Lumacaftor and Matrine: Possible Therapeutic Combination to Counteract the Inflammatory Process in Cystic Fibrosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture
2.3. Experimental Protocol
2.4. Indirect Immunofluorescence Analysis
2.5. Cytosol and Membrane Extracts
2.6. Total Protein Extraction and Western Blot Analysis
2.7. Measurement of Intracellular Reactive Oxygen Species (ROS)
2.8. Flow Cytometry Analysis
2.9. Determination of Hypodiploid Nuclei
2.10. Statistical Analysis
3. Results
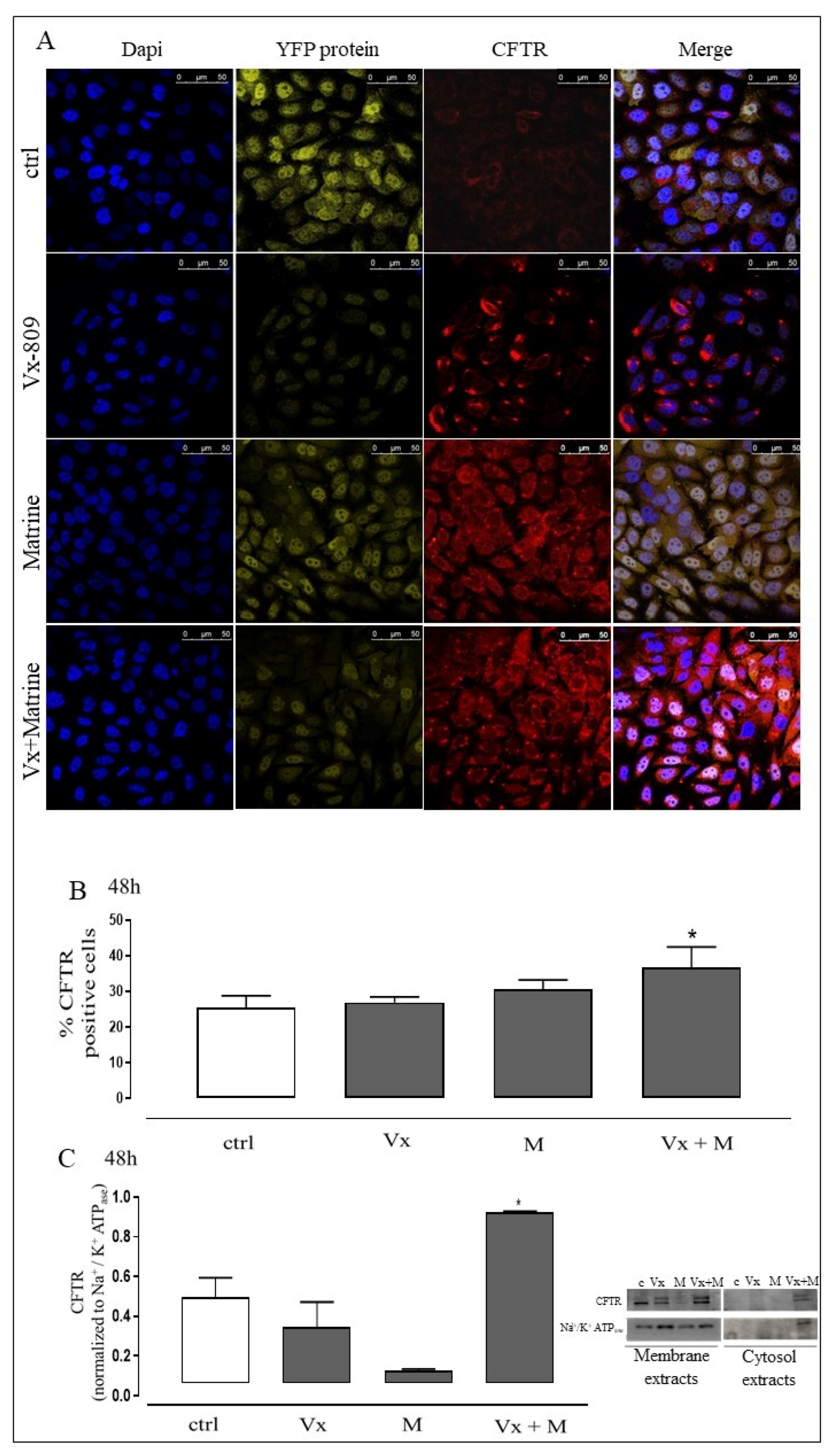
3.1. Vx-809 and Matrine Co-Treatment Increases CFTR Localization in Membrane
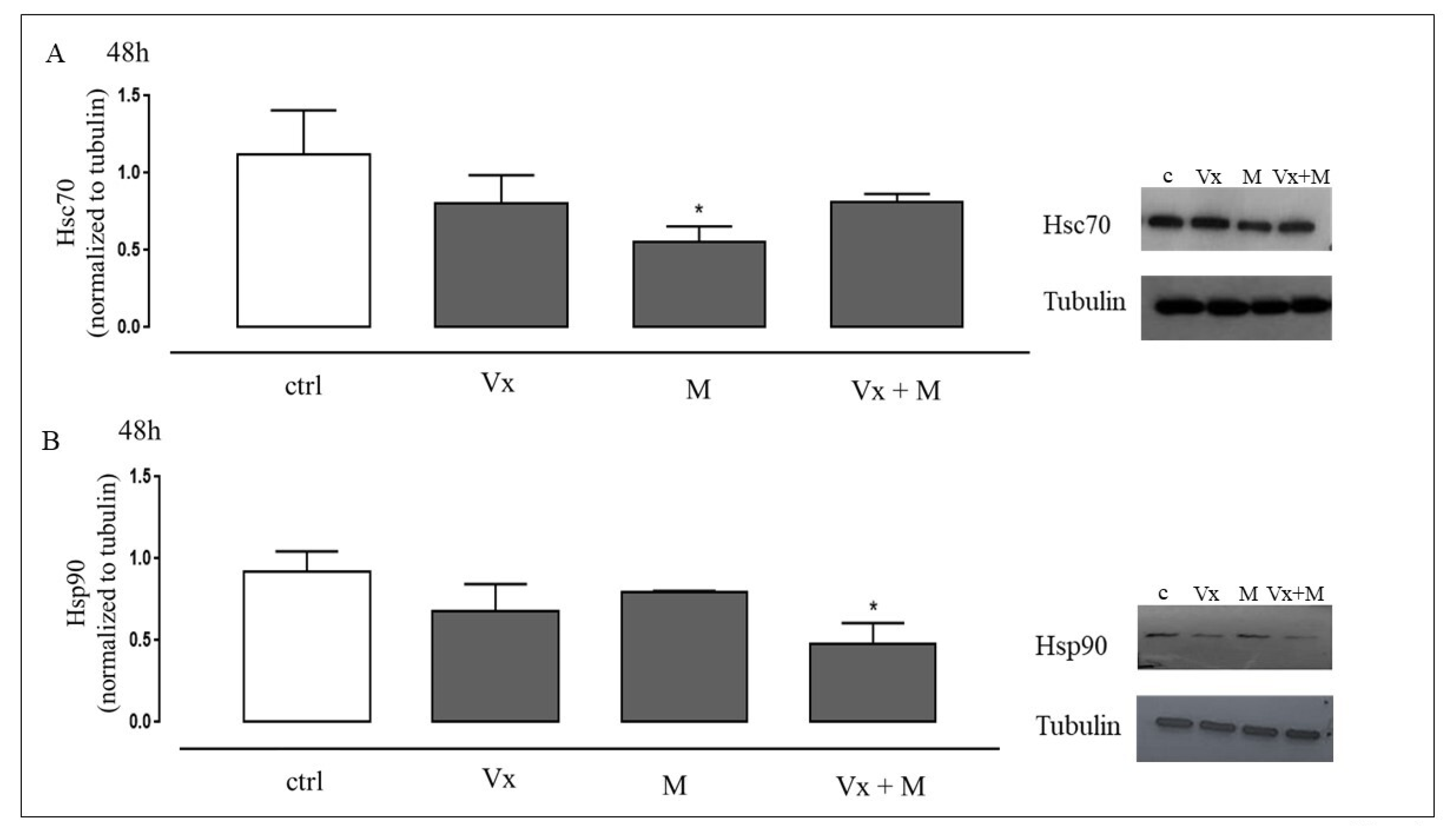
3.2. Vx-809 and Matrine Co-Treatment Interferes in the Molecular Trafficking
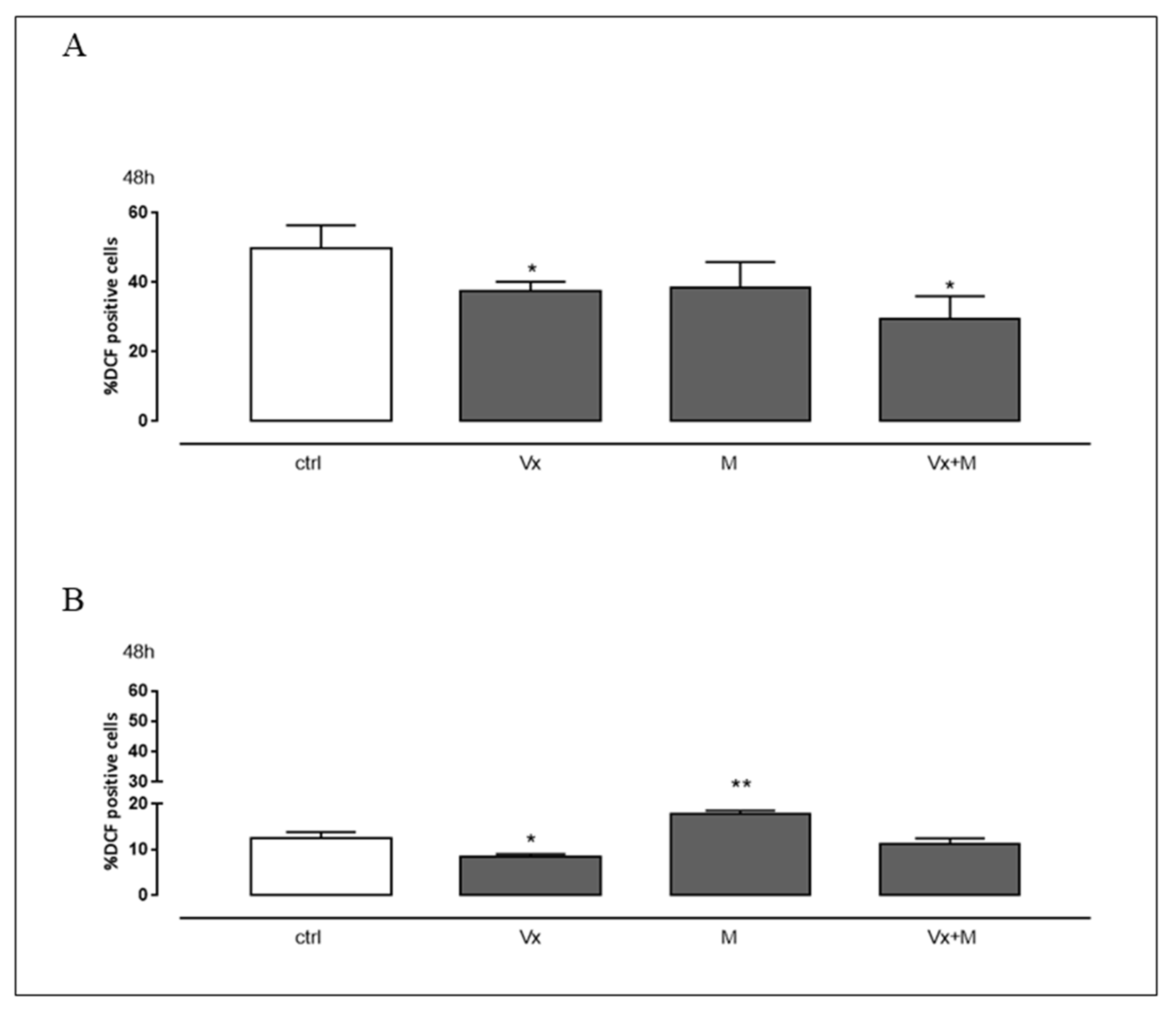
3.3. Vx-809 and Matrine Co-Treatment Reduces CF-Induced Oxidative Stress
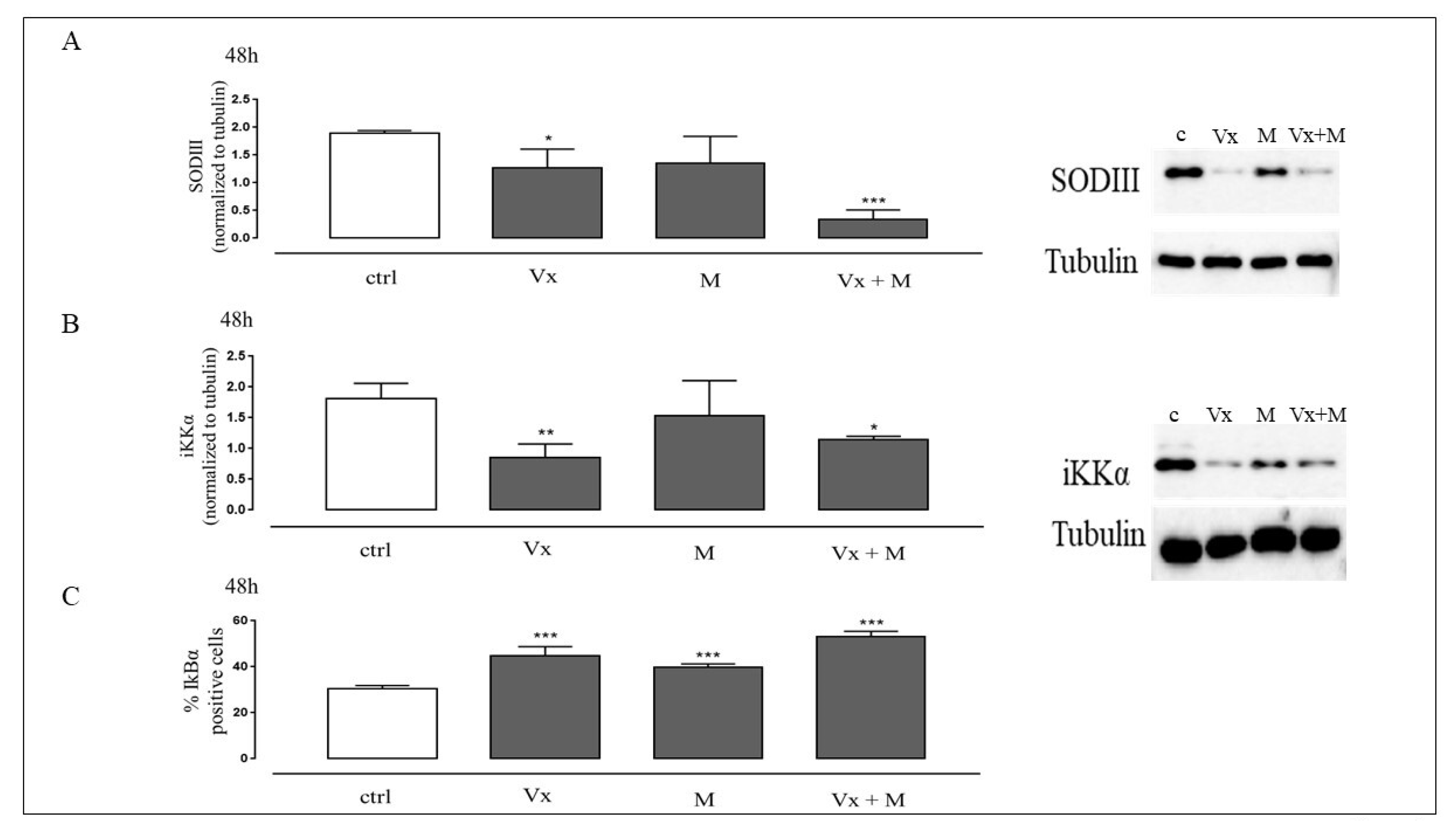
3.4. The Synergistic Effect of Vx-809 and Matrine Counteract ROS Signaling
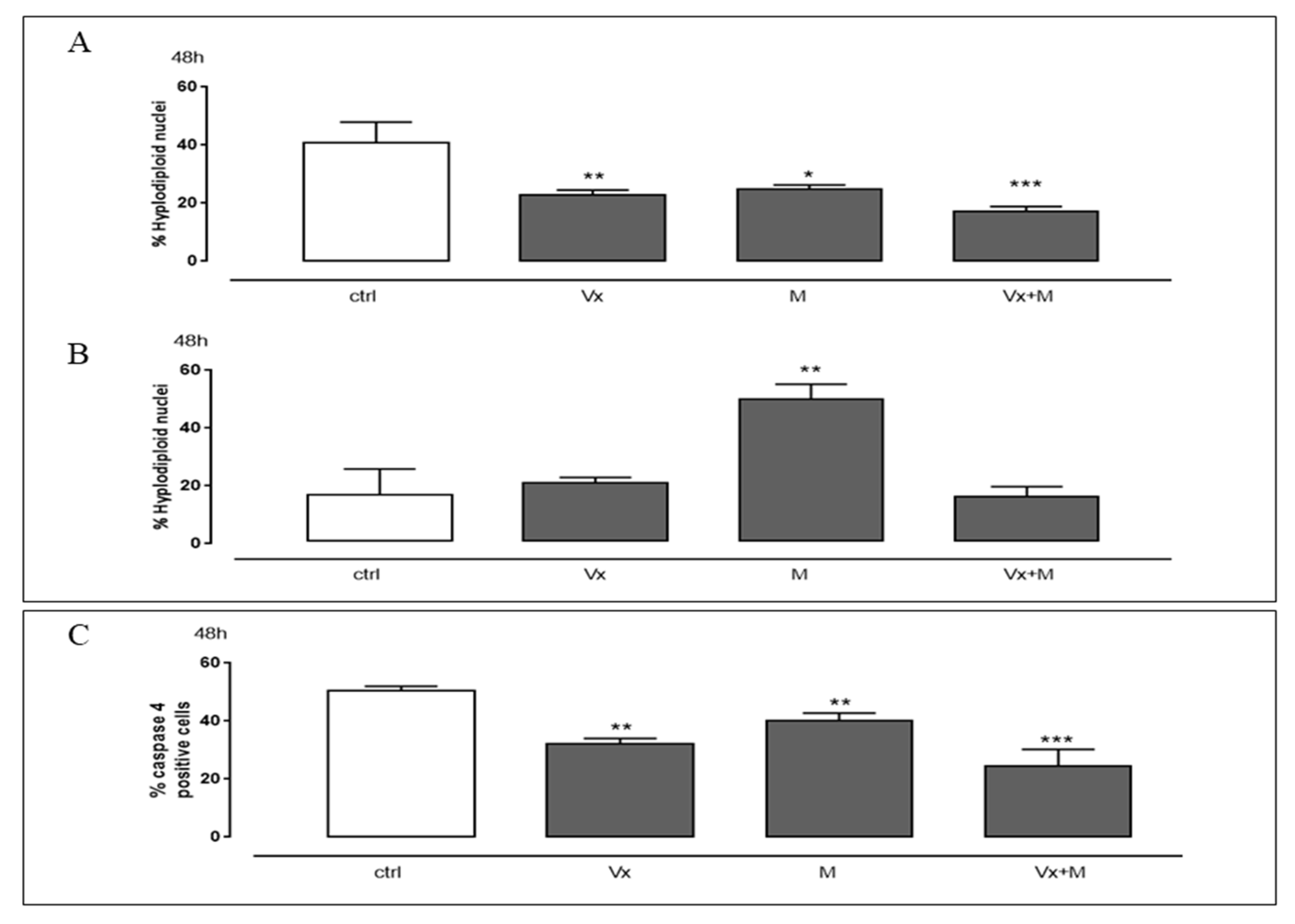
3.5. The “Corrector” of CFTR and the Quinolizidine Alkaloid Interfere with the Cell Death
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rafeeq, M.M.; Murad, H.A.S. Cystic fibrosis: Current therapeutic targets and future approaches. J. Transl. Med. 2017, 15, 84. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.C.; Alton, E.W.; Bush, A. Cystic fibrosis. BMJ 2007, 335, 1255–1259. [Google Scholar] [CrossRef] [PubMed]
- Sadowska-Bartosz, I.; Galiniak, S.; Bartosz, G.; Rachel, M. Oxidative modification of proteins in pediatric cystic fibrosis with bacterial infections. Oxid. Med. Cell. Longev. 2014, 389629. [Google Scholar] [CrossRef]
- Lopes-Pacheco, M. CFTR Modulators: The Changing Face of Cystic Fibrosis in the Era of Precision Medicine. Front. Pharmacol. 2020, 10, 1662. [Google Scholar] [CrossRef] [PubMed]
