Diagnostic Significance of Serum Galectin-3 in Hospitalized Patients with COVID-19—A Preliminary Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Protocol and Patients
2.2. Laboratory Tests
2.3. Statistical Analysis
3. Results
3.1. Characteristics of Patients
3.2. Association of Galectin-3 with the Severity of COVID-19
3.3. Correlations of Galectin-3 with Clinical Characteristics of Patients and Laboratory Test Results
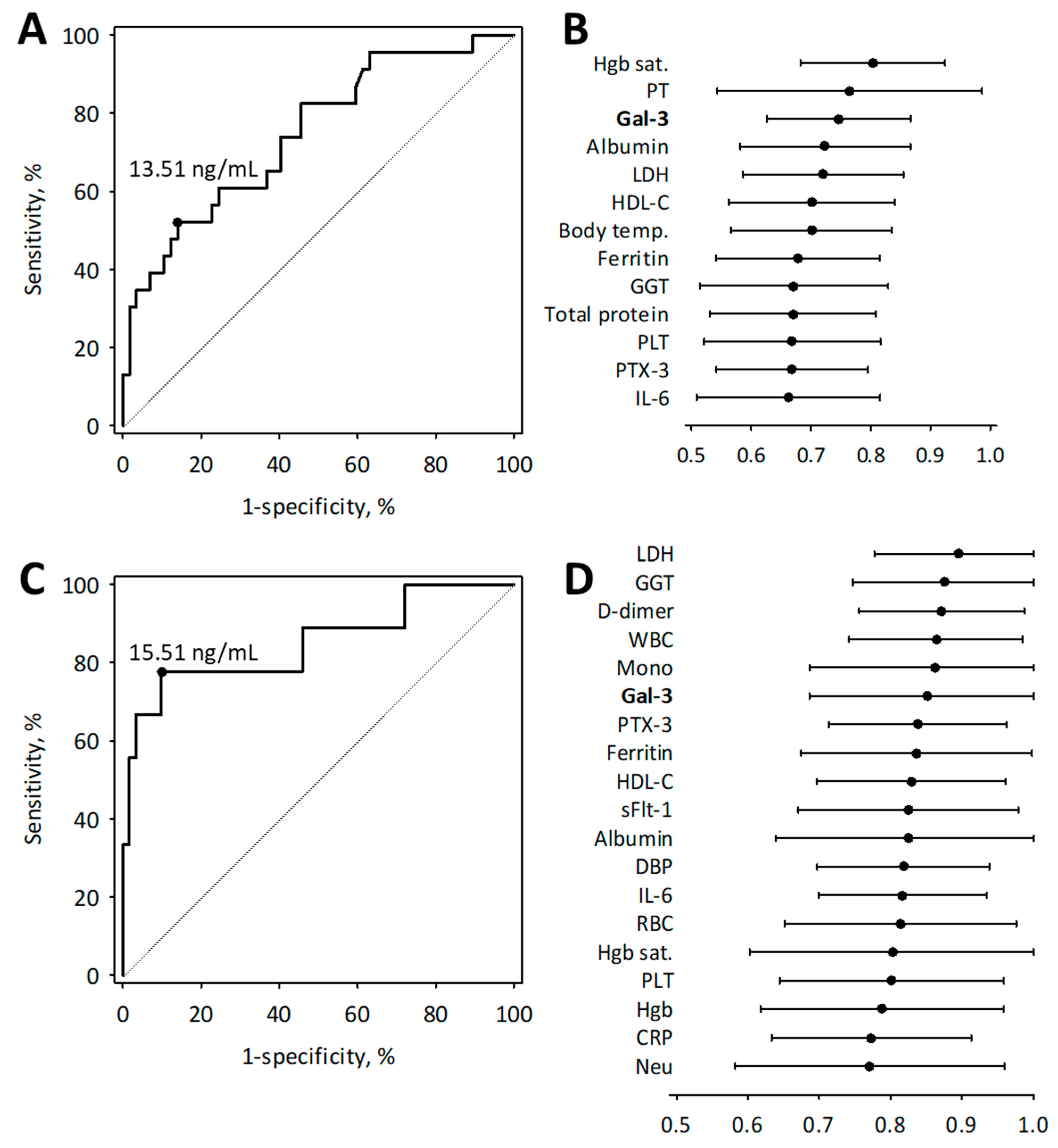
3.4. Diagnostic Usefulness of Galectin-3 in Moderate to Severe COVID-19
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Flisiak, R.; Parczewski, M.; Horban, A.; Jaroszewicz, J.; Kozielewicz, D.; Pawłowska, M.; Piekarska, A.; Simon, K.; Tomasiewicz, K.; Zarębska-Michaluk, D. Management of SARS-CoV-2 infection: Recommendations of the polish association of epidemiologists and infectiologists. Annex no. 2 as of October 13, 2020. Pol. Arch. Intern. Med. 2020, 130, 915–918. [Google Scholar] [CrossRef]
- Skevaki, C.; Fragkou, P.C.; Cheng, C.; Xie, M.; Renz, H. Laboratory characteristics of patients infected with the novel SARS-CoV-2 virus. J. Infect. 2020, 81, 205–212. [Google Scholar] [CrossRef]
- Pourbagheri-Sigaroodi, A.; Bashash, D.; Fateh, F.; Abolghasemi, H. Laboratory findings in COVID-19 diagnosis and prognosis. Clin. Chim. Acta 2020, 510, 475–482. [Google Scholar] [CrossRef]
- Moutchia, J.; Pokharel, P.; Kerri, A.; McGaw, K.; Uchai, S.; Nji, M.; Goodman, M. Clinical laboratory parameters associated with severe or critical novel coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. PLoS ONE 2020, 15, e0239802. [Google Scholar] [CrossRef]
- Favaloro, E.J.; Lippi, G. Recommendations for Minimal Laboratory Testing Panels in Patients with COVID-19: Potential for Prognostic Monitoring. Semin. Thromb. Hemost. 2020, 46, 379–382. [Google Scholar] [CrossRef] [Green Version]
- Schirinzi, A.; Pesce, F.; Laterza, R.; D’Alise, M.G.; Lovero, R.; Fontana, A.; Contino, R.; Di Serio, F. Pentraxin 3: Potential prognostic role in SARS-CoV-2 patients admitted to the emergency department. J. Infect. 2021, 82, 84–123. [Google Scholar] [CrossRef]
- Brunetta, E.; Folci, M.; Bottazzi, B.; De Santis, M.; Gritti, G.; Protti, A.; Mapelli, S.N.; Bonovas, S.; Piovani, D.; Leone, R.; et al. Macrophage expression and prognostic significance of the long pentraxin PTX3 in COVID-19. Nat. Immunol. 2021, 22, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka-Tojo, M. Vascular endothelial glycocalyx damage in COVID-19. Int. J. Mol. Sci. 2020, 21, 9712. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka-Tojo, M. Endothelial glycocalyx damage as a systemic inflammatory microvascular endotheliopathy in COVID-19. Biomed. J. 2020, 43, 399–413. [Google Scholar] [CrossRef]
