Proteolytic Cleavage of Receptor Tyrosine Kinases
Abstract
:1. Introduction
1.1. RTK Cleavage and Cancers
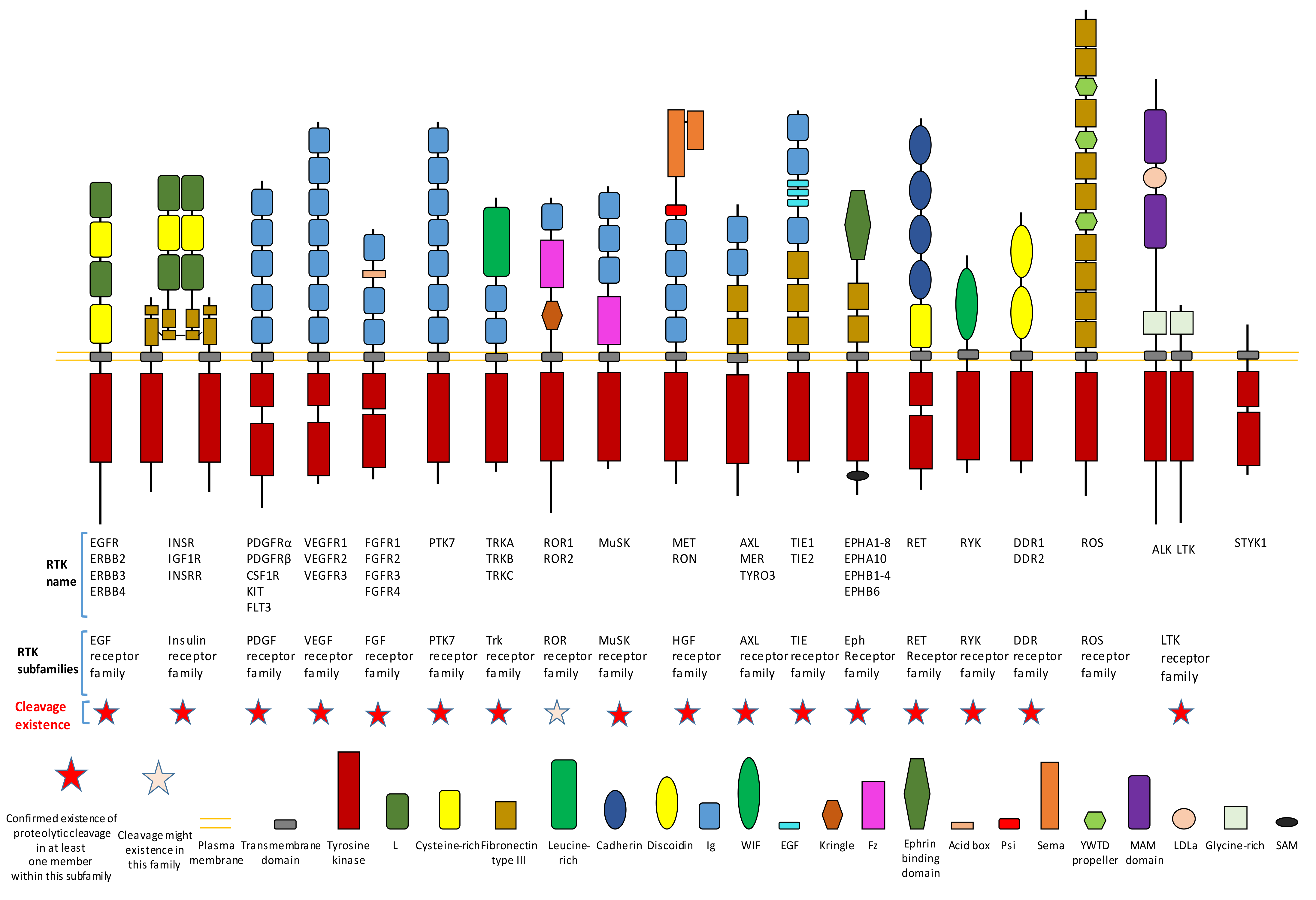
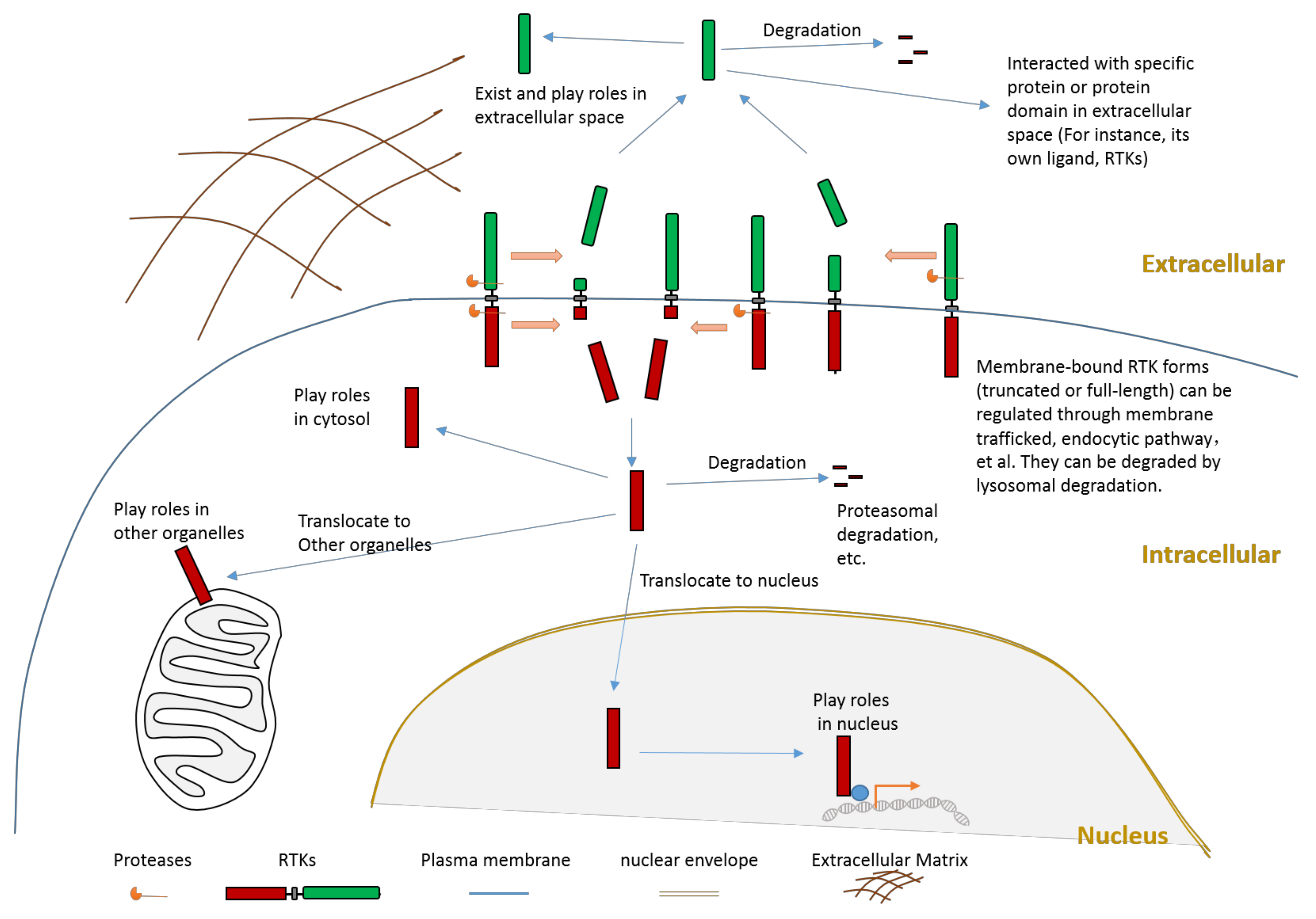
1.2. Some Characteristics of RTK Proteolytic Cleavage
2. Proteolytic Cleavage in Various Members of RTK
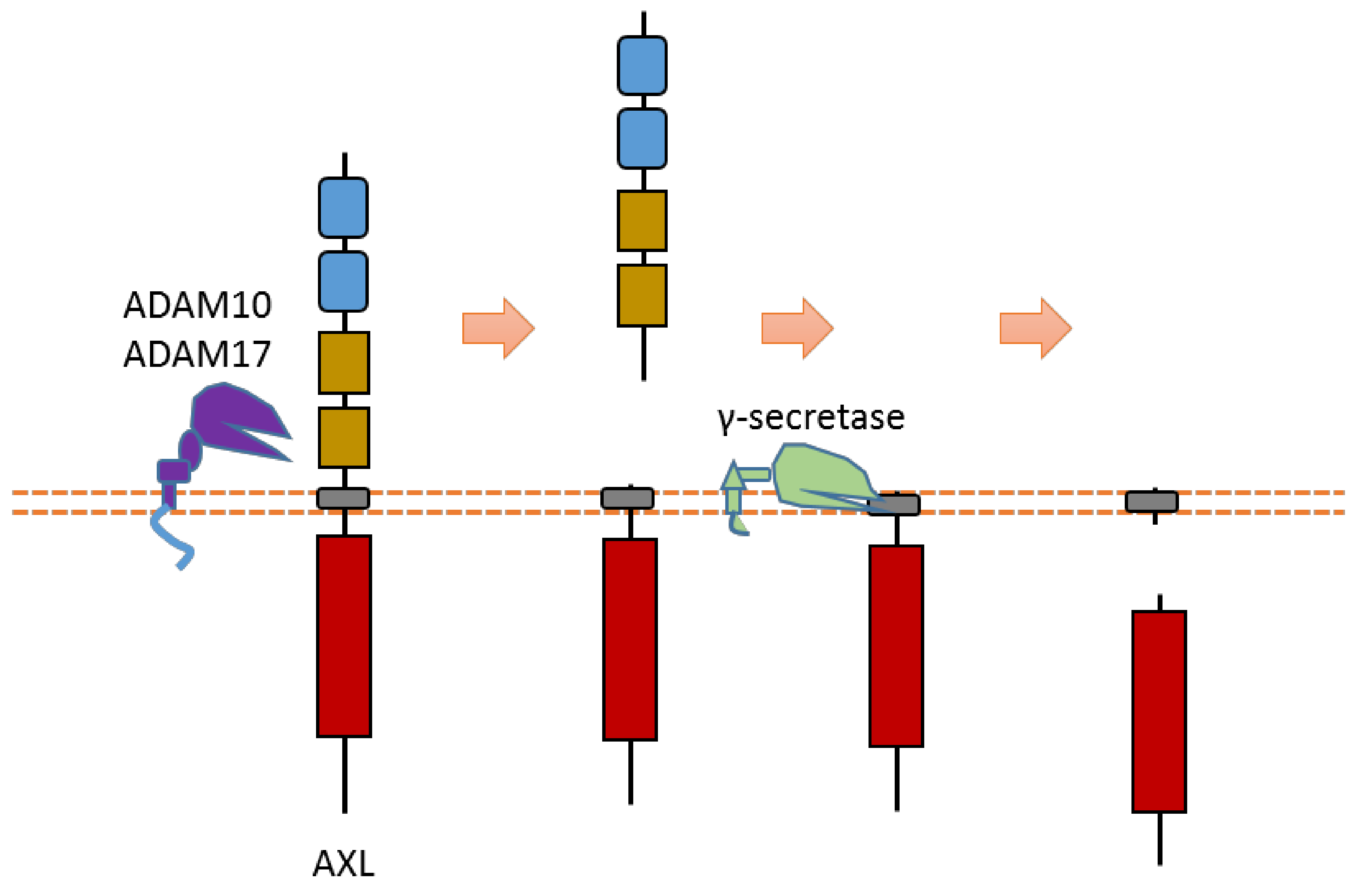
2.1. AXL
2.2. MET
2.3. Trk A, B, C
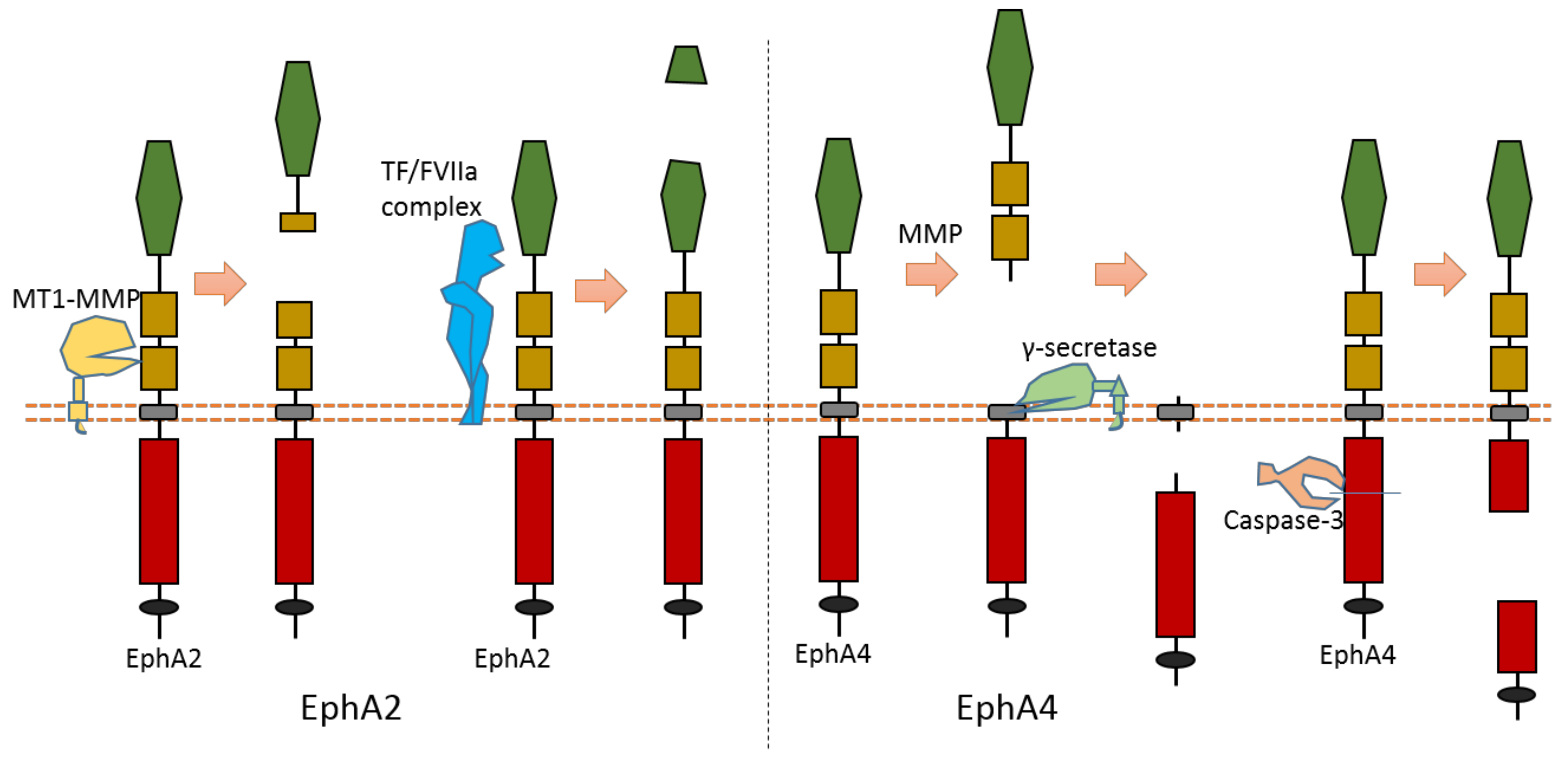
2.4. EphA2
2.5. EphA4
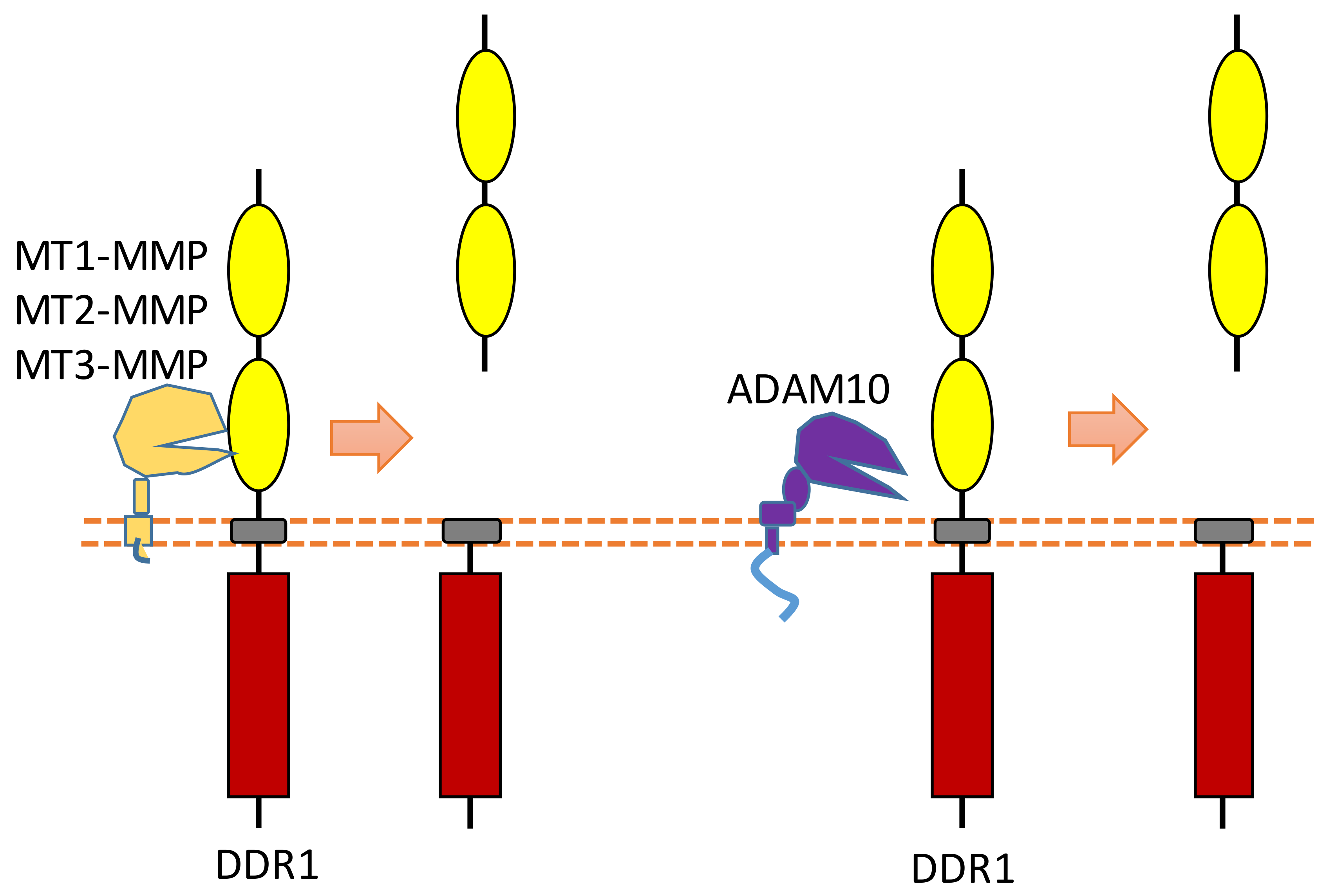
2.6. DDR1
2.7. FGFR1
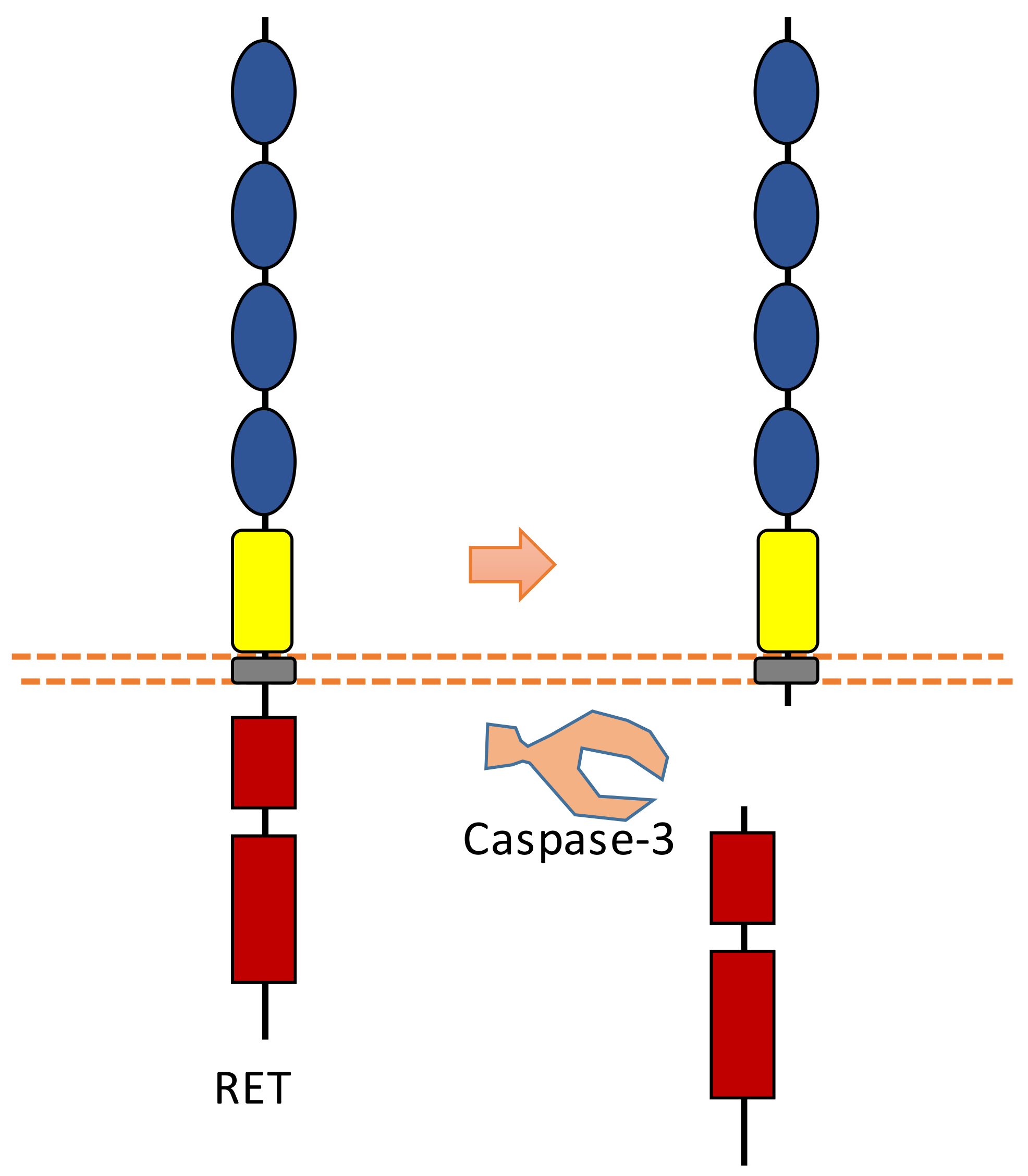
2.8. RET
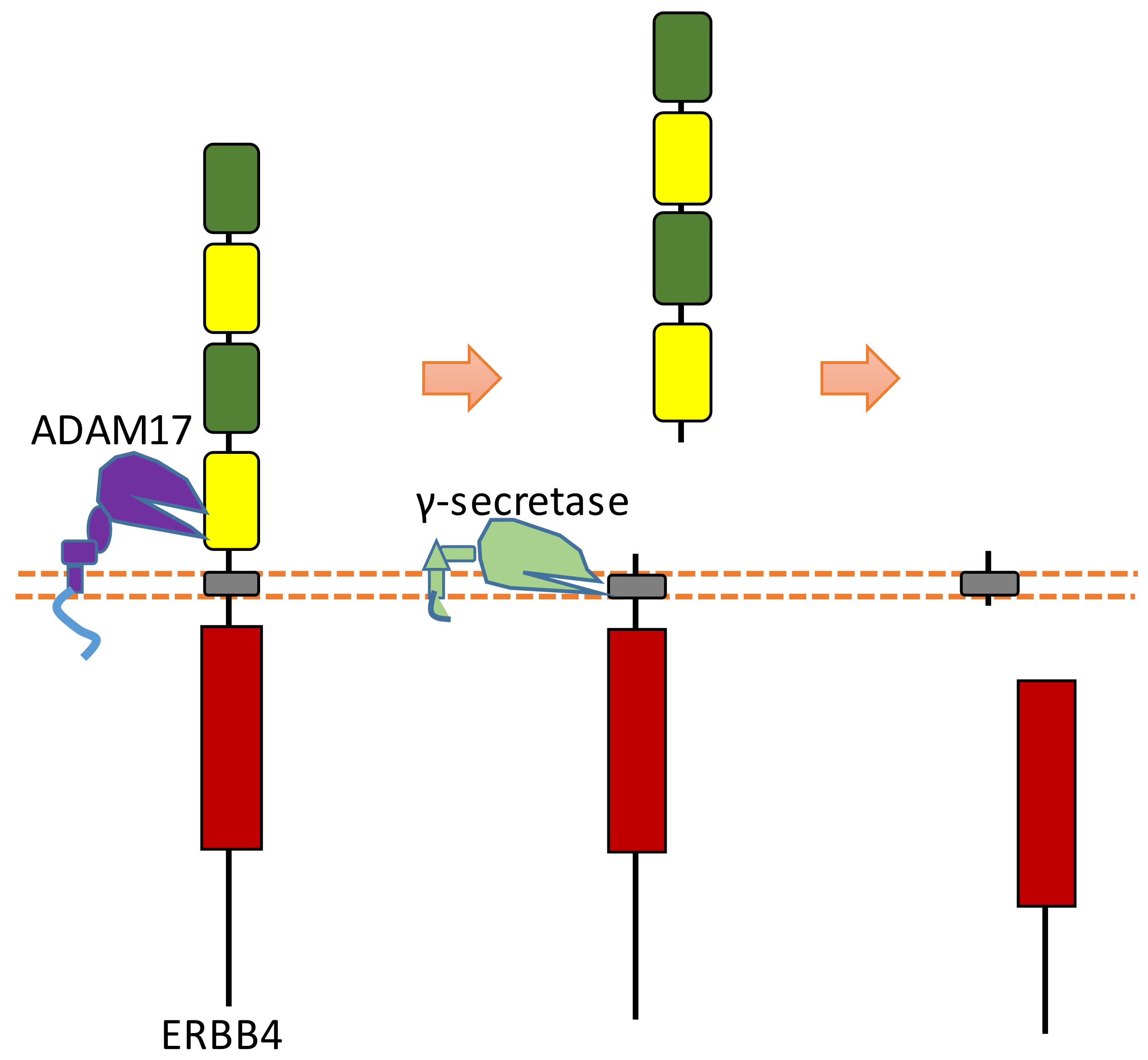
2.9. ERBB4
2.10. ERBB2
2.11. ALK
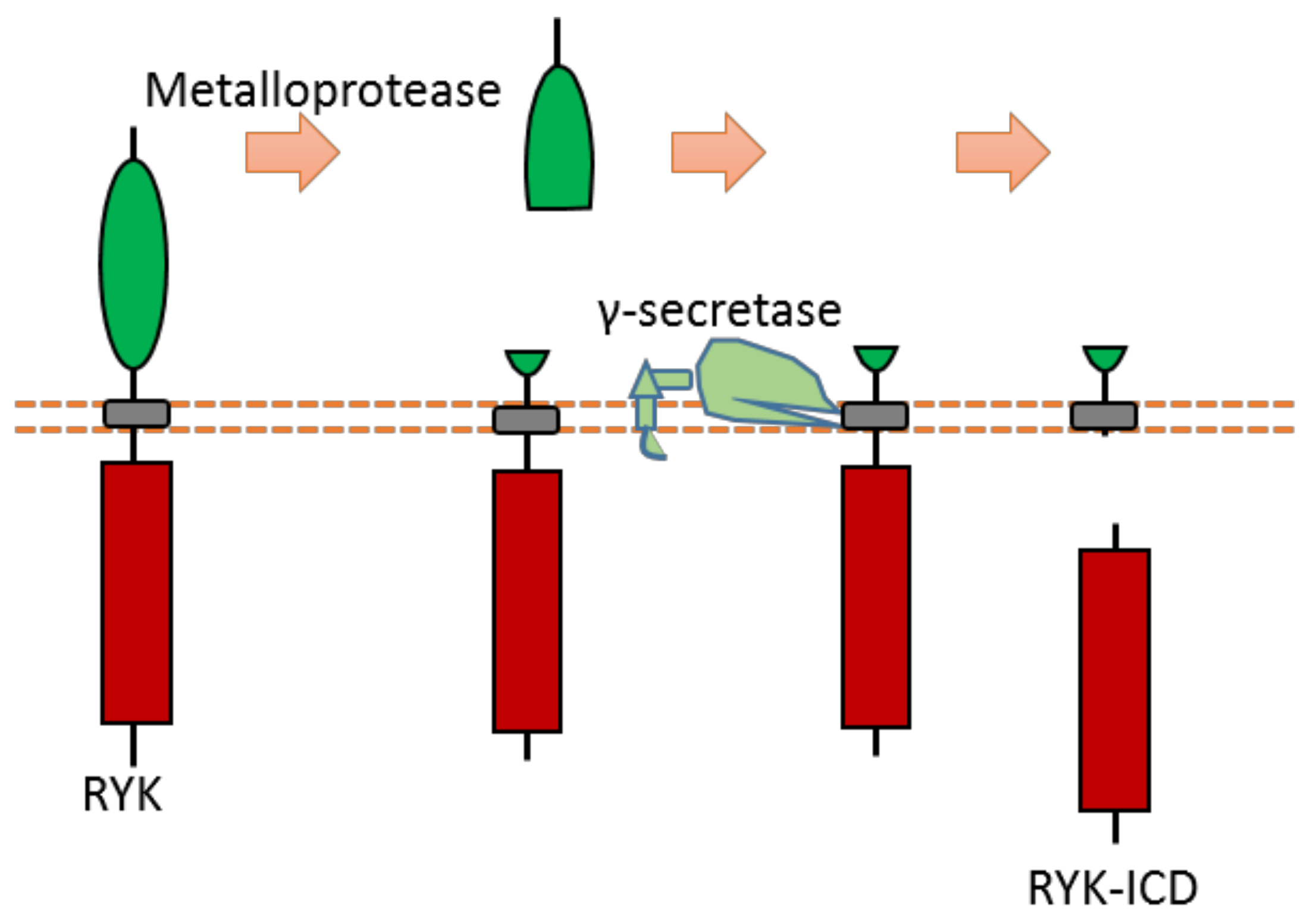
2.12. Ryk
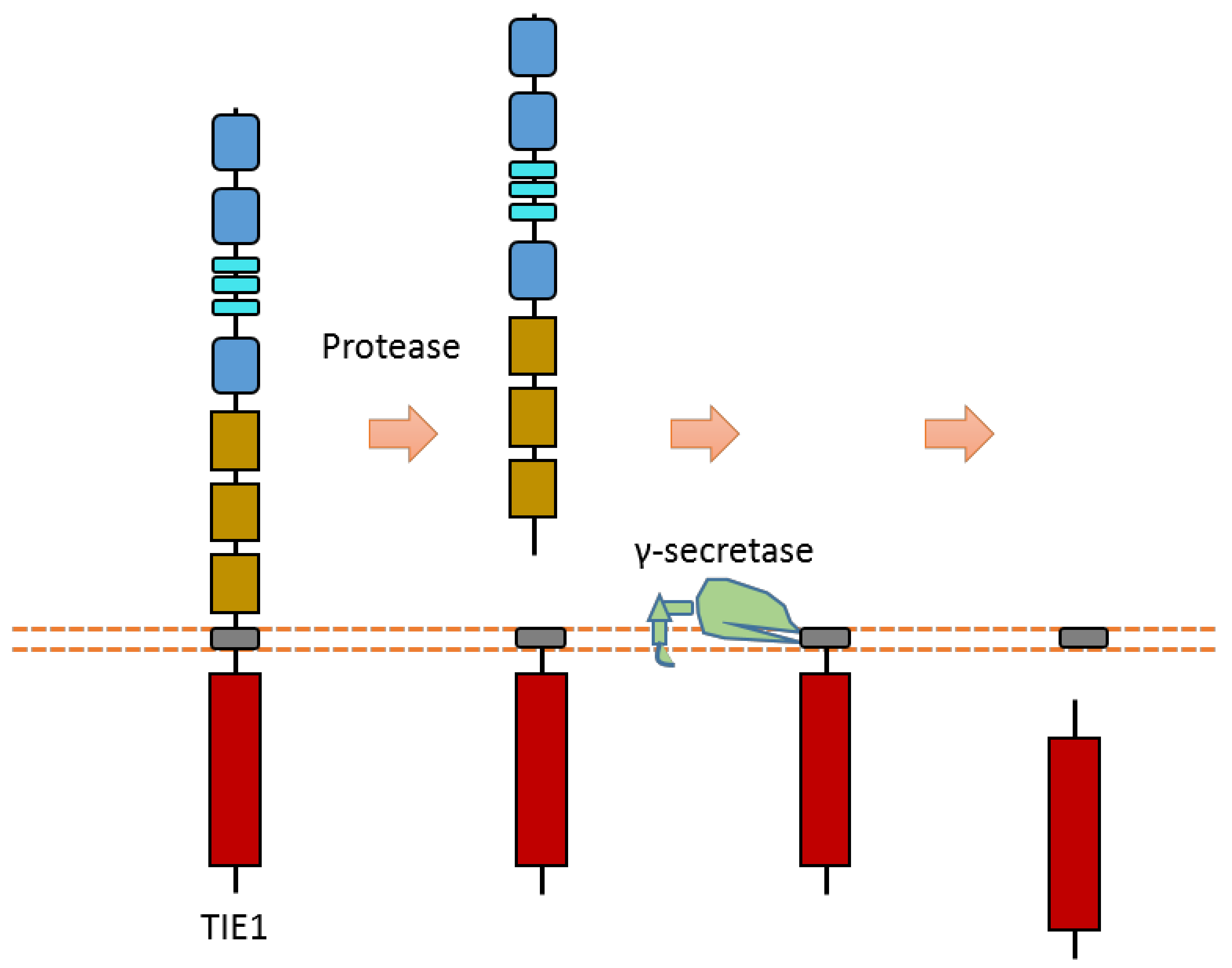
2.13. TIE1
2.14. VEGFR1
2.15. PTK7
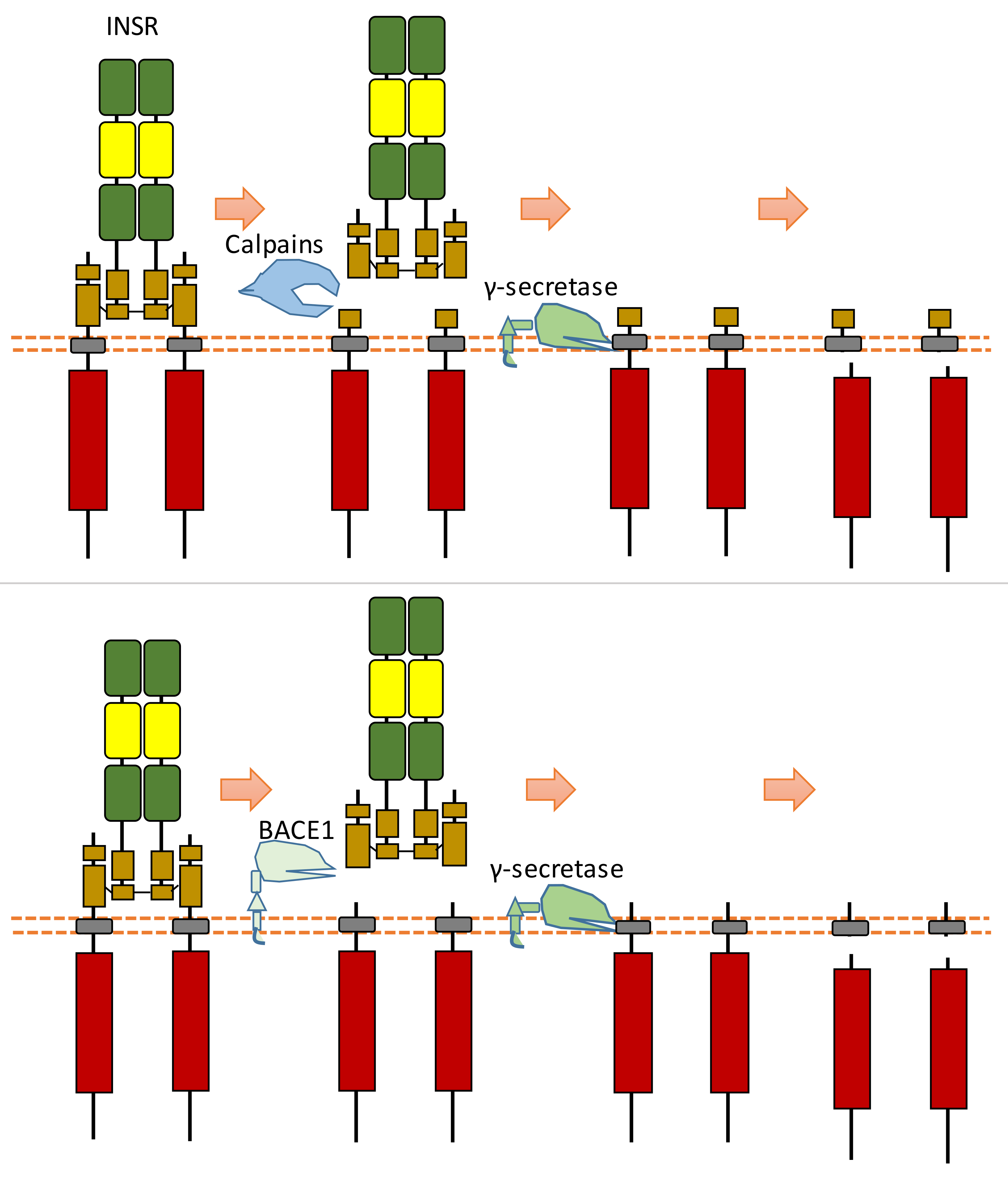
2.16. INSR
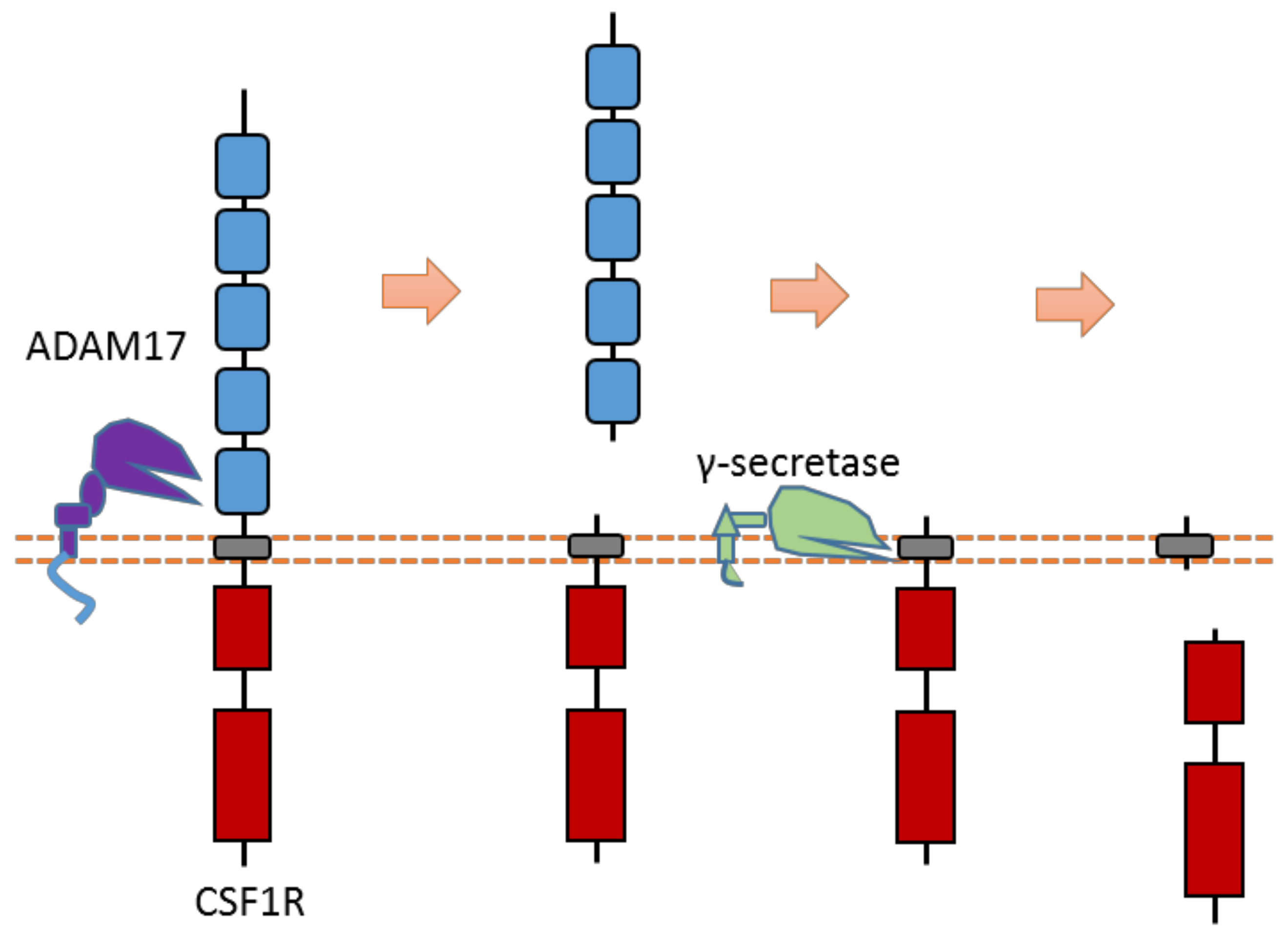
2.17. CSF1R
3. Summary
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lemmon, M.A.; Schlessinger, J. Cell signaling by receptor tyrosine kinases. Cell 2010, 141, 1117–1134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hubbard, S.R.; Miller, W.T. Receptor tyrosine kinases: Mechanisms of activation and signaling. Curr. Opin. Cell Biol. 2007, 19, 117–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casaletto, J.B.; McClatchey, A.I. Spatial regulation of receptor tyrosine kinases in development and cancer. Nat. Rev. Cancer 2012, 12, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.I.; Accili, D.; Cama, A.; Kadowaki, H.; Kadowaki, T.; Imano, E.; Sierra, M.L. Mutations in the insulin receptor gene in patients with genetic syndromes of insulin resistance. Adv. Exp. Med. Biol. 1991, 293, 197–213. [Google Scholar]
- Boucher, J.; Kleinridders, A.; Kahn, C.R. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb. Perspect. Biol. 2014, 6, a009191. [Google Scholar] [CrossRef] [Green Version]
- Du, Z.; Lovly, C.M. Mechanisms of receptor tyrosine kinase activation in cancer. Mol. Cancer 2018, 17, 58. [Google Scholar] [CrossRef]
- Bordeaux, M.C.; Forcet, C.; Granger, L.; Corset, V.; Bidaud, C.; Billaud, M.; Bredesen, D.E.; Edery, P.; Mehlen, P. The ret proto-oncogene induces apoptosis: A novel mechanism for hirschsprung disease. EMBO J. 2000, 19, 4056–4063. [Google Scholar] [CrossRef] [Green Version]
- Tulasne, D.; Deheuninck, J.; Lourenco, F.C.; Lamballe, F.; Ji, Z.; Leroy, C.; Puchois, E.; Moumen, A.; Maina, F.; Mehlen, P.; et al. Proapoptotic function of the met tyrosine kinase receptor through caspase cleavage. Mol. Cell. Biol. 2004, 24, 10328–10339. [Google Scholar] [CrossRef] [Green Version]
- Tauszig-Delamasure, S.; Yu, L.Y.; Cabrera, J.R.; Bouzas-Rodriguez, J.; Mermet-Bouvier, C.; Guix, C.; Bordeaux, M.C.; Arumae, U.; Mehlen, P. The trkc receptor induces apoptosis when the dependence receptor notion meets the neurotrophin paradigm. Proc. Natl. Acad. Sci. USA 2007, 104, 13361–13366. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Lavandeira, M.; Diaz-Rodriguez, E.; Garcia-Rendueles, M.E.; Rodrigues, J.S.; Perez-Romero, S.; Bravo, S.B.; Alvarez, C.V. Functional role of the ret dependence receptor, gfra co-receptors and ligands in the pituitary. Front. Horm. Res. 2010, 38, 127–138. [Google Scholar]
- Lefebvre, J.; Muharram, G.; Leroy, C.; Kherrouche, Z.; Montagne, R.; Ichim, G.; Tauszig-Delamasure, S.; Chotteau-Lelievre, A.; Brenner, C.; Mehlen, P.; et al. Caspase-generated fragment of the met receptor favors apoptosis via the intrinsic pathway independently of its tyrosine kinase activity. Cell Death Dis. 2013, 4, e871. [Google Scholar] [CrossRef] [PubMed]
- Mourali, J.; Benard, A.; Lourenco, F.C.; Monnet, C.; Greenland, C.; Moog-Lutz, C.; Racaud-Sultan, C.; Gonzalez-Dunia, D.; Vigny, M.; Mehlen, P.; et al. Anaplastic lymphoma kinase is a dependence receptor whose proapoptotic functions are activated by caspase cleavage. Mol. Cell. Biol. 2006, 26, 6209–6222. [Google Scholar] [CrossRef] [Green Version]
- Montagne, R.; Baranzelli, A.; Muharram, G.; Catherine, L.; Lesaffre, M.; Vinchent, A.; Kherrouche, Z.; Werkmeister, E.; Cortot, A.B.; Tulasne, D. Met receptor variant r970c favors calpain-dependent generation of a fragment promoting epithelial cell scattering. Oncotarget 2017, 8, 11268–11283. [Google Scholar] [CrossRef]
- Schelter, F.; Kobuch, J.; Moss, M.L.; Becherer, J.D.; Comoglio, P.M.; Boccaccio, C.; Kruger, A. A disintegrin and metalloproteinase-10 (adam-10) mediates dn30 antibody-induced shedding of the met surface receptor. J. Biol. Chem. 2010, 285, 26335–26340. [Google Scholar] [CrossRef] [Green Version]