- Ringshausen, F.C.; Hellmuth, T.; Dittrich, A.M. Evidence-based treatment of cystic fibrosis. Internist 2020, 61, 1212–1229. [Google Scholar] [CrossRef] [PubMed]
- Bross, P.; Corydon, T.J.; Andresen, B.S.; Jørgensen, M.M.; Bolund, L.; Gregersen, N. Protein misfolding and degradation in genetic diseases. Hum. Mutat. 1999, 14, 186–198. [Google Scholar] [CrossRef]
- O’Sullivan, B.P.; Freedman, S.D. Cystic fibrosis. Lancet 2009, 373, 1891–1904. [Google Scholar] [CrossRef]
- Ward, C.L.; Kopito, R.R. Intracellular turnover of cystic fibrosis transmembrane conductance regulator. Inefficient processing and rapid degradation of wild-type and mutant proteins. J. Biol. Chem. 1994, 269, 25710–25718. [Google Scholar] [CrossRef]
- Jensen, T.J.; Loo, M.A.; Pind, S.; Williams, D.B.; Goldberg, A.L.; Riordan, J.R. Multiple proteolytic systems, including the proteasome, contribute to CFTR processing. Cell 1995, 83, 129–135. [Google Scholar] [CrossRef]
- Ward, C.L.; Omura, S.; Kopito, R.R. Degradation of CFTR by the ubiquitin-proteasome pathway. Cell 1995, 83, 121–127. [Google Scholar] [CrossRef]
- Qu, B.H.; Thomas, P.J. Alteration of the cystic fibrosis transmembrane conductance regulator folding pathway—effects of the Delta F508 mutation on the thermodynamic stability and folding yield of NBD1. J. Biol. Chem. 1996, 271, 7261–7264. [Google Scholar] [CrossRef]
- Pankow, S.; Bamberger, C.; Calzolari, D.; Martínez-Bartolomé, S.; Lavallée-Adam, M.; Balch, W.E.; Yates, J.R., 3rd. ∆F508 CFTR interactome remodelling promotes rescue of cystic fibrosis. Nature 2015, 528, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Rottner, M.; Freyssinet, J.M.; Martínez, M.C. Mechanisms of the noxious inflammatory cycle in cystic fibrosis. Respir. Res. 2009, 10, 23. [Google Scholar] [CrossRef] [PubMed]
- Roesch, E.A.; Nichols, D.P.; Chmiel, J.F. Inflammation in cystic fibrosis: An update. Pediatr. Pulmonol. 2018, 53, S30–S50. [Google Scholar] [CrossRef] [PubMed]