- Iba, T.; Levy, J.H.; Levi, M.; Thachil, J. Coagulopathy in COVID-19. J. Thromb. Haemost. 2020, 18, 2103–2109. [Google Scholar] [CrossRef] [PubMed]
- Thachil, J.; Longstaff, C.; Favaloro, E.J.; Lippi, G.; Urano, T.; Kim, P.Y. The need for accurate D-dimer reporting in COVID-19: Communication from the ISTH SSC on fibrinolysis. J. Thromb. Haemost. 2020, 18, 2408–2411. [Google Scholar] [CrossRef]
- Smadja, D.M.; Guerin, C.L.; Chocron, R.; Yatim, N.; Boussier, J.; Gendron, N.; Khider, L.; Hadjadj, J.; Goudot, G.; Debuc, B.; et al. Angiopoietin-2 as a marker of endothelial activation is a good predictor factor for intensive care unit admission of COVID-19 patients. Angiogenesis 2020, 23, 611–620. [Google Scholar] [CrossRef]
- Dumnicka, P.; Sporek, M.; Mazur-Laskowska, M.; Ceranowicz, P.; Kuźniewski, M.; Drożdż, R.; Ambroży, T.; Olszanecki, R.; Kuśnierz-Cabala, B. Serum soluble fms-Like tyrosine kinase 1 (sFlt-1) predicts the severity of acute pancreatitis. Int. J. Mol. Sci. 2016, 17, 2038. [Google Scholar] [CrossRef] [Green Version]
- Dumnicka, P.; Kuśnierz-Cabala, B.; Sporek, M.; Mazur-Laskowska, M.; Gil, K.; Kuźniewski, M.; Ceranowicz, P.; Warzecha, Z.; Dembiński, A.; Bonior, J.; et al. Serum concentrations of angiopoietin-2 and soluble fms-like tyrosine kinase 1 (sFlt-1) are associated with coagulopathy among patients with acute pancreatitis. Int. J. Mol. Sci. 2017, 18, 753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Revilla, J.; Deierborg, T.; Venero, J.L.; Boza-Serrano, A. Hyperinflammation and Fibrosis in Severe COVID-19 Patients: Galectin-3, a Target Molecule to Consider. Front. Immunol. 2020, 11, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Madrigal-Matute, J.; Lindholt, J.S.; Fernandez-Garcia, C.E.; Benito-Martin, A.; Burillo, E.; Zalba, G.; Beloqui, O.; Llamas-Granda, P.; Ortiz, A.; Egido, J.; et al. Galectin-3, a biomarker linking oxidative stress and inflammation with the clinical outcomes of patients with atherothrombosis. J. Am. Heart Assoc. 2014, 3, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Fei, Y.; Yao, M.; Tao, J.; Deng, J.; Huang, B. Correlation between Galectin-3 and Early Herpes Zoster Neuralgia and Postherpetic Neuralgia: A Retrospective Clinical Observation. Pain Res. Manag. 2020, 2020, 8730918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, J.; Fu, C.; Cong, Z.; Peng, L.; Peng, Z.; Chen, T.; Wang, W.; Jiang, H.; Wei, Q.; Qin, C. Galectin-3 promotes caspase-independent cell death of HIV-1-infected macrophages. FEBS J. 2017, 284, 97–113. [Google Scholar] [CrossRef]
- Lin, C.Y.; Wang, W.H.; Huang, S.W.; Yeh, C.S.; Yuan, R.Y.; Yang, Z.S.; Urbina, A.N.; Tseng, S.P.; Lu, P.L.; Chen, Y.H.; et al. The examination of viral characteristics of HIV-1 CRF07_BC and its potential interaction with extracellular galectin-3. Pathogens 2020, 9, 425. [Google Scholar] [CrossRef] [PubMed]
