochemistry and pharmacology. Fitoterapia 2020, 143, 1–39. [Google Scholar] [CrossRef] [PubMed]
- Tongur, T.; Erkan, N.; Ayranci, E. Investigation of the composition and antioxidant activityof acetone and methanol extracts of Daphne sericea L. and Daphne gnidioides L. J. Food Sci. Technol. 2018, 55, 1396–1406. [Google Scholar] [CrossRef] [PubMed]
- Ulubelen, A.; Bucher, R.; Mabry, T.J. Flavone 5-O-glucosides from Daphne sericea. Phytochemistry 1982, 21, 801–803. [Google Scholar] [CrossRef]
- Kupchan, S.M.; Baxter, R.L. Mezerein: Antileukemic principle isolated from Daphne mezereum L. Science 1975, 21, 652–653. [Google Scholar] [CrossRef]
- Bahadırlı, N.P.; Türkmen, M. Essential oil compositions of Daphne sericea vahl. flowers with hydrodistillation method. Am. J. Essent. Oils Nat. Prod. 2020, 8, 6–9. [Google Scholar]
- Venditti, A. What is and what should never be: Artifacts, improbable phytochemicals, contaminants and natural products. Nat. Prod. Res. 2020, 34, 1014–1031. [Google Scholar] [CrossRef]
- Sciubba, F.; Di Cocco, M.E.; Gianferri, R.; Impellizzeri, D.; Mannina, L.; De Salvador, F.R.; Venditti, A.; Delfini, M. Metabolic profile of different Italian cultivars of hazelnut (Corylus avellana) by nuclear magnetic resonance spectroscopy. Nat. Prod. Res. 2014, 28, 1075–1081. [Google Scholar] [CrossRef]
- Venditti, A.; Serrilli, A.M.; Di Cecco, M.; Ciaschetti, G.; Bianco, A. Coumarins and other components of Daphne oleoides Schreb. subsp. oleoides from Majella National Park. Biochem. Syst. Ecol. 2019, 83, 39–46. [Google Scholar] [CrossRef]
- Tsai, Y.S.; Chen, C.T.; Yeh, H.C.; Li, H.T.; Chen, C.Y. Chemical constituents of the leaves of Elaeagnus grandifolia. Chem. Nat. Compd. 2019, 55, 334–336. [Google Scholar] [CrossRef]
- Nagase, H.; Omae, N.; Omori, A.; Nakagawasai, O.; Tadano, T.; Yokosuka, A.; Sashida, Y.; Mimaki, Y.; Tamakuni, T.; Ohizumi, Y. Nobiletin and its related flavonoids with CRE-dependent transcription-stimulating and neuritegenic activities. Biochem. Biophys. Res. Commun. 2005, 337, 1330–1336. [Google Scholar] [CrossRef]
- Kabouche, Z.; Benkiki, N.; Seguin, E.; Bruneau, C. A new dicoumarinyl ether and two rare furocoumarins from Ruta montana. Fitoterapia 2003, 74, 194–196. [Google Scholar] [CrossRef]
- Kort, R.; Vonk, H.; Xu, X.; Hoff, W.D.; Crielaard, W.; Hellingwerf, K.J. Evidence for trans-cis isomerization of the p-coumaric acid chromophores as the photochemical basis of the photocycle of photoactive yellow protein. FEBS Lett. 1996, 382, 73–78. [Google Scholar] [CrossRef]
- Xu, W.; Jin, H.; Zhang, W.; Hu, X.; Zhang, W.; Fu, J.; Su, J.; Yan, S.; Shen, Y. Chemical constituents from Daphne pedunculata. Chem. Nat. Compd. 2009, 45, 417–419. [Google Scholar] [CrossRef]
- Saha, R.; Ghosh, A.; Saha, B. Kinetics of micellar catalysis on oxidation of p-anisaldehyde to p-anisic acid in aqueous medium at room temperature. Chem. Eng. Sci. 2013, 99, 23–27. [Google Scholar] [CrossRef]
- Frezza, C.; Venditti, A.; Rossi, G.; Serafini, I.; Pitorri, M.; Ciccòla, A.; Foddai, S.; Bianco, A.; Serafini, M. Phytochemical study on the leaves of Wollemia nobilis. Biochem. Syst. Ecol. 2017, 74, 63–66. [Google Scholar] [CrossRef]