- Yasui, K.; Baba, A. Therapeutic potential of superoxide dismutase (SOD) for resolution of inflammation. Inflamm. Res. 2006, 55, 359–363. [Google Scholar] [CrossRef]
- Becker, M.N.; Sauer, M.S.; Muhlebach, M.S.; Hirsh, A.J.; Wu, Q.; Verghese, M.W.; Randell, S.H. Cytokine secretion by cystic fibrosis airway epithelial cells. Am. J. Respir. Crit. Care Med. 2004, 169, 645–653. [Google Scholar] [CrossRef]
- Weber, A.J.; Soong, G.; Bryan, R.; Saba, S.; Prince, A. Activation of NF-kappaB in airway epithelial cells is dependent on CFTR trafficking and Cl- channel function. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001, 281, L71–L78. [Google Scholar] [CrossRef]
- Velsor, L.W.; van Heeckeren, A.; Day, B.J. Antioxidant imbalance in the lungs of cystic fibrosis transmembrane conductance regulator protein mutant mice. Am. J. Physiol. Lung. Cell. Mol. Physiol. 2001, 281, L31–L38. [Google Scholar] [CrossRef]
- Ozben, T. Oxidative stress and apoptosis: Impact on cancer therapy. J. Pharm. Sci. 2007, 96, 2181–2196. [Google Scholar] [CrossRef]
- Vandivier, R.W.; Fadok, V.A.; Hoffmann, P.R.; Bratton, D.L.; Penvari, C.; Brown, K.K.; Brain, J.D.; Accurso, F.J.; Henson, P.M. Elastase-mediated phosphatidylserine receptor cleavage impairs apoptotic cell clearance in cystic fibrosis and bronchiectasis. J. Clin. Investig. 2002, 109, 661–670. [Google Scholar] [CrossRef]
- Ruffin, M.; Roussel, L.; Maillé, E.; Rousseau, S.; Brochiero, E. Vx-809/Vx-770 Treatment Reduces Inflammatory Response to Pseudomonas Aeruginosa in Primary Differentiated Cystic Fibrosis Bronchial Epithelial Cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2018, 314, L635–L641. [Google Scholar] [CrossRef] [PubMed]
- Spanò, V.; Montalbano, A.; Carbone, A.; Scudieri, P.; Galietta, L.J.V.; Barraja, P. An overview on chemical structures as ΔF508-CFTR correctors. Eur. J. Med. Chem. 2019, 180, 430–448. [Google Scholar] [CrossRef] [PubMed]
