Resveratrol Production in Yeast Hosts: Current Status and Perspectives
Abstract
:1. Introduction
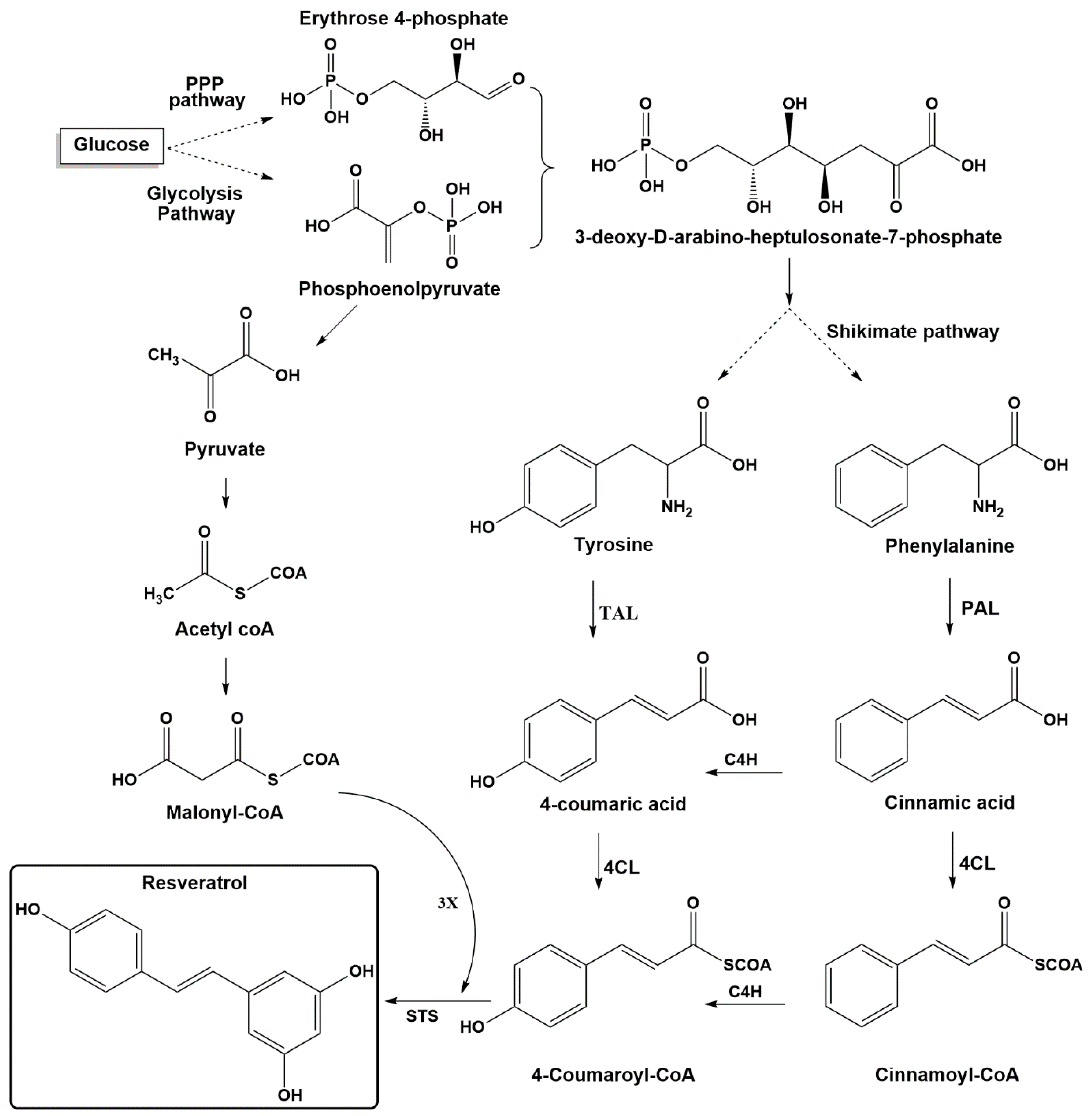
2. Resveratrol Biosynthesis in Nature
3. Resveratrol Production by Transgenic Yeasts
3.1. Pathway Engineering
3.2. Host Metabolic Engineering (Non-Pathway Genes)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 4CL | 4-coumaroyl-coA ligase |
| AAE13 | malonyl-CoA synthetase |
| Acetyl-CoA | acetyl-coenzyme A |
| Acetyl-ACP | acetyl-acyl carrier protein |
| ACC | acetyl-CoA carboxylase |
| ACS | acetyl-CoA synthase |
| ACK | acetate kinase |
| ADH | alcohol dehydrogenases |
| araE | arabinose transporter |
| ARO1 | multifunctional AROM complex |
| ARO2 | chorismate synthase |
| ARO3/ARO4/ARO5 | 3-deoxy- d-arabinoheptulosonate-7-phosphate (DAHP) synthase |
| ARO7 | chorismate mutase |
| ARO8 | aromatic amino acid aminotransferase I |
| ARO9 | aromatic amino acid aminotransferase II |
| ARO10 | transaminated amino acid decarboxylase |
| aroA | gene that encodes the 3-phospho-shikimate-1-carboxyvinyltransferase protein |
| aroB | gene that encodes the dehydroquinate synthase protein |
| aroC | gene that encodes the chorismate synthase protein |
| aroD | gene that encodes the dehydroquinate dehydratase protein |
| aroE | gene that encodes the shikimate dehydrogenase protein |
| aroG/aroF/aroH | genes that encode the DAHP synthase |
| aroK/aroL | genes that encode the shikimate kinase isoenzymes I/II |
| ATR2 | NADPH-cytochrome P450 reductase 2 |
| C4H | cinnamate 4-hydroxylase |
| CPR | cytochrome P450 reductase |
| CYB5 | cytochrome b5 |
| DAHP | 3-deoxy-d arabinoheptulosonate 7-phosphate |
| DAHPS | 3-deoxy-d arabinoheptulosonate 7-phosphate (DAHP) synthase |
| E4P | erythrose 4-phosphate |
| fabB/fabF | genes that encode the beta-ketoacyl-acp synthase I/II protein |
| fabD | gene that encodes the malonyl-CoA-acyl carrier protein transacylase |
| fabH | gene that encodes 3-oxoacyl carrier protein synthase III |
| fbr | feedback resistant |
| GRAS | generally recognized as safe |
| Malonyl-CoA | malonyl-coenzyme A |
| Malonyl-ACP | malonyl-acyl carrier protein |
| MatB | malonyl-CoA synthetase |
| MatC | malonate carrier protein |
| PAD | phenyl acrylic acid decarboxylase |
| PAL | phenylalanine ammonia lyase |
| PEP | phosphoenolpyruvate |
| PEPS | phosphoenolpyruvate synthase |
| PEX10 | peroxisomal biogenesis factor 10 |
| PHA2 | prephenate dehydratase |
| PPP | pentose phosphate pathway |
| PTA | phosphate acetyltransferase |
| PYK | pyruvate kinase |
| STS | stilbene synthase |
| TAL | tyrosine ammonia-lyase |
| TKT | transketolase |
| TRP2 | anthranilate synthase |
| TRP3 | indole-3-glycerol-phosphate synthase |
| tyrA/pheA | genes that encode the chorismate mutase protein |
| TyrA | chorismate mutase/prephenate dehydrogenase |
| tyrB | gene that encodes the tyrosine aminotransferase |
| TyrR | transcriptional regulatory protein |
| VST/RS | resveratrol synthase |
| xfpK/xpkA | phosphoketolase. |
References
- Jeandet, P.; Sobarzo-Sánchez, E.; Silva, A.S.; Clément, C.; Nabavi, S.F.; Battino, M.; Rasekhian, M.; Belwal, T.; Habtemariam, S.; Koffas, M. Whole-cell biocatalytic, enzymatic and green chemistry methods for the production of resveratrol and its derivatives. Biotechnol. Adv. 2020, 39, 107461. [Google Scholar] [CrossRef] [PubMed]
- Gugleva, V.; Zasheva, S.; Hristova, M.; Andonova, V. Topical use of resveratrol: Technological aspects. Pharmacia 2020, 67, 89. [Google Scholar] [CrossRef]
- Jeandet, P.; Delaunois, B.; Conreux, A.; Donnez, D.; Nuzzo, V.; Cordelier, S.; Clément, C.; Courot, E. Biosynthesis, metabolism, molecular engineering, and biological functions of stilbene phytoalexins in plants. Biofactors 2010, 36, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Rabesiaka, M.; Rakotondramasy-Rabesiaka, L.; Mabille, I.; Porte, C.; Havet, J.-L. Extraction of trans-resveratrol from red wine and optimization by response surface methodology. Sep. Purif. Technol. 2011, 81, 56–61. [Google Scholar] [CrossRef]
- Jeandet, P.; Douillet-Breuil, A.-C.; Bessis, R.; Debord, S.; Sbaghi, M.; Adrian, M. Phytoalexins from the Vitaceae: Biosynthesis, phytoalexin gene expression in transgenic plants, antifungal activity, and metabolism. J. Agric. Food Chem. 2002, 50, 2731–2741. [Google Scholar] [CrossRef]