- King, R.D.; Lubinski, J.M.; Friedman, H.M. Herpes simplex virus type 1 infection increases the carbohydrate binding activity and the secretion of cellular galectin-3. Arch. Virol. 2009, 154, 609–618. [Google Scholar] [CrossRef]
- Caniglia, J.L.; Asuthkar, S.; Tsung, A.J.; Guda, M.R.; Velpula, K.K. Immunopathology of galectin-3: An increasingly promising target in COVID-19. F1000Research 2020, 9, 1078. [Google Scholar] [CrossRef]
- Caniglia, J.L.; Guda, M.R.; Asuthkar, S.; Tsung, A.J.; Velpula, K.K. A potential role for Galectin-3 inhibitors in the treatment of COVID-19. PeerJ 2020, 8, e9392. [Google Scholar] [CrossRef] [PubMed]
- Buszko, M.; Park, J.H.; Verthelyi, D.; Sen, R.; Young, H.A.; Rosenberg, A.S. The dynamic changes in cytokine responses in COVID-19: A snapshot of the current state of knowledge. Nat. Immunol. 2020, 21, 1146–1151. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Lee, J.Y.; Yang, J.W.; Lee, K.H.; Effenberger, M.; Szpirt, W.; Kronbichler, A.; Shin, J., II. Immunopathogenesis and treatment of cytokine storm in COVID-19. Theranostics 2020, 11, 316–329. [Google Scholar] [CrossRef] [PubMed]
- Behloul, N.; Baha, S.; Shi, R.; Meng, J. Role of the GTNGTKR motif in the N-terminal receptor-binding domain of the SARS-CoV-2 spike protein. Virus Res. 2020, 286, 198058. [Google Scholar] [CrossRef] [PubMed]
- Pirone, L.; Del Gatto, A.; Di Gaetano, S.; Saviano, M.; Capasso, D.; Zaccaro, L.; Pedone, E. A multi-targeting approach to fight sars-cov-2 attachment. Front. Mol. Biosci. 2020, 7, 186. [Google Scholar] [CrossRef] [PubMed]
- Kazancioglu, S.; Yilmaz, F.M.; Bastug, A.; Ozbay, B.O.; Aydos, O.; Yücel, Ç.; Bodur, H.; Yilmaz, G. Assessment of Galectin-1, Galectin-3, and PGE2 Levels in Patients with COVID-19. Jpn. J. Infect. Dis. 2021. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- De Biasi, S.; Meschiari, M.; Gibellini, L.; Bellinazzi, C.; Borella, R.; Fidanza, L.; Gozzi, L.; Iannone, A.; Lo Tartaro, D.; Mattioli, M.; et al. Marked T cell activation, senescence, exhaustion and skewing towards TH17 in patients with COVID-19 pneumonia. Nat. Commun. 2020, 11, 1–17. [Google Scholar] [CrossRef]
- Díaz-Alvarez, L.; Ortega, E. The Many Roles of Galectin-3, a Multifaceted Molecule, in Innate Immune Responses against Pathogens. Mediators Inflamm. 2017, 2017, 9–12. [Google Scholar] [CrossRef] [Green Version]
- Filer, A.; Bik, M.; Parsonage, G.N.; Fitton, J.; Trebilcock, E.; Howlett, K.; Cook, M.; Raza, K.; Simmons, D.L.; Thomas, A.M.C.; et al. Galectin 3 induces a distinctive pattern of cytokine and chemokine production in rheumatoid synovial fibroblasts via selective signaling pathways. Arthritis Rheum. 2009, 60, 1604–1614. [Google Scholar] [CrossRef] [Green Version]
