Phytochemistry and Diverse Pharmacology of Genus Mimosa: A Review
Abstract
:1. Introduction
2. Materials and Methods
3. Chemical Profiling of Genus Mimosa
3.1. Qualitative and Quantitative Analysis of Phytochemicals in Genus Mimosa
3.2. Bioactive Constitutients of Genus Mimosa
3.2.1. M. tenuiflora
3.2.2. M. pigra
3.2.3. M. caesalpiniifolia
3.2.4. M. hamata
3.2.5. M. diplotricha
3.2.6. M. xanthocentra
3.2.7. M. hostilis
3.2.8. M. artemisiana
3.2.9. M. invisa
3.2.10. M. scabrella
3.2.11. M. somniam
3.2.12. M. pudica
Whole Plant (Tree) Phytochemicals
Aerial Part Phytochemicals
Leaf Phytochemicals
Root Phytochemicals
Seed Phytochemicals
Stem Phytochemicals
4. Pharmacological Activities of Genus Mimosa
4.1. Antiprotozoal Activity
4.2. Antimicrobial Activity
4.3. Antiviral Activity
4.4. Antioxidant Activity
4.5. Antiproliferative Activity
4.6. Cytotoxic Activity
5. Marker Compounds of Genus Mimosa
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Ahuchaogu, A.A.; Chukwu, O.J.; Echeme, J.O. Secondary Metabolites from Mimosa Pudica: Isolation, Purification and NMR Characterization. IOSR J. Appl. Chem. 2017, 10, 15–20. [Google Scholar] [CrossRef]
- Silva, A.S.; Araújo, S.B.; Souza, D.C.; Silva, F.A. Study of the Cu, Mn, Pb and Zn dynamics in soil, plants and bee pollen from the region of Teresina (PI), Brazil. An. Acad. Bras. Ciências 2012, 84, 881–889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muddiman, S.; Hodkinson, I.; Hollis, D. Legume-feeding psyllids of the genus Heteropsylla (Homoptera: Psylloidea). Bull. Entomol. Res. 1992, 82, 73–117. [Google Scholar] [CrossRef] [Green Version]
- Amalraj, T.; Ignacimuthu, S. Hyperglycemic effect of leaves of Mimosa pudica Linn. Fitoterapia 2002, 73, 351–352. [Google Scholar] [CrossRef]
- Camargo-Ricalde, S.L. Descripción, distribución, anatomía, composición química y usos de Mimosa tenuiflora (Fabaceae-Mimosoideae) en México. Rev. Biol. Trop. 2000, 48, 939–954. [Google Scholar] [PubMed]
- Galinato, M.I.; Moody, K.; Piggin, C.M. Upland Rice Weeds of South and Southeast Asia; IRRI: Los Baños, Philippine, 1999; p. 161. [Google Scholar]
- Hussain, N.; Modan, M.H.; Shabbir, S.G.; Zaidi, S. Antimicrobial principles in Mimosa hamata. J. Nat. Prod. 1979, 42, 525–527. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, A.; Mahmood, A.; Qureshi, R.A. Antimicrobial activities of three species of family mimosaceae. Pak. J. Pharm. Sci. 2012, 25, 203–206. [Google Scholar]
- Joyamma, V.; Rao, S.; Hrishikeshavan, H.; Aroor, A.; Kulkarni, D. Biochemical mechanisms and effects of Mimosa pudica (Linn) on experimental urolithiasis in rats. Indian J. Exp. Biol. 1990, 28, 237–240. [Google Scholar]
- Gupta, R.; Vairale, M.; Deshmukh, R.; Chaudhary, P.; Wate, S.R. Ethnomedicinal uses of some plants used by Gond tribe of Bhandara district, Maharashtra. Indian J. Tradit. Knowl. 2010, 9, 713–717. [Google Scholar]
- Lemes, P.G.; Castro, A.; Zanuncio, J. Oncideres ocularis (Coleoptera: Cerambycidae) Girdling Mimosa bimucronata (Fabaceae) in Brazil. Fla. Entomol. 2014, 97, 1240–1243. [Google Scholar] [CrossRef]
- Lorenzi, H. Árvores Brasileiras: Manual de Identificação e Cultivo de Plantas Arbóreas Nativas do Brasil, 2nd ed.; Editora Plantarum: Nova Odessa, SP, Brazil, 1998; p. 10. [Google Scholar]
- Jiang, Y.; Massiot, G.; Lavaud, C.; Teulon, J.-M.; Guéchot, C.; Haag-Berrurier, M.; Anton, R. Triterpenoid glycosides from the bark of Mimosa tenuiflora. Phytochemistry 1991, 30, 2357–2360. [Google Scholar] [CrossRef]
