The Histaminergic System in Neuropsychiatric Disorders
Abstract
:1. Introduction
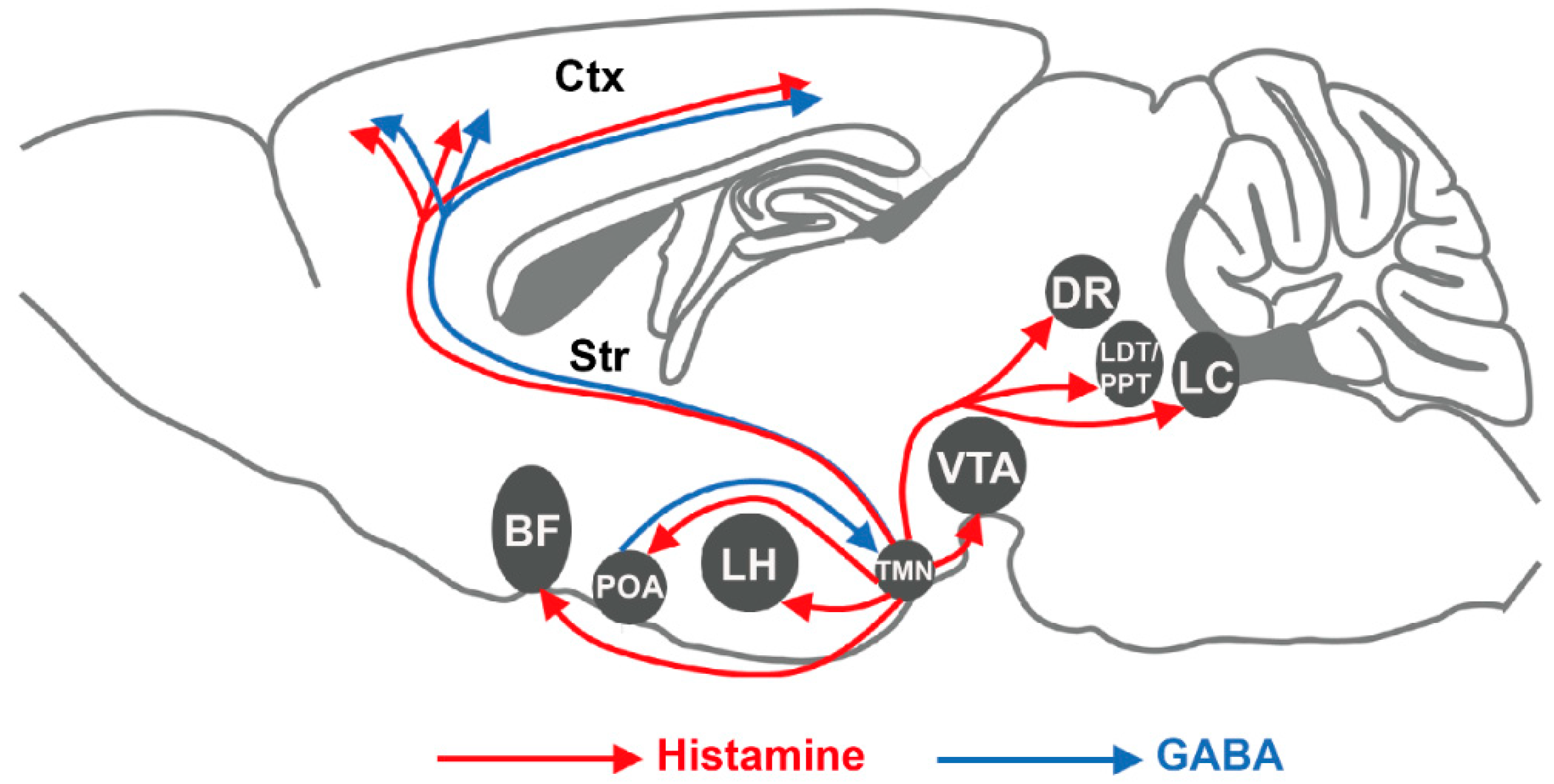
2. Histaminergic Signaling in the Brain
2.1. Histamine in the Brain
2.2. Histamine Receptors
2.2.1. Histamine H1 Receptor (H1R)
2.2.2. Histamine H2 Receptor (H2R)
2.2.3. Histamine H3 Receptor (H3R)
2.2.4. Histamine H4 Receptor (H4R)
3. The Histaminergic System in Neuropsychiatric Disorders
3.1. Sleep Disorders
3.2. Schizophrenia
3.3. Alzheimer’s Disease (AD)
3.4. Tourette’s Syndrome (TS)
3.5. Parkinson’s Disease (PD)
4. Concluding Remarks and Future Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Kessler, R.C.; Amminger, G.P.; Aguilar-Gaxiola, S.; Alonso, J.; Lee, S.; Ustün, T.B. Age of onset of mental disorders: A review of recent literature. Curr. Opin. Psychiatry 2007, 20, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.C.; Berglund, P.; Demler, O.; Jin, R.; Merikangas, K.R.; Walters, E.E. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry 2005, 62, 593–602. [Google Scholar] [CrossRef] [Green Version]
- Fineberg, N.A.; Haddad, P.M.; Carpenter, L.; Gannon, B.; Sharpe, R.; Young, A.H.; Joyce, E.; Rowe, J.; Wellsted, D.; Nutt, D.J.; et al. The size, burden and cost of disorders of the brain in the UK. J. Psychopharmacol. 2013, 27, 761–770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gooch, C.L.; Pracht, E.; Borenstein, A.R. The burden of neurological disease in the United States: A summary report and call to action. Ann. Neurol. 2017, 81, 479–484. [Google Scholar] [CrossRef]
- Schwartz, J.C.; Arrang, J.M.; Garbarg, M.; Pollard, H.; Ruat, M. Histaminergic transmission in the mammalian brain. Physiol. Rev. 1991, 71, 1–51. [Google Scholar] [CrossRef] [PubMed]
- Panula, P.; Nuutinen, S. The histaminergic network in the brain: Basic organization and role in disease. Nat. Rev. Neurosci. 2013, 14, 472–487. [Google Scholar] [CrossRef]
- Panula, P.; Sundvik, M.; Karlstedt, K. Developmental roles of brain histamine. Trends Neurosci. 2014, 37, 159–168. [Google Scholar] [CrossRef]
- Baronio, D.; Gonchoroski, T.; Castro, K.; Zanatta, G.; Gottfried, C.; Riesgo, R. Histaminergic system in brain disorders: Lessons from the translational approach and future perspectives. Ann. Gen. Psychiatry 2014, 13, 34. [Google Scholar] [CrossRef] [Green Version]
- Haas, H.L.; Sergeeva, O.A.; Selbach, O. Histamine in the nervous system. Physiol. Rev. 2008, 88, 1183–1241. [Google Scholar] [CrossRef]
- Haas H and Panula P, The role of histamine and the tuberomamillary nucleus in the nervous system. Nat. Rev. Neurosci. 2003, 4, 121–130. [CrossRef]
- Ericson, H.; Blomqvist, A.; Köhler, C. Origin of neuronal inputs to the region of the tuberomammillary nucleus of the rat brain. J. Comp. Neurol. 1991, 311, 45–64. [Google Scholar] [CrossRef]
- Yu, X.; Ye, Z.; Houston, C.M.; Zecharia, A.Y.; Ma, Y.; Zhang, Z.; Uygun, D.S.; Parker, S.; Vyssotski, A.L.; Yustos, R.; et al. Wakefulness Is Governed by GABA and Histamine Cotransmission. Neuron 2015, 87, 164–178. [Google Scholar] [CrossRef] [Green Version]
- Kukko-Lukjanov, T.K.; Panula, P. Subcellular distribution of histamine, GABA and galanin in tuberomamillary neurons in vitro. J. Chem. Neuroanat. 2003, 25, 279–292. [Google Scholar] [CrossRef]
- Trottier, S.; Chotard, C.; Traiffort, E.; Unmehopa, U.; Fisser, B.; Swaab, D.F.; Schwartz, J.C. Co-localization of histamine with GABA but not with galanin in the human tuberomamillary nucleus. Brain Res. 2002, 939, 52–64. [Google Scholar] [CrossRef]
- Brown, R.E.; Stevens, D.R.; Haas, H.L. The physiology of brain histamine. Prog. Neurobiol. 2001, 63, 637–672. [Google Scholar] [CrossRef]
- Takahashi, K.; Lin, J.S.; Sakai, K. Neuronal activity of histaminergic tuberomammillary neurons during wake-sleep states in the mouse. J. Neurosci. 2006, 26, 10292–10298. [Google Scholar] [CrossRef]
- Katoh, Y.; Niimi, M.; Yamamoto, Y.; Kawamura, T.; Morimoto-Ishizuka, T.; Sawada, M.; Takemori, H.; Yamatodani, A. Histamine production by cultured microglial cells of the mouse. Neurosci. Lett. 2001, 305, 181–184. [Google Scholar] [CrossRef]
- Huang, H.; Li, Y.; Liang, J.; Finkelman, F.D. Molecular Regulation of Histamine Synthesis. Front Immunol. 2018, 9, 1392. [Google Scholar] [CrossRef]
- Nuutinen, S.; Panula, P. Histamine in neurotransmission and brain diseases. Adv. Exp. Med. Biol. 2010, 709, 95–107. [Google Scholar] [PubMed]
- Slotkin, T.A.; Slepetis, R.J.; Weigel, S.J.; Whitmore, W.L. Effects of alpha-fluoromethylhistidine (FMH), an irreversible inhibitor of histidine decarboxylase, on development of brain histamine and catecholamine systems in the neonatal rat. Life Sci. 1983, 32, 2897–2903. [Google Scholar] [CrossRef]
- Hu, W.; Chen, Z. The roles of histamine and its receptor ligands in central nervous system disorders: An update. Pharmacol. Ther. 2017, 175, 116–132. [Google Scholar] [CrossRef]
