Smart Bioinks for the Printing of Human Tissue Models
Abstract
:1. Introduction
2. Types of Smart Bioinks
2.1. Nanoparticles
2.2. Microparticles and Microspheres
2.3. Microswimmers
2.4. Nano/Micro/Macrogels
2.5. General Hydrogel-Colloid Composite Bioinks
3. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Noorani, R. 3D Printing: Technology, Applications, and Selection, 1st ed.; CRC Press: Boca Raton, FL, USA, 2018; ISBN 978-1-4987-8376-7. [Google Scholar]
- Conner, B.P.; Manogharan, G.P.; Martof, A.N.; Rodomsky, L.M.; Rodomsky, C.M.; Jordan, D.C.; Limperos, J.W. Making sense of 3-D printing: Creating a map of additive manufacturing products and services. Addit. Manuf. 2014, 1–4, 64–76. [Google Scholar] [CrossRef]
- Xie, Z.; Gao, M.; Lobo, A.O.; Webster, T.J. 3D Bioprinting in Tissue Engineering for Medical Applications: The Classic and the Hybrid. Polymers 2020, 12, 1717. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, N.; Haddow, G.; Seymour, T.; Faulkner-Jones, A.; Shu, W. 3D bioprint me: A socioethical view of bioprinting human organs and tissues. J. Med. Ethics 2017, 43, 618–624. [Google Scholar] [CrossRef] [Green Version]
- Mandrycky, C.; Wang, Z.; Kim, K.; Kim, D.-H. 3D bioprinting for engineering complex tissues. Biotechnol. Adv. 2016, 34, 422–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, H.; Nowicki, M.; Fisher, J.P.; Zhang, L.G. 3D Bioprinting for Organ Regeneration. Adv. Healthc. Mater. 2017, 6, 1601118. [Google Scholar] [CrossRef] [Green Version]
- Vijayavenkataraman, S.; Lu, W.F.; Fuh, J.Y.H. 3D bioprinting of skin: A state-of-the-art review on modelling, materials, and processes. Biofabrication 2016, 8, 032001. [Google Scholar] [CrossRef]
- Freeman, F.E.; Burdis, R.; Kelly, D.J. Printing New Bones: From Print-and-Implant Devices to Bioprinted Bone Organ Precursors. Trends Mol. Med. 2021, 27, 700–711. [Google Scholar] [CrossRef] [PubMed]
- Hoch, E.; Tovar, G.E.M.; Borchers, K. Bioprinting of artificial blood vessels: Current approaches towards a demanding goal. Eur. J. Cardiothorac. Surg. 2014, 46, 767–778. [Google Scholar] [CrossRef] [PubMed]
- de la Vega, L.; Lee, C.; Sharma, R.; Amereh, M.; Willerth, S.M. 3D bioprinting models of neural tissues: The current state of the field and future directions. Brain Res. Bull. 2019, 150, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Roche, C.D.; Brereton, R.J.L.; Ashton, A.W.; Jackson, C.; Gentile, C. Current challenges in three-dimensional bioprinting heart tissues for cardiac surgery. Eur. J. Cardiothorac. Surg. 2020, 58, 500–510. [Google Scholar] [CrossRef]
- Aljohani, W.; Ullah, M.W.; Zhang, X.; Yang, G. Bioprinting and its applications in tissue engineering and regenerative medicine. Int. J. Biol. Macromol. 2018, 107, 261–275. [Google Scholar] [CrossRef]
- Zheng, J.-H.; Zhang, J.-K.; Tian, Y.-P.; Song, Y.-B.; Yang, Z.-W.; Huang, X.-H. A Stereological Study of Mouse Ovary Tissues for 3D Bioprinting Application. Cell. Mol. Bioeng. 2021, 14, 259–265. [Google Scholar] [CrossRef]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Gillispie, G.; Prim, P.; Copus, J.; Fisher, J.; Mikos, A.G.; Yoo, J.J.; Atala, A.; Lee, S.J. Assessment methodologies for extrusion-based bioink printability. Biofabrication 2020, 12, 022003. [Google Scholar] [CrossRef] [PubMed]
- Peper, S.; Neelam, A.; Thy, V.; Awad, K.; Varanasi, V. Rheologic Properties of a Novel Gelatin Methacrylate Bioink for 3D Printing Musculoskeletal Grafts. Int. J. Exerc. Sci. Conf. Proc. 2020, 2, 127. [Google Scholar]