- Ueda, M.; Takada, N.; Yamamura, S. Molecular approach to the nyctinastic movement of the plant controlled by a biological clock. Int. J. Mol. Sci. 2001, 2, 156–164. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, G.; Ferrari, R.R. Fruits of Mimosa foliolosa (Fabales: Fabaceae) as Sleeping Shelter for Megachile (Pseudocentron) botucatuna (Hymenoptera: Megachilidae). Neotrop. Entomol. 2012, 41, 518–520. [Google Scholar] [CrossRef]
- Baggio, A.J.; Carpanezzi, A.A. Exploração Seletiva do Sub-Bosque: Uma Alternativa para Aumentar a Rentabilidade dos Bracatingais; Embrapa Florestas-Circular Técnica (INFOTECA-E); Empresa Brasileira de Pesquisa Agropecuária: Brasilia, Brazil, 1998. [Google Scholar]
- Simon, M.F.; Grether, R.; de Queiroz, L.P.; Särkinen, T.E.; Dutra, V.F.; Hughes, C.E. The evolutionary history of Mimosa (Leguminosae): Toward a phylogeny of the sensitive plants. Am. J. Bot. 2011, 98, 1201–1221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nworgu, F.; Egbunike, G. Nutritional Potential of Centrosema pubescens Mimosa invisa and Pueraria phaseoloides Leaf Meals on Growth Performance Responses of Broiler Chickens. Am. J. Exp. Agric. 2013, 3, 506. [Google Scholar] [CrossRef] [Green Version]
- Erkan, G.; Şengül, K.; Kaya, S. Dyeing of white and indigo dyed cotton fabrics with Mimosa tenuiflora extract. J. Saudi Chem. Soc. 2014, 18, 139–148. [Google Scholar] [CrossRef] [Green Version]
- Silva, T.M.S.; dos Santos, F.P.; Evangelista-Rodrigues, A.; da Silva, E.M.S.; da Silva, G.S.; de Novais, J.S.; dos Santos, F.d.A.R.; Camara, C.A. Phenolic compounds, melissopalynological, physicochemical analysis and antioxidant activity of jandaíra (Melipona subnitida) honey. J. Food Compos. Anal. 2013, 29, 10–18. [Google Scholar] [CrossRef] [Green Version]
- Vasconcelos do Nascimento, C.; Albuquerque Pontes Filho, R.; Artur, A.; Costa, M. Application of poultry processing industry waste: A strategy for vegetation growth in degraded soil. Waste Manag. 2014, 36, 316–322. [Google Scholar] [CrossRef]
- Apolinário, V.; Dubeux, J., Jr.; Lira, M.; Luiz, R.; Mello, A.; Santos, M.; Sampaio, E.V.S.B.; Muir, J. Tree Legumes Provide Marketable Wood and Add Nitrogen in Warm-Climate Silvopasture Systems. Agron. J. 2015, 107, 1915–1921. [Google Scholar] [CrossRef]
- Chiocchio, I.; Mandrone, M.; Sanna, C.; Maxia, A.; Tacchini, M.; Poli, F. Screening of a hundred plant extracts as tyrosinase and elastase inhibitors, two enzymatic targets of cosmetic interest. Ind. Crops Prod. 2018, 122, 498–505. [Google Scholar] [CrossRef]
- Durgadevi, G.; Karthika, N. Screening of phytochemicals and pharmacological studies on Mimosa pudica L. Asian J. Innov. Res. 2018, 3, 19–28. [Google Scholar]
- do Nascimento, I.A.; Braz-Filho, R.; de Carvalho, M.G.; Mathias, L.; de Alcântara Fonseca, F. Flavonoides e Outros Compostos Isolados de Mimosa artemisiana Heringer e Paula. Quim. Nova 2012, 35, 2159–2164. [Google Scholar] [CrossRef] [Green Version]
- Mathew, J.; Joy, J.K.; Vazhacharickal, P.; Sajeshkumar, N.K. Phytochemical Analysis And Invitro Hemostatic Activity Of Mimosa Pudica, Hemigraphis Colorata And Chromolaena Odorata Leaf Extracts. CIBTech J. Pharm. Sci. 2016, 5, 16–34. [Google Scholar]