- Uchino, Y.; Woodward, A.M.; Mauris, J.; Peterson, K.; Verma, P.; Nilsson, U.J.; Rajaiya, J.; Argüeso, P. Galectin-3 is an amplifier of the interleukin-1β-mediated inflammatory response in corneal keratinocytes. Immunology 2018, 154, 490–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, W.C.; Chen, H.L.; Chen, H.Y.; Peng, K.P.; Lee, Y.; Huang, L.M.; Chang, L.Y.; Liu, F.T. Galectin-3 and Its genetic variation rs4644 modulate enterovirus 71 infection. PLoS ONE 2016, 11, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Kulkarni, R.; Prasad, A. Exosomes Derived from HIV-1 Infected DCs Mediate Viral trans-Infection via Fibronectin and Galectin-3. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, D.K.; Dowling, C.A.; Jeng, K.C.G.; Chen, J.T.; Yang, R.Y.; Liu, F.T. Galectin-3 expression is induced in cirrhotic liver and hepatocellular carcinoma. Int. J. Cancer 1999, 81, 519–526. [Google Scholar] [CrossRef]
- Bièche, I.; Asselah, T.; Laurendeau, I.; Vidaud, D.; Degot, C.; Paradis, V.; Bedossa, P.; Valla, D.C.; Marcellin, P.; Vidaud, M. Molecular profiling of early stage liver fibrosis in patients with chronic hepatitis C virus infection. Virology 2005, 332, 130–144. [Google Scholar] [CrossRef] [PubMed]
- Uluca, Ü.; Şen, V.; Ece, A.; Tan, İ.; Karabel, D.; Aktar, F.; Karabel, M.; Balık, H.; Güneş, A. Serum galectin-3 levels in children with chronic hepatitis B infection and inactive hepatitis B carriers. Med. Sci. Monit. 2015, 21, 1376–1380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, M.; Kumar, D.; Butty, V.; Singh, S.; Esteban, A.; Fink, G.R.; Ploegh, H.L.; Sehrawat, S. Galectin-3 Regulates γ-Herpesvirus Specific CD8 T Cell Immunity. iScience 2018, 9, 101–119. [Google Scholar] [CrossRef] [Green Version]
- Qin, C.; Zhou, L.; Hu, Z.; Zhang, S.; Yang, S.; Tao, Y.; Xie, C.; Ma, K.; Shang, K.; Wang, W.; et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. 2020, 71, 762–768. [Google Scholar] [CrossRef]
- Maynard, S.E.; Min, J.; Merchan, J.; Lim, K.; Li, J.; Mondal, S.; Libermann, T.A.; Morgan, J.P.; Sellke, F.W.; Stillman, I.E.; et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J. Clin. Investig. 2003, 111, 649–658. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Kaplan, N.; Wysocki, J.; Yang, W.; Lu, K.; Peng, H.; Batlle, D.; Lavker, R.M. The ACE2-deficient mouse: A model for a cytokine storm-driven inflammation. FASEB J. 2020, 34, 10505–10515. [Google Scholar] [CrossRef]
- Pine, A.B.; Meizlish, M.L.; Goshua, G.; Chang, C.H.; Zhang, H.; Bishai, J.; Bahel, P.; Patel, A.; Gbyli, R.; Kwan, J.M.; et al. Circulating markers of angiogenesis and endotheliopathy in COVID-19. Pulm. Circ. 2020, 10. [Google Scholar] [CrossRef]
