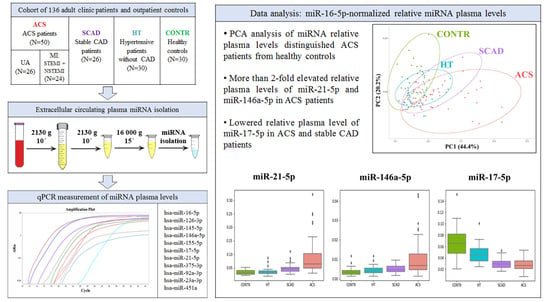
Circulating Extracellular miRNA Analysis in Patients with Stable CAD and Acute Coronary Syndromes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Plasma Collection and Storage
2.3. Hemolysis Assessment of Plasma Samples
2.4. Plasma miRNA Isolation
2.5. cDNA Synthesis and Quantitative PCR (qPCR) for miRNA Detection
2.6. Data Analysis
3. Results
3.1. Study Sample Characteristics
- ACS: 50 patients with ACS, including 26 patients with UA and 24 patients with MI (13 STEMI and 11 NSTEMI patients);
- SCAD: 26 patients with stable CAD;
- HT: 30 hypertensive patients without CAD;
- CONTR: 30 healthy controls.
3.2. Circulating Extracellular Plasma miRNA Levels
3.3. Extracellular Plasma miRNA Markers of SCAD and ACS
3.4. Influence of Age and Sex on Plasma miRNA Levels
3.5. Influence of Medication Therapies on Plasma miRNA Levels
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Sample Characteristics | Group Comparison | p-Value |
|---|---|---|
| Age | ACS vs. CONTR | 0.0000001122 |
| ACS vs. HT | 0.1048233616 | |
| ACS vs. SCAD | 0.0839889419 | |
| SCAD vs. CONTR | 0.0000000135 | |
| SCAD vs. HT | 0.0029000312 | |
| HT vs. CONTR | 0.0001067908 | |
| Total cholesterol, mmol/L | ACS vs. CONTR | 0.1155480592 |
| ACS vs. HT | 0.1449169304 | |
| ACS vs. SCAD | 0.0003161259 | |
| SCAD vs. CONTR | 0.0000189394 | |
| SCAD vs. HT | 0.0012925035 | |
| HT vs. CONTR | 0.0104547902 | |
| Triglycerides, mmol/L | ACS vs. CONTR | 0.0000007239 |
| ACS vs. HT | 0.0071015629 | |
| ACS vs. SCAD | 0.0306688370 | |
| SCAD vs. CONTR | 0.0000003456 | |
| SCAD vs. HT | 0.0005974758 | |
| HT vs. CONTR | 0.0053900319 | |
| LDL cholesterol, mmol/L | ACS vs. CONTR | 0.0002317522 |
| ACS vs. HT | 0.1048233616 | |
| ACS vs. SCAD | 0.0053309371 | |
| SCAD vs. CONTR | 0.0000022994 | |
| SCAD vs. HT | 0.0005974758 | |
| HT vs. CONTR | 0.0036770681 | |
| HDL cholesterol, mmol/L | ACS vs. CONTR | 0.0000001122 |
| ACS vs. HT | 0.0000028800 | |
| ACS vs. SCAD | 0.0000276922 | |
| SCAD vs. CONTR | 0.0519353179 | |
| SCAD vs. HT | 0.0369636147 | |
| HT vs. CONTR | 0.4208844542 | |
| HS | ACS vs. CONTR | 0.3345576652 |
| ACS vs. HT | 0.0364064683 | |
| ACS vs. SCAD | 0.1892085639 | |
| SCAD vs. CONTR | 0.0519353179 | |
| SCAD vs. HT | 0.0012561992 | |
| HT vs. CONTR | 0.0921457462 | |
| dCq (miR-23a-3p–miR-451a) | ACS vs. CONTR | 0.1127677221 |
| ACS vs. HT | 0.0043305646 | |
| ACS vs. SCAD | 0.2088928853 | |
| SCAD vs. CONTR | 0.3286657605 | |
| SCAD vs. HT | 0.0407942838 | |
| HT vs. CONTR | 0.0475028521 |
| Importance of Components | ||||||||
|---|---|---|---|---|---|---|---|---|
| PC1 | PC2 | PC3 | PC4 | PC5 | PC6 | PC7 | PC8 | |
| Standard deviation | 1.885 | 1.271 | 0.961 | 0.815 | 0.734 | 0.637 | 0.429 | 0.338 |
| Proportion of variance | 0.444 | 0.202 | 0.116 | 0.083 | 0.067 | 0.051 | 0.023 | 0.014 |
| Cumulative proportion | 0.444 | 0.646 | 0.762 | 0.845 | 0.912 | 0.963 | 0.986 | 1.000 |
| miRNA | miR- 126-3p | miR- 145-5p | miR- 146a-5p | miR- 155-5p | miR- 17-5p | miR- 21-5p | miR- 375-3p | miR- 92a-3p |
|---|---|---|---|---|---|---|---|---|
| Beta-blockers | 0.932 | 0.932 | 0.932 | 0.254 | 0.126 | 0.468 | 0.760 | 0.887 |
| Calcium channel blockers | 0.889 | 0.889 | 0.889 | 0.889 | 0.889 | 0.889 | 0.889 | 0.889 |
| Nitrates | 0.184 | 0.272 | 0.116 | 0.116 | 0.130 | 0.116 | 0.599 | 0.932 |
