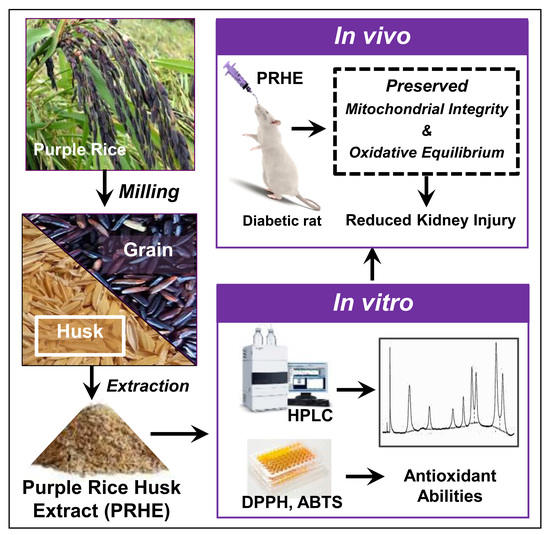
Protective Effects of Purple Rice Husk against Diabetic Nephropathy by Modulating PGC-1α/SIRT3/SOD2 Signaling and Maintaining Mitochondrial Redox Equilibrium in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Preparation of PRHE
2.2. Phytochemical Analysis, Quantification of Bioactive Compounds, and Evaluation of Antioxidant Capacity of PRHE
2.3. Animals and Experimental Protocols
2.4. Biochemical Assays
2.4.1. Determinations of Metabolic Indexes
2.4.2. Determinations of Renal Functions
2.4.3. Determinations of Renal Oxidative Stress
2.4.4. Determinations of Mitochondrial Functions
2.5. Histopathological Examinations
2.6. Western Blot Analysis
2.7. Statistical Analyses
3. Results
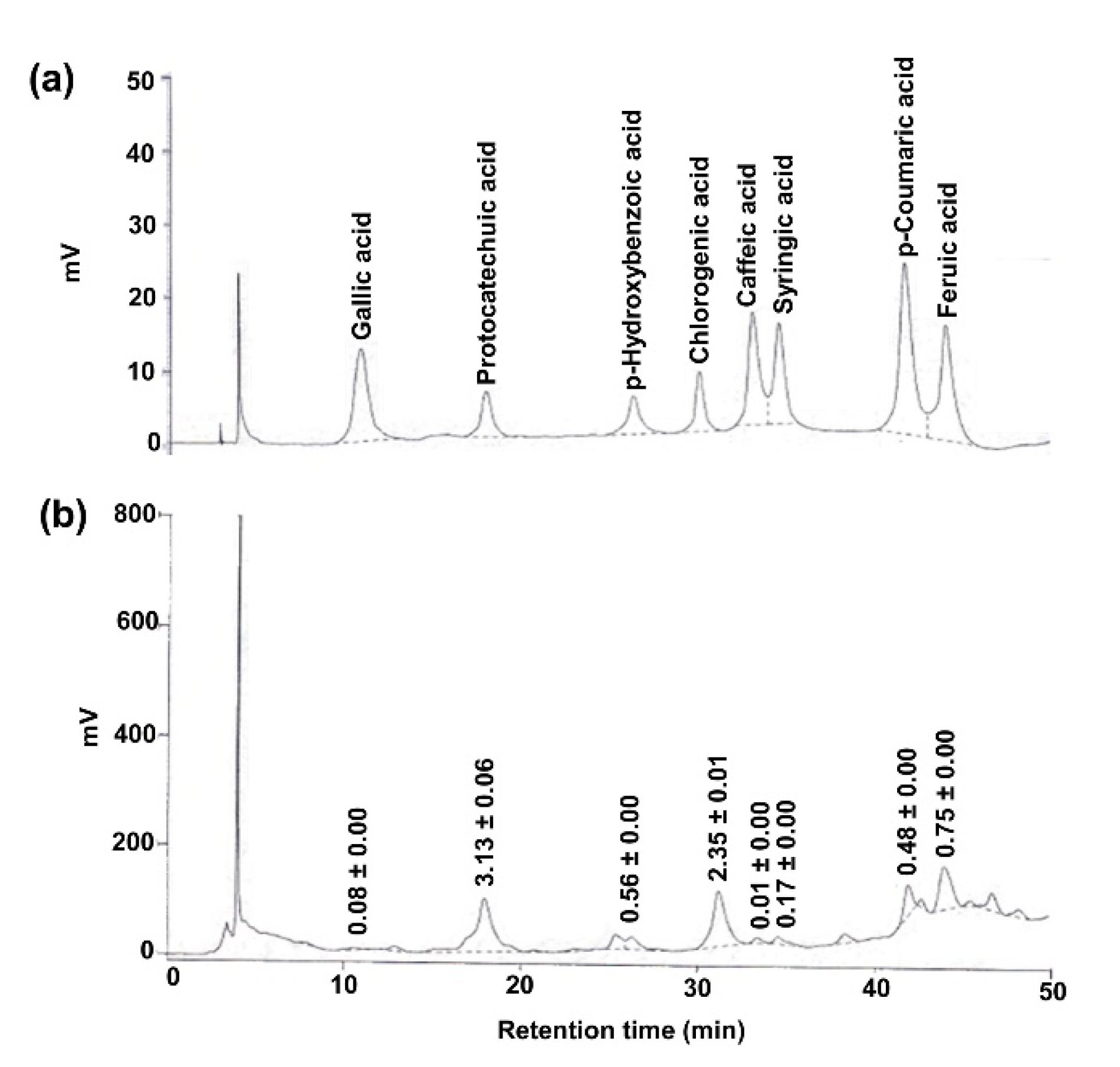
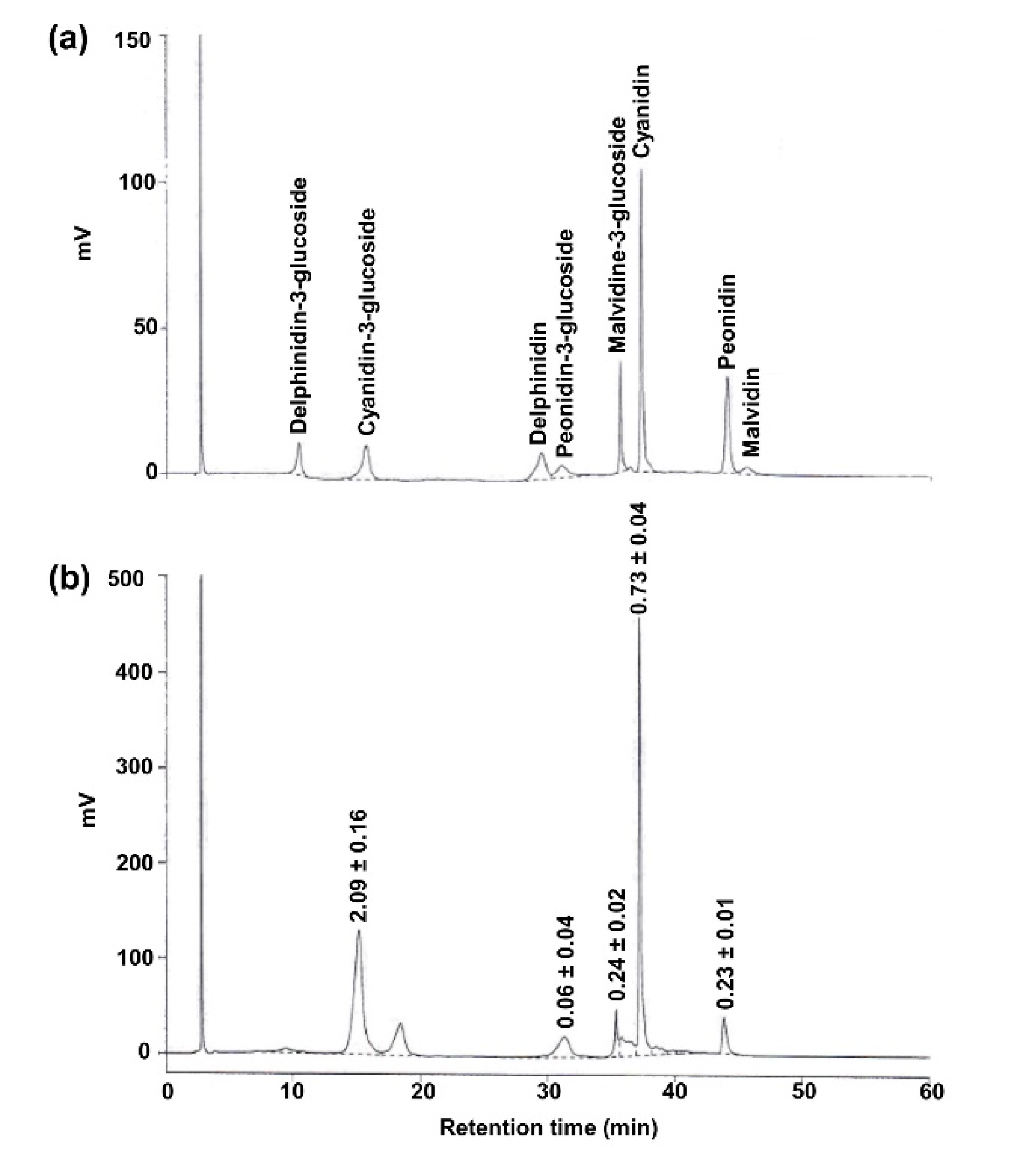
3.1. PRHE Possesses Antioxidant Capacity and Contains Protocatechuic Acid and Cyanidin-3-Glucoside as Major Phenolic-Based Compounds
3.2. PRHE Improves Diabetes-Induced Metabolic Alterations
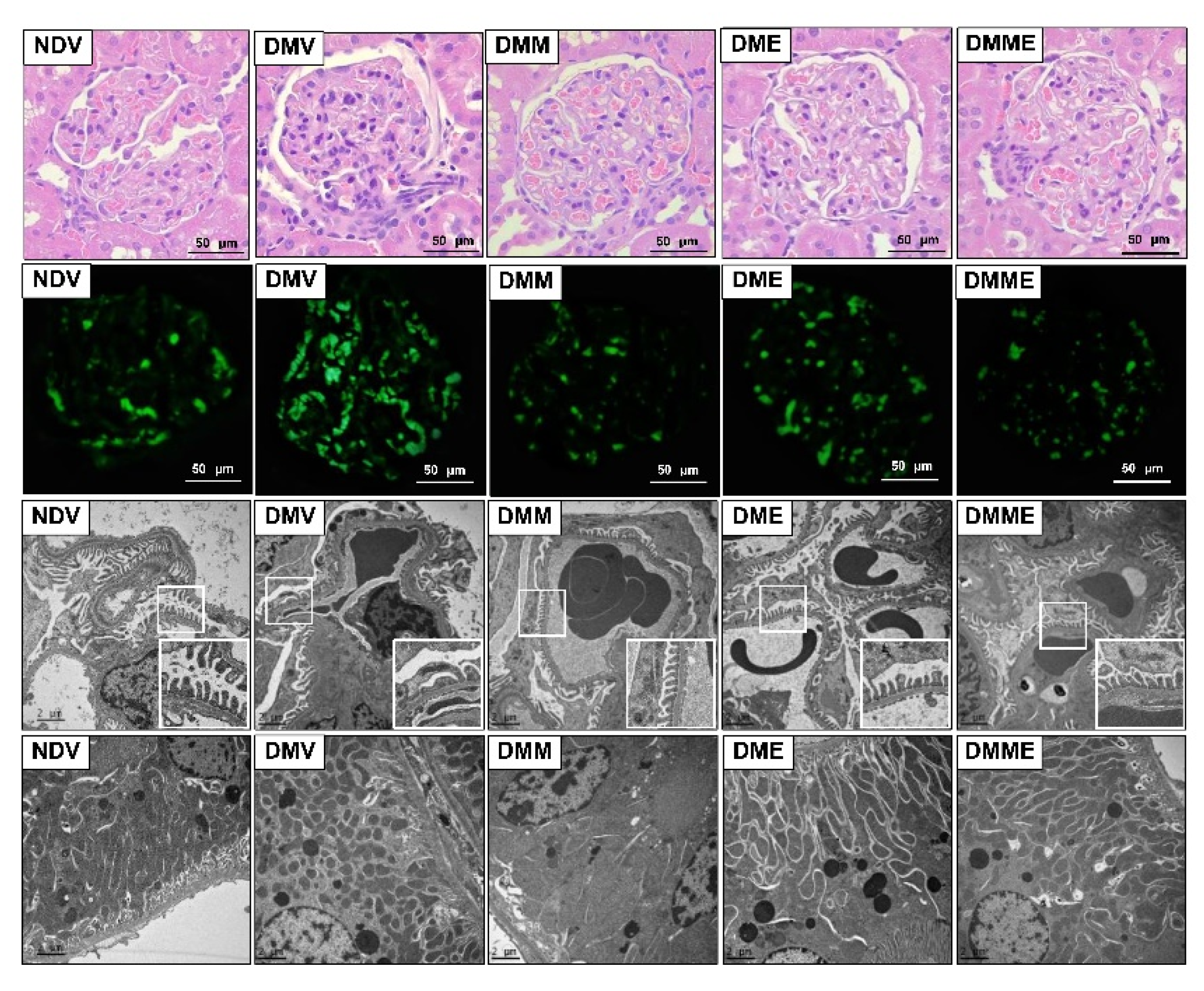
3.3. PRHE Ameliorates Diabetes-Induced Renal Functional and Structural Impairments
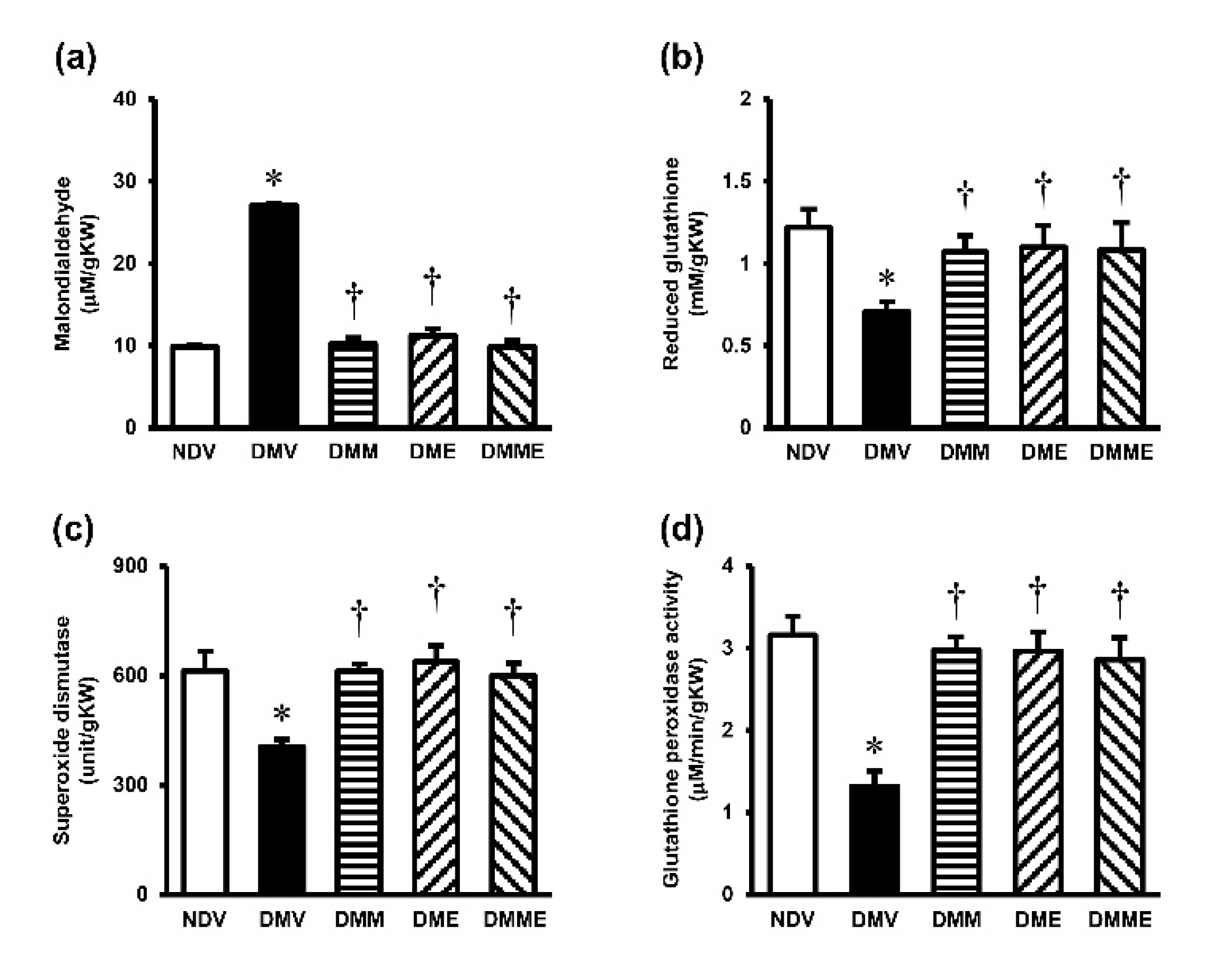
3.4. PRHE Attenuates Diabetes-Induced Renal Oxidative Stress and Mitochondrial Dysfunction
3.5. PRHE Modifies PGC-1α-SIRT3-Ac-SOD2-SOD2 Signaling Transduction
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Compositions | Normal Diet | High-Fat Diet | ||
|---|---|---|---|---|
| g/100 g Diet | % Energy | g/100 g Diet | % Energy | |
| Carbohydrates | 56.21 | 60.11 | 26.38 | 22.54 |
| Fat | 4.55 | 10.95 | 27.89 | 53.63 |
| Protein | 27.06 | 28.94 | 28.81 | 23.93 |
| Vitamin and mineral | 6.54 | - | 9.92 | - |
| Fiber | 3.43 | - | 4.32 | - |
| Total energy (%) | 100 | 100 | ||
| Caloric value (kcal/g) | 3.74 | 4.68 | ||
| Parameters | NDV | DMV | DME 150 | DME 300 | DME 600 |
|---|---|---|---|---|---|
