Ex Vivo Activation of Red Blood Cell Senescence by Plasma from Sickle-Cell Disease Patients: Correlation between Markers and Adhesion Consequences during Acute Disease Events
Abstract
1. Introduction
2. Study Design and Methods
3. Results
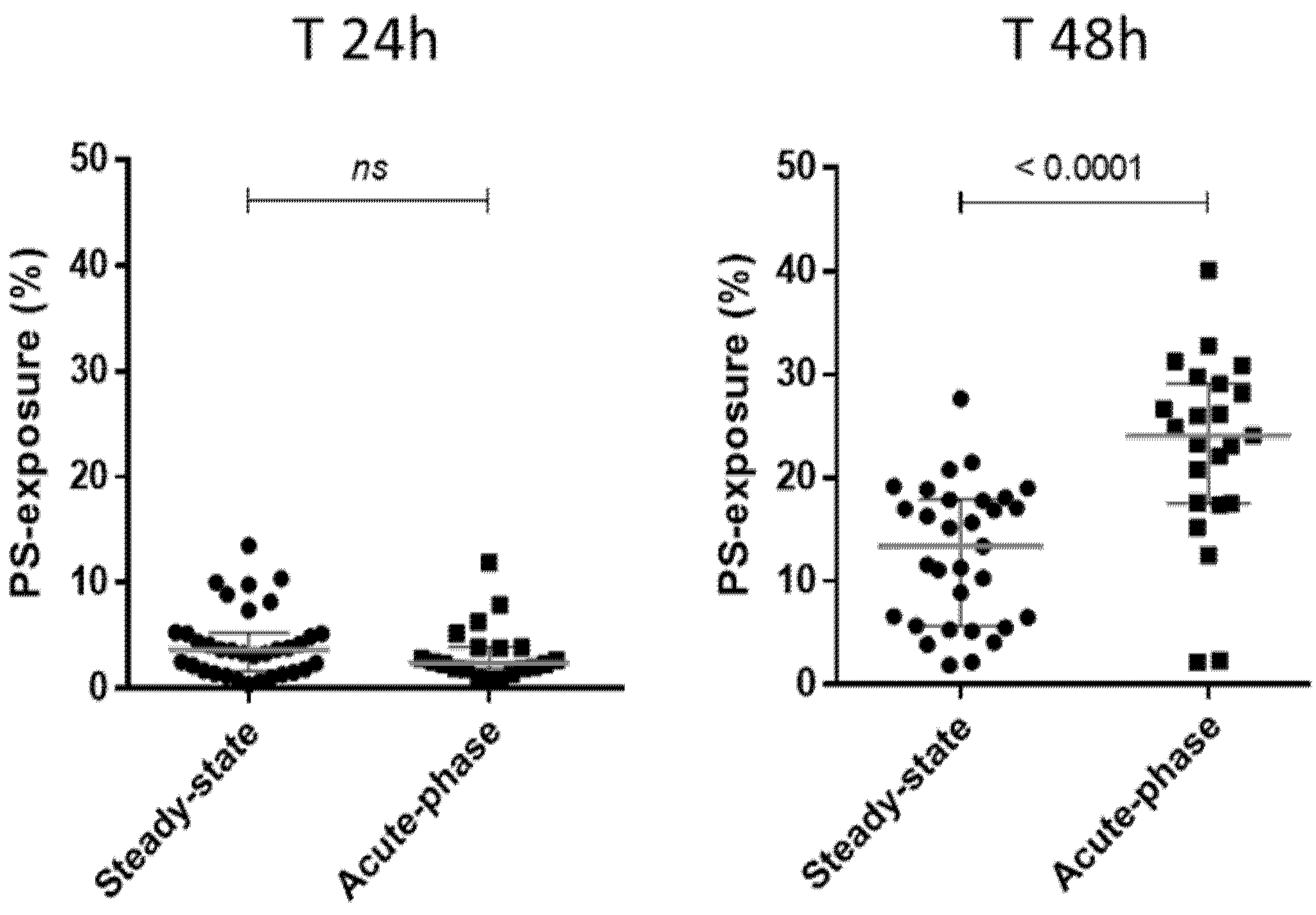
3.1. Exposure of Donor AA RBCs from Blood Units to Pathological Plasma from SCD Patients Leads to RBC Senescence Correlated with the Patients’ Clinical Status
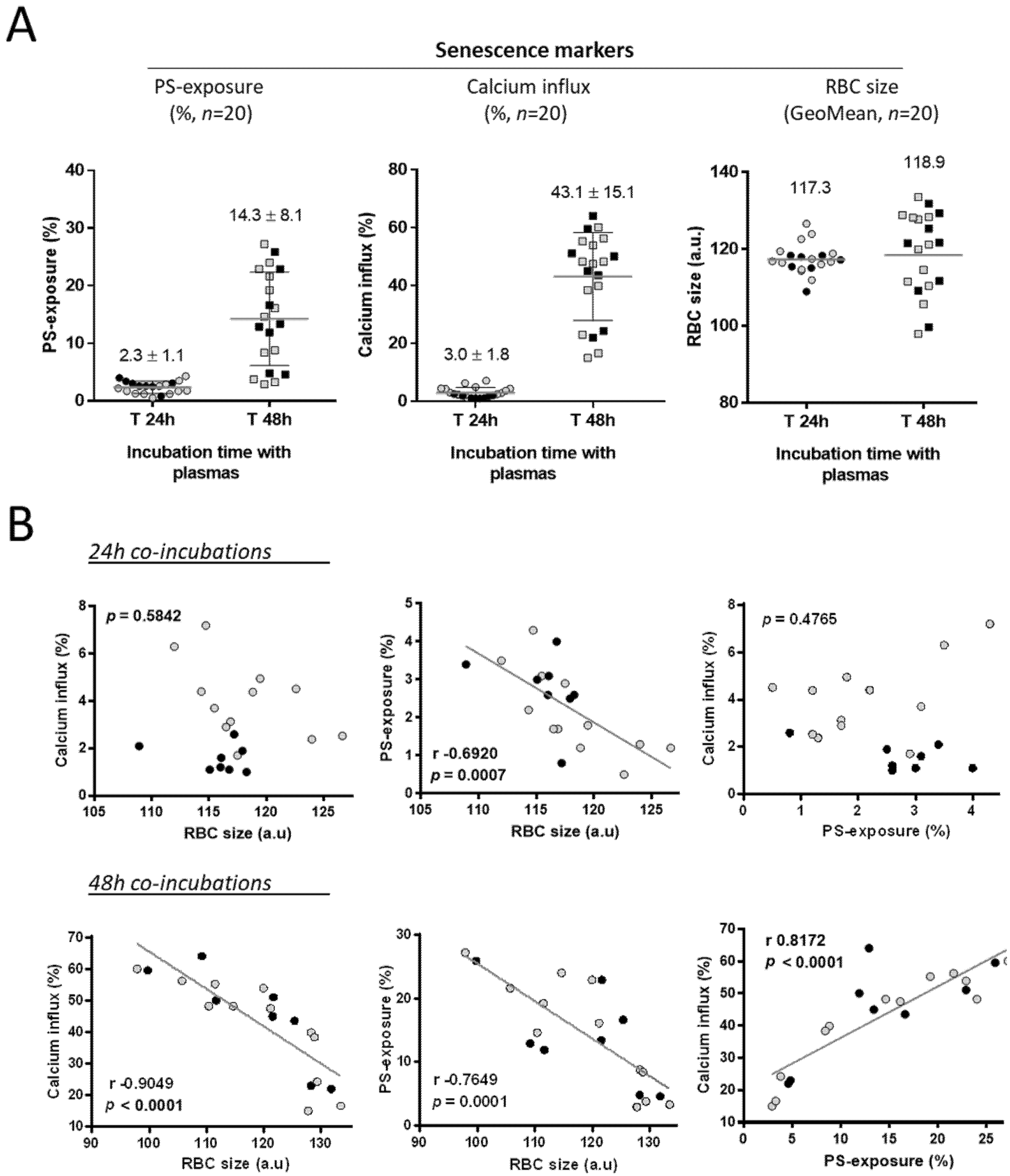
3.2. The Levels of Senescence Markers on AA RBCs from Blood Units Are Correlated after a 48 h Incubation Period with Plasma from SCD Patients
3.3. Senescence of AA RBCs Induced by SCD Plasma Samples Leads to in-Flow Adhesion to Thrombospondin
3.4. The Strength of Senescent RBC’ Adhesion to Thrombospondin Is Related to the Clinical Status of the Patient from Whom the Plasma Sample Was Collected
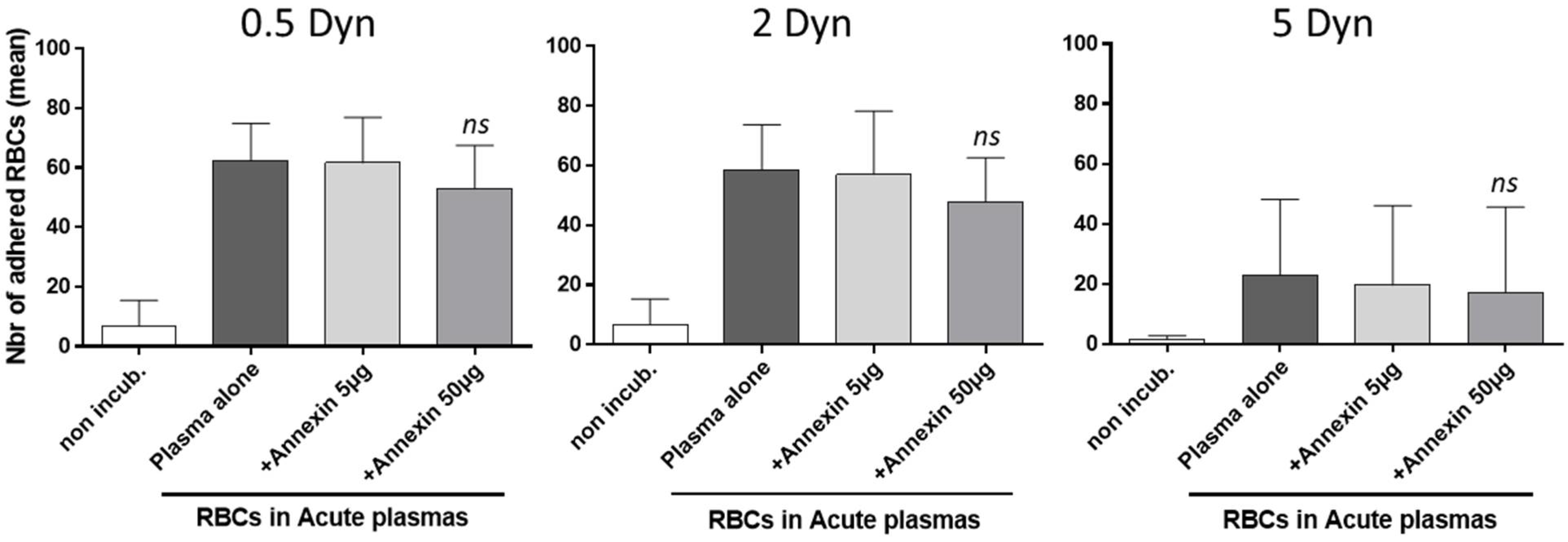
3.5. Adhesion to Thrombospondin of AA RBCs, Rendered Senescent by SCD Acute Plasma Samples, Is Not Reversed by an Annexin V Pretreatment
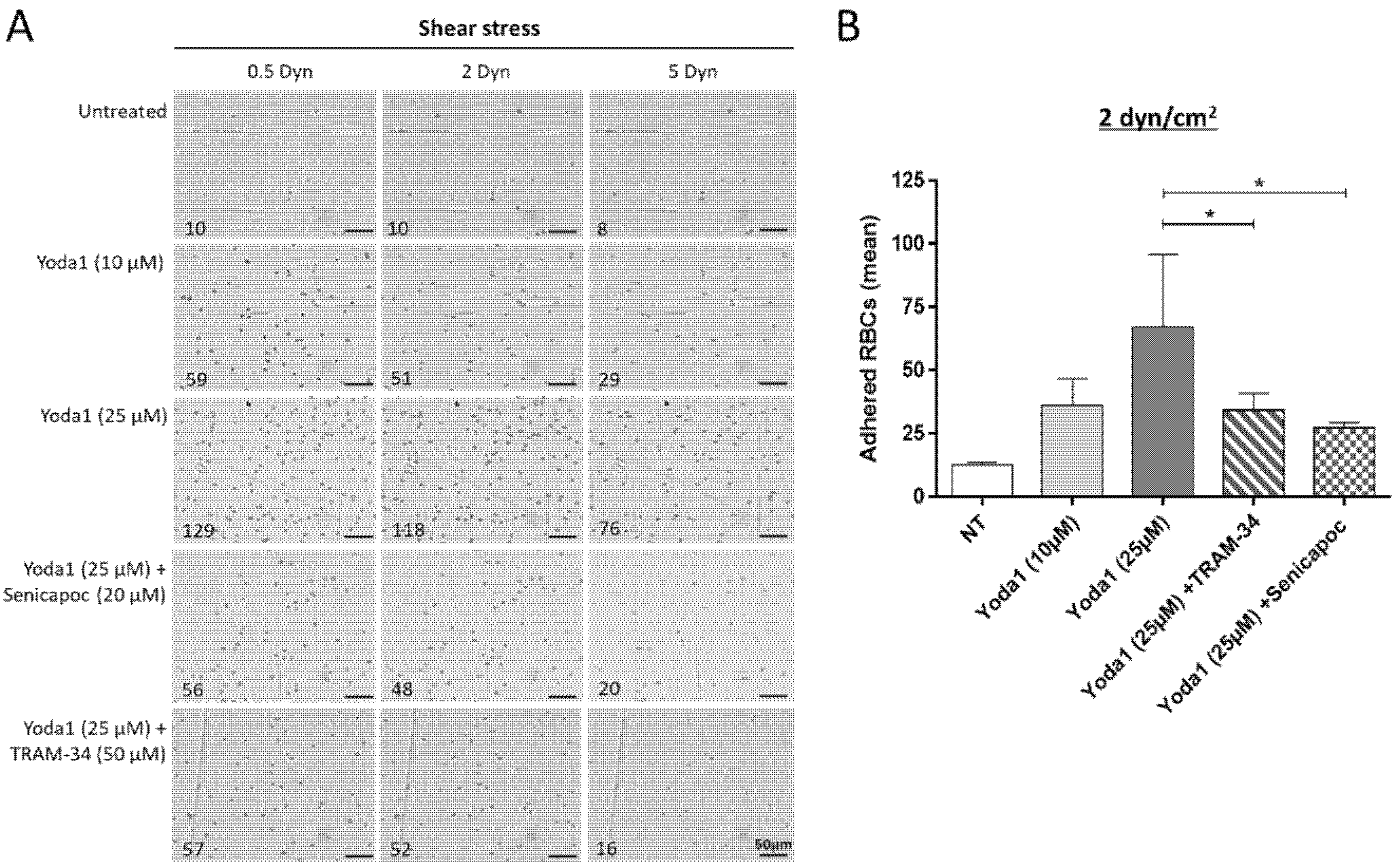
3.6. The Adhesion of Senescent RBCs to Thrombospondin Can Be Reproduced In Vitro with an Agonist of Piezo1 and Blocked by Gardos Channel Inhibitors
4. Discussion
5. Study Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Wanko, S.O.; Telen, M.J. Transfusion management in sickle cell disease. Hematol. Oncol. Clin. N. Am. 2005, 5, 803–826. [Google Scholar] [CrossRef] [PubMed]
- Karon, B.S.; van Buskirk, C.M.; Jaben, E.A.; Hoyer, J.D.; Thomas, D.D. Temporal sequence of major biochemical events during blood bank storage of packed red blood cells. Blood Transfus. 2012, 4, 453–461. [Google Scholar]
- Bennett-Guerrero, E.; Veldman, T.H.; Doctor, A.; Telen, M.J.; Ortel, T.L.; Reid, T.S.; Mulherin, M.A.; Zhu, H.; Buck, R.D.; Califf, R.M.; et al. Evolution of adverse changes in stored RBCs. Proc. Natl. Acad. Sci. USA 2007, 104, 17063–17068. [Google Scholar] [CrossRef] [PubMed]