- Venditti, A.; Frezza, C.; Maggi, F.; Lupidi, G.; Bramucci, M.; Quassinti, L.; Giuliani, C.; Cianfaglione, K.; Papa, F.; Serafini, M.; et al. Phytochemistry, micromorphology and bioactivities of Ajuga chamaepitys (L.) Schreb. (Lamiaceae, Ajugoideae): Two new harpagide derivatives and an unusual iridoid glycosides pattern. Fitoterapia 2016, 113, 35–43. [Google Scholar] [CrossRef]
- Venditti, A.; Maggi, F.; Quassinti, L.; Bramucci, M.; Lupidi, G.; Ornano, L.; Ballero, M.; Sanna, C.; Bruno, M.; Rosselli, S.; et al. Bioactive constituents of Juniperus turbinata Guss. from La Maddalena Archipelago. Chem. Biodivers. 2018, 15, 1–15. [Google Scholar] [CrossRef]
- Frezza, C.; Venditti, A.; Sciubba, F.; Tomai, P.; Antonetti, M.; Franceschin, M.; Di Cocco, M.E.; Gentili, A.; Delfini, M.; Serafini, M.; et al. Phytochemical profile of Euphorbia peplus L. collected in Central Italy and NMR semi-quantitative analysis of the diterpenoid fraction. J. Pharma Biomed. Anal. 2018, 160, 152–159. [Google Scholar] [CrossRef]
- Venditti, A.; Frezza, C.; Vincenti, F.; Brodella, A.; Sciubba, F.; Montesano, C.; Franceschin, M.; Sergi, M.; Foddai, S.; Di Cocco, M.E.; et al. A syn-ent-labdadiene derivative with a rare spiro-β-lactone function from the male cones of Wollemia nobilis. Phytochemistry 2019, 158, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Venditti, A.; Frezza, C.; Rossi, G.; Serafini, I.; Ciccola, A.; Sciubba, F.; Foddai, S.; Tomassini, L.; Bianco, A.; Serafini, M. A new bicyclic monoterpene glucoside and a new biflavone from the male reproduction organs of Wollemia nobilis. Fitoterapia 2019, 133, 62–69. [Google Scholar] [CrossRef]
- Venditti, A.; Ukwueze, S.E. A possible glycosidic benzophenone with full substitution on B-ring from Psidium guajava leaves. Nat. Prod. Res. 2017, 31, 739–741. [Google Scholar] [CrossRef] [PubMed]
- Di Sotto, A.; Vecchiato, M.; Abete, L.; Toniolo, C.; Giusti, A.M.; Mannina, L.; Locatelli, M.; Nicoletti, M.; Di Giacomo, S. Capsicum annuum L. var. Cornetto di Pontecorvo PDO: Polyphenolic profile and in vitro biological activities. J. Funct. Foods 2018, 40, 679–691. [Google Scholar] [CrossRef]
- Di Sotto, A.; Checconi, P.; Celestino, I.; Locatelli, M.; Carissimi, S.; De Angelis, M.; Rossi, V.; Limongi, D.; Toniolo, C.; Martinoli, L.; et al. Antiviral and antioxidant activity of a hydroalcoholic extract from Humulus lupulus L. Oxid. Med. Cell. Longev. 2018, 2018, 1–14. [Google Scholar] [CrossRef]
- Akowuah, G.A.; Zhari, I.; Norhayati, I.; Sadikun, A.; Khamsah, S.M. Sinensetin, eupatorin, 3′-hydroxy-5,6,7,4′-tetramethoxyflavone and rosmarinic acid contents and antioxidative effect of Orthosiphon stamineus from Malaysia. Food Chem. 2004, 87, 559–566. [Google Scholar] [CrossRef]
- Yam, M.F.; Lim, V.; Salman, I.M.; Ameer, O.Z.; Ang, L.F.; Rosidah, N.; Abdulkarim, M.F.; Abdullah, G.Z.; Basir, R.; Sadikun, A.; et al. HPLC and anti-inflammatory studies of the flavonoid rich chloroform extract fraction of Orthosiphon stamineus leaves. Molecules 2010, 15, 4452–4466. [Google Scholar] [CrossRef] [PubMed]