- Kitanaka, J.; Kitanaka, N.; Tsujimura, T.; Terada, N.; Takemura, M. Expression of diamine oxidase (histaminase) in guinea-pig tissues. Eur. J. Pharmacol. 2002, 437, 179–185. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Nakamura, T.; Yanai, K. Histaminergic neurons in the tuberomammillary nucleus as a control centre for wakefulness. Br. J. Pharmacol. 2021, 178, 750–769. [Google Scholar] [CrossRef]
- Panula, P.; Chazot, P.L.; Cowart, M.; Gutzmer, R.; Leurs, R.; Liu, W.L.; Stark, H.; Thurmond, R.L.; Haas, H.L. International Union of Basic and Clinical Pharmacology. XCVIII. Histamine Receptors. Pharmacol. Rev. 2015, 67, 601–655. [Google Scholar] [PubMed] [Green Version]
- Kárpáti, A.; Yoshikawa, T.; Naganuma, F.; Matsuzawa, T.; Kitano, H.; Yamada, Y.; Yokoyama, M.; Futatsugi, A.; Mikoshiba, K.; Yanai, K. Histamine H(1) receptor on astrocytes and neurons controls distinct aspects of mouse behaviour. Sci. Rep. 2019, 9, 16451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kárpáti, A.; Yoshikawa, T.; Nakamura, T.; Iida, T.; Matsuzawa, T.; Kitano, H.; Harada, R.; Yanai, K. Histamine elicits glutamate release from cultured astrocytes. J. Pharmacol. Sci. 2018, 137, 122–128. [Google Scholar] [CrossRef]
- Karlstedt, K.; Senkas, A.; Ahman, M.; Panula, P. Regional expression of the histamine H(2) receptor in adult and developing rat brain. Neuroscience 2001, 102, 201–208. [Google Scholar] [CrossRef]
- Pillot, C.; Heron, A.; Cochois, V.; Tardivel-Lacombe, J.; Ligneau, X.; Schwartz, J.C.; Arrang, J.M. A detailed mapping of the histamine H(3) receptor and its gene transcripts in rat brain. Neuroscience 2002, 114, 173–193. [Google Scholar] [CrossRef]
- Sallmen, T.; Lozada, A.F.; Anichtchik, O.V.; Beckman, A.L.; Panula, P. Increased brain histamine H3 receptor expression during hibernation in golden-mantled ground squirrels. BMC Neurosci. 2003, 4, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellenbroek, B.A.; Ghiabi, B. The other side of the histamine H3 receptor. Trends Neurosci. 2014, 37, 191–199. [Google Scholar] [CrossRef]
- Nieto-Alamilla, G.; Márquez-Gómez, R.; García-Gálvez, A.M.; Morales-Figueroa, G.E.; Arias-Montaño, J.A. The Histamine H3 Receptor: Structure, Pharmacology, and Function. Mol. Pharmacol. 2016, 90, 649–673. [Google Scholar] [CrossRef] [Green Version]
- Mariottini, C.; Scartabelli, T.; Bongers, G.; Arrigucci, S.; Nosi, D.; Leurs, R.; Chiarugi, A.; Blandina, P.; Pellegrini-Giampietro, D.E.; Passani, M.B. Activation of the histaminergic H3 receptor induces phosphorylation of the Akt/GSK-3 beta pathway in cultured cortical neurons and protects against neurotoxic insults. J. Neurochem. 2009, 110, 1469–1478. [Google Scholar] [CrossRef] [PubMed]
- Orr, E.; Quay, W.B. Hypothalamic 24-h rhythms in histamine, histidine, decarboxylase and histamine-N-methyltransferase. Endocrinology 1975, 96, 941–945. [Google Scholar] [CrossRef] [PubMed]
- Friedman, A.H.; Walker, C.A. Circadian rhythms in rat mid-brain and caudate nucleus biogenic amine levels. J. Physiol. 1968, 197, 77–85. [Google Scholar] [CrossRef]
- Ko, E.M.; Estabrooke, I.V.; McCarthy, M.; Scammell, T.E. Wake-related activity of tuberomammillary neurons in rats. Brain Res. 2003, 992, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Yanai, K. Studies on functional roles of the histaminergic neuron system by using pharmacological agents, knockout mice and positron emission tomography. Tohoku J. Exp. Med. 2001, 195, 197–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inoue, I.; Yanai, K.; Kitamura, D.; Taniuchi, I.; Kobayashi, T.; Niimura, K.; Watanabe, T.; Watanabe, T. Impaired locomotor activity and exploratory behavior in mice lacking histamine H1 receptors. Proc. Natl. Acad. Sci. USA 1996, 93, 13316–13320. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, K.; Suwa, H.; Ishikawa, T.; Kotani, H. Targeted disruption of H3 receptors results in changes in brain histamine tone leading to an obese phenotype. J. Clin. Investig. 2002, 110, 1791–1799. [Google Scholar] [CrossRef]
- Toyota, H.; Dugovic, C.; Koehl, M.; Laposky, A.D.; Weber, C.; Ngo, K.; Wu, Y.; Lee, D.H.; Yanai, K.; Sakurai, E.; et al. Behavioral characterization of mice lacking histamine H(3) receptors. Mol. Pharmacol. 2002, 62, 389–397. [Google Scholar] [CrossRef] [Green Version]
- Parmentier, R.; Ohtsu, H.; Djebbara-Hannas, Z.; Valatx, J.L.; Watanabe, T.; Lin, J.S. Anatomical, physiological, and pharmacological characteristics of histidine decarboxylase knock-out mice: Evidence for the role of brain histamine in behavioral and sleep-wake control. J. Neurosci. 2002, 22, 7695–7711. [Google Scholar] [CrossRef]
