Abstract
Various pathophysiological mechanisms have been implicated in hypertension, but those resulting in vascular dysfunction and remodeling are critical and may help to identify critical pharmacological targets. This mini-review article focuses on central mechanisms contributing to the vascular dysfunction and remodeling of hypertension, increased oxidative stress and impaired nitric oxide (NO) bioavailability, which enhance vascular matrix metalloproteinase (MMP) activity. The relationship between NO, MMP and oxidative stress culminating in the vascular alterations of hypertension is examined. While the alterations of hypertension are not fully attributable to these pathophysiological mechanisms, there is strong evidence that such mechanisms play critical roles in increasing vascular MMP expression and activity, thus resulting in abnormal degradation of extracellular matrix components, receptors, peptides, and intracellular proteins involved in the regulation of vascular function and structure. Imbalanced vascular MMP activity promotes vasoconstriction and impairs vasodilation, stimulating vascular smooth muscle cells (VSMC) to switch from contractile to synthetic phenotypes, thus facilitating cell growth or migration, which is associated with the deposition of extracellular matrix components. Finally, the protective effects of MMP inhibitors, antioxidants and drugs that enhance vascular NO activity are briefly discussed. Newly emerging therapies that address these essential mechanisms may offer significant advantages to prevent vascular remodeling in hypertensive patients.
1. Hypertension Is a Common Disease Leading to a Variety of Cardiovascular Complications and Presenting a Significant Public Health Problem
Cardiovascular diseases (CVDs) are the greatest causes of death globally. More people die from CVDs annually than any other cause [1]. CVDs include coronary heart disease, heart failure, stroke and hypertension. In 2017, 17.8 million deaths were attributed to CVDs globally, an estimated 31% of all deaths worldwide. Most CVD deaths are due to heart attacks or strokes [1]. High blood pressure, which is characterized by systolic blood pressure ≥140 mm Hg and diastolic blood pressure ≥90 mm Hg [2], is a significant risk factor for heart attacks and CVDs [1]. Therefore, hypertension is still a leading cause of death globally, accounting for 10.4 million deaths in 2017 [3].
2. Basic Pathophysiological Mechanisms Contributing to the Vascular Alterations of Hypertension
Hypertension is a complex multifactorial disorder involving pathophysiological mechanisms that interact with each other to promote functional and structural modifications of the cardiovascular system. Sustained high blood pressure results from the combination of various genetic and environmental alterations that have neuroendocrine, hemodynamic, inflammatory and tissue redox components. This mini-review article will focus on critical mechanisms contributing to the vascular remodeling of hypertension: increased oxidative stress both impairing NO bioavailability and enhancing vascular MMP activity.
Oxidative stress results from the overproduction or inhibited inactivation of reactive oxygen species (ROS), causing alterations in the redox state of proteins and other mediators [4]. The most critical ROS in the cardiovascular system are superoxide anions (O2−), hydrogen peroxide (H2O2), nitric oxide (NO) and peroxynitrite (ONOO−) [5]. Increased ROS formation triggers protein oxidation and activation of a cascade of cell signaling events, resulting in endothelial dysfunction and MMP activation [6,7,8]. In fact, there is strong evidence that the biochemical alterations mentioned here underlie critical mechanisms leading to endothelial dysfunction and vascular remodeling in hypertension.
MMPs are calcium and zinc-dependent enzymes that play a crucial role in the degradation of extracellular matrix components [9,10], membrane proteins [11,12,13], peptides [14,15] and intracellular proteins [16,17,18,19,20,21,22]. Proteolytic MMP activity increases blood pressure, causes vasoconstriction and endothelial dysfunction and promotes cell migration with vascular remodeling in hypertension [12,17,18,19,23,24,25,26,27,28,29,30,31]. In fact, it is now widely acknowledged that the interaction between ROS and MMPs tends to increase vasoconstriction and attenuate endothelial relaxation mediated by vasoactive agents [12,32,33]. Phenylephrine (PE), angiotensin II (Ang-II), and endothelin-1 (ET-1) promote vasoconstriction at least in part by inducing ROS production, and this mechanism is essential to maintain vasoconstriction [4,12,34,35,36,37,38]. Another important mechanism associated with increased oxidative stress is the reduced bioavailability of an endothelium-derived relaxing factor (EDRF) such as NO, which leads to endothelial dysfunction. This may result from NO rapidly reacting with several other ROS, particularly O2−, forming ONOO− [5], which enhances protein nitration and contributes to many pathophysiological mechanisms taking place in the vasculature of hypertensive subjects [4]. NO is a vasodilator produced by three synthases: endothelial (eNOS), neuronal (nNOS) and inducible (iNOS). These synthases convert L-arginine and molecular oxygen to produce NO and L-citrulline using the cofactors tetrahydrobiopterin (BH4), flavin adenine dinucleotide (FAD), flavin mononucleotide (FMN) and nicotinamide adenine dinucleotide phosphate (NAD) [39]. Impaired NO availability in hypertension is also due to increased concentrations of asymmetric dimethylarginine, which competes with L-arginine for endothelial NO synthase, inhibiting NO formation [40,41], or endothelial NO synthase uncoupling, thus making this enzyme another source of O2− instead of NO [42]. In contrast, the increase in iNOS expression has detrimental effects on hypertension, as the excess NO produced by this isoform reacts with O2−, forming ONOO−, and leads to oxidative damage. iNOS can also regulate arginase activity, reducing NO bioavailability by uncoupling eNOS, with enhanced production of O2− instead of NO [43]. Increased iNOS expression was found in aortas and hearts from rats [44] and from patients with heart failure [45,46]. There is also increased nitrotyrosine (a marker of ONOO− formation) in the aortas and hearts of hypertensive animals. Moreover, iNOS knockout mice submitted to myocardial infarction show lower mortality and less oxidative stress and cardiac remodeling when compared to wild-type mice [47,48].
These critical mechanisms involving increased MMP activity and tissue ROS concentrations, associated with impaired NO activity, interact with other cellular signaling pathways that cause both functional and proliferative alterations in the vasculature of hypertensive subjects.
3. An Overview of MMPs’ Regulation by Oxidative Stress and NO Bioavailability
MMPs are initially synthesized in a latent pro-form as zymogens. The basic structure of the catalytic portion of proteases consists of a catalytic domain with three histidine residues linked to a zinc atom (Zn2+) and a pro-domain containing a cysteine. In the inactive state, the cysteine residue interacts with the zinc ion, maintaining MMP latency [49]. Enzymatic activation is required to cleave the pro-domain and to expose the catalytic site [50]. MMP activation may also occur as a result of the disruption of zinc interaction with cysteine in the pro-domain [51,52,53], arising from enhanced ROS formation, for example [6,7,8].
It has been shown that changes in the tissue concentrations of O2−, H2O2 and ONOO− affect MMP-2 activity [7,8]. High concentrations of H2O2 and ONOO− decrease MMP-2 activity, whereas low concentrations of these ROS increase MMP-2 activity [7,8] (Figure 1). The antioxidant activity of the enzyme catalase protects against the loss of MMP-2 activity, induced by high concentrations of H2O2 [7]. Likewise, in the presence of glutathione, the increase in MMP-2 activity induced by ONOO− does not occur [8]. In this respect, ONOO− has been shown to activate the MMP-2 zymogen in a concentration-dependent manner, with low concentrations (<1 µM) increasing and high concentrations (3–100 µM) decreasing MMP-2 activity. This effect involves the ONOO− mediated S-glutathionylation of the critical cysteine residue of 72 kDa MMP-2 [8]. Interestingly, more complex interactions have been shown between ONOO− and glutathione, affecting MMP-2 phosphorylation, which is another post-translational modification of MMP-2, mediated by protein kinase C (PKC), which may affect MMP-2 activity [54,55]. The decrease in MMP-2 activity can result in physiological changes. Patients with MMP-2 deficiencies have bone, joint and congenital heart problems [56,57,58,59]. In some clinical situations with increased oxidative stress, such as sepsis, decreased MMP-2 activity has been shown in the heart perfusate and the left ventricles of rats after lipopolysaccharide (LPS) infusion [60]. It is known that MMP-2 plays a role in the dysfunction found in these organs. However, its role has yet to be further elucidated. Moreover, MMP-2 knockout mice show a pro-inflammatory phenotype [61,62,63]. On the other hand, increased MMP-2 activity also increases inflammation. These previous findings should be taken into consideration when testing MMP inhibitors, as excessive MMP inhibition may also promote inflammation. For a more in-depth review of this issue, please read [64].
The activation of MMP-2 by ONOO− leads to the formation of active 72 kDa intracellular MMP-2 [8], which is implicated in the degradation of critical intracellular targets in the contractile machinery of cardiomyocytes during ischemia and reperfusion, such as troponin I [22], alpha-actinin [21], titin [16] and light chain myosin [20], probably leading to heart failure. This is very important to the cardiovascular system as a whole, as the deleterious consequences of MMP-2 activation are not limited to the heart [65]. In fact, MMP-2 activation by oxidative stress has been shown in the aortas of two-kidney, one-clip (2K-1C) hypertensive rats in association with decreased calponin-1 expression, resulting in abnormal vascular smooth muscle cells’ (VSMCs’) proliferation and hypertrophic remodeling [17,18,19]. However, treatment with nonspecific inhibitor doxycycline, dose-dependently [66], prevented MMP-2 increased activity and calponin loss [17,19]. The antioxidant tempol, which previously was shown to decrease vascular MMP-2 activity in hypertension [28], prevented calponin-1 loss in 2K-1C hypertensive rats, possibly by decreasing MMP-2 S-glutathionylation [18]. Further supporting the relationship between an imbalanced redox state and abnormal MMP-2 activity, increased ROS concentrations have been shown to promote the formation of a truncated MMP-2 isoform (NTT-MMP-2) with 65 kDa located in the mitochondria and cytosol. Lovett et al. showed that NTT-MMP-2 overexpression in a cardiomyocyte downregulated the B-cell lymphoma-extra-large (BcL-xL) and heat shock protein family D1 (HSPD1), which are genes associated with resistance to oxidative stress [67] (Figure 1). Moreover, transgenic MMP-2 expression in mice shows increased lipid peroxidation in the heart after ischemia-reperfusion [68].
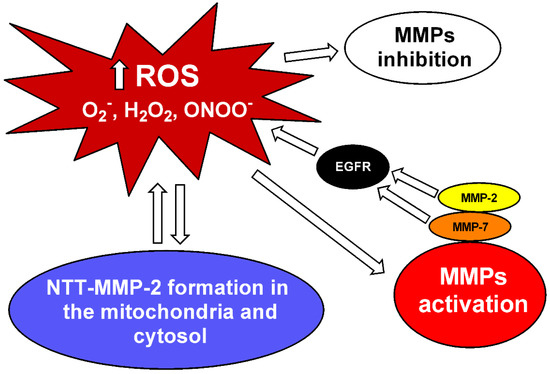
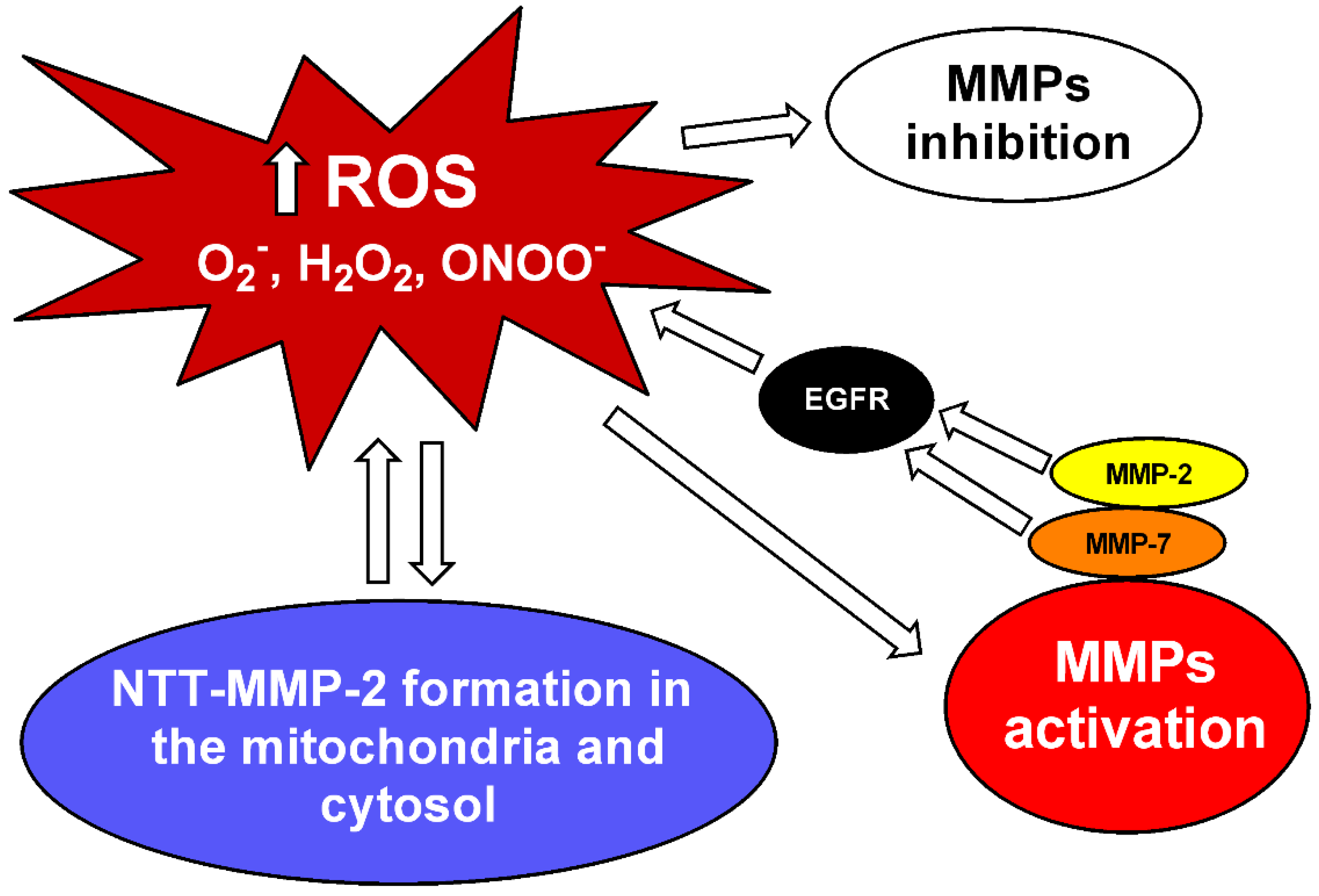

Figure 1.