- Takaoka, M. Of the phenolic substrate of hellebore (Veratrum grandiflorum Loes. fil.). J. Fac. Sci. Hokkaido Imper. Univ. 1940, 3, 1–16. [Google Scholar]
- Nonomura, S.; Kanagawa, H.; Makimoto, A. Chemical Constituents of Polygonaceous Plants. I. Studies on the Components of Ko-jo-kon. (Polygonum cuspidatum SIEB. et ZUCC.). Yakugaku Zasshi 1963, 83, 988–990. [Google Scholar] [CrossRef] [Green Version]
- Jeandet, P.; Bessis, R.; Gautheron, B. The production of resveratrol (3, 5, 4’-trihydroxystilbene) by grape berries in different developmental stages. Am. J. Enol. Vitic. 1991, 42, 41–46. [Google Scholar]
- Lyons, M.M.; Yu, C.; Toma, R.; Cho, S.Y.; Reiboldt, W.; Lee, J.; van Breemen, R. Resveratrol in raw and baked blueberries and bilberries. J. Agric. Food Chem. 2003, 51, 5867–5870. [Google Scholar] [CrossRef]
- Wang, Y.; Catana, F.; Yang, Y.; Roderick, R.; van Breemen, R.B. An LC-MS method for analyzing total resveratrol in grape juice, cranberry juice, and in wine. J. Agric. Food Chem. 2002, 50, 431–435. [Google Scholar] [CrossRef]
- Shrikanta, A.; Kumar, A.; Govindaswamy, V. Resveratrol content and antioxidant properties of underutilized fruits. J. Food Sci. Technol. 2015, 52, 383–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mudnic, I.; Budimir, D.; Modun, D.; Gunjaca, G.; Generalic, I.; Skroza, D.; Katalinic, V.; Ljubenkov, I.; Boban, M. Antioxidant and vasodilatory effects of blackberry and grape wines. J. Med. Food 2012, 15, 315–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sales, J.M.; Resurreccion, A.V. Resveratrol in peanuts. Crit. Rev. Food Sci. Nutr. 2014, 54, 734–770. [Google Scholar] [CrossRef] [PubMed]
- Pezzuto, J.M.; Moon, R.C.; Jang, M.-S.; Ouali, A.; Lin, S.; Barillas, K.S. Pharmaceutical Formulations of Resveratrol and Methods of Use Thereof. U.S. Patent US6414037B1, 2 July 2002. [Google Scholar]
- Ratz-Łyko, A.; Arct, J. Resveratrol as an active ingredient for cosmetic and dermatological applications: A review. J. Cosmet. Laser Ther. 2019, 21, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Pai, R.S. Recent advances of resveratrol in nanostructured based delivery systems and in the management of HIV/AIDS. J. Control. Release 2014, 194, 178–188. [Google Scholar] [CrossRef]
- Ndiaye, M.; Philippe, C.; Mukhtar, H.; Ahmad, N. The grape antioxidant resveratrol for skin disorders: Promise, prospects, and challenges. Arch. Biochem. Biophys. 2011, 508, 164–170. [Google Scholar] [CrossRef] [Green Version]
- Pangeni, R.; Sahni, J.K.; Ali, J.; Sharma, S.; Baboota, S. Resveratrol: Review on therapeutic potential and recent advances in drug delivery. Expert Opin. Drug Deliv. 2014, 11, 1285–1298. [Google Scholar] [CrossRef]
- Jeandet, P.; Delaunois, B.; Aziz, A.; Donnez, D.; Vasserot, Y.; Cordelier, S.; Courot, E. Metabolic engineering of yeast and plants for the production of the biologically active hydroxystilbene, resveratrol. J. Biomed. Biotechnol. 2012, 2012, 1–14. [Google Scholar] [CrossRef]
- Voloshyna, I.; Hussaini, S.M.; Reiss, A.B. Resveratrol in cholesterol metabolism and atherosclerosis. J. Med. Food 2012, 15, 763–773. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Butt, M.S.; Nadeem, M.; Peters, D.G.; Mubarak, M.S. Resveratrol as an anti-cancer agent: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1428–1447. [Google Scholar] [CrossRef]
- Varoni, E.M.; Lo Faro, A.F.; Sharifi-Rad, J.; Iriti, M. Anticancer molecular mechanisms of resveratrol. Front. Nutr. 2016, 3, 8. [Google Scholar] [CrossRef] [Green Version]
- Cai, H.; Scott, E.; Kholghi, A.; Andreadi, C.; Rufini, A.; Karmokar, A.; Britton, R.G.; Horner-Glister, E.; Greaves, P.; Jawad, D.; et al. Cancer chemoprevention: Evidence of a nonlinear dose response for the protective effects of resveratrol in humans and mice. Sci. Transl. Med. 2015, 7, ra117–ra298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carter, L.G.; D’Orazio, J.A.; Pearson, K.J. Resveratrol and cancer: Focus on in vivo evidence. Endocr. Relat. Cancer 2014, 21, R209–R225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Sá Coutinho, D.; Pacheco, M.T.; Frozza, R.L.; Bernardi, A. Anti-inflammatory effects of resveratrol: Mechanistic insights. Int. J. Mol. Sci. 2018, 19, 1812. [Google Scholar] [CrossRef] [Green Version]