- Pössl, A.; Hartzke, D.; Schmidts, T.M.; Runkel, F.E.; Schlupp, P. A targeted rheological bioink development guideline and its systematic correlation with printing behavior. Biofabrication 2021, 13, 035021. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yang, F.; Zhao, H.; Gao, Q.; Xia, B.; Fu, J. Research on the printability of hydrogels in 3D bioprinting. Sci. Rep. 2016, 6, 29977. [Google Scholar] [CrossRef] [PubMed]
- Freeman, F.E.; Kelly, D.J. Tuning Alginate Bioink Stiffness and Composition for Controlled Growth Factor Delivery and to Spatially Direct MSC Fate within Bioprinted Tissues. Sci. Rep. 2017, 7, 17042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bedell, M.L.; Melchiorri, A.J.; Aleman, J.; Skardal, A.; Mikos, A.G. A high-throughput approach to compare the biocompatibility of candidate bioink formulations. Bioprinting 2020, 17, e00068. [Google Scholar] [CrossRef]
- Mancha Sánchez, E.; Gómez-Blanco, J.C.; López Nieto, E.; Casado, J.G.; Macías-García, A.; Díaz Díez, M.A.; Carrasco-Amador, J.P.; Torrejón Martín, D.; Sánchez-Margallo, F.M.; Pagador, J.B. Hydrogels for Bioprinting: A Systematic Review of Hydrogels Synthesis, Bioprinting Parameters, and Bioprinted Structures Behavior. Front. Bioeng. Biotechnol. 2020, 8, 776. [Google Scholar] [CrossRef]
- Trampe, E.; Koren, K.; Akkineni, A.R.; Senwitz, C.; Krujatz, F.; Lode, A.; Gelinsky, M.; Kühl, M. Functionalized Bioink with Optical Sensor Nanoparticles for O2 Imaging in 3D-Bioprinted Constructs. Adv. Funct. Mater. 2018, 28, 1804411. [Google Scholar] [CrossRef]
- Weng, T.; Zhang, W.; Xia, Y.; Wu, P.; Yang, M.; Jin, R.; Xia, S.; Wang, J.; You, C.; Han, C.; et al. 3D bioprinting for skin tissue engineering: Current status and perspectives. J. Tissue Eng. 2021, 12, 204173142110285. [Google Scholar] [CrossRef] [PubMed]
- Lee, V.; Singh, G.; Trasatti, J.P.; Bjornsson, C.; Xu, X.; Tran, T.N.; Yoo, S.-S.; Dai, G.; Karande, P. Design and Fabrication of Human Skin by Three-Dimensional Bioprinting. Tissue Eng. Part C Methods 2014, 20, 473–484. [Google Scholar] [CrossRef] [Green Version]
- Albanna, M.; Binder, K.W.; Murphy, S.V.; Kim, J.; Qasem, S.A.; Zhao, W.; Tan, J.; El-Amin, I.B.; Dice, D.D.; Marco, J.; et al. In Situ Bioprinting of Autologous Skin Cells Accelerates Wound Healing of Extensive Excisional Full-Thickness Wounds. Sci. Rep. 2019, 9, 1856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, W.L.; Yeong, W.Y.; Naing, M.W. Polyelectrolyte gelatin-chitosan hydrogel optimized for 3D bioprinting in skin tissue engineering. Int. J. Bioprint. 2016, 2, 53–62. [Google Scholar] [CrossRef]
- Kim, B.S.; Gao, G.; Kim, J.Y.; Cho, D. 3D Cell Printing of Perfusable Vascularized Human Skin Equivalent Composed of Epidermis, Dermis, and Hypodermis for Better Structural Recapitulation of Native Skin. Adv. Healthc. Mater. 2019, 8, 1801019. [Google Scholar] [CrossRef]
- Min, D.; Lee, W.; Bae, I.-H.; Lee, T.R.; Croce, P.; Yoo, S.-S. Bioprinting of biomimetic skin containing melanocytes. Exp. Dermatol. 2018, 27, 453–459. [Google Scholar] [CrossRef]
- Hong, S.; Kim, J.S.; Jung, B.; Won, C.; Hwang, C. Coaxial bioprinting of cell-laden vascular constructs using a gelatin–tyramine bioink. Biomater. Sci. 2019, 7, 4578–4587. [Google Scholar] [CrossRef]
- Monferrer, E.; Martín-Vañó, S.; Carretero, A.; García-Lizarribar, A.; Burgos-Panadero, R.; Navarro, S.; Samitier, J.; Noguera, R. A three-dimensional bioprinted model to evaluate the effect of stiffness on neuroblastoma cell cluster dynamics and behavior. Sci. Rep. 2020, 10, 6370. [Google Scholar] [CrossRef] [Green Version]