MMP inhibition and interaction between MMPs and reactive oxygen species (ROS). Increased levels of superoxide (O2−), hydrogen peroxide (H2O2) and peroxynitrite (ONOO−) can activate matrix metalloproteinases (MMPs) [6,7,8]. MMP-2 and MMP-7 activate pro-oxidant pathways in the vascular tissue via epidermal growth factor receptor (EGFR) transactivation [12,69]. ROS can also lead to the formation of a truncated MMP-2 (NTT-MMP-2) with 65 kDa located in the mitochondria and cytosol, which downregulates genes associated with resistance to oxidative stress [67]. High ROS concentrations may decrease MMP activity [7,8].
Figure 1.
MMP inhibition and interaction between MMPs and reactive oxygen species (ROS). Increased levels of superoxide (O2−), hydrogen peroxide (H2O2) and peroxynitrite (ONOO−) can activate matrix metalloproteinases (MMPs) [6,7,8]. MMP-2 and MMP-7 activate pro-oxidant pathways in the vascular tissue via epidermal growth factor receptor (EGFR) transactivation [12,69]. ROS can also lead to the formation of a truncated MMP-2 (NTT-MMP-2) with 65 kDa located in the mitochondria and cytosol, which downregulates genes associated with resistance to oxidative stress [67]. High ROS concentrations may decrease MMP activity [7,8].

More recently, our group showed evidence supporting the idea that increased MMP-2 activity in VSMC directly activates pro-oxidant pathways. MMP-2 cleaves pro-heparin binding epidermal growth factor (HB-EGF) and soluble HB-EGF, in turn, transactivates the EGF receptor (EGFR). This kinase receptor activates phospholipase, PKC and NADPH oxidase, which increases ROS formation [12] (Figure 1). Barhoumi et al., using MMP-2 knockout mice, showed that MMP-2 is required for Ang-II to increase perivascular ROS formation in an animal model with the infusion of Ang-II (Figure 1). Interestingly, MMP-2 knockout impairs the vascular dysfunction and oxidative stress induced by Ang-II without affecting the increases in blood pressure induced by this mediator [32]. In addition to MMP-2, MMP-7 also activates pro-oxidant pathways in vascular tissue via EGFR transactivation [69] (Figure 1).
While the relationship between increased ROS concentrations and MMP activation is relatively clear, the same is not true with NO. Besides its vasodilatory effects, NO also inhibits platelet aggregation, VSMC migration and proliferation and is involved in the regulation of extracellular matrix composition [70]. The direct relation between NO and MMPs, especially MMP-9, seems to be conflicting, indicating a dual role of the molecule in MMP-9 regulation [71]. While NO can activate MMP-9 in rat retinal neurons, the NO donor spermine-NONOate decreases MMP-9 activity in endothelial and carcinoma co-cultures [72,73]. It is possible that this apparent discrepancy is explained by differences (low versus high) in NO concentrations [71]. There is clinical evidence supporting the notion that endogenous NO production, assessed by circulating nitrite concentrations, is inversely related to the circulating concentrations of MMP-2 and MMP-9 [74,75].
Overall, the studies discussed above support the idea that increased vascular oxidative stress has a direct relationship with imbalanced MMP activity, and this mechanism may involve impaired NO bioavailability. Together, these mechanisms may underlie both functional and structural alterations commonly found in the vessels of hypertensive subjects.
4. Evidence Indicating That Increased Vascular MMP Activity Promotes Vascular Dysfunction in Hypertension
Increased vascular MMP-2 expression and activity has been shown in a variety of hypertensive animal models, both in conductance and resistance arteries [27,76,77]. This biochemical alteration is associated with increased responsiveness to vasoconstrictors, such as PE, and reduced responsiveness to vasodilators, such as acetylcholine, both prevented by MMP inhibitors [19,27]. Confirming these findings, endothelial dysfunction is observed in wild-type mice after Ang-II treatment but not in MMP-2 knockout mice [32]. Given that antioxidant drugs and MMP inhibitors reverse these functional vascular alterations associated with increased vascular MMP-2 activity, it is now evident that increased ROS formation plays an important role in promoting these pathophysiological mechanisms in the vessels from hypertensive animals [12,26,28].
As discussed above, we have previously shown that MMP-2 cleavage of pro-HB-EGF results in the transactivation of the HB-EGF receptor and intracellular signaling, increasing ROS formation [12]. This mechanism may explain how increased MMP-2 activity increases the vascular reactivity to PE or other vasoconstrictors, including Ang-II, as previously shown [32], possibly by directly stimulating ROS formation [36] (Figure 2). Interestingly, while ROS formation is usually accepted as an upstream mechanism of MMP activation, there is now growing evidence indicating that MMP activation may be upstream of ROS production [12,33,68,69].
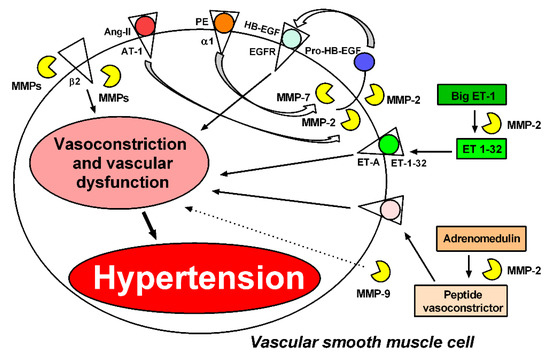
Figure 2.
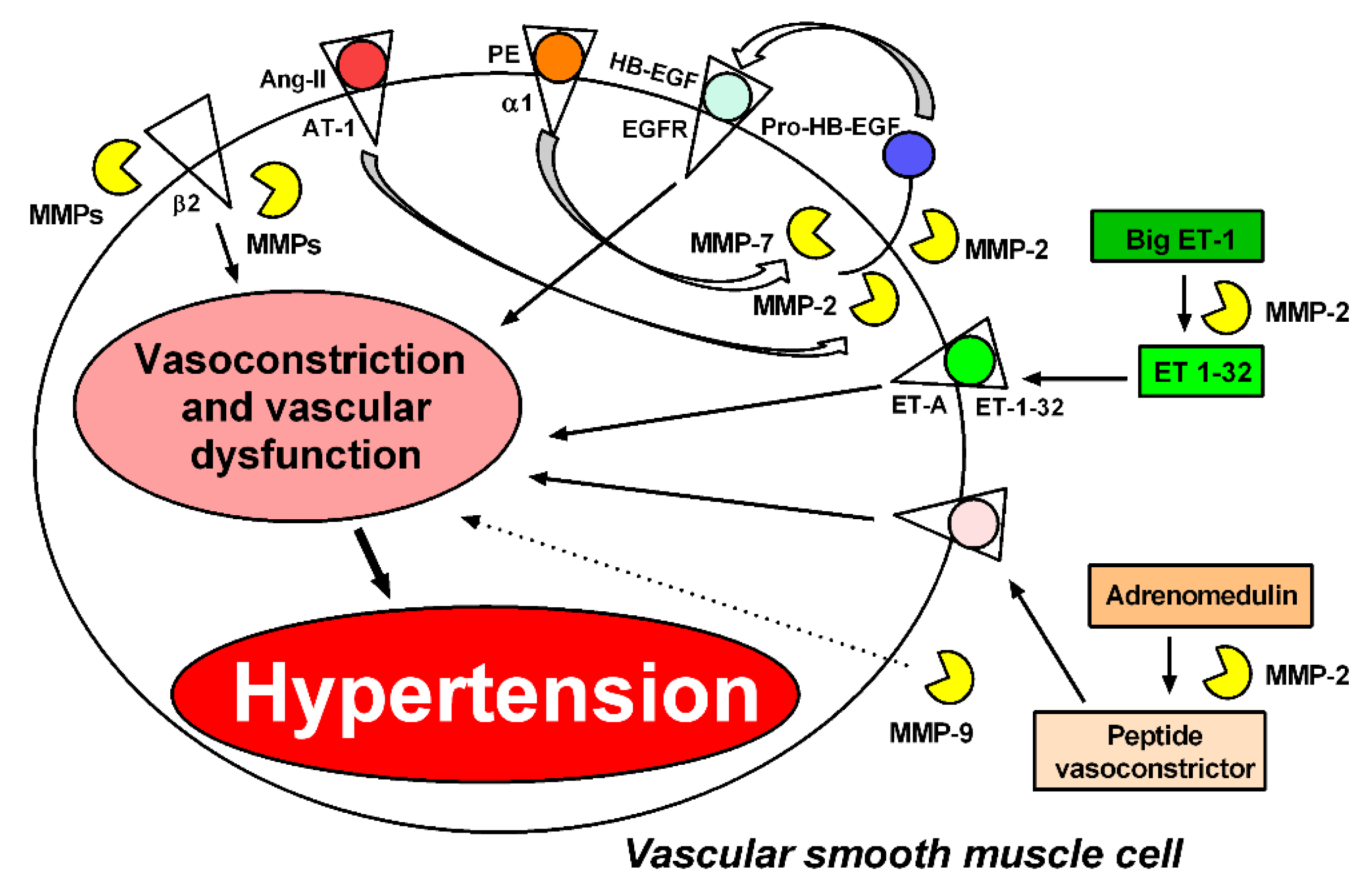
MMPs induce vasoconstriction and vascular dysfunction through different mechanisms. Phenylephrine (PE), angiotensin II (Ang-II), and endothelin-1 (ET-1) mediate vasoconstriction by generating reactive oxygen species (ROS); this mechanism is essential to maintain vasoconstriction. MMP-2 and MMP-7 are required for Ang-II and PE to induce vasoconstriction by transactivation of the epidermal growth factor receptor (EGFR) [12,69]. MMP-2 also induces vasoconstriction by cleaving big endothelin-1 (Big ET-1) to endothelin 1–32 (ET 1–32) [14]. The action of MMP-2 on adrenomedullin leads to the formation of metabolites with vasoconstrictive activity [78]. MMPs (not identified yet) cleave the beta-2 adrenergic receptor, contributing to increased arteriolar tone in spontaneously hypertensive rats [30]. MMP-9 can increase the vasoconstriction of arterioles and venules by a mechanism not yet clarified [30]. These processes may contribute to hypertension.