- Huang, Z.L.; Mochizuki, T.; Qu, W.M.; Hong, Z.Y.; Watanabe, T.; Urade, Y.; Hayaishi, O. Altered sleep-wake characteristics and lack of arousal response to H3 receptor antagonist in histamine H1 receptor knockout mice. Proc. Natl. Acad. Sci. USA 2006, 103, 4687–4692. [Google Scholar] [CrossRef] [Green Version]
- Gondard, E.; Anaclet, C.; Akaoka, H.; Guo, R.X.; Zhang, M.; Buda, C.; Franco, P.; Kotani, H.; Lin, J.S. Enhanced histaminergic neurotransmission and sleep-wake alterations, a study in histamine H3-receptor knock-out mice. Neuropsychopharmacology 2013, 38, 1015–1031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thakkar, M.M. Histamine in the regulation of wakefulness. Sleep Med. Rev. 2011, 15, 65–74. [Google Scholar] [CrossRef] [Green Version]
- Monti, J.M.; Pellejero, T.; Jantos, H. Effects of H1- and H2-histamine receptor agonists and antagonists on sleep and wakefulness in the rat. J. Neural. Transm. 1986, 66, 1–11. [Google Scholar] [CrossRef]
- Lin, J.S.; Sakai, K.; Vanni-Mercier, G.; Arrang, J.M.; Garbarg, M.; Schwartz, J.C.; Jouvet, M. Involvement of histaminergic neurons in arousal mechanisms demonstrated with H3-receptor ligands in the cat. Brain Res. 1990, 523, 325–330. [Google Scholar] [CrossRef]
- Williams, R.H.; Chee, M.J.; Kroeger, D.; Ferrari, L.L.; Maratos-Flier, E.; Scammell, T.E.; Arrigoni, E. Optogenetic-mediated release of histamine reveals distal and autoregulatory mechanisms for controlling arousal. J. Neurosci. 2014, 34, 6023–6029. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Weber, F.; Zhong, P.; Tan, C.L.; Nguyen, T.N.; Beier, K.; Hörmann, N.; Chang, W.-C.; Zhang, Z.; Do, J.P.; et al. Identification of preoptic sleep neurons using retrograde labelling and gene profiling. Nature 2017, 545, 477–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peyron, C.; Tighe, D.K.; Pol, A.N.V.D.; de Lecea, L.; Heller, H.C.; Sutcliffe, J.G.; Kilduff, T. Neurons Containing Hypocretin (Orexin) Project to Multiple Neuronal Systems. J. Neurosci. 1998, 18, 9996–10015. [Google Scholar] [CrossRef] [Green Version]
- Schöne, C.; Apergis-Schoute, J.; Sakurai, T.; Adamantidis, A.; Burdakov, D. Coreleased Orexin and Glutamate Evoke Nonredundant Spike Outputs and Computations in Histamine Neurons. Cell. Rep. 2014, 7, 697–704. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.-L.; Qu, W.-M.; Li, W.-D.; Mochizuki, T.; Eguchi, N.; Watanabe, T.; Urade, Y.; Hayaishi, O. Arousal effect of orexin A depends on activation of the histaminergic system. Proc. Natl. Acad. Sci. USA 2001, 98, 9965–9970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sundvik, M.; Kudo, H.; Toivonen, P.; Rozov, S.; Chen, Y.; Panula, P. The histaminergic system regulates wakefulness and orexin/hypocretin neuron development via histamine receptor H1 in zebrafish. FASEB J. 2011, 25, 4338–4347. [Google Scholar] [CrossRef]
- Venner, A.; Mochizuki, T.; De Luca, R.; Anaclet, C.; Scammell, T.E.; Saper, C.B.; Arrigoni, E.; Fuller, P.M. Reassessing the Role of Histaminergic Tuberomammillary Neurons in Arousal Control. J. Neurosci. 2019, 39, 8929–8939. [Google Scholar] [CrossRef]
- Fujita, A.A.; Bonnavion, P.; Wilson, M.M.; Mickelsen, L.L.; Bloit, J.J.; De Lecea, L.L.; Jackson, A.A. Hypothalamic Tuberomammillary Nucleus Neurons: Electrophysiological Diversity and Essential Role in Arousal Stability. J. Neurosci. 2017, 37, 9574–9592. [Google Scholar] [CrossRef] [PubMed]
- Takeda, N.; Inagaki, S.; Shiosaka, S.; Taguchi, Y.; Oertel, W.H.; Tohyama, M.; Watanabe, T.; Wada, H. Immunohistochemical evidence for the coexistence of histidine decarboxylase-like and glutamate decarboxylase-like immunoreactivities in nerve cells of the magnocellular nucleus of the posterior hypothalamus of rats. Proc. Natl. Acad. Sci. USA 1984, 81, 7647–7650. [Google Scholar] [CrossRef] [Green Version]
- Abdurakhmanova, S.; Grotell, M.; Kauhanen, J.; Linden, A.M.; Korpi, E.R.; Panula, P. Increased Sensitivity of Mice Lacking Extrasynaptic δ-Containing GABA(A) Receptors to Histamine Receptor 3 Antagonists. Front. Pharmacol. 2020, 11, 594. [Google Scholar] [CrossRef] [PubMed]
- Mickelsen, L.E.; Flynn, W.F.; Springer, K.; Wilson, L.; Beltrami, E.J.; Bolisetty, M.; Robson, P.; Jackson, A.C. Cellular taxonomy and spatial organization of the murine ventral posterior hypothalamus. Elife 2020, 9, e58901. [Google Scholar] [CrossRef] [PubMed]
- Vu, M.; Du, G.; Bayliss, D.A.; Horner, R.L. TASK Channels on Basal Forebrain Cholinergic Neurons Modulate Electrocortical Signatures of Arousal by Histamine. J. Neurosci. 2015, 35, 13555–13567. [Google Scholar] [CrossRef]
- Ericson, H.; Blomqvist, A.; Köhler, C. Brainstem afferents to the tuberomammillary nucleus in the rat brain with special reference to monoaminergic innervation. J. Comp. Neurol. 1989, 281, 169–192. [Google Scholar] [CrossRef]
- Scammell, T.E. The neurobiology, diagnosis, and treatment of narcolepsy. Ann. Neurol. 2003, 53, 154–166. [Google Scholar] [CrossRef]
