Effectiveness of N-Acetylcysteine in the Treatment of Renal Deterioration Caused by Long-Term Exposure to Bisphenol A
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Preparations
2.2. Experimental Designs
2.3. Biochemical Assays
2.3.1. Evaluations of Renal Functions
2.3.2. Evaluation of Renal Oxidative Stress
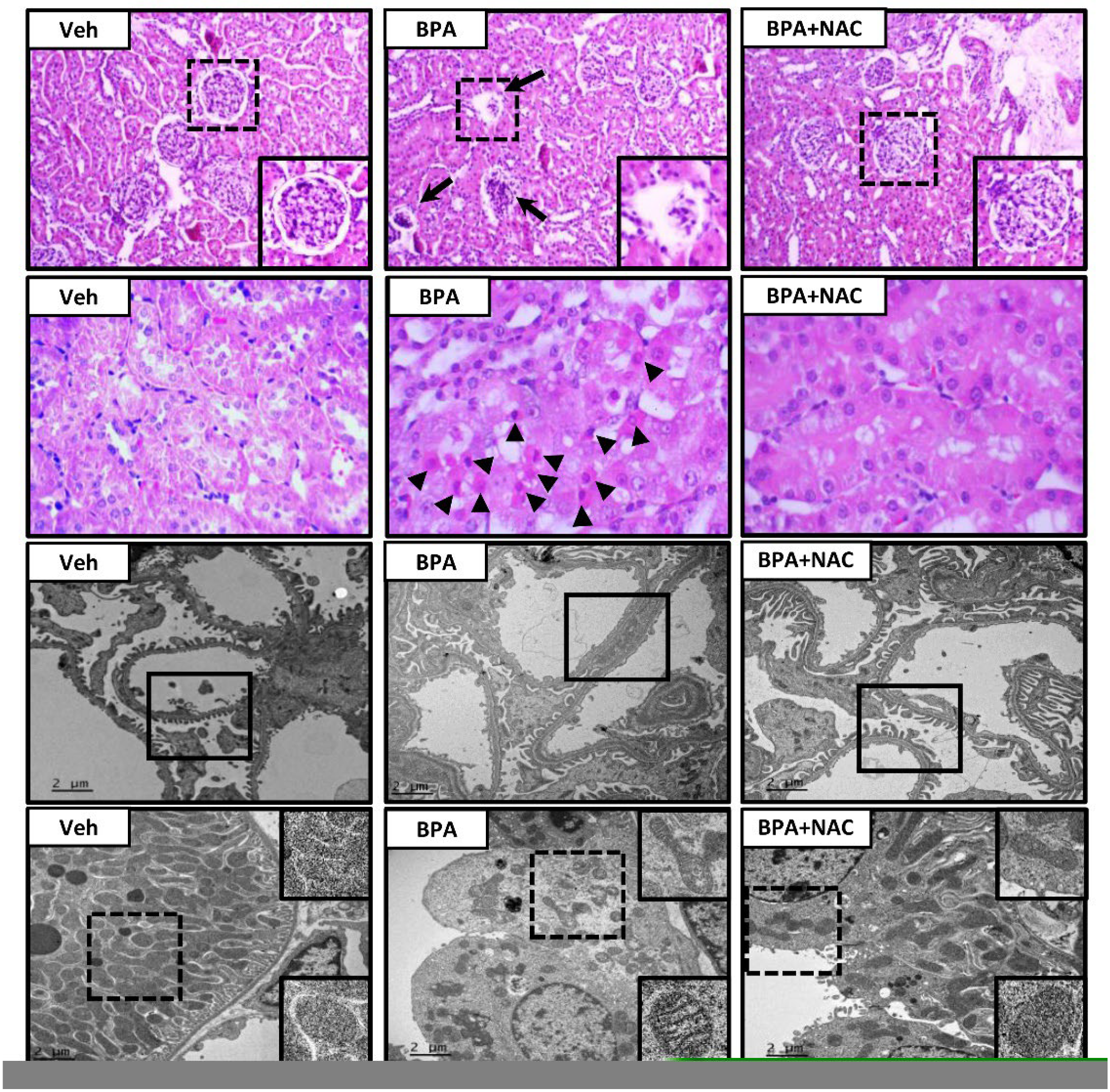
2.4. Histopathological Studies
2.5. Mitochondrial Studies
2.5.1. Preparation of Mitochondrial Proteins
2.5.2. Determination of Mitochondrial Reactive Oxygen Species (ROS) Production
2.5.3. Determination of Mitochondrial Membrane Potential
2.5.4. Determination of Mitochondrial Swelling
2.6. Western Blot Analysis
2.7. Statistical Analysis
3. Results
3.1. Effects of Long-Term BPA Exposure and NAC Treatment on Body Weight, Kidney Weight, Food and Water Intake
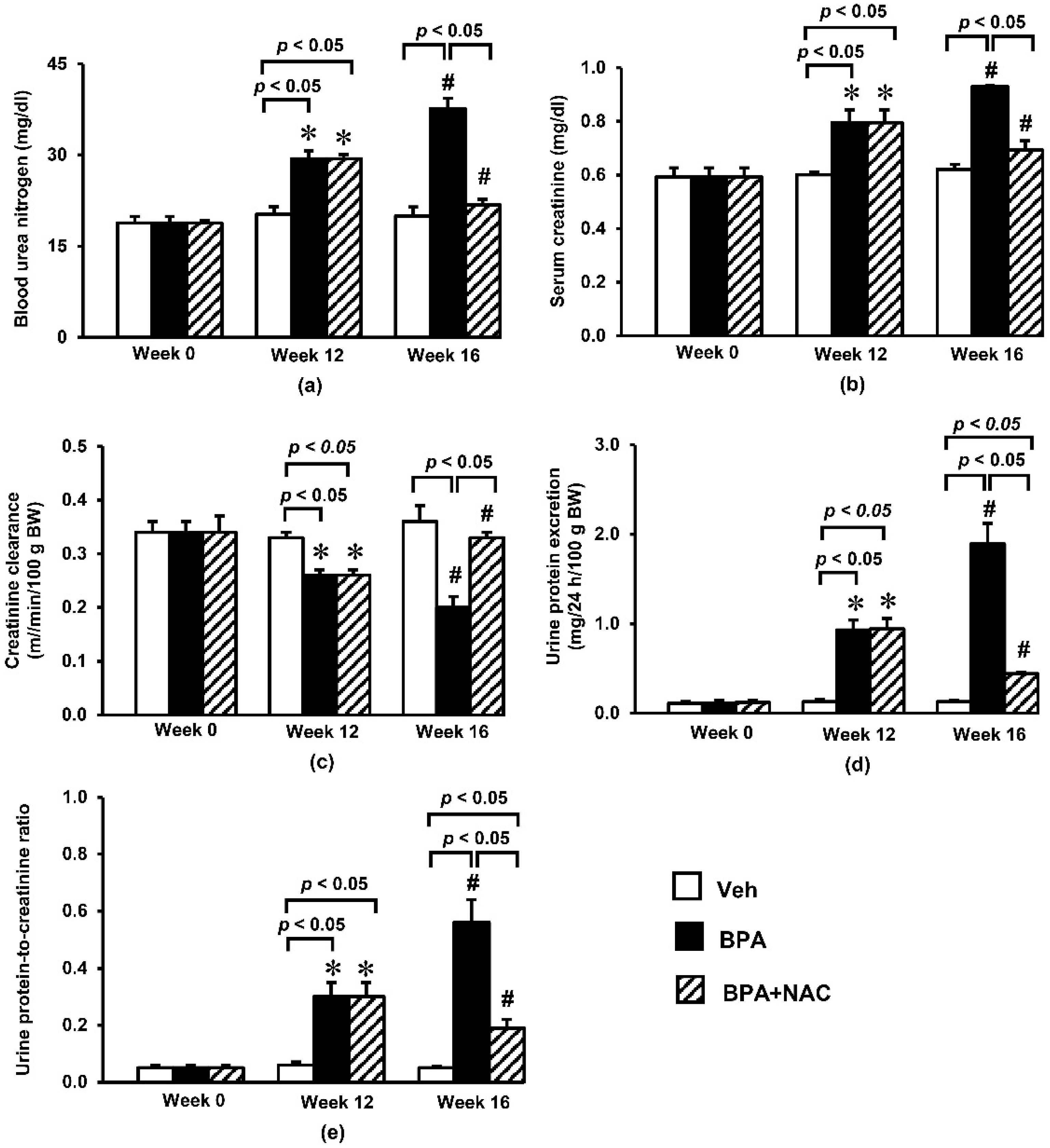
3.2. Effects of Long-Term BPA Exposure and NAC Treatment on Renal Function and Histopathology
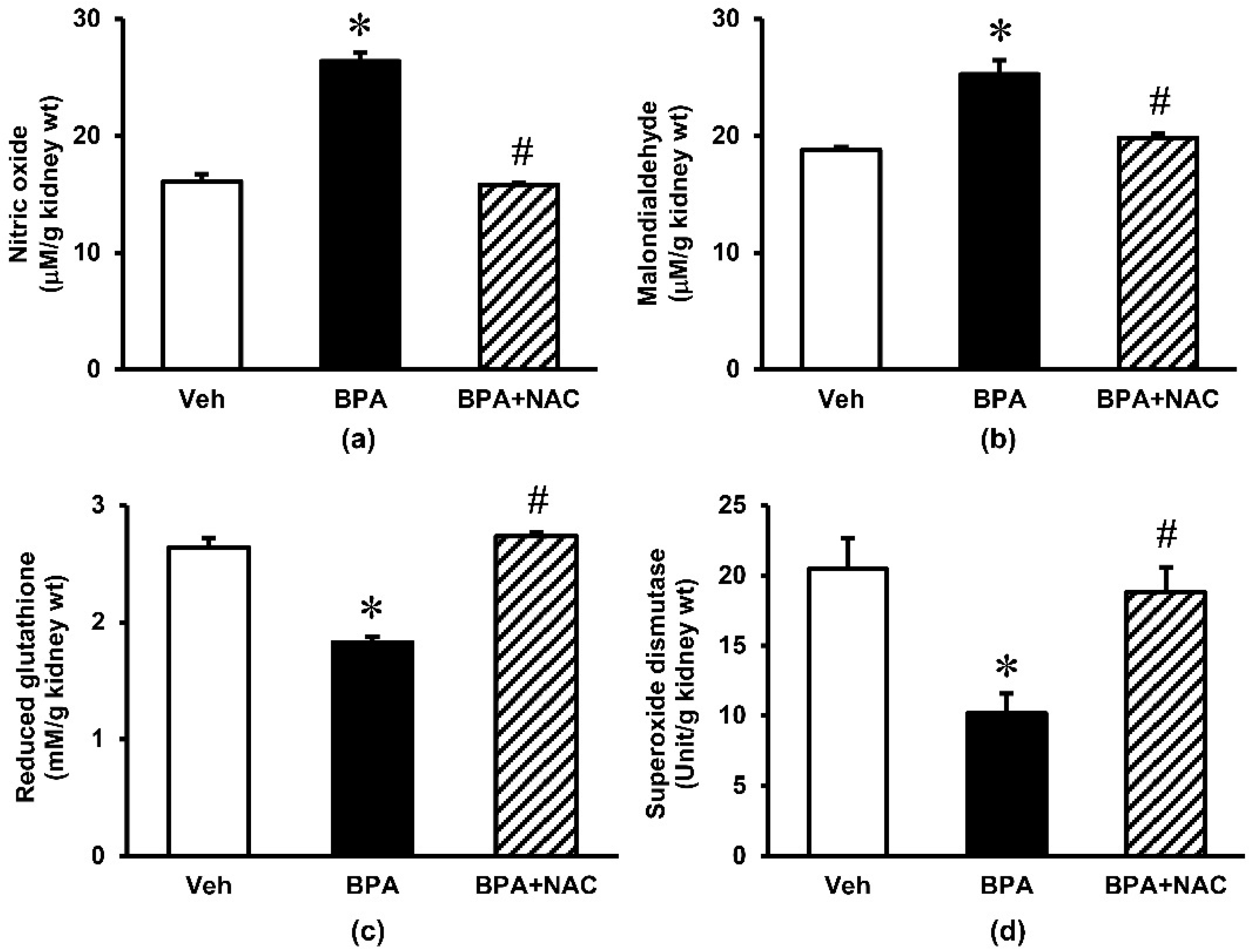
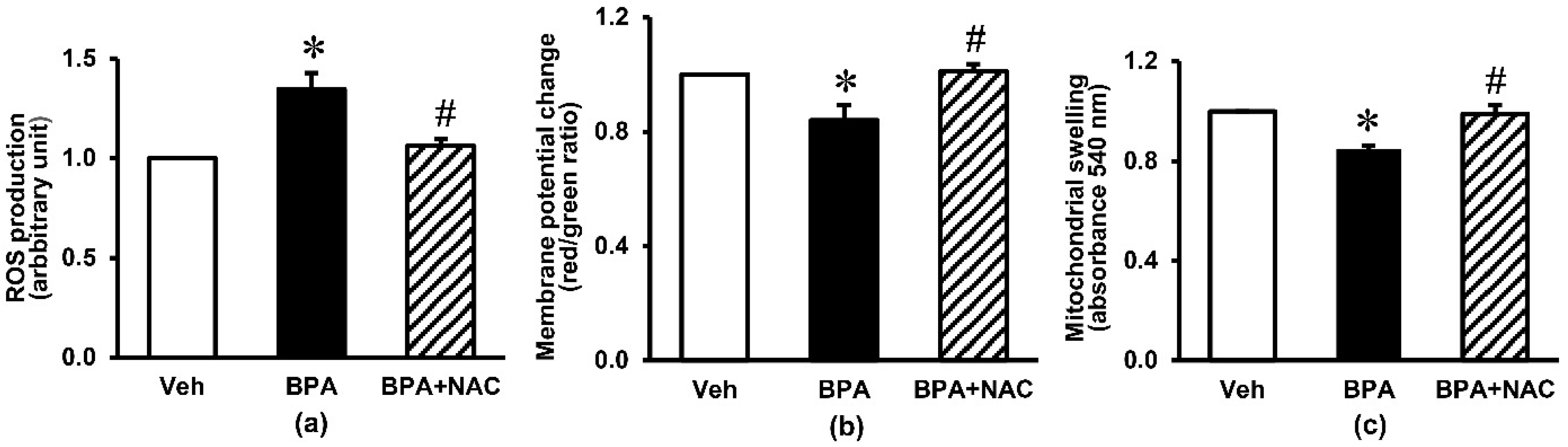
3.3. Effects of Long-Term BPA Exposure and NAC Treatment on Renal Oxidative Stress and Mitochondrial Function
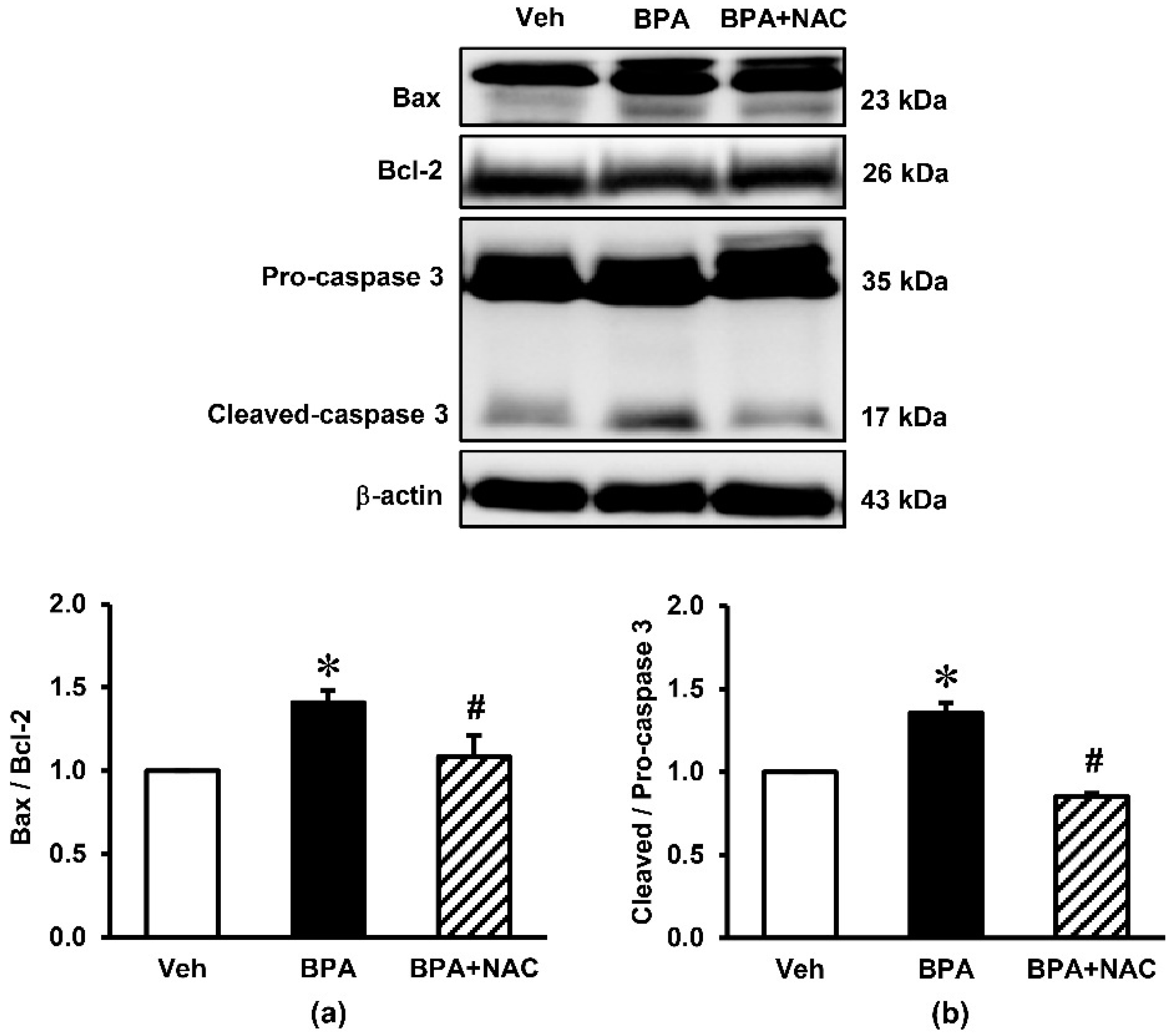
3.4. Effects of Long-Term BPA Exposure and NAC Treatment on Renal Cortical Expressions of Apoptotic Markers
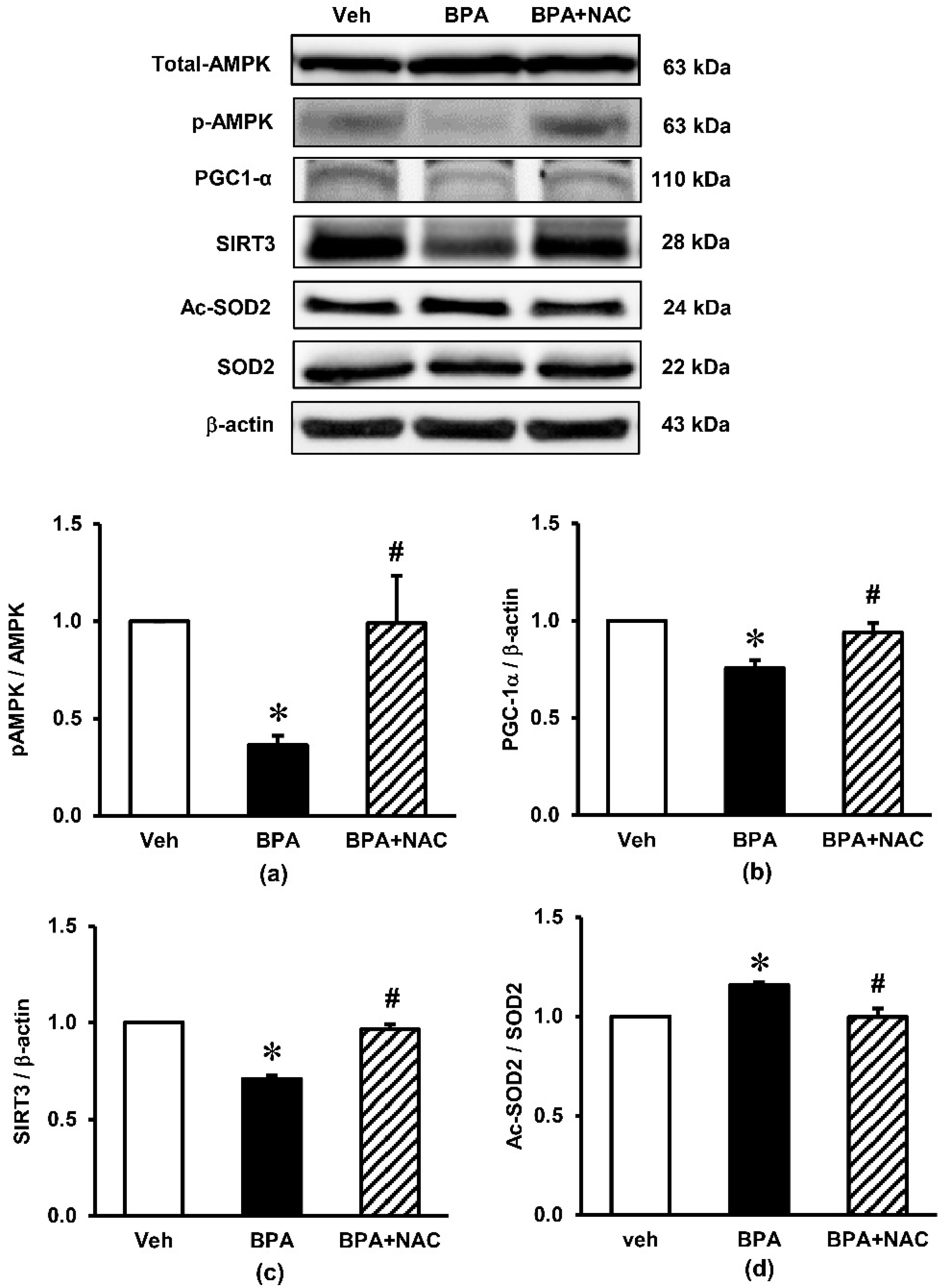
3.5. Effects of Long-Term BPA Exposure and NAC Treatment on Renal Cortical Expressions of Signaling Proteins Involved in AMPK-SIRT3-SOD2 Axis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kataria, A.; Trasande, L.; Trachtman, H. The effects of environmental chemicals on renal function. Nat. Rev. Nephrol. 2015, 11, 610–625. [Google Scholar] [CrossRef] [PubMed]