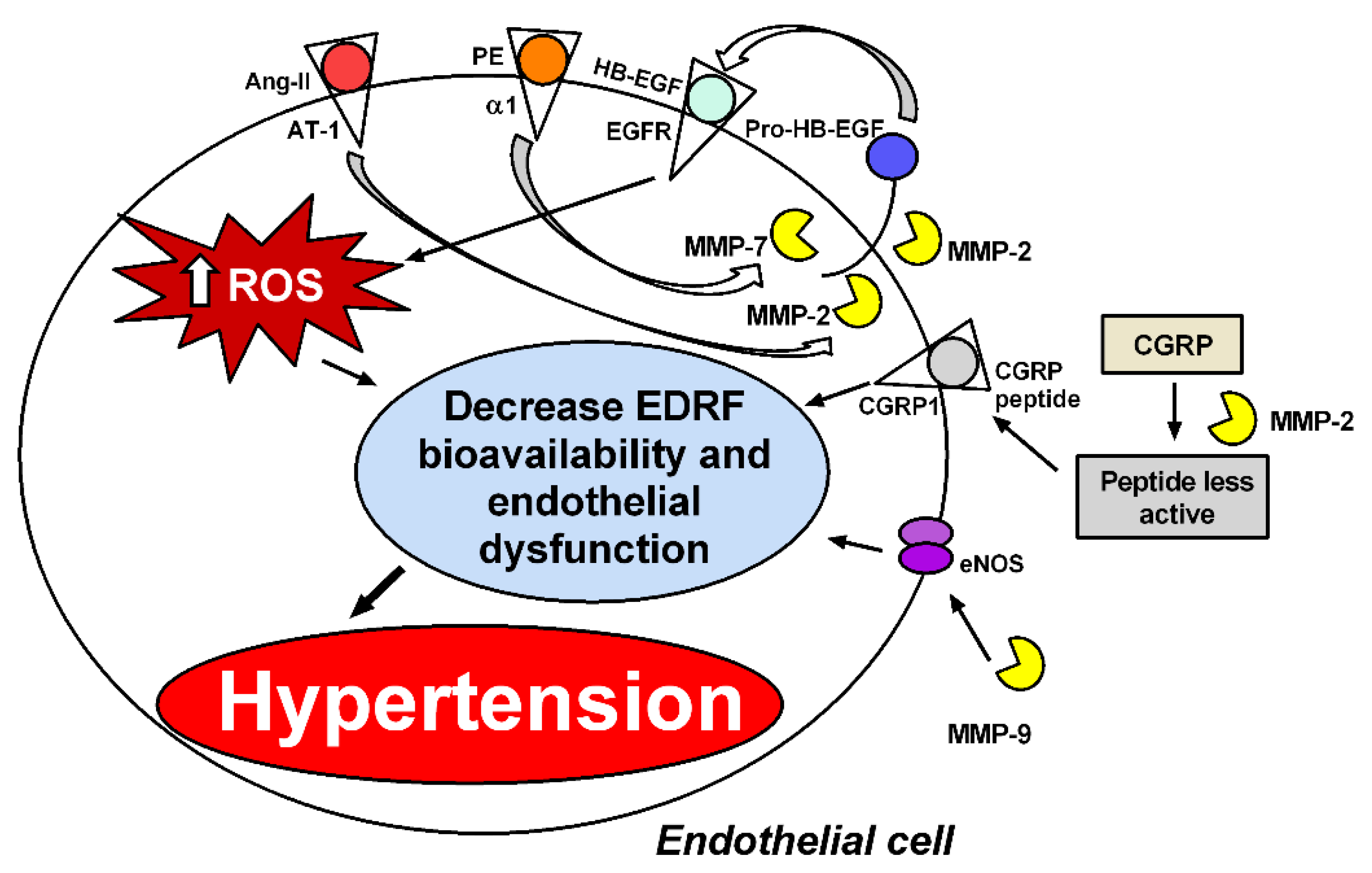
Increased ROS activity reduces NO bioavailability, particularly in hypertension [79], by mechanisms discussed above. The improvement in the endothelium-dependent vascular responses to acetylcholine reported in hypertensive rats treated with doxycycline may reflect improved NO activity resulting from lower NO inactivation by ROS when those animals are treated with this MMP inhibitor [26]. While most studies have implicated MMP-2 as a major cause of vascular dysfunction in hypertension, MMP-9 is apparently also involved. In fact, increased endothelium-dependent vasodilation is described in resistance arteries from MMP-9 knockout mice, and this finding is associated with increased expression of endothelial NO endothelial synthase [80]. In addition to causing increased ROS formation and reduced NO activity, imbalanced vascular MMP activity promotes vascular dysfunction by activating other mechanisms (Figure 3).
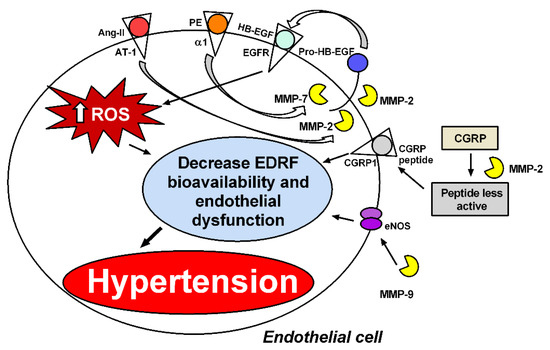
Figure 3.
MMPs induce endothelial dysfunction by different mechanisms. MMP-2 and MMP-7 are required for phenylephrine (PE) to induce reactive oxygen species (ROS) by transactivation of the epidermal growth factor receptor (EGFR) [12,69]. MMP-2 is also required for angiotensin II (Ang-II) to increase perivascular ROS formation [32]. MMP-2 cleaves the vasodilator peptide related to the calcitonin gene (CGRP) into peptides with more minor vasodilatory effects, and MMP-9 decreases endothelial nitric oxide synthase (eNOS) expression and vasodilatation in resistance arteries [80]. ROS decrease endothelium-derived relaxing factors’ (EDRFs’) bioavailability, such as NO, leading to endothelial dysfunction and hypertension.
MMP-mediated proteolytic activity degrades peptides and proteins (particularly receptors) that are involved in the control of vascular tone and blood pressure regulation, and, therefore, may promote hypertension. Rodrigues et al. have shown that MMPs cleave the beta-2 adrenergic receptor, contributing to increased arteriolar tone in spontaneously hypertensive rats (SHR) [30]. Interestingly, MMP-2 infusion also impairs responses to beta-1 adrenergic receptor stimulation [81]. However, the involvement of MMPs in blood pressure regulation remains unclear. Treatment with the nuclear factor kappaB (NF-kB) inhibitor pyrrolidine dithiocarbamate (PDTC) blocks this cleavage process, suggesting that NF-kB increases the activity of MMPs [82], resulting in cleavage of the beta-2 adrenergic receptor [31] (Figure 2).
MMP-2 and MMP-7 are the most widely studied MMPs involved in functional alterations of the cardiovascular system. Previous studies have shown that MMP-2 contributes to impairing blood pressure regulation by cleaving big endothelin-1 (Big ET-1) and generating a potent vasoconstrictor, endothelin 1–32 (ET 1–32) [14]. Moreover, MMP-2 degrades adrenomedullin, leading to the formation of less potent vasodilatory metabolites and some vasoconstrictors [78] (Figure 2). It has also been demonstrated in vitro that MMP-2 cleaves the vasodilator peptide related to the calcitonin gene (CGRP) into peptides with fewer vasodilatory effects [15] (Figure 3). In addition to MMP-2-mediated vasoconstriction and impaired vasodilation, MMP-7 has been shown to promote the vasoconstriction of resistance arteries. Hao et al. have shown that MMP-7 stimulates the vasoconstriction of mesenteric arteries by mechanisms that are dependent on EGFR transactivation, which are essential for maintaining vascular tone [69] (Figure 2). Further supporting this idea, in vivo injection of MMP-7 and MMP-9, individually or in combination, into microvessels of Wistar rats causes the rapid vasoconstriction of venules and arterioles [30] (Figure 2).
The studies discussed above show strong evidence that hypertension is associated with increased vascular MMP activity, resulting in impaired vascular function by a variety of mechanisms affecting vascular biology. Chronic hypertension, however, is associated with several structural alterations to the vasculature.
5. Structural Alterations in the Vasculature Associated with Increased MMP Activity
Structural modifications usually found in the arteries of hypertensive animals are considered adaptive responses to changes in hemodynamic or metabolic demands. However, the progression of such modifications causes maladaptive alterations that can culminate in the pathological vascular alterations observed in cardiovascular diseases [83]. In hypertension, the rearrangement of vascular wall components is caused by changes in blood-pressure-induced circumferential wall stress and blood-flow-induced shear stress, which lead to modifications of the vascular extracellular matrix composition and changes in the cellular secretion of endogenous cytokines, with enhanced sensitivity to circulating humoral factors [25,84].
Several studies correlate circulating MMP concentrations and hypertension [85,86,87,88]. The activation of MMPs, especially MMP-2 and MMP-9, is directly involved in the vascular remodeling observed in hypertension since they are responsible for the degradation of extracellular matrix proteins, including elastin and collagen, and promote migration and phenotypic alterations of VSMC in resistance and conductance arteries [84]. These modifications result in the accumulation of collagen degradation products and increase vascular wall stiffness [84]. Vascular remodeling is a hallmark of a vascular disease’s severity and progression and is critically associated with relevant clinical events and the prognosis [89].
6. Imbalanced Vascular MMP Activity Activates Critical Mechanisms Leading to Vascular Remodeling in Hypertension
While vascular hypertrophy, usually found in the vessels of hypertensive subjects, tends to normalize vascular wall tension, it involves major changes in the endothelial and VSMC, as well as in the composition of the extracellular matrix [90]. In essential hypertension, eutrophic remodeling occurs in small arteries (diameter < 300 μm), which is associated with a reduced lumen and external diameter, increasing the media thickness to lumen diameter (M/L) ratio without significant changes to the total amount of wall tissue (whole cross-sectional area is maintained) or vessel wall material [91]. Vascular remodeling of these arteries prevents an increase in wall stress at the level of arterioles and capillaries [92]. In conductance arteries, however, hypertrophic remodeling results in augmented M/L ratios and cross-sectional areas, which are associated with VSMC growth (increased volume) or hyperplasia (increased cell number), collagen deposition and elastin degradation. These alterations cause increased vessel stiffness and decreased arterial compliance, negatively impacting the myocardial work capacity and coronary perfusion [93].
In addition to the functional alterations promoted by MMPs in the vasculature that are discussed above, these proteases also play critical roles in the vascular remodeling elicited by stimuli such as oxidative stress, inflammation, Ang-II and hemodynamic forces in hypertension [94]. In fact, mechanical forces and vasoconstrictor agonists have synergistic effects on the expression and activity of MMPs in hypertension [95]. Increases in both the MMP-2 and MMP-9 concentrations and activity assessed in the vessels or in the plasma of hypertensive animals or humans are apparently not accompanied by corresponding increases in the activity of endogenous MMP inhibitors (the tissue inhibitors of matrix metalloproteinases), thus revealing imbalanced MMP activity in hypertension [77,85,86,88]. In addition to increasing the degradation of the extracellular matrix components, these alterations stimulate vascular smooth muscle cells to switch from contractile to synthetic phenotypes, thus facilitating cell growth or migration [96]. This phenotype switch, which is associated with variable deposition of extracellular matrix components (collagen, proteoglycan, fibronectin and elastin) [90] is prevented by MMP inhibitors [27,93].
Many bioactive molecules—such as Ang-II, ET-1, aldosterone and catecholamines—impact MMP activity and vascular remodeling, at least in part, because of their capacity to increase oxidative stress and to impair NO activity in the vascular wall [97]. Ang-II activates vascular NADPH oxidase in the VSMC, increasing O2− formation [98], and activates vascular MMP, as discussed above. ET-1 has been shown to enhance MMP-2 activity in mesangial cells, and the blockade of ET-1 type A receptors reduces MMP-2 and MMP-9 in the hearts of deoxycorticosterone acetate (DOCA)-salt hypertensive rats [99,100]. Although aldosterone apparently does not directly activate MMPs, it has been shown that eplerenone, a selective mineralocorticoid receptor antagonist, reduces ventricular MMP-2 and MMP-9 activities in dogs [101,102]. Moreover, the non-selective aldosterone antagonist, spironolactone, reduces vascular MMP-2 activity in hypertensive rats [103]. Catecholamine norepinephrine increases cardiac MMP-2 activity and collagen deposition [104], thus indicating that adrenergic receptors’ activation enhances MMP activity. In fact, the antagonist of β-adrenergic receptors, nebivolol, attenuates hypertension-induced vascular remodeling and increases vascular MMP-2 activity [105]. Similar effects are also reported in the hearts of hypertensive animals [106].
MMP-2 and MMP-9 are also responsible for activating cytokines, such as transforming growth factor-β (TGF-β), which are involved in collagen accumulation and profibrotic alterations in remodeling that are associated with hypertension [105]. Tumor necrosis factor-α (TNF-α) is another cytokine implicated in the vascular remodeling of hypertension, as it is essential for Ang-II-induced MMP-2 expression [107]. Finally, another mechanism that contributes to the VSMC phenotype switch in hypertension is the MMP-2-induced degradation of calponin-1, as recently shown [17,19].