- Codony-Servat, J.; Albanell, J.; Lopez-Talavera, J.C.; Arribas, J.; Baselga, J. Cleavage of the her2 ectodomain is a pervanadate-activable process that is inhibited by the tissue inhibitor of metalloproteases-1 in breast cancer cells. Cancer Res. 1999, 59, 1196–1201. [Google Scholar]
- Liu, P.C.; Liu, X.; Li, Y.; Covington, M.; Wynn, R.; Huber, R.; Hillman, M.; Yang, G.; Ellis, D.; Marando, C.; et al. Identification of adam10 as a major source of her2 ectodomain sheddase activity in her2 overexpressing breast cancer cells. Cancer Biol. Ther. 2006, 5, 657–664. [Google Scholar] [CrossRef] [Green Version]
- Tse, C.; Gauchez, A.S.; Jacot, W.; Lamy, P.J. Her2 shedding and serum her2 extracellular domain: Biology and clinical utility in breast cancer. Cancer Treat. Rev. 2012, 38, 133–142. [Google Scholar] [CrossRef]
- Wang, J.; Willumsen, N.; Zheng, Q.; Xue, Y.; Karsdal, M.A.; Bay-Jensen, A.C. Bringing cancer serological diagnosis to a new level: Focusing on her2, protein ectodomain shedding and neoepitope technology. Future Oncol. 2013, 9, 35–44. [Google Scholar] [CrossRef]
- Chen, L.M.; Chai, K.X. Proteolytic cleavages in the extracellular domain of receptor tyrosine kinases by membrane-associated serine proteases. Oncotarget 2017, 8, 56490–56505. [Google Scholar] [CrossRef]
- Molina, M.A.; Codony-Servat, J.; Albanell, J.; Rojo, F.; Arribas, J.; Baselga, J. Trastuzumab (herceptin), a humanized anti-her2 receptor monoclonal antibody, inhibits basal and activated her2 ectodomain cleavage in breast cancer cells. Cancer Res. 2001, 61, 4744–4749. [Google Scholar]
- Christianson, T.A.; Doherty, J.K.; Lin, Y.J.; Ramsey, E.E.; Holmes, R.; Keenan, E.J.; Clinton, G.M. Nh2-terminally truncated her-2/neu protein: Relationship with shedding of the extracellular domain and with prognostic factors in breast cancer. Cancer Res. 1998, 58, 5123–5129. [Google Scholar] [PubMed]
- Liu, X.; Fridman, J.S.; Wang, Q.; Caulder, E.; Yang, G.; Covington, M.; Liu, C.; Marando, C.; Zhuo, J.; Li, Y.; et al. Selective inhibition of adam metalloproteases blocks her-2 extracellular domain (ecd) cleavage and potentiates the anti-tumor effects of trastuzumab. Cancer Biol. Ther. 2006, 5, 648–656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feldinger, K.; Generali, D.; Kramer-Marek, G.; Gijsen, M.; Ng, T.B.; Wong, J.H.; Strina, C.; Cappelletti, M.; Andreis, D.; Li, J.L.; et al. Adam10 mediates trastuzumab resistance and is correlated with survival in her2 positive breast cancer. Oncotarget 2014, 5, 6633–6646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maatta, J.A.; Sundvall, M.; Junttila, T.T.; Peri, L.; Laine, V.J.; Isola, J.; Egeblad, M.; Elenius, K. Proteolytic cleavage and phosphorylation of a tumor-associated erbb4 isoform promote ligand-independent survival and cancer cell growth. Mol. Biol. Cell 2006, 17, 67–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ni, C.Y.; Murphy, M.P.; Golde, T.E.; Carpenter, G. Gamma -secretase cleavage and nuclear localization of erbb-4 receptor tyrosine kinase. Science 2001, 294, 2179–2181. [Google Scholar] [CrossRef]
- Hoeing, K.; Zscheppang, K.; Mujahid, S.; Murray, S.; Volpe, M.V.; Dammann, C.E.; Nielsen, H.C. Presenilin-1 processing of erbb4 in fetal type ii cells is necessary for control of fetal lung maturation. Biochim. Biophys. Acta 2011, 1813, 480–491. [Google Scholar] [CrossRef] [Green Version]
- Zscheppang, K.; Dork, T.; Schmiedl, A.; Jones, F.E.; Dammann, C.E. Neuregulin receptor erbb4 functions as a transcriptional cofactor for the expression of surfactant protein b in the fetal lung. Am. J. Respir. Cell Mol. Biol. 2011, 45, 761–767. [Google Scholar] [CrossRef]
- Zscheppang, K.; Konrad, M.; Zischka, M.; Huhn, V.; Dammann, C.E. Estrogen-induced upregulation of sftpb requires transcriptional control of neuregulin receptor erbb4 in mouse lung type ii epithelial cells. Biochim. Biophys. Acta 2011, 1813, 1717–1727. [Google Scholar] [CrossRef] [Green Version]
- Paatero, I.; Jokilammi, A.; Heikkinen, P.T.; Iljin, K.; Kallioniemi, O.P.; Jones, F.E.; Jaakkola, P.M.; Elenius, K. Interaction with erbb4 promotes hypoxia-inducible factor-1alpha signaling. J. Biol. Chem. 2012, 287, 9659–9671. [Google Scholar] [CrossRef] [Green Version]
- Chioni, A.M.; Grose, R. Fgfr1 cleavage and nuclear translocation regulates breast cancer cell behavior. J. Cell Biol. 2012, 197, 801–817. [Google Scholar] [CrossRef] [Green Version]
- Na, H.W.; Shin, W.S.; Ludwig, A.; Lee, S.T. The cytosolic domain of protein-tyrosine kinase 7 (ptk7), generated from sequential cleavage by a disintegrin and metalloprotease 17 (adam17) and gamma-secretase, enhances cell proliferation and migration in colon cancer cells. J. Biol. Chem. 2012, 287, 25001–25009. [Google Scholar] [CrossRef] [Green Version]