- Rezg, R.; El-Fazaa, S.; Gharbi, N.; Mornagui, B. Bisphenol A and human chronic diseases: Current evidences, possible mechanisms, and future perspectives. Environ. Int. 2014, 64, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Geens, T.; Aerts, D.; Berthot, C.; Bourguignon, J.P.; Goeyens, L.; Lecomte, P.; Maghuin-Rogister, G.; Pironnet, A.M.; Pussemier, L.; Scippo, M.L.; et al. A review of dietary and non-dietary exposure to bisphenol-A. Food Chem. Toxicol. 2012, 50, 3725–3740. [Google Scholar] [CrossRef] [PubMed]
- Rochester, J.R. Bisphenol A and human health: A review of the literature. Reprod. Toxicol. 2013, 42, 132–155. [Google Scholar] [CrossRef]
- Carchia, E.; Porreca, I.; Almeida, P.J.; D’Angelo, F.; Cuomo, D.; Ceccarelli, M.; De Felice, M.; Mallardo, M.; Ambrosino, C. Evaluation of low doses BPA-induced perturbation of glycemia by toxicogenomics points to a primary role of pancreatic islets and to the mechanism of toxicity. Cell Death Dis. 2015, 6, e1959. [Google Scholar] [CrossRef]
- Posnack, N.G. The adverse cardiac effects of Di(2-ethylhexyl)phthalate and Bisphenol A. Cardiovasc. Toxicol. 2014, 14, 339–357. [Google Scholar] [CrossRef]
- Jain, S.; Kumar, C.H.; Suranagi, U.D.; Mediratta, P.K. Protective effect of N-acetylcysteine on bisphenol A-induced cognitive dysfunction and oxidative stress in rats. Food Chem. Toxicol. 2011, 49, 1404–1409. [Google Scholar] [CrossRef]