| Food intake (g/day) | 20.54 ± 0.17 | 16.87 ± 0.43 * | 17.56 ± 0.76 * | 17.22 ± 0.56 * | 17.00 ± 0.37 * |
| Energy intake (kcal/day) | 76.82 ± 0.62 | 78.92 ± 1.99 * | 82.20 ± 3.55 | 80.59 ± 2.63 | 79.57 ± 1.01 |
| BW gain (%) | 41.60 ± 1.45 | 87.49 ± 4.92 * | 56.63 ± 3.87 *,† | 46.14 ± 5.12 † | 37.57 ± 4.41 † |
| Glucose (mg/dL) | 161.18 ± 10.98 | 433.99 ± 48.50 * | 283.00 ± 15.51*,† | 238.24 ± 3.72 *,† | 260.37 ± 22.51 *,† |
| Insulin (ng/mL) | 1.98 ± 0.12 | 4.57 ± 1.31 * | 3.88 ± 0.35 *,# | 1.92 ± 0.40 † | 2.74 ± 0.36 † |
| HOMA-IR | 0.80 ± 0.10 | 4.53 ± 0.79 * | 2.69 ± 0.20 *,†,# | 1.12 ± 0.22 † | 1.77 ± 0.35 † |
| Serum creatinine (mg/dL) | 0.45 ± 0.03 | 0.63 ± 0.02 * | 0.55 ± 0.10 | 0.48 ± 0.03 † | 0.52 ± 0.14 |
| Creatinine clearance (ml/min/g KW) | 0.97 ± 0.05 | 0.54 ± 0.03 * | 0.63 ± 0.16 * | 0.91 ± 0.04 † | 0.85 ± 0.15 † |
References
- Wu, M.; Li, S.; Yu, X.; Chen, W.; Ma, H.; Shao, C.; Zhang, Y.; Zhang, A.; Huang, S.; Jia, Z. Mitochondrial activity contributes to impaired renal metabolic homeostasis and renal pathology in STZ-induced diabetic mice. Am. J. Physiol. Renal Physiol. 2019, 317, F593–F605. [Google Scholar] [CrossRef] [PubMed]
- International Diabetes Federation. IDF Diabetes Atlas, 9th ed.; Brussels, Belgium, 2019; Available online: https://www.diabetesatlas.org (accessed on 10 June 2021).
- Saritsiri, S.; Mongkolchati, A.; Suksaroj, T. Prevalence of chronic kidney disease and related factors among diabetic patients in primary care, Bangkok, Thailand. J. Public Health Dev. 2021, 19, 1–8. [Google Scholar]
- Yu, S.M.; Bonventre, J.V. Acute kidney injury and progression of diabetic kidney disease. Adv. Chronic Kidney Dis. 2018, 25, 166–180. [Google Scholar] [CrossRef]