- Golubkov, V.S.; Prigozhina, N.L.; Zhang, Y.; Stoletov, K.; Lewis, J.D.; Schwartz, P.E.; Hoffman, R.M.; Strongin, A.Y. Protein-tyrosine pseudokinase 7 (ptk7) directs cancer cell motility and metastasis. J. Biol. Chem. 2014, 289, 24238–24249. [Google Scholar] [CrossRef] [Green Version]
- Pozner-Moulis, S.; Pappas, D.J.; Rimm, D.L. Met, the hepatocyte growth factor receptor, localizes to the nucleus in cells at low density. Cancer Res. 2006, 66, 7976–7982. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Lu, W.; Liu, S.; Yang, Q.; Carver, B.S.; Li, E.; Wang, Y.; Fazli, L.; Gleave, M.; Chen, Z. Crosstalk between nuclear met and sox9/beta-catenin correlates with castration-resistant prostate cancer. Mol. Endocrinol. 2014, 28, 1629–1639. [Google Scholar] [CrossRef] [Green Version]
- Barisione, G.; Fabbi, M.; Gino, A.; Queirolo, P.; Orgiano, L.; Spano, L.; Picasso, V.; Pfeffer, U.; Mosci, C.; Jager, M.J.; et al. Potential role of soluble c-met as a new candidate biomarker of metastatic uveal melanoma. JAMA Ophthalmol. 2015, 133, 1013–1021. [Google Scholar] [CrossRef] [Green Version]
- Nath, D.; Williamson, N.J.; Jarvis, R.; Murphy, G. Shedding of c-met is regulated by crosstalk between a g-protein coupled receptor and the egf receptor and is mediated by a timp-3 sensitive metalloproteinase. J. Cell Sci. 2001, 114, 1213–1220. [Google Scholar] [CrossRef]
- Athauda, G.; Giubellino, A.; Coleman, J.A.; Horak, C.; Steeg, P.S.; Lee, M.J.; Trepel, J.; Wimberly, J.; Sun, J.; Coxon, A.; et al. C-met ectodomain shedding rate correlates with malignant potential. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2006, 12, 4154–4162. [Google Scholar] [CrossRef] [Green Version]
- Foveau, B.; Ancot, F.; Leroy, C.; Petrelli, A.; Reiss, K.; Vingtdeux, V.; Giordano, S.; Fafeur, V.; Tulasne, D. Down-regulation of the met receptor tyrosine kinase by presenilin-dependent regulated intramembrane proteolysis. Mol. Biol. Cell 2009, 20, 2495–2507. [Google Scholar] [CrossRef] [Green Version]
- Montagne, R.; Berbon, M.; Doublet, L.; Debreuck, N.; Baranzelli, A.; Drobecq, H.; Leroy, C.; Delhem, N.; Porte, H.; Copin, M.C.; et al. Necrosis- and apoptosis-related met cleavages have divergent functional consequences. Cell Death Dis. 2015, 6, e1769. [Google Scholar] [CrossRef] [Green Version]
- O’Bryan, J.P.; Fridell, Y.W.; Koski, R.; Varnum, B.; Liu, E.T. The transforming receptor tyrosine kinase, axl, is post-translationally regulated by proteolytic cleavage. J. Biol. Chem. 1995, 270, 551–557. [Google Scholar] [CrossRef] [Green Version]
- Weinger, J.G.; Omari, K.M.; Marsden, K.; Raine, C.S.; Shafit-Zagardo, B. Up-regulation of soluble axl and mer receptor tyrosine kinases negatively correlates with gas6 in established multiple sclerosis lesions. Am. J. Pathol. 2009, 175, 283–293. [Google Scholar] [CrossRef] [Green Version]
- Orme, J.J.; Du, Y.; Vanarsa, K.; Mayeux, J.; Li, L.; Mutwally, A.; Arriens, C.; Min, S.; Hutcheson, J.; Davis, L.S.; et al. Heightened cleavage of axl receptor tyrosine kinase by adam metalloproteases may contribute to disease pathogenesis in sle. Clin. Immunol. 2016, 169, 58–68. [Google Scholar] [CrossRef] [Green Version]
- Miller, M.A.; Oudin, M.J.; Sullivan, R.J.; Wang, S.J.; Meyer, A.S.; Im, H.; Frederick, D.T.; Tadros, J.; Griffith, L.G.; Lee, H.; et al. Reduced proteolytic shedding of receptor tyrosine kinases is a post-translational mechanism of kinase inhibitor resistance. Cancer Discov. 2016, 6, 382–399. [Google Scholar] [CrossRef] [Green Version]
- Miller, M.A.; Meyer, A.S.; Beste, M.T.; Lasisi, Z.; Reddy, S.; Jeng, K.W.; Chen, C.H.; Han, J.; Isaacson, K.; Griffith, L.G.; et al. Adam-10 and -17 regulate endometriotic cell migration via concerted ligand and receptor shedding feedback on kinase signaling. Proc. Natl. Acad. Sci. USA 2013, 110, E2074–E2083. [Google Scholar] [CrossRef] [Green Version]
- Merlos-Suarez, A.; Ruiz-Paz, S.; Baselga, J.; Arribas, J. Metalloprotease-dependent protransforming growth factor-alpha ectodomain shedding in the absence of tumor necrosis factor-alpha-converting enzyme. J. Biol. Chem. 2001, 276, 48510–48517. [Google Scholar] [CrossRef] [Green Version]
- Sunnarborg, S.W.; Hinkle, C.L.; Stevenson, M.; Russell, W.E.; Raska, C.S.; Peschon, J.J.; Castner, B.J.; Gerhart, M.J.; Paxton, R.J.; Black, R.A.; et al. Tumor necrosis factor-alpha converting enzyme (tace) regulates epidermal growth factor receptor ligand availability. J. Biol. Chem. 2002, 277, 12838–12845. [Google Scholar] [CrossRef] [Green Version]
- Sahin, U.; Weskamp, G.; Kelly, K.; Zhou, H.M.; Higashiyama, S.; Peschon, J.; Hartmann, D.; Saftig, P.; Blobel, C.P. Distinct roles for adam10 and adam17 in ectodomain shedding of six egfr ligands. J. Cell Biol. 2004, 164, 769–779. [Google Scholar] [CrossRef] [Green Version]
