Microbe-Mediated Biosynthesis of Nanoparticles: Applications and Future Prospects
Abstract
1. Introduction
2. Strategies for Synthesis of Nanoparticles Using Microbes
2.1. Bacterial-Mediated Biosynthesis of Nanoparticles
2.1.1. Metal Nanoparticles
Silver Nanoparticles (AgNPs)
Gold Nanoparticles (AuNPs)
Other Important Metal Nanoparticles
2.1.2. Metal Oxide Nanoparticles
2.1.3. Organic Nanoparticles
2.2. Actinobacteria-Mediated Biosynthesis of Nanoparticles
3. Fungi- and Yeast-Mediated Biosynthesis of Nanoparticles
3.1. Metal Nanoparticles
3.2. Metal Oxide Nanoparticles
4. Algae-Mediated Biosynthesis of Nanoparticles
4.1. Metal Nanoparticles
4.2. Metal Oxide Nanoparticles
5. Virus-Mediated Biosynthesis of Nanoparticles
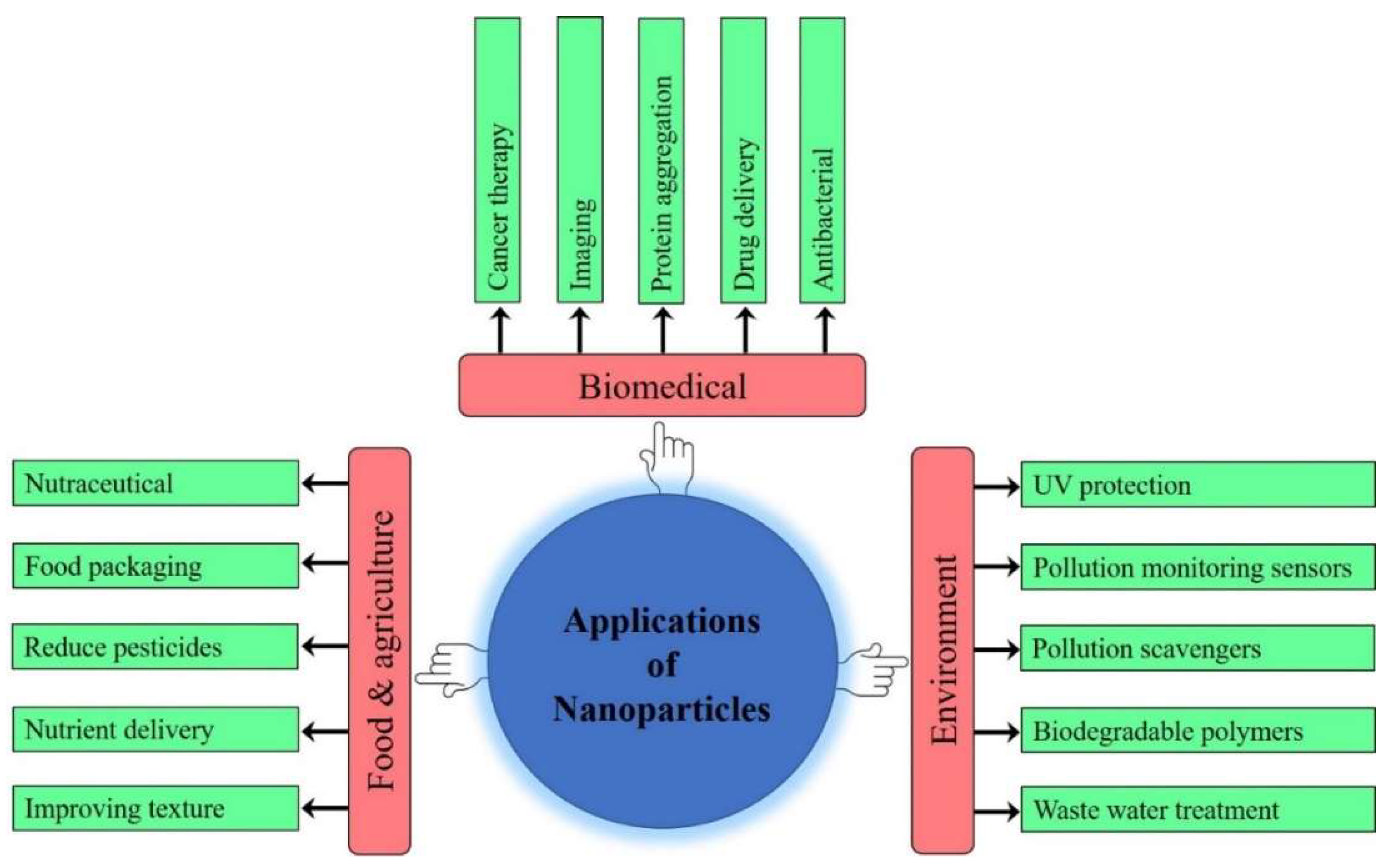
6. Applications of Nanoparticles
6.1. Biomedical Applications of Nanoparticles
6.2. Role of Nanoparticles in Drug Delivery and Diagnostics
6.3. Application of Nanoparticles in Food Industry
6.4. Applications of Nanoparticles in Agriculture
6.4.1. Nanoparticles as Fungicides
6.4.2. Nanoparticles as Fertilizers
6.4.3. Nanoparticles as Pesticides
6.4.4. Applications of Nanoparticles in Bioremediation
7. Future Perspectives and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Elfeky, A.S.; Salem, S.S.; Elzaref, A.S.; Owda, M.E.; Eladawy, H.A.; Saeed, A.M.; Awad, M.A.; Abou-Zeid, R.E.; Fouda, A. Multifunctional cellulose nanocrystal /metal oxide hybrid, photo-degradation, antibacterial and larvicidal activities. Carbohydr. Polym. 2020, 230, 115711. [Google Scholar] [CrossRef] [PubMed]
- Salem, S.S.; Fouda, A. Green synthesis of metallic nanoparticles and their prospective biotechnological applications: An overview. Biol. Trace Elem. Res. 2021, 199, 344–370. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Gahlawat, G.; Choudhury, A.R. A review on the biosynthesis of metal and metal salt nanoparticles by microbes. RSC Adv. 2019, 9, 12944–12967. [Google Scholar] [CrossRef]
- Grasso, G.; Zane, D.; Dragone, R. Microbial nanotechnology: Challenges and prospects for green biocatalytic synthesis of nanoscale materials for sensoristic and biomedical applications. Nanomaterials 2020, 10, 11. [Google Scholar] [CrossRef]
- Inshakova, E.; Inshakov, O. World market for nanomaterials: Structure and trends. In Proceedings of the MATEC Web of Conferences, Sevastopol, Russia, 11–15 September 2017; EDP Sciences: Les Ulis, France, 2017; Volume 129, p. 02013. [Google Scholar]
- Dobias, J.; Suvorova, E.I.; Bernier-Latmani, R. Role of proteins in controlling selenium nanoparticle size. Nanotechnology 2011, 22, 195605. [Google Scholar] [CrossRef]
- Shedbalkar, U.; Singh, R.; Wadhwani, S.; Gaidhani, S.; Chopade, B. Microbial synthesis of gold nanoparticles: Current status and future prospects. Adv. Colloid Interface Sci. 2014, 209, 40–48. [Google Scholar] [CrossRef]
- Albanese, A.; Tang, P.S.; Chan, W.C. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu. Rev. Biomed. Eng. 2012, 14, 1–16. [Google Scholar] [CrossRef]
- Arshad, A.; Iqbal, J.; Mansoor, Q.; Ahmed, I. Graphene/sio2 nanocomposites: The enhancement of photocatalytic and biomedical activity of sio2 nanoparticles by graphene. J. Appl. Phys. 2017, 121, 244901. [Google Scholar] [CrossRef]
- Nath, D.; Banerjee, P. Green nanotechnology—A new hope for medical biology. Environ. Toxicol. Pharmacol. 2013, 36, 997–1014. [Google Scholar] [CrossRef]
- Kharisov, B.I.; Kharissova, O.V.; Ortiz Mendez, U.; De La Fuente, I.G. Decoration of carbon nanotubes with metal nanoparticles: Recent trends. Synth. React. Inorg. Met. Chem. 2016, 46, 55–76. [Google Scholar] [CrossRef]
- You, H.; Yang, S.; Ding, B.; Yang, H. Synthesis of colloidal metal and metal alloy nanoparticles for electrochemical energy applications. Chem. Soc. Rev. 2013, 42, 2880–2904. [Google Scholar] [CrossRef]
- Shah, M.; Fawcett, D.; Sharma, S.; Tripathy, S.K.; Poinern, G.E.J. Green synthesis of metallic nanoparticles via biological entities. Materials 2015, 8, 7278–7308. [Google Scholar] [CrossRef]
- Fariq, A.; Khan, T.; Yasmin, A. Microbial synthesis of nanoparticles and their potential applications in biomedicine. J. Appl. Biomed. 2017, 15, 241–248. [Google Scholar] [CrossRef]
- Castro, L.; Blázquez, M.L.; González, F.G.; Ballester, A. Mechanism and applications of metal nanoparticles prepared by bio-mediated process. Rev. Adv. Sci. Eng. 2014, 3, 199–216. [Google Scholar] [CrossRef]
- Jang, H.D.; Kim, S.K.; Chang, H.; Choi, J.-H.; Cho, B.-G.; Jo, E.H.; Choi, J.-W.; Huang, J. Three-dimensional crumpled graphene-based platinum–gold alloy nanoparticle composites as superior electrocatalysts for direct methanol fuel cells. Carbon 2015, 93, 869–877. [Google Scholar] [CrossRef]
- Singh, P.; Kim, Y.-J.; Zhang, D.; Yang, D.-C. Biological synthesis of nanoparticles from plants and microorganisms. Trends Biotechnol. 2016, 34, 588–599. [Google Scholar] [CrossRef]
- Saravanan, M.; Barik, S.K.; MubarakAli, D.; Prakash, P.; Pugazhendhi, A. Synthesis of silver nanoparticles from bacillus brevis (ncim 2533) and their antibacterial activity against pathogenic bacteria. Microb. Pathog. 2018, 116, 221–226. [Google Scholar] [CrossRef]
- Buszewski, B.; Railean-Plugaru, V.; Pomastowski, P.; Rafińska, K.; Szultka-Mlynska, M.; Golinska, P.; Wypij, M.; Laskowski, D.; Dahm, H. Antimicrobial activity of biosilver nanoparticles produced by a novel streptacidiphilus durhamensis strain. J. Microbiol. Immunol. Infect. 2018, 51, 45–54. [Google Scholar] [CrossRef]
- Sanaeimehr, Z.; Javadi, I.; Namvar, F. Antiangiogenic and antiapoptotic effects of green-synthesized zinc oxide nanoparticles using sargassum muticum algae extraction. Cancer Nanotechnol. 2018, 9, 1–16. [Google Scholar] [CrossRef]
- He, S.; Zhang, Y.; Guo, Z.; Gu, N. Biological synthesis of gold nanowires using extract of rhodopseudomonas capsulata. Biotechnol. Prog. 2008, 24, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhou, L.; Yang, H.; Lv, R.; Tian, P.; Li, X.; Zhang, Y.; Chen, Z.; Lin, F. Self-assembled exopolysaccharide nanoparticles for bioremediation and green synthesis of noble metal nanoparticles. ACS Appl. Mater. Interfaces 2017, 9, 22808–22818. [Google Scholar] [CrossRef] [PubMed]
- Golmohammadi, H.; Morales-Narvaez, E.; Naghdi, T.; Merkoci, A. Nanocellulose in sensing and biosensing. Chem. Mater. 2017, 29, 5426–5446. [Google Scholar] [CrossRef]
- Shivaji, S.; Madhu, S.; Singh, S. Extracellular synthesis of antibacterial silver nanoparticles using psychrophilic bacteria. Process. Biochem. 2011, 46, 1800–1807. [Google Scholar] [CrossRef]
- Kang, S.H.; Bozhilov, K.N.; Myung, N.V.; Mulchandani, A.; Chen, W. Microbial synthesis of cds nanocrystals in genetically engineered E. Coli. Angew. Chem. 2008, 120, 5264–5267. [Google Scholar] [CrossRef]
- Chan, W.C.W. Nano research for COVID-19. ACS Nano 2020, 14, 3719–3720. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, H.; Chen, Z.-S.; Chen, G. Biosynthesis of Nanoparticles by Microorganisms and Their Applications. J. Nanomater. 2011, 2011, 1–16. [Google Scholar] [CrossRef]
- Das, V.L.; Thomas, R.; Varghese, R.T.; Soniya, E.; Mathew, J.; Radhakrishnan, E. Extracellular synthesis of silver nanoparticles by the bacillus strain cs 11 isolated from industrialized area. 3 Biotech 2014, 4, 121–126. [Google Scholar] [CrossRef]
- Yadav, A.; Kon, K.; Kratosova, G.; Duran, N.; Ingle, A.P.; Rai, M. Fungi as an efficient mycosystem for the synthesis of metal nanoparticles: Progress and key aspects of research. Biotechnol. Lett. 2015, 37, 2099–2120. [Google Scholar] [CrossRef]
- Al-Dhabi, N.A.; Mohammed Ghilan, A.-K.; Arasu, M.V. Characterization of silver nanomaterials derived from marine streptomyces sp. Al-dhabi-87 and its in vitro application against multidrug resistant and extended-spectrum beta-lactamase clinical pathogens. Nanomaterials 2018, 8, 279. [Google Scholar] [CrossRef]
- Marooufpour, N.; Alizadeh, M.; Hatami, M.; Lajayer, B.A. Biological Synthesis of Nanoparticles by Different Groups of Bacteria. In Microbial Nanobionics; Springer: Cham, Switzerland, 2019; pp. 63–85. [Google Scholar]