- Nishino, S.; Sakurai, E.; Nevsimalova, S.; Yoshida, Y.; Watanabe, T.; Yanai, K.; Mignot, E. Decreased CSF histamine in narcolepsy with and without low CSF hypocretin-1 in comparison to healthy controls. Sleep 2009, 32, 175–180. [Google Scholar] [CrossRef] [Green Version]
- Kanbayashi, T.; Kodama, T.; Kondo, H.; Satoh, S.; Inoue, Y.; Chiba, S.; Shimizu, T.; Nishino, S. CSF histamine contents in narcolepsy, idiopathic hypersomnia and obstructive sleep apnea syndrome. Sleep 2009, 32, 181–187. [Google Scholar] [CrossRef] [Green Version]
- Bassetti, C.L.; Baumann, C.; Dauvilliers, Y.; Croyal, M.; Robert, P.; Schwartz, J.-C. Cerebrospinal fluid histamine levels are decreased in patients with narcolepsy and excessive daytime sleepiness of other origin. J. Sleep Res. 2010, 19, 620–623. [Google Scholar] [CrossRef] [PubMed]
- Franco, P.; Dauvilliers, Y.; Inocente, C.O.; Guyon, A.; Villanueva, C.; Raverot, V.; Plancoulaine, S.; Lin, J. Impaired histaminergic neurotransmission in children with narcolepsy type 1. CNS Neurosci. Ther. 2018, 25, 386–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- John, J.; Thannickal, T.C.; McGregor, R.M.; Ramanathan, L.; Ohtsu, H.; Nishino, S.; Sakai, N.; Yamanaka, A.; Stone, C.; Cornford, M.; et al. Greatly increased numbers of histamine cells in human narcolepsy with cataplexy. Ann. Neurol. 2013, 74, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Valko, P.O.; Gavrilov, Y.; Yamamoto, M.; Reddy, H.; Haybaeck, J.; Mignot, E.; Baumann, C.; Scammell, T.E. Increase of histaminergic tuberomammillary neurons in narcolepsy. Ann. Neurol. 2013, 74, 794–804. [Google Scholar] [CrossRef]
- Dauvilliers, Y.; Delallée, N.; Jaussent, I.; Scholz, S.; Bayard, S.; Croyal, M.; Schwartz, J.-C.; Robert, P. Normal Cerebrospinal Fluid Histamine and tele-Methylhistamine Levels in Hypersomnia Conditions. Sleep 2012, 35, 1359–1366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Croyal, M.; Dauvilliers, Y.; Labeeuw, O.; Capet, M.; Schwartz, J.-C.; Robert, P. Histamine and tele-methylhistamine quantification in cerebrospinal fluid from narcoleptic subjects by liquid chromatography tandem mass spectrometry with precolumn derivatization. Anal. Biochem. 2010, 409, 28–36. [Google Scholar] [CrossRef]
- Lamb, Y.N. Pitolisant: A Review in Narcolepsy with or without Cataplexy. CNS Drugs 2020, 34, 207–218. [Google Scholar] [CrossRef]
- Dauvilliers, Y.; Arnulf, I.; Szakacs, Z.; Leu-Semenescu, S.; LeComte, I.; Scart-Gres, C.; LeComte, J.-M.; Schwartz, J.-C.; Bastuji, H.; Vieccherini, M.F.; et al. Long-term use of pitolisant to treat patients with narcolepsy: Harmony III Study. Sleep 2019, 42. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.S.; Dauvilliers, Y.; Arnulf, I.; Bastuji, H.; Anaclet, C.; Parmentier, R.; Kocher, L.; Yanagisawa, M.; Lehert, P.; Ligneau, X.; et al. An inverse agonist of the histamine H(3) receptor improves wakefulness in narcolepsy: Studies in orexin-/- mice and patients. Neurobiol. Dis. 2008, 30, 74–83. [Google Scholar] [CrossRef] [Green Version]
- Owen, M.J.; Sawa, A.; Mortensen, P.B. Schizophrenia. Lancet 2016, 388, 86–97. [Google Scholar] [CrossRef] [Green Version]
- Marder, S.R.; Cannon, T.D. Schizophrenia. N. Engl. J. Med. 2019, 381, 1753–1761. [Google Scholar] [CrossRef]
- McCutcheon, R.A.; Reis Marques, T.; Howes, O.D. Schizophrenia-An Overview. JAMA Psychiatry 2020, 77, 201–210. [Google Scholar] [CrossRef]
- Prell, G.D.; Green, J.P.; Kaufmann, C.A.; Khandelwal, J.K.; Morrishow, A.M.; Kirch, D.G.; Linnoila, M.; Wyatt, R.J. Histamine metabolites in cerebrospinal fluid of patients with chronic schizophrenia: Their relationships to levels of other aminergic transmitters and ratings of symptoms. Schizophr. Res. 1995, 14, 93–104. [Google Scholar] [CrossRef]
- Jin, C.Y.; Anichtchik, O.; Panula, P. Altered histamine H3 receptor radioligand binding in post-mortem brain samples from subjects with psychiatric diseases. Br. J. Pharmacol. 2009, 157, 118–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwabuchi, K.; Ito, C.; Tashiro, M.; Kato, M.; Kano, M.; Itoh, M.; Iwata, R.; Matsuoka, H.; Sato, M.; Yanai, K. Histamine H1 receptors in schizophrenic patients measured by positron emission tomography. Eur. Neuropsychopharmacol. 2005, 15, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Ito, C.; Hiraoka, K.; Tashiro, M.; Shibuya, K.; Funaki, Y.; Yoshikawa, T.; Iwata, R.; Matsuoka, H.; Yanai, K. Histamine H1 receptor occupancy by the new-generation antipsychotics olanzapine and quetiapine: A positron emission tomography study in healthy volunteers. Psychopharmacology 2015, 232, 3497–3505. [Google Scholar] [CrossRef] [PubMed]
- Kaminsky, R.; Moriarty, T.; Bodine, J.; Wolf, D.; Davidson, M. Effect of famotidine on deficit symptoms of schizophrenia. Lancet 1990, 335, 1351–1352. [Google Scholar] [CrossRef]
