Efficient Degradation of 2-Mercaptobenzothiazole and Other Emerging Pollutants by Recombinant Bacterial Dye-Decolorizing Peroxidases
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
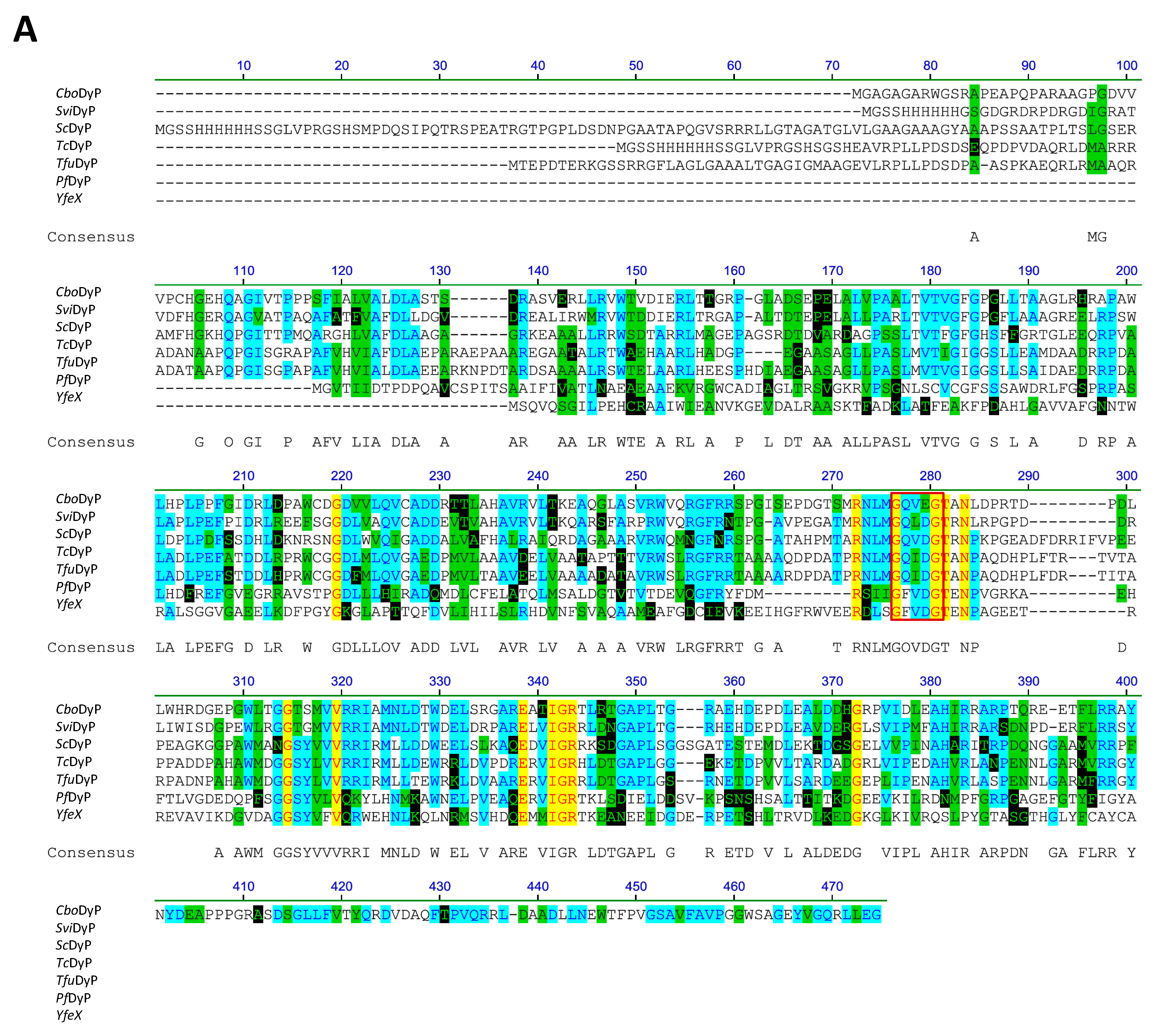
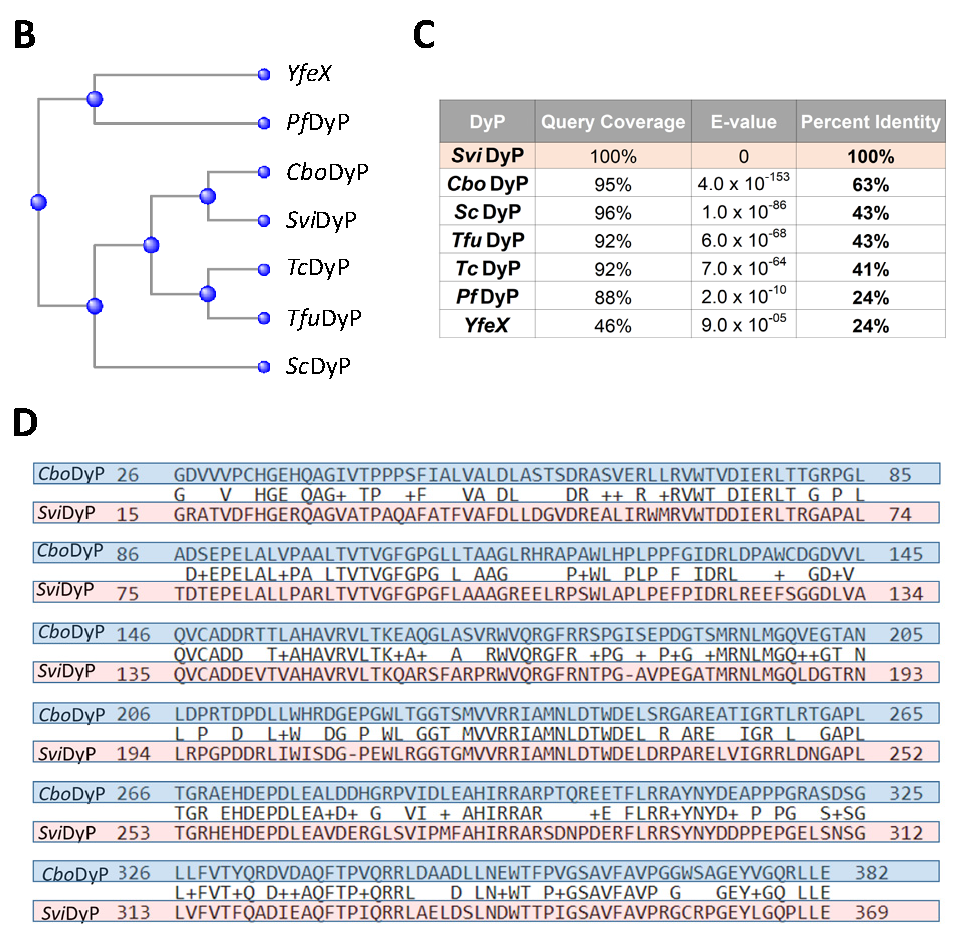
2.2. Sequence Alignment of Seven Recombinant Bacterial DyPs (rDyPs)
2.3. Determination of Optimal pH for the Seven rDyPs
2.4. Degradation of Emerging Pollutants by Recombinant DyPs
2.5. LCMSMS Based EPs Degradation Assay
2.6. LCMSMS Analysis of Products of MBT Degrdation
3. Results and Discussion
3.1. Sequence Alignments of the Recombinant Bacterial DyPs
3.2. Determination of Optimal pH for the Recombinant Bacterial DyPs
3.3. DyPs-Mediated Degradation of Emerging Pollutants
3.4. The Role of Redox Mediating Species
3.5. LCMSMS Analysis of MBT Intermediates Generated by SviDyP
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- La Farré, M.; Pérez, S.; Kantiani, L.; Barceló, D. Fate and toxicity of emerging pollutants, their metabolites and transformation products in the aquatic environment. TrAC Trends Anal. Chem. 2008, 27, 991–1007. [Google Scholar] [CrossRef]
- Kanaujiya, D.K.; Paul, T.; Sinharoy, A.; Pakshirajan, K. Biological Treatment Processes for the Removal of Organic Micropollutants from Wastewater: A Review. Curr. Pollut. Rep. 2019, 5, 112–128. [Google Scholar] [CrossRef]
- Gavrilescu, M.; Demnerová, K.; Aamand, J.; Agathos, S.; Fava, F. Emerging pollutants in the environment: Present and future challenges in biomonitoring, ecological risks and bioremediation. New Biotechnol. 2015, 32, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Nilsen, E.; Smalling, K.L.; Ahrens, L.; Gros, M.; Miglioranza, K.S.B.; Picó, Y.; Schoenfuss, H.L. Critical review: Grand challenges in assessing the adverse effects of contaminants of emerging concern on aquatic food webs: Challenges assessing contaminants of emerging concern. Environ. Toxicol Chem. 2019, 38, 46–60. [Google Scholar] [CrossRef]
- Rodriguez-Narvaez, O.M.; Peralta-Hernandez, J.M.; Goonetilleke, A.; Bandala, E.R. Treatment technologies for emerging contaminants in water: A review. Chem. Eng. J. 2017, 323, 361–380. [Google Scholar] [CrossRef]
- Sher, F.; Hanif, K.; Iqbal, S.Z.; Imran, M. Implications of advanced wastewater treatment: Electrocoagulation and electroflocculation of effluent discharged from a wastewater treatment plant. J. Water Process Eng. 2020, 33, 101101. [Google Scholar] [CrossRef]