- Jeevan, P.; Ramya, K.; Rena, A.E. Extracellular biosynthesis of silver nanoparticles by culture supernatant of pseudomonas aeruginosa. Indian J. Biotechnol. 2012, 11, 72–76. [Google Scholar]
- Fernández-Llamosas, H.; Castro, L.; Blázquez, M.L.; Díaz, E.; Carmona, M. Speeding up bioproduction of selenium nanoparticles by using vibrio natriegens as microbial factory. Sci. Rep. 2017, 7, 16046. [Google Scholar] [CrossRef]
- Waghmare, S.R.; Mulla, M.N.; Marathe, S.R.; Sonawane, K.D. Ecofriendly production of silver nanoparticles using candida utilis and its mechanistic action against pathogenic microorganisms. 3 Biotech 2015, 5, 33–38. [Google Scholar] [CrossRef]
- Kalabegishvili, T.L.; Kirkesali, E.I.; Rcheulishvili, A.N.; Ginturi, E.N.; Murusidze, I.G.; Pataraya, D.T.; Gurielidze, M.A.; Tsertsvadze, G.I.; Gabunia, V.N.; Lomidze, L.G. Synthesis of gold nanoparticles by some strains of arthrobacter genera. J. Mater. Sci. Eng. A Struct. Mater. Prop. Microstruct. Process. 2012, 2, 164–173. [Google Scholar]
- Du, L.; Jiang, H.; Liu, X.; Wang, E. Biosynthesis of gold nanoparticles assisted by escherichia coli dh5α and its application on direct electrochemistry of hemoglobin. Electrochem. Commun. 2007, 9, 1165–1170. [Google Scholar] [CrossRef]
- Suresh, A.K.; Pelletier, D.A.; Wang, W.; Broich, M.L.; Moon, J.W.; Gu, B.; Allison, D.P.; Joy, D.C.; Phelps, T.J.; Doktycz, M.J. Biofabrication of discrete spherical gold nanoparticles using the metal-reducing bacterium shewanella oneidensis. Acta Biomater. 2011, 7, 2148–2152. [Google Scholar] [CrossRef]
- Bao, H.; Lu, Z.; Cui, X.; Qiao, Y.; Guo, J.; Anderson, J.M.; Li, C.M. Extracellular microbial synthesis of biocompatible cdte quantum dots. Acta Biomater. 2010, 6, 3534–3541. [Google Scholar] [CrossRef]
- Wang, T.; Yang, L.; Zhang, B.; Liu, J. Extracellular biosynthesis and transformation of selenium nanoparticles and application in h2o2 biosensor. Colloids Surf. B Biointerfaces 2010, 80, 94–102. [Google Scholar] [CrossRef]
- Ramya, S.; Shanmugasundaram, T.; Balagurunathan, R. Biomedical potential of actinobacterially synthesized selenium nanoparticles with special reference to anti-biofilm, anti-oxidant, wound healing, cytotoxic and anti-viral activities. J. Trace Elem. Med. Biol. 2015, 32, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Roychoudhury, P.; Gopal, P.K.; Paul, S.; Pal, R. Cyanobacteria assisted biosynthesis of silver nanoparticles—A potential antileukemic agent. J. Appl. Phycol. 2016, 28, 3387–3394. [Google Scholar] [CrossRef]
- Shao, W.; Liu, H.; Liu, X.; Sun, H.; Wang, S.; Zhang, R. Ph-responsive release behavior and anti-bacterial activity of bacterial cellulose-silver nanocomposites. Int. J. Biol. Macromol. 2015, 76, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Torres, S.; Campos, V.; León, C.; Rodríguez-Llamazares, S.; Rojas, S.; Gonzalez, M.; Smith, C.; Mondaca, M. Biosynthesis of selenium nanoparticles by pantoea agglomerans and their antioxidant activity. J. Nanopart. Res. 2012, 14, 1236. [Google Scholar] [CrossRef]
- Kalishwaralal, K.; Banumathi, E.; Pandian, S.R.K.; Deepak, V.; Muniyandi, J.; Eom, S.H.; Gurunathan, S. Silver nanoparticles inhibit vegf induced cell proliferation and migration in bovine retinal endothelial cells. Colloids Surf. B Biointerfaces 2009, 73, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Kalishwaralal, K.; Deepak, V.; Pandian, S.R.K.; Kottaisamy, M.; BarathManiKanth, S.; Kartikeyan, B.; Gurunathan, S. Biosynthesis of silver and gold nanoparticles using brevibacterium casei. Colloids Surf. B Biointerfaces 2010, 77, 257–262. [Google Scholar] [CrossRef]
- Ahmad, M.S.; Yasser, M.M.; Sholkamy, E.N.; Ali, A.M.; Mehanni, M.M. Anticancer activity of biostabilized selenium nanorods synthesized by streptomyces bikiniensis strain ess_ama-1. Int. J. Nanomed. 2015, 10, 3389. [Google Scholar]
- Gurunathan, S.; Han, J.W.; Eppakayala, V.; Kim, J.-H. Green synthesis of graphene and its cytotoxic effects in human breast cancer cells. Int. J. Nanomed. 2013, 8, 1015. [Google Scholar] [CrossRef]
- Suresh, A.K.; Pelletier, D.A.; Wang, W.; Moon, J.-W.; Gu, B.; Mortensen, N.P.; Allison, D.P.; Joy, D.C.; Phelps, T.J.; Doktycz, M.J. Silver nanocrystallites: Biofabrication using shewanella oneidensis, and an evaluation of their comparative toxicity on gram-negative and gram-positive bacteria. Environ. Sci. Technol. 2010, 44, 5210–5215. [Google Scholar] [CrossRef]
- Manivasagan, P.; Alam, M.S.; Kang, K.-H.; Kwak, M.; Kim, S.-K. Extracellular synthesis of gold bionanoparticles by nocardiopsis sp. And evaluation of its antimicrobial, antioxidant and cytotoxic activities. Bioprocess Biosyst. Eng. 2015, 38, 1167–1177. [Google Scholar] [CrossRef]
- Mondal, A.H.; Yadav, D.; Ali, A.; Khan, N.; Jin, J.O.; Haq, Q.M.R. Anti-bacterial and anti-candidal activity of silver nanoparticles biosynthesized using citrobacter spp. Ms5 culture supernatant. Biomolecules 2020, 10, 944. [Google Scholar] [CrossRef]
- Mondal, A.H.; Yadav, D.; Mitra, S.; Mukhopadhyay, K. Biosynthesis of silver nanoparticles using culture supernatant of shewanella sp. Ary1 and their antibacterial activity. Int. J. Nanomed. 2020, 15, 8295–8310. [Google Scholar] [CrossRef]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef]
- Bridges, K.; Kidson, A.; Lowbury, E.; Wilkins, M. Gentamicin-and silver-resistant pseudomonas in a burns unit. Br. Med. J. 1979, 1, 446–449. [Google Scholar] [CrossRef]
- Haefeli, C.; Franklin, C.; Hardy, K.E. Plasmid-determined silver resistance in pseudomonas stutzeri isolated from a silver mine. J. Bacteriol. 1984, 158, 389–392. [Google Scholar] [CrossRef]
- NVKV Prasad, T.; Subba Rao Kambala, V.; Naidu, R. A critical review on biogenic silver nanoparticles and their antimicrobial activity. Curr. Nanosci. 2011, 7, 531–544. [Google Scholar] [CrossRef]
- Iravani, S. Bacteria in nanoparticle synthesis: Current status and future prospects. Int. Sch. Res. Not. 2014, 2014, 359316. [Google Scholar] [CrossRef]
- Klaus, T.; Joerger, R.; Olsson, E.; Granqvist, C.-G. Silver-based crystalline nanoparticles, microbially fabricated. Proc. Natl. Acad. Sci. USA 1999, 96, 13611–13614. [Google Scholar] [CrossRef]
- Nair, B.; Pradeep, T. Coalescence of nanoclusters and formation of submicron crystallites assisted by lactobacillus strains. Cryst. Growth Des. 2002, 2, 293–298. [Google Scholar] [CrossRef]
- Shahverdi, A.R.; Minaeian, S.; Shahverdi, H.R.; Jamalifar, H.; Nohi, A.-A. Rapid synthesis of silver nanoparticles using culture supernatants of enterobacteria: A novel biological approach. Process Biochem. 2007, 42, 919–923. [Google Scholar] [CrossRef]
- Shahverdi, A.R.; Fakhimi, A.; Shahverdi, H.R.; Minaian, S. Synthesis and effect of silver nanoparticles on the antibacterial activity of different antibiotics against staphylococcus aureus and escherichia coli. Nanomedicine 2007, 3, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Kalimuthu, K.; Suresh Babu, R.; Venkataraman, D.; Bilal, M.; Gurunathan, S. Biosynthesis of silver nanocrystals by bacillus licheniformis. Colloids Surf. B Biointerfaces 2008, 65, 150–153. [Google Scholar] [CrossRef]
- Parikh, R.Y.; Singh, S.; Prasad, B.L.; Patole, M.S.; Sastry, M.; Shouche, Y.S. Extracellular synthesis of crystalline silver nanoparticles and molecular evidence of silver resistance from morganella sp.: Towards understanding biochemical synthesis mechanism. Chembiochem 2008, 9, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, N.; Daneshpajouh, S.; Seyedbagheri, S.; Atashdehghan, R.; Abdi, K.; Sarkar, S.; Minaian, S.; Shahverdi, H.R.; Shahverdi, A.R. Biological synthesis of very small silver nanoparticles by culture supernatant of klebsiella pneumonia: The effects of visible-light irradiation and the liquid mixing process. Mater. Res. Bull. 2009, 44, 1415–1421. [Google Scholar] [CrossRef]
- Nanda, A.; Saravanan, M. Biosynthesis of silver nanoparticles from staphylococcus aureus and its antimicrobial activity against mrsa and mrse. Nanomedicine 2009, 5, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, R.; O’Mullane, A.P.; Parikh, R.Y.; Smooker, P.M.; Bhargava, S.K.; Bansal, V. Bacterial kinetics-controlled shape-directed biosynthesis of silver nanoplates using morganella psychrotolerans. Langmuir 2011, 27, 714–719. [Google Scholar] [CrossRef]
