Periodontal Inflamed Surface Area Mediates the Link between Homocysteine and Blood Pressure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Periodontal Examination
- Calculation of mean CAL and gingival recession for each particular tooth;
- Linear mean CAL and gingival recession is used to calculate PESA;
- For each tooth, PISA is calculated through the multiplication of PESA by the proportion of sites around the tooth with BOP;
- The sum of all individual PISA and PESA scores, in mm2, provides an overall area, respectively, for each participant.
2.3. Plasma Homocysteine Measurement
2.4. Covariates
2.5. Data Management, Test Methods and Analysis
3. Results
3.1. Characteristics of the Study Sample
3.2. Correlation Estimates of Hcy Compared to CRP
3.3. Mediation Analyses
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Škovierová, H.; Vidomanová, E.; Mahmood, S.; Sopková, J.; Drgová, A.; Červeňová, T.; Halašová, E.; Lehotský, J. The Molecular and Cellular Effect of Homocysteine Metabolism Imbalance on Human Health. Int. J. Mol. Sci. 2016, 17, 1733. [Google Scholar] [CrossRef]
- Al Mutairi, F. Hyperhomocysteinemia: Clinical Insights. J. Cent. Nerv. Syst. Dis. 2020, 12, 117957352096223. [Google Scholar] [CrossRef]
- Refsum, H.; Smith, A.D.; Ueland, P.M.; Nexo, E.; Clarke, R.; McPartlin, J.; Johnston, C.; Engbaek, F.; Schneede, J.; McPartlin, C.; et al. Facts and Recommendations about Total Homocysteine Determinations: An Expert Opinion. Clin. Chem. 2004, 50, 3–32. [Google Scholar] [CrossRef] [Green Version]
- Perła-Kaján, J.; Twardowski, T.; Jakubowski, H. Mechanisms of Homocysteine Toxicity in Humans. Amino Acids 2007, 32, 561–572. [Google Scholar] [CrossRef]
- Kang, S.S.; Wong, P.W.K.; Malinow, M.R. Hyperhomocyst(e)Inemia as a Risk Factor for Occlusive Vascular Disease. Annu. Rev. Nutr. 1992, 12, 279–298. [Google Scholar] [CrossRef]
- McCully, K.S. Chemical Pathology of Homocysteine I. Atherogenesis. Ann. Clin. Lab. Sci. 1993, 23, 477–493. [Google Scholar] [PubMed]
- Wald, D.S.; Morris, J.K.; Law, M.; Wald, N.J. Folic Acid, Homocysteine, and Cardiovascular Disease: Judging Causality in the Face of Inconclusive Trial Evidence Evidence from Patients with Homocystinuria. BMJ 2006, 333, 1114–1117. [Google Scholar] [CrossRef] [Green Version]
- Stühlinger, M.C.; Tsao, P.S.; Her, J.H.; Kimoto, M.; Balint, R.F.; Cooke, J.P. Homocysteine Impairs the Nitric Oxide Synthase Pathway Role of Asymmetric Dimethylarginine. Circulation 2001, 104, 2569–2575. [Google Scholar] [CrossRef]
- Fournier, P.; Fourcade, J.; Roncalli, J.; Salvayre, R.; Galinier, M.; Caussé, E. Homocysteine in Chronic Heart Failure. Clin. Lab. 2015, 61. [Google Scholar] [CrossRef]
- Kornman, K.S.; Duff, G.W. Candidate Genes as Potential Links between Periodontal and Cardiovascular Diseases. Ann. Periodontol./Am. Acad. Periodontol. 2001, 6, 48–57. [Google Scholar] [CrossRef]
- Darveau, R.P. Periodontitis: A Polymicrobial Disruption of Host Homeostasis. Nat. Rev. Microbiol. 2010, 8, 481–490. [Google Scholar] [CrossRef]
- Hajishengallis, G. Periodontitis: From Microbial Immune Subversion to Systemic Inflammation. Nat. Rev. Immunol. 2015, 15, 30–44. [Google Scholar] [CrossRef]
- Montebugnoli, L.; Servidio, D.; Miaton, R.A.; Prati, C.; Tricoci, P.; Melloni, C. Poor Oral Health Is Associated with Coronary Heart Disease and Elevated Systemic Inflammatory and Haemostatic Factors. J. Clin. Periodontol. 2004, 31, 25–29. [Google Scholar] [CrossRef]
- Joseph, R.; Nath, S.G.; Joseraj, M.G. Elevated Plasma Homocysteine Levels in Chronic Periodontitis: A Hospital-Based Case-Control Study. J. Periodontol. 2011, 82, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, S.; Prabhuji, M.L.V.; Karthikeyan, B.V. Effect of Non-Surgical Periodontal Therapy on Plasma Homocysteine Levels in Indian Population with Chronic Periodontitis: A Pilot Study. J. Clin. Periodontol. 2015, 42, 221–227. [Google Scholar] [CrossRef]
- Muñoz Aguilera, E.; Suvan, J.; Buti, J.; Czesnikiewicz-Guzik, M.; Barbosa Ribeiro, A.; Orlandi, M.; Guzik, T.J.; Hingorani, A.D.; Nart, J.; D’Aiuto, F. Periodontitis Is Associated with Hypertension: A Systematic Review and Meta-Analysis. Cardiovasc. Res. 2020, 116, 28–39. [Google Scholar] [CrossRef] [PubMed]
- NHANES Questionnaires, Datasets, and Related Documentation. Available online: https://wwwn.cdc.gov/nchs/nhanes/ (accessed on 6 March 2021).