| ACEI | 0.653 | 0.994 | 0.994 | 0.994 | 0.994 | 0.994 | 0.014 | 0.994 |
| Diuretics | 0.996 | 0.996 | 0.728 | 0.996 | 0.996 | 0.210 | 0.512 | 0.996 |
| Antiplatelet drugs | 0.351 | 0.572 | 0.351 | 0.529 | 0.604 | 0.572 | 0.529 | 0.529 |
| Anticoagulants | 0.424 | 0.924 | 0.540 | 0.424 | 0.424 | 0.424 | 0.891 | 0.038 |
| Statins | 0.366 | 0.366 | 0.366 | 0.366 | 0.366 | 0.426 | 0.366 | 0.366 |


References
- Roth, G.A.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, Regional, and National Age-Sex-Specific Mortality for 282 Causes of Death in 195 Countries and Territories, 1980–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. The Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef] [Green Version]
- Sanchis-Gomar, F.; Perez-Quilis, C.; Leischik, R.; Lucia, A. Epidemiology of Coronary Heart Disease and Acute Coronary Syndrome. Ann. Transl. Med. 2016, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics—2020 Update: A Report From the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef]
- Maddox, T.M.; Stanislawski, M.A.; Grunwald, G.K.; Bradley, S.M.; Ho, P.M.; Tsai, T.T.; Patel, M.R.; Sandhu, A.; Valle, J.; Magid, D.J.; et al. Nonobstructive Coronary Artery Disease and Risk of Myocardial Infarction. JAMA 2014, 312, 1754–1763. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tan, G.-J.; Han, L.-N.; Bai, Y.-Y.; He, M.; Liu, H.-B. Novel Biomarkers for Cardiovascular Risk Prediction. J. Geriatr. Cardiol. 2017, 14, 135–150. [Google Scholar] [CrossRef]
- Del Val Martin, D.; Sanmartín Fernández, M.; Zamorano Gómez, J.L. Biomarkers in Acute Coronary Syndrome. IJC Metab. Endocr. 2015, 8, 20–23. [Google Scholar] [CrossRef] [Green Version]
- Korley, F.K.; Jaffe, A.S. Preparing the United States for High-Sensitivity Cardiac Troponin Assays. J. Am. Coll. Cardiol. 2013, 61, 1753–1758. [Google Scholar] [CrossRef] [Green Version]
- McMahon, C.G.; Lamont, J.V.; Curtin, E.; McConnell, R.I.; Crockard, M.; Kurth, M.J.; Crean, P.; Fitzgerald, S.P. Diagnostic Accuracy of Heart-Type Fatty Acid-Binding Protein for the Early Diagnosis of Acute Myocardial Infarction. Am. J. Emerg. Med. 2012, 30, 267–274. [Google Scholar] [CrossRef]
- Gami, B.N.; Patel, D.S.; Haridas, N.; Chauhan, K.P.; Shah, H.; Trivedi, A. Utility of Heart-Type Fatty Acid Binding Protein as a New Biochemical Marker for the Early Diagnosis of Acute Coronary Syndrome. J. Clin. Diagn. Res. 2015, 9, BC22–BC24. [Google Scholar] [CrossRef] [PubMed]
- Kabekkodu, S.P.; Mananje, S.R.; Saya, R.P. A Study on the Role of Heart Type Fatty Acid Binding Protein in the Diagnosis of Acute Myocardial Infarction. J. Clin. Diagn. Res. 2016, 10, OC07-10. [Google Scholar] [CrossRef]
- McCarthy, C.P.; McEvoy, J.W.; Januzzi, J.L. Biomarkers in Stable Coronary Artery Disease. Am. Heart J. 2018, 196, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Bronze-da-Rocha, E. MicroRNAs Expression Profiles in Cardiovascular Diseases. Biomed. Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reid, G.; Kirschner, M.B.; van Zandwijk, N. Circulating MicroRNAs: Association with Disease and Potential Use as Biomarkers. Crit. Rev. Oncol. Hematol. 2011, 80, 193–208. [Google Scholar] [CrossRef] [PubMed]
- Kondkar, A.A.; Abu-Amero, K.K. Utility of Circulating MicroRNAs as Clinical Biomarkers for Cardiovascular Diseases. Available online: https://www.hindawi.com/journals/bmri/2015/821823/ (accessed on 13 January 2021).