- Prakash, A.; Sharma, S.; Ahmad, N.; Ghosh, A.; Sinha, P. Synthesis of agnps by bacillus cereus bacteria and their antimicrobial potential. J. Biomater. Nanobiotechnol. 2011, 2, 155. [Google Scholar] [CrossRef]
- Sunkar, S.; Nachiyar, C.V. Biogenesis of antibacterial silver nanoparticles using the endophytic bacterium bacillus cereus isolated from garcinia xanthochymus. Asian Pac. J. Trop. Biomed. 2012, 2, 953–959. [Google Scholar] [CrossRef]
- Lin, Z.-Y.; Fu, J.-K.; Wu, J.-M.; Liu, Y.-Y.; Cheng, H. Preliminary study on the mechanism of non-enzymatic bioreduction of precious metal ions. Acta Phys. Chim. Sin. 2001, 17, 477–480. [Google Scholar] [CrossRef]
- Yong, P.; Rowson, N.A.; Farr, J.P.G.; Harris, I.R.; Macaskie, L.E. Bioreduction and biocrystallization of palladium by desulfovibrio desulfuricans ncimb 8307. Biotechnol. Bioeng. 2002, 80, 369–379. [Google Scholar] [CrossRef]
- Hulkoti, N.I.; Taranath, T. Biosynthesis of nanoparticles using microbes—A review. Colloids Surf. B Biointerfaces 2014, 121, 474–483. [Google Scholar] [CrossRef]
- Zhang, D.; Ma, X.-L.; Gu, Y.; Huang, H.; Zhang, G.-W. Green synthesis of metallic nanoparticles and their potential applications to treat cancer. Front. Chem. 2020, 8, 799. [Google Scholar] [CrossRef]
- Kulkarni, R.R.; Shaiwale, N.S.; Deobagkar, D.N.; Deobagkar, D.D. Synthesis and extracellular accumulation of silver nanoparticles by employing radiation-resistant deinococcus radiodurans, their characterization, and determination of bioactivity. Int. J. Nanomed. 2015, 10, 963. [Google Scholar]
- Yumei, L.; Yamei, L.; Qiang, L.; Jie, B. Rapid biosynthesis of silver nanoparticles based on flocculation and reduction of an exopolysaccharide from arthrobacter sp. B4: Its antimicrobial activity and phytotoxicity. J. Nanomater. 2017, 2017, 9703614. [Google Scholar] [CrossRef]
- Iravani, S.; Varma, R.S. Biofactories: Engineered nanoparticles via genetically engineered organisms. Green Chem. 2019, 21, 4583–4603. [Google Scholar] [CrossRef]
- Wing-ShanáLin, I. Biosynthesis of silver nanoparticles from silver (i) reduction by the periplasmic nitrate reductase c-type cytochrome subunit napc in a silver-resistant e. Ácoli. Chem. Sci. 2014, 5, 3144–3150. [Google Scholar]
- Ramanathan, R.; Field, M.R.; O’Mullane, A.P.; Smooker, P.M.; Bhargava, S.K.; Bansal, V. Aqueous phase synthesis of copper nanoparticles: A link between heavy metal resistance and nanoparticle synthesis ability in bacterial systems. Nanoscale 2013, 5, 2300–2306. [Google Scholar] [CrossRef]
- Yuan, Q.; Bomma, M.; Xiao, Z. Enhanced silver nanoparticle synthesis by escherichia coli transformed with candida albicans metallothionein gene. Materials 2019, 12, 4180. [Google Scholar] [CrossRef]
- Fu, J.-Z.; Liu, Y.-Y.; Gu, P.-Y.; Shang, D.-L.; Lin, Z.-Y.; Yao, B.-X.; Weng, S.-Z. Spectroscopic charcterization on the biosorption and bioreduction of ag(i) by lactobacillus sp.A09*. Acta Phys. -Chim. Sin. 2000, 16, 779–782. [Google Scholar] [CrossRef]
- Ahmad, A.; Mukherjee, P.; Senapati, S.; Mandal, D.; Khan, M.I.; Kumar, R.; Sastry, M. Extracellular biosynthesis of silver nanoparticles using the fungus fusarium oxysporum. Colloids Surf. B Biointerfaces 2003, 28, 313–318. [Google Scholar] [CrossRef]
- Lengke, M.F.; Fleet, M.E.; Southam, G. Biosynthesis of silver nanoparticles by filamentous cyanobacteria from a silver(i) nitrate complex. Langmuir 2007, 23, 2694–2699. [Google Scholar] [CrossRef]
- Whiteley, C.; Govender, Y.; Riddin, T.; Rai, M. Enzymatic Synthesis of Platinum Nanoparticles: Prokaryote and Eukaryote systems. In Metal Nanoparticles in Microbiology; Springer: Berlin/Heidelberg, Germany, 2011; pp. 103–134. [Google Scholar]
- Beveridge, T.; Murray, R. Sites of metal deposition in the cell wall of bacillus subtilis. J. Bacteriol. 1980, 141, 876–887. [Google Scholar] [CrossRef]
- Konishi, Y.; Ohno, K.; Saitoh, N.; Nomura, T.; Nagamine, S. Microbial synthesis of gold nanoparticles by metal reducing bacterium. Trans. Mater. Res. Soc. Jpn. 2004, 29, 2341–2343. [Google Scholar]
- Husseiny, M.; Abd El-Aziz, M.; Badr, Y.; Mahmoud, M. Biosynthesis of gold nanoparticles using pseudomonas aeruginosa. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2007, 67, 1003–1006. [Google Scholar] [CrossRef] [PubMed]
- Sneha, K.; Sathishkumar, M.; Mao, J.; Kwak, I.; Yun, Y.-S. Corynebacterium glutamicum-mediated crystallization of silver ions through sorption and reduction processes. Chem. Eng. J. 2010, 162, 989–996. [Google Scholar] [CrossRef]
- Liu, C.-J.; Wang, C.-H.; Chen, S.-T.; Chen, H.-H.; Leng, W.-H.; Chien, C.-C.; Wang, C.-L.; Kempson, I.M.; Hwu, Y.; Lai, T.-C. Enhancement of cell radiation sensitivity by pegylated gold nanoparticles. Phys. Med. Biol. 2010, 55, 931. [Google Scholar] [CrossRef] [PubMed]
- Correa-Llantén, D.N.; Muñoz-Ibacache, S.A.; Castro, M.E.; Muñoz, P.A.; Blamey, J.M. Gold nanoparticles synthesized by geobacillus sp. Strain id17 a thermophilic bacterium isolated from deception island, antarctica. Microb. Cell Fact. 2013, 12, 75. [Google Scholar] [CrossRef]
- Srinath, B.; Namratha, K.; Byrappa, K. Eco-friendly synthesis of gold nanoparticles by bacillus subtilis and their environmental applications. Adv. Sci. Lett. 2018, 24, 5942–5946. [Google Scholar] [CrossRef]
- Lengke, M.F.; Fleet, M.E.; Southam, G. Morphology of gold nanoparticles synthesized by filamentous cyanobacteria from gold(i)−thiosulfate and gold(iii)−chloride complexes. Langmuir 2006, 22, 2780–2787. [Google Scholar] [CrossRef] [PubMed]
- Brayner, R.; Barberousse, H.; Hemadi, M.; Djedjat, C.; Yéprémian, C.; Coradin, T.; Livage, J.; Fiévet, F.; Couté, A. Cyanobacteria as bioreactors for the synthesis of au, ag, pd, and pt nanoparticles via an enzyme-mediated route. J. Nanosci. Nanotechnol. 2007, 7, 2696–2708. [Google Scholar] [CrossRef]
- Govindaraju, K.; Basha, S.K.; Kumar, V.G.; Singaravelu, G. Silver, gold and bimetallic nanoparticles production using single-cell protein (spirulina platensis) geitler. J. Mater. Sci. 2008, 43, 5115–5122. [Google Scholar] [CrossRef]
- Attaran, N.; Eshghi, H.; Rahimizadeh, M.; Mashreghi, M.; Bakavoli, M. Genetically modified luminescent bacteria ralostonia solanacerum, pseudomonas syringae, pseudomonas savastanoi, and wild type bacterium vibrio fischeri in biosynthesis of gold nanoparticles from gold chloride trihydrate. Artif. Cells Nanomed. Biotechnol. 2016, 44, 263–269. [Google Scholar] [CrossRef]
- Brock, T.D.; Gustafson, J. Ferric iron reduction by sulfur- and iron-oxidizing bacteria. Appl. Environ. Microbiol. 1976, 32, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.E. Bacterial tellurite resistance. Trends Microbiol. 1999, 7, 111–115. [Google Scholar] [CrossRef]
- Lloyd, J.R.; Ridley, J.; Khizniak, T.; Lyalikova, N.N.; Macaskie, L.E. Reduction of technetium by desulfovibrio desulfuricans: Biocatalyst characterization and use in a flowthrough bioreactor. Appl. Environ. Microbiol. 1999, 65, 2691–2696. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R.; Stolz, J.F.; Nord, G.L.; Phillips, E.J. Anaerobic production of magnetite by a dissimilatory iron-reducing microorganism. Nature 1987, 330, 252–254. [Google Scholar] [CrossRef]
- Mullen, M.; Wolf, D.; Ferris, F.; Beveridge, T.; Flemming, C.; Bailey, G. Bacterial sorption of heavy metals. Appl. Environ. Microbiol. 1989, 55, 3143–3149. [Google Scholar] [CrossRef]
- Watson, J.; Ellwood, D.; Soper, A.; Charnock, J. Nanosized strongly-magnetic bacterially-produced iron sulfide materials. J. Magn. Magn. Mater. 1999, 203, 69–72. [Google Scholar] [CrossRef]
- Bharde, A.; Wani, A.; Shouche, Y.; Joy, P.A.; Prasad, B.L.V.; Sastry, M. Bacterial aerobic synthesis of nanocrystalline magnetite. J. Am. Chem. Soc. 2005, 127, 9326–9327. [Google Scholar] [CrossRef]
- Prasad, K.; Jha, A.K.; Kulkarni, A. Lactobacillus assisted synthesis of titanium nanoparticles. Nanoscale Res. Lett. 2007, 2, 248–250. [Google Scholar] [CrossRef]