- Villa, E.; Critelli, R.; Lasagni, S.; Melegari, A.; Curatolo, A.; Celsa, C.; Romagnoli, D.; Melegari, G.; Pivetti, A.; Di Marco, L.; et al. Dynamic angiopoietin-2 assessment predicts survival and chronic course in hospitalized patients with COVID-19. Blood Adv. 2021, 5, 662–673. [Google Scholar] [CrossRef]
- Norata, G.D.; Garlanda, C.; Catapano, A.L. The Long Pentraxin PTX3: A Modulator of the Immunoinflammatory Response in Atherosclerosis and Cardiovascular Diseases. Trends Cardiovasc. Med. 2010, 20, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Fornai, F.; Carrizzo, A.; Forte, M.; Ambrosio, M.; Damato, A.; Ferrucci, M.; Biagioni, F.; Busceti, C.; Puca, A.A.; Vecchione, C. The inflammatory protein Pentraxin 3 in cardiovascular disease. Immun. Ageing 2016, 13, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Ristagno, G.; Fumagalli, F.; Bottazzi, B.; Mantovani, A.; Olivari, D.; Novelli, D.; Latini, R. Pentraxin 3 in cardiovascular disease. Front. Immunol. 2019, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hara, A.; Niwa, M.; Noguchi, K.; Kanayama, T.; Niwa, A.; Matsuo, M.; Hatano, Y.; Tomita, H. Galectin-3 as a next-generation biomarker for detecting early stage of various diseases. Biomolecules 2020, 10, 389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pugliese, G.; Iacobini, C.; Pesce, C.M.; Menini, S. Galectin-3: An emerging all-out player in metabolic disorders and their complications. Glycobiology 2015, 25, 136–150. [Google Scholar] [CrossRef] [Green Version]
- Ho, J.E.; Liu, C.; Lyass, A.; Courchesne, P.; Pencina, M.J.; Vasan, R.S.; Larson, M.G.; Levy, D. Galectin-3, a marker of cardiac fibrosis, predicts incident heart failure in the community. J. Am. Coll. Cardiol. 2012, 60, 1249–1256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Boer, R.A.; van Veldhuisen, D.J.; Gansevoort, R.T.; Muller Kobold, A.C.; van Gilst, W.H.; Hillege, H.L.; Bakker, S.J.L.; van der Harst, P. The fibrosis marker galectin-3 and outcome in the general population. J. Intern. Med. 2012, 272, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, G.; Iacobini, C.; Ricci, C.; Blasetti Fantauzzi, C.; Menini, S. Galectin-3 in diabetic patients. Clin. Chem. Lab. Med. 2014, 52, 1413–1423. [Google Scholar] [CrossRef] [PubMed]


| Characteristic | Values (n = 70) |
|---|---|
| Age, years | 58 (49; 67) |
| Male sex, n (%) | 27 (38.6) |
| BMI, kg/m2 * | 28.4 (4.2) |
| Body temperature, °C | 37.8 (36.4; 38.6) |
| Systolic blood pressure, mmHg | 135 (20) |
| Diastolic blood pressure, mmHg | 85 (12) |
| Heart rate, beats/min | 89 (15) |
| Hemoglobin oxygen saturation, % | 97 (94; 98) |
| Symptoms: | |