- Gupta Shashi, K.; Claudia, B.; Thomas, T. Circulating MicroRNAs as Biomarkers and Potential Paracrine Mediators of Cardiovascular Disease. Circ. Cardiovasc. Genet. 2010, 3, 484–488. [Google Scholar] [CrossRef] [Green Version]
- Creemers Esther, E.; Tijsen Anke, J.; Pinto Yigal, M.; van Rooij, E. Circulating MicroRNAs. Circ. Res. 2012, 110, 483–495. [Google Scholar] [CrossRef]
- Janika, V.; Thomas, T. Circulating Noncoding RNAs as Biomarkers of Cardiovascular Disease and Injury. Circ. Res. 2017, 120, 381–399. [Google Scholar] [CrossRef]
- Ahlin, F.; Arfvidsson, J.; Vargas, K.G.; Stojkovic, S.; Huber, K.; Wojta, J. MicroRNAs as Circulating Biomarkers in Acute Coronary Syndromes: A Review. Vasc. Pharmacol. 2016, 81, 15–21. [Google Scholar] [CrossRef]
- Deddens, J.C.; Colijn, J.M.; Oerlemans, M.I.F.J.; Pasterkamp, G.; Chamuleau, S.A.; Doevendans, P.A.; Sluijter, J.P.G. Circulating MicroRNAs as Novel Biomarkers for the Early Diagnosis of Acute Coronary Syndrome. J. Cardiovasc. Trans. Res. 2013, 6, 884–898. [Google Scholar] [CrossRef]
- Kaur, A.; Mackin, S.T.; Schlosser, K.; Wong, F.L.; Elharram, M.; Delles, C.; Stewart, D.J.; Dayan, N.; Landry, T.; Pilote, L. Systematic Review of MicroRNA Biomarkers in Acute Coronary Syndrome and Stable Coronary Artery Disease. Cardiovasc. Res. 2020, 116, 1113–1124. [Google Scholar] [CrossRef]
- Feinberg, M.W.; Moore, K.J. MicroRNA Regulation of Atherosclerosis. Circ. Res. 2016, 118, 703–720. [Google Scholar] [CrossRef] [Green Version]
- Chamorro-Jorganes, A.; Lee, M.Y.; Araldi, E.; Landskroner-Eiger, S.; Fernández-Fuertes, M.; Sahraei, M.; Quiles del Rey, M.; van Solingen, C.; Yu, J.; Fernández-Hernando, C.; et al. VEGF-Induced Expression of MiR-17–92 Cluster in Endothelial Cells Is Mediated by ERK/ELK1 Activation and Regulates Angiogenesis. Circ. Res. 2016, 118, 38–47. [Google Scholar] [CrossRef]
- Bonauer, A.; Carmona, G.; Iwasaki, M.; Mione, M.; Koyanagi, M.; Fischer, A.; Burchfield, J.; Fox, H.; Doebele, C.; Ohtani, K.; et al. MicroRNA-92a Controls Angiogenesis and Functional Recovery of Ischemic Tissues in Mice. Science 2009, 324, 1710–1713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Aurora, A.B.; Johnson, B.A.; Qi, X.; McAnally, J.; Hill, J.A.; Richardson, J.A.; Bassel-Duby, R.; Olson, E.N. An Endothelial-Specific MicroRNA Governs Vascular Integrity and Angiogenesis. Dev. Cell 2008, 15, 261–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fish, J.E.; Santoro, M.M.; Morton, S.U.; Yu, S.; Yeh, R.-F.; Wythe, J.D.; Bruneau, B.G.; Stainier, D.Y.R.; Srivastava, D. MiR-126 Regulates Angiogenic Signaling and Vascular Integrity. Dev. Cell 2008, 15, 272–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Li, Q.; Hosen, M.R.; Zietzer, A.; Flender, A.; Levermann, P.; Schmitz, T.; Frühwald, D.; Goody, P.; Nickenig, G.; et al. Atherosclerotic Conditions Promote the Packaging of Functional MicroRNA-92a-3p Into Endothelial Microvesicles. Circ. Res. 2019, 124, 575–587. [Google Scholar] [CrossRef]
- Xue, S.; Liu, D.; Zhu, W.; Su, Z.; Zhang, L.; Zhou, C.; Li, P. Circulating MiR-17-5p, MiR-126-5p and MiR-145-3p Are Novel Biomarkers for Diagnosis of Acute Myocardial Infarction. Front. Physiol. 2019, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamada, H.; Suzuki, K.; Fujii, R.; Kawado, M.; Hashimoto, S.; Watanabe, Y.; Iso, H.; Fujino, Y.; Wakai, K.; Tamakoshi, A. Circulating MiR-21, MiR-29a, and MiR-126 Are Associated with Premature Death Risk Due to Cancer and Cardiovascular Disease: The JACC Study. Sci. Rep. 2021, 11, 5298. [Google Scholar] [CrossRef]
- Faraoni, I.; Antonetti, F.R.; Cardone, J.; Bonmassar, E. MiR-155 Gene: A Typical Multifunctional MicroRNA. Biochimica Biophysica Acta BBA Mol. Basis Dis. 2009, 1792, 497–505. [Google Scholar] [CrossRef]