- Edwards, D.R.; Handsley, M.M.; Pennington, C.J. The adam metalloproteinases. Mol. Asp. Med. 2008, 29, 258–289. [Google Scholar] [CrossRef]
- Di Marco, E.; Pierce, J.H.; Fleming, T.P.; Kraus, M.H.; Molloy, C.J.; Aaronson, S.A.; Di Fiore, P.P. Autocrine interaction between tgf alpha and the egf-receptor: Quantitative requirements for induction of the malignant phenotype. Oncogene 1989, 4, 831–838. [Google Scholar] [PubMed]
- Gullick, W.J. Prevalence of aberrant expression of the epidermal growth factor receptor in human cancers. Br. Med Bull. 1991, 47, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Barnard, J.A.; Beauchamp, R.D.; Russell, W.E.; Dubois, R.N.; Coffey, R.J. Epidermal growth factor-related peptides and their relevance to gastrointestinal pathophysiology. Gastroenterology 1995, 108, 564–580. [Google Scholar] [CrossRef]
- Baselga, J.; Mendelsohn, J.; Kim, Y.M.; Pandiella, A. Autocrine regulation of membrane transforming growth factor-alpha cleavage. J. Biol. Chem. 1996, 271, 3279–3284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diaz-Rodriguez, E.; Montero, J.C.; Esparis-Ogando, A.; Yuste, L.; Pandiella, A. Extracellular signal-regulated kinase phosphorylates tumor necrosis factor alpha-converting enzyme at threonine 735: A potential role in regulated shedding. Mol. Biol. Cell 2002, 13, 2031–2044. [Google Scholar] [CrossRef] [PubMed]
- Soond, S.M.; Everson, B.; Riches, D.W.; Murphy, G. Erk-mediated phosphorylation of thr735 in tnfalpha-converting enzyme and its potential role in tace protein trafficking. J. Cell Sci. 2005, 118, 2371–2380. [Google Scholar] [CrossRef] [Green Version]
- Hattori, M.; Osterfield, M.; Flanagan, J.G. Regulated cleavage of a contact-mediated axon repellent. Science 2000, 289, 1360–1365. [Google Scholar] [CrossRef]
- Janes, P.W.; Saha, N.; Barton, W.A.; Kolev, M.V.; Wimmer-Kleikamp, S.H.; Nievergall, E.; Blobel, C.P.; Himanen, J.P.; Lackmann, M.; Nikolov, D.B. Adam meets eph: An adam substrate recognition module acts as a molecular switch for ephrin cleavage in trans. Cell 2005, 123, 291–304. [Google Scholar] [CrossRef] [Green Version]
- Georgakopoulos, A.; Litterst, C.; Ghersi, E.; Baki, L.; Xu, C.; Serban, G.; Robakis, N.K. Metalloproteinase/presenilin1 processing of ephrinb regulates ephb-induced src phosphorylation and signaling. EMBO J. 2006, 25, 1242–1252. [Google Scholar] [CrossRef] [Green Version]
- Ieguchi, K.; Tomita, T.; Omori, T.; Komatsu, A.; Deguchi, A.; Masuda, J.; Duffy, S.L.; Coulthard, M.G.; Boyd, A.; Maru, Y. Adam12-cleaved ephrin-a1 contributes to lung metastasis. Oncogene 2014, 33, 2179–2190. [Google Scholar] [CrossRef] [Green Version]
- Ieguchi, K.; Tomita, T.; Takao, T.; Omori, T.; Mishima, T.; Shimizu, I.; Tognolini, M.; Lodola, A.; Tsunoda, T.; Kobayashi, S.; et al. Analysis of adam12-mediated ephrin-a1 cleavage and its biological functions. Int. J. Mol. Sci. 2021, 22. [Google Scholar]
- Wilhelmsen, K.; van der Geer, P. Phorbol 12-myristate 13-acetate-induced release of the colony-stimulating factor 1 receptor cytoplasmic domain into the cytosol involves two separate cleavage events. Mol. Cell. Biol. 2004, 24, 454–464. [Google Scholar] [CrossRef] [Green Version]
- Cai, J.; Chen, Z.; Ruan, Q.; Han, S.; Liu, L.; Qi, X.; Boye, S.L.; Hauswirth, W.W.; Grant, M.B.; Boulton, M.E. Gamma-secretase and presenilin mediate cleavage and phosphorylation of vascular endothelial growth factor receptor-1. J. Biol. Chem. 2011, 286, 42514–42523. [Google Scholar] [CrossRef] [Green Version]
- Vahidi, A.; Glenn, G.; van der Geer, P. Identification and mutagenesis of the tace and gamma-secretase cleavage sites in the colony-stimulating factor 1 receptor. Biochem. Biophys. Res. Commun. 2014, 450, 782–787. [Google Scholar] [CrossRef]
- Xu, J.; Litterst, C.; Georgakopoulos, A.; Zaganas, I.; Robakis, N.K. Peptide ephb2/ctf2 generated by the gamma-secretase processing of ephb2 receptor promotes tyrosine phosphorylation and cell surface localization of n-methyl-d-aspartate receptors. J. Biol. Chem. 2009, 284, 27220–27228. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Wan, J.; Yang, Z.; Lei, X.; Niu, Q.; Jiang, L.; Passtoors, W.M.; Zang, A.; Fraering, P.C.; Wu, F. Regulated intramembrane proteolysis of the axl receptor kinase generates an intracellular domain that localizes in the nucleus of cancer cells. Faseb J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2017, 31, 1382–1397. [Google Scholar] [CrossRef] [Green Version]
- Merilahti, J.A.M.; Ojala, V.K.; Knittle, A.M.; Pulliainen, A.T.; Elenius, K. Genome-wide screen of gamma-secretase-mediated intramembrane cleavage of receptor tyrosine kinases. Mol. Biol. Cell 2017, 28, 3123–3131. [Google Scholar] [CrossRef]