- Cunningham, D.P.; Lundie, L. Precipitation of cadmium by clostridium thermoaceticum. Appl. Environ. Microbiol. 1993, 59, 7–14. [Google Scholar] [CrossRef]
- Sweeney, R.Y.; Mao, C.; Gao, X.; Burt, J.L.; Belcher, A.M.; Georgiou, G.; Iverson, B.L. Bacterial biosynthesis of cadmium sulfide nanocrystals. Chem. Biol. 2004, 11, 1553–1559. [Google Scholar] [CrossRef]
- Varshney, R.; Bhadauria, S.; Gaur, M.; Pasricha, R. Copper nanoparticles synthesis from electroplating industry effluent. Nano Biomed. Eng. 2011, 3, 115–119. [Google Scholar] [CrossRef]
- Visha, P.; Nanjappan, K.; Selvaraj, P.; Jayachandran, S.; Elango, A.; Kumaresan, G. Biosynthesis and structural characteristics of selenium nanoparticles using lactobacillus acidophilus bacteria by wet sterilization process. Int. J. Adv. Vet. Sci. Technol. 2015, 4, 178–183. [Google Scholar]
- Yan, L.; Da, H.; Zhang, S.; López, V.M.; Wang, W. Bacterial magnetosome and its potential application. Microbiol. Res. 2017, 203, 19–28. [Google Scholar] [CrossRef]
- Vargas, G.; Cypriano, J.; Correa, T.; Leão, P.; Bazylinski, D.A.; Abreu, F. Applications of magnetotactic bacteria, magnetosomes and magnetosome crystals in biotechnology and nanotechnology: Mini-review. Molecules 2018, 23, 2438. [Google Scholar] [CrossRef]
- Alphandery, E.; Faure, S.; Seksek, O.; Guyot, F.; Chebbi, I. Chains of magnetosomes extracted from amb-1 magnetotactic bacteria for application in alternative magnetic field cancer therapy. ACS Nano 2011, 5, 6279–6296. [Google Scholar] [CrossRef]
- Roda, A.; Cevenini, L.; Borg, S.; Michelini, E.; Calabretta, M.M.; Schüler, D. Bioengineered bioluminescent magnetotactic bacteria as a powerful tool for chip-based whole-cell biosensors. Lab Chip. 2013, 13, 4881–4889. [Google Scholar] [CrossRef]
- Boucher, M.; Geffroy, F.; Prévéral, S.; Bellanger, L.; Selingue, E.; Adryanczyk-Perrier, G.; Pean, M.; Lefèvre, C.; Pignol, D.; Ginet, N. Genetically tailored magnetosomes used as mri probe for molecular imaging of brain tumor. Biomaterials 2017, 121, 167–178. [Google Scholar] [CrossRef]
- Zonaro, E.; Piacenza, E.; Presentato, A.; Monti, F.; Dell’Anna, R.; Lampis, S.; Vallini, G. Ochrobactrum sp. Mpv1 from a dump of roasted pyrites can be exploited as bacterial catalyst for the biogenesis of selenium and tellurium nanoparticles. Microb. Cell Fact. 2017, 16, 215. [Google Scholar] [CrossRef]
- Ahmed, E.; Kalathil, S.; Shi, L.; Alharbi, O.; Wang, P. Synthesis of ultra-small platinum, palladium and gold nanoparticles by shewanella loihica pv-4 electrochemically active biofilms and their enhanced catalytic activities. J. Saudi Chem. Soc. 2018, 22, 919–929. [Google Scholar] [CrossRef]
- Chen, Y.L.; Tuan, H.Y.; Tien, C.W.; Lo, W.H.; Liang, H.C.; Hu, Y.C. Augmented biosynthesis of cadmium sulfide nanoparticles by genetically engineered escherichia coli. Biotechnol. Prog. 2009, 25, 1260–1266. [Google Scholar] [CrossRef]
- Jung, J.H.; Park, T.J.; Lee, S.Y.; Seo, T.S. Homogeneous biogenic paramagnetic nanoparticle synthesis based on a microfluidic droplet generator. Angew. Chem. Int. Ed. 2012, 51, 5634–5637. [Google Scholar] [CrossRef]
- Kolinko, I.; Lohße, A.; Borg, S.; Raschdorf, O.; Jogler, C.; Tu, Q.; Pósfai, M.; Tompa, E.; Plitzko, J.M.; Brachmann, A. Biosynthesis of magnetic nanostructures in a foreign organism by transfer of bacterial magnetosome gene clusters. Nat. Nanotechnol. 2014, 9, 193–197. [Google Scholar] [CrossRef]
- Choi, Y.; Park, T.J.; Lee, D.C.; Lee, S.Y. Recombinant escherichia coli as a biofactory for various single-and multi-element nanomaterials. Proc. Natl. Acad. Sci. USA 2018, 115, 5944–5949. [Google Scholar] [CrossRef]
- Dhoondia, Z.H.; Chakraborty, H. Lactobacillus mediated synthesis of silver oxide nanoparticles. Nanomater. Nanotechnol. 2012, 2, 15. [Google Scholar] [CrossRef]
- Li, R.; Chen, Z.; Ren, N.; Wang, Y.; Wang, Y.; Yu, F. Biosynthesis of silver oxide nanoparticles and their photocatalytic and antimicrobial activity evaluation for wound healing applications in nursing care. J. Photochem. Photobiol. B Biol. 2019, 199, 111593. [Google Scholar] [CrossRef]
- Vithiya, K.; Kumar, R.; Sen, S. Antimicrobial activity of biosynthesized silver oxide nanoparticles. J. Pure Appl. Microbiol 2014, 4, 3263–3268. [Google Scholar]
- Murthy, S.K. Nanoparticles in modern medicine: State of the art and future challenges. Int. J. Nanomed. 2007, 2, 129. [Google Scholar]
- Hassan, S.E.-D.; Fouda, A.; Radwan, A.A.; Salem, S.S.; Barghoth, M.G.; Awad, M.A.; Abdo, A.M.; El-Gamal, M.S. Endophytic actinomycetes streptomyces spp mediated biosynthesis of copper oxide nanoparticles as a promising tool for biotechnological applications. JBIC J. Biol. Inorg. Chem. 2019, 24, 377–393. [Google Scholar] [CrossRef]
- Bukhari, S.I.; Hamed, M.M.; Al-Agamy, M.H.; Gazwi, H.S.; Radwan, H.H.; Youssif, A.M. Biosynthesis of copper oxide nanoparticles using streptomyces mhm38 and its biological applications. J. Nanomater. 2021, 2021, 6693302. [Google Scholar] [CrossRef]
- Yusof, H.M.; Mohamad, R.; Zaidan, U.H.; Samsudin, A.A. Biosynthesis of zinc oxide nanoparticles by cell-biomass and supernatant of lactobacillus plantarum ta4 and its antibacterial and biocompatibility properties. Sci. Rep. 2020, 10, 19996. [Google Scholar] [CrossRef] [PubMed]
- Ogunyemi, S.O.; Zhang, M.; Abdallah, Y.; Ahmed, T.; Qiu, W.; Ali, M.; Yan, C.; Yang, Y.; Chen, J.; Li, B. The bio-synthesis of three metal oxide nanoparticles (zno, mno2, and mgo) and their antibacterial activity against the bacterial leaf blight pathogen. Front. Microbiol. 2020, 11, 3099. [Google Scholar] [CrossRef] [PubMed]
- Irshad, M.A.; Nawaz, R.; ur Rehman, M.Z.; Adrees, M.; Rizwan, M.; Ali, S.; Ahmad, S.; Tasleem, S. Synthesis, characterization and advanced sustainable applications of titanium dioxide nanoparticles: A review. Ecotoxicol. Environ. Saf. 2021, 212, 111978. [Google Scholar] [CrossRef] [PubMed]
- Pant, B.; Park, M.; Park, S.-J. Tio2 nps assembled into a carbon nanofiber composite electrode by a one-step electrospinning process for supercapacitor applications. Polymers 2019, 11, 899. [Google Scholar] [CrossRef] [PubMed]
- Irshad, M.A.; Nawaz, R.; ur Rehman, M.Z.; Imran, M.; Ahmad, J.; Ahmad, S.; Inam, A.; Razzaq, A.; Rizwan, M.; Ali, S. Synthesis and characterization of titanium dioxide nanoparticles by chemical and green methods and their antifungal activities against wheat rust. Chemosphere 2020, 258, 127352. [Google Scholar] [CrossRef]
- Khan, R.; Fulekar, M. Biosynthesis of titanium dioxide nanoparticles using bacillus amyloliquefaciens culture and enhancement of its photocatalytic activity for the degradation of a sulfonated textile dye reactive red 31. J. Colloid Interface Sci. 2016, 475, 184–191. [Google Scholar] [CrossRef]
- Fulekar, J.; Dutta, D.P.; Pathak, B.; Fulekar, M.H. Novel microbial and root mediated green synthesis of tio2 nanoparticles and its application in wastewater remediation. J. Chem. Technol. Biotechnol. 2018, 93, 736–743. [Google Scholar] [CrossRef]
- Taran, M.; Rad, M.; Alavi, M. Biosynthesis of tio2 and zno nanoparticles by halomonas elongata ibrc-m 10214 in different conditions of medium. BioImpacts 2018, 8, 81. [Google Scholar] [CrossRef]
- Ağçeli, G.K.; Hammachi, H.; Kodal, S.P.; Cihangir, N.; Aksu, Z. A novel approach to synthesize tio 2 nanoparticles: Biosynthesis by using streptomyces sp. Hc1. J. Inorg. Organomet. Polym. Mater. 2020, 30, 3221–3229. [Google Scholar] [CrossRef]
- Sundaram, P.A.; Augustine, R.; Kannan, M. Extracellular biosynthesis of iron oxide nanoparticles by bacillus subtilis strains isolated from rhizosphere soil. Biotechnol. Bioprocess Eng. 2012, 17, 835–840. [Google Scholar] [CrossRef]
- Majeed, S.; Danish, M.; Ibrahim, M.N.M.; Sekeri, S.H.; Ansari, M.T.; Nanda, A.; Ahmad, G. Bacteria Mediated Synthesis of Iron Oxide Nanoparticles and Their Antibacterial, Antioxidant, Cytocompatibility Properties. J. Clust. Sci. 2020, 1–12. [Google Scholar] [CrossRef]
- Gahlawat, G.; Shikha, S.; Chaddha, B.S.; Chaudhuri, S.R.; Mayilraj, S.; Choudhury, A.R. Microbial glycolipoprotein-capped silver nanoparticles as emerging antibacterial agents against cholera. Microb. Cell Fact. 2016, 15, 25. [Google Scholar] [CrossRef]