MMP-1, MMP-2, MMP-3 and MMP-9 are the MMPs most frequently found at increased concentrations in hypertensive patients [68,108,109,110,111]. In fact, increased plasma levels of MMP-9 correlate with increased cardiovascular risk, myocardial infarct mortality, cardiac remodeling and dysfunction [112,113,114,115,116]. MMP-2 is also associated with cardiac failure, and both genetic polymorphisms commonly found in the MMP-2 gene [117,118,119,120,121,122] and increased plasma MMP-2 concentrations [123,124,125,126,127,128] are linked to heart failure. On the other hand, MMP-9 and its endogenous inhibitor, tissue inhibitor of metalloproteinase (TIMP)-1, when measured in serum, have been shown at increased concentrations. The MMP-9/TIMP-1 ratio (an index of net MMP-9 activity) is associated with great arterial stiffness, as assessed by carotid-femoral pulse wave velocity, but not with muscular arterial elasticity, as assessed by carotid-radial pulse wave velocity (CRPWV), in patients with hypertension [110]. Yasmin et al. showed increased MMP-2, MMP-9 and serum elastase activity in hypertensive patients. These findings were associated with aortic stiffness; the higher MMP-9 levels were an independent predictor of aortic stiffness [111]. Zhou et al. showed that polymorphism of MMP-9 in never-treated hypertensive patients was associated with increased blood pressure and aortic stiffness [129]. In addition, Stakos et al. showed that aortic stiffness in patients with arterial hypertension was associated with higher proMMP-1 and a higher proMMP-1/TIMP-1 ratio [109]. Together, these studies clearly indicate the involvement of various MMPs in hypertension.
7. MMP Inhibition as a Therapeutic Strategy to Prevent Vascular Remodeling in Hypertension
The huge number of studies indicating that vascular remodeling of hypertension is associated with abnormal vascular MMP activity, matrix deposition and oxidative stress and decreased NO activity suggest that these critical mechanisms are important pharmacological targets to explore in order to prevent or to treat the vascular alterations of hypertension. In the sections above, we discussed a variety of studies using MMP inhibitors or antioxidant drugs that may blunt MMP activation.
While MMP inhibitors or antioxidants directly interfere with vascular MMP activation, many other studies show that other commonly used drugs may also attenuate vascular MMP activation and prevent the vascular remodeling of hypertension by exerting pleiotropic effects not directly associated with their canonical mechanism of action (Figure 4). For example, the antagonist of β1-adrenergic receptors, nebivolol, decreases vascular MMP-2 activity and attenuates hypertension-induced vascular remodeling and collagen deposition by mechanisms apparently independent of β1 adrenoceptor-blocking properties, probably related to diminished NADPH oxidase activity decreasing ROS formation [103,106]. Additional examples of drugs exerting such beneficial pleiotropic effects include some dihydropyridines—such as nifedipine, nimodipine and amlodipine—which have antioxidant effects and downregulate MMP [130,131,132], or losartan, an antagonist of Ang-II type 1 receptors [133]. Other drugs with antioxidant effects, such as quercetin, also exert anti-MMP and anti-remodeling effects in hypertension [29].
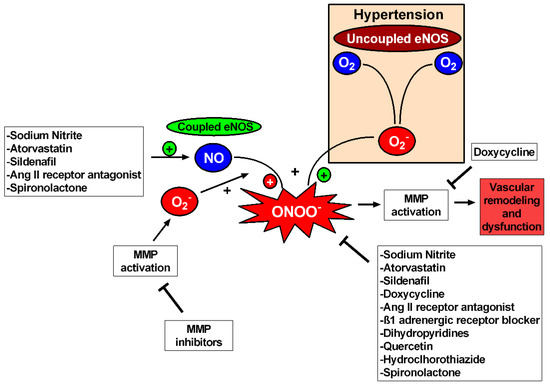
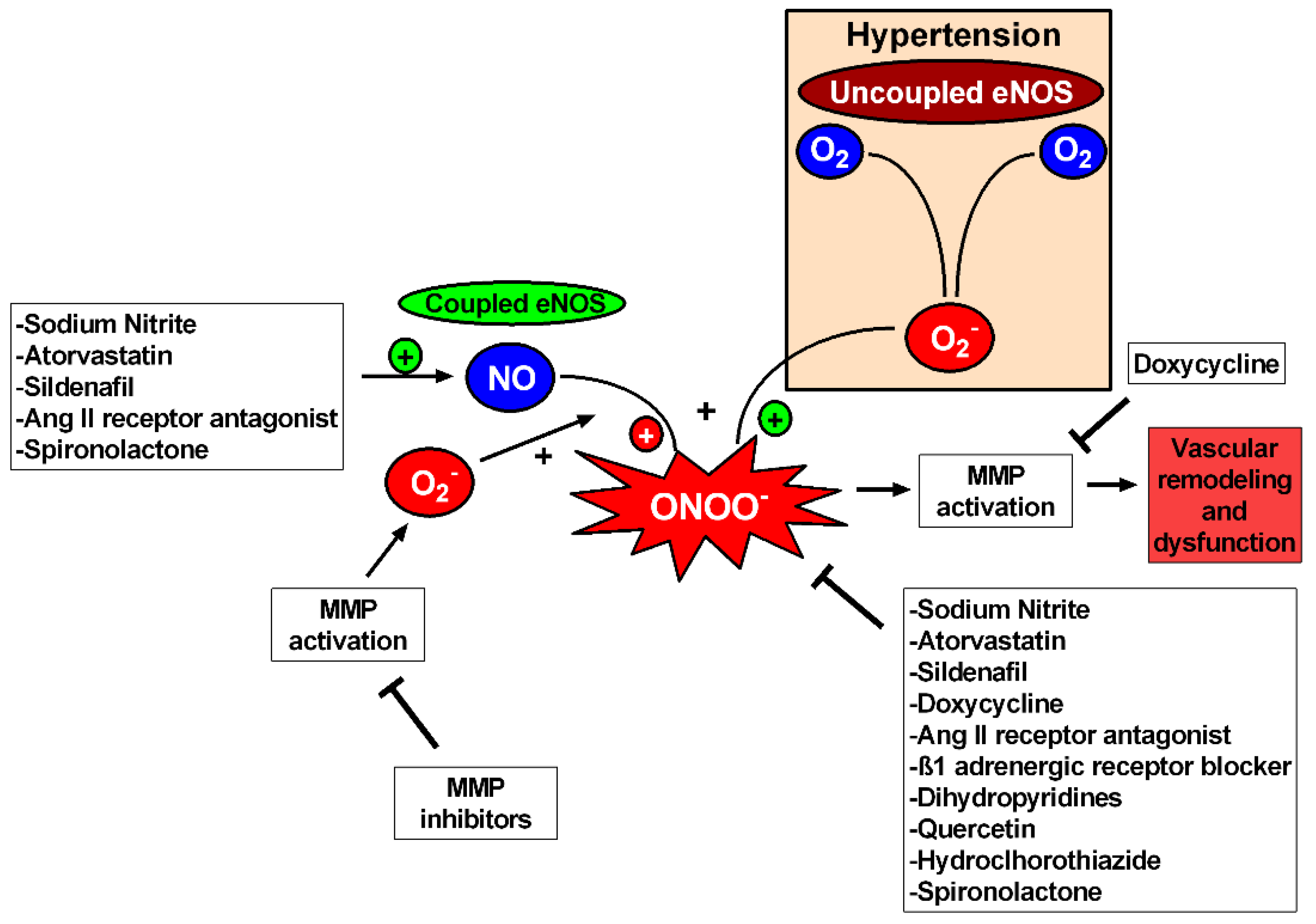
Figure 4.
Oxidative stress inhibition reduces MMP activation in hypertension. Uncoupled eNOS and MMPs produce superoxide (O2−) that uses NO for peroxynitrite (ONOO−) production causing MMP activation and consequently vascular remodeling and dysfunction. Thus, MMP inhibitors and drugs with antioxidant properties can inhibit MMP activation, improving vascular function and reverting vascular remodeling. Doxycycline has antioxidant effects but also relies on direct inhibition of the active forms of MMPs. Drugs capable of augmenting NO bioavailability are also related to MMP inhibition, but further studies are needed to establish the relation between augmentation in NO (and NO-related species) and MMPs in hypertension.
Finally, recent studies have shown that therapeutic strategies to increase NO activity include statins or the phosphodiesterase-5 inhibitor, sildenafil [134]. Inorganic nitrite [135,136] or nitrate [96] supplementation may also exert antioxidant effects and diminish MMP-2 activity and the vascular remodeling of hypertension. Importantly, oral nitrite treatment prevents hypertension-induced vascular oxidative stress and remodeling independently of its anti-hypertensive effects [135], thus suggesting that these beneficial effects are not dependent on blood pressure. Together, these studies strongly suggest a relationship between increased NO availability, decreased oxidative stress, attenuated vascular MMP activity and protection against vascular remodeling in hypertension.
First-generation MMP inhibitors are nonspecific. They act by binding to zinc in the catalytic domain present in all MMPs, thus decreasing its selectivity. These compounds have chemical instability bonding [137,138]. The low selectivity of these compounds is attributable to the fact that zinc is present in other proteases, such as disintegrin and metalloproteinases (ADAMs) and zincins [139,140]. While these inhibitors decrease the activity of MMPs that would be overexpressed, they also decrease the activity of MMPs that are not related to the pathophysiology of the disease. For this reason, first-generation MMP inhibitors are not the best pharmacological choice in clinical trials. MMP inhibitors based on small molecules that bind to the S1 region near the zinc ion in the catalytic site have been developed, thus allowing more selectivity and specificity to particular substrates [137,138]. Others MMP inhibitors—based on peptides, proteins, antibodies and, more recently, targeted mutations in TIMPs—have increased specificity and selectivity [137,138]. Doxycycline is currently the only FDA-approved MMP inhibitor available in the clinic for periodontites treatment [141,142,143]. It is known as a broad-spectrum MMP inhibitor but preferentially inhibits MMP-2, MMP-9 and MMP-8. It is a weak MMP-1 inhibitor and does not inhibit MMP-3 or MMP-7 [144,145,146,147]. Another inhibitor is Ilmostat (GM6001), which has good specificity for MMP-1, MMP-2 and MMP-9 and has already undergone phase-I and phase-II clinical trials [148]. Prinomastat (AG-3340) has specificity for MMP-1, MMP-2 and MMP-9, as shown in a phase-III clinical study [149]. There is the GA-5745/andecaliximab antibody, with selective activity for MMP-9, which has already undergone phase I, II and III clinical studies [150,151,152]. However, it has not yet been tested in cardiovascular disease trials. With respect to the use of MMP inhibitors, it is suggested that normalizing MMP levels instead of completely inhibiting them is better because there are important physiological roles played by these proteases. Excessive MMP inhibition may lead to bone, joint, inflammatory and cardiovascular complications [61,62,63].
It has not yet been established whether eNOS cofactors such as FAD, FMN and BH4 can interfere with MMP activity. However, supplementation with enzyme cofactors directly increases eNOS enzyme activity and NO production [153,154]. It is possible that increasing these cofactors decreases MMP activity in hypertension because NO can modulate MMP activity [71,72,73,74]. Additionally, a study showed that MMP-2 cleaves heat shock protein 90 (HSP90), another cofactor for eNOS activity, and MMP-2 colocalizes with HSP90 and eNOS, suggesting a direct interaction between MMP-2 and eNOS [155]. Therefore, supplementation with eNOS cofactors could represent an exciting, non-tested therapy to control abnormal MMP activity in hypertension.
In conclusion, it is now clear that imbalanced vascular MMP activity promotes vascular dysfunction and a variety of structural alterations, resulting in vascular remodeling in hypertension. The protective effects of MMP inhibitors, antioxidants and drugs that enhance vascular NO activity have recently been becoming more evident and new therapies are emerging that address these important mechanisms, which may offer significant advantages to prevent the vascular remodeling of hypertensive patients.
Author Contributions
All authors have designed and wrote the original manuscript. A.F.P.; R.I.M.B. and J.E.T.-S. wrote, reviewed and edited the manuscript. J.E.T.-S. and R.F.G. supervised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by Fundação de Aparo a Pesquisa do Estado de São Paulo (FAPESP Grant number 2014-23946-0), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that there are no conflict of interest.