- Thorp, E.; Vaisar, T.; Subramanian, M.; Mautner, L.; Blobel, C.; Tabas, I. Shedding of the mer tyrosine kinase receptor is mediated by adam17 protein through a pathway involving reactive oxygen species, protein kinase cdelta, and p38 mitogen-activated protein kinase (mapk). J. Biol. Chem. 2011, 286, 33335–33344. [Google Scholar] [CrossRef] [Green Version]
- Duplaquet, L.; Leroy, C.; Vinchent, A.; Paget, S.; Lefebvre, J.; Vanden Abeele, F.; Lancel, S.; Giffard, F.; Paumelle, R.; Bidaux, G.; et al. Control of cell death/survival balance by the met dependence receptor. eLife 2020, 9. [Google Scholar] [CrossRef]
- Ichim, G.; Genevois, A.L.; Menard, M.; Yu, L.Y.; Coelho-Aguiar, J.M.; Llambi, F.; Jarrosson-Wuilleme, L.; Lefebvre, J.; Tulasne, D.; Dupin, E.; et al. The dependence receptor trkc triggers mitochondria-dependent apoptosis upon cobra-1 recruitment. Mol. Cell 2013, 51, 632–646. [Google Scholar] [CrossRef] [Green Version]
- Cabrera, N.; Diaz-Rodriguez, E.; Becker, E.; Martin-Zanca, D.; Pandiella, A. Trka receptor ectodomain cleavage generates a tyrosine-phosphorylated cell-associated fragment. J. Cell Biol. 1996, 132, 427–436. [Google Scholar] [CrossRef] [Green Version]
- Diaz-Rodriguez, E.; Cabrera, N.; Esparis-Ogando, A.; Montero, J.C.; Pandiella, A. Cleavage of the trka neurotrophin receptor by multiple metalloproteases generates signalling-competent truncated forms. Eur. J. Neurosci. 1999, 11, 1421–1430. [Google Scholar] [CrossRef] [Green Version]
- Vidaurre, O.G.; Gascon, S.; Deogracias, R.; Sobrado, M.; Cuadrado, E.; Montaner, J.; Rodriguez-Pena, A.; Diaz-Guerra, M. Imbalance of neurotrophin receptor isoforms trkb-fl/trkb-t1 induces neuronal death in excitotoxicity. Cell Death Dis. 2012, 3, e256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeronimo-Santos, A.; Vaz, S.H.; Parreira, S.; Rapaz-Lerias, S.; Caetano, A.P.; Buee-Scherrer, V.; Castren, E.; Valente, C.A.; Blum, D.; Sebastiao, A.M.; et al. Dysregulation of trkb receptors and bdnf function by amyloid-beta peptide is mediated by calpain. Cereb. Cortex 2015, 25, 3107–3121. [Google Scholar] [CrossRef] [Green Version]
- Danelon, V.; Montroull, L.E.; Unsain, N.; Barker, P.A.; Masco, D.H. Calpain-dependent truncated form of trkb-fl increases in neurodegenerative processes. Mol. Cell. Neurosci. 2016, 75, 81–92. [Google Scholar] [CrossRef]
- Tejeda, G.S.; Ayuso-Dolado, S.; Arbeteta, R.; Esteban-Ortega, G.M.; Vidaurre, O.G.; Diaz-Guerra, M. Brain ischaemia induces shedding of a bdnf-scavenger ectodomain from trkb receptors by excitotoxicity activation of metalloproteinases and gamma-secretases. J. Pathol. 2016, 238, 627–640. [Google Scholar] [CrossRef] [Green Version]
- Xia, Y.; Wang, Z.H.; Liu, P.; Edgington-Mitchell, L.; Liu, X.; Wang, X.C.; Ye, K. Trkb receptor cleavage by delta-secretase abolishes its phosphorylation of app, aggravating alzheimer’s disease pathologies. Mol. Psychiatry 2020. [Google Scholar] [CrossRef] [PubMed]
- Nievergall, E.; Lackmann, M.; Janes, P.W. Eph-dependent cell-cell adhesion and segregation in development and cancer. Cell. Mol. Life Sci. Cmls 2012, 69, 1813–1842. [Google Scholar] [CrossRef]
- Tandon, M.; Vemula, S.V.; Mittal, S.K. Emerging strategies for epha2 receptor targeting for cancer therapeutics. Expert Opin. Ther. Targets 2011, 15, 31–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker-Daniels, J.; Coffman, K.; Azimi, M.; Rhim, J.S.; Bostwick, D.G.; Snyder, P.; Kerns, B.J.; Waters, D.J.; Kinch, M.S. Overexpression of the epha2 tyrosine kinase in prostate cancer. Prostate 1999, 41, 275–280. [Google Scholar] [CrossRef]
- Zelinski, D.P.; Zantek, N.D.; Stewart, J.C.; Irizarry, A.R.; Kinch, M.S. Epha2 overexpression causes tumorigenesis of mammary epithelial cells. Cancer Res. 2001, 61, 2301–2306. [Google Scholar]
- Martini, G.; Cardone, C.; Vitiello, P.P.; Belli, V.; Napolitano, S.; Troiani, T.; Ciardiello, D.; Della Corte, C.M.; Morgillo, F.; Matrone, N.; et al. Epha2 is a predictive biomarker of resistance and a potential therapeutic target for improving antiepidermal growth factor receptor therapy in colorectal cancer. Mol. Cancer Ther. 2019, 18, 845–855. [Google Scholar] [CrossRef] [Green Version]
- Kikuchi, K.; Kozuka-Hata, H.; Oyama, M.; Seiki, M.; Koshikawa, N. Identification of proteolytic cleavage sites of epha2 by membrane type 1 matrix metalloproteinase on the surface of cancer cells. Methods Mol. Biol. 2018, 1731, 29–37. [Google Scholar]