- Zordoky, B.N.M.; Robertson, I.M.; Dyck, J.R.B. Preclinical and clinical evidence for the role of resveratrol in the treatment of cardiovascular diseases. Biochim. Biophys. Acta Mol. Basis Dis. 2015, 1852, 1155–1177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malaguarnera, L. Influence of resveratrol on the immune response. Nutrients 2019, 11, 946. [Google Scholar] [CrossRef] [Green Version]
- Prysyazhna, O.; Wolhuter, K.; Switzer, C.; Santos, C.; Yang, X.; Lynham, S.; Shah, A.M.; Eaton, P.; Burgoyne, J.R. Blood pressure lowering by the antioxidant resveratrol is counterintuitively mediated by oxidation of cGMP-dependent protein kinase. Circulation 2019, 140, 126–137. [Google Scholar] [CrossRef]
- Rao, Y.L.; Ganaraja, B.; Joy, T.; Pai, M.M.; Ullal, S.D.; Murlimanju, B.V. Neuroprotective effects of resveratrol in Alzheimer’s disease. Front. Biosci. 2020, 12, 139–149. [Google Scholar]
- Bastianetto, S.; Ménard, C.; Quirion, R. Neuroprotective action of resveratrol. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2015, 1852, 1195–1201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rege, S.D.; Geetha, T.; Griffin, G.D.; Broderick, T.L.; Babu, J.R. Neuroprotective effects of resveratrol in Alzheimer disease pathology. Front. Aging Neurosci. 2014, 6, 218. [Google Scholar] [CrossRef] [Green Version]
- Singh, N.; Agrawal, M.; Doré, S. Neuroprotective Properties and Mechanisms of Resveratrol in in Vitro and in Vivo Experimental Cerebral Stroke Models. ACS Chem. Neurosci. 2013, 4, 1151–1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adhikari, N.; Prasad Aryal, Y.; Jung, J.-K.; Ha, J.-H.; Choi, S.-Y.; Kim, J.-Y.; Lee, T.-H.; Kim, S.-H.; Yamamoto, H.; Suh, J.-Y.; et al. Resveratrol enhances bone formation by modulating inflammation in the mouse periodontitis model. J. Periodontal Res. 2021, in press. [Google Scholar] [CrossRef]
- Jeandet, P.; Clément, C.; Tisserant, L.-P.; Crouzet, J.; Courot, É. Use of grapevine cell cultures for the production of phytostilbenes of cosmetic interest. Comptes Rendus Chim. 2016, 19, 1062–1070. [Google Scholar] [CrossRef] [Green Version]
- Tsai, M.-J.; Lu, I.J.; Fu, Y.-S.; Fang, Y.-P.; Huang, Y.-B.; Wu, P.-C. Nanocarriers enhance the transdermal bioavailability of resveratrol: In-vitro and in-vivo study. Colloids Surf. B Biointerfaces 2016, 148, 650–656. [Google Scholar] [CrossRef]
- Pelliccia, M.; Giannella, A.; Giannella, J. Use of Resveratrol for the Treatment of Exfoliative Eczema, Acne and Psoriasis. U.S. Patent US20010056071A1, 27 December 2001. [Google Scholar]
- Baxter, R.A. Anti-aging properties of resveratrol: Review and report of a potent new antioxidant skin care formulation. J. Cosmet. Dermatol. 2008, 7, 2–7. [Google Scholar] [CrossRef]
- Zhou, X.; Ruan, Q.; Ye, Z.; Chu, Z.; Xi, M.; Li, M.; Hu, W.; Guo, X.; Yao, P.; Xie, W. Resveratrol accelerates wound healing by attenuating oxidative stress-induced impairment of cell proliferation and migration. Burns 2021, 47, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.-H.; Peng, C.; Zhang, H. Polydatin: A review of pharmacology and pharmacokinetics. Pharm. Biol. 2013, 51, 1347–1354. [Google Scholar] [CrossRef]
- Lv, M.; Liu, K.; Fu, S.; Li, Z.; Yu, X. Pterostilbene attenuates the inflammatory reaction induced by ischemia/reperfusion in rat heart. Mol. Med. Rep. 2015, 11, 724–728. [Google Scholar] [CrossRef] [Green Version]
- Aldawsari, F.S.; Velázquez-Martínez, C.A. 3,4′,5-trans-Trimethoxystilbene; a natural analogue of resveratrol with enhanced anticancer potency. Investig. New Drugs 2015, 33, 775–786. [Google Scholar] [CrossRef]
- McCormack, D.E.; Mannal, P.; McDonald, D.; Tighe, S.; Hanson, J.; McFadden, D. Genomic analysis of pterostilbene predicts its antiproliferative effects against pancreatic cancer in vitro and in vivo. J. Gastrointest. Surg. 2012, 16, 1136–1143. [Google Scholar] [CrossRef] [Green Version]
- Sale, S.; Verschoyle, R.D.; Boocock, D.; Jones, D.; Wilsher, N.; Ruparelia, K.C.; Potter, G.A.; Farmer, P.B.; Steward, W.P.; Gescher, A.J. Pharmacokinetics in mice and growth-inhibitory properties of the putative cancer chemopreventive agent resveratrol and the synthetic analogue trans 3, 4, 5, 4′-tetramethoxystilbene. Br. J. Cancer 2004, 90, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Chen, L.; Liu, X.; Li, X.; Cao, Y.; Bai, Y.; Qi, F. Pterostilbene inhibits amyloid-β-induced neuroinflammation in a microglia cell line by inactivating the NLRP3/caspase-1 inflammasome pathway. J. Cell. Biochem. 2018, 119, 7053–7062. [Google Scholar] [CrossRef]