- Sher, F.; Hanif, K.; Rafey, A.; Khalid, U.; Zafar, A.; Ameen, M.; Lima, E.C. Removal of micropollutants from municipal wastewater using different types of activated carbons. J. Environ. Manag. 2021, 278, 111302. [Google Scholar] [CrossRef] [PubMed]
- Kausar, A.; Sher, F.; Hazafa, A.; Javed, A.; Sillanpää, M.; Iqbal, M. Biocomposite of sodium-alginate with acidified clay for wastewater treatment: Kinetic, equilibrium and thermodynamic studies. Int. J. Biol. Macromol. 2020, 161, 1272–1285. [Google Scholar] [CrossRef]
- Khan, N.A.; Khan, S.U.; Ahmed, S.; Farooqi, I.H.; Yousefi, M.; Mohammadi, A.A.; Changani, F. Recent trends in disposal and treatment technologies of emerging-pollutants- A critical review. TrAC Trends Anal. Chem. 2020, 122, 115744. [Google Scholar] [CrossRef]
- Cheng, N.; Wang, B.; Wu, P.; Lee, X.; Xing, Y.; Chen, M.; Gao, B. Adsorption of emerging contaminants from water and wastewater by modified biochar: A review. Environ. Pollut. 2021, 273, 116448. [Google Scholar] [CrossRef]
- Mohapatra, D.P.; Kirpalani, D.M. Advancement in treatment of wastewater: Fate of emerging contaminants. Can. J. Chem. Eng. 2019, 97, 2621–2631. [Google Scholar] [CrossRef]
- Tufail, A.; Price, W.E.; Mohseni, M.; Pramanik, B.K.; Hai, F.I. A critical review of advanced oxidation processes for emerging trace organic contaminant degradation: Mechanisms, factors, degradation products, and effluent toxicity. J. Water Process Eng. 2021, 40, 101778. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, X.; Han, J.; Gong, H.; Meng, L.; Mei, X.; Sun, Y.; Qi, L.; Gan, L. Degradation of emerging contaminants by sono-Fenton process with in situ generated H2O2 and the improvement by P25-mediated visible light irradiation. J. Hazard. Mater. 2020, 391, 122229. [Google Scholar] [CrossRef]
- Bilal, M.; Ashraf, S.S.; Barceló, D.; Iqbal, H.M.N. Biocatalytic degradation/redefining “removal” fate of pharmaceutically active compounds and antibiotics in the aquatic environment. Sci. Total Environ. 2019, 691, 1190–1211. [Google Scholar] [CrossRef] [PubMed]
- Akay, C.; Tezel, U. Biotransformation of Acetaminophen by intact cells and crude enzymes of bacteria: A comparative study and modelling. Sci. Total Environ. 2020, 703, 134990. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Rasheed, T.; Iqbal, H.M.N.; Yan, Y. Peroxidases-assisted removal of environmentally-related hazardous pollutants with reference to the reaction mechanisms of industrial dyes. Sci. Total Environ. 2018, 644, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Alneyadi, A.H.; Rauf, M.A.; Ashraf, S.S. Oxidoreductases for the remediation of organic pollutants in water—A critical review. Crit. Rev. Biotechnol. 2018, 38, 971–988. [Google Scholar] [CrossRef] [PubMed]