- Eriksson, O.; Ramstrom, M.; Hornaeus, K.; Bergquist, J.; Mokhtari, D.; Siegbahn, A. The eph tyrosine kinase receptors ephb2 and epha2 are novel proteolytic substrates of tissue factor/coagulation factor viia. J. Biol. Chem. 2014, 289, 32379–32391. [Google Scholar] [CrossRef] [Green Version]
- Eriksson, O.; Thulin, A.; Asplund, A.; Hegde, G.; Navani, S.; Siegbahn, A. Cross-talk between the tissue factor/coagulation factor viia complex and the tyrosine kinase receptor epha2 in cancer. BMC Cancer 2016, 16, 341. [Google Scholar] [CrossRef] [Green Version]
- Inoue, E.; Deguchi-Tawarada, M.; Togawa, A.; Matsui, C.; Arita, K.; Katahira-Tayama, S.; Sato, T.; Yamauchi, E.; Oda, Y.; Takai, Y. Synaptic activity prompts gamma-secretase-mediated cleavage of epha4 and dendritic spine formation. J. Cell Biol. 2009, 185, 551–564. [Google Scholar] [CrossRef] [Green Version]
- Gatto, G.; Morales, D.; Kania, A.; Klein, R. Epha4 receptor shedding regulates spinal motor axon guidance. Curr. Biol. CB 2014, 24, 2355–2365. [Google Scholar] [CrossRef] [Green Version]
- Defourny, J.; Peuckert, C.; Kullander, K.; Malgrange, B. Epha4-adam10 interplay patterns the cochlear sensory epithelium through local disruption of adherens junctions. iScience 2019, 11, 246–257. [Google Scholar] [CrossRef] [Green Version]
- Furne, C.; Ricard, J.; Cabrera, J.R.; Pays, L.; Bethea, J.R.; Mehlen, P.; Liebl, D.J. Ephrinb3 is an anti-apoptotic ligand that inhibits the dependence receptor functions of epha4 receptors during adult neurogenesis. Biochim. Biophys. Acta 2009, 1793, 231–238. [Google Scholar] [CrossRef] [Green Version]
- Vogel, W.; Gish, G.D.; Alves, F.; Pawson, T. The discoidin domain receptor tyrosine kinases are activated by collagen. Mol. Cell 1997, 1, 13–23. [Google Scholar] [CrossRef]
- Juskaite, V.; Corcoran, D.S.; Leitinger, B. Collagen induces activation of ddr1 through lateral dimer association and phosphorylation between dimers. eLife 2017, 6, e25716. [Google Scholar] [CrossRef]
- Vogel, W.F. Ligand-induced shedding of discoidin domain receptor 1. FEBS Lett. 2002, 514, 175–180. [Google Scholar] [CrossRef] [Green Version]
- Fu, H.L.; Sohail, A.; Valiathan, R.R.; Wasinski, B.D.; Kumarasiri, M.; Mahasenan, K.V.; Bernardo, M.M.; Tokmina-Roszyk, D.; Fields, G.B.; Mobashery, S.; et al. Shedding of discoidin domain receptor 1 by membrane-type matrix metalloproteinases. J. Biol. Chem. 2013, 288, 12114–12129. [Google Scholar] [CrossRef] [Green Version]
- Shitomi, Y.; Thogersen, I.B.; Ito, N.; Leitinger, B.; Enghild, J.J.; Itoh, Y. Adam10 controls collagen signaling and cell migration on collagen by shedding the ectodomain of discoidin domain receptor 1 (ddr1). Mol. Biol. Cell 2015, 26, 659–673. [Google Scholar] [CrossRef]
- Levi, E.; Fridman, R.; Miao, H.Q.; Ma, Y.S.; Yayon, A.; Vlodavsky, I. Matrix metalloproteinase 2 releases active soluble ectodomain of fibroblast growth factor receptor 1. Proc. Natl. Acad. Sci. USA 1996, 93, 7069–7074. [Google Scholar] [CrossRef] [Green Version]
- Loeb, C.R.; Harris, J.L.; Craik, C.S. Granzyme b proteolyzes receptors important to proliferation and survival, tipping the balance toward apoptosis. J. Biol. Chem. 2006, 281, 28326–28335. [Google Scholar] [CrossRef] [Green Version]
- Degnin, C.R.; Laederich, M.B.; Horton, W.A. Ligand activation leads to regulated intramembrane proteolysis of fibroblast growth factor receptor 3. Mol. Biol. Cell 2011, 22, 3861–3873. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, J.R.; Bouzas-Rodriguez, J.; Tauszig-Delamasure, S.; Mehlen, P. Ret modulates cell adhesion via its cleavage by caspase in sympathetic neurons. J. Biol. Chem. 2011, 286, 14628–14638. [Google Scholar] [CrossRef] [Green Version]
- Donnelly, C.R.; Gabreski, N.A.; Suh, E.B.; Chowdhury, M.; Pierchala, B.A. Non-canonical ret signaling augments p75-mediated cell death in developing sympathetic neurons. J. Cell Biol. 2018, 217, 3237–3253. [Google Scholar] [CrossRef]
- Rio, C.; Buxbaum, J.D.; Peschon, J.J.; Corfas, G. Tumor necrosis factor-alpha-converting enzyme is required for cleavage of erbb4/her4. J. Biol. Chem. 2000, 275, 10379–10387. [Google Scholar] [CrossRef] [Green Version]
- Merilahti, J.A.M.; Elenius, K. Gamma-secretase-dependent signaling of receptor tyrosine kinases. Oncogene 2018, 38, 151–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Q.C.; Tikhomirov, O.; Zhou, W.; Carpenter, G. Ectodomain cleavage of erbb-4: Characterization of the cleavage site and m80 fragment. J. Biol. Chem. 2003, 278, 38421–38427. [Google Scholar] [CrossRef] [Green Version]
- Roskoski, R., Jr. The erbb/her family of protein-tyrosine kinases and cancer. Pharmacol. Res. 2014, 79, 34–74. [Google Scholar] [CrossRef] [PubMed]