- Council of Europe. Guide to the Preparation, Use and Quality Assurance of Blood Components. Recommendation n°R(95)15 on the Preparation, Use and Quality Assurance of Blood Components, 14th ed.; Council of Europe Publishing: Strasbourg, France, 2008. [Google Scholar]
- Hess, J.R. An update of solutions for red cell storage. Vox Sang 2006, 91, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Luten, M.; Roerdinkholder-Stoelwinder, B.; Schaap, N.P.; de Grip, W.J.; Bos, H.J.; Bosman, G.J. Survival of red blood cells after transfusion: A comparison between red cells concentrates of different storage periods. Transfusion 2008, 48, 1478–1485. [Google Scholar] [CrossRef] [PubMed]
- Janz, D.R.; Zhao, Z.; Koyama, T.; May, A.K.; Bernard, G.R.; Bastarache, J.A.; Young, P.P.; Ware, L.B. Longer storage duration of red blood cells is associated with an increased risk of acute lung injury in patients with sepsis. Ann. Intensive Care 2013, 3, 33. [Google Scholar] [CrossRef]
- Bosman, G.J.; Willekens, F.L.; Were, J.M. Erythrocyte aging: A more than superficial resemblance to apoptosis? Cell Physiol. Biochem. 2005, 16, 1–8. [Google Scholar] [CrossRef]
- Chadebech, P.; Habibi, A.; Nzouakou, R.; Bachir, D.; Meunier-Costes, N.; Bonin, P.; Rodet, M.; Chami, B.; Galacteros, F.; Bierling, P.; et al. Delayed hemolytic transfusion reaction in sickle cell disease patients: Evidence of an emerging syndrome with suicidal red blood cell death. Transfusion 2009, 9, 1785–1792. [Google Scholar] [CrossRef]
- Lang, K.S.; Duranton, C.; Poehlmann, H.; Myssina, S.; Bauer, C.; Lang, F.; Wieder, T.; Huber, S.M. Cation channels trigger apoptotic death of erythrocytes. Cell Death Differ. 2003, 10, 249–256. [Google Scholar] [CrossRef]
- Gatidis, S.; Föller, M.; Lang, F. Hemin-induced suicidal erythrocyte death. Ann. Hematol. 2009, 8, 721–726. [Google Scholar] [CrossRef]