- Tran, Q.T.N.; Tan, D.W.S.; Wong, W.S.F.; Chai, C.L.L. From irreversible to reversible covalent inhibitors: Harnessing the andrographolide scaffold for anti-inflammatory action. Eur. J. Med. Chem. 2021, 209, 112943. [Google Scholar] [CrossRef] [PubMed]
- Carbone, A.; Montalbano, A.; Spanò, V.; Musante, I.; Galietta, L.J.V.; Barraja, P. Furocoumarins as multi-target agents in the treatment of cystic fibrosis. Eur. J. Med. Chem. 2019, 180, 283–290. [Google Scholar] [CrossRef]
- Spanò, V.; Venturini, A.; Genovese, M.; Barreca, M.; Raimondi, M.V.; Montalbano, A.; Galietta, L.J.V.; Barraja, P. Current development of CFTR potentiators in the last decade. Eur. J. Med. Chem. 2020, 204, 112631. [Google Scholar] [CrossRef]
- Keating, D.; Marigowda, G.; Burr, L.; Daines, C.; Mall, M.A.; McKone, E.F.; Ramsey, B.W.; Rowe, S.M.; Sass, L.A.; Tullis, E.; et al. VX16-445-001 Study Group.VX-445-Tezacaftor-Ivacaftor in Patients with Cystic Fibrosis and One or Two Phe508del Alleles. N. Engl. J. Med. 2018, 379, 1612–1620. [Google Scholar] [CrossRef]
- Veit, G.; Roldan, A.; Hancock, M.A.; Da Fonte, D.F.; Xu, H.; Hussein, M.; Frenkiel, S.; Matouk, E.; Velkov, T.; Lukacs, G.L. Allosteric folding correction of F508del and rare CFTR mutants by elexacaftor-tezacaftor-ivacaftor (Trikafta) combination. JCI Insight 2020, 5, e139983. [Google Scholar] [CrossRef]
- Laselva, O.; Bartlett, C.; Gunawardena, T.N.A.; Ouyang, H.; Eckford, P.D.W.; Moraes, T.J.; Bear, C.E.; Gonska, T. Rescue of multiple class II CFTR mutations by elexacaftor+ tezacaftor+ivacaftor mediated in part by the dual activities of Elexacaftor as both corrector and potentiator. Eur. Respir. J. 2020, 10, 2002774. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, X.; Qiu, C.; Liu, F.; Liu, P.; Liu, Z. Matrine suppresses AGE-induced HAEC injury by inhibiting ROS-mediated NRLP3 inflammasome activation. Eur. J. Pharmacol. 2018, 822, 207–211. [Google Scholar] [CrossRef]