- Mehta, A.; Sidhu, C.; Pinnaka, A.K.; Choudhury, A.R. Extracellular polysaccharide production by a novel osmotolerant marine strain of alteromonas macleodii and its application towards biomineralization of silver. PLoS ONE 2014, 9, e98798. [Google Scholar] [CrossRef]
- Raj, R.; Dalei, K.; Chakraborty, J.; Das, S. Extracellular polymeric substances of a marine bacterium mediated synthesis of cds nanoparticles for removal of cadmium from aqueous solution. J. Colloid Interface Sci. 2016, 462, 166–175. [Google Scholar] [CrossRef]
- Saxena, I.M.; Dandekar, T.; Brown, R. Mechanisms in cellulose biosynthesis. Mol. Biol. 2004. Available online: http://www.esf.edu/outreach/pd/2000/cellulose/saxena.pdf (accessed on 13 June 2021).
- Pourreza, N.; Golmohammadi, H.; Naghdi, T.; Yousefi, H. Green in-situ synthesized silver nanoparticles embedded in bacterial cellulose nanopaper as a bionanocomposite plasmonic sensor. Biosens. Bioelectron. 2015, 74, 353–359. [Google Scholar] [CrossRef]
- Morales-Narváez, E.; Golmohammadi, H.; Naghdi, T.; Yousefi, H.; Kostiv, U.; Horak, D.; Pourreza, N.; Merkoçi, A. Nanopaper as an optical sensing platform. ACS Nano 2015, 9, 7296–7305. [Google Scholar] [CrossRef]
- Ortega, F.G.; Fernández-Baldo, M.A.; Fernández, J.G.; Serrano, M.J.; Sanz, M.I.; Díaz-Mochón, J.J.; Lorente, J.A.; Raba, J. Study of antitumor activity in breast cell lines using silver nanoparticles produced by yeast. Int. J. Nanomed. 2015, 10, 2021. [Google Scholar]
- Karthik, L.; Kumar, G.; Kirthi, A.V.; Rahuman, A.A.; Bhaskara Rao, K.V. Streptomyces sp. Lk3 mediated synthesis of silver nanoparticles and its biomedical application. Bioprocess Biosyst. Eng. 2014, 37, 261–267. [Google Scholar] [CrossRef]
- Abd-Elnaby, H.M.; Abo-Elala, G.M.; Abdel-Raouf, U.M.; Hamed, M.M. Antibacterial and anticancer activity of extracellular synthesized silver nanoparticles from marine streptomyces rochei mhm13. Egypt. J. Aquat. Res. 2016, 42, 301–312. [Google Scholar] [CrossRef]
- Wypij, M.; Czarnecka, J.; Świecimska, M.; Dahm, H.; Rai, M.; Golinska, P. Synthesis, characterization and evaluation of antimicrobial and cytotoxic activities of biogenic silver nanoparticles synthesized from streptomyces xinghaiensis of1 strain. World J. Microbiol. Biotechnol. 2018, 34, 23. [Google Scholar] [CrossRef]
- Bakhtiari-Sardari, A.; Mashreghi, M.; Eshghi, H.; Behnam-Rasouli, F.; Lashani, E.; Shahnavaz, B. Comparative evaluation of silver nanoparticles biosynthesis by two cold-tolerant streptomyces strains and their biological activities. Biotechnol. Lett. 2020, 42, 1985–1999. [Google Scholar] [CrossRef]
- Ahmad, A.; Senapati, S.; Khan, M.I.; Kumar, R.; Sastry, M. Extracellular biosynthesis of monodisperse gold nanoparticles by a novel extremophilic actinomycete, thermomonospora sp. Langmuir 2003, 19, 3550–3553. [Google Scholar] [CrossRef]
- Ahmad, A.; Senapati, S.; Khan, M.I.; Kumar, R.; Ramani, R.; Srinivas, V.; Sastry, M. Intracellular synthesis of gold nanoparticles by a novel alkalotolerant actinomycete, rhodococcus species. Nanotechnology 2003, 14, 824. [Google Scholar] [CrossRef]
- He, S.; Guo, Z.; Zhang, Y.; Zhang, S.; Wang, J.; Gu, N. Biosynthesis of gold nanoparticles using the bacteria rhodopseudomonas capsulata. Mater. Lett. 2007, 61, 3984–3987. [Google Scholar] [CrossRef]
- Ranjitha, V.R.; Rai, V.R. Actinomycetes mediated synthesis of gold nanoparticles from the culture supernatant of streptomyces griseoruber with special reference to catalytic activity. 3 Biotech 2017, 7, 299. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.E.L.D.; Salem, S.S.; Fouda, A.; Awad, M.A.; El-Gamal, M.S.; Abdo, A.M. New approach for antimicrobial activity and bio-control of various pathogens by biosynthesized copper nanoparticles using endophytic actinomycetes. J. Radiat. Res. Appl. Sci. 2018, 11, 262–270. [Google Scholar] [CrossRef]
- Mishra, A.; Kumari, M.; Pandey, S.; Chaudhry, V.; Gupta, K.; Nautiyal, C. Biocatalytic and antimicrobial activities of gold nanoparticles synthesized by trichoderma sp. Bioresour. Technol. 2014, 166, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Metuku, R.P.; Pabba, S.; Burra, S.; Gudikandula, K.; Charya, M.S. Biosynthesis of silver nanoparticles from schizophyllum radiatum he 863742.1: Their characterization and antimicrobial activity. 3 Biotech 2014, 4, 227–234. [Google Scholar] [CrossRef]
- Bhargava, A.; Jain, N.; Khan, M.A.; Pareek, V.; Dilip, R.V.; Panwar, J. Utilizing metal tolerance potential of soil fungus for efficient synthesis of gold nanoparticles with superior catalytic activity for degradation of rhodamine b. J. Environ. Manag. 2016, 183, 22–32. [Google Scholar] [CrossRef]
- Rajput, S.; Werezuk, R.; Lange, R.M.; McDermott, M.T. Fungal isolate optimized for biogenesis of silver nanoparticles with enhanced colloidal stability. Langmuir 2016, 32, 8688–8697. [Google Scholar] [CrossRef]
- Gericke, M.; Pinches, A. Microbial production of gold nanoparticles. Gold Bull. 2006, 39, 22–28. [Google Scholar] [CrossRef]
- Birla, S.S.; Gaikwad, S.C.; Gade, A.K.; Rai, M.K. Rapid synthesis of silver nanoparticles from fusarium oxysporum by optimizing physicocultural conditions. Sci. World J. 2013, 2013, 796018. [Google Scholar] [CrossRef] [PubMed]
- Kitching, M.; Choudhary, P.; Inguva, S.; Guo, Y.; Ramani, M.; Das, S.K.; Marsili, E. Fungal surface protein mediated one-pot synthesis of stable and hemocompatible gold nanoparticles. Enzym. Microb. Technol. 2016, 95, 76–84. [Google Scholar] [CrossRef] [PubMed]
- El Domany, E.B.; Essam, T.M.; Ahmed, A.E.; Farghali, A.A. Biosynthesis physico-chemical optimization of gold nanoparticles as anti-cancer and synergetic antimicrobial activity using pleurotus ostreatus fungus. J. Appl. Pharm. Sci. 2018, 8, 119–128. [Google Scholar]
- Neethu, S.; Midhun, S.J.; Sunil, M.; Soumya, S.; Radhakrishnan, E.; Jyothis, M. Efficient visible light induced synthesis of silver nanoparticles by penicillium polonicum ara 10 isolated from chetomorpha antennina and its antibacterial efficacy against salmonella enterica serovar typhimurium. J. Photochem. Photobiol. B Biol. 2018, 180, 175–185. [Google Scholar] [CrossRef]
- Clarance, P.; Luvankar, B.; Sales, J.; Khusro, A.; Agastian, P.; Tack, J.C.; Al Khulaifi, M.M.; Al-Shwaiman, H.A.; Elgorban, A.M.; Syed, A.; et al. Green synthesis and characterization of gold nanoparticles using endophytic fungi fusarium solani and its in-vitro anticancer and biomedical applications. Saudi J. Biol. Sci. 2020, 27, 706–712. [Google Scholar] [CrossRef]
- Munawer, U.; Raghavendra, V.B.; Ningaraju, S.; Krishna, K.L.; Ghosh, A.R.; Melappa, G.; Pugazhendhi, A. Biofabrication of gold nanoparticles mediated by the endophytic cladosporium species: Photodegradation, in vitro anticancer activity and in vivo antitumor studies. Int. J. Pharm. 2020, 588, 119729. [Google Scholar] [CrossRef]
- Ramos, M.M.; Morais, E.d.S.; Sena, I.d.S.; Lima, A.L.; de Oliveira, F.R.; de Freitas, C.M.; Fernandes, C.P.; de Carvalho, J.C.T.; Ferreira, I.M. Silver nanoparticle from whole cells of the fungi trichoderma spp. Isolated from brazilian amazon. Biotechnol. Lett. 2020, 42, 833–843. [Google Scholar] [CrossRef]
- Apte, M.; Sambre, D.; Gaikawad, S.; Joshi, S.; Bankar, A.; Kumar, A.R.; Zinjarde, S. Psychrotrophic yeast yarrowia lipolytica ncyc 789 mediates the synthesis of antimicrobial silver nanoparticles via cell-associated melanin. AMB Express 2013, 3, 32. [Google Scholar] [CrossRef]
- Zhang, X.; Qu, Y.; Shen, W.; Wang, J.; Li, H.; Zhang, Z.; Li, S.; Zhou, J. Biogenic synthesis of gold nanoparticles by yeast magnusiomyces ingens lh-f1 for catalytic reduction of nitrophenols. Colloids Surf. A Physicochem. Eng. Asp. 2016, 497, 280–285. [Google Scholar] [CrossRef]
- Eugenio, M.; Müller, N.; Frasés, S.; Almeida-Paes, R.; Lima, L.M.T.; Lemgruber, L.; Farina, M.; de Souza, W.; Sant’Anna, C. Yeast-derived biosynthesis of silver/silver chloride nanoparticles and their antiproliferative activity against bacteria. RSC Adv. 2016, 6, 9893–9904. [Google Scholar] [CrossRef]