- Aekplakorn, W.; Chariyalertsak, S.; Kessomboon, P.; Sangthong, R.; Inthawong, R.; Putwatana, P.; Taneepanichskul, S. Thai National Health Examination Survey IV Study Group. Prevalence and management of diabetes and metabolic risk factors in Thai adults: The Thai National Health Examination Survey IV, 2009. Diabetes Care. 2011, 34, 1980–1985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aekplakorn, W.; Chariyalertsak, S.; Kessomboon, P.; Assanangkornchai, S.; Taneepanichskul, S.; Putwatana, P. Prevalence of Diabetes and Relationship with Socioeconomic Status in the Thai Population: National Health Examination Survey, 2004–2014. J. Diabetes Res. 2018, 2018, 1654530. [Google Scholar] [CrossRef]
- Liu, W.; Liu, J.; Triplett, L.; Leach, J.E.; Wang, G.L. Novel insights into rice innate immunity against bacterial and fungal pathogens. Annu. Rev. Phytopathol. 2014, 52, 213–241. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Aal, E.S.M.; Young, J.C.; Rabalski, I. Anthocyanin composition in black, blue, pink, purple, and red cereal grains. J. Agric. Food Chem. 2006, 54, 4696–4704. [Google Scholar] [CrossRef]
- Deng, G.F.; Xu, X.R.; Zhang, Y.; Li, D.; Gan, R.Y.; Li, H.B. Phenolic compounds and bioactivities of pigmented rice. Crit. Rev. Food Sci. Nutr. 2013, 53, 296–306. [Google Scholar] [CrossRef]
- Sohail, M.; Rakha, A.; Butt, M.S.; Iqbal, M.J.; Rashid, S. Rice bran nutraceutics: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2017, 57, 3771–3780. [Google Scholar] [CrossRef]
- Butsat, S.; Siriamornpun, S. Antioxidant capacities and phenolic compounds of the husk, bran and endosperm of Thai rice. Food Chem. 2010, 119, 606–613. [Google Scholar] [CrossRef]
- Chariyakornkul, A.; Punvittayagul, C.; Taya, S.; Wongpoomchai, W. Inhibitory effect of purple rice husk extract on AFB1-induced micronucleus formation in rat liver through modulation of xenobiotic metabolizing enzymes. BMC Complement. Altern. Med. 2019, 19, 237. [Google Scholar] [CrossRef] [Green Version]