- Mei, Q.; Rao, J.; Bei, H.P.; Liu, Y.; Zhao, X. 3D Bioprinting Photo-Crosslinkable Hydrogels for Bone and Cartilage Repair. Int. J. Bioprint. 2021, 7, 367. [Google Scholar] [CrossRef]
- Zhang, K.; Feng, Q.; Xu, J.; Xu, X.; Tian, F.; Yeung, K.W.K.; Bian, L. Self-Assembled Injectable Nanocomposite Hydrogels Stabilized by Bisphosphonate-Magnesium (Mg2+ ) Coordination Regulates the Differentiation of Encapsulated Stem Cells via Dual Crosslinking. Adv. Funct. Mater. 2017, 27, 1701642. [Google Scholar] [CrossRef]
- de Windt, T.S.; Vonk, L.A.; Slaper-Cortenbach, I.C.M.; van den Broek, M.P.H.; Nizak, R.; van Rijen, M.H.P.; de Weger, R.A.; Dhert, W.J.A.; Saris, D.B.F. Allogeneic Mesenchymal Stem Cells Stimulate Cartilage Regeneration and Are Safe for Single-Stage Cartilage Repair in Humans upon Mixture with Recycled Autologous Chondrons: Allogeneic MSCs Stimulate Cartilage Repair in the Knee. Stem Cells 2017, 35, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Levato, R.; Webb, W.R.; Otto, I.A.; Mensinga, A.; Zhang, Y.; van Rijen, M.; van Weeren, R.; Khan, I.M.; Malda, J. The bio in the ink: Cartilage regeneration with bioprintable hydrogels and articular cartilage-derived progenitor cells. Acta Biomater. 2017, 61, 41–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cadena, M.; Ning, L.; King, A.; Hwang, B.; Jin, L.; Serpooshan, V.; Sloan, S.A. 3D Bioprinting of Neural Tissues. Adv. Healthc. Mater. 2021, 10, 2001600. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Zhang, J.; Du, T.; Xu, X.; Deng, X.; Chen, S.; Liu, J.; Chen, Y.; Liu, X.; Xiong, M.; et al. Rapid 3D printing of functional nanoparticle-enhanced conduits for effective nerve repair. Acta Biomater. 2019, 90, 49–59. [Google Scholar] [CrossRef]
- Sharma, R.; Smits, I.P.M.; De La Vega, L.; Lee, C.; Willerth, S.M. 3D Bioprinting Pluripotent Stem Cell Derived Neural Tissues Using a Novel Fibrin Bioink Containing Drug Releasing Microspheres. Front. Bioeng. Biotechnol. 2020, 8, 57. [Google Scholar] [CrossRef]
- Marsh, A.C.; Apu, E.H.; Bunn, M.; Contag, C.H.; Ashammakhi, N.; Chatzistavrou, X. A New Bioink for Improved 3D Bioprinting of Bone-Like Constructs. bioRxiv 2021, 1, 1–25. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [Green Version]
- Heid, S.; Boccaccini, A.R. Advancing bioinks for 3D bioprinting using reactive fillers: A review. Acta Biomater. 2020, 113, 1–22. [Google Scholar] [CrossRef]
- Gao, G.; Schilling, A.F.; Hubbell, K.; Yonezawa, T.; Truong, D.; Hong, Y.; Dai, G.; Cui, X. Improved properties of bone and cartilage tissue from 3D inkjet-bioprinted human mesenchymal stem cells by simultaneous deposition and photocrosslinking in PEG-GelMA. Biotechnol. Lett. 2015, 37, 2349–2355. [Google Scholar] [CrossRef]
- Doschak, M.R.; Kucharski, C.M.; Wright, J.E.I.; Zernicke, R.F.; Uludaǧ, H. Improved Bone Delivery of Osteoprotegerin by Bisphosphonate Conjugation in a Rat Model of Osteoarthritis. Mol. Pharm. 2009, 6, 634–640. [Google Scholar] [CrossRef]
- Park, D.; Park, C.-W.; Choi, Y.; Lin, J.; Seo, D.-H.; Kim, H.-S.; Lee, S.Y.; Kang, I.-C. A novel small-molecule PPI inhibitor targeting integrin αvβ3-osteopontin interface blocks bone resorption in vitro and prevents bone loss in mice. Biomaterials 2016, 98, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Teno, N.; Masuya, K.; Ehara, T.; Kosaka, T.; Miyake, T.; Irie, O.; Hitomi, Y.; Matsuura, N.; Umemura, I.; Iwasaki, G.; et al. Effect of cathepsin K inhibitors on bone resorption. J. Med. Chem. 2008, 51, 5459–5462. [Google Scholar] [CrossRef] [PubMed]