- Huang, M.; Xin, W. Matrine inhibiting pancreatic cells epithelial-mesenchymal transition and invasion through ROS/NF-κB/MMPs pathway. Life Sci. 2018, 192, 55–61. [Google Scholar] [CrossRef]
- Basile, A.; Pascale, M.; Franceschelli, S.; Nieddu, E.; Mazzei, M.T.; Fossa, P.; Turco, M.C.; Mazzei, M. Matrine modulates HSC70 levels and rescues ΔF508-CFTR. J. Cell. Physiol. 2012, 227, 3317–3323. [Google Scholar] [CrossRef]
- Marengo, B.; Speciale, A.; Senatore, L.; Garibaldi, S.; Musumeci, F.; Nieddu, E.; Pollarolo, B.; Pronzato, M.A.; Schenone, S.; Mazzei, M.; et al. Matrine in Association With FD-2 Stimulates F508del-cystic Fibrosis Transmembrane Conductance Regulator Activity in the Presence of Corrector VX809. Mol. Med. Rep. 2017, 16, 8849–8853. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guo, S.; Chen, Y.; Pang, C.; Wang, X.; Shi, S.; Zhang, H.; An, H.; Zhan, Y. Matrine is a novel inhibitor of the TMEM16A chloride channel with antilung adenocarcinoma effects. J. Cell. Physiol. 2019, 234, 8698–8708. [Google Scholar] [CrossRef]
- Stanton, B.A.; Coutermarsh, B.; Barnaby, R.; Hogan, D. Pseudomonas aeruginosa Reduces VX-809 Stimulated F508del-CFTR Chloride Secretion by Airway Epithelial Cells. PLoS ONE 2015, 10, e0127742. [Google Scholar] [CrossRef]
- Franceschelli, S.; Bruno, A.P.; Festa, M.; Falco, A.; Gionti, E.; d’Avenia, M.; De Marco, M.; Basile, A.; Iorio, V.; Marzullo, L.; et al. BAG3 Protein Is Involved in Endothelial Cell Response to Phenethyl Isothiocyanate. Oxid. Med. Cell. Longev. 2018, 2018, 5967890. [Google Scholar] [CrossRef] [PubMed]
- Pecoraro, M.; Ciccarelli, M.; Fiordelisi, A.; Iaccarino, G.; Pinto, A.; Popolo, A. Diazoxide Improves Mitochondrial Connexin 43 Expression in a Mouse Model of Doxorubicin-Induced Cardiotoxicity. Int. J. Mol. Sci. 2018, 19, 757. [Google Scholar] [CrossRef] [PubMed]