- Khan, S.; Beigh, S.; Chaudhari, B.P.; Sharma, S.; Aliul Hasan Abdi, S.; Ahmad, S.; Ahmad, F.; Parvez, S.; Raisuddin, S. Mitochondrial dysfunction induced by Bisphenol A is a factor of its hepatotoxicity in rats. Environ. Toxicol. 2016, 31, 1922–1934. [Google Scholar] [CrossRef]
- Gassman, N.R. Induction of oxidative stress by bisphenol A and its pleiotropic effects. Environ. Mol. Mutagen 2017, 58, 60–71. [Google Scholar] [CrossRef]
- Kobroob, A.; Peerapanyasut, W.; Chattipakorn, N.; Wongmekiat, O. Damaging Effects of Bisphenol A on the Kidney and the Protection by Melatonin: Emerging Evidences from In Vivo and In Vitro Studies. Oxid Med. Cell Longev. 2018, 2018, 3082438. [Google Scholar] [CrossRef]
- Trasande, L.; Attina, T.M.; Trachtman, H. Bisphenol A exposure is associated with low-grade urinary albumin excretion in children of the United States. Kidney Int. 2013, 83, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Bi, Y.; Qi, L.; Wang, T.; Xu, M.; Huang, Y.; Xu, Y.; Chen, Y.; Lu, J.; Wang, W.; et al. Exposure to bisphenol A is associated with low-grade albuminuria in Chinese adults. Kidney Int. 2012, 81, 1131–1139. [Google Scholar] [CrossRef] [PubMed]
- Cusumano, G.; Romagnoli, J.; Liuzzo, G.; Ciavarella, L.P.; Severino, A.; Copponi, G.; Manchi, M.; Giubilato, S.; Zannoni, G.F.; Stigliano, E.; et al. N-Acetylcysteine and High-Dose Atorvastatin Reduce Oxidative Stress in an Ischemia-Reperfusion Model in the Rat Kidney. Transpl. Proc. 2015, 47, 2757–2762. [Google Scholar] [CrossRef]