References
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; American Heart Association Council; et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar]
- Unger, T.; Borghi, C.; Charchar, F.; Khan, N.A.; Poulter, N.R.; Prabhakaran, D.; Ramirez, A.; Schlaich, M.; Stergiou, G.S.; Tomaszewski, M.; et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension 2020, 75, 1334–1357. [Google Scholar] [CrossRef]
- GBD 2017 Risk Factor Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1923–1994. [Google Scholar]
- Touyz, R.M.; Rios, F.J.; Alves-Lopes, R.; Neves, K.B.; Camargo, L.L.; Montezano, A.C. Oxidative Stress: A Unifying Paradigm in Hypertension. Can. J. Cardiol. 2020, 36, 659–670. [Google Scholar] [CrossRef]
- Costa, T.J.; Barros, P.R.; Arce, C.; Santos, J.D.; Da Silva-Neto, J.; Egea, G.; Dantas, A.P.; Tostes, R.C.; Jimenez-Altayo, F. The homeostatic role of hydrogen peroxide, superoxide anion and nitric oxide in the vasculature. Free Radic. Biol. Med. 2020, 162, 615–635. [Google Scholar] [CrossRef]
- Migita, K.; Maeda, Y.; Abiru, S.; Komori, A.; Yokoyama, T.; Takii, Y.; Nakamura, M.; Yatsuhashi, H.; Eguchi, K.; Ishibashi, H. Peroxynitrite-mediated matrix metalloproteinase-2 activation in human hepatic stellate cells. FEBS Lett. 2005, 579, 3119–3125. [Google Scholar] [CrossRef]
- Rajagopalan, S.; Meng, X.P.; Ramasamy, S.; Harrison, D.G.; Galis, Z.S. Reactive oxygen species produced by macrophage-derived foam cells regulate the activity of vascular matrix metalloproteinases in vitro. Implications for atherosclerotic plaque stability. J. Clin. Investig. 1996, 98, 2572–2579. [Google Scholar] [CrossRef] [PubMed]
- Viappiani, S.; Nicolescu, A.C.; Holt, A.; Sawicki, G.; Crawford, B.D.; Leon, H.; Van Mulligen, T.; Schulz, R. Activation and modulation of 72kDa matrix metalloproteinase-2 by peroxynitrite and glutathione. Biochem. Pharmacol. 2009, 77, 826–834. [Google Scholar] [CrossRef]
- Nagase, H.; Visse, R.; Murphy, G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 2006, 69, 562–573. [Google Scholar] [CrossRef]
- Woessner, J.F., Jr. Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991, 5, 2145–2154. [Google Scholar] [CrossRef]
- Lucchesi, P.A.; Sabri, A.; Belmadani, S.; Matrougui, K. Involvement of metalloproteinases 2/9 in epidermal growth factor receptor transactivation in pressure-induced myogenic tone in mouse mesenteric resistance arteries. Circulation 2004, 110, 3587–3593. [Google Scholar] [CrossRef] [PubMed]
- Prado, A.F.; Pernomian, L.; Azevedo, A.; Costa, R.A.P.; Rizzi, E.; Ramos, J.; Paes Leme, A.F.; Bendhack, L.M.; Tanus-Santos, J.E.; Gerlach, R.F. Matrix metalloproteinase-2-induced epidermal growth factor receptor transactivation impairs redox balance in vascular smooth muscle cells and facilitates vascular contraction. Redox Biol. 2018, 18, 181–190. [Google Scholar] [CrossRef]
- Smiljanic, K.; Obradovic, M.; Jovanovic, A.; Djordjevic, J.; Dobutovic, B.; Jevremovic, D.; Marche, P.; Isenovic, E.R. Thrombin stimulates VSMC proliferation through an EGFR-dependent pathway: Involvement of MMP-2. Mol. Cell. Biochem. 2014, 396, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Patron, C.; Radomski, M.W.; Davidge, S.T. Vascular matrix metalloproteinase-2 cleaves big endothelin-1 yielding a novel vasoconstrictor. Circ. Res. 1999, 85, 906–911. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Patron, C.; Stewart, K.G.; Zhang, Y.; Koivunen, E.; Radomski, M.W.; Davidge, S.T. Vascular matrix metalloproteinase-2-dependent cleavage of calcitonin gene-related peptide promotes vasoconstriction. Circ. Res. 2000, 87, 670–676. [Google Scholar] [CrossRef]
- Ali, M.A.; Cho, W.J.; Hudson, B.; Kassiri, Z.; Granzier, H.; Schulz, R. Titin is a target of matrix metalloproteinase-2: Implications in myocardial ischemia/reperfusion injury. Circulation 2010, 122, 2039–2047. [Google Scholar] [CrossRef] [PubMed]
- Belo, V.A.; Parente, J.M.; Tanus-Santos, J.E.; Castro, M.M. Matrix metalloproteinase (MMP)-2 decreases calponin-1 levels and contributes to arterial remodeling in early hypertension. Biochem. Pharmacol. 2016, 118, 50–58. [Google Scholar] [CrossRef]
- Blascke de Mello, M.M.; Parente, J.M.; Schulz, R.; Castro, M.M. Matrix metalloproteinase (MMP)-2 activation by oxidative stress decreases aortic calponin-1 levels during hypertrophic remodeling in early hypertension. Vascul. Pharmacol. 2019, 116, 36–44. [Google Scholar] [CrossRef]
- Parente, J.M.; Pereira, C.A.; Oliveira-Paula, G.H.; Tanus-Santos, J.E.; Tostes, R.C.; Castro, M.M. Matrix Metalloproteinase-2 Activity is Associated with Divergent Regulation of Calponin-1 in Conductance and Resistance Arteries in Hypertension-induced Early Vascular Dysfunction and Remodelling. Basic Clin. Pharmacol. Toxicol. 2017, 121, 246–256. [Google Scholar] [CrossRef]
- Sawicki, G.; Leon, H.; Sawicka, J.; Sariahmetoglu, M.; Schulze, C.J.; Scott, P.G.; Szczesna-Cordary, D.; Schulz, R. Degradation of myosin light chain in isolated rat hearts subjected to ischemia-reperfusion injury: A new intracellular target for matrix metalloproteinase-2. Circulation 2005, 112, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Sung, M.M.; Schulz, C.G.; Wang, W.; Sawicki, G.; Bautista-Lopez, N.L.; Schulz, R. Matrix metalloproteinase-2 degrades the cytoskeletal protein alpha-actinin in peroxynitrite mediated myocardial injury. J. Mol. Cell. Cardiol. 2007, 43, 429–436. [Google Scholar] [CrossRef]
- Wang, W.; Schulze, C.J.; Suarez-Pinzon, W.L.; Dyck, J.R.; Sawicki, G.; Schulz, R. Intracellular action of matrix metalloproteinase-2 accounts for acute myocardial ischemia and reperfusion injury. Circulation 2002, 106, 1543–1549. [Google Scholar] [CrossRef]
- Azevedo, A.; Prado, A.F.; Antonio, R.C.; Issa, J.P.; Gerlach, R.F. Matrix metalloproteinases are involved in cardiovascular diseases. Basic. Clin. Pharmacol. Toxicol. 2014, 115, 301–314. [Google Scholar] [CrossRef]
- Bouvet, C.; Gilbert, L.A.; Girardot, D.; deBlois, D.; Moreau, P. Different involvement of extracellular matrix components in small and large arteries during chronic NO synthase inhibition. Hypertension 2005, 45, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Brown, I.A.M.; Diederich, L.; Good, M.E.; DeLalio, L.J.; Murphy, S.A.; Cortese-Krott, M.M.; Hall, J.L.; Le, T.H.; Isakson, B.E. Vascular Smooth Muscle Remodeling in Conductive and Resistance Arteries in Hypertension. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1969–1985. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.M.; Rizzi, E.; Ceron, C.S.; Guimaraes, D.A.; Rodrigues, G.J.; Bendhack, L.M.; Gerlach, R.F.; Tanus-Santos, J.E. Doxycycline ameliorates 2K-1C hypertension-induced vascular dysfunction in rats by attenuating oxidative stress and improving nitric oxide bioavailability. Nitric Oxide 2012, 26, 162–168. [Google Scholar] [CrossRef]
- Castro, M.M.; Rizzi, E.; Figueiredo-Lopes, L.; Fernandes, K.; Bendhack, L.M.; Pitol, D.L.; Gerlach, R.F.; Tanus-Santos, J.E. Metalloproteinase inhibition ameliorates hypertension and prevents vascular dysfunction and remodeling in renovascular hypertensive rats. Atherosclerosis 2008, 198, 320–331. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.M.; Rizzi, E.; Rodrigues, G.J.; Ceron, C.S.; Bendhack, L.M.; Gerlach, R.F.; Tanus-Santos, J.E. Antioxidant treatment reduces matrix metalloproteinase-2-induced vascular changes in renovascular hypertension. Free Radic. Biol. Med. 2009, 46, 1298–1307. [Google Scholar] [CrossRef]
- Pereira, S.C.; Parente, J.M.; Belo, V.A.; Mendes, A.S.; Gonzaga, N.A.; do Vale, G.T.; Ceron, C.S.; Tanus-Santos, J.E.; Tirapelli, C.R.; Castro, M.M. Quercetin decreases the activity of matrix metalloproteinase-2 and ameliorates vascular remodeling in renovascular hypertension. Atherosclerosis 2018, 270, 146–153. [Google Scholar] [CrossRef]
- Rodrigues, S.F.; Tran, E.D.; Fortes, Z.B.; Schmid-Schonbein, G.W. Matrix metalloproteinases cleave the beta2-adrenergic receptor in spontaneously hypertensive rats. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H25–H35. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.I.; Schmid-Schonbein, G.W. Nuclear factor kappa B and matrix metalloproteinase induced receptor cleavage in the spontaneously hypertensive rat. Hypertension 2011, 57, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Barhoumi, T.; Fraulob-Aquino, J.C.; Mian, M.O.R.; Ouerd, S.; Idris-Khodja, N.; Huo, K.G.; Rehman, A.; Caillon, A.; Dancose-Giambattisto, B.; Ebrahimian, T.; et al. Matrix metalloproteinase-2 knockout prevents angiotensin II-induced vascular injury. Cardiovasc. Res. 2017, 113, 1753–1762. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Du, M.; Lopez-Campistrous, A.; Fernandez-Patron, C. Agonist-induced activation of matrix metalloproteinase-7 promotes vasoconstriction through the epidermal growth factor-receptor pathway. Circ. Res. 2004, 94, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, P.; Kossmann, S.; Munzel, T.; Daiber, A. Redox regulation of cardiovascular inflammation—Immunomodulatory function of mitochondrial and Nox-derived reactive oxygen and nitrogen species. Free Radic. Biol. Med. 2017, 109, 48–60. [Google Scholar] [CrossRef]
- Forrester, S.J.; Booz, G.W.; Sigmund, C.D.; Coffman, T.M.; Kawai, T.; Rizzo, V.; Scalia, R.; Eguchi, S. Angiotensin II Signal Transduction: An Update on Mechanisms of Physiology and Pathophysiology. Physiol. Rev. 2018, 98, 1627–1738. [Google Scholar] [CrossRef]
- Nguyen Dinh Cat, A.; Montezano, A.C.; Burger, D.; Touyz, R.M. Angiotensin II, NADPH oxidase, and redox signaling in the vasculature. Antioxid. Redox Signal. 2013, 19, 1110–1120. [Google Scholar] [CrossRef]
- Santillo, M.; Colantuoni, A.; Mondola, P.; Guida, B.; Damiano, S. NOX signaling in molecular cardiovascular mechanisms involved in the blood pressure homeostasis. Front. Physiol. 2015, 6, 194. [Google Scholar] [CrossRef]
- Thomas, D.D.; Ridnour, L.A.; Isenberg, J.S.; Flores-Santana, W.; Switzer, C.H.; Donzelli, S.; Hussain, P.; Vecoli, C.; Paolocci, N.; Ambs, S.; et al. The chemical biology of nitric oxide: Implications in cellular signaling. Free Radic. Biol. Med. 2008, 45, 18–31. [Google Scholar] [CrossRef]
- Liu, X.; Xu, X.; Shang, R.; Chen, Y. Asymmetric dimethylarginine (ADMA) as an important risk factor for the increased cardiovascular diseases and heart failure in chronic kidney disease. Nitric Oxide 2018, 78, 113–120. [Google Scholar] [CrossRef]
