Synthesis and Biological Evaluation of Benzo[b]thiophene Acylhydrazones as Antimicrobial Agents against Multidrug-Resistant Staphylococcus aureus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemistry
2.1.1. General Information
2.1.2. General Procedure for Ethyl 6-halogenobenzo[b]thiophene-2-carboxylate (4)
Ethyl 6-chlorobenzo[b]thiophene-2-carboxylate (4a)
Ethyl 6-fluorobenzo[b]thiophene-2-carboxylate (4b)
2.1.3. Procedure for Ethyl 6-(trifluoromethyl)benzo[b]thiophene-2-carboxylate (4c)
Ethyl 6-(trifluoromethyl)benzo[b]thiophene-2-carboxylate (4c)
2.1.4. Procedure for Ethyl 6-chlorobenzofuran-2-carboxylate (4d)
Ethyl 6-chlorobenzofuran-2-carboxylate (4d)
2.1.5. General Procedure for Benzo[b]thiophene-2-carboxylic Acids (1)
6-Chlorobenzo[b]thiophene-2-carboxylic acid (1b)
6-Fluorobenzo[b]thiophene-2-carboxylic acid (1c)
6-(Trifluoromethyl)benzo[b]thiophene-2-carboxylic acid (1d)
6-Chlorobenzofuran-2-carboxylic acid (1e)
2.1.6. General Procedure for Tert-butyl 2-(benzo[b]thiophene-2-carbonyl)hydrazine-1-carboxylates (2)
Tert-butyl-2-(benzo[b]thiophene-2-carbonyl)hydrazine-1-carboxylate (2a)
Tert-butyl-2-(6-chlorobenzo[b]thiophene-2-carbonyl)hydrazine-1-carboxylate (2b)
Tert-butyl-2-(6-fluorobenzo[b]thiophene-2-carbonyl)hydrazine-1-carboxylate (2c)
Tert-butyl-2-(6-(trifluoromethyl)benzo[b]thiophene-2-carbonyl)hydrazine-1-carboxylate (2d)
Tert-butyl-2-(6-chlorobenzofuran-2-carbonyl)hydrazine-1-carboxylate (2e)
2.1.7. General Procedure for N-Acylhydrazones Derivatives (I, II and III)
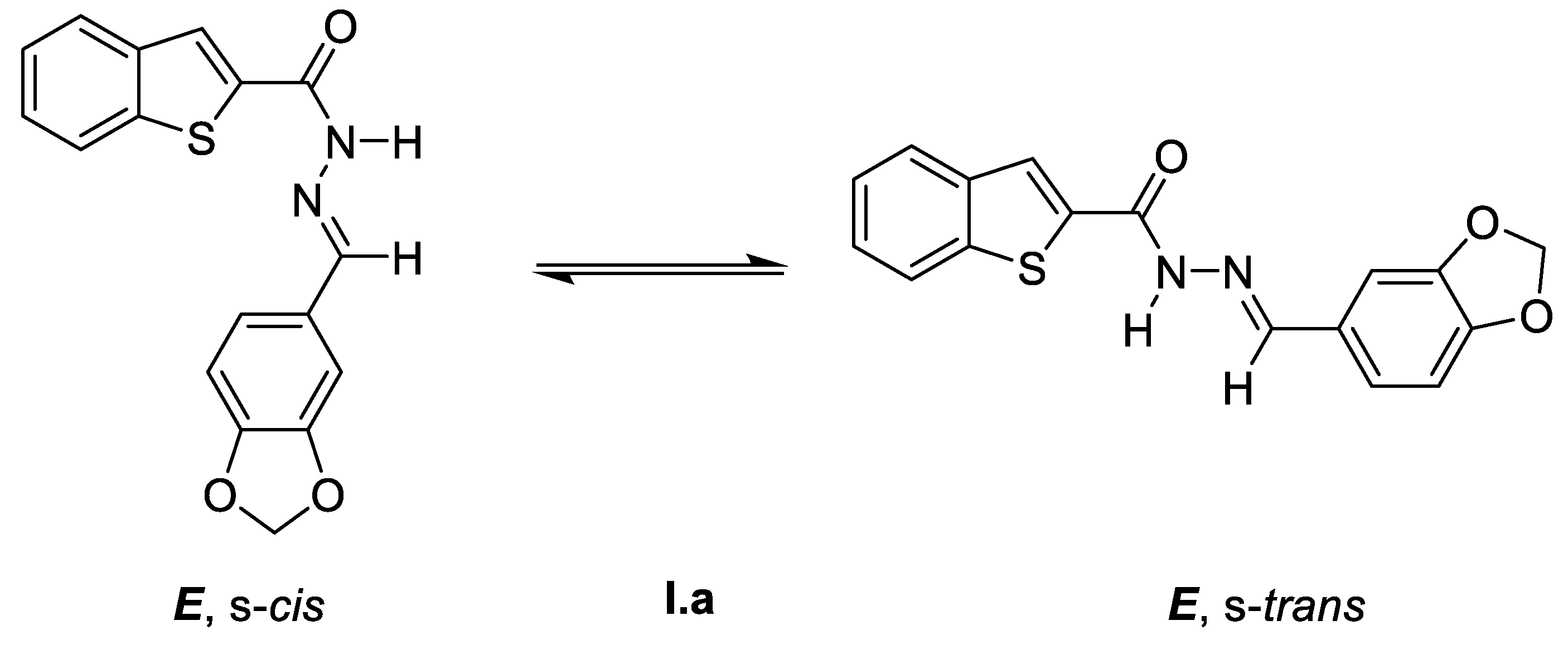
(E)-N’-(Benzo[d][1,3]dioxol-5-ylmethylene)benzo[b]thiophene-2-carbohydrazide (I.a)
(E)-N’-(4-(Dimethylamino)benzylidene)benzo[b]thiophene-2-carbohydrazide (I.b)
(E)-N’-(Pyridin-2-ylmethylene)benzo[b]thiophene-2-carbohydrazide (I.c)
(E)-N’-(Pyridin-3-ylmethylene)benzo[b]thiophene-2-carbohydrazide (I.d)
(E)-N’-(Pyridin-4-ylmethylene)benzo[b]thiophene-2-carbohydrazide (I.e)
(E)-N’-(3-Hydroxybenzylidene)benzo[b]thiophene-2-carbohydrazide (I.f)
(E)-N’-(4-Hydroxybenzylidene)benzo[b]thiophene-2-carbohydrazide (I.g)
(E)-N’-(4-Hydroxy-3-methoxybenzylidene)benzo[b]thiophene-2-carbohydrazide (I.h)
(E)-N’-(4-Chlorobenzylidene)benzo[b]thiophene-2-carbohydrazide (I.i)
(E)-N’-(4-Fluorobenzylidene)benzo[b]thiophene-2-carbohydrazide (I.j)
(E)-N’-(3-Nitrobenzylidene)benzo[b]thiophene-2-carbohydrazide (I.k)