- Peerapanyasut, W.; Kobroob, A.; Palee, S.; Chattipakorn, N.; Wongmekiat, O. Activation of sirtuin 3 and maintenance of mitochondrial integrity by N-acetylcysteine protects against bisphenol A-induced kidney and liver toxicity in rats. Int. J. Mol. Sci. 2019, 11, 267. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Xiang, H.; Liu, J.; Chen, Y.; He, R.R.; Liu, B. Mitochondrial sirtuin 3: New emerging biological function and therapeutic target. Theranostics 2020, 10, 8315–8342. [Google Scholar] [CrossRef]
- Benigni, A.; Perico, L.; Macconi, D. Mitochondrial Dynamics is linked to longevity and protects from end-organ Injury: The emerging role of sirtuin 3. Antioxid. Redox Signal. 2016, 25, 185–199. [Google Scholar] [CrossRef]
- Sagoo, M.K.; Gnudi, L. Diabetic nephropathy: Is there a role for oxidative stress? Free Radic. Biol. Med. 2018, 116, 50–63. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.Y.; Tang, C.Y. Determination of total phenolic and flavonoid content in selected fruits and vegetables as well as their stimulatory effects on mouse splenocyte proliferation. Food Chem. 2007, 101, 140–147. [Google Scholar] [CrossRef]
- Sutharut, J.; Sudarat, J. Total anthocyanin content and antioxidant activity of germinated colored rice. Int. Food Res. J. 2012, 19, 215–221. [Google Scholar]
- Umnajkitikorn, K.; Faiyue, B.; Saengnil, K. Enhancing antioxidant properties of germinated Thai rice (Oryza sativa L.) cv. Kum Doi Saket with salinity. J. Rice Res. 2013, 1, 103. [Google Scholar] [CrossRef]
- Srinivasan, K.; Viswanad, B.; Asrat, L.; Kaul, C.L.; Ramarao, P. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: A model for type 2 diabetes and pharmacological screening. Pharmacol. Res. 2005, 52, 313–320. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [Green Version]
- Kobroob, A.; Peerapanyasut, W.; Chattipakorn, N.; Wongmekiat, O. Damaging effects of bisphenol A on the kidney and the protection by melatonin: Emerging evidences from in vivo and in vitro studies. Oxid. Med. Cell. Longev. 2018, 2018, 3082438. [Google Scholar] [CrossRef]