- Chadebech, P.; Bodivit, G.; Razazi, K.; de Vassoigne, C.; Pellé, L.; Burin-des-Roziers, N.; Bocquet, T.; Bierling, P.; Djoudi, R.; Mekontso-Dessap, A.; et al. Red blood cells for transfusion in patients with sepsis: Respective roles of unit age and exposure to recipient plasma. Transfusion 2017, 8, 1898–1904. [Google Scholar] [CrossRef] [PubMed]
- Kuypers, F.A.; de Jong, K. The role of phosphatidylserine in recognition and removal of erythrocytes. Cell Mol. Biol. 2004, 2, 147–158. [Google Scholar]
- Pantaleo, A.; Giribaldi, G.; Mannu, F.; Arese, P.; Turrini, F. Naturally occurring anti-band 3 antibodies and red blood cell removal under physiological and pathological conditions. Autoimmun. Rev. 2008, 6, 457–462. [Google Scholar] [CrossRef]
- Klei, T.R.L.; de Back, D.Z.; Asif, P.J.; Verkuijlen, P.J.J.H.; Veldthuis, M.; Ligthart, P.C.; Berghuis, J.; Clifford, E.; Beuger, B.M.; van den Berg, T.K.; et al. Glycophorin-C sialylation regulates Lu/BCAM adhesive capacity during erythrocyte aging. Blood Adv. 2018, 1, 14–24. [Google Scholar] [CrossRef]
- Kuck, L.; Peart, J.N.; Simmonds, M.J. Active modulation of human erythrocyte mechanics. Am. J. Physiol. Cell Physiol. 2020, 2, C250–C257. [Google Scholar] [CrossRef]
- Föller, M.; Lang, F. Ion transport in eryptosis, the suicidal death of erythrocytes. Front. Cell Dev. Biol. 2020, 8, 597. [Google Scholar] [CrossRef]
- Burger, P.; Kostova, E.; Bloem, E.; Hilarius-Stokman, P.; Meijer, A.B.; van den Berg, T.K.; Verhoeven, A.J.; de Korte, D.; van Bruggen, R. Potassium leakage primes stored erythrocytes for phosphatidylserine exposure and shedding of pro-coagulant vesicles. Br. J. Haematol. 2013, 160, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Borst, O.; Abed, M.; Alesutan, I.; Towhid, S.T.; Syed, S.M.; Föller, M.; Gawaz, M.; Lang, F. Dynamic adhesion of eryptotic erythrocytes to endothelial cells via CXCL16/SR-PSOX. Am. J. Physiol. Cell Physiol. 2012, 4, C644–C651. [Google Scholar] [CrossRef] [PubMed]
- Setty, B.N.; Kulkarni, S.; Stuart, M.J. Role of erythrocyte phosphatidylserine in sickle red cell-endothelial adhesion. Blood 2002, 5, 1564–1571. [Google Scholar] [CrossRef] [PubMed]