- Lachat, C.; Hawwash, D.; Ocké, M.C.; Berg, C.; Forsum, E.; Hörnell, A.; Larsson, C.; Sonestedt, E.; Wirfält, E.; Åkesson, A.; et al. Strengthening the Reporting of Observational Studies in Epidemiology—Nutritional Epidemiology (STROBE-Nut): An Extension of the STROBE Statement. PLOS Med. 2016, 13, e1002036. [Google Scholar] [CrossRef] [Green Version]
- Dye, B.A.; Thornton-Evans, G. A Brief History of National Surveillance Efforts for Periodontal Disease in the United States. J. Periodontol. 2007, 78, 1373–1379. [Google Scholar] [CrossRef] [PubMed]
- Eke, P.I.; Page, R.C.; Wei, L.; Thornton-Evans, G.; Genco, R.J. Update of the Case Definitions for Population-Based Surveillance of Periodontitis. J. Periodontol. 2012, 83, 1449–1454. [Google Scholar] [CrossRef]
- Hujoel, P.P.; White, B.A.; García, R.I.; Listgarten, M.A. The Dentogingival Epithelial Surface Area Revisited. J. Periodontal. Res. 2001, 36, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Nesse, W.; Abbas, F.; Van Der Ploeg, I.; Spijkervet, F.K.L.; Dijkstra, P.U.; Vissink, A. Periodontal Inflamed Surface Area: Quantifying Inflammatory Burden. J. Clin. Periodontol. 2008, 35, 668–673. [Google Scholar] [CrossRef] [Green Version]
- NHANES 2001-2002 Data Documentation, Codebook, and Frequencies. Available online: https://wwwn.cdc.gov/Nchs/Nhanes/2001-2002/L06_2_B.htm (accessed on 10 November 2020).
- Shipchandler, M.; Moore, E. Rapid, Fully Automated Measurement of Plasma Homocyst(e)Ine with the Abbott IMx Analyzer. Clin. Chem. 1995, 41, 991–994. [Google Scholar] [CrossRef]
- NHANES 2003-2004 Data Documentation, Codebook, and Frequencies. Available online: https://wwwn.cdc.gov/Nchs/Nhanes/2003-2004/L06MH_C.htm (accessed on 10 November 2020).