- Suman, R.K.; Ray Mohanty, I.; Borde, M.K.; Maheshwari, U.; Deshmukh, Y.A. Development of an experimental model of diabetes co-existing with metabolic syndrome in rats. Adv. Pharmacol. Sci. 2016, 2016, 9463476. [Google Scholar] [CrossRef] [Green Version]
- Mythili, M.D.; Vyas, R.; Akila, G.; Gunasekaran, S. Effect of streptozotocin on the ultrastructure of rat pancreatic islets. Microsc. Res. Tech. 2004, 63, 274–281. [Google Scholar] [CrossRef]
- Boue, S.M.; Daigle, K.W.; Chen, M.H.; Cao, H.; Heiman, M.L. Antidiabetic potential of purple and red rice (Oryza sativa L.) bran extracts. J. Agric. Food Chem. 2016, 64, 5345–5353. [Google Scholar] [CrossRef]
- Talagavadi, V.; Rapisarda, P.; Galvano, F.; Pelicci, P.; Giorgio, M. Cyanidin-3-O-β-glucoside and protocatechuic acid activate AMPK/mTOR/S6K pathway and improve glucose homeostasis in mice. J. Func. Foods. 2016, 21, 338–348. [Google Scholar] [CrossRef]
- Scazzocchio, B.; Filesi, C.; Gaudio, I.D.; Archivio, M.D.; Santangelo, C.; Iacovelli, A.; Galvano, F.; Pluchinotta, F.R.; Giovannini, C.; Masella, R. Protocatechuic acid activates key components of insulin signaling pathway mimicking insulin activity. Mol. Nutr. Food Res. 2015, 59, 1472–1481. [Google Scholar] [CrossRef]
- Sasaki, R.; Nishimura, N.; Hoshino, H.; Isa, Y.; Kadowaki, M.; Ichi, T.; Tanaka, A.; Nishiumi, S.; Fukuda, I.; Ashida, H.; et al. Cyanidin 3-glucoside ameliorates hyperglycemia and insulin sensitivity due to down regulation of retinol binding protein 4 expression in diabetic mice. Biochem. Pharmacol. 2007, 74, 1619–1627. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, F.; Yang, S.; Chen, B.; Shi, J. Protocatechuic acid ameliorates high glucose-induced extracellular matrix accumulation in diabetic nephropathy. Biomed. Pharmacother. 2018, 98, 18–22. [Google Scholar] [CrossRef]
- Wei, P.Z.; Szeto, C.C. Mitochondrial dysfunction in diabetic kidney disease. Clin. Chim. Acta 2019, 496, 108–116. [Google Scholar] [CrossRef]