- Lang, K.S.; Lang, P.A.; Bauer, C.; Duranton, C.; Wieder, T.; Huber, S.M.; Lang, F. Mechanisms of suicidal erythrocyte death. Cell Physiol. Biochem. 2005, 15, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.; Lang, K.S.; Lang, P.A.; Huber, S.M.; Wieder, T. Mechanisms and significance of eryptosis. Antioxid. Redox Signal 2006, 8, 1183–1192. [Google Scholar] [CrossRef]
- Chadebech, P.; de Ménorval, M.A.; Bodivit, G.; Mekontso-Dessap, A.; Pakdaman, S.; Jouard, A.; Galactéros, F.; Bierling, P.; Habibi, A.; Pirenne, F. Evidence for benefits of the use fresh and cryopreserved blood to transfuse patients with acute sickle cell disease. Transfusion 2016, 56, 1730–1738. [Google Scholar] [CrossRef]
- Closse, C.; Dachary-Prigent, J.; Boisseau, M.R. Phosphatidylserine-related adhesion of human erythrocytes to vascular endothelium. Br. J. Haematol. 1999, 107, 300–302. [Google Scholar] [CrossRef]
- Fens, M.H.; van Wijk, R.; Andringa, G.; van Rooijen, K.L.; Dijstelbloem, H.M.; Rasmussen, J.T.; de Vooght, K.M.K.; Schiffelers, R.M.; Gaillard, C.A.J.M.; van Solinge, W.W. A role for activated endothelial cells in red blood cell clearance: Implications for vasopathology. Haematologica 2012, 97, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Noizat-Pirenne, F.; Habibi, A.; Mekontso-Dessap, A.; Razazi, K.; Chadebech, P.; Mahevas, M.; Vingert, B.; Bierling, P.; Galactéros, F.; Bartolucci, P.; et al. The use of rituximab to prevent severe delayed haemolytic transfusion reaction in immunized patients with sickle cell disease. Vox Sang. 2015, 3, 262–267. [Google Scholar] [CrossRef]
- Chaar, V.; Laurance, S.; Lapoumeroulie, C.; Cochet, S.; De Grandis, M.; Colin, Y.; Elion, J.; Le Van Kim, C.; El-Nemer, W. Hydroxycarbamide decreases sickle reticulocyte adhesion to resting endothelium by inhibiting endothelial lutheran/basal cell adhesion molecule (Lu/BCAM) through phosphodiesterase 4A activation. J. Biol. Chem. 2014, 16, 11512–11521. [Google Scholar] [CrossRef] [PubMed]