- Oliveira, F.F.M.; Barbosa, K.M.K.M.; de Oliveira, G.F.; Dantas, I.M.; Camacho, R.G.V. Micropropagação de Mimosa caesalpiniaefolia Benth. a partir de segmentos nodais e ápices caulinares. Rev. Caatinga 2007, 20, 152–159. [Google Scholar]
- Nana, F.; Sandjo, L.P.; Keumedjio, F.; Kuete, V.; Ngadjui, B.T. A new fatty aldol ester from the aerial part of Mimosa invisa (Mimosaceae). Nat. Prod. Res. 2012, 26, 1831–1836. [Google Scholar] [CrossRef]
- Tunna, T.; Zaidul, I.; Ahmed, Q.; Ghafoor, K.; Al-Juhaimi, F.; Uddin, M.; Hasan, M.; Ferdous, S. Analyses and profiling of extract and fractions of neglected weed Mimosa pudica Linn. traditionally used in Southeast Asia to treat diabetes. S. Afr. J. Bot. 2015, 99, 144–152. [Google Scholar] [CrossRef]
- Sutar, N.; Sutar, U.N.; Behera, B.C. Antidiabetic activity of the leaves of Mimosa pudica Linn. in albino rats. J. Herb. Med. Toxicol. 2009, 3, 123–126. [Google Scholar]
- Saraswat, R.; Pokharkar, R. GCMS studies of mimosa pudica. Int. J. PharmTech Res. 2012, 4, 93–98. [Google Scholar]
- Saxena, R.; Sharma, R.; Chandra Nandy, B.; Hardainiyan, S. The Study Of Phenolic Compounds And Antioxidant Potential Of Crude Extract And Fractions Of Mimosa Hamata. Int. Res. J. Pharm. 2017, 8, 83–86. [Google Scholar] [CrossRef]
- Almalki, M. In-Vitro Antioxidant Properties of the Leaf Extract of Mimosa pudica Linn. Indian J. Sci. Technol. 2016, 9, 1–6. [Google Scholar] [CrossRef]
- Parmar, F.; Kushawaha, N.; Highland, H.; George, L.-B. In vitro antioxidant and anticancer activity of Mimosa pudica linn extract and L-Mimosine on lymphoma daudi cells. Cancer Cell 2015, 1, 100–104. [Google Scholar]
- Silva, M.J.D.; Vilegas, W.; da Silva, M.A.; de Moura, C.F.G.; Ribeiro, F.A.P.; da Silva, V.H.P.; Ribeiro, D.A. Mimosa (Mimosa caesalpiniifolia) prevents oxidative DNA damage induced by cadmium exposure in Wistar rats. Toxicol. Mech. Methods 2014, 24, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Chandarana, M.; Singh, R.; Jasrai, Y. Determination of the phenolic and flavonoid contents in Mimosa hamataWilld., as well as their radical scavenging activity. Int. J. Pharmacogn. Phitochem. 2013, 28, 1121–1126. [Google Scholar]
- Borneo, R.; León, A.; Aguirre, A.; Ribotta, P.; Cantero, J. Antioxidant capacity of medicinal plants from the Province of Cordoba (Argentina) and their in vitro testing in model food system. Food Chem. 2009, 112, 664–670. [Google Scholar] [CrossRef]
- Prathima, C.; Shashikumara, T.T.; Jayanthi, M.K. Evaluation of anticonvulsant activity of Mimosa pudica root linn in swiss albino mice. Int. J. Pharm. Pharm. Sci. 2016, 8, 49–52. [Google Scholar]
- Jiang, Y.; You, X.-Y.; Fu, K.-L.; Yin, W.-L. Effects of Extract from Mangifera indica Leaf on Monosodium Urate Crystal-Induced Gouty Arthritis in Rats. Evid. Based Complement. Altern. Med. 2012, 2012, 967573. [Google Scholar] [CrossRef] [Green Version]
- Sampaio Octaviano de Souza, R.; Albuquerque, U.; Monteiro, J.; Lúcia Cavalcanti de Amorim, E. Jurema-Preta (Mimosa tenuiflora [Willd.] Poir.): A Review of its Traditional Use, Phytochemistry and Pharmacology. Braz. Arch. Biol. Technol. 2008, 51, 937–947. [Google Scholar] [CrossRef] [Green Version]
- Patel, N.K.; Bairwa, K.; Gangwal, R.; Jaiswal, G.; Jachak, S.M.; Sangamwar, A.T.; Bhutani, K.K. 2′-Hydroxy flavanone derivatives as an inhibitors of pro-inflammatory mediators: Experimental and molecular docking studies. Bioorgan. Med. Chem. Lett. 2015, 25, 1952–1955. [Google Scholar] [CrossRef] [PubMed]