- Labbaye, C.; Testa, U. The Emerging Role of MIR-146A in the Control of Hematopoiesis, Immune Function and Cancer. J. Hematol. Oncol. 2012, 5, 13. [Google Scholar] [CrossRef] [Green Version]
- Xue, S.; Zhu, W.; Liu, D.; Su, Z.; Zhang, L.; Chang, Q.; Li, P. Circulating MiR-26a-1, MiR-146a and MiR-199a-1 Are Potential Candidate Biomarkers for Acute Myocardial Infarction. Mol. Med. 2019, 25, 18. [Google Scholar] [CrossRef]
- Faccini, J.; Ruidavets, J.-B.; Cordelier, P.; Martins, F.; Maoret, J.-J.; Bongard, V.; Ferrières, J.; Roncalli, J.; Elbaz, M.; Vindis, C. Circulating MiR-155, MiR-145 and Let-7c as Diagnostic Biomarkers of the Coronary Artery Disease. Sci. Rep. 2017, 7, 42916. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C. MicroRNA-145 in Vascular Smooth Muscle Cell Biology. Cell Cycle 2009, 8, 3469–3473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Y.; Liu, X.; Yang, J.; Lin, Y.; Xu, D.-Z.; Lu, Q.; Deitch, E.A.; Huo, Y.; Delphin, E.S.; Zhang, C. MicroRNA-145, a Novel Smooth Muscle Cell Phenotypic Marker and Modulator, Controls Vascular Neointimal Lesion Formation. Circ. Res. 2009, 105, 158–166. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, C. MicroRNA-21 in Cardiovascular Disease. J. Cardiovasc. Transl. Res. 2010, 3, 251–255. [Google Scholar] [CrossRef] [Green Version]
- Baulina, N.; Osmak, G.; Kiselev, I.; Matveeva, N.; Kukava, N.; Shakhnovich, R.; Kulakova, O.; Favorova, O. NGS-Identified Circulating MiR-375 as a Potential Regulating Component of Myocardial Infarction Associated Network. J. Mol. Cell. Cardiol. 2018, 121, 173–179. [Google Scholar] [CrossRef]
- Binderup, H.G.; Madsen, J.S.; Heegaard, N.H.H.; Houlind, K.; Andersen, R.F.; Brasen, C.L. Quantification of MicroRNA Levels in Plasma—Impact of Preanalytical and Analytical Conditions. PLoS ONE 2018, 13, e0201069. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.H.; Yi, H.S.; Kim, Y.; Kroh, E.M.; Chien, J.W.; Eaton, K.D.; Goodman, M.T.; Tait, J.F.; Tewari, M.; Pritchard, C.C. Plasma Processing Conditions Substantially Influence Circulating MicroRNA Biomarker Levels. PLoS ONE 2013, 8, e64795. [Google Scholar] [CrossRef] [Green Version]
- Felekkis, K.; Papaneophytou, C. Challenges in Using Circulating Micro-RNAs as Biomarkers for Cardiovascular Diseases. Int. J. Mol. Sci. 2020, 21, 561. [Google Scholar] [CrossRef] [Green Version]
- Glinge, C.; Clauss, S.; Boddum, K.; Jabbari, R.; Jabbari, J.; Risgaard, B.; Tomsits, P.; Hildebrand, B.; Kääb, S.; Wakili, R.; et al. Stability of Circulating Blood-Based MicroRNAs—Pre-Analytic Methodological Considerations. PLoS ONE 2017, 12, e0167969. [Google Scholar] [CrossRef]
- Kumar, D.; Narang, R.; Sreenivas, V.; Rastogi, V.; Bhatia, J.; Saluja, D.; Srivastava, K. Circulatory MiR-133b and MiR-21 as Novel Biomarkers in Early Prediction and Diagnosis of Coronary Artery Disease. Genes 2020, 11, 164. [Google Scholar] [CrossRef] [Green Version]
- Kirschner, M.B.; Edelman, J.J.B.; Kao, S.C.-H.; Vallely, M.P.; Van Zandwijk, N.; Reid, G. The Impact of Hemolysis on Cell-Free MicroRNA Biomarkers. Front. Genet. 2013, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonald, J.S.; Milosevic, D.; Reddi, H.V.; Grebe, S.K.; Algeciras-Schimnich, A. Analysis of Circulating MicroRNA: Preanalytical and Analytical Challenges. Clin. Chem. 2011, 57, 833–840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pritchard, C.C.; Kroh, E.; Wood, B.; Arroyo, J.D.; Dougherty, K.J.; Miyaji, M.M.; Tait, J.F.; Tewari, M. Blood Cell Origin of Circulating MicroRNAs: A Cautionary Note for Cancer Biomarker Studies. Cancer Prev. Res. 2012, 5, 492–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krammer, T.L.; Mayr, M.; Hackl, M. MicroRNAs as Promising Biomarkers of Platelet Activity in Antiplatelet Therapy Monitoring. Int. J. Mol. Sci. 2020, 21, 561. [Google Scholar] [CrossRef] [PubMed]