- Bartolucci, P.; Chaar, V.; Picot, J.; Bachir, D.; Habibi, A.; Fauroux, C.; Galactéros, F.; Colin, Y.; Le Van Kim, C.; El Nemer, W. Decreased sickle red blood cell adhesion to laminin by hydroxyurea is associated with inhibition of Lu/BCAM protein phosphorylation. Blood 2010, 12, 2152–2159. [Google Scholar] [CrossRef]
- Di Liberto, G.; Kiger, L.; Marden, M.C.; Boyer, L.; Poitrine, F.C.; Conti, M.; Rakotoson, M.G.; Habibi, A.; Khorgami, S.; Vingert, B.; et al. Dense red blood cell and oxygen desaturation in sickle-cell disease. Am. J. Hematol. 2016, 10, 1008–1013. [Google Scholar] [CrossRef] [PubMed]
- Rakotoson, M.G.; Di Liberto, G.; Audureau, E.; Habibi, A.; Fauroux, C.; Khorgami, S.; Hulin, A.; Loric, S.; Noizat-Pirenne, F.; Galacteros, F.; et al. Biological parameters predictive of percent dense red blood cell decrease under hydroxyurea. Orphanet J. Rare Dis. 2015, 10, 57. [Google Scholar] [CrossRef]
- Lang, F.; Lang, K.S.; Lang, P.A.; Huber, S.M.; Wieder, T. Osmotic shock-induced suicidal death of erythrocytes. Acta Physiol. 2006, 1-2, 191–198. [Google Scholar] [CrossRef]
- Kempe, D.S.; Akel, A.; Lang, P.A.; Hermle, T.; Biswas, R.; Muresanu, J.; Friedrich, B.; Dreischer, P.; Wolz, C.; Schumacher, U.; et al. Suicidal erythrocyte death in sepsis. J. Mol. Med. 2007, 3, 273–281. [Google Scholar] [CrossRef]
- Liu, C.; Marshall, P.; Schreibman, I.; Vu, A.; Gai, W.; Whitlow, M. Interaction between terminal complement proteins C5b-7 and anionic phospholipids. Blood 1999, 7, 2297–2301. [Google Scholar] [CrossRef]
- Haynes, J., Jr.; Obiako, B.; Hester, R.B.; Baliga, B.S.; Stevens, T. Activated neutrophil-mediated sickle red blood cell adhesion to lung vascular endothelium: Role of phosphatidylserine-exposed sickle red blood cells. Am. J. Physiol. Heart Circ. Physiol. 2008, 1, H379–H385. [Google Scholar] [CrossRef][Green Version]
- Kaul, D.K.; Finnegan, E.; Barabino, G.A. Sickle red cell-endothelium interactions. Microcirculation 2009, 16, 97–111. [Google Scholar] [CrossRef]