- Arrighi, I.; Mark, S.; Alvisi, M.; von Rechenberg, B.; Hubbell, J.A.; Schense, J.C. Bone healing induced by local delivery of an engineered parathyroid hormone prodrug. Biomaterials 2009, 30, 1763–1771. [Google Scholar] [CrossRef]
- Rodan, G.A.; Martin, T.J. Therapeutic Approaches to Bone Diseases. Science 2000, 289, 1508–1514. [Google Scholar] [CrossRef]
- Pei, M.; Mao, J.; Xu, W.; Zhou, Y.; Xiao, P. Photocrosslinkable chitosan hydrogels and their biomedical applications. J. Polym. Sci. Part Polym. Chem. 2019, 57, 1862–1871. [Google Scholar] [CrossRef]
- Yi, H.-G.; Kim, H.; Kwon, J.; Choi, Y.-J.; Jang, J.; Cho, D.-W. Application of 3D bioprinting in the prevention and the therapy for human diseases. Signal Transduct. Target. Ther. 2021, 6, 177. [Google Scholar] [CrossRef]
- Narducci, D. An Introduction to Nanotechnologies: What’s in it for Us? Vet. Res. Commun. 2007, 31, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.; Takhistov, P.; McClements, D.J. Functional Materials in Food Nanotechnology. J. Food Sci. 2006, 71, R107–R116. [Google Scholar] [CrossRef] [Green Version]
- Saini, R.; Saini, S.; Sharma, S. Nanotechnology: The future medicine. J. Cutan. Aesthetic Surg. 2010, 3, 32–33. [Google Scholar] [CrossRef]
- Rizvi, S.A.A.; Saleh, A.M. Applications of nanoparticle systems in drug delivery technology. Saudi Pharm. J. 2018, 26, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Gungor-Ozkerim, P.S.; Inci, I.; Zhang, Y.S.; Khademhosseini, A.; Dokmeci, M.R. Bioinks for 3D bioprinting: An overview. Biomater. Sci. 2018, 6, 915–946. [Google Scholar] [CrossRef] [Green Version]
- Skardal, A.; Zhang, J.; McCoard, L.; Oottamasathien, S.; Prestwich, G.D. Dynamically Crosslinked Gold Nanoparticle-Hyaluronan Hydrogels. Adv. Mater. 2010, 22, 4736–4740. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Shi, D.; Gu, Y.; Kaneko, T.; Zhang, L.; Zhang, H.; Kaneko, D.; Chen, M. Injectable and Near-Infrared-Responsive Hydrogels Encapsulating Dopamine-Stabilized Gold Nanorods with Long Photothermal Activity Controlled for Tumor Therapy. Biomacromolecules 2019, 20, 3375–3384. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Schilling, A.F.; Yonezawa, T.; Wang, J.; Dai, G.; Cui, X. Bioactive nanoparticles stimulate bone tissue formation in bioprinted three-dimensional scaffold and human mesenchymal stem cells. Biotechnol. J. 2014, 9, 1304–1311. [Google Scholar] [CrossRef]
- Catros, S.; Fricain, J.-C.; Guillotin, B.; Pippenger, B.; Bareille, R.; Remy, M.; Lebraud, E.; Desbat, B.; Amédée, J.; Guillemot, F. Laser-assisted bioprinting for creating on-demand patterns of human osteoprogenitor cells and nano-hydroxyapatite. Biofabrication 2011, 3, 025001. [Google Scholar] [CrossRef] [PubMed]
- Castro, N.J.; O’Brien, J.; Zhang, L.G. Integrating biologically inspired nanomaterials and table-top stereolithography for 3D printed biomimetic osteochondral scaffolds. Nanoscale 2015, 7, 14010–14022. [Google Scholar] [CrossRef]
- Ye, L.; Wang, J.; Liao, C.; Li, S.; Fang, Y.; Yang, Z.; Hu, Y.; Guo, B. 3D Printed Composite Scaffolds Incorporating Ruthenium Complex–Loaded Liposomes as a Delivery System to Prevent the Proliferation of MG-63 Cells. Macromol. Mater. Eng. 2019, 304, 1900295. [Google Scholar] [CrossRef]
- Shu, T.; Shen, Q.; Su, L.; Zhang, X.; Serpe, M.J. In Situ Synthesis of CuS Nanoparticle-Doped Poly( N-isopropylacrylamide)-Based Microgels for Near-Infrared Triggered Photothermal Therapy. ACS Appl. Nano Mater. 2018, 1, 1776–1783. [Google Scholar] [CrossRef]