- Mehta, V.S.; Ram, D. Role of ranitidine in negative symptoms of schizophrenia-an open label study. Asian J. Psychiatr. 2014, 12, 150–154. [Google Scholar] [CrossRef]
- Ligneau, X.; Landais, L.; Perrin, D.; Piriou, J.; Uguen, M.; Denis, E.; Robert, P.; Parmentier, R.; Anaclet, C.; Lin, J.-S.; et al. Brain histamine and schizophrenia: Potential therapeutic applications of H3-receptor inverse agonists studied with BF2.649. Biochem. Pharmacol. 2007, 73, 1215–1224. [Google Scholar] [CrossRef] [Green Version]
- Southam, E.; Cilia, J.; Gartlon, J.E.; Woolley, M.L.; Lacroix, L.P.; Jennings, C.A.; Cluderay, J.E.; Reavill, C.; Rourke, C.; Wilson, D.M.; et al. Preclinical investigations into the antipsychotic potential of the novel histamine H3 receptor antagonist GSK207040. Psychopharmacology 2008, 201, 483–494. [Google Scholar] [CrossRef]
- Brown, J.W.; Whitehead, C.A.; Basso, A.M.; Rueter, L.E.; Zhang, M. Preclinical evaluation of non-imidazole histamine H3 receptor antagonists in comparison to atypical antipsychotics for the treatment of cognitive deficits associated with schizophrenia. Int. J. Neuropsychopharmacol. 2013, 16, 889–904. [Google Scholar] [CrossRef]
- Poyurovsky, M.; Fuchs, C.; Pashinian, A.; Levi, A.; Weizman, R.; Weizman, A. Reducing antipsychotic-induced weight gain in schizophrenia: A double-blind placebo-controlled study of reboxetine–betahistine combination. Psychopharmacology 2012, 226, 615–622. [Google Scholar] [CrossRef]
- Barak, N.; Beck, Y.; Albeck, J.H. A Randomized, Double-Blind, Placebo-Controlled Pilot Study of Betahistine to Counteract Olanzapine-Associated Weight Gain. J. Clin. Psychopharmacol. 2016, 36, 253–256. [Google Scholar] [CrossRef]
- Fang, Q.; Hu, W.-W.; Wang, X.-F.; Yang, Y.; Lou, G.-D.; Jin, M.-M.; Yan, H.-J.; Zeng, W.-Z.; Shen, Y.; Zhang, S.-H.; et al. Histamine up-regulates astrocytic glutamate transporter 1 and protects neurons against ischemic injury. Neuropharmacology 2013, 77, 156–166. [Google Scholar] [CrossRef]
- Liao, R.; Chen, Y.; Cheng, L.; Fan, L.; Chen, H.; Wan, Y.; You, Y.; Zheng, Y.; Jiang, L.; Chen, Z.; et al. Histamine H1 Receptors in Neural Stem Cells Are Required for the Promotion of Neurogenesis Conferred by H3 Receptor Antagonism following Traumatic Brain Injury. Stem Cell Rep. 2019, 12, 532–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, L.; Xu, C.; Wang, L.; An, D.; Jiang, L.; Zheng, Y.; Xu, Y.; Wang, Y.; Wang, Y.; Zhang, K.; et al. Histamine H1 receptor deletion in cholinergic neurons induces sensorimotor gating ability deficit and social impairments in mice. Nat. Commun. 2021, 12, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Abeysinghe, A.; Deshapriya, R.; Udawatte, C. Alzheimer’s disease; a review of the pathophysiological basis and therapeutic interventions. Life Sci. 2020, 256, 117996. [Google Scholar] [CrossRef]
- Vaz, M.; Silvestre, S. Alzheimer’s disease: Recent treatment strategies. Eur. J. Pharmacol. 2020, 887, 173554. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef] [PubMed]
- Mazurkiewicz-Kwilecki, I.M.; Nsonwah, S. Changes in the regional brain histamine and histidine levels in postmortem brains of Alzheimer patients. Can. J. Physiol. Pharmacol. 1989, 67, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Cacabelos, R.; Yamatodani, A.; Niigawa, H.; Hariguchi, S.; Tada, K.; Nishimura, T.; Wada, H.; Brandeis, L.; Pearson, J. Brain histamine in Alzheimer’s disease. Methods Find Exp. Clin. Pharmacol. 1989, 11, 353–360. [Google Scholar]
- Panula, P.; Rinne, J.; Kuokkanen, K.; Eriksson, K.; Sallmen, T.; Kalimo, H.; Relja, M. Neuronal histamine deficit in Alzheimer’s disease. Neuroscience 1997, 82, 993–997. [Google Scholar] [CrossRef]
- Shan, L.; Bossers, K.; Unmehopa, U.; Bao, A.-M.; Swaab, D.F. Alterations in the histaminergic system in Alzheimer’s disease: A postmortem study. Neurobiol. Aging 2012, 33, 2585–2598. [Google Scholar] [CrossRef] [PubMed]
- Shan, L.; Swaab, D.F.; Bao, A.-M. Neuronal histaminergic system in aging and age-related neurodegenerative disorders. Exp. Gerontol. 2013, 48, 603–607. [Google Scholar] [CrossRef]
- Higuchi, M.; Yanai, K.; Okamura, N.; Meguro, K.; Arai, H.; Itoh, M.; Iwata, R.; Ido, T.; Watanabe, T.; Sasaki, H. Histamine H1 receptors in patients with Alzheimer’s disease assessed by positron emission tomography. Neuroscience 2000, 99, 721–729. [Google Scholar] [CrossRef]
- Provensi, G.; Costa, A.; Izquierdo, I.; Blandina, P.; Passani, M.B. Brain histamine modulates recognition memory: Possible implications in major cognitive disorders. Br. J. Pharmacol. 2018, 177, 539–556. [Google Scholar] [CrossRef]
- Provensi, G.; Passani, M.B.; Costa, A.; Izquierdo, I.; Blandina, P. Neuronal histamine and the memory of emotionally salient events. Br. J. Pharmacol. 2018, 177, 557–569. [Google Scholar] [CrossRef]