- Rother, R.P.; Bell, L.; Hillmen, P.; Gladwin, M.T. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: A novel mechanism of human disease. JAMA 2005, 293, 1653–1662. [Google Scholar] [CrossRef] [PubMed]
- Potoka, K.P.; Gladwin, M.T. Vasculopathy and pulmonary hypertension in sickle cell disease. Am. J. Physiol. Lung Cell Mol. Physiol. 2015, 308, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Neidlinger, N.A.; Larkin, S.K.; Bhagat, A.; Victorino, G.P.; Kuypers, F.A. Hydrolysis of phosphatidylserine-exposing red blood cells by secretory phospholipase A2 generates lysophosphatidic acid and results in vascular dysfunction. J. Biol. Chem. 2006, 2, 775–781. [Google Scholar] [CrossRef]
- Lang, P.A.; Beringer, O.; Nicolay, J.P.; Amon, O.; Kempe, D.S.; Hermle, T.; Attanasio, P.; Akel, A.; Schäfer, R.; Friedrich, B.; et al. Suicidal death of erythrocytes in recurrent hemolytic uremic syndrome. J. Mol. Med. 2006, 5, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Klei, T.R.L.; Dalimot, J.J.; Beuger, B.M.; Veldthuis, M.; Ichou, F.A.; Verkuijlen, P.J.J.H.; Seignette, I.M.; Ligthart, P.C.; Kuijpers, T.W.; van Zwieten, R.; et al. The Gardos effect drives erythrocyte senescence and leads to Lu/BCAM and CD44 adhesion molecule activation. Blood Adv. 2020, 4, 6218–6229. [Google Scholar] [CrossRef] [PubMed]
- Rogers, S.; Lew, V.L. PIEZO1 and the mechanism of the long circulatory longevity of human red blood cells. PLoS Comput. Biol. 2021, 17, e1008496. [Google Scholar] [CrossRef] [PubMed]
- Wadud, R.; Hannemann, A.; Rees, D.C.; Brewin, J.N.; Gibson, J.S. Yoda1 and phosphatidylserine exposure in red cells from patients with sickle cell anaemia. Sci. Rep. 2020, 10, 20110. [Google Scholar] [CrossRef]
- Patel, A.; Demolombe, S.; Honoré, E. An alternative to force. Elife 2015, 4, e08659. [Google Scholar] [CrossRef]