- Elahian, F.; Reiisi, S.; Shahidi, A.; Mirzaei, S.A. High-throughput bioaccumulation, biotransformation, and production of silver and selenium nanoparticles using genetically engineered pichia pastoris. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 853–861. [Google Scholar] [CrossRef]
- Sriramulu, M.; Sumathi, S. Biosynthesis of palladium nanoparticles using saccharomyces cerevisiae extract and its photocatalytic degradation behaviour. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 025018. [Google Scholar] [CrossRef]
- Ansari, M.A.; Khan, H.M.; Khan, A.A.; Pal, R.; Cameotra, S.S. Antibacterial potential of al 2 o 3 nanoparticles against multidrug resistance strains of staphylococcus aureus isolated from skin exudates. J. Nanopart. Res. 2013, 15, 1970. [Google Scholar] [CrossRef]
- Suryavanshi, P.; Pandit, R.; Gade, A.; Derita, M.; Zachino, S.; Rai, M. Colletotrichum sp.-mediated synthesis of sulphur and aluminium oxide nanoparticles and its in vitro activity against selected food-borne pathogens. Lebenson Wiss. Technol. 2017, 81, 188–194. [Google Scholar] [CrossRef]
- Fouda, A.; Saad, E.; Salem, S.S.; Shaheen, T.I. In-vitro cytotoxicity, antibacterial, and uv protection properties of the biosynthesized zinc oxide nanoparticles for medical textile applications. Microb. Pathog. 2018, 125, 252–261. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Fouda, A.; Abdel-Rahman, M.A.; Hassan, S.E.-D.; El-Gamal, M.S.; Salem, S.S.; Shaheen, T.I. Fungal strain impacts the shape, bioactivity and multifunctional properties of green synthesized zinc oxide nanoparticles. Biocatal. Agric. Biotechnol. 2019, 19, 101103. [Google Scholar] [CrossRef]
- Sumanth, B.; Lakshmeesha, T.R.; Ansari, M.A.; Alzohairy, M.A.; Udayashankar, A.C.; Shobha, B.; Niranjana, S.R.; Srinivas, C.; Almatroudi, A. Mycogenic synthesis of extracellular zinc oxide nanoparticles from xylaria acuta and its nanoantibiotic potential. Int. J. Nanomed. 2020, 15, 8519. [Google Scholar] [CrossRef]
- Ashajyothi, C.; Harish, K.H.; Dubey, N.; Chandrakanth, R.K. Antibiofilm activity of biogenic copper and zinc oxide nanoparticles-antimicrobials collegiate against multiple drug resistant bacteria: A nanoscale approach. J. Nanostruct. Chem. 2016, 6, 329–341. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Abu-Elghait, M.; Ahmed, N.E.; Salem, S.S. Eco-friendly mycogenic synthesis of zno and cuo nanoparticles for in vitro antibacterial, antibiofilm, and antifungal applications. Biol. Trace Elem. Res. 2020, 1–12. [Google Scholar] [CrossRef]
- Badawy, A.A.; Abdelfattah, N.A.; Salem, S.S.; Awad, M.F.; Fouda, A. Efficacy assessment of biosynthesized copper oxide nanoparticles (cuo-nps) on stored grain insects and their impacts on morphological and physiological traits of wheat (triticum aestivum l.) plant. Biology 2021, 10, 233. [Google Scholar] [CrossRef]
- Fouda, A.; Salem, S.S.; Wassel, A.R.; Hamza, M.F.; Shaheen, T.I. Optimization of green biosynthesized visible light active cuo/zno nano-photocatalysts for the degradation of organic methylene blue dye. Heliyon 2020, 6, e04896. [Google Scholar] [CrossRef]
- Peiris, M.; Guansekera, T.; Jayaweera, P.; Fernando, S. Tio 2 nanoparticles from baker’s yeast: A potent antimicrobial. J. Microbiol. Biotechnol. 2018, 28, 1664–1670. [Google Scholar] [CrossRef]
- Chinnaperumal, K.; Govindasamy, B.; Paramasivam, D.; Dilipkumar, A.; Dhayalan, A.; Vadivel, A.; Sengodan, K.; Pachiappan, P. Bio-pesticidal effects of trichoderma viride formulated titanium dioxide nanoparticle and their physiological and biochemical changes on helicoverpa armigera (hub.). Pestic Biochem. Physiol. 2018, 149, 26–36. [Google Scholar] [CrossRef]
- Manimaran, K.; Murugesan, S.; Ragavendran, C.; Balasubramani, G.; Natarajan, D.; Ganesan, A.; Seedevi, P. Biosynthesis of tio 2 nanoparticles using edible mushroom (pleurotus djamor) extract: Mosquito larvicidal, histopathological, antibacterial and anticancer effect. J. Clust. Sci. 2020, 1–12. [Google Scholar] [CrossRef]
- Ali, A.; Hira Zafar, M.Z.; ul Haq, I.; Phull, A.R.; Ali, J.S.; Hussain, A. Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. Nanotechnol. Sci. Appl. 2016, 9, 49. [Google Scholar] [CrossRef]
- Bhargava, A.; Jain, N.; Barathi, M.; Akhtar, M.S.; Yun, Y.-S.; Panwar, J. Synthesis, Characterization and Mechanistic Insights of Mycogenic Iron Oxide Nanoparticles. J. Nanopart. Res. 2013, 15, 2031. [Google Scholar] [CrossRef]
- Mahanty, S.; Bakshi, M.; Ghosh, S.; Chatterjee, S.; Bhattacharyya, S.; Das, P.; Das, S.; Chaudhuri, P. Green synthesis of iron oxide nanoparticles mediated by filamentous fungi isolated from sundarban mangrove ecosystem, india. Bionanoscience 2019, 9, 637–651. [Google Scholar] [CrossRef]
- Mahanty, S.; Bakshi, M.; Ghosh, S.; Gaine, T.; Chatterjee, S.; Bhattacharyya, S.; Das, S.; Das, P.; Chaudhuri, P. Mycosynthesis of iron oxide nanoparticles using manglicolous fungi isolated from indian sundarbans and its application for the treatment of chromium containing solution: Synthesis, adsorption isotherm, kinetics and thermodynamics study. Environ. Nanotechnol. Monit. Manag. 2019, 12, 100276. [Google Scholar] [CrossRef]
- Vijayanandan, A.S.; Balakrishnan, R.M. Biosynthesis of cobalt oxide nanoparticles using endophytic fungus aspergillus nidulans. J. Environ. Manag. 2018, 218, 442–450. [Google Scholar] [CrossRef]
- Fawcett, D.; Verduin, J.J.; Shah, M.; Sharma, S.B.; Poinern, G.E.J. A review of current research into the biogenic synthesis of metal and metal oxide nanoparticles via marine algae and seagrasses. J. Nanosci. 2017, 2017, 8013850. [Google Scholar] [CrossRef]
- Dhas, T.S.; Kumar, V.G.; Karthick, V.; Angel, K.J.; Govindaraju, K. Facile synthesis of silver chloride nanoparticles using marine alga and its antibacterial efficacy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 120, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Edison, T.N.J.I.; Atchudan, R.; Kamal, C.; Lee, Y.R. Caulerpa racemosa: A marine green alga for eco-friendly synthesis of silver nanoparticles and its catalytic degradation of methylene blue. Bioprocess Biosyst. Eng. 2016, 39, 1401–1408. [Google Scholar] [CrossRef] [PubMed]
- da Silva Ferreira, V.; ConzFerreira, M.E.; Lima, L.M.T.R.; Frasés, S.; de Souza, W.; Sant’Anna, C. Green production of microalgae-based silver chloride nanoparticles with antimicrobial activity against pathogenic bacteria. Enzym. Microb. Technol. 2017, 97, 114–121. [Google Scholar] [CrossRef]
- Kim, D.-Y.; Saratale, R.G.; Shinde, S.; Syed, A.; Ameen, F.; Ghodake, G. Green synthesis of silver nanoparticles using laminaria japonica extract: Characterization and seedling growth assessment. J. Clean. Prod. 2018, 172, 2910–2918. [Google Scholar] [CrossRef]
- Fatima, R.; Priya, M.; Indurthi, L.; Radhakrishnan, V.; Sudhakaran, R. Biosynthesis of silver nanoparticles using red algae portieria hornemannii and its antibacterial activity against fish pathogens. Microb. Pathog. 2020, 138, 103780. [Google Scholar] [CrossRef]
- Bhuyar, P.; Rahim, M.H.A.; Sundararaju, S.; Ramaraj, R.; Maniam, G.P.; Govindan, N. Synthesis of silver nanoparticles using marine macroalgae padina sp. And its antibacterial activity towards pathogenic bacteria. Beni-Suef Univ. J. Basic Appl. Sci. 2020, 9, 3. [Google Scholar] [CrossRef]
- Senapati, S.; Syed, A.; Moeez, S.; Kumar, A.; Ahmad, A. Intracellular synthesis of gold nanoparticles using alga tetraselmis kochinensis. Mater. Lett. 2012, 79, 116–118. [Google Scholar] [CrossRef]
- Singh, M.; Kalaivani, R.; Manikandan, S.; Sangeetha, N.; Kumaraguru, A.K. Facile green synthesis of variable metallic gold nanoparticle using padina gymnospora, a brown marine macroalga. Appl. Nanosci. 2013, 3, 145–151. [Google Scholar] [CrossRef]
- Dahoumane, S.A.; Yéprémian, C.; Djédiat, C.; Couté, A.; Fiévet, F.; Coradin, T.; Brayner, R. Improvement of kinetics, yield, and colloidal stability of biogenic gold nanoparticles using living cells of euglena gracilis microalga. J. Nanopart. Res. 2016, 18, 79. [Google Scholar] [CrossRef]