- Cai, H.; Harrison, D.G. Endothelial dysfunction in cardiovascular diseases: The role of oxidant stress. Circ. Res. 2000, 87, 840–844. [Google Scholar] [CrossRef] [PubMed]
- Forstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 837a–837d. [Google Scholar] [CrossRef]
- Oliveira-Paula, G.H.; Lacchini, R.; Tanus-Santos, J.E. Inducible nitric oxide synthase as a possible target in hypertension. Curr. Drug Targets 2014, 15, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Stathopulos, P.B.; Lu, X.; Shen, J.; Scott, J.A.; Hammond, J.R.; McCormack, D.G.; Arnold, J.M.; Feng, Q. Increased L-arginine uptake and inducible nitric oxide synthase activity in aortas of rats with heart failure. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H859–H867. [Google Scholar] [CrossRef]
- Ferreiro, C.R.; Chagas, A.C.; Carvalho, M.H.; Dantas, A.P.; Scavone, C.; Souza, L.C.; Buffolo, E.; da Luz, P.L. Expression of inducible nitric oxide synthase is increased in patients with heart failure due to ischemic disease. Braz. J. Med. Biol. Res. 2004, 37, 1313–1320. [Google Scholar] [CrossRef][Green Version]
- Haywood, G.A.; Tsao, P.S.; Von der Leyen, H.E.; Mann, M.J.; Keeling, P.J.; Trindade, P.T.; Lewis, N.P.; Byrne, C.D.; Rickenbacher, P.R.; Bishopric, N.H.; et al. Expression of inducible nitric oxide synthase in human heart failure. Circulation 1996, 93, 1087–1094. [Google Scholar] [CrossRef]
- Feng, Q.; Lu, X.; Jones, D.L.; Shen, J.; Arnold, J.M. Increased inducible nitric oxide synthase expression contributes to myocardial dysfunction and higher mortality after myocardial infarction in mice. Circulation 2001, 104, 700–704. [Google Scholar] [CrossRef]
- Liu, Y.H.; Carretero, O.A.; Cingolani, O.H.; Liao, T.D.; Sun, Y.; Xu, J.; Li, L.Y.; Pagano, P.J.; Yang, J.J.; Yang, X.P. Role of inducible nitric oxide synthase in cardiac function and remodeling in mice with heart failure due to myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H2616–H2623. [Google Scholar] [CrossRef]
- Van Wart, H.E.; Birkedal-Hansen, H. The cysteine switch: A principle of regulation of metalloproteinase activity with potential applicability to the entire matrix metalloproteinase gene family. Proc. Natl. Acad. Sci. USA 1990, 87, 5578–5582. [Google Scholar] [CrossRef]
- Page-McCaw, A.; Ewald, A.J.; Werb, Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat. Rev. Mol. Cell. Biol. 2007, 8, 221–233. [Google Scholar] [CrossRef]
- Fliss, H.; Menard, M. Hypochlorous acid-induced mobilization of zinc from metalloproteins. Arch. Biochem. Biophys. 1991, 287, 175–179. [Google Scholar] [CrossRef]
- Fliss, H.; Menard, M. Oxidant-induced mobilization of zinc from metallothionein. Arch. Biochem. Biophys. 1992, 293, 195–199. [Google Scholar] [CrossRef]
- Vallee, B.L.; Auld, D.S. Zinc coordination, function, and structure of zinc enzymes and other proteins. Biochemistry 1990, 29, 5647–5659. [Google Scholar] [CrossRef]
- Sariahmetoglu, M.; Crawford, B.D.; Leon, H.; Sawicka, J.; Li, L.; Ballermann, B.J.; Holmes, C.; Berthiaume, L.G.; Holt, A.; Sawicki, G.; et al. Regulation of matrix metalloproteinase-2 (MMP-2) activity by phosphorylation. FASEB J. 2007, 21, 2486–2495. [Google Scholar] [CrossRef]
- Jacob-Ferreira, A.L.; Kondo, M.Y.; Baral, P.K.; James, M.N.; Holt, A.; Fan, X.; Schulz, R. Phosphorylation status of 72 kDa MMP-2 determines its structure and activity in response to peroxynitrite. PLoS ONE 2013, 8, e71794. [Google Scholar]
- Castberg, F.C.; Kjaergaard, S.; Mosig, R.A.; Lobl, M.; Martignetti, C.; Martignetti, J.A.; Myrup, C.; Zak, M. Multicentric osteolysis with nodulosis and arthropathy (MONA) with cardiac malformation, mimicking polyarticular juvenile idiopathic arthritis: Case report and literature review. Eur. J. Pediatr. 2013, 172, 1657–1663. [Google Scholar] [CrossRef]
- Martignetti, J.A.; Aqeel, A.A.; Sewairi, W.A.; Boumah, C.E.; Kambouris, M.; Mayouf, S.A.; Sheth, K.V.; Eid, W.A.; Dowling, O.; Harris, J.; et al. Mutation of the matrix metalloproteinase 2 gene (MMP2) causes a multicentric osteolysis and arthritis syndrome. Nat. Genet. 2001, 28, 261–265. [Google Scholar] [CrossRef]
- Mosig, R.A.; Dowling, O.; DiFeo, A.; Ramirez, M.C.; Parker, I.C.; Abe, E.; Diouri, J.; Aqeel, A.A.; Wylie, J.D.; Oblander, S.A.; et al. Loss of MMP-2 disrupts skeletal and craniofacial development and results in decreased bone mineralization, joint erosion and defects in osteoblast and osteoclast growth. Hum. Mol. Genet. 2007, 16, 1113–1123. [Google Scholar] [CrossRef] [PubMed]
- Tuysuz, B.; Mosig, R.; Altun, G.; Sancak, S.; Glucksman, M.J.; Martignetti, J.A. A novel matrix metalloproteinase 2 (MMP2) terminal hemopexin domain mutation in a family with multicentric osteolysis with nodulosis and arthritis with cardiac defects. Eur. J. Hum. Genet. 2009, 17, 565–572. [Google Scholar] [CrossRef]
- Lalu, M.M.; Gao, C.Q.; Schulz, R. Matrix metalloproteinase inhibitors attenuate endotoxemia induced cardiac dysfunction: A potential role for MMP-9. Mol. Cell. Biochem. 2003, 251, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Berry, E.; Hernandez-Anzaldo, S.; Ghomashchi, F.; Lehner, R.; Murakami, M.; Gelb, M.H.; Kassiri, Z.; Wang, X.; Fernandez-Patron, C. Matrix metalloproteinase-2 negatively regulates cardiac secreted phospholipase A2 to modulate inflammation and fever. J. Am. Heart Assoc. 2015, 4, e1868. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Patron, C.; Leung, D. Emergence of a metalloproteinase/phospholipase A2 axis of systemic inflammation. Metalloproteinases Med. 2015, 2, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Anzaldo, S.; Berry, E.; Brglez, V.; Leung, D.; Yun, T.J.; Lee, J.S.; Filep, J.G.; Kassiri, Z.; Cheong, C.; Lambeau, G.; et al. Identification of a Novel Heart-Liver Axis: Matrix Metalloproteinase-2 Negatively Regulates Cardiac Secreted Phospholipase A2 to Modulate Lipid Metabolism and Inflammation in the Liver. J. Am. Heart Assoc. 2015, 4, e2553. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Patron, C.; Kassiri, Z.; Leung, D. Modulation of Systemic Metabolism by MMP-2: From MMP-2 Deficiency in Mice to MMP-2 Deficiency in Patients. Compr. Physiol. 2016, 6, 1935–1949. [Google Scholar]
- Rizzi, E.; Ceron, C.S.; Guimaraes, D.A.; Prado, C.M.; Rossi, M.A.; Gerlach, R.F.; Tanus-Santos, J.E. Temporal changes in cardiac matrix metalloproteinase activity, oxidative stress, and TGF-beta in renovascular hypertension-induced cardiac hypertrophy. Exp. Mol. Pathol. 2012, 94, 1–9. [Google Scholar] [CrossRef]
- Guimaraes, D.A.; Rizzi, E.; Ceron, C.S.; Oliveira, A.M.; Oliveira, D.M.; Castro, M.M.; Tirapelli, C.R.; Gerlach, R.F.; Tanus-Santos, J.E. Doxycycline dose-dependently inhibits MMP-2-mediated vascular changes in 2K1C hypertension. Basic Clin. Pharmacol. Toxicol. 2011, 108, 318–325. [Google Scholar] [CrossRef]
- Lovett, D.H.; Mahimkar, R.; Raffai, R.L.; Cape, L.; Maklashina, E.; Cecchini, G.; Karliner, J.S. A novel intracellular isoform of matrix metalloproteinase-2 induced by oxidative stress activates innate immunity. PLoS ONE 2012, 7, e34177. [Google Scholar] [CrossRef]
- Zhou, H.Z.; Ma, X.; Gray, M.O.; Zhu, B.Q.; Nguyen, A.P.; Baker, A.J.; Simonis, U.; Cecchini, G.; Lovett, D.H.; Karliner, J.S. Transgenic MMP-2 expression induces latent cardiac mitochondrial dysfunction. Biochem. Biophys. Res. Commun. 2007, 358, 189–195. [Google Scholar] [CrossRef]
- Hao, L.; Nishimura, T.; Wo, H.; Fernandez-Patron, C. Vascular responses to alpha1-adrenergic receptors in small rat mesenteric arteries depend on mitochondrial reactive oxygen species. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 819–825. [Google Scholar] [CrossRef]
- Naseem, K.M. The role of nitric oxide in cardiovascular diseases. Mol. Aspects Med. 2005, 26, 33–65. [Google Scholar] [CrossRef]
- O’Sullivan, S.; Medina, C.; Ledwidge, M.; Radomski, M.W.; Gilmer, J.F. Nitric oxide-matrix metaloproteinase-9 interactions: Biological and pharmacological significance--NO and MMP-9 interactions. Biochim. Biophys. Acta 2014, 1843, 603–617. [Google Scholar] [CrossRef] [PubMed]
- Manabe, S.; Gu, Z.; Lipton, S.A. Activation of matrix metalloproteinase-9 via neuronal nitric oxide synthase contributes to NMDA-induced retinal ganglion cell death. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4747–4753. [Google Scholar] [CrossRef] [PubMed]
- Phillips, P.G.; Birnby, L.M. Nitric oxide modulates caveolin-1 and matrix metalloproteinase-9 expression and distribution at the endothelial cell/tumor cell interface. Am. J. Physiol. Lung Cell Mol. Physiol. 2004, 286, L1055–L1065. [Google Scholar] [CrossRef] [PubMed]
- Demacq, C.; Metzger, I.F.; Gerlach, R.F.; Tanus-Santos, J.E. Inverse relationship between markers of nitric oxide formation and plasma matrix metalloproteinase-9 levels in healthy volunteers. Clin. Chim. Acta 2008, 394, 72–76. [Google Scholar] [CrossRef]
- Metzger, I.F.; Sandrim, V.C.; Tanus-Santos, J.E. Endogenous nitric oxide formation correlates negatively with circulating matrix metalloproteinase (MMP)-2 and MMP-9 levels in black subjects. Mol. Cell. Biochem. 2012, 360, 393–399. [Google Scholar] [CrossRef]
- El-Hawary, S.S.; Sobeh, M.; Badr, W.K.; Abdelfattah, M.A.O.; Ali, Z.Y.; El-Tantawy, M.E.; Rabeh, M.A.; Wink, M. HPLC-PDA-MS/MS profiling of secondary metabolites from Opuntia ficus-indica cladode, peel and fruit pulp extracts and their antioxidant, neuroprotective effect in rats with aluminum chloride induced neurotoxicity. Saudi J. Biol. Sci. 2020, 27, 2829–2838. [Google Scholar] [CrossRef]
- Castro, M.M.; Rizzi, E.; Prado, C.M.; Rossi, M.A.; Tanus-Santos, J.E.; Gerlach, R.F. Imbalance between matrix metalloproteinases and tissue inhibitor of metalloproteinases in hypertensive vascular remodeling. Matrix Biol. 2010, 29, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.; Oh, H.R.; Unsworth, E.J.; Bregonzio, C.; Saavedra, J.M.; Stetler-Stevenson, W.G.; Cuttitta, F. Matrix metalloproteinase-2 cleavage of adrenomedullin produces a vasoconstrictor out of a vasodilator. Biochem. J. 2004, 383, 413–418. [Google Scholar] [CrossRef]
- Incalza, M.A.; D’Oria, R.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vascul. Pharmacol. 2018, 100, 1–19. [Google Scholar] [CrossRef]
- Su, J.; Palen, D.I.; Lucchesi, P.A.; Matrougui, K. Mice lacking the gene encoding for MMP-9 and resistance artery reactivity. Biochem. Biophys. Res. Commun. 2006, 349, 1177–1181. [Google Scholar] [CrossRef] [PubMed]