- Al-Maqdi, K.A.; Hisaindee, S.; Rauf, M.A.; Ashraf, S.S. Detoxification and degradation of sulfamethoxazole by soybean peroxidase and UV + H2O2 remediation approaches. Chem. Eng. J. 2018, 352, 450–458. [Google Scholar] [CrossRef]
- Huber, C.; Preis, M.; Harvey, P.J.; Grosse, S.; Letzel, T.; Schröder, P. Emerging pollutants and plants—Metabolic activation of diclofenac by peroxidases. Chemosphere 2016, 146, 435–441. [Google Scholar] [CrossRef]
- Yang, L.; Shi, Y.; Li, J.; Fang, L.; Luan, T. Transformation of aqueous sulfonamides under horseradish peroxidase and characterization of sulfur dioxide extrusion products from sulfadiazine. Chemosphere 2018, 200, 164–172. [Google Scholar] [CrossRef]
- Leng, Y.; Bao, J.; Xiao, H.; Song, D.; Du, J.; Mohapatra, S.; Werner, D.; Wang, J. Transformation mechanisms of tetracycline by horseradish peroxidase with/without redox mediator ABTS for variable water chemistry. Chemosphere 2020, 258, 127306. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G. Treatment of aqueous pentachlorophenol by horseradish peroxidase and hydrogen peroxide. Water Res. 2000, 34, 1629–1637. [Google Scholar] [CrossRef]
- Ashe, B.; Nguyen, L.N.; Hai, F.I.; Lee, D.-J.; van de Merwe, J.P.; Leusch, F.D.L.; Price, W.E.; Nghiem, L.D. Impacts of redox-mediator type on trace organic contaminants degradation by laccase: Degradation efficiency, laccase stability and effluent toxicity. Int. Biodeterior. Biodegrad. 2016, 113, 169–176. [Google Scholar] [CrossRef]
- Alneyadi, A.H.; Ashraf, S.S. Differential enzymatic degradation of thiazole pollutants by two different peroxidases—A comparative study. Chem. Eng. J. 2016, 303, 529–538. [Google Scholar] [CrossRef]
- Rahmanpour, R.; Bugg, T.D.H. Chapter 14. Structure and Reactivity of the Dye-decolorizing Peroxidase (DyP) Family. In Metallobiology; Raven, E., Dunford, B., Eds.; Royal Society of Chemistry: Cambridge, UK, 2015; pp. 334–357. ISBN 978-1-84973-911-5. [Google Scholar]
- Zubieta, C.; Krishna, S.S.; Kapoor, M.; Kozbial, P.; McMullan, D.; Axelrod, H.L.; Miller, M.D.; Abdubek, P.; Ambing, E.; Astakhova, T.; et al. Crystal structures of two novel dye-decolorizing peroxidases reveal a β-barrel fold with a conserved heme-binding motif. Proteins 2007, 69, 223–233. [Google Scholar] [CrossRef]