- Abdel-Wahab, W.M.; Moussa, F.I.; Saad, N.A. Synergistic protective effect of N-acetylcysteine and taurine against cisplatin-induced nephrotoxicity in rats. Drug Des. Devel. Ther. 2017, 11, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Le, J.W.; Zhu, J.H. Protective Effect of N-acetylcysteine pretreatment on acute kidney injury in septic rats. J. Surg. Res. 2020, 254, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, U.; Keller, F. Prophylaxis of contrast-induced nephrotoxicity. BioMed Res. Int. 2014, 2014, 308316. [Google Scholar] [CrossRef]
- Yuan, C.; Wang, L.; Zhu, L.; Ran, B.; Xue, X.; Wang, Z. N-acetylcysteine alleviated bisphenol A-induced testicular DNA hypermethylation of rare minnow (Gobiocypris rarus) by increasing cysteine contents. Ecotoxicol. Environ. Saf. 2019, 173, 243–250. [Google Scholar] [CrossRef]
- Ge, L.C.; Chen, Z.J.; Liu, H.; Zhang, K.S.; Su, Q.; Ma, X.Y.; Huang, H.B.; Zhao, Z.D.; Wang, Y.Y.; Giesy, J.P.; et al. Signaling related with biphasic effects of bisphenol A (BPA) on Sertoli cell proliferation: A comparative proteomic analysis. Biochim. Biophys. Acta 2014, 1840, 2663–2673. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Kaur, T.; Singla, S.K. Protective effects of N-acetylcysteine against hyperoxaluria induced mitochondrial dysfunction in male wistar rats. Mol. Cell Biochem. 