- Weckmann, M.; Thiele, D.; Liboschik, L.; Bahmer, T.; Pech, M.; Dittrich, A.M.; Fuchs, O.; Happle, C.; Schaub, B.; Ricklefs, I.; et al. Cytokine levels in children and adults with wheezing and asthma show specific patterns of variability over time. Clin. Exp. Immunol. 2020, 204, 152–164. [Google Scholar] [CrossRef]
- Cau, S.B.; Guimaraes, D.A.; Rizzi, E.; Ceron, C.S.; Souza, L.L.; Tirapelli, C.R.; Gerlach, R.F.; Tanus-Santos, J.E. Pyrrolidine dithiocarbamate down-regulates vascular matrix metalloproteinases and ameliorates vascular dysfunction and remodelling in renovascular hypertension. Br. J. Pharmacol. 2011, 164, 372–381. [Google Scholar] [CrossRef]
- Belo, V.A.; Guimaraes, D.A.; Castro, M.M. Matrix Metalloproteinase 2 as a Potential Mediator of Vascular Smooth Muscle Cell Migration and Chronic Vascular Remodeling in Hypertension. J. Vasc. Res. 2015, 52, 221–231. [Google Scholar] [CrossRef]
- Galis, Z.S.; Khatri, J.J. Matrix metalloproteinases in vascular remodeling and atherogenesis: The good, the bad, and the ugly. Circ. Res. 2002, 90, 251–262. [Google Scholar] [CrossRef]
- Xie, S.H.; Rabbani, S.; Ness-Jensen, E.; Lagergren, J. Circulating Levels of Inflammatory and Metabolic Biomarkers and Risk of Esophageal Adenocarcinoma and Barrett Esophagus: Systematic Review and Meta-analysis. Cancer Epidemiol. Biomark. Prev. 2020, 29, 2109–2118. [Google Scholar] [CrossRef]
- Raafat, K.; El-Darra, N.; Saleh, F.A. Gastroprotective and anti-inflammatory effects of Prunus cerasus phytochemicals and their possible mechanisms of action. J. Tradit. Complement. Med. 2020, 10, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Ritter, A.M.; de Faria, A.P.; Barbaro, N.; Sabbatini, A.R.; Correa, N.B.; Brunelli, V.; Amorim, R.; Modolo, R.; Moreno, H. Crosstalk between obesity and MMP-9 in cardiac remodelling -a cross-sectional study in apparent treatment-resistant hypertension. Blood Pressure 2017, 26, 122–129. [Google Scholar] [CrossRef]
- Tayebjee, M.H.; Nadar, S.; Blann, A.D.; Gareth Beevers, D.; MacFadyen, R.J.; Lip, G.Y. Matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 in hypertension and their relationship to cardiovascular risk and treatment: A substudy of the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT). Am. J. Hypertens. 2004, 17, 764–769. [Google Scholar] [CrossRef]
- O’Leary, D.H.; Polak, J.F.; Kronmal, R.A.; Manolio, T.A.; Burke, G.L.; Wolfson, S.K., Jr. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N. Engl. J. Med. 1999, 340, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Naiki, T. Adaptation and remodeling of vascular wall; biomechanical response to hypertension. J. Mech. Behav. Biomed. Mater. 2009, 2, 3–19. [Google Scholar] [CrossRef]
- Wang, X.; Khalil, R.A. Matrix Metalloproteinases, Vascular Remodeling, and Vascular Disease. Adv. Pharmacol. 2018, 81, 241–330. [Google Scholar] [PubMed]
- Lee, R.M.; Dickhout, J.G.; Sandow, S.L. Vascular structural and functional changes: Their association with causality in hypertension: Models, remodeling and relevance. Hypertens. Res. 2017, 40, 311–323. [Google Scholar] [CrossRef]
- Castro, M.M.; Tanus-Santos, J.E.; Gerlach, R.F. Matrix metalloproteinases: Targets for doxycycline to prevent the vascular alterations of hypertension. Pharmacol. Res. 2011, 64, 567–572. [Google Scholar] [CrossRef]
- Renna, N.F.; de Las Heras, N.; Miatello, R.M. Pathophysiology of vascular remodeling in hypertension. Int. J. Hypertens. 2013, 2013, 808353. [Google Scholar] [CrossRef]
- Feihl, F.; Liaudet, L.; Levy, B.I.; Waeber, B. Hypertension and microvascular remodelling. Cardiovasc. Res. 2008, 78, 274–285. [Google Scholar] [CrossRef]
- Miller, A.; Carr, S.; Rabbitts, T.; Ali, H. Multimeric antibodies with increased valency surpassing functional affinity and potency thresholds using novel formats. MAbs 2020, 12, 1752529. [Google Scholar] [CrossRef] [PubMed]
- Tsuruda, T.; Costello-Boerrigter, L.C.; Burnett, J.C., Jr. Matrix metalloproteinases: Pathways of induction by bioactive molecules. Heart Fail. Rev. 2004, 9, 53–61. [Google Scholar] [CrossRef]
- Griendling, K.K.; Minieri, C.A.; Ollerenshaw, J.D.; Alexander, R.W. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ. Res. 1994, 74, 1141–1148. [Google Scholar] [CrossRef]
- Kitamura, A.; Kagami, S.; Urushihara, M.; Kondo, S.; Yoshizumi, M.; Tamaki, T.; Kuroda, Y. Endothelin-1 is a potent stimulator of alpha1beta1 integrin-mediated collagen matrix remodeling by rat mesangial cells. Biochem. Biophys. Res. Commun. 2002, 299, 555–561. [Google Scholar] [CrossRef]
- Ammarguellat, F.Z.; Gannon, P.O.; Amiri, F.; Schiffrin, E.L. Fibrosis, matrix metalloproteinases, and inflammation in the heart of DOCA-salt hypertensive rats: Role of ET(A) receptors. Hypertension 2002, 39, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Brilla, C.G.; Zhou, G.; Matsubara, L.; Weber, K.T. Collagen metabolism in cultured adult rat cardiac fibroblasts: Response to angiotensin II and aldosterone. J. Mol. Cell. Cardiol. 1994, 26, 809–820. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, G.; Morita, H.; Mishima, T.; Sharov, V.G.; Todor, A.; Tanhehco, E.J.; Rudolph, A.E.; McMahon, E.G.; Goldstein, S.; Sabbah, H.N. Effects of long-term monotherapy with eplerenone, a novel aldosterone blocker, on progression of left ventricular dysfunction and remodeling in dogs with heart failure. Circulation 2002, 106, 2967–2972. [Google Scholar] [CrossRef] [PubMed]
- Ceron, C.S.; Castro, M.M.; Rizzi, E.; Montenegro, M.F.; Fontana, V.; Salgado, M.C.; Gerlach, R.F.; Tanus-Santos, J.E. Spironolactone and hydrochlorothiazide exert antioxidant effects and reduce vascular matrix metalloproteinase-2 activity and expression in a model of renovascular hypertension. Br. J. Pharmacol. 2010, 160, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Briest, W.; Holzl, A.; Rassler, B.; Deten, A.; Leicht, M.; Baba, H.A.; Zimmer, H.G. Cardiac remodeling after long term norepinephrine treatment in rats. Cardiovasc. Res. 2001, 52, 265–273. [Google Scholar] [CrossRef]
- Khafipour, A.; Eissa, N.; Munyaka, P.M.; Rabbi, M.F.; Kapoor, K.; Kermarrec, L.; Khafipour, E.; Bernstein, C.N.; Ghia, J.E. Denosumab Regulates Gut Microbiota Composition and Cytokines in Dinitrobenzene Sulfonic Acid (DNBS)-Experimental Colitis. Front. Microbiol. 2020, 11, 1405. [Google Scholar] [CrossRef]
- Rizzi, E.; Guimaraes, D.A.; Ceron, C.S.; Prado, C.M.; Pinheiro, L.C.; Martins-Oliveira, A.; Gerlach, R.F.; Tanus-Santos, J.E. Beta1-Adrenergic blockers exert antioxidant effects, reduce matrix metalloproteinase activity, and improve renovascular hypertension-induced cardiac hypertrophy. Free Radic. Biol. Med. 2014, 73, 308–317. [Google Scholar] [CrossRef]
- Mattos, B.R.; Bonacio, G.F.; Vitorino, T.R.; Garcia, V.T.; Amaral, J.H.; Dellalibera-Joviliano, R.; Franca, S.C.; Tanus-Santos, J.E.; Rizzi, E. TNF-alpha inhibition decreases MMP-2 activity, reactive oxygen species formation and improves hypertensive vascular hypertrophy independent of its effects on blood pressure. Biochem. Pharmacol. 2020, 180, 114121. [Google Scholar] [CrossRef]
- McNulty, M.; Mahmud, A.; Spiers, P.; Feely, J. Collagen type-I degradation is related to arterial stiffness in hypertensive and normotensive subjects. J. Hum. Hypertens. 2006, 20, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Stakos, D.A.; Tziakas, D.N.; Chalikias, G.K.; Mitrousi, K.; Tsigalou, C.; Boudoulas, H. Associations between collagen synthesis and degradation and aortic function in arterial hypertension. Am. J. Hypertens. 2010, 23, 488–494. [Google Scholar] [CrossRef]
- Tan, J.; Hua, Q.; Xing, X.; Wen, J.; Liu, R.; Yang, Z. Impact of the metalloproteinase-9/tissue inhibitor of metalloproteinase-1 system on large arterial stiffness in patients with essential hypertension. Hypertens. Res. 2007, 30, 959–963. [Google Scholar] [CrossRef]
- Yasmin, S.W.; McEniery, C.M.; Wallace, S.; Dakham, Z.; Pulsalkar, P.; Maki-Petaja, K.; Ashby, M.J.; Cockcroft, J.R.; Wilkinson, I.B. Matrix metalloproteinase-9 (MMP-9), MMP-2, and serum elastase activity are associated with systolic hypertension and arterial stiffness. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 372. [Google Scholar] [CrossRef]
- Kelly, D.; Cockerill, G.; Ng, L.L.; Thompson, M.; Khan, S.; Samani, N.J.; Squire, I.B. Plasma matrix metalloproteinase-9 and left ventricular remodelling after acute myocardial infarction in man: A prospective cohort study. Eur. Heart J. 2007, 28, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Somuncu, M.U.; Pusuroglu, H.; Karakurt, H.; Bolat, I.; Karakurt, S.T.; Demir, A.R.; Isiksacan, N.; Akgul, O.; Surgit, O. The prognostic value of elevated matrix metalloproteinase-9 in patients undergoing primary percutaneous coronary intervention for ST-elevation myocardial infarction: A two-year prospective study. Rev. Port. Cardiol. 2020, 39, 267–276. [Google Scholar] [CrossRef]
- Squire, I.B.; Evans, J.; Ng, L.L.; Loftus, I.M.; Thompson, M.M. Plasma MMP-9 and MMP-2 following acute myocardial infarction in man: Correlation with echocardiographic and neurohumoral parameters of left ventricular dysfunction. J. Card. Fail. 2004, 10, 328–333. [Google Scholar] [CrossRef]
- Zhu, J.J.; Zhao, Q.; Qu, H.J.; Li, X.M.; Chen, Q.J.; Liu, F.; Chen, B.D.; Yang, Y.N. Usefulness of plasma matrix metalloproteinase-9 levels in prediction of in-hospital mortality in patients who received emergent percutaneous coronary artery intervention following myocardial infarction. Oncotarget 2017, 8, 105809–105818. [Google Scholar] [CrossRef]
- Blankenberg, S.; Rupprecht, H.J.; Poirier, O.; Bickel, C.; Smieja, M.; Hafner, G.; Meyer, J.; Cambien, F.; Tiret, L.; AtheroGene, I. Plasma concentrations and genetic variation of matrix metalloproteinase 9 and prognosis of patients with cardiovascular disease. Circulation 2003, 107, 1579–1585. [Google Scholar] [CrossRef]
- Hua, Y.; Song, L.; Wu, N.; Xie, G.; Lu, X.; Fan, X.; Meng, X.; Gu, D.; Yang, Y. Polymorphisms of MMP-2 gene are associated with systolic heart failure prognosis. Clin. Chim. Acta 2009, 404, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Vasku, A.; Goldbergova, M.; Holla, L.I.; Spinarova, L.; Spinar, J.; Vitovec, J.; Vacha, J. Two MMP-2 promoter polymorphisms (−790T/G and −735C/T) in chronic heart failure. Clin. Chem. Lab. Med. 2003, 41, 1299–1303. [Google Scholar] [CrossRef]