- Li, Y.-R.; Li, S.; Lin, C.-C. Effect of resveratrol and pterostilbene on aging and longevity. BioFactors 2018, 44, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Nivelle, L.; Hubert, J.; Courot, E.; Jeandet, P.; Aziz, A.; Nuzillard, J.-M.; Renault, J.-H.; Clément, C.; Martiny, L.; Delmas, D.; et al. Anti-cancer activity of resveratrol and derivatives produced by grapevine cell suspensions in a 14 L stirred bioreactor. Molecules 2017, 22, 474. [Google Scholar] [CrossRef] [PubMed]
- González-Sarrías, A.; Gromek, S.; Niesen, D.; Seeram, N.P.; Henry, G.E. Resveratrol Oligomers Isolated from Carex Species Inhibit Growth of Human Colon Tumorigenic Cells Mediated by Cell Cycle Arrest. J. Agric. Food Chem. 2011, 59, 8632–8638. [Google Scholar] [CrossRef] [PubMed]
- Barjot, C.; Tournaire, M.; Castagnino, C.; Vigor, C.; Vercauteren, J.; Rossi, J.-F. Evaluation of antitumor effects of two vine stalk oligomers of resveratrol on a panel of lymphoid and myeloid cell lines: Comparison with resveratrol. Life Sci. 2007, 81, 1565–1574. [Google Scholar] [CrossRef]
- Muhtadi; Hakim, E.H.; Juliawaty, L.D.; Syah, Y.M.; Achmad, S.A.; Latip, J.; Ghisalberti, E.L. Cytotoxic resveratrol oligomers from the tree bark of Dipterocarpus hasseltii. Fitoterapia 2006, 77, 550–555. [Google Scholar] [CrossRef]
- Chowdhury, S.A.; Kishino, K.; Satoh, R.; Hashimoto, K.; Kikuchi, H.; Nishikawa, H.; Shirataki, Y.; Sakagami, H. Tumor-specificity and apoptosis-inducing activity of stilbenes and flavonoids. Anticancer. Res. 2005, 25, 2055–2063. [Google Scholar]
- Nivelle, L.; Hubert, J.; Courot, E.; Borie, N.; Renault, J.-H.; Nuzillard, J.-M.; Harakat, D.; Clément, C.; Martiny, L.; Delmas, D.; et al. Cytotoxicity of labruscol, a new resveratrol dimer produced by grapevine cell suspensions, on human skin melanoma cancer cell line HT-144. Molecules 2017, 22, 1940. [Google Scholar] [CrossRef] [Green Version]
- Rohaiza, S.; Yaacob, W.; Din, L.; Nazlina, I. Cytotoxic oligostilbenes from Shorea hopeifolia. Afr. J. Pharm. Pharmacol. 2011, 5, 1272–1277. [Google Scholar] [CrossRef] [Green Version]
- Nabavi, S.M.; Šamec, D.; Tomczyk, M.; Milella, L.; Russo, D.; Habtemariam, S.; Suntar, I.; Rastrelli, L.; Daglia, M.; Xiao, J.; et al. Flavonoid biosynthetic pathways in plants: Versatile targets for metabolic engineering. Biotechnol. Adv. 2020, 38, 107316. [Google Scholar] [CrossRef] [PubMed]
- Langcake, P.; Pryce, R.J. The production of resveratrol and the viniferins by grapevines in response to ultraviolet irradiation. Phytochemistry 1977, 16, 1193–1196. [Google Scholar] [CrossRef]
- Keylor, M.H.; Matsuura, B.S.; Stephenson, C.R.J. Chemistry and biology of resveratrol-derived natural products. Chem. Rev. 2015, 115, 8976–9027. [Google Scholar] [CrossRef]
- Shen, T.; Wang, X.-N.; Lou, H.-X. Natural stilbenes: An overview. Nat. Prod. Rep. 2009, 26, 916–935. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Zhang, H.-J.; Xuan, L.-J.; Zhang, J.; Xu, Y.-M.; Bai, D.-L. Stilbenoids: Chemistry and bioactivities. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 453–646. [Google Scholar]
- Vannozzi, A.; Dry, I.B.; Fasoli, M.; Zenoni, S.; Lucchin, M. Genome-wide analysis of the grapevine stilbene synthase multigenic family: Genomic organization and expression profiles upon biotic and abiotic stresses. BMC Plant Biol. 2012, 12, 130. [Google Scholar] [CrossRef]
- Jeandet, P.; Clément, C.; Cordelier, S. Regulation of resveratrol biosynthesis in grapevine: New approaches for disease resistance? J. Exp. Bot. 2019, 70, 375–378. [Google Scholar] [CrossRef]
- Vannozzi, A.; Wong, D.C.J.; Höll, J.; Hmmam, I.; Matus, J.T.; Bogs, J.; Ziegler, T.; Dry, I.; Barcaccia, G.; Lucchin, M. Combinatorial regulation of stilbene synthase genes by wrky and myb transcription factors in grapevine (Vitis vinifera L.). Plant Cell Physiol. 2018, 59, 1043–1059. [Google Scholar] [CrossRef]
- Höll, J.; Vannozzi, A.; Czemmel, S.; D’Onofrio, C.; Walker, A.R.; Rausch, T.; Lucchin, M.; Boss, P.K.; Dry, I.B.; Bogs, J. The R2R3-MYB transcription factors MYB14 and MYB15 regulate stilbene biosynthesis in Vitis vinifera. Plant Cell 2013, 25, 4135–4149. [Google Scholar] [CrossRef] [Green Version]