- Ogola, H.J.O.; Kamiike, T.; Hashimoto, N.; Ashida, H.; Ishikawa, T.; Shibata, H.; Sawa, Y. Molecular Characterization of a Novel Peroxidase from the Cyanobacterium Anabaena sp. Strain PCC 7120. Appl. Environ. Microbiol. 2009, 75, 7509–7518. [Google Scholar] [CrossRef]
- Sugano, Y.; Muramatsu, R.; Ichiyanagi, A.; Sato, T.; Shoda, M. DyP, a Unique Dye-decolorizing Peroxidase, Represents a Novel Heme Peroxidase Family. J. Biol. Chem. 2007, 282, 36652–36658. [Google Scholar] [CrossRef]
- Lončar, N.; Drašković, N.; Božić, N.; Romero, E.; Simić, S.; Opsenica, I.; Vujčić, Z.; Fraaije, M.W. Expression and Characterization of a Dye-Decolorizing Peroxidase from Pseudomonas Fluorescens Pf0-1. Catalysts 2019, 9, 463. [Google Scholar] [CrossRef]
- Lambertz, C.; Ece, S.; Fischer, R.; Commandeur, U. Progress and obstacles in the production and application of recombinant lignin-degrading peroxidases. Bioengineered 2016, 7, 145–154. [Google Scholar] [CrossRef]
- Colpa, D.I.; Fraaije, M.W. High overexpression of dye decolorizing peroxidase TfuDyP leads to the incorporation of heme precursor protoporphyrin IX. J. Mol. Catal. B Enzym. 2016, 134, 372–377. [Google Scholar] [CrossRef]
- Colpa, D.I.; Fraaije, M.W.; van Bloois, E. DyP-type peroxidases: A promising and versatile class of enzymes. J. Ind. Microbiol. Biotechnol. 2014, 41, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Langbehn, R.K.; Michels, C.; Soares, H.M. Antibiotics in wastewater: From its occurrence to the biological removal by environmentally conscious technologies. Environ. Pollut. 2021, 275, 116603. [Google Scholar] [CrossRef] [PubMed]
- Vasilachi, I.C.; Asiminicesei, D.M.; Fertu, D.I.; Gavrilescu, M. Occurrence and Fate of Emerging Pollutants in Water Environment and Options for Their Removal. Water 2021, 13, 181. [Google Scholar] [CrossRef]
- Kasonga, T.K.; Coetzee, M.A.A.; Kamika, I.; Ngole-Jeme, V.M.; Benteke Momba, M.N. Endocrine-disruptive chemicals as contaminants of emerging concern in wastewater and surface water: A review. J. Environ. Manag. 2021, 277, 111485. [Google Scholar] [CrossRef] [PubMed]
- Almaqdi, K.A.; Morsi, R.; Alhayuti, B.; Alharthi, F.; Ashraf, S.S. LC-MSMS based screening of emerging pollutant degradation by different peroxidases. BMC Biotechnol. 2019, 19, 83. [Google Scholar] [CrossRef]
- Chen, C.; Li, T. Bacterial dye-decolorizing peroxidases: Biochemical properties and biotechnological opportunities. Phys. Sci. Rev. 2016, 1. [Google Scholar] [CrossRef]