- Riccardi, C.; Nicoletti, I. Analysis of apoptosis by propidium iodide staining and flow cytometry. Nat. Protoc. 2006, 1, 1458–1461. [Google Scholar] [CrossRef] [PubMed]
- Galietta, L.J.; Haggie, P.M.; Verkman, A.S. Green fluorescent protein-based halide indicators with improved chloride and iodide affinities. FEBS Lett. 2001, 499, 220–224. [Google Scholar] [CrossRef]
- Chanoux, R.A.; Rubenstein, R.C. Molecular Chaperones as Targets to Circumvent the CFTR Defect in Cystic Fibrosis. Front. Pharmacol. 2012, 3, 137. [Google Scholar] [CrossRef]
- Morel, Y.; Barouki, R. Influence du stress oxydant sur la régulation des gènes. Méd. Sci. 1998, 14, 713–721. [Google Scholar] [CrossRef]
- Tan, C.; Qian, X.; Jia, R.; Wu, M.; Liang, Z. Matrine induction of reactive oxygen species activates p38 leading to caspase-dependent cell apoptosis in non-small cell lung cancer cells. Oncol. Rep. 2013, 30, 2529–2535. [Google Scholar] [CrossRef]
- Jiang, J.H.; Pi, J.; Jin, H.; Yang, F.; Cai, J.Y. Chinese herb medicine matrine induce apoptosis in human esophageal squamous cancer KYSE-150 cells through increasing reactive oxygen species and inhibiting mitochondrial function. Pathol. Res. Pract. 2018, 214, 691–699. [Google Scholar] [CrossRef]
- Fukai, T.; Ushio-Fukai, M. Superoxide Dismutases: Role in Redox Signaling, Vascular Function, and Diseases. Antioxid. Redox Signal. 2011, 15, 1583–1606. [Google Scholar] [CrossRef]
- Valdivieso, Á.G.; Dugour, A.V.; Sotomayor, V.; Clauzure, M.; Figueroa, J.M.; Santa-Coloma, T.A. N-acetyl cysteine reverts the proinflammatory state induced by cigarette smoke extract in lung Calu-3 cells. Redox Biol. 2018, 16, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Hagler, J.; Palombella, V.J.; Melandri, F.; Scherer, D.; Ballard, D.; Maniatis, T. Signal-induced site-specific phosphorylation targets I kappa B alpha to the ubiquitin-proteasome pathway. Genes. Dev. 1995, 9, 1586–1597. [Google Scholar] [CrossRef]