- Rimando, A.M.; Pan, Z.; Polashock, J.J.; Dayan, F.E.; Mizuno, C.S.; Snook, M.E.; Liu, C.-J.; Baerson, S.R. In planta production of the highly potent resveratrol analogue pterostilbene via stilbene synthase and O-methyltransferase co-expression. Plant Biotechnol. J. 2012, 10, 269–283. [Google Scholar] [CrossRef]
- Hall, D.; De Luca, V. Mesocarp localization of a bi-functional resveratrol/hydroxycinnamic acid glucosyltransferase of Concord grape (Vitis labrusca). Plant J. 2007, 49, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Liu, J. Resveratrol: A review of plant sources, synthesis, stability, modification and food application. J. Sci. Food Agric. 2020, 100, 1392–1404. [Google Scholar] [CrossRef]
- Delaunois, B.; Cordelier, S.; Conreux, A.; Clément, C.; Jeandet, P. Molecular engineering of resveratrol in plants. Plant Biotechnol. J. 2009, 7, 2–12. [Google Scholar] [CrossRef]
- Donnez, D.; Jeandet, P.; Clément, C.; Courot, E. Bioproduction of resveratrol and stilbene derivatives by plant cells and microorganisms. Trends Biotechnol. 2009, 27, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Fan, E.; Zhang, K.; Zhu, M.; Wang, Q. Obtaining resveratrol: From chemical synthesis to biotechnological production. Mini-Rev. Org. Chem. 2010, 7, 272–281. [Google Scholar] [CrossRef]
- Sun, X.; Shen, X.; Jain, R.; Lin, Y.; Wang, J.; Sun, J.; Yan, Y.; Yuan, Q. Synthesis of chemicals by metabolic engineering of microbes. Chem. Soc. Rev. 2015, 44, 3760–3785. [Google Scholar] [CrossRef] [PubMed]
- Braga, A.; Ferreira, P.; Oliveira, J.; Rocha, I.; Faria, N. Heterologous production of resveratrol in bacterial hosts: Current status and perspectives. World J. Microbiol. Biotechnol. 2018, 34, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madzak, C. Yarrowia lipolytica: Recent achievements in heterologous protein expression and pathway engineering. Appl. Microbiol. Biotechnol. 2015, 99, 4559–4577. [Google Scholar] [CrossRef] [PubMed]
- Rainha, J.; Gomes, D.; Rodrigues, L.R.; Rodrigues, J.L. Synthetic biology approaches to engineer Saccharomyces cerevisiae towards the industrial production of valuable polyphenolic compounds. Life 2020, 10, 56. [Google Scholar] [CrossRef] [PubMed]
- Thapa, S.B.; Pandey, R.P.; Park, Y.I.; Sohng, J.K. Biotechnological advances in resveratrol production and its chemical diversity. Molecules 2019, 24, 2571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Yang, Y.; Yan, Y. Bioproduction of Resveratrol. In Biotechnology of Natural Products; Schwab, W., Lange, B.M., Wüst, M., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 61–79. [Google Scholar]
- Groenewald, M.; Boekhout, T.; Neuvéglise, C.; Gaillardin, C.; Van Dijck, P.W.; Wyss, M. Yarrowia lipolytica: Safety assessment of an oleaginous yeast with a great industrial potential. Crit. Rev. Microbiol. 2014, 40, 187–206. [Google Scholar] [CrossRef]
- Beopoulos, A.; Chardot, T.; Nicaud, J.-M. Yarrowia lipolytica: A model and a tool to understand the mechanisms implicated in lipid accumulation. Biochimie 2009, 91, 692–696. [Google Scholar] [CrossRef] [PubMed]
- Madzak, C. Engineering Yarrowia lipolytica for use in biotechnological applications: A review of major achievements and recent innovations. Mol. Biotechnol. 2018, 60, 621–635. [Google Scholar] [CrossRef]
- Becker, J.V.; Armstrong, G.O.; Van Der Merwe, M.J.; Lambrechts, M.G.; Vivier, M.A.; Pretorius, I.S. Metabolic engineering of Saccharomyces cerevisiae for the synthesis of the wine-related antioxidant resveratrol. FEMS Yeast Res. 2003, 4, 79–85. [Google Scholar] [CrossRef] [Green Version]
- Beekwilder, J.; Wolswinkel, R.; Jonker, H.; Hall, R.; de Vos, C.H.R.; Bovy, A. Production of resveratrol in recombinant microorganisms. Appl. Environ. Microbiol. 2006, 72, 5670–5672. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Li, S.-Z.; Li, J.; Pan, X.; Cahoon, R.E.; Jaworski, J.G.; Wang, X.; Jez, J.M.; Chen, F.; Yu, O. Using unnatural protein fusions to engineer resveratrol biosynthesis in yeast and mammalian cells. J. Am. Chem. Soc. 2006, 128, 13030–13031. [Google Scholar] [CrossRef]