- Hollmen, M.; Liu, P.; Kurppa, K.; Wildiers, H.; Reinvall, I.; Vandorpe, T.; Smeets, A.; Deraedt, K.; Vahlberg, T.; Joensuu, H.; et al. Proteolytic processing of erbb4 in breast cancer. PLoS ONE 2012, 7, e39413. [Google Scholar] [CrossRef] [PubMed]
- Muraoka-Cook, R.S.; Sandahl, M.; Husted, C.; Hunter, D.; Miraglia, L.; Feng, S.M.; Elenius, K.; Earp, H.S., 3rd. The intracellular domain of erbb4 induces differentiation of mammary epithelial cells. Mol. Biol. Cell 2006, 17, 4118–4129. [Google Scholar] [CrossRef] [Green Version]
- Yuan, C.X.; Lasut, A.L.; Wynn, R.; Neff, N.T.; Hollis, G.F.; Ramaker, M.L.; Rupar, M.J.; Liu, P.; Meade, R. Purification of her-2 extracellular domain and identification of its cleavage site. Protein Expr. Purif. 2003, 29, 217–222. [Google Scholar] [CrossRef]
- Strohecker, A.M.; Yehiely, F.; Chen, F.; Cryns, V.L. Caspase cleavage of her-2 releases a bad-like cell death effector. J. Biol. Chem. 2008, 283, 18269–18282. [Google Scholar] [CrossRef] [Green Version]
- Van Veelen, W.; Le, N.H.; Helvensteijn, W.; Blonden, L.; Theeuwes, M.; Bakker, E.R.; Franken, P.F.; van Gurp, L.; Meijlink, F.; van der Valk, M.A.; et al. Beta-catenin tyrosine 654 phosphorylation increases wnt signalling and intestinal tumorigenesis. Gut 2011, 60, 1204–1212. [Google Scholar] [CrossRef]
- Moog-Lutz, C.; Degoutin, J.; Gouzi, J.Y.; Frobert, Y.; Brunet-de Carvalho, N.; Bureau, J.; Creminon, C.; Vigny, M. Activation and inhibition of anaplastic lymphoma kinase receptor tyrosine kinase by monoclonal antibodies and absence of agonist activity of pleiotrophin. J. Biol. Chem. 2005, 280, 26039–26048. [Google Scholar] [CrossRef] [Green Version]
- Degoutin, J.; Brunet-de Carvalho, N.; Cifuentes-Diaz, C.; Vigny, M. Alk (anaplastic lymphoma kinase) expression in drg neurons and its involvement in neuron-schwann cells interaction. Eur. J. Neurosci. 2009, 29, 275–286. [Google Scholar] [CrossRef]
- Hovens, C.M.; Stacker, S.A.; Andres, A.C.; Harpur, A.G.; Ziemiecki, A.; Wilks, A.F. Ryk, a receptor tyrosine kinase-related molecule with unusual kinase domain motifs. Proc. Natl. Acad. Sci. USA 1992, 89, 11818–11822. [Google Scholar] [CrossRef] [Green Version]
- Trivier, E.; Ganesan, T.S. Ryk, a catalytically inactive receptor tyrosine kinase, associates with ephb2 and ephb3 but does not interact with af-6. J. Biol. Chem. 2002, 277, 23037–23043. [Google Scholar] [CrossRef] [Green Version]
- Halford, M.M.; Macheda, M.L.; Parish, C.L.; Takano, E.A.; Fox, S.; Layton, D.; Nice, E.; Stacker, S.A. A fully human inhibitory monoclonal antibody to the wnt receptor ryk. PLoS ONE 2013, 8, e75447. [Google Scholar] [CrossRef] [Green Version]
- Lyu, J.; Yamamoto, V.; Lu, W. Cleavage of the wnt receptor ryk regulates neuronal differentiation during cortical neurogenesis. Dev. Cell 2008, 15, 773–780. [Google Scholar] [CrossRef] [Green Version]
- Tourette, C.; Farina, F.; Vazquez-Manrique, R.P.; Orfila, A.M.; Voisin, J.; Hernandez, S.; Offner, N.; Parker, J.A.; Menet, S.; Kim, J.; et al. The wnt receptor ryk reduces neuronal and cell survival capacity by repressing foxo activity during the early phases of mutant huntingtin pathogenicity. PLoS Biol. 2014, 12, e1001895. [Google Scholar] [CrossRef] [Green Version]
- Lyu, J.; Wesselschmidt, R.L.; Lu, W. Cdc37 regulates ryk signaling by stabilizing the cleaved ryk intracellular domain. J. Biol. Chem. 2009, 284, 12940–12948. [Google Scholar] [CrossRef] [Green Version]
- Chang, W.H.; Choi, S.H.; Moon, B.S.; Cai, M.; Lyu, J.; Bai, J.; Gao, F.; Hajjali, I.; Zhao, Z.; Campbell, D.B.; et al. Smek1/2 is a nuclear chaperone and cofactor for cleaved wnt receptor ryk, regulating cortical neurogenesis. Proc. Natl. Acad. Sci. USA 2017, 114, E10717–E10725. [Google Scholar] [CrossRef] [Green Version]
- Marron, M.B.; Singh, H.; Tahir, T.A.; Kavumkal, J.; Kim, H.Z.; Koh, G.Y.; Brindle, N.P. Regulated proteolytic processing of tie1 modulates ligand responsiveness of the receptor-tyrosine kinase tie2. J. Biol. Chem. 2007, 282, 30509–30517. [Google Scholar] [CrossRef] [Green Version]
- Singh, H.; Tahir, T.A.; Alawo, D.O.; Issa, E.; Brindle, N.P. Molecular control of angiopoietin signalling. Biochem. Soc. Trans. 2011, 39, 1592–1596. [Google Scholar] [CrossRef]
- Mueller, S.B.; Kontos, C.D. Tie1: An orphan receptor provides context for angiopoietin-2/tie2 signaling. J. Clin. Investig. 2016, 126, 3188–3191. [Google Scholar] [CrossRef] [Green Version]
- Cai, J.; Jiang, W.G.; Grant, M.B.; Boulton, M. Pigment epithelium-derived factor inhibits angiogenesis via regulated intracellular proteolysis of vascular endothelial growth factor receptor 1. J. Biol. Chem. 2006, 281, 3604–3613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, J.; Qi, X.; Kociok, N.; Skosyrski, S.; Emilio, A.; Ruan, Q.; Han, S.; Liu, L.; Chen, Z.; Bowes Rickman, C.; et al. Beta-secretase (bace1) inhibition causes retinal pathology by vascular dysregulation and accumulation of age pigment. EMBO Mol. Med. 2012, 4, 980–991. [Google Scholar] [CrossRef] [PubMed]