- Cheresh, P.; Kim, S.J.; Tulasiram, S.; David, K.W. Oxidative Stress and Pulmonary Fibrosis. Biochim. Biophys. Acta. 2013, 1832, 1028–1040. [Google Scholar] [CrossRef] [PubMed]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Imaizumi, K.; Miyoshi, K.; Katayama, T.; Yoneda, T.; Taniguchi, M.; Kudo, T.; Tohyama, M. The unfolded protein response and Alzheimer’s disease. Biochim. Biophys. Acta. 2001, 1536, 85–96. [Google Scholar] [CrossRef]
- Hitomi, J.; Katayama, T.; Eguchi, Y.; Kudo, T.; Taniguchi, M.; Koyama, Y.; Manabe, T.; Yamagishi, S.; Bando, Y.; Imaizumi, K.; et al. Involvement of caspase-4 in endoplasmic reticulum stress-induced apoptosis and Aβ-induced cell death. J. Cell. Biol. 2004, 165, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Trinh, N.T.N.; Bilodeau, C.; Maillé, E.; Ruffin, M.; Quintal, M.C.; Desrosiers, M.Y.; Rousseau, S.; Brochiero, E. Deleterious impact of Pseudomonas aeruginosa on cystic fibrosis transmembrane conductance regulator function and rescue in airway epithelial cells. Eur. Respir. J. 2015, 45, 1590–1602. [Google Scholar] [CrossRef] [PubMed]
- Laselva, O.; Stone, T.A.; Bear, C.E.; Deber, C.M. Anti-Infectives Restore ORKAMBI® Rescue of F508del-CFTR Function in Human Bronchial Epithelial Cells Infected with Clinical Strains of P. aeruginosa. Biomolecules 2020, 10, 334. [Google Scholar] [CrossRef]
- Folkerts, G.; Kloek, J.; Muijsers, R.B.; Nijkamp, F.P. Reactive nitrogen and oxygen species in airway inflammation. Eur. J. Pharmacol. 2001, 429, 251–262. [Google Scholar] [CrossRef]