- Lee, M.; Bae, K.; Levinson, C.; Zenobi-Wong, M. Nanocomposite bioink exploits dynamic covalent bonds between nanoparticles and polysaccharides for precision bioprinting. Biofabrication 2020, 12, 025025. [Google Scholar] [CrossRef] [PubMed]
- Chimene, D.; Peak, C.W.; Gentry, J.L.; Carrow, J.K.; Cross, L.M.; Mondragon, E.; Cardoso, G.B.; Kaunas, R.; Gaharwar, A.K. Nanoengineered Ionic–Covalent Entanglement (NICE) Bioinks for 3D Bioprinting. ACS Appl. Mater. Interfaces 2018, 10, 9957–9968. [Google Scholar] [CrossRef] [PubMed]
- Bakht, S.M.; Pardo, A.; Gómez-Florit, M.; Reis, R.L.; Domingues, R.M.A.; Gomes, M.E. Engineering next-generation bioinks with nanoparticles: Moving from reinforcement fillers to multifunctional nanoelements. J. Mater. Chem. B 2021, 9, 5025–5038. [Google Scholar] [CrossRef] [PubMed]
- Fujioka-Kobayashi, M.; Ota, M.S.; Shimoda, A.; Nakahama, K.; Akiyoshi, K.; Miyamoto, Y.; Iseki, S. Cholesteryl group- and acryloyl group-bearing pullulan nanogel to deliver BMP2 and FGF18 for bone tissue engineering. Biomaterials 2012, 33, 7613–7620. [Google Scholar] [CrossRef]
- Luo, Z.; Zhang, S.; Pan, J.; Shi, R.; Liu, H.; Lyu, Y.; Han, X.; Li, Y.; Yang, Y.; Xu, Z.; et al. Time-responsive osteogenic niche of stem cells: A sequentially triggered, dual-peptide loaded, alginate hybrid system for promoting cell activity and osteo-differentiation. Biomaterials 2018, 163, 25–42. [Google Scholar] [CrossRef]
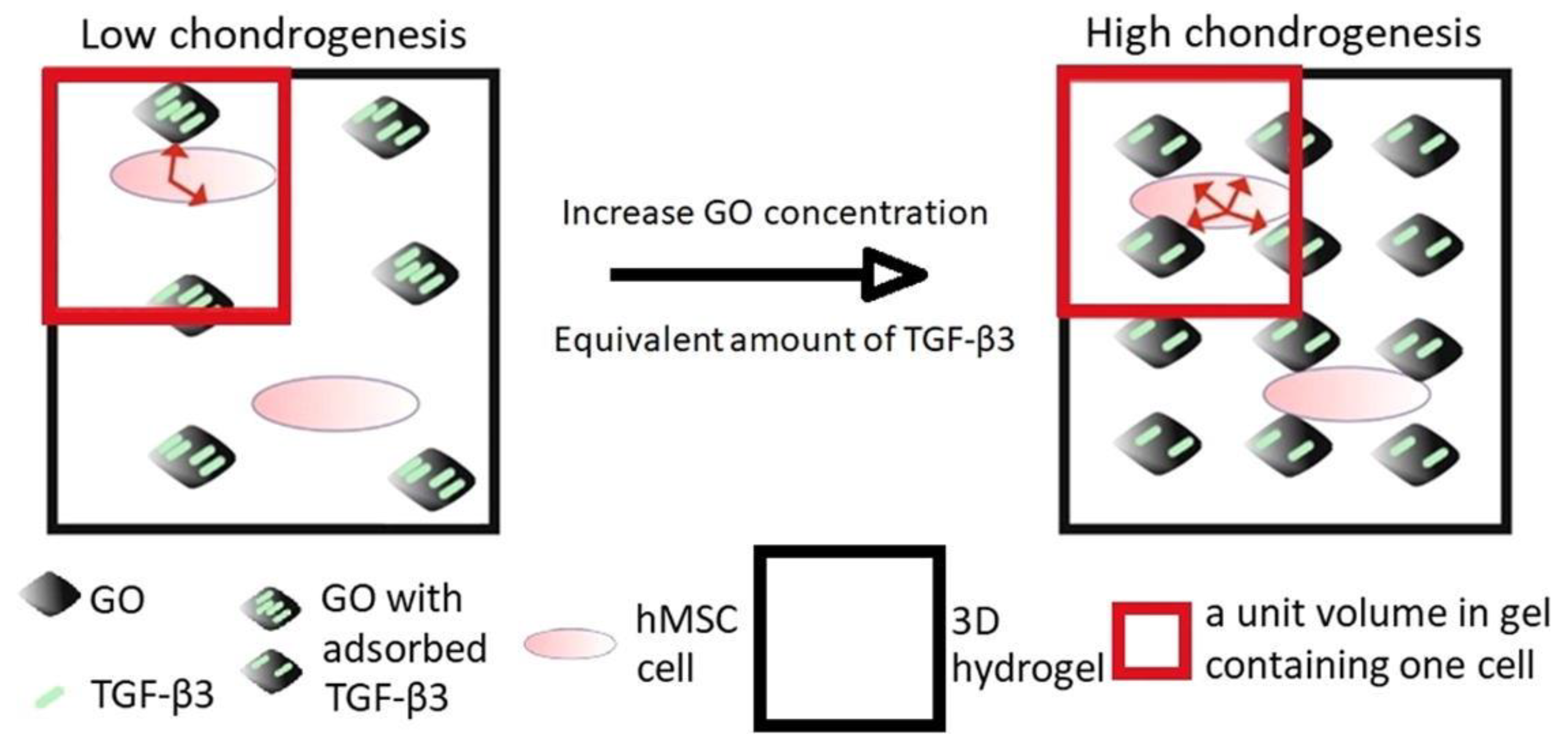
- Zhou, M.; Lozano, N.; Wychowaniec, J.K.; Hodgkinson, T.; Richardson, S.M.; Kostarelos, K.; Hoyland, J.A. Graphene oxide: A growth factor delivery carrier to enhance chondrogenic differentiation of human mesenchymal stem cells in 3D hydrogels. Acta Biomater. 2019, 96, 271–280. [Google Scholar] [CrossRef]
- Kohane, D.S. Microparticles and nanoparticles for drug delivery. Biotechnol. Bioeng. 2007, 96, 203–209. [Google Scholar] [CrossRef]
- Zhang, J.; Saltzman, M. Engineering biodegradable nanoparticles for drug and gene delivery. Chem. Eng. Prog. 2013, 109, 25–30. [Google Scholar]
- Daly, A.C.; Riley, L.; Segura, T.; Burdick, J.A. Hydrogel microparticles for biomedical applications. Nat. Rev. Mater. 2020, 5, 20–43. [Google Scholar] [CrossRef]
- Kim, M.K.; Jeong, W.; Lee, S.M.; Kim, J.B.; Jin, S.; Kang, H.-W. Decellularized extracellular matrix-based bio-ink with enhanced 3D printability and mechanical properties. Biofabrication 2020, 12, 025003. [Google Scholar] [CrossRef]
- Neufurth, M.; Wang, X.; Wang, S.; Steffen, R.; Ackermann, M.; Haep, N.D.; Schröder, H.C.; Müller, W.E.G. 3D printing of hybrid biomaterials for bone tissue engineering: Calcium-polyphosphate microparticles encapsulated by polycaprolactone. Acta Biomater. 2017, 64, 377–388. [Google Scholar] [CrossRef]
- Sun, Y.; You, Y.; Jiang, W.; Wu, Q.; Wang, B.; Dai, K. Generating ready-to-implant anisotropic menisci by 3D-bioprinting protein-releasing cell-laden hydrogel-polymer composite scaffold. Appl. Mater. Today 2020, 18, 100469. [Google Scholar] [CrossRef]
- Wang, Y.-L.; Hu, J.-J. Sub-100-micron calcium-alginate microspheres: Preparation by nitrogen flow focusing, dependence of spherical shape on gas streams and a drug carrier using acetaminophen as a model drug. Carbohydr. Polym. 2021, 269, 118262. [Google Scholar] [CrossRef]