- Ramakrishna, M.; Rajesh Babu, D.; Gengan, R.M.; Chandra, S.; Nageswara Rao, G. Green synthesis of gold nanoparticles using marine algae and evaluation of their catalytic activity. J. Nanostruct. Chem. 2016, 6, 1–13. [Google Scholar] [CrossRef]
- González-Ballesteros, N.; Prado-López, S.; Rodríguez-González, J.B.; Lastra, M.; Rodríguez-Argüelles, M.C. Green synthesis of gold nanoparticles using brown algae cystoseira baccata: Its activity in colon cancer cells. Colloids Surf. B Biointerfaces 2017, 153, 190–198. [Google Scholar] [CrossRef]
- Momeni, S.; Nabipour, I. A simple green synthesis of palladium nanoparticles with sargassum alga and their electrocatalytic activities towards hydrogen peroxide. Appl. Biochem. Biotechnol. 2015, 176, 1937–1949. [Google Scholar] [CrossRef]
- Arsiya, F.; Sayadi, M.H.; Sobhani, S. Green synthesis of palladium nanoparticles using chlorella vulgaris. Mater. Lett. 2017, 186, 113–115. [Google Scholar] [CrossRef]
- Sayadi, M.H.; Salmani, N.; Heidari, A.; Rezaei, M.R. Bio-synthesis of palladium nanoparticle using spirulina platensis alga extract and its application as adsorbent. Surf. Interfaces 2018, 10, 136–143. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Kannan, C.; Annadurai, G. Green synthesis of silver nanoparticles using marine brown algae turbinaria conoides and its antibacterial activity. Int. J. Pharma Bio Sci. 2012, 3, 502–510. [Google Scholar]
- Khalafi, T.; Buazar, F.; Ghanemi, K. Phycosynthesis and enhanced photocatalytic activity of zinc oxide nanoparticles toward organosulfur pollutants. Sci. Rep. 2019, 9, 6866. [Google Scholar] [CrossRef]
- Taghizadeh, S.-M.; Lal, N.; Ebrahiminezhad, A.; Moeini, F.; Seifan, M.; Ghasemi, Y.; Berenjian, A. Green and economic fabrication of zinc oxide (zno) nanorods as a broadband uv blocker and antimicrobial agent. Nanomaterials 2020, 10, 530. [Google Scholar] [CrossRef]
- El-Belely, E.F.; Farag, M.; Said, H.A.; Amin, A.S.; Azab, E.; Gobouri, A.A.; Fouda, A. Green synthesis of zinc oxide nanoparticles (zno-nps) using arthrospira platensis (class: Cyanophyceae) and evaluation of their biomedical activities. Nanomaterials 2021, 11, 95. [Google Scholar] [CrossRef]
- Sharma, M.; Behl, K.; Nigam, S.; Joshi, M. TiO2-go nanocomposite for photocatalysis and environmental applications: A green synthesis approach. Vacuum 2018, 156, 434–439. [Google Scholar] [CrossRef]
- El-Kassas, H.Y.; Aly-Eldeen, M.A.; Gharib, S.M. Green synthesis of iron oxide (Fe3O4) nanoparticles using two selected brown seaweeds: Characterization and application for lead bioremediation. Acta Oceanol. Sin. 2016, 35, 89–98. [Google Scholar] [CrossRef]
- Kobayashi, M.; Tomita, S.; Sawada, K.; Shiba, K.; Yanagi, H.; Yamashita, I.; Uraoka, Y. Chiral meta-molecules consisting of gold nanoparticles and genetically engineered tobacco mosaic virus. Opt. Express 2012, 20, 24856–24863. [Google Scholar] [CrossRef]
- Mao, C.; Flynn, C.E.; Hayhurst, A.; Sweeney, R.; Qi, J.; Georgiou, G.; Iverson, B.; Belcher, A.M. Viral assembly of oriented quantum dot nanowires. Proc. Natl. Acad. Sci. USA 2003, 100, 6946–6951. [Google Scholar] [CrossRef] [PubMed]
- Slocik, J.M.; Naik, R.R.; Stone, M.O.; Wright, D.W. Viral templates for gold nanoparticle synthesis. J. Mater. Chem. 2005, 15, 749–753. [Google Scholar] [CrossRef]
- Cao, J.; Guenther, R.H.; Sit, T.L.; Opperman, C.H.; Lommel, S.A.; Willoughby, J.A. Loading and release mechanism of red clover necrotic mosaic virus derived plant viral nanoparticles for drug delivery of doxorubicin. Small 2014, 10, 5126–5136. [Google Scholar] [CrossRef]
- Czapar, A.E.; Zheng, Y.R.; Riddell, I.A.; Shukla, S.; Awuah, S.G.; Lippard, S.J.; Steinmetz, N.F. Tobacco mosaic virus delivery of phenanthriplatin for cancer therapy. ACS Nano 2016, 10, 4119–4126. [Google Scholar] [CrossRef] [PubMed]
- Le, D.H.T.; Lee, K.L.; Shukla, S.; Commandeur, U.; Steinmetz, N.F. Potato virus x, a filamentous plant viral nanoparticle for doxorubicin delivery in cancer therapy. Nanoscale 2017, 9, 2348–2357. [Google Scholar] [CrossRef]
- Chen, C.C.; Stark, M.; Baikoghli, M.; Cheng, R.H. Surface functionalization of hepatitis e virus nanoparticles using chemical conjugation methods. J. Vis. Exp. 2018, 57020. [Google Scholar] [CrossRef]
- Thangavelu, R.M.; Ganapathy, R.; Ramasamy, P.; Krishnan, K. Fabrication of virus metal hybrid nanomaterials: An ideal reference for bio semiconductor. Arab. J. Chem. 2020, 13, 2750–2765. [Google Scholar] [CrossRef]
- Mandeep, P.S. Microbial nanotechnology for bioremediation of industrial wastewater. Front. Microbiol. 2020, 11, 590631. [Google Scholar] [CrossRef]
- Seil, J.T.; Webster, T.J. Antimicrobial applications of nanotechnology: Methods and literature. Int. J. Nanomed. 2012, 7, 2767. [Google Scholar]
- Sondi, I.; Salopek-Sondi, B. Silver nanoparticles as antimicrobial agent: A case study on e. Coli as a model for gram-negative bacteria. J. Colloid Interface Sci. 2004, 275, 177–182. [Google Scholar] [CrossRef]
- Banu, A.; Rathod, V.; Ranganath, E. Silver nanoparticle production by rhizopus stolonifer and its antibacterial activity against extended spectrum β-lactamase producing (esbl) strains of enterobacteriaceae. Mater. Res. Bull. 2011, 46, 1417–1423. [Google Scholar] [CrossRef]
- Singh, P.; Kim, Y.J.; Singh, H.; Wang, C.; Hwang, K.H.; Farh Mel, A.; Yang, D.C. Biosynthesis, characterization, and antimicrobial applications of silver nanoparticles. Int. J. Nanomed. 2015, 10, 2567–2577. [Google Scholar] [CrossRef]
- Elbeshehy, E.K.; Elazzazy, A.M.; Aggelis, G. Silver nanoparticles synthesis mediated by new isolates of bacillus spp., nanoparticle characterization and their activity against bean yellow mosaic virus and human pathogens. Front. Microbiol. 2015, 6, 453. [Google Scholar] [CrossRef]
- Mahmoud, W.M.; Abdelmoneim, T.S.; Elazzazy, A.M. The impact of silver nanoparticles produced by bacillus pumilus as antimicrobial and nematicide. Front. Microbiol. 2016, 7, 1746. [Google Scholar] [CrossRef]
- Malarkodi, C.; Chitra, K.; Rajeshkumar, S.; Gnanajobitha, G.; Paulkumar, K.; Vanaja, M.; Annadurai, G. Novel eco-friendly synthesis of titanium oxide nanoparticles by using planomicrobium sp. And its antimicrobial evaluation. Der Pharm. Sin. 2013, 4, 59–66. [Google Scholar]
- Raghupathi, K.R.; Koodali, R.T.; Manna, A.C. Size-dependent bacterial growth inhibition and mechanism of antibacterial activity of zinc oxide nanoparticles. Langmuir 2011, 27, 4020–4028. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; Nada, A.A.; Hassabo, A.G.; Eid, B.M.; El-Deen, A.M.N.; Abou-Zeid, N.Y. Effect of different capping agents on physicochemical and antimicrobial properties of zno nanoparticles. Chem. Pap. 2017, 71, 1365–1375. [Google Scholar] [CrossRef]
- Mohammadi, F.M.; Ghasemi, N. Influence of temperature and concentration on biosynthesis and characterization of zinc oxide nanoparticles using cherry extract. J. Nanostruct. Chem. 2018, 8, 93–102. [Google Scholar] [CrossRef]
- Jain, D.; Shivani, A.A.B.; Singh, H.; Daima, H.K.; Singh, M.; Mohanty, S.R.; Stephen, B.J.; Singh, A. Microbial fabrication of zinc oxide nanoparticles and evaluation of their antimicrobial and photocatalytic properties. Front. Chem. 2020, 8, 778. [Google Scholar] [CrossRef]
- Jabir, N.R.; Tabrez, S.; Ashraf, G.M.; Shakil, S.; Damanhouri, G.A.; Kamal, M.A. Nanotechnology-based approaches in anticancer research. Int. J. Nanomed. 2012, 7, 4391–4408. [Google Scholar] [CrossRef]
- Sutradhar, K.B.; Amin, M. Nanotechnology in cancer drug delivery and selective targeting. Int. Sch. Res. Notices 2014, 2014, 939378. [Google Scholar] [CrossRef]
- Borse, V.; Kaler, A.; Banerjee, U.C. Microbial synthesis of platinum nanoparticles and evaluation of their anticancer activity. Int. J. Emerg. Trends Electr. Electron 2015, 11, 2320–9569. [Google Scholar]
- El-Batal, A.; Al Tamie, M. Biosynthesis of gold nanoparticles using marine streptomyces cyaneus and their antimicrobial, antioxidant and antitumor (in vitro) activities. J. Chem. Pharm. Res. 2015, 7, 1020–1036. [Google Scholar]
- Salouti, M.; Ahangari, A. Nanoparticle Based Drug Delivery Systems for Treatment of Infectious Diseases. In Application of Nanotechnology in Drug Delivery; InTech: London, UK, 2014; pp. 155–192. [Google Scholar] [CrossRef]