- Bagdany, M.; Veit, G.; Fukuda, R.; Avramescu, R.; Okiyoneda, T.; Baaklini, I.; Singh, J.; Sovak, G.; Xu, H.; Apaja, M.P.; et al. Chaperones rescue the energetic landscape of mutant CFTR at single molecule and in cell. Nat. Commun. 2017, 8, 398. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell. Longev. 2016, 7432797. [Google Scholar] [CrossRef]
- Kim, S.J.; Zhang, Z.; Hitomi, E.; Lee, Y.C.; Mukherjee, A.B. Endoplasmic reticulum stress-induced caspase-4 activation mediates apoptosis and neurodegeneration in INCL. Hum. Mol. Genet. 2006, 15, 1826–1834. [Google Scholar] [CrossRef] [PubMed]
- Regnis, J.A.; Robinson, M.; Bailey, D.L.; Cook, P.; Hooper, P.; Chan, H.K.; Gonda, I.; Bautovich, G.; Bye, P.T. Mucociliary clearance in patients with cystic fibrosis and in normal subjects. Am. J. Respir. Crit. Care Med. 1994, 150, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Danahay, H.; Gosling, M. TMEM16A: An Alternative Approach to Restoring Airway Anion Secretion in Cystic Fibrosis? Int. J. Mol. Sci. 2020, 21, 2386. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pecoraro, M.; Franceschelli, S.; Pascale, M. Lumacaftor and Matrine: Possible Therapeutic Combination to Counteract the Inflammatory Process in Cystic Fibrosis. Biomolecules 2021, 11, 422. https://doi.org/10.3390/biom11030422
Pecoraro M, Franceschelli S, Pascale M. Lumacaftor and Matrine: Possible Therapeutic Combination to Counteract the Inflammatory Process in Cystic Fibrosis. Biomolecules. 2021; 11(3):422. https://doi.org/10.3390/biom11030422
Chicago/Turabian StylePecoraro, Michela, Silvia Franceschelli, and Maria Pascale. 2021. "Lumacaftor and Matrine: Possible Therapeutic Combination to Counteract the Inflammatory Process in Cystic Fibrosis" Biomolecules 11, no. 3: 422. https://doi.org/10.3390/biom11030422
APA StylePecoraro, M., Franceschelli, S., & Pascale, M. (2021). Lumacaftor and Matrine: Possible Therapeutic Combination to Counteract the Inflammatory Process in Cystic Fibrosis. Biomolecules, 11(3), 422. https://doi.org/10.3390/biom11030422