- Tan, Y.J.; Tan, X.; Yeong, W.Y.; Tor, S.B. Hybrid microscaffold-based 3D bioprinting of multi-cellular constructs with high compressive strength: A new biofabrication strategy. Sci. Rep. 2016, 6, 39140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Huang, D.; Wang, L.; Hou, J.; Zhang, H.; Li, Y.; Zhong, S.; Wang, Y.; Wu, Y.; Huang, W. 3D bioprinted multiscale composite scaffolds based on gelatin methacryloyl (GelMA)/chitosan microspheres as a modular bioink for enhancing 3D neurite outgrowth and elongation. J. Colloid Interface Sci. 2020, 574, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.H.; McKinney, J.; Miller, T.; Bongiorno, T.; McDevitt, T.C. Gelatin methacrylate microspheres for controlled growth factor release. Acta Biomater. 2015, 13, 101–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stefan, L.; Jeannie, S.; Michael, D.; Peter, M. 3D printing of polymeric microspheres for tissue engineering scaffolds. Front. Bioeng. Biotechnol. 2016, 4. [Google Scholar] [CrossRef]
- Vhora, I.; Khatri, N.; Misra, A. Applications of Polymers in Parenteral Drug Delivery. In Applications of Polymers in Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2021; pp. 221–261. ISBN 978-0-12-819659-5. [Google Scholar]
- Wang, X.; Hu, C.; Schurz, L.; De Marco, C.; Chen, X.; Pané, S.; Nelson, B.J. Surface-Chemistry-Mediated Control of Individual Magnetic Helical Microswimmers in a Swarm. ACS Nano 2018, 12, 6210–6217. [Google Scholar] [CrossRef]
- Wang, X.; Qin, X.-H.; Hu, C.; Terzopoulou, A.; Chen, X.-Z.; Huang, T.-Y.; Maniura-Weber, K.; Pané, S.; Nelson, B.J. 3D Printed Enzymatically Biodegradable Soft Helical Microswimmers. Adv. Funct. Mater. 2018, 28, 1804107. [Google Scholar] [CrossRef]
- Mahon, E.; Salvati, A.; Baldelli Bombelli, F.; Lynch, I.; Dawson, K.A. Designing the nanoparticle–biomolecule interface for “targeting and therapeutic delivery. J. Control. Release 2012, 161, 164–174. [Google Scholar] [CrossRef]
- Bunea, A.-I.; Taboryski, R. Recent Advances in Microswimmers for Biomedical Applications. Micromachines 2020, 11, 1048. [Google Scholar] [CrossRef]
- An, Y.; Hubbell, J.A. Intraarterial protein delivery via intimally-adherent bilayer hydrogels. J. Control. Release 2000, 64, 205–215. [Google Scholar] [CrossRef]
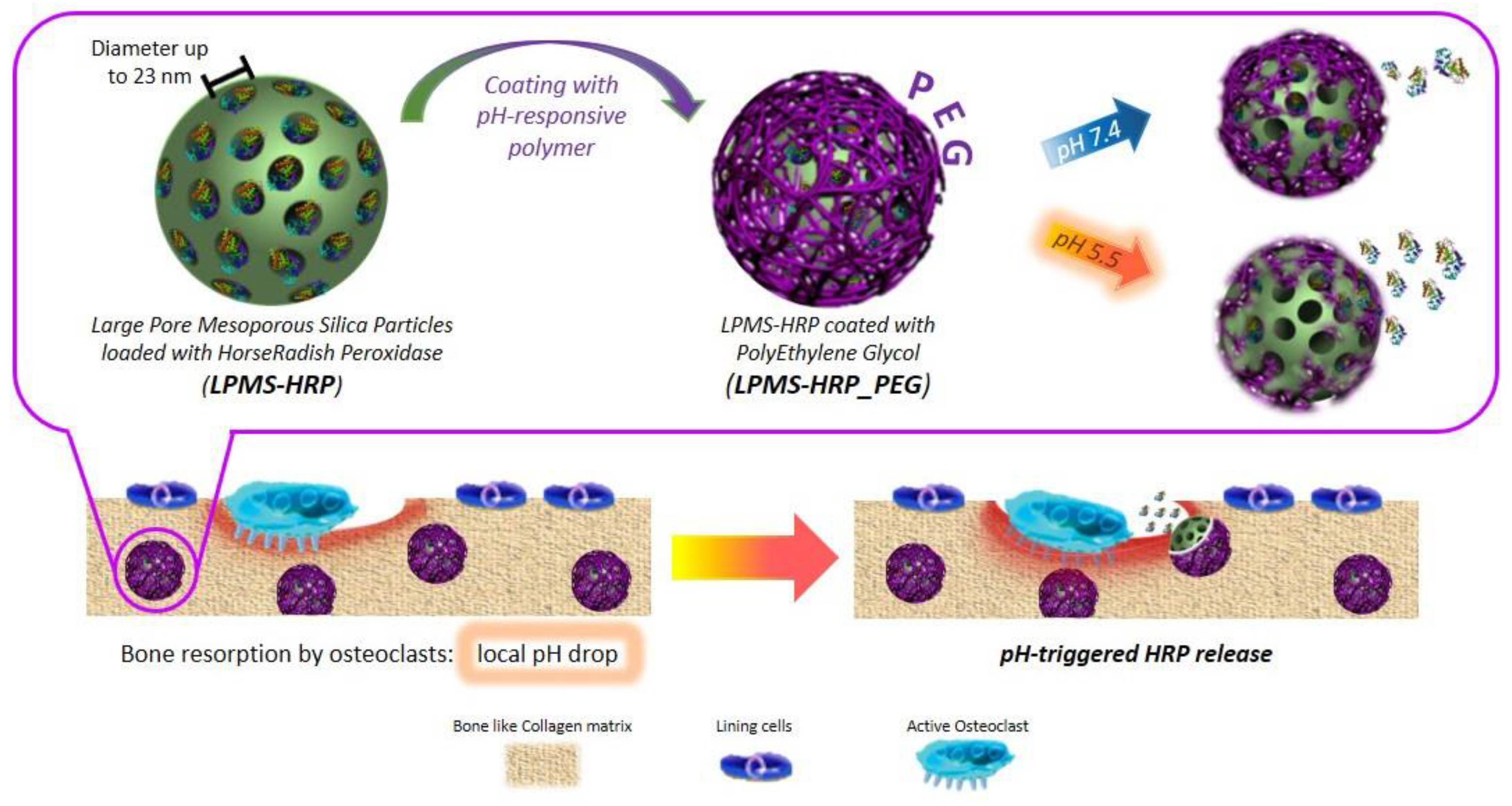
- Banche-Niclot, F.; Montalbano, G.; Fiorilli, S.; Vitale-Brovarone, C. PEG-Coated Large Mesoporous Silicas as Smart Platform for Protein Delivery and Their Use in a Collagen-Based Formulation for 3D Printing. Int. J. Mol. Sci. 2021, 22, 1718. [Google Scholar] [CrossRef] [PubMed]
- Highley, C.B.; Song, K.H.; Daly, A.C.; Burdick, J.A. Jammed Microgel Inks for 3D Printing Applications. Adv. Sci. 2019, 6, 1801076. [Google Scholar] [CrossRef] [Green Version]
- Gramlich, W.M.; Kim, I.L.; Burdick, J.A. Synthesis and orthogonal photopatterning of hyaluronic acid hydrogels with thiol-norbornene chemistry. Biomaterials 2013, 34, 9803–9811. [Google Scholar] [CrossRef] [Green Version]
- Vega, S.L.; Kwon, M.Y.; Song, K.H.; Wang, C.; Mauck, R.L.; Han, L.; Burdick, J.A. Combinatorial hydrogels with biochemical gradients for screening 3D cellular microenvironments. Nat. Commun. 2018, 9, 614. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Miao, Y.; Zhang, J.; Wu, J.P.; Kirk, T.B.; Xu, J.; Ma, D.; Xue, W. Three-dimensional printing of shape memory hydrogels with internal structure for drug delivery. Mater. Sci. Eng. C 2018, 84, 44–51. [Google Scholar] [CrossRef]
- Wu, Y.; Heikal, L.; Ferns, G.; Ghezzi, P.; Nokhodchi, A.; Maniruzzaman, M. 3D Bioprinting of Novel Biocompatible Scaffolds for Endothelial Cell Repair. Polymers 2019, 11, 1924. [Google Scholar] [CrossRef] [Green Version]