- Boushey, C.J.; Beresford, S.A.; Omenn, G.S.; Motulsky, A.G. A Quantitative Assessment of Plasma Homocysteine as a Risk Factor for Vascular Disease. Probable Benefits of Increasing Folic Acid Intakes. JAMA 1995, 274, 1049–1057. [Google Scholar] [CrossRef]
- Ueland, P.M.; Refsum, H.; Stabler, S.P.; Malinow, M.R.; Andersson, A.; Allen, R.H. Total Homocysteine in Plasma or Serum: Methods and Clinical Applications. Clin. Chem. 1993, 39, 1764–1779. [Google Scholar] [CrossRef]
- Ostchega, Y.; Prineas, R.J.; Paulose-Ram, R.; Grim, C.M.; Willard, G.; Collins, D. National Health and Nutrition Examination Survey 1999-2000: Effect of Observer Training and Protocol Standardization on Reducing Blood Pressure Measurement Error. J. Clin. Epidemiol. 2003, 56, 768–774. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Cardiology and the European Society of Hypertension. J. Hypertens 2018, 36, 1953–2041. [Google Scholar] [CrossRef] [Green Version]
- Mills, K.T.; Bundy, J.D.; Kelly, T.N.; Reed, J.E.; Kearney, P.M.; Reynolds, K.; Chen, J.; He, J. Global Disparities of Hypertension Prevalence and Control: A Systematic Analysis of Population-Based Studies From 90 Countries. Circulation 2016, 134, 441–450. [Google Scholar] [CrossRef]
- Botelho, J.; Machado, V.; Proença, L.; Mendes, J.J. The 2018 Periodontitis Case Definition Improves Accuracy Performance of Full-Mouth Partial Diagnostic Protocols. Sci. Rep. 2020, 10, 7093. [Google Scholar] [CrossRef]
- Nakano, Y.; Yoshimura, M.; Koga, T. Correlation between Oral Malodor and Periodontal Bacteria. Microbes Infect. 2002, 4, 679–683. [Google Scholar] [CrossRef]
- Ryder, M.I. Comparison of Neutrophil Functions in Aggressive and Chronic Periodontitis. Periodontology 2000 2010, 53, 124–137. [Google Scholar] [CrossRef]
- Yaegaki, K.; Sanada, K. Biochemical and Clinical Factors Influencing Oral Malodor in Periodontal Patients. J. Periodontol. 1992, 63, 783–789. [Google Scholar] [CrossRef]
- Botelho, J.; Machado, V.; Hussain, S.B.; Zehra, S.A.; Proença, L.; Orlandi, M.; Mendes, J.J.; D’Aiuto, F. Periodontitis and Circulating Blood Cell Profiles: A Systematic Review and Meta-Analysis. Exp. Hematol. 2021, 93, 1–13. [Google Scholar] [CrossRef]
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal Diseases. Nat. Rev. Dis. Primers 2017, 3, 17038. [Google Scholar] [CrossRef]