- Appierto, V.; Callari, M.; Cavadini, E.; Morelli, D.; Daidone, M.G.; Tiberio, P. A Lipemia-Independent NanoDrop®-Based Score to Identify Hemolysis in Plasma and Serum Samples. Bioanalysis 2014, 6, 1215–1226. [Google Scholar] [CrossRef] [PubMed]
- Urbich, C.; Kuehbacher, A.; Dimmeler, S. Role of MicroRNAs in Vascular Diseases, Inflammation, and Angiogenesis. Cardiovasc. Res. 2008, 79, 581–588. [Google Scholar] [CrossRef] [Green Version]
- Max, K.E.A.; Bertram, K.; Akat, K.M.; Bogardus, K.A.; Li, J.; Morozov, P.; Ben-Dov, I.Z.; Li, X.; Weiss, Z.R.; Azizian, A.; et al. Human Plasma and Serum Extracellular Small RNA Reference Profiles and Their Clinical Utility. Proc. Natl. Acad. Sci. USA 2018, 115, E5334–E5343. [Google Scholar] [CrossRef] [Green Version]
- Godoy, P.M.; Bhakta, N.R.; Barczak, A.J.; Cakmak, H.; Fisher, S.; MacKenzie, T.C.; Patel, T.; Price, R.W.; Smith, J.F.; Woodruff, P.G.; et al. Large Differences in Small RNA Composition Between Human Biofluids. Cell Rep. 2018, 25, 1346–1358. [Google Scholar] [CrossRef] [Green Version]
- Blondal, T.; Jensby Nielsen, S.; Baker, A.; Andreasen, D.; Mouritzen, P.; Wrang Teilum, M.; Dahlsveen, I.K. Assessing Sample and MiRNA Profile Quality in Serum and Plasma or Other Biofluids. Methods 2013, 59, S1–S6. [Google Scholar] [CrossRef]
- Willeit, P.; Zampetaki, A.; Dudek, K.; Kaudewitz, D.; King, A.; Kirkby, N.S.; Crosby-Nwaobi, R.; Prokopi, M.; Drozdov, I.; Langley, S.R.; et al. Circulating MicroRNAs as Novel Biomarkers for Platelet Activation. Circ. Res. 2013, 112, 595–600. [Google Scholar] [CrossRef] [Green Version]
- Sunderland, N.; Skroblin, P.; Barwari, T.; Huntley, R.P.; Lu, R.; Joshi, A.; Lovering, R.C.; Mayr, M. MicroRNA Biomarkers and Platelet Reactivity. Circ. Res. 2017, 120, 418–435. [Google Scholar] [CrossRef] [Green Version]
- De Boer, H.C.; van Solingen, C.; Prins, J.; Duijs, J.M.G.J.; Huisman, M.V.; Rabelink, T.J.; van Zonneveld, A.J. Aspirin Treatment Hampers the Use of Plasma MicroRNA-126 as a Biomarker for the Progression of Vascular Disease. Eur. Heart J. 2013, 34, 3451–3457. [Google Scholar] [CrossRef]
- Duttagupta, R.; Jiang, R.; Gollub, J.; Getts, R.C.; Jones, K.W. Impact of Cellular MiRNAs on Circulating MiRNA Biomarker Signatures. PLoS ONE 2011, 6, e20769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Alessandra, Y.; Chiesa, M.; Carena, M.C.; Beltrami, A.P.; Rizzo, P.; Buzzetti, M.; Ricci, V.; Ferrari, R.; Fucili, A.; Livi, U.; et al. Differential Role of Circulating MicroRNAs to Track Progression and Pre-Symptomatic Stage of Chronic Heart Failure: A Pilot Study. Biomedicines 2020, 8, 597. [Google Scholar] [CrossRef]
- D’Alessandra, Y.; Carena, M.C.; Spazzafumo, L.; Martinelli, F.; Bassetti, B.; Devanna, P.; Rubino, M.; Marenzi, G.; Colombo, G.I.; Achilli, F.; et al. Diagnostic Potential of Plasmatic MicroRNA Signatures in Stable and Unstable Angina. PLoS ONE 2013, 8, e80345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammad Mahmoud Hammad, R.; Hamed, D.H.E.D.; Eldosoky, M.A.E.R.; Ahmad, A.A.E.S.; Osman, H.M.; Abd Elgalil, H.M.; Mahmoud Hassan, M.M. Plasma MicroRNA-21, MicroRNA-146a and IL-13 Expression in Asthmatic Children. Innate Immun. 2018, 24, 171–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muñoz-San Martín, M.; Reverter, G.; Robles-Cedeño, R.; Buxò, M.; Ortega, F.J.; Gómez, I.; Tomàs-Roig, J.; Celarain, N.; Villar, L.M.; Perkal, H.; et al. Analysis of MiRNA Signatures in CSF Identifies Upregulation of MiR-21 and MiR-146a/b in Patients with Multiple Sclerosis and Active Lesions. J. Neuroinflammation 2019, 16, 220. [Google Scholar] [CrossRef] [Green Version]