(E)-N’-(4-Nitrobenzylidene)benzo[b]thiophene-2-carbohydrazide (I.l)
(E)-2-((2-(Benzo[b]thiophene-2-carbonyl)hydrazono)methyl)benzoic acid (I.m)
(E)-3-((2-(Benzo[b]thiophene-2-carbonyl)hydrazineylidene)methyl)benzoic acid (I.n)
(E)-4-((2-(Benzo[b]thiophene-2-carbonyl)hydrazono)methyl)benzoic acid (I.o)
(E)-N’-(Benzo[d][1,3]dioxol-5-ylmethylene)-6-chlorobenzo[b]thiophene-2-carbohydrazide (II.a)
(E)-6-Chloro-N’-(pyridin-2-ylmethylene)benzo[b]thiophene-2-carbohydrazide (II.b)
(E)-N’-(Benzo[d][1,3]dioxol-5-ylmethylene)-6-fluorobenzo[b]thiophene-2-carbohydrazide (II.c)
(E)-6-Fluoro-N’-(pyridin-2-ylmethylene)benzo[b]thiophene-2-carbohydrazide (II.d)
(E)-N’-((1H-Imidazol-4-yl)methylene)-6-chlorobenzo[b]thiophene-2-carbohydrazide (III.a)
(E)-6-Chloro-N’-(furan-2-ylmethylene)benzo[b]thiophene-2-carbohydrazide (III.b)
(E)-6-Chloro-N’-((5-(hydroxymethyl)furan-2-yl)methylene)benzo[b]thiophene-2-carbohydrazide (III.c)
(E)-6-Chloro-N’-(pyridin-2-ylmethylene)benzofuran-2-carbohydrazide (III.e)
(E)-N’-(Pyridin-2-ylmethylene)-6-(trifluoromethyl)benzo[b]thiophene-2-carbohydrazide (III.f)
2.1.8. Procedure for N’-(Pyridin-2-ylmethyl)benzo[b]thiophene-2-carbohydrazide (I.p)
N’-(Pyridin-2-ylmethyl)benzo[b]thiophene-2-carbohydrazide (I.p)
2.1.9. Procedure for Pyridine 2-aldoxime (6)
Pyridine 2-aldoxime (6)
2.1.10. Procedure for (E)-Picolinaldehyde O-(6-chlorobenzo[b]thiophene-2-carbonyl) oxime (III.d)
(E)-Picolinaldehyde O-(6-chlorobenzo[b]thiophene-2-carbonyl) oxime (III.d)
2.2. Biological Assays
2.2.1. Minimum Inhibitory Concentration (MIC) Evaluation
2.2.2. Cytotoxicity Assay
3. Results and Discussion
3.1. Synthesis and Biological Evaluation of Series I—Benzo[b]thiophene-2-Acylhydrazones
3.2. Synthesis and Biological Evaluation of Series II—6-Halogenobenzo[b]thiophene-2-Acylhydrazones
3.3. Synthesis and Biological Evaluation of Series III—Structural Modifications of II.b
3.4. Cytotoxicity Assay of II.b
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bradley, J.S.; Guidos, R.; Baragona, S.; Bartlett, J.G.; Rubinstein, E.; Zhanel, G.G.; Tino, M.D.; Pompliano, D.L.; Tally, F.; Tipirneni, P.; et al. Anti-infective research and development--problems, challenges, and solutions. Lancet Infect. Dis. 2007, 7, 68–78. [Google Scholar] [CrossRef]