- Petrucci, R.H.; Harwood, W.S.; Herring, F.G.; Madura, J.D. General Chemistry: Principles and Modern Applications, 9th ed.; Pearson/Prentice Hall: Upper Saddle River, NJ, USA, 2007; ISBN 978-0-13-149330-8. [Google Scholar]
- Michel, R.; Auzély-Velty, R. Hydrogel-Colloid Composite Bioinks for Targeted Tissue-Printing. Biomacromolecules 2020, 21, 2949–2965. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Janarthanan, G.; Tran, H.N.; Ham, H.J.; Yoon, J.; Noh, I. Bioink homogeneity control during 3D bioprinting of multicomponent micro/nanocomposite hydrogel for even tissue regeneration using novel twin screw extrusion system. Chem. Eng. J. 2021, 415, 128971. [Google Scholar] [CrossRef]
- Wang, J.; Xie, B.; Zhu, Z.; Xie, G.; Luo, B. 3D-printed construct from hybrid suspension as spatially and temporally controlled protein delivery system. J. Biomater. Appl. 2021, 36, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Willson, K.; Atala, A.; Yoo, J.J. Bioprinting Au Natural: The Biologics of Bioinks. Biomolecules 2021, 11, 1593. [Google Scholar] [CrossRef] [PubMed]
- Ashammakhi, N.; Ahadian, S.; Xu, C.; Montazerian, H.; Ko, H.; Nasiri, R.; Barros, N.; Khademhosseini, A. Bioinks and bioprinting technologies to make heterogeneous and biomimetic tissue constructs. Mater. Today Biol. 2019, 1, 100008. [Google Scholar] [CrossRef]
- Levato, R.; Visser, J.; Planell, J.A.; Engel, E.; Malda, J.; Mateos-Timoneda, M.A. Biofabrication of tissue constructs by 3D bioprinting of cell-laden microcarriers. Biofabrication 2014, 6, 035020. [Google Scholar] [CrossRef]
- Jose, R.R.; Rodriguez, M.J.; Dixon, T.A.; Omenetto, F.; Kaplan, D.L. Evolution of Bioinks and Additive Manufacturing Technologies for 3D Bioprinting. ACS Biomater. Sci. Eng. 2016, 2, 1662–1678. [Google Scholar] [CrossRef] [PubMed]
- Hölzl, K.; Lin, S.; Tytgat, L.; Van Vlierberghe, S.; Gu, L.; Ovsianikov, A. Bioink properties before, during and after 3D bioprinting. Biofabrication 2016, 8, 032002. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Song, S.-J.; Lee, J.Y.; Jang, H.; Choi, J.; Sun, K.; Park, Y. Cellular behavior in micropatterned hydrogels by bioprinting system depended on the cell types and cellular interaction. J. Biosci. Bioeng. 2013, 116, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Datta, P.; Ayan, B.; Ozbolat, V.; Sosnoski, D.; Ozbolat, I.T. 3D bioprinting for drug discovery and development in pharmaceutics. Acta Biomater. 2017, 57, 26–46. [Google Scholar] [CrossRef] [PubMed]
- Haleem, A.; Javaid, M.; Singh, R.P.; Suman, R. Significant roles of 4D printing using smart materials in the field of manufacturing. Adv. Ind. Eng. Polym. Res. 2021, 4, 301–311. [Google Scholar] [CrossRef]
- Li, Y.-C.; Zhang, Y.S.; Akpek, A.; Shin, S.R.; Khademhosseini, A. 4D bioprinting: The next-generation technology for biofabrication enabled by stimuli-responsive materials. Biofabrication 2016, 9, 012001. [Google Scholar] [CrossRef] [Green Version]
- Tibbits, S. 4D Printing: Multi-Material Shape Change. Archit. Des. 2014, 84, 116–121. [Google Scholar] [CrossRef]
- Wan, Z.; Zhang, P.; Liu, Y.; Lv, L.; Zhou, Y. Four-dimensional bioprinting: Current developments and applications in bone tissue engineering. Acta Biomater. 2020, 101, 26–42. [Google Scholar] [CrossRef] [PubMed]
- Subash, A.; Kandasubramanian, B. 4D printing of shape memory polymers. Eur. Polym. J. 2020, 134, 109771. [Google Scholar] [CrossRef]