- Beber, A.R.; Polina, E.R.; Biolo, A.; Santos, B.L.; Gomes, D.C.; La Porta, V.L.; Olsen, V.; Clausell, N.; Rohde, L.E.; Santos, K.G. Matrix Metalloproteinase-2 Polymorphisms in Chronic Heart Failure: Relationship with Susceptibility and Long-Term Survival. PLoS ONE 2016, 11, e0161666. [Google Scholar] [CrossRef]
- Delgado-Enciso, I.; Gonzalez-Hernandez, N.A.; Baltazar-Rodriguez, L.M.; Millan-Guerrero, R.O.; Newton-Sanchez, O.; Bayardo-Noriega, A.; Aleman-Mireles, A.; Enriquez-Maldonado, I.G.; Anaya-Carrillo, M.J.; Rojas-Martinez, A.; et al. Association of matrix metalloproteinase-2 gene promoter polymorphism with myocardial infarction susceptibility in a Mexican population. J. Genet. 2009, 88, 249–252. [Google Scholar] [CrossRef][Green Version]
- Mizon-Gerard, F.; de Groote, P.; Lamblin, N.; Hermant, X.; Dallongeville, J.; Amouyel, P.; Bauters, C.; Helbecque, N. Prognostic impact of matrix metalloproteinase gene polymorphisms in patients with heart failure according to the aetiology of left ventricular systolic dysfunction. Eur. Heart J. 2004, 25, 688–693. [Google Scholar] [CrossRef][Green Version]
- Perez-Hernandez, N.; Vargas-Alarcon, G.; Martinez-Rodriguez, N.; Martinez-Rios, M.A.; Pena-Duque, M.A.; Pena-Diaz Ade, L.; Valente-Acosta, B.; Posadas-Romero, C.; Medina, A.; Rodriguez-Perez, J.M. The matrix metalloproteinase 2-1575 gene polymorphism is associated with the risk of developing myocardial infarction in Mexican patients. J. Atheroscler. Thromb. 2012, 19, 718–727. [Google Scholar]
- Biolo, A.; Fisch, M.; Balog, J.; Chao, T.; Schulze, P.C.; Ooi, H.; Siwik, D.; Colucci, W.S. Episodes of acute heart failure syndrome are associated with increased levels of troponin and extracellular matrix markers. Circ. Heart Fail. 2010, 3, 44–50. [Google Scholar] [CrossRef]
- Nilsson, L.; Hallen, J.; Atar, D.; Jonasson, L.; Swahn, E. Early measurements of plasma matrix metalloproteinase-2 predict infarct size and ventricular dysfunction in ST-elevation myocardial infarction. Heart 2012, 98, 31–36. [Google Scholar] [CrossRef]
- Shirakabe, A.; Asai, K.; Hata, N.; Yokoyama, S.; Shinada, T.; Kobayashi, N.; Tomita, K.; Tsurumi, M.; Matsushita, M.; Mizuno, K. Immediate administration of atorvastatin decreased the serum MMP-2 level and improved the prognosis for acute heart failure. J. Cardiol. 2012, 59, 374–382. [Google Scholar] [CrossRef]
- Yamazaki, T.; Lee, J.D.; Shimizu, H.; Uzui, H.; Ueda, T. Circulating matrix metalloproteinase-2 is elevated in patients with congestive heart failure. J. Cardiol. 2004, 6, 41–45. [Google Scholar] [CrossRef]
- George, J.; Patal, S.; Wexler, D.; Roth, A.; Sheps, D.; Keren, G. Circulating matrix metalloproteinase-2 but not matrix metalloproteinase-3, matrix metalloproteinase-9, or tissue inhibitor of metalloproteinase-1 predicts outcome in patients with congestive heart failure. Am. Heart J. 2005, 150, 484–487. [Google Scholar] [CrossRef]
- Peeters, S.A.; Engelen, L.; Buijs, J.; Jorsal, A.; Parving, H.H.; Tarnow, L.; Rossing, P.; Schalkwijk, C.G.; Stehouwer, C.D.A. Plasma matrix metalloproteinases are associated with incident cardiovascular disease and all-cause mortality in patients with type 1 diabetes: A 12-year follow-up study. Cardiovasc. Diabetol. 2017, 16, 55. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Feely, J.; Spiers, J.P.; Mahmud, A. Matrix metalloproteinase-9 polymorphism contributes to blood pressure and arterial stiffness in essential hypertension. J. Hum. Hypertens. 2007, 21, 861–867. [Google Scholar] [CrossRef]
- Champtiaux, N.; Liote, F.; El Karoui, K.; Vigneau, C.; Miceli, C.; Cornec-Le Gall, E.; Remy, P.; Choukroun, G.; Fakhouri, F.; Garrouste, C.; et al. Spondyloarthritis-Associated IgA Nephropathy. Spondyloarthritis-Associated IgA. Nephropathy 2020, 5, 813–820. [Google Scholar]
- Ansari, S.; Azarmehr, N.; Barmoudeh, Z.; Moslemi, Z.; Ghahremani, H.; Mirzaei, A.; Salehpour, Z.; Rabani, M.R.; Doustimotlagh, A.H. Evaluation of the protective potential of hydroalcoholic extract of Thymus daenensis on acetaminophen-induced nephrotoxicity in rats. Heliyon 2020, 6, e03898. [Google Scholar] [CrossRef]
- Marcal, D.M.; Rizzi, E.; Martins-Oliveira, A.; Ceron, C.S.; Guimaraes, D.A.; Gerlach, R.F.; Tanus-Santos, J.E. Comparative study on antioxidant effects and vascular matrix metalloproteinase-2 downregulation by dihydropyridines in renovascular hypertension. Naunyn Schmiedebergs Arch. Pharmacol. 2011, 383, 35–44. [Google Scholar] [CrossRef]
- Martins-Oliveira, A.; Castro, M.M.; Oliveira, D.M.; Rizzi, E.; Ceron, C.S.; Guimaraes, D.; Reis, R.I.; Costa-Neto, C.M.; Casarini, D.E.; Ribeiro, A.A.; et al. Contrasting effects of aliskiren versus losartan on hypertensive vascular remodeling. Int. J. Cardiol. 2013, 167, 1199–1205. [Google Scholar] [CrossRef]
- Guimaraes, D.A.; Rizzi, E.; Ceron, C.S.; Martins-Oliveira, A.; Gerlach, R.F.; Shiva, S.; Tanus-Santos, J.E. Atorvastatin and sildenafil decrease vascular TGF-beta levels and MMP-2 activity and ameliorate arterial remodeling in a model of renovascular hypertension. Redox Biol. 2015, 6, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, E.; Amaral, J.H.; Guimaraes, D.A.; Conde-Tella, S.O.; Pinheiro, L.C.; Gerlach, R.F.; Castro, M.M.; Tanus-Santos, J.E. Nitrite treatment downregulates vascular MMP-2 activity and inhibits vascular remodeling in hypertension independently of its antihypertensive effects. Free Radic. Biol. Med. 2019, 130, 234–243. [Google Scholar] [CrossRef]
- Amaral, J.H.; Rizzi, E.S.; Alves-Lopes, R.; Pinheiro, L.C.; Tostes, R.C.; Tanus-Santos, J.E. Antioxidant and antihypertensive responses to oral nitrite involves activation of the Nrf2 pathway. Free Radic. Biol. Med. 2019, 141, 261–268. [Google Scholar] [CrossRef]
- Liu, J.; Khalil, R.A. Matrix Metalloproteinase Inhibitors as Investigational and Therapeutic Tools in Unrestrained Tissue Remodeling and Pathological Disorders. Prog. Mol. Biol. Transl. Sci. 2017, 148, 355–420. [Google Scholar] [PubMed]
- Raeeszadeh-Sarmazdeh, M.; Do, L.D.; Hritz, B.G. Metalloproteinases and Their Inhibitors: Potential for the Development of New Therapeutics. Cells 2020, 9, 1313. [Google Scholar] [CrossRef]
- Coussens, L.M.; Tinkle, C.L.; Hanahan, D.; Werb, Z. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell 2000, 103, 481–490. [Google Scholar] [CrossRef]
- Egeblad, M.; Werb, Z. New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer 2002, 2, 161–174. [Google Scholar] [CrossRef]
- Baer, P.N. Periostat: Low-dose doxycycline. A commentary. Periodontal Clin. Investig. 1998, 20, 5. [Google Scholar]
- Caton, J.G. Evaluation of Periostat for patient management. Compend. Contin. Educ. Dent. 1999, 20, 451–456, 458–460, 462; quiz 463. [Google Scholar]
- Novak, M.J.; Johns, L.P.; Miller, R.C.; Bradshaw, M.H. Adjunctive benefits of subantimicrobial dose doxycycline in the management of severe, generalized, chronic periodontitis. J. Periodontol. 2002, 73, 762–769. [Google Scholar] [CrossRef]
- Golub, L.M.; Lee, H.M.; Ryan, M.E.; Giannobile, W.V.; Payne, J.; Sorsa, T. Tetracyclines inhibit connective tissue breakdown by multiple non-antimicrobial mechanisms. Adv. Dent. Res. 1998, 12, 12–26. [Google Scholar] [CrossRef]
- Kivela-Rajamaki, M.; Maisi, P.; Srinivas, R.; Tervahartiala, T.; Teronen, O.; Husa, V.; Salo, T.; Sorsa, T. Levels and molecular forms of MMP-7 (matrilysin-1) and MMP-8 (collagenase-2) in diseased human peri-implant sulcular fluid. J Periodontal Res. 2003, 38, 583–590. [Google Scholar] [CrossRef]
- Nip, L.H.; Uitto, V.J.; Golub, L.M. Inhibition of epithelial cell matrix metalloproteinases by tetracyclines. J. Periodontal Res. 1993, 28, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.N., Jr.; Mickler, E.A.; Hasty, K.A.; Brandt, K.D. Specificity of inhibition of matrix metalloproteinase activity by doxycycline: Relationship to structure of the enzyme. Arthritis Rheum. 1999, 42, 1140–1146. [Google Scholar] [CrossRef]
- Kocer, S.S.; Walker, S.G.; Zerler, B.; Golub, L.M.; Simon, S.R. Metalloproteinase inhibitors, nonantimicrobial chemically modified tetracyclines, and ilomastat block Bacillus anthracis lethal factor activity in viable cells. Infect. Immun. 2005, 73, 7548–7557. [Google Scholar] [CrossRef]
- Deryugina, E.I.; Ratnikov, B.I.; Strongin, A.Y. Prinomastat, a hydroxamate inhibitor of matrix metalloproteinases, has a complex effect on migration of breast carcinoma cells. Int. J. Cancer 2003, 104, 533–541. [Google Scholar] [CrossRef]
- Gossage, D.L.; Cieslarova, B.; Ap, S.; Zheng, H.; Xin, Y.; Lal, P.; Chen, G.; Smith, V.; Sundy, J.S. Phase 1b Study of the Safety, Pharmacokinetics, and Disease-related Outcomes of the Matrix Metalloproteinase-9 Inhibitor Andecaliximab in Patients With Rheumatoid Arthritis. Clin. Ther. 2018, 40, 156–165e5. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Bhandari, B.R.; Randall, C.; Younes, Z.H.; Romanczyk, T.; Xin, Y.; Wendt, E.; Chai, H.; McKevitt, M.; Zhao, S.; et al. Andecaliximab [Anti-matrix Metalloproteinase-9] Induction Therapy for Ulcerative Colitis: A Randomised, Double-Blind, Placebo-Controlled, Phase 2/3 Study in Patients With Moderate to Severe Disease. J. Crohns Colitis 2018, 12, 1021–1029. [Google Scholar] [CrossRef]
- Schreiber, S.; Siegel, C.A.; Friedenberg, K.A.; Younes, Z.H.; Seidler, U.; Bhandari, B.R.; Wang, K.; Wendt, E.; McKevitt, M.; Zhao, S.; et al. A Phase 2, Randomized, Placebo-Controlled Study Evaluating Matrix Metalloproteinase-9 Inhibitor, Andecaliximab, in Patients With Moderately to Severely Active Crohn’s Disease. J. Crohns Colitis 2018, 12, 1014–1020. [Google Scholar] [CrossRef]
- Ahmad, A.; Dempsey, S.K.; Daneva, Z.; Azam, M.; Li, N.; Li, P.L.; Ritter, J.K. Role of Nitric Oxide in the Cardiovascular and Renal Systems. Int. J. Mol. Sci. 2018, 19, 2605. [Google Scholar] [CrossRef] [PubMed]
- Muhl, H.; Pfeilschifter, J. Tetrahydrobiopterin is a limiting factor of nitric oxide generation in interleukin 1 beta-stimulated rat glomerular mesangial cells. Kidney Int. 1994, 46, 1302–1306. [Google Scholar] [CrossRef] [PubMed]
- Nagareddy, P.R.; Rajput, P.S.; Vasudevan, H.; McClure, B.; Kumar, U.; Macleod, K.M.; McNeill, J.H. Inhibition of matrix metalloproteinase-2 improves endothelial function and prevents hypertension in insulin-resistant rats. Br. J. Pharmacol. 2012, 165, 705–715. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).