- Hossain, G.S.; van Thienen, J.V.; Werstuck, G.H.; Zhou, J.; Sood, S.K.; Dickhout, J.G.; de Koning, A.B.L.; Tang, D.; Wu, D.; Falk, E.; et al. TDAG51 Is Induced by Homocysteine, Promotes Detachment-Mediated Programmed Cell Death, and Contributes to the Cevelopment of Atherosclerosis in Hyperhomocysteinemia. J. Biol. Chem. 2003, 278, 30317–30327. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Cai, Y.; Adachi, M.T.; Oshiro, S.; Aso, T.; Kaufman, R.J.; Kitajima, S. Homocysteine Induces Programmed Cell Death in Human Vascular Endothelial Cells through Activation of the Unfolded Protein Response. J. Biol. Chem. 2001, 276, 35867–35874. [Google Scholar] [CrossRef] [Green Version]
- Ganguly, P.; Alam, S.F. Role of Homocysteine in the Development of Cardiovascular Disease. Nutr. J. 2015, 14. [Google Scholar] [CrossRef] [Green Version]
- Velsko, I.M.; Chukkapalli, S.S.; Rivera, M.F.; Lee, J.-Y.; Chen, H.; Zheng, D.; Bhattacharyya, I.; Gangula, P.R.; Lucas, A.R.; Kesavalu, L. Active Invasion of Oral and Aortic Tissues by Porphyromonas Gingivalis in Mice Causally Links Periodontitis and Atherosclerosis. PLoS ONE 2014, 9, e97811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmlund, A.; Lampa, E.; Lind, L. Poor Response to Periodontal Treatment May Predict Future Cardiovascular Disease. J. Dent. Res. 2017, 96, 768–773. [Google Scholar] [CrossRef]
- Keceli, H.G.; Ercan, N.; Karsiyaka Hendek, M.; Kisa, U.; Mesut, B.; Olgun, E. The Effect of the Systemic Folic Acid Intake as an Adjunct to Scaling and Root Planing on Clinical Parameters and Homocysteine and C-Reactive Protein Levels in Gingival Crevicular Fluid of Periodontitis Patients: A Randomized Placebo-Controlled Clinical t. J. Clin. Periodontol. 2020, 47, 602–613. [Google Scholar] [CrossRef]
- Tyagi, N.; Sedoris, K.C.; Steed, M.; Ovechkin, A.V.; Moshal, K.S.; Tyagi, S.C. Mechanisms of Homocysteine-Induced Oxidative Stress. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H2649–H2656. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Andrukhov, O.; Rausch-Fan, X. Oxidative Stress and Antioxidant System in Periodontitis. Front. Physiol. 2017, 8, 910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Machado, V.; Botelho, J.; Mascarenhas, P.; Cavacas, M.A.; Alves, R.; Mendes, J.J. Partial Recording Protocols Performance on the Assessment of Periodontitis Severity and Extent: Bias Magnitudes, Sensibility, and Specificity. Rev. Port. De Estomatol. Med. Dent. E Cir. Maxilofac. 2018, 59, 145–153. [Google Scholar] [CrossRef]
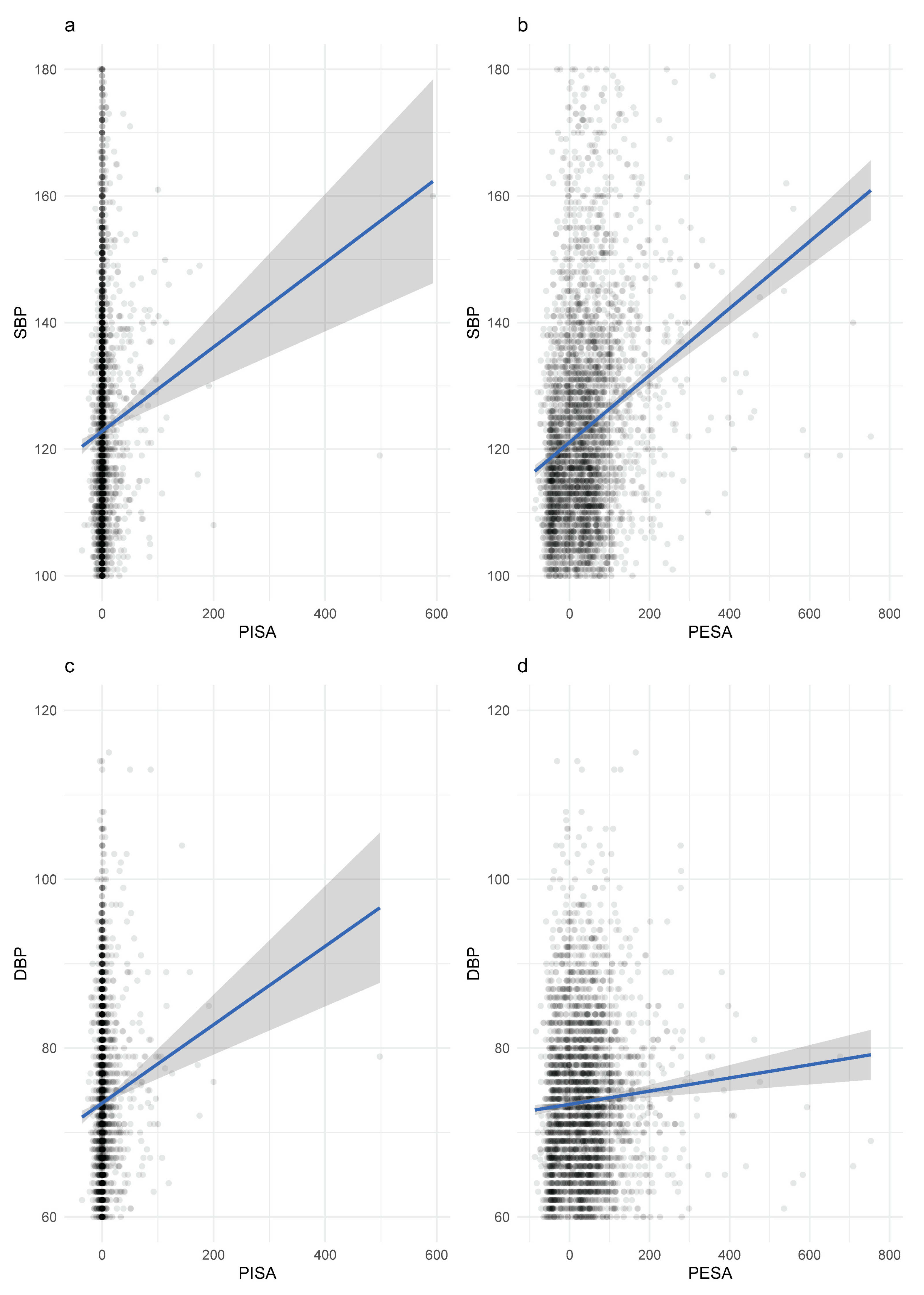
| Variable | No Periodontitis (n = 3287) | Periodontitis (n = 734) | p-Value | Overall (n = 4021) |
|---|---|---|---|---|
| Age (years), mean (SD) | 38.4 (17.8) | 56.8 (15.8) | <0.001 | 41.7 (18.9) |