- Omlor, A.J.; Nguyen, J.; Bals, R.; Dinh, Q.T. Nanotechnology in respiratory medicine. Respir. Res. 2015, 16, 64. [Google Scholar] [CrossRef]
- Kundu, D.; Hazra, C.; Chatterjee, A.; Chaudhari, A.; Mishra, S. Extracellular biosynthesis of zinc oxide nanoparticles using rhodococcus pyridinivorans nt2: Multifunctional textile finishing, biosafety evaluation and in vitro drug delivery in colon carcinoma. J. Photochem. Photobiol. B Biol. 2014, 140, 194–204. [Google Scholar] [CrossRef]
- Kumar, S.A.; Peter, Y.-A.; Nadeau, J.L. Facile biosynthesis, separation and conjugation of gold nanoparticles to doxorubicin. Nanotechnology 2008, 19, 495101. [Google Scholar] [CrossRef]
- Khan, S.A.; Gambhir, S.; Ahmad, A. Extracellular biosynthesis of gadolinium oxide (gd2o3) nanoparticles, their biodistribution and bioconjugation with the chemically modified anticancer drug taxol. Beilstein J. Nanotechnol. 2014, 5, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Tallury, P.; Malhotra, A.; Byrne, L.M.; Santra, S. Nanobioimaging and sensing of infectious diseases. Adv. Drug Deliv. Rev. 2010, 62, 424–437. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.; Zubair, S.; Tufail, S.; Sherwani, A.; Sajid, M.; Raman, S.C.; Azam, A.; Owais, M. Fungus-mediated biological synthesis of gold nanoparticles: Potential in detection of liver cancer. Int. J. Nanomed. 2011, 6, 2305–2319. [Google Scholar] [CrossRef]
- Shukla, K. Nanotechnology and emerging trends in dairy foods: The inside story to food additives and ingredients. Int. J. Nanosci. Nanotechnol. 2012, 1, 41–58. [Google Scholar]
- Espitia, P.J.P.; Soares, N.d.F.F.; dos Reis Coimbra, J.S.; de Andrade, N.J.; Cruz, R.S.; Medeiros, E.A.A. Zinc oxide nanoparticles: Synthesis, antimicrobial activity and food packaging applications. Food Bioproc. Tech. 2012, 5, 1447–1464. [Google Scholar] [CrossRef]
- Rajamanickam, U.; Mylsamy, P.; Viswanathan, S.; Muthusamy, P. Biosynthesis of Zinc Nanoparticles Using Actinomycetes for Antibacterial Food Packaging. In Proceedings of the International Conference on nutrition and food sciences IPCBEE, Singapore, 23–24 July 2012. [Google Scholar]
- Prasad, R.; Kumar, V.; Prasad, K.S. Nanotechnology in sustainable agriculture: Present concerns and future aspects. Afr. J. Biotechnol. 2014, 13, 705–713. [Google Scholar]
- Huang, J.; Lin, L.; Sun, D.; Chen, H.; Yang, D.; Li, Q. Bio-inspired synthesis of metal nanomaterials and applications. Chem. Soc. Rev. 2015, 44, 6330–6374. [Google Scholar] [CrossRef]
- Kaur, P.; Thakur, R.; Duhan, J.S.; Chaudhury, A. Management of wilt disease of chickpea in vivo by silver nanoparticles biosynthesized by rhizospheric microflora of chickpea (cicer arietinum). J. Chem. Technol. Biotechnol. 2018, 93, 3233–3243. [Google Scholar] [CrossRef]
- Bisinoti, M.C.; Moreira, A.B.; Melo, C.A.; Fregolente, L.G.; Bento, L.R.; dos Santos, J.V.; Ferreira, O.P. Application of Carbon-Based Nanomaterials as Fertilizers in Soils. In Nanomaterials Applications for Environmental Matrices: Water, Soil and Air; do Nascimento, R.F., Nascimento, R.F., Ferreira, O.P., Paula, A.J., Neto, V.O.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 305–333. [Google Scholar] [CrossRef]
- Guo, H.; White, J.C.; Wang, Z.; Xing, B. Nano-enabled fertilizers to control the release and use efficiency of nutrients. Curr. Opin. Environ. Sci. Health 2018, 6, 77–83. [Google Scholar] [CrossRef]
- Subbarao, C.V.; Kartheek, G.; Sirisha, D. Slow release of potash fertilizer through polymer coating. Int. J. Appl. Sci. Eng. 2013, 11, 25–30. [Google Scholar]
- Duhan, J.S.; Kumar, R.; Kumar, N.; Kaur, P.; Nehra, K.; Duhan, S. Nanotechnology: The new perspective in precision agriculture. Biotechnol. Rep. 2017, 15, 11–23. [Google Scholar] [CrossRef]
- Rai, M.; Ingle, A. Role of nanotechnology in agriculture with special reference to management of insect pests. Appl. Microbiol. Biotechnol. 2012, 94, 287–293. [Google Scholar] [CrossRef]
- Goswami, A.; Roy, I.; Sengupta, S.; Debnath, N. Novel applications of solid and liquid formulations of nanoparticles against insect pests and pathogens. Thin Solid Films 2010, 519, 1252–1257. [Google Scholar] [CrossRef]
- Athanassiou, C.; Kavallieratos, N.; Benelli, G.; Losic, D.; Rani, P.U.; Desneux, N. Nanoparticles for pest control: Current status and future perspectives. J. Pest Sci. 2018, 91, 1–15. [Google Scholar] [CrossRef]
- Rawtani, D.; Khatri, N.; Tyagi, S.; Pandey, G. Nanotechnology-based recent approaches for sensing and remediation of pesticides. J. Environ. Manag. 2018, 206, 749–762. [Google Scholar] [CrossRef]
- Wang, L.; Li, X.; Zhang, G.; Dong, J.; Eastoe, J. Oil-in-water nanoemulsions for pesticide formulations. J. Colloid Interface Sci. 2007, 314, 230–235. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, T.; Wu, K.; Oikawa, K.; Hidaka, H.; Serpone, N. Photoassisted degradation of dye pollutants. 3. Degradation of the cationic dye rhodamine b in aqueous anionic surfactant/tio2 dispersions under visible light irradiation: Evidence for the need of substrate adsorption on tio2 particles. Environ. Sci. Technol. 1998, 32, 2394–2400. [Google Scholar] [CrossRef]
- Kharissova, O.V.; Dias, H.R.; Kharisov, B.I.; Pérez, B.O.; Pérez, V.M.J. The greener synthesis of nanoparticles. Trends Biotechnol. 2013, 31, 240–248. [Google Scholar] [CrossRef]
- Sharma, K.; Singh, G.; Kumar, M.; Bhalla, V. Silver nanoparticles: Facile synthesis and their catalytic application for the degradation of dyes. RSC Adv. 2015, 5, 25781–25788. [Google Scholar] [CrossRef]
- Suvith, V.; Philip, D. Catalytic degradation of methylene blue using biosynthesized gold and silver nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 118, 526–532. [Google Scholar] [CrossRef]
- Panáček, A.; Prucek, R.; Hrbáč, J.; Nevečná, T.; Šteffková, J.; Zbořil, R.; Kvítek, L. Polyacrylate-Assisted Size Control of Silver Nanoparticles and Their Catalytic Activity. Chem. Mater. 2014, 26, 1332–1339. [Google Scholar] [CrossRef]
- Koul, B.; Taak, P. (Eds.) Biotechnological Strategies for Effective Remediation of Polluted Soils; Springer: Singapore, 2018; pp. 77–84. [Google Scholar]
- Hamida, R.S.; Albasher, G.; Bin-Meferij, M.M. Oxidative stress and apoptotic responses elicited by nostoc-synthesized silver nanoparticles against different cancer cell lines. Cancers 2020, 12, 2099. [Google Scholar] [CrossRef]
- Hamida, R.S.; Ali, M.A.; Goda, D.A.; Khalil, M.I.; Al-Zaban, M.I. Novel biogenic silver nanoparticle-induced reactive oxygen species inhibit the biofilm formation and virulence activities of methicillin-resistant staphylococcus aureus (mrsa) strain. Front. Bioeng. Biotechnol. 2020, 8, 433. [Google Scholar] [CrossRef]
- Hamida, R.S.; Ali, M.A.; Redhwan, A.; Bin-Meferij, M.M. Cyanobacteria–a promising platform in green nanotechnology: A review on nanoparticles fabrication and their prospective applications. Int. J. Nanomed. 2020, 15, 6033. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Y.-Y.; Huang, J.; Chen, C.-Y.; Wang, Z.-X.; Xie, H. Silver nanoparticles: Synthesis, medical applications and biosafety. Theranostics 2020, 10, 8996. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koul, B.; Poonia, A.K.; Yadav, D.; Jin, J.-O. Microbe-Mediated Biosynthesis of Nanoparticles: Applications and Future Prospects. Biomolecules 2021, 11, 886. https://doi.org/10.3390/biom11060886
Koul B, Poonia AK, Yadav D, Jin J-O. Microbe-Mediated Biosynthesis of Nanoparticles: Applications and Future Prospects. Biomolecules. 2021; 11(6):886. https://doi.org/10.3390/biom11060886
Chicago/Turabian StyleKoul, Bhupendra, Anil Kumar Poonia, Dhananjay Yadav, and Jun-O Jin. 2021. "Microbe-Mediated Biosynthesis of Nanoparticles: Applications and Future Prospects" Biomolecules 11, no. 6: 886. https://doi.org/10.3390/biom11060886
APA StyleKoul, B., Poonia, A. K., Yadav, D., & Jin, J.-O. (2021). Microbe-Mediated Biosynthesis of Nanoparticles: Applications and Future Prospects. Biomolecules, 11(6), 886. https://doi.org/10.3390/biom11060886