- Semaming, Y.; Kumfu, S.; Pannangpetch, P.; Chattipakorn, S.C.; Chattipakorn, N. Protocatechuic acid exerts a cardioprotective effect in type 1 diabetic rats. J. Endocrinol. 2014, 223, 13–23. [Google Scholar] [CrossRef] [Green Version]
- Semaming, Y.; Sripetchwandee, J.; Sa-Nguanmoo, P.; Pintana, H.; Pannangpetch, P.; Chattipakorn, N.; Chattipakorn, S.C. Protocatechuic acid protects brain mitochondrial function in streptozotocin-induced diabetic rats. Appl. Physiol. Nutr. Metab. 2015, 40, 1078–1081. [Google Scholar] [CrossRef]
- Liobikas, J.; Skemiene, K.; Trumbeckaite, S.; Borutaite, V. Anthocyanins in cardioprotection: A path through mitochondria. Pharmacol. Res. 2016, 113, 808–815. [Google Scholar] [CrossRef]
- Wei, J.; Wu, H.; Zhang, H.; Li, F.; Chen, S.; Hou, B.; Shi, Y.; Zhao, L.; Duan, H. Anthocyanins inhibit high glucose-induced renal tubular cell apoptosis caused by oxidative stress in db/db mice. Int. J. Mol. Med. 2018, 41, 1608–1618. [Google Scholar] [CrossRef]
- Foretz, M.; Guigas, B.; Bertrand, L.; Pollak, M.; Viollet, B. Metformin: From mechanisms of action to therapies. Cell Metab. 2014, 20, 953–966. [Google Scholar] [CrossRef] [Green Version]
- Kitada, M.; Ogura, Y.; Monno, I.; Koya, D. Sirtuins and type 2 diabetes: Role in inflammation, oxidative stress, and mitochondrial function. Front. Endocrinol. 2019, 10, 187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, J.; Liu, X.; Su, C.; Wu, F.; Sun, J.; Zhang, J.; Yang, X.; Zhang, C.; Zhou, Z.; Zhang, X.; et al. Inhibition of mitochondrial oxidative damage improves reendothelialization capacity of endothelial progenitor cells via SIRT3 (Sirtuin3)-enhanced SOD2 (Superoxide dismutase 2) deacetylation in hypertension. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1682–1698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogura, Y.; Kitada, M.; Monno, I.; Kanasaki, K.; Watanabe, A.; Koya, D. Renal mitochondrial oxidative stress is enhanced by the reduction of Sirt3 activity in Zucker diabetic fatty rats. Redox Rep. 2018, 23, 153–159. [Google Scholar] [CrossRef] [Green Version]