- Laavola, M.; Nieminen, R.; Yam, M.F.; Sadikun, A.; Asmawi, M.Z.; Basir, R.; Welling, J.; Vapaatalo, H.; Korhonen, R.; Moilanen, E. Flavonoids eupatorin and sinensetin present in Orthosiphon stamineus leaves inhibit inflammatory gene expression and STAT1 activation. Planta Med. 2012, 78, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Ismail, Z. Quantification and enrichment of sinensetin in the leaves of Orthosiphon stamineus. Arab. J. Chem. 2016, 9, 1338–1341. [Google Scholar] [CrossRef]
- Liang, S.; Tian, J.-M.; Feng, Y.; Liu, X.-H.; Xiong, Z.; Zhang, W.-D. Flavonoids from Daphne aurantiaca and their inhibitory activities against nitric oxide production. Chem. Pharm. Bull. 2011, 59, 653–656. [Google Scholar] [CrossRef]
- Xu, W.C.; Shen, J.G.; Jiang, J.Q. Phytochemical and biological studies of the plants from the genus Daphne. Chem. Biodivers. 2011, 8, 1215–1233. [Google Scholar] [CrossRef]
- Huang, S.Z.; Ma, Q.Y.; Liu, Y.Q.; Zhou, J.; Zhao, Y.X. Chemical constituents from Daphne acutiloba. China J. Chin. Mat. Med. 2013, 38, 64–69. [Google Scholar]
- Venditti, A.; Sanna, C.; Lorenzetti, L.M.; Ballero, M.; Bianco, A. New coumarinyl ethers in Daphne oleoides SCHREB. collected from Sardinia Island. Chem. Biodivers. 2017, 14, 1–8. [Google Scholar] [CrossRef]
- Niwa, M.; Sugino, H.; Takashima, S.; Sakai, T.; Wu, Y.-C.; Wu, T.-S.; Kuoh, C.-S. A new coumarin glucoside from Daphne arisanensis. Chem. Pharm. Bull. 1991, 39, 2422–2424. [Google Scholar] [CrossRef]
- Wei, Z.; Weidong, Z.; Tingzhao, L.; Runhui, L.; Peng, F.; Huiliang, L. Phenolic constituents from Daphne odora var. atrocaulis. Nat. Prod. Res. Dev. 2005, 17, 26–28. [Google Scholar]
- Hu, X.-J.; Jin, H.-Z.; Yan, L.; Zhang, W.-D. Chemical constituents of Daphne retusa. Nat. Prod. Res. Dev. 2011, 23, 20–24. [Google Scholar]
- Malafronte, N.; Vassallo, A.; Dal Piaz, F.; Bader, A.; Braca, A.; De Tommasi, N. Biflavonoids from Daphne linearifolia Hart. Phytochem. Lett. 2012, 5, 621–625. [Google Scholar] [CrossRef]
- Liang, S.; Shen, Y.-H.; Tian, J.-M.; Wu, Z.-J.; Jin, H.-Z.; Zhang, W.-D.; Yan, S.-K. Phenylpropanoids from Daphne feddei and their inhibitory activities against NO production. J. Nat. Prod. 2008, 71, 1902–1905. [Google Scholar] [CrossRef]
- Chen, Y.C.; Ma, Q.Y.; Huang, S.Z.; Guo, Z.K.; Zhao, Y.X.; Hua, Y. Analyses on chemical constituents of Daphne holosericea. J. Southwest For. Univ. 2014, 34, 97–101. [Google Scholar]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Müller, L.; Fröhlich, K.; Böhm, V. Comparative antioxidant activities of carotenoids measured by ferric reducing antioxidant power (FRAP), ABTS+ bleaching assay (αTEAC), DPPH assay and peroxyl radical scavenging assay. Food Chem. 2011, 129, 139–148. [Google Scholar] [CrossRef]
- Khodadadian, Z.; Hassanpour-Ezatti, M.; Mousavi, S.Z.; Asgarpanah, J. Analgesic and anti-inflammatory potential of aerial parts of the Daphne mucronate Royle extract in mice: Opioid-independent action. Asian Pac. J. Trop. Biomed. 2016, 6, 198–201. [Google Scholar] [CrossRef]