- Borsato, D.M.; Prudente, A.S.; Döll-Boscardin, P.M.; Borsato, A.V.; Luz, C.F.; Maia, B.H.; Cabrini, D.A.; Otuki, M.F.; Miguel, M.D.; Farago, P.V. Topical anti-inflammatory activity of a monofloral honey of Mimosa scabrella provided by Melipona marginata during winter in Southern Brazil. J. Med. Food 2014, 17, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Bitencourt, M.; Conceição Jerônimo de Souza Lima, M.; Torres-Rêgo, M.; Fernandes, J.; Silva-Júnior, A.; Tambourgi, D.; Maria Zucolotto, S.; Fernandes-Pedrosa, M. Neutralizing Effects of Mimosa tenuiflora Extracts against Inflammation Caused by Tityus serrulatus Scorpion Venom. BioMed Res. Int. 2014, 2014, 378235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruz, M.P.; Andrade, C.M.F.; Silva, K.O.; de Souza, E.P.; Yatsuda, R.; Marques, L.M.; David, J.P.; David, J.M.; Napimoga, M.H.; Clemente-Napimoga, J.T. Antinoceptive and Anti-inflammatory Activities of the Ethanolic Extract, Fractions and Flavones Isolated from Mimosa tenuiflora (Willd.) Poir (Leguminosae). PLoS ONE 2016, 11, e0150839. [Google Scholar] [CrossRef] [Green Version]
- Kumar Vikram, P.; Malvi, R.; Jain, D. evaluation of analgesic and anti-inflammatory potential of mimosa pudica linn. Int. J. Curr. Pharm. Res. 2015, 4, 47–50. [Google Scholar]
- Ganguly, M.; Devi, N.; Mahanta, R.; Borthakur, M.K. Effect of Mimosa pudica root extract on vaginal estrous and serum hormones for screening of antifetility activity in albino mice. Contraception 2008, 76, 482–485. [Google Scholar] [CrossRef]
- Le Tran, Q.; Tezuka, Y.; Ueda, J.-Y.; Nhan, N.; Maruyama, Y.; Begum, K.; Kim, H.; Wataya, Y.; Kim Tran, Q.; Kadota, S. In Vitro Antiplasmodial Activity of Antimalarial Medicinal Plants Used in Vietnamese Traditional Medicine. J. Ethnopharmacol. 2003, 86, 249–252. [Google Scholar] [CrossRef]
- Marimuthu, S.; Rahuman, A.; Rajakumar, G.; Thirunavukkarasu, S.; Kirthi, V.; Chidambaram, J.; Bagavan, A.; Abduz Zahir, A.; Elango, G.; Kamaraj, D.C. Evaluation of green synthesized silver nanoparticles against parasites. Parasitol. Res. 2010, 108, 1541–1549. [Google Scholar] [CrossRef] [PubMed]
- Paul, J.; Khan, S.; Mohammed, S.; Asdaq, S. Wound healing evaluation of chloroform and methanolic extracts of mimosa pudica roots in rats. Int. J. Biol. Med. Res. 2010, 1, 223–227. [Google Scholar]
- Jose, J.; Dhanya, A.; Haridas, K.R.; Kumar, T.S.; Jayaraman, S.; Variyar, E.J.; Sudhakaran, S. Structural characterization of a novel derivative of myricetin from Mimosa pudica as an anti-proliferative agent for the treatment of cancer. Biomed. Pharmacother. 2016, 84, 1067–1077. [Google Scholar] [CrossRef]
- Monção, B.N.; Araújo, Q.B.; Silva, D.J.; Lima, J.D.; Ferreira, M.P.; Airoldi, P.F.; Pessoa, C.; Citó, M.A. Assessing Chemical Constituents of Mimosa caesalpiniifolia Stem Bark: Possible Bioactive Components Accountable for the Cytotoxic Effect of M. caesalpiniifolia on Human Tumour Cell Lines. Molecules 2015, 20, 4204–4224. [Google Scholar] [CrossRef] [Green Version]
- Molina, M.; Contreras, C.; Tellez-Alcantara, P. Mimosa pudica may possess antidepressant actions in the rat. Phytomedicine 1999, 6, 319–323. [Google Scholar] [CrossRef]
- Saifuddin Khalid, M.; Shah, J.; Suresh, D.K.; Singh, R.; Narasimha Reddy, I.V.; Kumar, S. Evaluation of anti-diarrhoeal potential of ethanolic extract of Mimosa pudica leaves. Int. J. Green Pharm. 2011, 5, 75. [Google Scholar] [CrossRef]