| Gender, % (n) | ||||
| Males | 46.2 (1520) | 61.6 (452) | <0.001 | 49.0 (1972) |
| Females | 53.8 (1767) | 38.4 (282) | 51.0 (2049) | |
| Race/ethnicity, % (n) | ||||
| Mexican American | 21.0 (690) | 26.0 (191) | 0.001 | 21.9 (881) |
| Non-Hispanic White | 3.4 (111) | 4.0 (29) | 3.5 (140) | |
| Non-Hispanic Black | 50.0 (1643) | 41.6 (305) | 48.4 (1948) | |
| Other Hispanic | 21.8 (715) | 24.0 (176) | 22.2 (891) | |
| Other races | 3.9 (128) | 4.5 (33) | 4.0 (161) | |
| Education level, % (n) | ||||
| <High school | 24.2 (797) | 37.9 (278) | <0.001 | 26.7 (1075) |
| High school | 25.6 (842) | 26.8 (197) | 25.8 (1039) | |
| >High school | 50.1 (1647) | 35.1 (258) | 47.4 (1905) | |
| Smoking status, % (n) | ||||
| Never | 63.9 (2100) | 37.5 (275) | <0.001 | 59.1 (2375) |
| Former | 18.3 (603) | 31.5 (231) | 20.7 (834) | |
| Current | 17.8 (584) | 31.1 (228) | 20.2 (812) | |
| BMI (kg/m2), mean (SD) | 27.7 (7.0) | 28.5 (7.2) | 0.011 | 27.9 (7.1) |
| Hcy (µmol/L), mean (SD) | 8.2 (3.9) | 9.8 (4.0) | <0.001 | 8.5 (4.0) |
| Hcy elevated level category, % (n) | 2.8 (92) | 6.1 (45) | <0.001 | 3.4 (137) |
| CRP (mg/dL), mean (SD) | 0.4 (0.8) | 0.5 (0.8) | <0.001 | 0.4 (0.8) |
| Chronic medical conditions, mean (SD) | 0.8 (1.5) | 1.4 (2.7) | <0.001 | 0.9 (1.8) |
| Diabetes Mellitus, n (%) | 5.0 (164) | 14.7 (108) | <0.001 | 6.8 (272) |
| Hypertension, n (%) | 12.1 (399) | 29.4 (216) | <0.001 | 15.3 (615) |
| Mean SBP (mmHg), mean (SD) | 120.0 (17.6) | 132.0 (21.2) | <0.001 | 122.2 (18.9) |
| Mean DBP (mmHg), mean (SD) | 69.4 (11.7) | 72.0 (11.9) | <0.001 | 69.8 (11.8) |
| PISA (mm2), mean (SD) | 0.8 (7.9) | 13.5 (38.4) | <0.001 | 3.1 (18.5) |
| PESA (mm2), mean (SD) | 17.3 (51.4) | 118.0 (107.0) | <0.001 | 35.6 (75.7) |
| Vitamin B12 (pg/mL), mean (SD) | 550.0 (1120.0) | 554.0 (521.0) | 0.880 | 550.9 (1037.1) |
| Folate (ng/mL), mean (SD) | 12.6 (7.8) | 14.6 (26.3) | 0.040 | 12.9 (13.3) |
| Variable | CRP (mg/dL) | Homocysteine (µmol/L) |
|---|---|---|
| Age (years) | 0.036 * | 0.326 ** |
| Hcy (µmol/L) | −0.019 | - |
| BMI (g/m2) | 0.215 ** | 0.003 |
| SBP (mmHg) | 0.037 * | 0.274 ** |
| DBP (mmHg) | 0.015 | 0.124 ** |
| PISA (mm2) | 0.030 | 0.054 ** |
| PESA (mm2) | 0.031 | 0.177 ** |
| WBC (109/L) | 0.154 ** | −0.026 |
| Segmented Neutrophils (109/L) | 0.143 ** | −0.035 * |
| Vitamin B12 (pg/mL) | 0.006 | −0.058 ** |
| Folate (ng/mL) | −0.011 | −0.045 ** |
| Model | Homocysteine | |
|---|---|---|
| PISA | PESA | |
| 1 | 0.012 ** (0.003) | 0.009 ** (0.001) |
| 2 | 0.005 (0.003) | 0.002 * (0.001) |