- Dai, H.; Kaneko, K.; Kato, H.; Fujii, S.; Jing, Y.; Xu, A.; Sakurai, E.; Kato, M.; Okamura, N.; Kuramasu, A.; et al. Selective cognitive dysfunction in mice lacking histamine H1 and H2 receptors. Neurosci. Res. 2007, 57, 306–313. [Google Scholar] [CrossRef]
- Zlomuzica, A.; Ruocco, L.; Sadile, A.; Huston, J.; Dere, E. Histamine H1 receptor knockout mice exhibit impaired spatial memory in the eight-arm radial maze. Br. J. Pharmacol. 2009, 157, 86–91. [Google Scholar] [CrossRef] [Green Version]
- Zlomuzica, A.; Dere, D.; Dere, E. The histamine H1 receptor and recollection-based discrimination in a temporal order memory task in the mouse. Pharmacol. Biochem. Behav. 2013, 111, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Dere, E.; Zlomuzica, A.; Viggiano, D.; Ruocco, L.; Watanabe, T.; Sadile, A.; Huston, J.; De Souza-Silva, M. Episodic-like and procedural memory impairments in histamine H1 Receptor knockout mice coincide with changes in acetylcholine esterase activity in the hippocampus and dopamine turnover in the cerebellum. Neuroscience 2008, 157, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Yanai, K.; Son, L.Z.; Endou, M.; Sakurai, E.; Nakagawasai, O.; Tadano, T.; Kisara, K.; Inoue, I.; Watanabe, T. Behavioural characterization and amounts of brain monoamines and their metabolites in mice lacking histamine H1 receptors. Neuroscience 1998, 87, 479–487. [Google Scholar] [CrossRef]
- Schneider, E.H.; Neumann, D.; Seifert, R. Modulation of behavior by the histaminergic system: Lessons from H1R-and H2R-deficient mice. Neurosci. Biobehav. Rev. 2014, 42, 252–266. [Google Scholar] [CrossRef] [PubMed]
- Da Silveira, C.K.B.; Furini, C.R.; Benetti, F.; da Cruz Monteiro, S.; Izquierdo, I. The role of histamine receptors in the consolidation of object recognition memory. Neurobiol. Learn Mem. 2013, 103, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Chen, J.Q.; Kamei, C. Effect of H1-antagonists on spatial memory deficit evaluated by 8-arm radial maze in rats. Acta Pharmacol. Sin. 2001, 22, 609–613. [Google Scholar]
- Hasenöhrl, R.U.; Weth, K.; Huston, J.P. Intraventricular infusion of the histamine H(1) receptor antagonist chlorpheniramine improves maze performance and has anxiolytic-like effects in aged hybrid Fischer 344xBrown Norway rats. Exp. Brain Res. 1999, 128, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Dere, E.; De Souza-Silva, M.A.; Topic, B.; Spieler, R.E.; Haas, H.L.; Huston, J.P. Histidine-Decarboxylase Knockout Mice Show Deficient Nonreinforced Episodic Object Memory, Improved Negatively Reinforced Water-Maze Performance, and Increased Neo- and Ventro-Striatal Dopamine Turnover. Learn. Mem. 2003, 10, 510–519. [Google Scholar] [CrossRef] [Green Version]
- Acevedo, S.F.; Ohtsu, H.; Benice, T.S.; Rizk-Jackson, A.; Raber, J. Age-dependent measures of anxiety and cognition in male histidine decarboxylase knockout (Hdc-/-) mice. Brain Res. 2006, 1071, 113–123. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, S.; Zhu, Y.; Fu, Q.; Zhu, Y.; Gong, Y.; Ohtsu, H.; Luo, J.; Wei, E.; Chen, Z. Improved learning and memory of contextual fear conditioning and hippocampal CA1 long-term potentiation in histidine decarboxylase knock-out mice. Hippocampus 2007, 17, 634–641. [Google Scholar] [CrossRef]
- Gong, Y.-X.; Shou, W.-T.; Feng, B.; Zhang, W.-P.; Wang, H.-J.; Ohtsu, H.; Chen, Z. Ameliorating effect of histamine on impairment of cued fear extinction induced by morphine withdrawal in histidine decarboxylase gene knockout mice. Acta Pharmacol. Sin. 2010, 31, 1431–1437. [Google Scholar] [CrossRef] [Green Version]
- Schneider, E.H.; Neumann, D.; Seifert, R. Modulation of behavior by the histaminergic system: Lessons from HDC-, H3R- and H4R-deficient mice. Neurosci. Biobehav. Rev. 2014, 47, 101–121. [Google Scholar] [CrossRef] [PubMed]
- Rizk, A.; Curley, J.; Robertson, J.; Raber, J. Anxiety and cognition in histamine H3 receptor-/- mice. Eur. J. Neurosci. 2004, 19, 1992–1996. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-W.; Hu, W.-W.; Chen, Z.; Zhang, L.-S.; Shen, H.-Q.; Timmerman, H.; Leurs, R.; Yanai, K. Effect of the histamine H3-antagonist clobenpropit on spatial memory deficits induced by MK-801 as evaluated by radial maze in Sprague–Dawley rats. Behav. Brain Res. 2004, 151, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Orsetti, M.; Ghi, P.; Di Carlo, G. Histamine H(3)-receptor antagonism improves memory retention and reverses the cognitive deficit induced by scopolamine in a two-trial place recognition task. Behav. Brain Res. 2001, 124, 235–242. [Google Scholar] [CrossRef]
- Zlomuzica, A.; Dere, D.; Binder, S.; Silva, M.A.D.S.; Huston, J.P.; Dere, E. Neuronal histamine and cognitive symptoms in Alzheimer’s disease. Neuropharmacology 2015, 106, 135–145. [Google Scholar] [CrossRef]