- Stephan, F.; De Salvatore, R.; Henrik, F.; Thomas, S.; Ariane, F.; Christoph, L.; Michael, W.; Hamm Christian, W.; Tino, R.; Marga, M.-A.; et al. Circulating MicroRNAs in Patients With Coronary Artery Disease. Circ. Res. 2010, 107, 677–684. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Hao, J.; Sun, X.; Zhang, Y.; Wei, Q. Circulating Pro-Angiogenic Micro-Ribonucleic Acid in Patients with Coronary Heart Disease. Interact. Cardio Vasc. Thorac. Surg. 2018, 27, 336–342. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Xu, L.; Hu, Q.; Yang, S.; Zhang, B.; Jiang, H. MiR-17-5p as Circulating Biomarkers for the Severity of Coronary Atherosclerosis in Coronary Artery Disease. Int. J. Cardiol. 2015, 197, 123–124. [Google Scholar] [CrossRef] [PubMed]
- Xiang, M.; Zeng, Y.; Yang, R.; Xu, H.; Chen, Z.; Zhong, J.; Xie, H.; Xu, Y.; Zeng, X. U6 Is Not a Suitable Endogenous Control for the Quantification of Circulating MicroRNAs. Biochem. Biophys. Res. Commun. 2014, 454, 210–214. [Google Scholar] [CrossRef]
- Pierre-Emmanuel, R.; Anne-Clémence, V.; Nicolas, A.; Gilles, C.; Alain, S.; Alain, T.; Boulanger Chantal, M.; Christian, W.; Sebastian, M. Microparticles, Vascular Function, and Atherothrombosis. Circ. Res. 2011, 109, 593–606. [Google Scholar] [CrossRef]
- Polakovičová, M.; Musil, P.; Laczo, E.; Hamar, D.; Kyselovič, J. Circulating MicroRNAs as Potential Biomarkers of Exercise Response. Int. J. Mol. Sci. 2016, 17, 1553. [Google Scholar] [CrossRef]
- Siracusa, J.; Koulmann, N.; Banzet, S. Circulating MyomiRs: A New Class of Biomarkers to Monitor Skeletal Muscle in Physiology and Medicine. J. Cachexia Sarcopenia Muscle 2018, 9, 20–27. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Orekhov, A.N.; Bobryshev, Y.V. Cardiac-Specific MiRNA in Cardiogenesis, Heart Function, and Cardiac Pathology (with Focus on Myocardial Infarction). J. Mol. Cell. Cardiol. 2016, 94, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Ewing, L.E.; Koturbash, I.; Gurley, B.J.; Miousse, I.R. MicroRNAs as Biomarkers for Liver Injury: Current Knowledge, Challenges and Future Prospects. Food Chem Toxicol 2017, 110, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Karlsen, T.A.; Aae, T.F.; Brinchmann, J.E. Robust Profiling of MicroRNAs and IsomiRs in Human Plasma Exosomes across 46 Individuals. Sci. Rep. 2019, 9, 19999. [Google Scholar] [CrossRef]
- Jansen, F.; Stumpf, T.; Proebsting, S.; Franklin, B.S.; Wenzel, D.; Pfeifer, P.; Flender, A.; Schmitz, T.; Yang, X.; Fleischmann, B.K.; et al. Intercellular Transfer of MiR-126-3p by Endothelial Microparticles Reduces Vascular Smooth Muscle Cell Proliferation and Limits Neointima Formation by Inhibiting LRP6. J. Mol. Cell Cardiol. 2017, 104, 43–52. [Google Scholar] [CrossRef]
- Nakaoka, H.; Hirono, K.; Yamamoto, S.; Takasaki, I.; Takahashi, K.; Kinoshita, K.; Takasaki, A.; Nishida, N.; Okabe, M.; Ce, W.; et al. MicroRNA-145-5p and MicroRNA-320a Encapsulated in Endothelial Microparticles Contribute to the Progression of Vasculitis in Acute Kawasaki Disease. Sci. Rep. 2018, 8, 1016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cordes, K.R.; Sheehy, N.T.; White, M.P.; Berry, E.C.; Morton, S.U.; Muth, A.N.; Lee, T.-H.; Miano, J.M.; Ivey, K.N.; Srivastava, D. MiR-145 and MiR-143 Regulate Smooth Muscle Cell Fate and Plasticity. Nature 2009, 460, 705–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elia, L.; Quintavalle, M.; Zhang, J.; Contu, R.; Cossu, L.; Latronico, M.V.G.; Peterson, K.L.; Indolfi, C.; Catalucci, D.; Chen, J.; et al. The Knockout of MiR-143 and -145 Alters Smooth Muscle Cell Maintenance and Vascular Homeostasis in Mice: Correlates with Human Disease. Cell Death Differ. 2009, 16, 1590–1598. [Google Scholar] [CrossRef]