| 3 | 0.005 (0.003) | 0.002 * (0.001) |
| 4 | 0.004 (0.003) | 0.002 * (0.001) |
| 5 | 0.004 (0.003) | 0.002 * (0.001) |
| 6 | 0.004 (0.003) | 0.002 * (0.001) |
| 7 | 0.003 (0.003) | 0.002 * (0.001) |
| 8 | 0.003 (0.003) | 0.002 * (0.001) |
| Exposure: Hcy/Outcome: SBP Mediator: PISA | |||
| Effect | Estimate | SE | p-value |
| a (exposure → mediator) | 0.156 | 0.076 | 0.041 |
| b (mediator → outcome) | 0.076 | 0.017 | <0.001 |
| c (total effect) | 0.069 | 0.006 | <0.001 |
| c′ (direct effect) | 0.012 | 0.003 | <0.001 |
| ab (mediated effect) | 0.012 | 0.006 | <0.001 |
| ab/c (PISA percentage mediated) = 17.4% | |||
| Exposure: Hcy/Outcome: SBP Mediator: PESA | |||
| Effect | Estimate | SE | p-value |
| a (exposure → mediator) | 0.006 | 0.001 | <0.001 |
| b (mediator → outcome) | 1.176 | 0.074 | <0.001 |
| c (total effect) | 0.900 | 0.063 | <0.001 |
| c′ (direct effect) | 0.892 | 0.063 | <0.001 |
| ab (mediated effect) | 0.008 | 0.001 | <0.001 |
| ab/c (PISA percentage mediated) = 0.9% | |||
| Exposure: Hcy/Outcome: DBP Mediator: PISA | |||
| Effect | Estimate | SE | p-value |
| a (exposure → mediator) | 0.219 | 0.074 | 0.003 |
| b (mediator → outcome) | 0.035 | 0.010 | 0.001 |
| c (total effect) | 0.049 | 0.006 | <0.001 |
| c′ (direct effect) | 0.041 | 0.005 | <0.001 |
| ab (mediated effect) | 0.008 | 0.003 | 0.017 |
| ab/c (PISA percentage mediated) = 16.3% | |||
| Exposure: Hcy/Outcome: DBP Mediator: PESA | |||
| Effect | Estimate | SE | p-value |
| a (exposure → mediator) | 3.222 | 0.297 | <0.001 |
| b (mediator → outcome) | 0.011 | 0.002 | <0.001 |
| c (total effect) | 0.072 | 0.009 | <0.001 |
| c′ (direct effect) | 0.038 | 0.005 | <0.001 |
| ab (mediated effect) | 0.034 | 0.008 | <0.001 |
| ab/c (PISA percentage mediated) = 47.2% | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Botelho, J.; Machado, V.; Leira, Y.; Proença, L.; Mendes, J.J. Periodontal Inflamed Surface Area Mediates the Link between Homocysteine and Blood Pressure. Biomolecules 2021, 11, 875. https://doi.org/10.3390/biom11060875
Botelho J, Machado V, Leira Y, Proença L, Mendes JJ. Periodontal Inflamed Surface Area Mediates the Link between Homocysteine and Blood Pressure. Biomolecules. 2021; 11(6):875. https://doi.org/10.3390/biom11060875
Chicago/Turabian StyleBotelho, João, Vanessa Machado, Yago Leira, Luís Proença, and José João Mendes. 2021. "Periodontal Inflamed Surface Area Mediates the Link between Homocysteine and Blood Pressure" Biomolecules 11, no. 6: 875. https://doi.org/10.3390/biom11060875