- Trantas, E.; Panopoulos, N.; Ververidis, F. Metabolic engineering of the complete pathway leading to heterologous biosynthesis of various flavonoids and stilbenoids in Saccharomyces cerevisiae. Metab. Eng. 2009, 11, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Sydor, T.; Schaffer, S.; Boles, E. Considerable increase in resveratrol production by recombinant industrial yeast strains with use of rich medium. Appl. Environ. Microbiol. 2010, 76, 3361–3363. [Google Scholar] [CrossRef] [Green Version]
- Shin, S.-Y.; Han, N.S.; Park, Y.-C.; Kim, M.-D.; Seo, J.-H. Production of resveratrol from p-coumaric acid in recombinant Saccharomyces cerevisiae expressing 4-coumarate: Coenzyme A ligase and stilbene synthase genes. Enzym. Microb. Technol. 2011, 48, 48–53. [Google Scholar] [CrossRef]
- Wang, Y.; Halls, C.; Zhang, J.; Matsuno, M.; Zhang, Y.; Yu, O. Stepwise increase of resveratrol biosynthesis in yeast Saccharomyces cerevisiae by metabolic engineering. Metab. Eng. 2011, 13, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.-Y.; Jung, S.-M.; Kim, M.-D.; Han, N.S.; Seo, J.-H. Production of resveratrol from tyrosine in metabolically engineered Saccharomyces cerevisiae. Enzym. Microb. Technol. 2012, 51, 211–216. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, O. Synthetic scaffolds increased resveratrol biosynthesis in engineered yeast cells. J. Biotechnol. 2012, 157, 258–260. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, H.; Yu, O. A plant malonyl-CoA synthetase enhances lipid content and polyketide yield in yeast cells. Appl. Microbiol. Biotechnol. 2014, 98, 5435–5447. [Google Scholar] [CrossRef]
- Sun, P.; Liang, J.-L.; Kang, L.-Z.; Huang, X.-Y.; Huang, J.-J.; Ye, Z.-W.; Guo, L.-Q.; Lin, J.-F. Increased resveratrol production in wines using engineered wine strains Saccharomyces cerevisiae EC1118 and relaxed antibiotic or auxotrophic selection. Biotechnol. Prog. 2015, 31, 650–655. [Google Scholar] [CrossRef]
- Li, M.; Kildegaard, K.R.; Chen, Y.; Rodriguez, A.; Borodina, I.; Nielsen, J. De novo production of resveratrol from glucose or ethanol by engineered Saccharomyces cerevisiae. Metab. Eng. 2015, 32, 1–11. [Google Scholar] [CrossRef]
- Li, M.; Schneider, K.; Kristensen, M.; Borodina, I.; Nielsen, J. Engineering yeast for high-level production of stilbenoid antioxidants. Sci. Rep. 2016, 6, 36827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villa-Ruano, N.; Rivera, A.; Rubio-Rosas, E.; Landeta-Cortés, G.; Varela-Caselis, J.L.; Romero-Arenas, O. Comparative activity of six recombinant stilbene synthases in yeast for resveratrol production. Appl. Sci. 2020, 10, 4847. [Google Scholar] [CrossRef]
- Yuan, S.-F.; Yi, X.; Johnston, T.G.; Alper, H.S. De novo resveratrol production through modular engineering of an Escherichia coli–Saccharomyces cerevisiae co-culture. Microb. Cell Factories 2020, 19, 143. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.L.; Xue, Z.; Zhu, Q.Q. Method for the Production of Resveratrol in a Recombinant Oleaginous Microorganism. U.S. Patent USOO7772444B2, 10 August 2010. [Google Scholar]
- Palmer, C.M.; Miller, K.K.; Nguyen, A.; Alper, H.S. Engineering 4-coumaroyl-CoA derived polyketide production in Yarrowia lipolytica through a β-oxidation mediated strategy. Metab. Eng. 2020, 57, 174–181. [Google Scholar] [CrossRef]
- He, Q.; Szczepańska, P.; Yuzbashev, T.; Lazar, Z.; Ledesma-Amaro, R. De novo production of resveratrol from glycerol by engineering different metabolic pathways in Yarrowia lipolytica. Metab. Eng. Commun. 2020, 11, e00146. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Ma, J.; Zhu, Y.; Ding, X.; Xu, P. Engineering Yarrowia lipolytica as a chassis for de novo synthesis of five aromatic-derived natural products and chemicals. ACS Synth. Biol. 2020, 9, 2096–2106. [Google Scholar] [CrossRef] [PubMed]
- Sáez-Sáez, J.; Wang, G.; Marella, E.R.; Sudarsan, S.; Cernuda Pastor, M.; Borodina, I. Engineering the oleaginous yeast Yarrowia lipolytica for high-level resveratrol production. Metab. Eng. 2020, 62, 51–61. [Google Scholar] [CrossRef]
- Wang, L.; Deng, A.; Zhang, Y.; Liu, S.; Liang, Y.; Bai, H.; Cui, D.; Qiu, Q.; Shang, X.; Yang, Z.; et al. Efficient CRISPR–Cas9 mediated multiplex genome editing in yeasts. Biotechnol. Biofuels 2018, 11, 277. [Google Scholar] [CrossRef]