- Punekar, N.S. ENZYMES: Catalysis, Kinetics and Mechanisms; Springer: Singapore, 2018; ISBN 9789811307843. [Google Scholar]
- Habib, M.H.; Rozeboom, H.J.; Fraaije, M.W. Characterization of a New DyP-Peroxidase from the Alkaliphilic Cellulomonad, Cellulomonas bogoriensis. Molecules 2019, 24, 1208. [Google Scholar] [CrossRef]
- Schultz, G.; Henion, J. Fundamentals of LC-MS/MS for Regulated Bioanalysis. In Regulated Bioanalysis: Fundamentals and Practice; Rocci, M.L., Lowes, S., Eds.; AAPS Advances in the Pharmaceutical Sciences Series; Springer International Publishing: Cham, Switzerland, 2017; Volume 26, pp. 103–120. ISBN 978-3-319-54800-5. [Google Scholar]
- Yu, W.; Liu, W.; Huang, H.; Zheng, F.; Wang, X.; Wu, Y.; Li, K.; Xie, X.; Jin, Y. Application of a Novel Alkali-Tolerant Thermostable DyP-Type Peroxidase from Saccharomonospora viridis DSM 43017 in Biobleaching of Eucalyptus Kraft Pulp. PLoS ONE 2014, 9, e110319. [Google Scholar] [CrossRef]
- Lueangjaroenkit, P.; Teerapatsakul, C.; Sakka, K.; Sakka, M.; Kimura, T.; Kunitake, E.; Chitradon, L. Two Manganese Peroxidases and a Laccase of Trametes polyzona KU-RNW027 with Novel Properties for Dye and Pharmaceutical Product Degradation in Redox Mediator-Free System. Mycobiology 2019, 47, 217–229. [Google Scholar] [CrossRef]
- Karim, Z.; Husain, Q. Redox-mediated oxidation and removal of aromatic amines from polluted water by partially purified bitter gourd (Momordica charantia) peroxidase. Int. Biodeterior. Biodegrad. 2009, 63, 587–593. [Google Scholar] [CrossRef]
- Umamaheswari, B.; Rajaram, R. Microaerobic degradation of 2-Mercaptobenzothiazole present in industrial wastewater. J. Hazard. Mater. 2017, 321, 773–781. [Google Scholar] [CrossRef]
- Qin, Y.; Li, H.; Lu, J.; Ding, Y.; Ma, C.; Liu, X.; Liu, Z.; Huo, P.; Yan, Y. Photocatalytic degradation of 2-Mercaptobenzothiazole by a novel Bi2WO6 nanocubes/In(OH)3 photocatalyst: Synthesis process, degradation pathways, and an enhanced photocatalytic performance mechanism study. Appl. Surf. Sci. 2019, 481, 1313–1326. [Google Scholar] [CrossRef]
- Zhu, Z.; Yu, Y.; Dong, H.; Liu, Z.; Li, C.; Huo, P.; Yan, Y. Intercalation Effect of Attapulgite in g-C3N4 Modified with Fe3O4 Quantum Dots To Enhance Photocatalytic Activity for Removing 2-Mercaptobenzothiazole under Visible Light. ACS Sustain. Chem. Eng. 2017, 5, 10614–10623. [Google Scholar] [CrossRef]