- Costa, P.D.C.; Costa, D.C.S.; Correia, T.R.; Gaspar, V.M.; Mano, J.F. Natural Origin Biomaterials for 4D Bioprinting Tissue-Like Constructs. Adv. Mater. Technol. 2021, 6, 2100168. [Google Scholar] [CrossRef]

| Bioink Formulation | Application | Bioprinting Technique | Bioactivity | Advantages | Disadvantages | References |
|---|---|---|---|---|---|---|
| Bioprinted polyelectrolyte gelatin-CS (PGC) hydrogels with neonatal human foreskin fibroblasts (FBs) | Dermis constructs in which collagen and some blood vessels are produced | Extrusion | Cell viability was not quantified—images show high levels of viability | Good biocompatibility Good Attachment and proliferation | No functional assays were performed | [26] |
| Gelatin polymerized with thrombin loaded with human umbilical vein endothelial cells | Fully perfused vascularized 3D-bioprinted skin model | Extrusion and inkjet printing | Viability was not quantified, but the layers of skin were observed | Great printability Rapid gel–sol transition Recapitulation of skin phenotype and successful perfusion was achieved | The thrombin in the vascular bioink could partially crosslink the dECM–fibrinogen bioink during the incubation at 37 °C No information is provided on how long these constructs can be maintained in culture | [27] |
| Melanocytes (MCs) and Kupffer cells (KCs) on top of a bioprinted dermal layer consisting of a fibroblast-containing collagen hydrogel | Induced skin pigmentation upon subsequent air–liquid interface culture, creating a melanocytes-containing epidermal layer | Pneumatically driven extrusion-based printing | Cell viability was not quantified Histology showed the appropriate phenotypes were maintained | Good mechanical rigidity while having the printed cells kept in each layer at a designated depth | The use of immortalized KC could interfere with the proper differentiation of the KC (and incomplete stratification of the epidermis). | [28] |
| Gelatin–tyramine bioink encapsulated FBs (HDF and NIH-3T3) and HUVECs | Bioprinting endothelial cell-encapsulating gelatin–PEG–tyramine sheath was cultured in vitro and checked for blood vessel-like tissue formation | Coaxial-nozzle-based | High cell viability ranging from 80–95% | High biocompatibility and biodegradability Short gelling time Produced a relevant structure | Structure was only maintained in culture for eight days | [29] |
| Gelatin methacrylate and methacrylated alginate with neuroblastoma cells | Quantify and localize the effects of physical-chemical communication signals between tumour cells and the surrounding biomaterial stiffness over time | Extrusion | Cell proliferation (~30%) was observed | Measurements carried out in human tumours, mice tumours and hydrogels are comparable at room temperature | High elasticity in these hydrogels (Low Young’s Modulus) | [30] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maan, Z.; Masri, N.Z.; Willerth, S.M. Smart Bioinks for the Printing of Human Tissue Models. Biomolecules 2022, 12, 141. https://doi.org/10.3390/biom12010141
Maan Z, Masri NZ, Willerth SM. Smart Bioinks for the Printing of Human Tissue Models. Biomolecules. 2022; 12(1):141. https://doi.org/10.3390/biom12010141
Chicago/Turabian StyleMaan, Zeina, Nadia Z. Masri, and Stephanie M. Willerth. 2022. "Smart Bioinks for the Printing of Human Tissue Models" Biomolecules 12, no. 1: 141. https://doi.org/10.3390/biom12010141
APA StyleMaan, Z., Masri, N. Z., & Willerth, S. M. (2022). Smart Bioinks for the Printing of Human Tissue Models. Biomolecules, 12(1), 141. https://doi.org/10.3390/biom12010141