- Balakrishnan, N.; Bhaskar, V.; Jayakar, B.; Sangameswaran, B. Antibacterial activity of Mimosa pudica, Aegle marmelos and Sida cordifolia. Pharmacogn. Mag. 2006, 2, 198–199. [Google Scholar]
- Sowmya, A.; Ananthi, T. Hypolipidemic activity of Mimosa pudica Linn on butter induced hyperlipidemia in rats. Asian J. Res. Pharm. Sci. 2011, 1, 123–126. [Google Scholar]
- Purkayastha, A.; Chakravarty, P.; Dewan, B. hypolipidemic effect of ethanolic extract of mimosa pudica leaves on dyslipidemia following hepatic injury induced by carbon tetrachloride in albino rats. Int. J. Pharm. Sci. Res. 2016, 7, 3284–3290. [Google Scholar]
- Kumaresan, R.; Veerakumar, S.; Elango, V. A study on hepatoprotective activity of Mimosa pudica in albino rats. Int. J. Pharm. Phytochem. Res. 2015, 7, 337–339. [Google Scholar]
- Lin, L.-C.; Chiou, C.-T.; Cheng, J.-J. 5-Deoxyflavones with cytotoxic activity from Mimosa diplotricha. J. Nat. Prod. 2011, 74, 2001–2004. [Google Scholar] [CrossRef]
- Jain, S.; Jain, R.; Vlietinck, A. In vivo and in vitro antimicrobial efficacy of Mimosa hamata. Indian J. Biotechnol. 2004, 3, 271–273. [Google Scholar]
- Mehta, B.K.; Savita Sharma, K.; Dubey, A. 4-Ethylgallic acid from two Mimosa species. Phytochemistry 1988, 27, 3004–3005. [Google Scholar] [CrossRef]
- Mehmood, N.; Zubair, M.; Rızwan, K.; Rasool, N.; Shahid, M.; Ahmad, V. Antioxidant, antimicrobial and phytochemical analysis of Cichorium intybus seeds extract and various organic fractions. Iran. J. Pharm. Res. 2012, 11, 1145–1151. [Google Scholar]
- Aslam, F.; Riaz, M.; Zubair, M.; Rizwan, K.; Abbas, M.; Bukhari, T.H.; Bukhari, I.H. Antioxidant, haemolytic activities and GC-MS profiling of Carissa carandas roots. Int. J. Phytomed. 2012, 3, 567–578. [Google Scholar]
- Bari, M.N.; Zubair, M.; Rizwan, K.; Rasool, N.; Bukhari, I.H.; Akram, S.; Bokhari, T.H.; Shahid, M.; Hameed, M.; Ahmad, V.U. Biological activities of Opuntia Monacantha cladodes. J. Chem. Soc. Pak. 2012, 34, 990–995. [Google Scholar]
- Imran, M.; Rasool, N.; Rizwan, K.; Zubair, M.; Riaz, M.; Zia-Ul-Haq, M.; Rana, U.A.; Nafady, A.; Jaafar, H.Z. Chemical composition and Biological studies of Ficus benjamina. Chem. Cent. J. 2014, 8, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizwan, K.; Zubair, M.; Rasool, N.; Mahmood, A.; Ercisli, S.; Tareen, R.B.; Zia-Ul-Haq, M. Compositional studies and antioxidant potential of Fruit of Zizyphus oxyphylla EDGEW. Oxid. Commun. 2016, 39, 1309–1322. [Google Scholar]
- Ashraf, S.N.; Zubair, M.; Rizwan, K.; Tareen, R.B.; Rasool, N.; Zia-Ul-Haq, M.; Ercisli, S. Compositional studies and Biological activities of Perovskia abrotanoides Kar. oils. Biol. Res. 2014, 47, 12. [Google Scholar] [CrossRef] [Green Version]
- Rasool, N.; Tehseen, H.; Riaz, M.; Rizwan, K.; Zubair, M.; Mahmood, Y.; Iqbal, M.; Bukhari, I.H. Cytotoxicity studies and antioxidant potential of Acacia nilotica roots. Int. J. Chem. Biochem. Sci. 2013, 3, 34–41. [Google Scholar]
- Rasool, N.; Afzal, S.; Riaz, M.; Rashid, U.; Rizwan, K.; Zubair, M.; Ali, S.; Shahid, M. Evaluation of antioxidant activity, cytotoxic studies and GC-MS profiling of matthiola incana (stock flower). Legume Res. Int. J. 2013, 36, 21–32. [Google Scholar]
- Khan, S.A.; Rasool, N.; Riaz, M.; Nadeem, R.; Rashid, U.; Rizwan, K.; Zubair, M.; Bukhari, I.H.; Gulzar, T. Evaluation of Antioxidant and Cytotoxicity Studies of Clerodendrum inerme. Asian J. Chem. 2013, 25, 7457–7462. [Google Scholar] [CrossRef]