2015, 405, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Peerapanyasut, W.; Thamprasert, K.; Wongmekiat, O. Ubiquinol supplementation protects against renal ischemia and reperfusion injury in rats. Free Radic. Res. 2014, 48, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Peerapanyasut, W.; Kobroob, A.; Palee, S.; Chattipakorn, N.; Wongmekiat, O. Bisphenol A aggravates renal ischemia-reperfusion injury by disrupting mitochondrial homeostasis and N-acetylcysteine mitigates the injurious outcomes. IUBMB Life 2020, 72, 758–770. [Google Scholar] [CrossRef] [PubMed]
- Olea-Herrero, N.; Arenas, M.I.; Munoz-Moreno, C.; Moreno-Gomez-Toledano, R.; Gonzalez-Santander, M.; Arribas, I.; Bosch, R.J. Bisphenol-A induces podocytopathy with proteinuria in mice. J. Cell Physiol. 2014, 229, 2057–2066. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, O.; Farokhi, F.; Banan Khojasteh, S.M.; Ozi, S.A. The effect of Bisphenol A on serum parameters and morphology of kidney’s tissue. Biol. Forum 2015, 7, 79–90. [Google Scholar]
- Ji, Z.Z.; Xu, Y.C. Melatonin protects podocytes from angiotensin II-induced injury in an in vitro diabetic nephropathy model. Mol. Med. Rep. 2016, 14, 920–926. [Google Scholar] [CrossRef] [PubMed]
- El-Missiry, M.A.; Othman, A.I.; Al-Abdan, M.A.; El-Sayed, A.A. Melatonin ameliorates oxidative stress, modulates death receptor pathway proteins, and protects the rat cerebrum against bisphenol-A-induced apoptosis. J. Neurol. Sci. 2014, 347, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, V.; Afsharian, P.; Shahhoseini, M.; Kalantar, S.M.; Moini, A. A Review on Various Uses of N-Acetyl Cysteine. Cell