- De Franceschi, L.; Franco, R.S.; Bertoldi, M.; Brugnara, C.; Matté, A.; Siciliano, A.; Wieschhaus, A.J.; Chishti, A.H.; Joiner, C.H. Pharmacological inhibition of calpain-1 prevents red cell dehydration and reduces Gardos channel activity in a mouse model of sickle cell disease. FASEB J. 2013, 2, 750–759. [Google Scholar] [CrossRef]
- Nwankwo, J.O.; Lei, J.; Xu, J.; Rivera, A.; Gupta, K.; Chishti, A.H. Genetic inactivation of calpain-1 attenuates pain sensitivity in a humanized mouse model of sickle cell disease. Haematologica 2016, 10, e397–e400. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aglialoro, F.; Hofsink, N.; Hofman, M.; Brandhorst, N.; van den Akker, E. Inside out integrin activation mediated by PIEZO1 signaling in erythroblasts. Front. Physiol. 2020, 11, 958. [Google Scholar] [CrossRef] [PubMed]
- Cartron, J.P.; Elion, J. Erythroid adhesion molecules in sickle cell disease: Effect of hydroxyurea. Transfus Clin. Biol. 2008, 1–2, 39–50. [Google Scholar] [CrossRef]
- Novelli, E.M.; Kato, G.J.; Ragni, M.V.; Zhang, Y.; Hildesheim, M.E.; Nouraie, M.; Barge, S.; Meyer, M.P.; Hassett, A.C.; Gordeuk, V.R.; et al. Plasma thrombospondin-1 is increased during acute sickle cell vaso-occlusive events and associated with acute chest syndrome, hydroxyurea therapy, and lower hemolytic rates. Am. J. Hematol. 2012, 3, 326–330. [Google Scholar] [CrossRef]
- Lee, K.; Gane, P.; Roudot-Thoraval, F.; Godeau, B.; Bachir, D.; Bernaudin, F.; Cartron, J.P.; Galactéros, F.; Bierling, P. The nonexpression of CD36 on reticulocytes and mature red blood cells does not modify the clinical course of patients with sickle cell anemia. Blood 2001, 4, 966–971. [Google Scholar] [CrossRef]
- Le Toriellec, E.; Muralitharan, V.; Chadebech, P.; Jouard, A.; Ansart-Pirenne, H.; Pirenne, F.; Tournamille, C.; Croisille, L. New molecular basis associated with CD36-negative phenotype in the sub-Saharan African population. Transfusion 2020, 11, 2482–2488. [Google Scholar] [CrossRef]