- Zubair, M.; Rizwan, K.; Rashid, U.; Saeed, R.; Saeed, A.A.; Rasool, N.; Riaz, M. GC/MS profiling, in vitro antioxidant, antimicrobial and haemolytic activities of Smilax macrophylla leaves. Arab. J. Chem. 2017, 9, S1435–S1442. [Google Scholar] [CrossRef] [Green Version]
- Zia-Ul-Haq, M.; Stanković, M.S.; Rizwan, K.; Feo, V.D. Grewia asiatica L., a food plant with multiple uses. Molecules 2013, 18, 2663–2682. [Google Scholar] [CrossRef]
- Zubair, M.; Bibi, Z.; Rizwan, K.; Rasool, N.; Zahoor, A.F.; Riaz, M. In Vitro Antimicrobial and Haemolytic Studies of Bambusa arundinaceae leaves. J. Appl. Pharm. Sci. 2013, 3, 111–115. [Google Scholar]
- Riaz, M.; Rasool, N.; Bukhari, I.H.; Shahid, M.; Zubair, M.; Rizwan, K.; Rashid, U. In vitro antimicrobial, antioxidant, cytotoxicity and GC-MS analysis of Mazus goodenifolius. Molecules 2012, 17, 14275–14287. [Google Scholar] [CrossRef] [Green Version]
- Rizwan, K.; Zubair, M.; Rasool, N.; Riaz, M.; Zia-Ul-Haq, M.; de Feo, V. Phytochemical and biological studies of Agave attenuata. Int. J. Mol. Sci. 2012, 13, 6440–6451. [Google Scholar] [CrossRef] [PubMed]
- Riaz, M.; Rasool, N.; Bukhari, I.; Rizwan, K.; Zubair, M.; Javed, F.; Altaf, A.; Qayyum, H. Antioxidant, Antimicrobial Activity and GC-MS analysis of Russelia equsetiformis Essential Oils. Oxid. Commun. 2012, 35, 1027–1037. [Google Scholar]
- Sharma, P.V.; Ahmad, Z.A. Present status of research on genus: Mimosa. Anc. Sci. Life 1987, 7, 21–29. [Google Scholar]
- Singh, R.; Jasrai, Y.T. Mimosa hamata (Willd.), a plant with antipathogenic properties. Int. J. Med. Aromat. Plants 2012, 2, 677–682. [Google Scholar]
- Jasuja, N.D.; Saxena, R.; Chandra, S.; Bhargava, S.; Joshi, S. Pharmacological evaluation of an ethnomedicinal and endangered desert plant: Mimosa hamata. J. Biol. Sci. 2014, 14, 52–59. [Google Scholar] [CrossRef] [Green Version]
- Muhammad, G.; Hussain, M.A.; Jantan, I.; Bukhari, S.N.A. Mimosa pudica L., a high-value medicinal plant as a source of bioactives for pharmaceuticals. Compr. Rev. Food Sci. Food Saf. 2016, 15, 303–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanaye, M.; Joglekar, C.; Pagare, N. Mimosa—A brief overview. J. Pharmacogn. Phytochem. 2015, 4, 182–187. [Google Scholar]
- Majeed, I.; Rizwan, K.; Ashar, A.; Rasheed, T.; Amarowicz, R.; Kausar, H.; Zia-Ul-Haq, M.; Marceanu, L.G. A Comprehensive Review of the Ethnotraditional Uses and Biological and Pharmacological Potential of the Genus Mimosa. Int. J. Mol. Sci. 2021, 22, 7463. [Google Scholar] [CrossRef]
- Haddad, H.; Seyed Hoseini, E.; Nikzad, H.; Hossein Aarabi, M. Pharmacological properties of medicinal herbs by focus on secondary metabolites. Life Sci. J. 2012, 9, 509–520. [Google Scholar]
- Oliveira, L.M.B.; Macedo, I.T.F.; Vieira, L.S.; Camurça-Vasconcelos, A.L.F.; Tomé, A.R.; Sampaio, R.A.; Louvandini, H.; Bevilaqua, C.M.L. Effects of Mimosa tenuiflora on larval establishment of Haemonchus contortus in sheep. Vet. Parasitol. 2013, 196, 341–346. [Google Scholar] [CrossRef] [Green Version]
- Racadio, S.P. The Medicinal Prospects of Makahiya (Mimosa Pudica Linn) Plant. Adv. Life Sci. 2016, 6, 7–12. [Google Scholar]
- Ahuchaogu, A.; Chukwu, J.; Obike, A.; Ugonna Oha, T.; Bull, J.; Echeme, O. Quantitative Determination of Secondary Metabolites and Antibacterial Activity of Mimosa Pudica. Int. J. Med. Plants Nat. Prod. 2017, 3, 2454–7999. [Google Scholar]