- Bardgett, M.E.; Davis, N.N.; Schultheis, P.J.; Griffith, M.S. Ciproxifan, an H3 receptor antagonist, alleviates hyperactivity and cognitive deficits in the APP Tg2576 mouse model of Alzheimer’s disease. Neurobiol. Learn Mem. 2011, 95, 64–72. [Google Scholar] [CrossRef] [Green Version]
- Nathan, P.J.; Boardley, R.; Scott, N.; Berges, A.; Maruff, P.; Sivananthan, T.; Upton, N.; Lowy, M.T.; Nestor, P.J.; Lai, R. The safety, tolerability, pharmacokinetics and cognitive effects of GSK239512, a selective histamine H₃ receptor antagonist in patients with mild to moderate Alzheimer’s disease: A preliminary investigation. Curr. Alzheimer Res. 2013, 10, 240–251. [Google Scholar] [CrossRef]
- Grove, R.A.; Harrington, C.M.; Mahler, A.; Beresford, I.; Maruff, P.; Lowy, M.T.; Nicholls, A.P.; Boardley, R.L.; Berges, A.C.; Nathan, P.J.; et al. A randomized, double-blind, placebo-controlled, 16-week study of the H3 receptor antagonist, GSK239512 as a monotherapy in subjects with mild-to-moderate Alzheimer’s disease. Curr. Alzheimer Res. 2014, 11, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Haig, G.M.; Pritchett, Y.; Meier, A.; Othman, A.A.; Hall, C.; Gault, L.M.; Lenz, R.A. A Randomized Study of H3 Antagonist ABT-288 in Mild-To-Moderate Alzheimer’s Dementia1. J. Alzheimer Dis. 2014, 42, 959–971. [Google Scholar] [CrossRef]
- Egan, M.; Yaari, R.; Liu, L.; Ryan, M.; Peng, Y.; Lines, C.; Michelson, D. Pilot randomized controlled study of a histamine receptor inverse agonist in the symptomatic treatment of AD. Curr. Alzheimer Res. 2012, 9, 481–490. [Google Scholar] [CrossRef]
- Hallett, M. Tourette Syndrome: Update. Brain Dev. 2015, 37, 651–655. [Google Scholar] [CrossRef] [Green Version]
- Efron, D.; Dale, R.C. Tics and Tourette syndrome. J. Paediatr. Child Health 2018, 54, 1148–1153. [Google Scholar] [CrossRef]
- Ercan-Sencicek, A.G.; Stillman, A.A.; Ghosh, A.K.; Bilguvar, K.; O’Roak, B.J.; Mason, C.E.; Abbott, T.; Gupta, A.; King, R.A.; Pauls, D.L.; et al. L-Histidine Decarboxylase and Tourette’s Syndrome. N. Engl. J. Med. 2010, 362, 1901–1908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez, T.V.; Sanders, S.; Yurkiewicz, I.R.; Ercan-Sencicek, A.G.; Kim, Y.-S.; Fishman, D.O.; Raubeson, M.J.; Song, Y.; Yasuno, K.; Ho, W.S.; et al. Rare Copy Number Variants in Tourette Syndrome Disrupt Genes in Histaminergic Pathways and Overlap with Autism. Biol. Psychiatry 2012, 71, 392–402. [Google Scholar] [CrossRef] [Green Version]
- Karagiannidis, I.; Dehning, S.; Sandor, P.; Tarnok, Z.; Rizzo, R.; Wolanczyk, T.; Madruga-Garrido, M.; Hebebrand, J.; Nöthen, M.; Lehmkuhl, G.; et al. Support of the histaminergic hypothesis in Tourette Syndrome: Association of the histamine decarboxylase gene in a large sample of families. J. Med. Genet. 2013, 50, 760–764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baldan, L.C.; Williams, K.A.; Gallezot, J.-D.; Pogorelov, V.; Rapanelli, M.; Crowley, M.; Anderson, G.M.; Loring, E.; Gorczyca, R.; Billingslea, E.; et al. Histidine Decarboxylase Deficiency Causes Tourette Syndrome: Parallel Findings in Humans and Mice. Neuron 2014, 81, 77–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rapanelli, M.; Frick, L.; Bito, H.; Pittenger, C. Histamine modulation of the basal ganglia circuitry in the development of pathological grooming. Proc. Natl. Acad. Sci. USA 2017, 114, 6599–6604. [Google Scholar] [CrossRef] [Green Version]
- Rapanelli, M.; Frick, L.; Pogorelov, V.; Ohtsu, H.; Bito, H.; Pittenger, C. Histamine H3R receptor activation in the dorsal striatum triggers stereotypies in a mouse model of tic disorders. Transl. Psychiatry 2017, 7, e1013. [Google Scholar] [CrossRef]
- Raza, C.; Anjum, R.; Shakeel, N.U.A. Parkinson’s disease: Mechanisms, translational models and management strategies. Life Sci. 2019, 226, 77–90. [Google Scholar] [CrossRef]
- Radhakrishnan, D.M.; Goyal, V. Parkinson’s disease: A review. Neurol. India 2018, 66, s26–s35. [Google Scholar] [PubMed]
- Braak, H.; Del Tredici, K.; Rüb, U.; de Vos, R.A.; Steur, E.N.J.; Braak, E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 2003, 24, 197–211. [Google Scholar] [CrossRef]
- Shan, L.; Liu, C.-Q.; Balesar, R.; Hofman, M.A.; Bao, A.-M.; Swaab, D.F. Neuronal histamine production remains unaltered in Parkinson’s disease despite the accumulation of Lewy bodies and Lewy neurites in the tuberomamillary nucleus. Neurobiol. Aging 2012, 33, 1343–1344. [Google Scholar] [CrossRef]
- Nakamura, S.; Ohnishi, K.; Nishimura, M.; Suenaga, T.; Akiguchi, I.; Kimura, J.; Kimura, T. Large neurons in the tuberomammillary nucleus in patients with Parkinson’s disease and multiple system atrophy. Neurology 1996, 46, 1693–1696. [Google Scholar] [CrossRef] [PubMed]
- Rinne, J.O.; Anichtchik, O.; Eriksson, K.S.; Kaslin, J.; Tuomisto, L.; Kalimo, H.; Röyttä, M.; Panula, P. Increased brain histamine levels in Parkinson’s disease but not in multiple system atrophy. J. Neurochem. 2002, 81, 954–960. [Google Scholar] [CrossRef]