- Bissinger, R.; Petkova-Kirova, P.; Mykhailova, O.; Oldenborg, P.A.; Novikova, E.; Donkor, D.A.; Dietz, T.; Bhuyan, A.A.M.; Sheffield, W.P.; Grau, M.; et al. Thrombospondin-1/CD47 signaling modulates transmembrane cation conductance, survival, and deformability of human red blood cells. Cell Commun. Signal 2020, 1, 155. [Google Scholar] [CrossRef]
- Novelli, E.M.; Little-Ihrig, L.; Knupp, H.E.; Rogers, N.M.; Yao, M.; Baust, J.J.; Meijles, D.; Croix, C.M.S.; Ross, M.A.; Pagano, P.J.; et al. Vascular TSP1-CD47 signaling promotes sickle cell-associated arterial vasculopathy and pulmonary hypertension in mice. Am. J. Physiol. Cell. Mol. Physiol. 2019, 316, L1150–L1164. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chadebech, P.; Bodivit, G.; Di Liberto, G.; Jouard, A.; Vasseur, C.; Pirenne, F.; Bartolucci, P. Ex Vivo Activation of Red Blood Cell Senescence by Plasma from Sickle-Cell Disease Patients: Correlation between Markers and Adhesion Consequences during Acute Disease Events. Biomolecules 2021, 11, 963. https://doi.org/10.3390/biom11070963
Chadebech P, Bodivit G, Di Liberto G, Jouard A, Vasseur C, Pirenne F, Bartolucci P. Ex Vivo Activation of Red Blood Cell Senescence by Plasma from Sickle-Cell Disease Patients: Correlation between Markers and Adhesion Consequences during Acute Disease Events. Biomolecules. 2021; 11(7):963. https://doi.org/10.3390/biom11070963
Chicago/Turabian StyleChadebech, Philippe, Gwellaouen Bodivit, Gaétana Di Liberto, Alicia Jouard, Corinne Vasseur, France Pirenne, and Pablo Bartolucci. 2021. "Ex Vivo Activation of Red Blood Cell Senescence by Plasma from Sickle-Cell Disease Patients: Correlation between Markers and Adhesion Consequences during Acute Disease Events" Biomolecules 11, no. 7: 963. https://doi.org/10.3390/biom11070963
APA StyleChadebech, P., Bodivit, G., Di Liberto, G., Jouard, A., Vasseur, C., Pirenne, F., & Bartolucci, P. (2021). Ex Vivo Activation of Red Blood Cell Senescence by Plasma from Sickle-Cell Disease Patients: Correlation between Markers and Adhesion Consequences during Acute Disease Events. Biomolecules, 11(7), 963. https://doi.org/10.3390/biom11070963