- Sheeba, D.G.; Gomathi, K.S.; Citarasu, D. Anti-Mycobacterial and Phytochemical Investigation of Methanol Extracts of Few Medicinal Plants. J. Chem. Pharm. Sci. 2015, 8, 480–486. [Google Scholar]
- Mahadevan, M.V.; Ramaswamy, R.S.; Banumathi, V. Mimosa pudica exerts neuroprotection against mpp+ induced neurotoxicity in shsy5y cell lines-an in vitro model of anti-parkinsonism. Int. J. Pharm. Pharm. Sci. 2016, 9, 21. [Google Scholar]
- Ramesh, S.; Karthikeyan, K.; Chandran, C. Photochemical screening and pharmacognostic studies on Mimosa pudica L. (Sensitive plant). Int. J. Fauna Biol. Stud. 2017, 4, 170–175. [Google Scholar]
- Chinnathambi, A.; Sathasivam, A. Analysis of the phytochemical constituents of Mimosa pudica and determination of their antimicrobial activity. In Proceedings of the Biotech Congress, Beijing, China, 13–15 July 2015; p. 61. [Google Scholar]
- Harborne, J.B.; Williams, C.A. Anthocyanins and other flavonoids. Nat. Prod. Rep. 2001, 18, 310–333. [Google Scholar] [CrossRef]
- Nagarajan, K.; Saravanararaja, M.; Aruna Devi, P.S. Antibacterial Efficiency of Fabaceae Plants of a Tropical Freshwater Lake. ScieXplore Int. J. Res. Sci. 2015, 2, 13. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.H.; Choo, C.; Watawana, M.I.; Jayawardena, N.; Waisundara, V.Y. An appraisal of eighteen commonly consumed edible plants as functional food based on their antioxidant and starch hydrolase inhibitory activities. J. Sci. Food Agric. 2015, 95, 2956–2964. [Google Scholar] [CrossRef] [PubMed]
- Ittiyavirah, S.P.; Pullochal, I. Adaptogenic and nootropic activity of Mimosa pudica in albino wistar rats. Int. J. Nutr. Pharmacol. Neurol. Dis. 2014, 4, 231. [Google Scholar] [CrossRef]
- Ao, S.; Kc, O.; Ukwueze, S. Evaluation of the antidiabetic potential of ethanol root extract of Mimosa pigra Linn (Fabaceae) in alloxan-induced diabetic albino rats. Int. J. Curr. Res. 2015, 7, 15577–15581. [Google Scholar]
- Shorinwa Olusayo, A.; Nnamdi, E.; Afieroho Ozadheoghene, E. Evaluation of mimosa pigra roots on haematological and biochemical parameters of albino rats. World J. Pharm. Res. 2016, 5, 810–822. [Google Scholar]
- Rosado-Vallado, M.; Brito-Loeza, W.; Mena-Rejon, G.; Quintero-Marmol, E.; Flores-Guido, J. Antimicrobial activity of Fabaceae species used in Yucatan traditional medicine. Fitoterapia 2000, 71, 570–573. [Google Scholar] [CrossRef]
- Saxena, R.; Sharma, R.; Nandy, B.; Jasuja, D.N. Qualitative and quantitative estimation of bioactive compounds in Mimosa hamata. Int. J. Pharm. Pharm. Sci. 2014, 6, 72–75. [Google Scholar]
- Manosroi, J.; Zaruwa, M.; Manosroi, A. Potent Hypoglycemic Effect of Nigerian Anti-Diabetic Medicinal Plants. J. Complement. Integr. Med. 2011, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, N.; Carrillo-Hormaza, L.; Pujol, A.; Álzate, F.; Osorio, E.; Lara-Guzman, O. Antioxidant capacity and phenolic content of commonly used anti-inflammatory medicinal plants in Colombia. Ind. Crops Prod. 2015, 70, 272–279. [Google Scholar] [CrossRef]
- Seraglio, S.K.T.; Valese, A.C.; Daguer, H.; Bergamo, G.; Azevedo, M.S.; Gonzaga, L.V.; Fett, R.; Costa, A.C.O. Development and validation of a LC-ESI-MS/MS method for the determination of phenolic compounds in honeydew honeys with the diluted-and-shoot approach. Food Res. Int. 2016, 87, 60–67. [Google Scholar] [CrossRef]