- Hergenreider, E.; Heydt, S.; Tréguer, K.; Boettger, T.; Horrevoets, A.J.G.; Zeiher, A.M.; Scheffer, M.P.; Frangakis, A.S.; Yin, X.; Mayr, M.; et al. Atheroprotective Communication between Endothelial Cells and Smooth Muscle Cells through MiRNAs. Nat. Cell Biol. 2012, 14, 249–256. [Google Scholar] [CrossRef]
- Zhelankin, A.V.; Vasiliev, S.V.; Stonogina, D.A.; Babalyan, K.A.; Sharova, E.I.; Doludin, Y.V.; Shchekochikhin, D.Y.; Generozov, E.V.; Akselrod, A.S. Elevated Plasma Levels of Circulating Extracellular MiR-320a-3p in Patients with Paroxysmal Atrial Fibrillation. Int. J. Mol. Sci. 2020, 21, 3485. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandra, Y.; Devanna, P.; Limana, F.; Straino, S.; Di Carlo, A.; Brambilla, P.G.; Rubino, M.; Carena, M.C.; Spazzafumo, L.; De Simone, M.; et al. Circulating MicroRNAs Are New and Sensitive Biomarkers of Myocardial Infarction. Eur. Heart J. 2010, 31, 2765–2773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.; de Ronde, M.W.J.; Kok, M.G.M.; Beijk, M.A.; Winter, R.J.D.; van der Wal, A.C.; Sondermeijer, B.M.; Meijers, J.C.M.; Creemers, E.E.; Pinto-Sietsma, S.-J. MiR-223-3p and MiR-122-5p as Circulating Biomarkers for Plaque Instability. Open Heart 2020, 7, e001223. [Google Scholar] [CrossRef] [PubMed]




| Assay Name | Assay ID | Mature miRNA Sequence | Type of miRNA |
|---|---|---|---|
| hsa-miR-16-5p | 477860_mir | UAGCAGCACGUAAAUAUUGGCG | Normalization control |
| hsa-miR-23a-3p | 478532_mir | AUCACAUUGCCAGGGAUUUCC | Hemolysis assessment |
| hsa-miR-451a | 478107_mir | AAACCGUUACCAUUACUGAGUU | Hemolysis assessment |
| hsa-miR-126-3p | 477887_mir | UCGUACCGUGAGUAAUAAUGCG | Candidate to ACS |
| hsa-miR-145-5p | 477916_mir | GUCCAGUUUUCCCAGGAAUCCCU | Candidate to ACS |
| hsa-miR-146a-5p | 478399_mir | UGAGAACUGAAUUCCAUGGGUU | Candidate to ACS |
| hsa-miR-155-5p | 477927_mir | UUAAUGCUAAUCGUGAUAGGGGUU | Candidate to ACS |
| hsa-miR-17-5p | 478447_mir | CAAAGUGCUUACAGUGCAGGUAG | Candidate to ACS |
| hsa-miR-21-5p | 477975_mir | UAGCUUAUCAGACUGAUGUUGA | Candidate to ACS |
| hsa-miR-375-3p | 478074_mir | UUUGUUCGUUCGGCUCGCGUGA | Candidate to ACS |
| hsa-miR-92a-3p | 477827_mir | UAUUGCACUUGUCCCGGCCUGU | Candidate to ACS |
| Group ID | CONTR | HT | SCAD | ACS | Total |
|---|---|---|---|---|---|
| Number of patients | 30 | 30 | 26 | 50 | 136 |
| Mean age (SD), years | 47.3 (5.6) | 57.7 (9.5) | 64.9 (7.5) | 61.1 (10.9) | 58.1 (10.8) |
| Gender (male/female) | 15/15 | 17/13 | 15/11 | 40/10 | 87/49 |
| Type 2 DM presence | 0 | 0 | 6 | 13 | 19 |
| Hyperlipidemia | 0 | 7 | 22 | 35 | 64 |
| HT presence | 0 | 30 | 24 | 42 | 96 |
| AF presence | 0 | 0 | 1 | 2 | 3 |
| MI history | 0 | 0 | 0 | 16 | 16 |
| Coronary atherosclerosis | 0 | 0 | 15 | 34 | 49 |
| Blood lipid Profile | |||||
| Total cholesterol, mmol/L, mean (SD) | 4.55 (0.9) | 5.19 (0.98) | 5.34 (1.25) | 4.95 (1.37) | 4.96 (1.18) |
| Triglycerides, mmol/L, mean (SD) | 1.13 (0.31) | 1.46 (0.5) | 1.61 (0.55) | 1.81 (0.72) | 1.53 (0.62) |
| LDL cholesterol, mmol/L, mean (SD) | 2.14 (0.56) | 2.69 (0.67) | 2.98 (1.03) | 2.98 (1.16) | 2.7 (0.96) |
| HDL cholesterol, mmol/L, mean (SD) | 1.86 (0.51) | 1.84 (0.5) | 1.53 (0.59) | 1.06 (0.28) | 1.51 (0.57) |
| Medication Therapies | |||||
| Beta-blockers | 2 | 15 | 23 | 24 | 64 |
| Calcium channel blockers | 0 | 4 | 13 | 4 | 21 |
| ACEI | 0 | 12 | 12 | 32 | 56 |
| Diuretics | 0 | 4 | 9 | 16 | 29 |
| Nitrates | 0 | 1 | 9 | 2 | 12 |
| Antiplatelet drugs | 0 | 9 | 17 | 42 | 68 |