| Cough, n (%) | 41 (58.6) |
| Weakness, n (%) | 40 (57.1) |
| Dyspnea, n (%) | 29 (41.4) |
| Muscle pain, n (%) | 19 (27.1) |
| Headache or dizziness, n (%) | 15 (21.4) |
| Loss of taste, n (%) | 15 (21.4) |
| Loss of smell, n (%) | 14 (20.0) |
| Sore throat, n (%) | 13 (18.6) |
| Shivers, n (%) | 12 (17.1) |
| Diarrhea, n (%) | 12 (17.1) |
| Nausea or vomiting, n (%) | 8 (11.4) |
| Abdominal pain, n (%) | 4 (5.7) |
| Comorbidities: | |
| Hypertension, n (%) | 44 (62.9) |
| Diabetes mellitus, n (%) | 17 (24.3) |
| Thyroid disease, n (%) | 15 (21.4) |
| COPD or asthma, n (%) | 8 (11.4) |
| Charlson comorbidity index, points | 2 (1; 4) |
| Laboratory Test | n 1 | Values | Reference Range |
|---|---|---|---|
| RBC, × 106/µL | 66 | 4.31 (4.07; 4.52) | F: 4.0–5.0; M: 4.5–5.9 |
| Blood hemoglobin, g/dL | 66 | 12.7 (12.3; 13.5) | F: 12–16; M: 14–18 |
| Hematocrit, % | 66 | 38.0 (36.4; 40.5) | F: 37–47; M: 40–54 |
| WBC, × 103/µL | 66 | 5.96 (4.70; 6.69) | 4.5–10.0 |
| Neutrophils, × 103/µL | 62 | 3.49 (2.62; 4.11) | 1.8–7.7 |
| Lymphocytes, × 103/µL | 62 | 1.50 (1.25; 1.87) | 1.0–4.5 |
| Neutrophil/lymphocyte ratio | 62 | 2.16 (1.55; 3.55) | Not available |
| Monocytes, × 103/µL | 62 | 0.51 (0.41; 0.64) | 0.1–0.8 |
| Platelet count, × 103/µL | 66 | 246 (202; 333) | 140–440 |
| Alanine aminotransferase, U/L | 68 | 37 (20; 62) | F: 10–35; M: 10–50 |
| Aspartate aminotransferase, U/L | 66 | 26 (18; 47) | F: 10–35; M: 10–50 |
| Gamma-glutamyl transpeptidase, U/L | 64 | 40 (22; 80) | F: 5–36; M: 8–61 |
| Alkaline phosphatase, U/L | 65 | 64 (55; 88) | F: 35–104; M: 40–129 |
| Lactate dehydrogenase, U/L | 63 | 179 (130; 244) | F: 135–214; M: 135–225 |
| Total bilirubin, µmol/L | 65 | 6.40 (4.70; 8.56) | < 21.0 |
| Glucose, mmol/L | 61 | 5.37 (4.75; 6.38) | 3.30–5.60 |
| Urea, mmol/L | 69 | 5.40 (4.24; 6.81) | 2.76–8.07 |
| Creatinine, µmol/L | 68 | 67.6 (60.0; 88.8) | F: 44–80; M: 62–106 |
| Lactate, mmol/L | 6 | 1.20 (0.90; 1.50) | 0.5–2.2 |
| Albumin, g/L | 63 | 38.8 (5.6) | 35–52 |
| Total protein, g/L | 63 | 65.9 (6.1) | 66–87 |
| Sodium, mmol/L | 63 | 140 (139; 142) | 136–145 |
| Potassium, mmol/L | 68 | 4.53 (0.60) | 3.50–5.10 |
| Total cholesterol, mmol/L | 62 | 4.78 (1.23) | 3.2–5.2 |
| HDL-cholesterol, mmol/L | 62 | 1.04 (0.27) | F: > 1.2; M: > 1.0 |
| LDL-cholesterol, mmol/L | 62 | 2.61 (1.03) | < 3.4 |
| Triglycerides, mmol/L | 62 | 1.63 (1.33; 1.94) | < 2.26 |
| Creatinine kinase, U/L | 64 | 42 (29; 70) | F: 26–192; M: 39–308 |
| Cardiac troponin I, ng/L | 10 | 2.95 (2.00; 4.98) | < 47.3 |
| Myoglobin, µg/L | 7 | 46.2 (36.4; 103.8) | < 110.0 |
| NT-proBNP, pg/mL | 9 | 119 (80; 507) | < 125 (< 75 years); < 450 (≥ 75 years) |
| Iron, µmol/L | 64 | 17.0 (11.6; 22.1) | 5.83–34.5 |