- Chen, R.; Yang, S.; Zhang, L.; Zhou, Y.J. Advanced strategies for production of natural products in yeast. iScience 2020, 23, 100879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, H.; Wood, K.V.; Morgan, J.A. Metabolic engineering of the phenylpropanoid pathway in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2005, 71, 2962–2969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez, A.; Kildegaard, K.R.; Li, M.; Borodina, I.; Nielsen, J. Establishment of a yeast platform strain for production of p-coumaric acid through metabolic engineering of aromatic amino acid biosynthesis. Metab. Eng. 2015, 31, 181–188. [Google Scholar] [CrossRef] [Green Version]
- Shrestha, A.; Pandey, R.P.; Sohng, J.K. Biosynthesis of resveratrol and piceatannol in engineered microbial strains: Achievements and perspectives. Appl. Microbiol. Biotechnol. 2019, 103, 2959–2972. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lin, Y.; Li, L.; Linhardt, R.J.; Yan, Y. Regulating malonyl-CoA metabolism via synthetic antisense RNAs for enhanced biosynthesis of natural products. Metab. Eng. 2015, 29, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Shao, D.; Shi, J.; Huang, Q.; Yang, H.; Jin, M. Strategies for enhancing resveratrol production and the expression of pathway enzymes. Appl. Microbiol. Biotechnol. 2016, 100, 7407–7421. [Google Scholar] [CrossRef]
- Lim, C.G.; Fowler, Z.L.; Hueller, T.; Schaffer, S.; Koffas, M.A. High-yield resveratrol production in engineered Escherichia coli. Appl. Environ. Microbiol. 2011, 77, 3451–3460. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Yu, O.; Du, G.; Zhou, J.; Chen, J. Fine-tuning of the fatty acid pathway by synthetic antisense RNA for enhanced (2S)-naringenin production from L-tyrosine in Escherichia coli. Appl. Environ. Microbiol. 2014, 80, 7283–7292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, J.-l.; Guo, L.-q.; Lin, J.-f.; He, Z.-q.; Cai, F.-j.; Chen, J.-f. A novel process for obtaining pinosylvin using combinatorial bioengineering in Escherichia coli. World J. Microbiol. Biotechnol. 2016, 32, 102. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Du, G.; Chen, J.; Zhou, J. Enhancing flavonoid production by systematically tuning the central metabolic pathways based on a CRISPR interference system in Escherichia coli. Sci. Rep. 2015, 5, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Na, D.; Yoo, S.M.; Chung, H.; Park, H.; Park, J.H.; Lee, S.Y. Metabolic engineering of Escherichia coli using synthetic small regulatory RNAs. Nat. Biotechnol. 2013, 31, 170–174. [Google Scholar] [CrossRef]
- Zha, W.; Rubin-Pitel, S.B.; Shao, Z.; Zhao, H. Improving cellular malonyl-CoA level in Escherichia coli via metabolic engineering. Metab. Eng. 2009, 11, 192–198. [Google Scholar] [CrossRef]
- Lütke-Eversloh, T.; Stephanopoulos, G. L-tyrosine production by deregulated strains of Escherichia coli. Appl. Microbiol. Biotechnol. 2007, 75, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Bulter, T.; Bernstein, J.R.; Liao, J.C. A perspective of metabolic engineering strategies: Moving up the systems hierarchy. Biotechnol. Bioeng. 2003, 84, 815–821. [Google Scholar] [CrossRef] [PubMed]


| Yeast/Parent Strain | Pathway Genes (Source) | Pathway/Host Engineering | Genetic System | Precursor | Titer (mg/L) | Scale | Year of Publication | Reference |
|---|---|---|---|---|---|---|---|---|
| S. cerevisiae FY23 | 4CL216 (P. trichocarpa × P. deltoides) VTS1 (V. vinifera) | - | Episomal plasmid | p-Coumaric acid | 0.00145 | Flask | 2003 | [77] |
| S. cerevisiae CEN-PK113-3B | 4CL2 (N. tabacum) STS (V. vinifera) | - | One copy genome integration | p-Coumaric acid | 5.8 | Flask | 2006 | [78] |
| S. cerevisiae WAT11 | TAL (R. sphaeroides) 4CL (A. thaliana)::STS (V. vinifera) | - | Episomal plasmid | p-Coumaric acid | 5.25 | Flask | 2006 | [79] |
| S. cerevisiae YPH499 | PAL, CPRa (P. trichocarpa × P. deltoides) C4H, 4CL (G. max) STS (V. vinifera) | - | Episomal plasmid | Phenylalanine p-Coumaric acid | 0.29 0.31 | Flask | 2009 | [80] |
| Industrial Brazilian yeast (S. cerevisiae) | 4CL1 (A. thaliana) STS (V. vinifera) | - | Episomal plasmid | p-Coumaric acid | 262–391 | Flask | 2010 | [81] |
| S. cerevisiae W303-1A | 4CL1 (A. thaliana) STS (A. hypogaea) | PAD1 knockout | Episomal plasmid | p-Coumaric acid | 3.1 | Flask | 2011 | [82] |