J. 2017, 19, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Elbini Dhouib, I.; Jallouli, M.; Annabi, A.; Gharbi, N.; Elfazaa, S.; Lasram, M.M. A minireview on N-acetylcysteine: An old drug with new approaches. Life Sci. 2016, 151, 359–363. [Google Scholar] [CrossRef]
- Samuni, Y.; Goldstein, S.; Dean, O.M.; Berk, M. The chemistry and biological activities of N-acetylcysteine. Biochim. Biophys. Acta 2013, 1830, 4117–4129. [Google Scholar] [CrossRef]
- Minamiyama, Y.; Ichikawa, H.; Takemura, S.; Kusunoki, H.; Naito, Y.; Yoshikawa, T. Generation of reactive oxygen species in sperms of rats as an earlier marker for evaluating the toxicity of endocrine-disrupting chemicals. Free Radic. Res. 2010, 44, 1398–1406. [Google Scholar] [CrossRef]
- Yu, L.; Gong, B.; Duan, W.; Fan, C.; Zhang, J.; Li, Z.; Xue, X.; Xu, Y.; Meng, D.; Li, B.; et al. Melatonin ameliorates myocardial ischemia/reperfusion injury in type 1 diabetic rats by preserving mitochondrial function: Role of AMPK-PGC-1alpha-SIRT3 signaling. Sci. Rep. 2017, 7, 41337. [Google Scholar] [CrossRef]
- Perico, L.; Morigi, M.; Benigni, A. Mitochondrial Sirtuin 3 and Renal Diseases. Nephron 2016, 134, 14–19. [Google Scholar] [CrossRef]
- Benigni, A.; Perico, L.; Macconi, D. Mitochondrial Dynamics Is Linked to Longevity and Protects from End-Organ Injury: The Emerging Role of Sirtuin 3. Antioxid. Redox Signal. 2016, 25, 185–199. [Google Scholar] [CrossRef]