- San-Martín, M.I.; Escapa, A.; Alonso, R.M.; Canle, M.; Morán, A. Degradation of 2-mercaptobenzothizaole in microbial electrolysis cells: Intermediates, toxicity, and microbial communities. Sci. Total Environ. 2020, 733, 139155. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Qiu, J.; Gao, Y.; Stefan, M.I.; Li, X.F. Nontargeted identification and predicted toxicity of new byproducts generated from UV treatment of water containing micropollutant 2-mercaptobenzothiazole. Water Res. 2021, 188, 116542. [Google Scholar] [CrossRef] [PubMed]
- De Wever, H.; De Moor, K.; Verachtert, H. Toxicity of 2-mercaptobenzothiazole towards bacterial growth and respiration. Appl. Microbiol. Biotechnol. 1994, 42, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Sorahan, T. Cancer risks in chemical production workers exposed to 2-mercaptobenzothiazole. Occup. Environ. Med. 2008, 66, 269–273. [Google Scholar] [CrossRef] [PubMed]






| # | Category | Emerging Pollutants (EPs) | Structure | Retention Time (min) | MRM Transition (m/z) | Fragmentor Voltage (V) | Collision Energy (V) | Polarity |
|---|---|---|---|---|---|---|---|---|
| 1 | Antibiotic | Sulfamethoxazole |  | 11.2 | 254 → 156 | 135 | 20 | Positive |
| 2 | Antibiotic | Trimethoprim |  | 8.4 | 291 → 230 | 135 | 20 | Positive |
| 3 | Antibiotic | Norfloxacin |  | 10.1 | 320 → 302 | 135 | 20 | Positive |
| 4 | Antibiotic | Chloram-phenicol |  | 12.1 | 321 → 152 | 0 | 20 | Negative |
| 5 | Antibiotic | 3-Methyl-2(3H)-benzothiazolone |  | 13.7 | 202 →175 | 135 | 30 | Positive |
| 6 | Antibiotic | Penicillin GK |  | 13.7 | 335 → 160 | 0 | 10 | Positive |
| 7 | Antibiotic | Lincomycin-HCl |  | 4.8 | 407 → 359 | 135 | 20 | Positive |
| 8 | Antibiotic | Roxithro-mycin |  | 15.0 | 837 → 680 | 135 | 20 | Positive |
| 9 | Anti-oxidant | Caffeic acid |  | 6.4 | 181 → 163 | 135 | 20 | Positive |
| 10 | Anti-seizure drug | Levetiracetam |  | 2.5 | 171 → 126 | 0 | 10 | Positive |
| 11 | Anti-seizure drug | Phenytoin |  | 14.4 | 253 → 182 | 135 | 10 | Positive |
| 12 | Anti-depressant | Venlafaxine-HCl |  | 12.0 | 278 → 260 | 135 | 10 | Positive |
| 13 | Beta-blocker | Atenolol |  | 1.6 | 171 → 126 | 0 | 10 | Positive |
| 14 | Diuretic drug | Hydrochloro-thiazide |  | 3.7 | 296 → 269 | 140 | 20 | Negative |
| 15 | Diuretic drug | Furosemide |  | 14.6 | 329 → 285 | 140 | 15 | Negative |
| 16 | Flocculation agent | Acrylamide |  | 0.8 | 72 → 55 | 50 | 10 | Positive |
| 17 | Fungicide (or its derivative) | Thiabendazole |  | 5.2 | 267 → 190 | 135 | 20 | Positive |
| 18 | Herbicide | Prometryn |  | 14.4 | 166 →123 | 135 | 30 | Positive |
| 19 | Herbicide | 2-methyl-4-chlorophenoxyacetic acid (MCPA) |  | 15.8 | 242 → 158 | 135 | 30 | Positive |