| S. cerevisiae WAT11 | TAL (R. sphaeroides) 4CL::STS, 4CL1 (A. thaliana)-STS (V. vinifera) fusion enzyme | Expression of araE transporter (E. coli) | One copy genome integration | Tyrosine, p-Coumaric acid Grape Juice | 3.1 2.3 3.44 | Shake flask | 2011 | [83] |
| S. cerevisiae W303-1A | PAL (R. toruloides) C4H, 4CL1 (A. thaliana) STS (A. hypogaea) | Overexpression of ACC1 | Episomal plasmid | Tyrosine | 5.8 | Batch bioreactor | 2012 | [84] |
| S. cerevisiae WAT11 | 4CL1 (A. thaliana) STS (V. vinifera) | Synthetic scaffold | Episomal plasmid | p-Coumaric acid | 14.4 | Flask | 2012 | [85] |
| S. cerevisiae WAT11 | 4CL::STS, 4CL1 (A. thaliana)-STS (V. vinifera) fusion enzyme | Overexpression of: AAE13 | One copy genome integration | p-Coumaric acid | Up to 3.7 | Flask | 2014 | [86] |
| S. cerevisiae EC1118 | 4CL (A. thaliana) STS (V. vinifera) | - | Episomal plasmids | p-coumaric acid | 8.249 | Flask | 2015 | [87] |
| S. cerevisiae CEN. PK102-5B | TAL (H. aurantiacus) TAL (F. johnsoniae) 4CL1 and 4CL2 (A. thaliana) RS (V. vinifera) | Overexpression of ARO4fbr, ARO7fbr, and ACC1 | Multiple copy genome integration | Glucose Ethanol | 415.65 531.41 | Fed-batch bioreactor Fed-batch bioreactor | 2015 | [88] |
| S. cerevisiae CEN. PK102-5B | PAL2, C4H, 4CL2 (A. thaliana) VST1 (V. vinifera) | Overexpression of ARO4fbr, ARO7fbr, ACC1, CYB5 (S. cerevisiae), ATR2 (A. thaliana), ACS (S. enterica), and deletion of aro10 | Multiple-copy genome integration | Glucose Ethanol | 812 755 | Fed-batch bioreactor Fed-batch bioreactor | 2016 | [89] |
| S. cerevisiae W303 | 4CL1 (P. appendiculatum) STS (P. henryana) STS (P. cuspidatum) STS (M. alba var. atropurpurea) STS (R. tataricum) STS (V. vinifera) STS (A. hypogaea) One STS gene for each yeast line | - | Episomal plasmids | p-Coumaric acid | 23.7–39.9 | Batch bioreactor | 2020 | [90] |
| Co-culture of E. coli NEB10β and S. cerevisiae BY4741 | TAL (T. cutaneum) | Overexpression of aroG and tyrA in a tyrR knockout strain | Bacterial Expression Vectors | Glucose | 36 | Co-culture fermentation | 2020 | [91] |
| 4CL (A. thaliana) STS (V. vinifera) | Overexpression of: ACC1 | One copy genome integration | p-Coumaric acid (secreted from E. coli) | |||||
| Y. lipolytica ATCC 20362 | PAL/TAL (R.glutinis) 4CL (S. coelicolor) STS (V. vinifera) | - | l-tyrosine | 1.46 | 2010 | [92] | ||
| Y. lipolytica | 4CL (N. tabacum) STS (A. hypogaea) | Overexpression of: ACC1, PEX10 | Randomly genome integration | p-Coumaric acid | 48.7 | Flask | 2020 | [93] |
| Y. lipolytica Po1d (wt), derived from W29 | TAL (F. johnsoniae) PAL (V. vinifera) C4H, 4CL1 (A. thaliana) VST (V. vinifera) | - | Multiple copy genome integration | Glycerol | 430 | Bioreactor | 2020 | [94] |
| Y. lipolytica Po1fk derived from W29 | TAL (R. toruloides) 4CL (P. crispum) STS (V. vinifera) | ARO4fbr (S. cerevisiae) aroGfbr (E. coli) xfpK (B. breve) xpkA (A. capsulatum) Overexpression: of ARO1, ARO2, ARO3, ARO4, ARO5, TKT Deletion of: TRP2, TRP3, ARO8, ARO9, PYK, PHA2 | One copy genome integration | Glucose | 12.67 | Flask | 2020 | [95] |
| Y. lipolytica ST6512 (W29) | TAL (F. johnsoniae) 4CL1(A. thaliana) VST1 (V. vinifera) | Overexpression of: ARO4fbr and ARO7fbr | Multiple copy genome integration | Glucose Glucose | 409 12355 | Flask Fed-batch bioreactor | 2020 | [96] |
| Ogataea polymorpha | TAL (H. aurantiacus) 4CL (A. thaliana) STS (V. vinifera) | - | CRISPR–Cas9-assisted multiplex genome editing, multi-copy integration | Tyrosine | 97.23 | Flask | 2018 | [97] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, G.G.; Yan, J.; Xu, L.; Yang, M.; Yan, Y. Resveratrol Production in Yeast Hosts: Current Status and Perspectives. Biomolecules 2021, 11, 830. https://doi.org/10.3390/biom11060830
Ibrahim GG, Yan J, Xu L, Yang M, Yan Y. Resveratrol Production in Yeast Hosts: Current Status and Perspectives. Biomolecules. 2021; 11(6):830. https://doi.org/10.3390/biom11060830
Chicago/Turabian StyleIbrahim, Gehad G., Jinyong Yan, Li Xu, Min Yang, and Yunjun Yan. 2021. "Resveratrol Production in Yeast Hosts: Current Status and Perspectives" Biomolecules 11, no. 6: 830. https://doi.org/10.3390/biom11060830
APA StyleIbrahim, G. G., Yan, J., Xu, L., Yang, M., & Yan, Y. (2021). Resveratrol Production in Yeast Hosts: Current Status and Perspectives. Biomolecules, 11(6), 830. https://doi.org/10.3390/biom11060830