- Li, X.; Song, S.; Xu, M.; Hua, Y.; Ding, Y.; Shan, X.; Meng, G.; Wang, Y. Sirtuin3 deficiency exacerbates carbon tetrachloride-induced hepatic injury in mice. J. Biochem. Mol. Toxicol. 2019, 33, e22249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, X.; Li, N.; Zhang, J.; Yang, J.; Bu, P. Sirtuin 3 deficiency aggravates contrast-induced acute kidney injury. J. Transl. Med. 2018, 16, 313. [Google Scholar] [CrossRef] [PubMed]
- Morigi, M.; Perico, L.; Rota, C.; Longaretti, L.; Conti, S.; Rottoli, D.; Novelli, R.; Remuzzi, G.; Benigni, A. Sirtuin 3-dependent mitochondrial dynamic improvements protect against acute kidney injury. J. Clin Investig. 2015, 125, 715–726. [Google Scholar] [CrossRef]
- Kong, X.; Wang, R.; Xue, Y.; Liu, X.; Zhang, H.; Chen, Y.; Fang, F.; Chang, Y. Sirtuin 3, a new target of PGC-1alpha, plays an important role in the suppression of ROS and mitochondrial biogenesis. PLoS ONE 2010, 5, e11707. [Google Scholar] [CrossRef] [PubMed]
- Kahn, B.B.; Alquier, T.; Carling, D.; Hardie, D.G. AMP-activated protein kinase: Ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005, 1, 15–25. [Google Scholar] [CrossRef]
| Parameters | Week 0 | Week 12 | Week 16 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Veh | BPA | BPA+NAC | Veh | BPA | BPA + NAC | Veh | BPA | BPA + NAC | |
| BW (g) | 135.0 | 135.0 | 134.0 | 459.17 | 415.00 | 415.83 | 466.67 | 396.67 | 395.00 |
| ±1.83 | ±0.62 | ±1.53 | ±5.07 ‡ | ±14.61 *,‡ | ±10.12 *,‡ | ±10.54 | ±4.94 * | ±10.17 * | |
| Food intake | - | - | - | 20.69 | 19.58 | 19.58 | 17.74 | 17.71 | 17.80 |
| (g/day) | ±0.82 | ±0.29 | ±0.28 | ±1.09 | ±0.36 | ±0.12 | |||