- Spellberg, B.; Powers, J.H.; Brass, E.P.; Miller, L.G.; Edwards, J.E., Jr. Trends in antimicrobial drug development: Implications for the future. Clin. Infect. Dis. 2004, 38, 1279–1286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neill, J.O. Antimicrobial resistance: Tackling a crisis for the health and wealth of nations. In Review Paper-Tackling a Crisis for the Health and Wealth of Nations; HM Government Wellcome Trust: London, UK, 2014; pp. 1–20. [Google Scholar]
- Rasko, D.A.; Sperandio, V. Anti-virulence strategies to combat bacteria-mediated disease. Nat. Rev. Drug Discov. 2010, 9, 117–128. [Google Scholar] [CrossRef]
- Keri, R.S.; Chand, K.; Budagumpi, S.; Balappa Somappa, S.; Patil, S.A.; Nagaraja, B.M. An overview of benzo[b]thiophene-based medicinal chemistry. Eur. J. Med. Chem. 2017, 138, 1002–1033. [Google Scholar] [CrossRef]
- Hadda, T.B.; Srivastava, S.; Das, B.; Salgado-Zamora, H.; Shaheen, U.; Bader, A.; Naseer, M.M. POM analyses of antimicrobial activity of some 2,3-armed 4,5,6,7-tetrahydro-1-benzothiophenes: Favourable and unfavourable physico-chemical parameters in design of antibacterial and mycolytic agents. Med. Chem. Res. 2014, 23, 995–1003. [Google Scholar] [CrossRef]
- Kumara, T.H.S.; Mahadevan, K.M.; Harishkumar, H.N.; Padmashali, B.; Naganagowda, G. Synthesis of Benzo[b]thiophene Substituted Carbamates, Ureas, Semicarbazides, and Pyrazoles and Their Antimicrobial and Analgesic Activity. Phosphorus Sulfur Silicon Relat. Elem. 2009, 184, 1866–1879. [Google Scholar] [CrossRef]
- Penthala, N.R.; Sonar, V.N.; Horn, J.; Leggas, M.; Yadlapalli, J.S.; Crooks, P.A. Synthesis and evaluation of a series of benzothiophene acrylonitrile analogs as anticancer agents. Medchemcomm 2013, 4, 1073–1078. [Google Scholar] [CrossRef] [Green Version]
- Moinet, G.; Leriche, C.; Kergoat, M. Antidiabetic Compounds Comprising Benzofuran and Benzothiophene Derivatives. U.S. Patent 7375130B2, 20 May 2008. [Google Scholar]
- Berrade, L.; Aisa, B.; Ramirez, M.J.; Galiano, S.; Guccione, S.; Moltzau, L.R.; Levy, F.O.; Nicoletti, F.; Battaglia, G.; Molinaro, G.; et al. Novel benzo[b]thiophene derivatives as new potential antidepressants with rapid onset of action. J. Med. Chem. 2011, 54, 3086–3090. [Google Scholar] [CrossRef]
- Fakhr, I.M.; Radwan, M.A.; el-Batran, S.; Abd el-Salam, O.M.; el-Shenawy, S.M. Synthesis and pharmacological evaluation of 2-substituted benzo[b]thiophenes as anti-inflammatory and analgesic agents. Eur. J. Med. Chem. 2009, 44, 1718–1725. [Google Scholar] [CrossRef]