| Ferritin, µg/L | 64 | 216 (123; 526) | 13–400 |
| Prothrombin time, s | 24 | 10.9 (10.5; 11.4) | 10.4–13.0 |
| D-dimer, mg/L | 37 | 0.48 (0.28; 1.15) | < 0.55 |
| C-reactive protein, mg/L | 69 | 4.79 (1.48; 23.90) | < 0.5 |
| Procalcitonin, ng/mL | 20 | ND (ND; 0.08) | < 0.10 |
| Interleukin-6, pg/mL | 62 | 3.77 (1.50; 16.71) | < 7.0 |
| Pentraxin-3, ng/mL | 70 | 2.37 (2.02; 3.48) | 1.47–4.25 2 |
| sFlt-1, pg/mL | 64 | 128 (111; 148) | 63.3–108.3 2,3 |
| Galectin-3, ng/mL | 70 | 9.41 (7.74; 14.22) | 5.49–12.77 2 |
| Characteristic | Pneumonia (n = 23) | No Pneumonia (n = 47) | p |
|---|---|---|---|
| Body temperature, °C | 38.5 (37.8; 39.0) | 36.8 (36.3; 38.5) | 0.007 |
| Hemoglobin saturation, % | 94 (88; 96) | 97 (96; 98) | < 0.001 |
| Platelet count, × 103/µL | 301 (223; 403) | 232 (190; 300) | 0.029 |
| Gamma-glutamyl transpeptidase, U/L | 66 (42; 198) | 34 (21; 73) | 0.035 |
| Lactate dehydrogenase, U/L | 221 (175; 316) | 158 (124; 209) | 0.005 |
| Prothrombin time, s | 11.4 (10.8; 12.1) | 10.8 (10.5; 11.2) | 0.047 |
| Ferritin, µg/L | 327 (197; 712) | 176 (103; 351) | 0.026 |
| Interleukin-6, pg/mL | 7.01 (2.54; 55.64) | 2.86 (1.50; 10.45) | 0.037 |
| HDL-cholesterol, mmol/L | 0.89 (0.78; 1.01) | 1.04 (0.89; 1.26) | 0.014 |
| Total protein, g/L | 63.3 (5.5) | 67.1 (6.0) | 0.031 |
| Albumin, g/L | 35.5 (5.9) | 40.2 (4.9) | 0.005 |
| Galectin-3, ng/mL | 13.30 (8.93; 17.38) | 8.55 (6.73; 10.98) | 0.001 |
| Pentraxin-3, ng/mL | 2.92 (2.19; 4.77) | 2.28 (1.84; 3.21) | 0.032 |
| ICU Treatment (n = 9) | No ICU Treatment (n = 61) | p | |
|---|---|---|---|
| Diastolic blood pressure, mmHg | 74 (8) | 86 (12) | 0.002 |
| Hemoglobin saturation, % | 92 (81; 95) | 97 (95; 98) | 0.003 |
| Hematocrit, % | 33.4 (29.5; 38.4) | 38.2 (36.5; 40.7) | 0.021 |
| Hemoglobin, g/dL | 11.3 (9.9; 12.6) | 12.9 (12.3; 13.8) | 0.014 |
| RBC, × 106/µL | 3.66 (3.41; 4.27) | 4.31 (4.08; 4.70) | 0.007 |
| WBC, × 103/µL | 10.60 (6.24; 11.46) | 5.84 (4.45; 6.61) | 0.002 |
| Neutrophil count, × 103/µL | 5.73 (3.55; 8.53) | 3.32 (2.56; 3.94) | 0.044 |
| Monocyte count, × 103/µL | 0.93 (0.81; 1.10) | 0.50 (0.40; 0.58) | 0.004 |
| Platelet count, × 103/µL | 403 (272; 478) | 232 (189; 308) | 0.010 |
| C-reactive protein, mg/L | 18.2 (5.9; 71.1) | 3.6 (1.2; 17.5) | 0.009 |
| Gamma-glutamyl transpeptidase, U/L | 253 (117; 308) | 35 (21; 74) | 0.006 |
| Lactate dehydrogenase, U/L | 322 (225; 548) | 164 (129; 221) | 0.001 |
| D-dimer, mg/L | 1.62 (0.90; 2.14) | 0.32 (0.25; 0.78) | 0.003 |
| Ferritin, µg/L | 766 (526; 1235) | 209 (111; 474) | 0.007 |
| Interleukin-6, pg/mL | 16.98 (6.46; 99.90) | 3.42 (1.50; 15.92) | 0.006 |
| HDL-cholesterol, mmol/L | 0.82 (0.69; 0.90) | 1.03 (0.88; 1.20) | 0.009 |
| Albumin, g/L | 32 (6) | 40 (5) | 0.006 |