- Anichtchik, O.; Rinne, J.O.; Kalimo, H.; Panula, P. An Altered Histaminergic Innervation of the Substantia Nigra in Parkinson’s Disease. Exp. Neurol. 2000, 163, 20–30. [Google Scholar] [CrossRef]
- Shan, L.; Bossers, K.; Luchetti, S.; Balesar, R.; Lethbridge, N.; Chazot, P.L.; Bao, A.-M.; Swaab, D.F. Alterations in the histaminergic system in the substantia nigra and striatum of Parkinson’s patients: A postmortem study. Neurobiol. Aging 2012, 33, 1488.e1–1488.e13. [Google Scholar] [CrossRef]
- Liu, C.-Q.; Chen, Z.; Liu, F.-X.; Hu, D.-N.; Luo, J.-H. Involvement of brain endogenous histamine in the degeneration of dopaminergic neurons in 6-hydroxydopamine-lesioned rats. Neuropharmacology 2007, 53, 832–841. [Google Scholar] [CrossRef]
- Liu, C.-Q.; Hu, D.-N.; Liu, F.-X.; Chen, Z.; Luo, J.-H. Apomorphine-induced turning behavior in 6-hydroxydopamine lesioned rats is increased by histidine and decreased by histidine decarboxylase, histamine H1 and H2 receptor antagonists, and an H3 receptor agonist. Pharmacol. Biochem. Behav. 2008, 90, 325–330. [Google Scholar] [CrossRef]
- Johnston, T.H.; Van Der Meij, A.; Brotchie, J.M.; Fox, S.H. Effect of histamine H2 receptor antagonism on levodopa-induced dyskinesia in the MPTP-macaque model of Parkinson’s disease. Mov. Disord. 2010, 25, 1379–1390. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Cui, G.; Wang, X.; Yue, X.; Shi, H.; Shen, X.; Zhang, Z. Ranitidine reduced levodopa-induced dyskinesia in a rat model of Parkinson’s disease. Neuropsychiatr. Dis. Treat. 2013, 10, 39–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nowak, P.; Noras, Ł.; Jochem, J.; Szkilnik, R.; Brus, H.; Körőssy, E.; Drab, J.; Kostrzewa, R.M.; Brus, R. Histaminergic Activity in a Rodent Model of Parkinson’s Disease. Neurotox. Res. 2009, 15, 246–251. [Google Scholar] [CrossRef]
- Masini, D.; Aguiar, C.L.; Bonito-Oliva, A.; Papadia, D.; Andersson, R.; Fisahn, A.; Fisone, G. The histamine H3 receptor antagonist thioperamide rescues circadian rhythm and memory function in experimental parkinsonism. Transl. Psychiatry 2017, 7, e1088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molinari, S.P.; Kaminski, R.; Rocco, A.; Yahr, M.D. The use of famotidine in the treatment of Parkinson’s disease: A pilot study. J. Neural Transm. 1995, 9, 243–247. [Google Scholar] [CrossRef]
- Schwartz, J.-C. The histamine H3 receptor: From discovery to clinical trials with pitolisant. Br. J. Pharmacol. 2011, 163, 713–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Disorder | Ligands | Target | Phase | NCT Number |
|---|---|---|---|---|
| Narcolepsy | TS-091 | H3R inverse agonist/antagonist | II | NCT03267303 |
| GSK189254 | H3R inverse agonist/antagonist | II | NCT00366080 | |
| JNJ-17216498 | H3R antagonist | II | NCT00424931 | |
| BF2.649 | H3R inverse agonist/antagonist | III | NCT01638403 | |
| BF2.649 | H3R inverse agonist/antagonist | III | NCT01399606 | |
| BF2.649 | H3R inverse agonist/antagonist | III | NCT01067222 | |
| BF2.649 | H3R inverse agonist/antagonist | III | NCT01800045 | |
| PF-03654746 | H3R antagonist | II | NCT01006122 | |
| Schizophrenia | Famotidine | H2R antagonist | IV | NCT00565175 |
| BF2.649 | H3R inverse agonist/antagonist | II | NCT00690274 | |
| GSK239512 | H3R inverse agonist/antagonist | II | NCT01009060 | |
| MK0249 | H3R inverse agonist/antagonist | II | NCT00506077 | |
| ABT-288 | H3R inverse agonist/antagonist | II | NCT01077700 | |
| Alzheimer’s disease | GSK239512 | H3R inverse agonist/antagonist | II | NCT01009255 |
| MK0249 | H3R inverse agonist/antagonist | II | NCT00420420 | |
| PF-03654746 | H3R antagonist | I | NCT01028911 | |
| ABT-288 | H3R inverse agonist/antagonist | II | NCT01018875 | |
| Tourette’s syndrome | AZD5213 | H3R inverse agonist/antagonist | II | NCT01904773 |
| Parkinson’s disease | Famotidine | H2R antagonist | II | NCT01937078 |
| BF2.649 | H3R inverse agonist/antagonist | III | NCT01036139 | |
| BF2.649 | H3R inverse agonist/antagonist | III | NCT01066442 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, L.; Liu, J.; Chen, Z. The Histaminergic System in Neuropsychiatric Disorders. Biomolecules 2021, 11, 1345. https://doi.org/10.3390/biom11091345
Cheng L, Liu J, Chen Z. The Histaminergic System in Neuropsychiatric Disorders. Biomolecules. 2021; 11(9):1345. https://doi.org/10.3390/biom11091345
Chicago/Turabian StyleCheng, Li, Jiaying Liu, and Zhong Chen. 2021. "The Histaminergic System in Neuropsychiatric Disorders" Biomolecules 11, no. 9: 1345. https://doi.org/10.3390/biom11091345
APA StyleCheng, L., Liu, J., & Chen, Z. (2021). The Histaminergic System in Neuropsychiatric Disorders. Biomolecules, 11(9), 1345. https://doi.org/10.3390/biom11091345