- Pathak, C.; Ranjan Singh, R.; Yadav, S.; Kapoor, N.; Raina, V.; Gupta, S.; Surolia, A. Evaluation of benzothiophene carboxamides as analgesics and anti-inflammatory agents. IUBMB Life 2014, 66, 201–211. [Google Scholar] [CrossRef]
- Thota, S.; Rodrigues, D.A.; Pinheiro, P.S.M.; Lima, L.M.; Fraga, C.A.M.; Barreiro, E.J. N-Acylhydrazones as drugs. Bioorg. Med. Chem. Lett. 2018, 28, 2797–2806. [Google Scholar] [CrossRef]
- Fedi, V.; Altamura, M.; Catalioto, R.M.; Giannotti, D.; Giolitti, A.; Giuliani, S.; Guidi, A.; Harmat, N.J.; Lecci, A.; Meini, S.; et al. Discovery of a new series of potent and selective linear tachykinin NK2 receptor antagonists. J. Med. Chem. 2007, 50, 4793–4807. [Google Scholar] [CrossRef]
- Mueller, C.E.; Pegurier, C.; Deligny, M.L.R.; El-Tayeb, A.; Hockemeyer, J.; Ledecq, M.; Mercier, J.; Provins, L.; Boshta, N.M.; Bhattarai, S. (aza) indole-, benzothiophene-, and benzofuran-3-sulfonamides. WO Patent 2018122232, 5 July 2018. [Google Scholar]
- Cheng, K.; Wang, X.; Yin, H. Small-molecule inhibitors of the TLR3/dsRNA complex. J. Am. Chem. Soc. 2011, 133, 3764–3767. [Google Scholar] [CrossRef] [Green Version]
- Tan, Y.J.; Li, M.; Gunawan, G.A.; Nyantakyi, S.A.; Dick, T.; Go, M.L.; Lam, Y. Amide-Amine Replacement in Indole-2-carboxamides Yields Potent Mycobactericidal Agents with Improved Water Solubility. ACS Med. Chem. Lett. 2021, 12, 704–712. [Google Scholar] [CrossRef]
- Li, Z.; Xu, Q.; Yu, C.; Yee, C.; Gwaltney, I.S.L.; Metcalf, B.W.; Richards, S.; Lardy, M.A.; Setti, L.; Sham, H. Pyrazolopyridine Pyrazolopyrimidine and Related Compounds. U.S. Patent 2015315198, 5 November 2015. [Google Scholar]
- Chen, W.; Zhou, Z.H.; Chen, H.B. Efficient synthesis of chiral benzofuryl β-amino alcohols via a catalytic asymmetric Henry reaction. Org. Biomol. Chem. 2017, 15, 1530–1536. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.; Yu, W.; Song, D.; Zhang, W.; Guo, J.; Zhu, J.; Ren, Y.; Kong, L. Discovery of fluorescent coumarin-benzo[b]thiophene 1, 1-dioxide conjugates as mitochondria-targeting antitumor STAT3 inhibitors. Eur. J. Med. Chem. 2019, 174, 236–251. [Google Scholar] [CrossRef]