| Anticoagulants | 0 | 3 | 9 | 16 | 28 |
| Statins | 1 | 11 | 20 | 41 | 73 |
| Hemolysis Assessment of Plasma Samples | |||||
| HS (SD) | 0.122 (0.045) | 0.144 (0.053) | 0.103 (0.033) | 0.121 (0.054) | 0.123 (0.050) |
| dCq(miR-23a-3p–miR-451a) (SD) | 11.1 (0.98) | 11.58 (1.09) | 11.03 (1.12) | 10.72 (1.22) | 11.05 (1.16) |
| miRNA | Type of Comparison | Group Comparison | |||||
|---|---|---|---|---|---|---|---|
| ACS vs. CONTR | ACS vs. HT | ACS vs. SCAD | SCAD vs. CONTR | SCAD vs. HT | HT vs. CONTR | ||
| miR-126-3p | FC | 1.53 | 1.75 | 1.26 | 1.21 | 1.39 | 0.87 |
| p < 0.05 (M–W) | * | ||||||
| p < 0.05 (M–W_corr) | |||||||
| p < 0.1 (MLR) | |||||||
| miR-145-5p | FC | 0.93 | 1.36 | 0.99 | 0.94 | 1.37 | 0.68 |
| p < 0.05 (M–W) | |||||||
| p < 0.05 (M–W_corr) | |||||||
| p < 0.1 (MLR) | |||||||
| miR-146a-5p | FC | 2.7 | 2.25 | 1.97 | 1.37 | 1.14 | 1.2 |
| p < 0.05 (M–W) | * | * | * | * | |||
| p < 0.05 (M–W_corr) | * | * | |||||
| p < 0.1 (MLR) | * | * | * | ||||
| miR-155-5p | FC | 1.8 | 1.72 | 1.61 | 1.12 | 1.07 | 1.05 |
| p < 0.05 (M–W) | |||||||
| p < 0.05 (M–W_corr) | |||||||
| p < 0.1 (MLR) | |||||||
| miR-17-5p | FC | 0.42 | 0.6 | 0.93 | 0.45 | 0.64 | 0,7 |
| p < 0.05 (M–W) | * | * | * | * | * | ||
| p < 0.05 (M–W_corr) | * | * | * | * | |||
| p < 0.1 (MLR) | * | * | * | * | * | ||
| miR-21-5p | FC | 2.69 | 2.42 | 1.79 | 1.5 | 1.36 | 1.11 |
| p < 0.05 (M–W) | * | * | * | * | * | ||
| p < 0.05 (M–W_corr) | * | * | * | * | * | ||
| p < 0.1 (MLR) | * | * | * | * | |||
| miR-375-3p | FC | 0.45 | 0.94 | 0.79 | 0.57 | 1.18 | 0.48 |
| p < 0.05 (M–W) | * | ||||||
| p < 0.05 (M–W_corr) | * | ||||||
| p < 0.1 (MLR) | * | * | |||||
| miR-92a-3p | FC | 1.15 | 1.25 | 1.27 | 0.91 | 0.98 | 0.93 |
| p < 0.05 (M–W) | * | * | |||||
| p < 0.05 (M–W_corr) | * | * | |||||
| p < 0.1 (MLR) | |||||||
| Medication Therapy | Number of Patients | Statistically Significant Change in Relative Plasma miRNA Level (p < 0.05) | |
|---|---|---|---|
| Not Receiving Therapy | Receiving Therapy | ||
| Beta-blockers | 29 | 47 | miR-92a-3p↓ |
| Calcium channel blockers | 59 | 17 | − |
| ACEI | 32 | 44 | miR-375-3p↓ |
| Diuretics | 51 | 25 | miR-126-3p↑, miR-21-5p↑ |
| Nitrates | 65 | 11 | − |
| Antiplatelet drugs | 17 | 59 | − |
| Anticoagulants | 51 | 25 | miR-17-5p↓ |
| Statins | 15 | 61 | miR-126-3p↓ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhelankin, A.V.; Stonogina, D.A.; Vasiliev, S.V.; Babalyan, K.A.; Sharova, E.I.; Doludin, Y.V.; Shchekochikhin, D.Y.; Generozov, E.V.; Akselrod, A.S. Circulating Extracellular miRNA Analysis in Patients with Stable CAD and Acute Coronary Syndromes. Biomolecules 2021, 11, 962. https://doi.org/10.3390/biom11070962
Zhelankin AV, Stonogina DA, Vasiliev SV, Babalyan KA, Sharova EI, Doludin YV, Shchekochikhin DY, Generozov EV, Akselrod AS. Circulating Extracellular miRNA Analysis in Patients with Stable CAD and Acute Coronary Syndromes. Biomolecules. 2021; 11(7):962. https://doi.org/10.3390/biom11070962
Chicago/Turabian StyleZhelankin, Andrey V., Daria A. Stonogina, Sergey V. Vasiliev, Konstantin A. Babalyan, Elena I. Sharova, Yurii V. Doludin, Dmitry Y. Shchekochikhin, Eduard V. Generozov, and Anna S. Akselrod. 2021. "Circulating Extracellular miRNA Analysis in Patients with Stable CAD and Acute Coronary Syndromes" Biomolecules 11, no. 7: 962. https://doi.org/10.3390/biom11070962
APA StyleZhelankin, A. V., Stonogina, D. A., Vasiliev, S. V., Babalyan, K. A., Sharova, E. I., Doludin, Y. V., Shchekochikhin, D. Y., Generozov, E. V., & Akselrod, A. S. (2021). Circulating Extracellular miRNA Analysis in Patients with Stable CAD and Acute Coronary Syndromes. Biomolecules, 11(7), 962. https://doi.org/10.3390/biom11070962