| 20 | Herbicide | Fluometuron |  | 15.0 | 201 → 125 | 47 | 13 | Positive |
| 21 | Histamine H₂ receptor antagonist | Cimetidine |  | 1.6 | 233 → 72 | 135 | 30 | Positive |
| 22 | Insect repellent | N, N-Diethyl-meta-toluamide (DEET) |  | 15.3 | 192 → 119 | 135 | 30 | Positive |
| 23 | Keratolytic agent | Salicylic acid |  | 11.6 | 139 → 121 | 50 | 10 | Positive |
| 24 | Lipid regulating agent | Gemfibrozil |  | 20.3 | 251 → 129 | 0 | 40 | Positive |
| 25 | Non-steroidal anti-inflammatory drugs (NSAID) | Meloxicam |  | 16.7 | 352 → 115 | 135 | 6 | Positive |
| 26 | NSAID | Ibuprofen |  | 19.0 | 207 → 161 | 135 | 20 | Positive |
| 27 | Phyto estrogen | Biochanin A |  | 17.5 | 285 → 152 | 0 | 30 | Positive |
| 28 | Pain killer | Paracetamol |  | 1.9 | 152 → 110 | 0 | 10 | Positive |
| 29 | Rubber additive | 2-(methylthio) benzothiazole (MTBT) |  | 17.1 | 182 → 167 | 135 | 30 | Positive |
| 30 | Stimulant | Caffeine |  | 6.8 | 195 → 138 | 135 | 30 | Positive |
| 31 | Vulcanization agent | 2-Mercapto benzothiazole (MBT) |  | 13.3 | 168 → 135 | 135 | 30 | Positive |
| DyPs | YfeX | TfuDyP | PfDyP B2 | TcDyP | ScDyP | SviDyP | CboDyP | |
|---|---|---|---|---|---|---|---|---|
| 31 EPs | ||||||||
| 2-Mercaptobenzothiazole | - | + | + | - | - | ++++ | ++++ | |
| Gemfibrozil | ++ | + | + | +++ | - | - | - | |
| Caffeic Acid | - | - | - | - | - | +++ | ++ | |
| Acrylamide | ++ | ++ | ++ | + | - | + | - | |
| Biochanin A | ++ | + | + | - | + | - | - | |
| 3-Methyl-2-benzothiazolinone | - | - | - | - | - | + | ++ | |
| (4-Chloro-2-methylphenoxy) acetic acid | - | - | - | - | - | + | ++ | |
| Venlafaxine | - | - | - | - | - | ++ | - | |
| Ibuprofen | + | + | + | - | - | + | - | |
| Fluometuron | - | - | - | - | - | + | + | |
| Cimetidine | - | + | + | - | + | - | - | |
| Salicylic acid | - | - | - | - | - | + | + | |
| Chloramphenicol | - | - | - | + | + | - | - | |
| Lincomycin hydrochloride | - | - | - | - | - | + | - | |
| DEET | - | - | - | - | - | - | + | |
| Paracetamol | - | - | - | - | - | + | - | |
| 2-(Methylthio) benzothiazole | - | + | - | - | - | - | - | |
| Sulfamethoxazole | - | - | - | - | - | + | - | |
| Levetiracetam | - | - | - | - | - | - | - | |
| Caffeine | - | - | - | - | - | - | - | |
| Thiabendazole | - | - | - | - | - | - | - | |
| Prometryn | - | - | - | - | - | - | - | |
| Phenytoin | - | - | - | - | - | - | - | |
| Atenolol | - | - | - | - | - | - | - | |
| Trimethoprim | - | - | - | - | - | - | - | |