- Gensini, M.; Altamura, M.; Dimoulas, T.; Fedi, V.; Giannotti, D.; Giuliani, S.; Guidi, A.; Harmat, N.J.; Meini, S.; Nannicini, R.; et al. Modulation on C- and N-terminal moieties of a series of potent and selective linear tachykinin NK(2) receptor antagonists. ChemMedChem 2010, 5, 65–78. [Google Scholar] [CrossRef]
- Kim, B.K.; Ko, H.; Jeon, E.S.; Ju, E.S.; Jeong, L.S.; Kim, Y.C. 2,3,4-Trihydroxybenzyl-hydrazide analogues as novel potent coxsackievirus B3 3C protease inhibitors. Eur. J. Med. Chem. 2016, 120, 202–216. [Google Scholar] [CrossRef] [PubMed]
- Younus Wani, M.; Athar, F.; Salauddin, A.; Mohan Agarwal, S.; Azam, A.; Choi, I.; Roouf Bhat, A. Novel terpene based 1,4,2-dioxazoles: Synthesis, characterization, molecular properties and screening against Entamoeba histolytica. Eur. J. Med. Chem. 2011, 46, 4742–4752. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-P.; Liu, J.; Zhang, J.-X.; Huang, J.-H.; Wan, C.-Z.; Li, C.-H.; You, X.-Z. Synthesis and ferroelectric properties of platinum (II) complexes with chiral isoxazoline ligand. Polyhedron 2013, 60, 85–92. [Google Scholar] [CrossRef]
- Leonczak, P.; Gao, L.J.; Ramadori, A.T.; Lescrinier, E.; Rozenski, J.; De Jonghe, S.; Herdewijn, P. Synthesis and structure-activity relationship studies of 2-(1,3,4-oxadiazole-2(3H)-thione)-3-amino-5-arylthieno [2,3-b]pyridines as inhibitors of DRAK2. ChemMedChem 2014, 9, 2587–2601. [Google Scholar] [CrossRef] [PubMed]
- Palla, G.; Predieri, G.; Domiano, P.; Vignali, C.; Turner, W. Conformational behaviour and E/Z isomerization of N-acyl and N-aroylhydrazones. Tetrahedron 1986, 42, 3649–3654. [Google Scholar] [CrossRef]
- Kumar, P.; Kadyan, K.; Duhan, M.; Sindhu, J.; Singh, V.; Saharan, B.S. Design, synthesis, conformational and molecular docking study of some novel acyl hydrazone based molecular hybrids as antimalarial and antimicrobial agents. Chem. Cent. J. 2017, 11, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
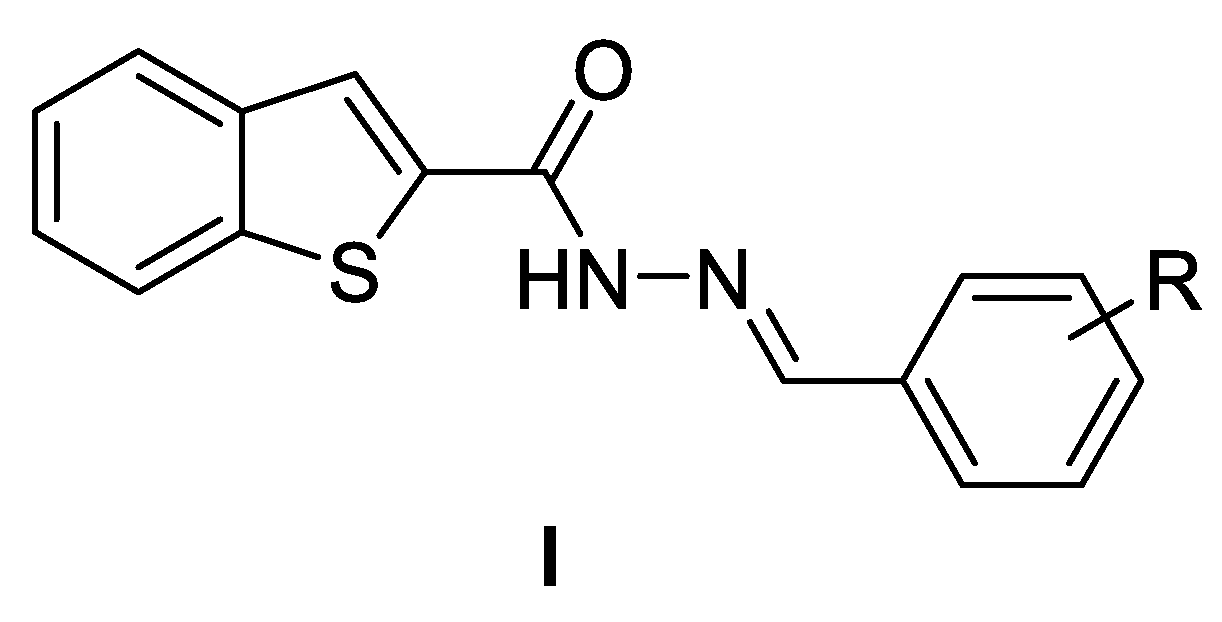








| Name | Structure | LogP | MIC (µg/mL) | ||
|---|---|---|---|---|---|
| ATCC 29213 a | SF8300 b | ST20171643 c | |||
| I.a |  | 4.20 | 32 | 64 | 16 |
| I.b |  | 4.41 | >256 | >256 | >256 |
| I.c |  | 3.13 | 16 | 16 | 16 |
| I.d |  | 3.07 | >256 | >256 | >256 |
| I.e |  | 3.02 | >256 | >256 | >256 |
| I.f |  | 3.80 | >256 | >256 | ND |
| I.g |  | 3.83 | >256 | >256 | >256 |
| I.h |  | 3.64 | >256 | >256 | >256 |