- Jing, E.; Emanuelli, B.; Hirschey, M.D.; Boucher, J.; Lee, K.Y.; Lombard, D.; Verdin, E.M.; Kahn, C.R. Sirtuin-3 (Sirt3) regulates skeletal muscle metabolism and insulin signaling via altered mitochondrial oxidation and reactive oxygen species production. Proc. Natl. Acad. Sci. USA 2011, 108, 14608–14613. [Google Scholar] [CrossRef] [Green Version]
- Locatelli, M.; Zoja, C.; Zanchi, C.; Corna, D.; Villa, S.; Bolognini, S.; Novelli, R.; Perico, L.; Remuzzi, G.; Benigni, A.; et al. Manipulating Sirtuin 3 pathway ameliorates renal damage in experimental diabetes. Sci. Rep. 2020, 10, 8418. [Google Scholar] [CrossRef]
- Wang, C.; Yang, Y.; Zhang, Y.; Liu, J.; Zhenjun Yao, Z.; Zhang, C. Protective effects of metformin against osteoarthritis through upregulation of SIRT3-mediated PINK1/Parkin-dependent mitophagy in primary chondrocytes. Biosci. Trends. 2018, 12, 605–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Driver, C.; Bamitale, K.D.S.; Kazi, A.; Olla, M.; Nyane, N.A.; Owira, P.M.O. Cardioprotective effects of metformin. J. Cardiovasc. Pharmacol. 2018, 72, 121–127. [Google Scholar] [CrossRef] [PubMed]


| Parameters | NDV | DMV | DMM | DME | DMME |
|---|---|---|---|---|---|
| Food intake (g/day) | 20.83 ± 0.26 a | 17.58 ± 0.53 b | 17.57 ± 0.34 b | 17.05 ± 0.38 b | 17.64 ± 0.49 b |
| Energy intake (kcal/day) | 77.90 ± 0.98 a | 82.25 ± 2.46 a | 82.22 ± 1.59 a | 79.79 ± 1.78 a | 82.56 ± 2.30 a |
| BW gain (%) | 39.17 ± 1.81 a | 76.22 ± 7.78 b | 49.90 ± 3.05 a | 46.61 ± 3.36 a | 44.48 ± 4.75 a |
| VF/100 g BW | 5.91 ± 0.62 a | 13.33 ± 0.69 b | 10.60 ± 0.40 c | 9.26 ± 0.69 c | 10.08 ± 0.56 c |
| Total cholesterol (mg/mL) | 61.19 ± 1.77 a | 107.60 ± 3.07 b | 63.00 ± 2.17 a | 61.92 ± 5.31 a | 72.55 ± 8.60 a |