| Water intake | - | - | - | 20.12 | 20.45 | 20.27 | 20.52 | 21.58 | 20.30 |
| (ml/day) | ±1.27 | ±1.35 | ±1.02 | ±1.12 | ±0.96 | ±1.59 | |||
| KW | - | - | - | - | - | - | 2.85 | 2.32 | 2.24 |
| (g) | ±0.07 | ±0.01 * | ±0.04 * | ||||||
| KW/BW | - | - | - | - | - | - | 0.61 | 0.59 | 0.59 |
| (* 100) | ±0.02 | ±0.01 | ±0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobroob, A.; Peerapanyasut, W.; Kumfu, S.; Chattipakorn, N.; Wongmekiat, O. Effectiveness of N-Acetylcysteine in the Treatment of Renal Deterioration Caused by Long-Term Exposure to Bisphenol A. Biomolecules 2021, 11, 655. https://doi.org/10.3390/biom11050655
Kobroob A, Peerapanyasut W, Kumfu S, Chattipakorn N, Wongmekiat O. Effectiveness of N-Acetylcysteine in the Treatment of Renal Deterioration Caused by Long-Term Exposure to Bisphenol A. Biomolecules. 2021; 11(5):655. https://doi.org/10.3390/biom11050655
Chicago/Turabian StyleKobroob, Anongporn, Wachirasek Peerapanyasut, Sirinart Kumfu, Nipon Chattipakorn, and Orawan Wongmekiat. 2021. "Effectiveness of N-Acetylcysteine in the Treatment of Renal Deterioration Caused by Long-Term Exposure to Bisphenol A" Biomolecules 11, no. 5: 655. https://doi.org/10.3390/biom11050655
APA StyleKobroob, A., Peerapanyasut, W., Kumfu, S., Chattipakorn, N., & Wongmekiat, O. (2021). Effectiveness of N-Acetylcysteine in the Treatment of Renal Deterioration Caused by Long-Term Exposure to Bisphenol A. Biomolecules, 11(5), 655. https://doi.org/10.3390/biom11050655