| I.i |  | 4.98 | 128 | 128 | 128 |
| I.j |  | 4.47 | >256 | >256 | 128 |
| I.k |  | 4.24 | >256 | >256 | ND |
| I.l |  | 4.26 | 64 | 64 | 32 |
| I.m |  | 3.83 | 256 | >256 | >256 |
| I.n |  | 4.19 | >256 | >256 | 256 |
| I.o |  | 4.22 | >256 | >256 | 256 |
| I.p |  | 2.33 | 64 | 64 | 64 |
| Name | Structure | LogP | MIC (µg/mL) | ||
|---|---|---|---|---|---|
| ATCC 29213 a | SF8300 b | ST20171643 c | |||
| II.a |  | 4.85 | >256 | >256 | >256 |
| II.b |  | 3.79 | 4 | 4 | 4 |
| II.c |  | 4.33 | >256 | >256 | >256 |
| II.d |  | 3.27 | 32 | 16 | 16 |
| Name | Structure | LogP | MIC (µg/mL) | ||
|---|---|---|---|---|---|
| ATCC 29213 a | SF8300 b | ST20171643 c | |||
| III.a |  | 3.19 | 256 | 64 | 64 |
| III.b |  | 4.22 | >256 | 256 | 256 |
| III.c |  | 3.79 | >256 | 256 | 256 |
| III.d |  | 3.89 | >256 | >256 | >256 |
| III.e |  | 3.15 | >256 | 256 | 128 |
| III.f |  | 4.01 | 32 | 8 | 16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbier, T.; Barbry, A.; Magand, J.; Badiou, C.; Davy, F.; Baudouin, A.; Queneau, Y.; Dumitrescu, O.; Lina, G.; Soulère, L. Synthesis and Biological Evaluation of Benzo[b]thiophene Acylhydrazones as Antimicrobial Agents against Multidrug-Resistant Staphylococcus aureus. Biomolecules 2022, 12, 131. https://doi.org/10.3390/biom12010131
Barbier T, Barbry A, Magand J, Badiou C, Davy F, Baudouin A, Queneau Y, Dumitrescu O, Lina G, Soulère L. Synthesis and Biological Evaluation of Benzo[b]thiophene Acylhydrazones as Antimicrobial Agents against Multidrug-Resistant Staphylococcus aureus. Biomolecules. 2022; 12(1):131. https://doi.org/10.3390/biom12010131
Chicago/Turabian StyleBarbier, Thibaut, Alexia Barbry, Jérémy Magand, Cédric Badiou, Floriane Davy, Anne Baudouin, Yves Queneau, Oana Dumitrescu, Gérard Lina, and Laurent Soulère. 2022. "Synthesis and Biological Evaluation of Benzo[b]thiophene Acylhydrazones as Antimicrobial Agents against Multidrug-Resistant Staphylococcus aureus" Biomolecules 12, no. 1: 131. https://doi.org/10.3390/biom12010131
APA StyleBarbier, T., Barbry, A., Magand, J., Badiou, C., Davy, F., Baudouin, A., Queneau, Y., Dumitrescu, O., Lina, G., & Soulère, L. (2022). Synthesis and Biological Evaluation of Benzo[b]thiophene Acylhydrazones as Antimicrobial Agents against Multidrug-Resistant Staphylococcus aureus. Biomolecules, 12(1), 131. https://doi.org/10.3390/biom12010131