- Kupeli, E.; Tosun, A.; Yesilada, E. Assessment of anti-inflammatory and antinociceptive activities of Daphne pontica L. (Thymelaeaceae). J. Ethnopharmacol. 2007, 113, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Süntar, I.; KüpeliAkkol, E.; Keles, H.; Yesilada, E.; Sarker, S.D.; Arroo, R. Efficacy of Daphne oleoides subsp. kurdica used for wound healing: Identification of active compounds through bioassay guided isolation technique. J. Ethnopharmacol. 2012, 141, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Atay-Balkan, I.; Taşkın, T.; Doğan, H.T.; Deniz, I.; Akaydın, G. A comparative investigation on the in vitro anti-inflammatory, antioxidant and antimicrobial potentials of subextracts from the aerial parts of Daphne oleoides Schreb. subsp. oleoides. Ind. Crop Prod. 2017, 95, 695–703. [Google Scholar] [CrossRef]
- Toniolo, C.; Nicoletti, M.; Maggi, F.; Venditti, A. HPTLC determination of chemical composition variability in raw materials used in botanicals. Nat. Prod. Res. 2014, 28, 119–126. [Google Scholar] [CrossRef]
- Zengin, G.; Arkan, T.; Aktumsek, A.; Guler, G.O.; Cakmak, Y.S. A study on antioxidant capacities and fatty acid compositions of two Daphne species from Turkey: New sources of antioxidants and essential fatty acids. J. Food. Biochem. 2012, 37, 646–653. [Google Scholar] [CrossRef]
- Li, Z.; Chen, X.; Liu, Z.; Ye, W.; Li, L.; Qian, L.; Ding, H.; Li, P.; Aung, L.H.H. Recent advances: Molecular mechanism of RNA oxidation and its role in various diseases. Front. Mol. Biosci. 2020, 7, 1–7. [Google Scholar] [CrossRef]
- Quintanar, L.; Lim, M.H. Metal ions and degenerative diseases. J. Biol. Inorg. Chem. 2019, 24, 1137–1139. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, oxidative stress, and antioxidants: Back and forth in the pathophysiology of chronic diseases. Front. Physiol. 2020, 11, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Nagai, R.; Murray, D.B.; Metz, T.O.; Baynes, J.W. Chelation: A fundamental mechanism of action of AGE inhibitors, AGE breakers, and other inhibitors of diabetes complications. Diabetes 2012, 61, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Di Sotto, A.; Locatelli, M.; Macone, A.; Toniolo, C.; Cesa, S.; Carradori, S.; Eufemi, M.; Mazzanti, G.; Di Giacomo, S. Hypoglycemic, antiglycation, and cytoprotective properties of a phenol-rich extract from waste peel of Punica granatum L. var. Dente di Cavallo DC2. Molecules 2019, 24, 3103. [Google Scholar] [CrossRef] [PubMed]
- Grael, C.F.F.; Kanashiro, A.; Kabeya, L.M.; Jordão, C.O.; Takeara, R.; Gobbo-Neto, L.; Polizello, A.C.M.; Lucisano-Valim, Y.M.; Lopes, N.P.; Lopes, J.L.C. In vitro study of antioxidant and scavenger properties of phenolic compounds from Lychnophora species. Química Nova 2010, 33, 867–870. [Google Scholar] [CrossRef]
- Kim, D.-S.; Lim, S.-B. Semi-continuous subcritical water extraction of flavonoids from Citrus unshiu peel: Their antioxidant and enzyme inhibitory activities. Antioxidants 2020, 9, 360. [Google Scholar] [CrossRef] [PubMed]
- Es-Safi, N.-E.; Kollmann, A.; Khlifi, S.; Ducrot, P.-H. Antioxidative effect of compounds isolated from Globularia alypum L. structure–activity relationship. LWT Food Sci. Technol. 2007, 40, 1246–1252. [Google Scholar] [CrossRef]
- Qiu, J.; Gao, F.; Shen, G.; Li, C.; Han, X.; Zhao, Q.; Zhao, D.; Hua, X.; Pang, Y. Metabolic engineering of the phenylpropanoid pathway enhances the antioxidant capacity of Saussurea involucrata. PLoS ONE 2013, 8, e70665. [Google Scholar] [CrossRef] [PubMed]
- Kiliç, I.; Yeşiloğlu, Y. Spectroscopic studies on the antioxidant activity of p-coumaric acid. Spectrochim. Acta Part A 2013, 115, 719–724. [Google Scholar]