| sFlt-1, pg/mL | 183 (131; 260) | 124 (107; 143) | 0.006 |
| Galectin-3, ng/mL | 23.46 (15.51; 27.80) | 8.93 (7.58; 12.97) | 0.001 |
| Pentraxin-3, ng/mL | 4.77 (2.92; 8.17) | 2.30 (1.88; 3.21) | 0.001 |
| Variable | Galectin-3 | |
|---|---|---|
| R | p | |
| Diastolic blood pressure | −0.30 | 0.012 |
| Hemoglobin saturation | −0.36 | 0.002 |
| BMI | 0.43 | 0.006 |
| Charlson comorbidity index | 0.27 | 0.021 |
| Hematocrit | −0.33 | 0.007 |
| Hemoglobin | −0.28 | 0.022 |
| RBC | −0.42 | < 0.001 |
| Aspartate aminotransferase | 0.25 | 0.040 |
| Gamma-glutamyl transpeptidase | 0.26 | 0.036 |
| Lactate dehydrogenase | 0.55 | < 0.001 |
| Sodium | −0.25 | 0.049 |
| Urea | 0.33 | 0.006 |
| HDL-cholesterol | −0.38 | 0.003 |
| Triglycerides | 0.27 | 0.031 |
| Total protein | −0.29 | 0.022 |
| Albumin | −0.41 | 0.001 |
| Ferritin | 0.51 | < 0.001 |
| C-reactive protein | 0.28 | 0.018 |
| Interleukin-6 | 0.57 | < 0.001 |
| Pentraxin-3 | 0.54 | < 0.001 |
| sFlt-1 | 0.48 | < 0.001 |
| Independent Variable | Dependent Variable | |||
|---|---|---|---|---|
| Pneumonia | Treatment in ICU | |||
| OR (95% CI) | p | OR (95% CI) | p | |
| Galectin-3 | 1.19 (1.06–1.34) | 0.002 | 1.31 (1.11–1.53) | 0.001 |
| Age | 1.01 (0.95–1.07) | 0.7 | 1.00 (0.91–1.11) | 0.9 |
| Charlson comorbidity index | 0.86 (0.59–1.24) | 0.4 | 0.94 (0.54–1.65) | 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuśnierz-Cabala, B.; Maziarz, B.; Dumnicka, P.; Dembiński, M.; Kapusta, M.; Bociąga-Jasik, M.; Winiarski, M.; Garlicki, A.; Grodzicki, T.; Kukla, M. Diagnostic Significance of Serum Galectin-3 in Hospitalized Patients with COVID-19—A Preliminary Study. Biomolecules 2021, 11, 1136. https://doi.org/10.3390/biom11081136
Kuśnierz-Cabala B, Maziarz B, Dumnicka P, Dembiński M, Kapusta M, Bociąga-Jasik M, Winiarski M, Garlicki A, Grodzicki T, Kukla M. Diagnostic Significance of Serum Galectin-3 in Hospitalized Patients with COVID-19—A Preliminary Study. Biomolecules. 2021; 11(8):1136. https://doi.org/10.3390/biom11081136
Chicago/Turabian StyleKuśnierz-Cabala, Beata, Barbara Maziarz, Paulina Dumnicka, Marcin Dembiński, Maria Kapusta, Monika Bociąga-Jasik, Marek Winiarski, Aleksander Garlicki, Tomasz Grodzicki, and Michał Kukla. 2021. "Diagnostic Significance of Serum Galectin-3 in Hospitalized Patients with COVID-19—A Preliminary Study" Biomolecules 11, no. 8: 1136. https://doi.org/10.3390/biom11081136
APA StyleKuśnierz-Cabala, B., Maziarz, B., Dumnicka, P., Dembiński, M., Kapusta, M., Bociąga-Jasik, M., Winiarski, M., Garlicki, A., Grodzicki, T., & Kukla, M. (2021). Diagnostic Significance of Serum Galectin-3 in Hospitalized Patients with COVID-19—A Preliminary Study. Biomolecules, 11(8), 1136. https://doi.org/10.3390/biom11081136