| Triglycerides (mg/mL) | 52.25 ± 4.68 a | 103.50 ± 4.47 b | 62.51 ± 3.64 a | 57.34 ± 2.24 a | 65.82 ± 7.64 a |
| Glucose (mg/dL) | 163.77 ± 7.14 a | 386.47 ± 43.31 b | 233.41 ± 19.18 a | 223.82 ± 9.43 a | 230.06 ± 29.79 a |
| Insulin (ng/mL) | 1.92 ± 0.15 a | 4.14 ± 0.90 b | 1.90 ± 0.21 a | 1.82 ± 0.30 a | 1.94 ± 0.31 a |
| HOMA-IR | 0.78 ± 0.08 a | 3.79 ± 0.69 b | 1.06 ± 0.09 a | 1.01 ± 0.17 a | 1.04 ± 0.14 a |
| Parameters | NDV | DMV | DMM | DME | DMME |
|---|---|---|---|---|---|
| KW/BW (g/100 g BW) | 0.43 ± 0.02 a | 0.56 ± 0.01 b | 0.44± 0.01 a | 0.47 ± 0.03 a | 0.48 ± 0.02 a |
| Serum creatinine (mg/dL) | 0.41 ± 0.04 a | 0.63 ± 0.03 b | 0.45 ± 0.02 a | 0.47 ± 0.02 a | 0.47 ± 0.02 a |
| Creatinine clearance (mL/min/g KW) | 0.95 ± 0.04 a | 0.55 ± 0.03 b | 0.97 ± 0.01 a | 0.94 ± 0.03 a | 0.98 ± 0.03 a |
| Urine microalbumin (mg/g creatinine) | 22.75 ± 4.56 a | 83.37 ± 16.62 b | 40.40 ± 11.74 a | 43.22 ± 16.09 a | 41.12 ± 16.11 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wongmekiat, O.; Lailerd, N.; Kobroob, A.; Peerapanyasut, W. Protective Effects of Purple Rice Husk against Diabetic Nephropathy by Modulating PGC-1α/SIRT3/SOD2 Signaling and Maintaining Mitochondrial Redox Equilibrium in Rats. Biomolecules 2021, 11, 1224. https://doi.org/10.3390/biom11081224
Wongmekiat O, Lailerd N, Kobroob A, Peerapanyasut W. Protective Effects of Purple Rice Husk against Diabetic Nephropathy by Modulating PGC-1α/SIRT3/SOD2 Signaling and Maintaining Mitochondrial Redox Equilibrium in Rats. Biomolecules. 2021; 11(8):1224. https://doi.org/10.3390/biom11081224
Chicago/Turabian StyleWongmekiat, Orawan, Narissara Lailerd, Anongporn Kobroob, and Wachirasek Peerapanyasut. 2021. "Protective Effects of Purple Rice Husk against Diabetic Nephropathy by Modulating PGC-1α/SIRT3/SOD2 Signaling and Maintaining Mitochondrial Redox Equilibrium in Rats" Biomolecules 11, no. 8: 1224. https://doi.org/10.3390/biom11081224