- Maksymiak, M.M.; Zięba, M.; Orchel, A.; Musiał-Kulik, M.; Kowalczuk, M.; Adamus, G. Bioactive (co)oligoesters as potential delivery systems of p-anisic acid for cosmetic purposes. Materials 2020, 13, 4153. [Google Scholar] [CrossRef]
- Gülçin, I. Antioxidant activity of caffeic acid (3,4-dihydroxycinnamic acid). Toxicology 2006, 217, 213–220. [Google Scholar] [CrossRef]
- Ivanova, A.; Gerasimova, E.; Gazizullina, E. Study of antioxidant properties of agents from the perspective of their action mechanisms. Molecules 2020, 25, 4251. [Google Scholar] [CrossRef]
- Montecillo-Aguado, M.; Tirado-Rodriguez, B.; Tong, Z.; Vega, O.M.; Morales-Martínez, M.; Abkenari, S.; Pedraza-Chaverri, J.; Huerta-Yepez, S. Importance of the role of ω-3 and ω-6 polyunsaturated fatty acids in the progression of brain cancer. Brain Sci. 2020, 10, 381. [Google Scholar] [CrossRef] [PubMed]
- Michelis, F.; Tiligada, E.; Skaltsa, H.; Lazari, D.; Skaltsounis, A.L.; Delitheos, A. Effects of the flavonoid pilloin isolated from Marrubium cylleneum on mitogen-induced lymphocyte transformation. Pharm. Biol. 2002, 40, 245–248. [Google Scholar] [CrossRef]
- Choi, C.-H.; Sun, K.-H.; An, C.-S.; Yoo, J.-C.; Hahm, K.-S.; Leed, I.-H.; Sohng, J.-K.; Kim, Y.-C. Reversal of P-glycoprotein-mediated multidrug resistance by 5,6,7,3′,4′-pentamethoxyflavone (sinensetin). Biochem. Biophys. Res. Commun. 2002, 295, 832–840. [Google Scholar] [CrossRef]
- Qawoogh, S.S.; Shahiwal, A. Identification of potential anticancer phytochemicals against colorectal cancer by structure-based docking studies. J. Recept. Sign. Transduct. 2020, 40, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Xia, N. Syringin exhibits anti-cancer effects in HeLa human cervical cancer cells by inducing apoptosis, cell cycle arrest and inhibition of cell migration. Bangladesh J. Pharmacol. 2016, 11, 838–843. [Google Scholar] [CrossRef]
- Lall, N.; Kishore, N.; Binneman, B.; Twilley, D.; van de Venter, M.; du Plessis-Stoman, D. Cytotoxicity of syringin and 4-methoxycinnamyl alcohol isolated from Foeniculum vulgare on selected human cell lines. Nat. Prod. Res. 2015, 29, 1752–1756. [Google Scholar] [CrossRef] [PubMed]
- Jaganathan, S.K.; Supriyanto, E.; Mandal, M. Events associated with apoptotic effect of p-coumaric acid in HCT-15 colon cancer cells. World J. Gastroenterol. 2013, 19, 7726–7734. [Google Scholar] [CrossRef]
- Pelinson, L.P.; Assmann, C.E.; Palma, T.V.; Mânica da Cruz, I.B.; Mainardi Pillat, M.; Mânica, M.; Stefanello, N.; Cezimbra Weis, G.C.; de Oliveira Alves, A.; Melazzo de Andrade, C.; et al. Antiproliferative and apoptotic effects of caffeic acid on SK-Mel-28 human melanoma cancer cells. Mol. Biol. Rep. 2019, 46, 2085–2092. [Google Scholar] [CrossRef]
- Rezakhani, N.; Goliaei, B.; Parivar, K.; Nikoofar, A.R. Effects of X-irradiation and sinensetin on apoptosis induction in MDA-MB-231 human breast cancer cells. Int. J. Rad. Res. 2020, 18, 75–82. [Google Scholar]
- Khan, S.S.; Iqbal, M.A.; Majid, A.M.S.A. Effect of crystallization of caffeic acid enhanced stability and dual biological efficacy. Cogent Biol. 2016, 2, 1–8. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).