- Raikwar, N.S.; Liu, K.Z.; Thomas, C.P. N-terminal cleavage and release of the ectodomain of flt1 is mediated via adam10 and adam 17 and regulated by vegfr2 and the flt1 intracellular domain. PLoS ONE 2014, 9, e112794. [Google Scholar] [CrossRef] [PubMed]
- Swendeman, S.; Mendelson, K.; Weskamp, G.; Horiuchi, K.; Deutsch, U.; Scherle, P.; Hooper, A.; Rafii, S.; Blobel, C.P. Vegf-a stimulates adam17-dependent shedding of vegfr2 and crosstalk between vegfr2 and erk signaling. Circ. Res. 2008, 103, 916–918. [Google Scholar] [CrossRef] [PubMed]
- Han, K.Y.; Chang, J.H.; Lee, H.; Azar, D.T. Proangiogenic interactions of vascular endothelial mmp14 with vegf receptor 1 in vegfa-mediated corneal angiogenesis. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3313–3322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, K.Y.; Chang, J.H.; Azar, D.T. Mmp14-containing exosomes cleave vegfr1 and promote vegfa-induced migration and proliferation of vascular endothelial cells. Investig. Ophthalmol. Vis. Sci. 2019, 60, 2321–2329. [Google Scholar] [CrossRef]
- Han, K.Y.; Dugas-Ford, J.; Lee, H.; Chang, J.H.; Azar, D.T. Mmp14 cleavage of vegfr1 in the cornea leads to a vegf-trap antiangiogenic effect. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5450–5456. [Google Scholar] [CrossRef] [Green Version]
- Golubkov, V.S.; Aleshin, A.E.; Strongin, A.Y. Potential relation of aberrant proteolysis of human protein tyrosine kinase 7 (ptk7) chuzhoi by membrane type 1 matrix metalloproteinase (mt1-mmp) to congenital defects. J. Biol. Chem. 2011, 286, 20970–20976. [Google Scholar] [CrossRef] [Green Version]
- Golubkov, V.S.; Strongin, A.Y. Insights into ectodomain shedding and processing of protein-tyrosine pseudokinase 7 (ptk7). J. Biol. Chem. 2012, 287, 42009–42018. [Google Scholar] [CrossRef] [Green Version]
- Yuasa, T.; Amo-Shiinoki, K.; Ishikura, S.; Takahara, M.; Matsuoka, T.; Kaneto, H.; Kuroda, A.; Matsuhisa, M.; Hashida, S. Sequential cleavage of insulin receptor by calpain 2 and gamma-secretase impairs insulin signalling. Diabetologia 2016, 59, 2711–2721. [Google Scholar] [CrossRef]
- Meakin, P.J.; Mezzapesa, A.; Benabou, E.; Haas, M.E.; Bonardo, B.; Grino, M.; Brunel, J.M.; Desbois-Mouthon, C.; Biddinger, S.B.; Govers, R.; et al. The beta secretase bace1 regulates the expression of insulin receptor in the liver. Nat. Commun. 2018, 9, 1306. [Google Scholar] [CrossRef] [Green Version]
- Kasuga, K.; Kaneko, H.; Nishizawa, M.; Onodera, O.; Ikeuchi, T. Generation of intracellular domain of insulin receptor tyrosine kinase by gamma-secretase. Biochem. Biophys. Res. Commun. 2007, 360, 90–96. [Google Scholar] [CrossRef]
- Soluble Insulin Receptor Study Group. Soluble insulin receptor ectodomain is elevated in the plasma of patients with diabetes. Diabetes 2007, 56, 2028–2035. [Google Scholar] [CrossRef] [Green Version]
- El Mesallamy, H.O.; Hamdy, N.M.; Mostafa, D.M.; Amin, A.I. The serine protease granzyme b as an inflammatory marker, in relation to the insulin receptor cleavage in human obesity and type 2 diabetes mellitus. J. Interferon Cytokine Res. Off. J. Int. Soc. Interferon Cytokine Res. 2014, 34, 179–186. [Google Scholar] [CrossRef]
- Cruz, A.C.; Frank, B.T.; Edwards, S.T.; Dazin, P.F.; Peschon, J.J.; Fang, K.C. Tumor necrosis factor-alpha-converting enzyme controls surface expression of c-kit and survival of embryonic stem cell-derived mast cells. J. Biol. Chem. 2004, 279, 5612–5620. [Google Scholar] [CrossRef] [Green Version]
- Mendelson, K.; Swendeman, S.; Saftig, P.; Blobel, C.P. Stimulation of platelet-derived growth factor receptor beta (pdgfrbeta) activates adam17 and promotes metalloproteinase-dependent cross-talk between the pdgfrbeta and epidermal growth factor receptor (egfr) signaling pathways. J. Biol. Chem. 2010, 285, 25024–25032. [Google Scholar] [CrossRef] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, H. Proteolytic Cleavage of Receptor Tyrosine Kinases. Biomolecules 2021, 11, 660. https://doi.org/10.3390/biom11050660
Huang H. Proteolytic Cleavage of Receptor Tyrosine Kinases. Biomolecules. 2021; 11(5):660. https://doi.org/10.3390/biom11050660
Chicago/Turabian StyleHuang, Hao. 2021. "Proteolytic Cleavage of Receptor Tyrosine Kinases" Biomolecules 11, no. 5: 660. https://doi.org/10.3390/biom11050660
APA StyleHuang, H. (2021). Proteolytic Cleavage of Receptor Tyrosine Kinases. Biomolecules, 11(5), 660. https://doi.org/10.3390/biom11050660