| Hydrochlorothiazide | - | - | - | - | - | - | - | |
| Furosemide | - | - | - | - | - | - | - | |
| Penicillin | - | - | - | - | - | - | - | |
| Meloxicam | - | - | - | - | - | - | - | |
| Roxithromycin | - | - | - | - | - | - | - | |
| rDyPs | Emerging Pollutants | % Remaining (No HOBT) | % Remaining (+ HOBT) |
|---|---|---|---|
| YfeX | Phenytoin | - | ++ |
| PfDyP B2 | Gemfibrozil | + | +++ |
| Roxithromycin | - | ++ | |
| SviDyP | Prometryn | - | +++ |
| CboDyP | Penicillin | - | ++ |
| Intermediates (m/z) | Without HOBT | With HOBT |
|---|---|---|
| 123 | √ | √ |
| 125 | √ | √ |
| 158 | Not detected | √ |
| 167 | √ | √ |
| 171 | √ | √ |
| 300 | √ | √ |
| Biological | Chemical | Process/Agent | Ion Mass (m/z) | References |
|---|---|---|---|---|
| √ | Bacterial strain, Alcaligenes sp. CSMB1 | 95, 106, 123, 136, 150, 151, 165 | [44] | |
| √ | SBP | 120, 136, 182, 301, 332 | [24] | |
| CPO | ||||
| √ | SviDyP | 123, 125, 158, 167 171, 300 | This study | |
| √ | Photodegradation by Fe3O4-QDs@g-C3N4/ATP | 82, 110, 114, 125, 146, 171 | [45,46] | |
| √ | Photodegradation by 9- Bi2WO6/In (OH)3 composite | |||
| √ | √ | Eurobacteria and Graphene-based anode and stainless-steel cathode | 93, 94, 108, 109, 110, 125, 126, 135, 151, 158, 167, 169, 174, 183, 187, 199, 215, 231, 268, 283, 284, 300, 332, 364 | [47] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsadik, A.; Athamneh, K.; Yousef, A.F.; Shah, I.; Ashraf, S.S. Efficient Degradation of 2-Mercaptobenzothiazole and Other Emerging Pollutants by Recombinant Bacterial Dye-Decolorizing Peroxidases. Biomolecules 2021, 11, 656. https://doi.org/10.3390/biom11050656
Alsadik A, Athamneh K, Yousef AF, Shah I, Ashraf SS. Efficient Degradation of 2-Mercaptobenzothiazole and Other Emerging Pollutants by Recombinant Bacterial Dye-Decolorizing Peroxidases. Biomolecules. 2021; 11(5):656. https://doi.org/10.3390/biom11050656
Chicago/Turabian StyleAlsadik, Aya, Khawlah Athamneh, Ahmed F. Yousef, Iltaf Shah, and Syed Salman Ashraf. 2021. "Efficient Degradation of 2-Mercaptobenzothiazole and Other Emerging Pollutants by Recombinant Bacterial Dye-Decolorizing Peroxidases" Biomolecules 11, no. 5: 656. https://doi.org/10.3390/biom11050656
APA StyleAlsadik, A., Athamneh, K., Yousef, A. F., Shah, I., & Ashraf, S. S. (2021). Efficient Degradation of 2-Mercaptobenzothiazole and Other Emerging Pollutants by Recombinant Bacterial Dye-Decolorizing Peroxidases. Biomolecules, 11(5), 656. https://doi.org/10.3390/biom11050656