- Nandipati, M.C.; Sumalatha, G.; Baburao, C.; Babu, J.R.; Sridevi, C. Antitumor activity of mimosa rubicaulis lam against ehrlich ascites carcinoma in swiss albino mice. Int. J. Pharm. Sci. Res. 2014, 5, 1514. [Google Scholar]
- Wadood, A.; Ghufran, M.; Jamal, S.B.; Naeem, M.; Khan, A.; Ghaffar, R. Phytochemical analysis of medicinal plants occurring in local area of Mardan. Biochem. Anal Biochem. 2013, 2, 4. [Google Scholar] [CrossRef]
- Dominguez, X.A.; Garcia, G.S.; Williams, H.J.; Ortiz, C.; Scott, A.I.; Reibenspies, J.H. Kukulkanins A and B, new chalcones from Mimosa tenuefolia. J. Nat. Prod. 1989, 52, 864–867. [Google Scholar] [CrossRef]
- Bautista, E.; Calzada, F.; Ortega, A.; Yépez-Mulia, L. Antiprotozoal activity of flavonoids isolated from Mimosa tenuiflora (Fabaceae-Mimosoideae). J. Mex. Chem. Soc. 2011, 55, 251–253. [Google Scholar]
- León, L.; Maldonado, E.; Cruz, A.; Ortega, A. Tenuiflorins A-C: New 2-Phenoxychromones from the Leaves of Mimosa tenuiflora. Planta Med. 2004, 70, 536–539. [Google Scholar] [CrossRef] [PubMed]
- Gardner, D.; Riet-Correa, F.; Lemos, D.; Welch, K.; Pfister, J.; Panter, K. Teratogenic effects of Mimosa tenuiflora in a rat model and possible role of N-methyl-and N, N-dimethyltryptamine. J. Agric. Food Chem. 2014, 62, 7398–7401. [Google Scholar] [CrossRef]
- Meckes-Lozoya, M.; Lozoya, X.; Marles, R.J.; Soucy-Breau, C.; Sen, A.; Arnason, J.T. N, N-dimethyltryptamine alkaloid in Mimosa tenuiflora bark (tepescohuite). Arch. Investig. Med. 1990, 21, 175. [Google Scholar]
- Anton, R.; Jiang, Y.; Weniger, B.; Beck, J.; Rivier, L. Pharmacognosy of Mimosa tenuiflora (willd.) poiret. J. Ethnopharmacol. 1993, 38, 145–152. [Google Scholar] [CrossRef]
- Jiang, Y.; Weniger, B.; Haag-Berrurier, M.; Anton, R.; Beck, J.P.; Italiano, L. Effects of saponins from Mimosa tenuiflora on lymphoma cells and lymphocytes. Phytother. Res. 1992, 6, 310–313. [Google Scholar] [CrossRef]
- Vepsäläinen, J.J.; Auriola, S.; Tukiainen, M.; Ropponen, N.; Callaway, J. Isolation and characterization of yuremamine, a new phytoindole. Planta Med. 2005, 71, 1053–1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calvert, M.B.; Sperry, J. Bioinspired total synthesis and structural revision of yuremamine, an alkaloid from the entheogenic plant Mimosa tenuiflora. Chem. Commun. 2015, 51, 6202–6205. [Google Scholar] [CrossRef]
- Okonkwo, C.J.; Njoku, O.U.; Okonkwo, T.J.; Afieroho, O.E.; Proksch, P. Two new acylated flavonol glycosides from Mimosa pigra L. leaves sub-family Mimosoideae. Future J. Pharm. Sci. 2016, 2, 71–75. [Google Scholar] [CrossRef]
- Englert, J.; Weniger, B.; Lobstein, A.; Anton, R.; Krempp, E.; Guillaume, D.; Leroy, Y. Triterpenoid saponins from Mimosa pigra. J. Nat. Prod. 1995, 58, 1265–1269. [Google Scholar] [CrossRef]
- Rakotomalala, G.; Agard, C.; Tonnerre, P.; Tesse, A.; Derbré, S.; Michalet, S.; Hamzaoui, J.; Rio, M.; Cario-Toumaniantz, C.; Richomme, P. Extract from Mimosa pigra attenuates chronic experimental pulmonary hypertension. J. Ethnopharmacol. 2013, 148, 106–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, M.; Moura, L.; Mendes, M.; Arcanjo, D.; Monção, N.; Araújo, B.; Lopes, J.; Silva-Filho, J.; Fernandes, R.; Oliveira, R. Hypotensive and vasorelaxant effects induced by the ethanolic extract of the Mimosa caesalpiniifolia Benth.(Mimosaceae) inflorescences in normotensive rats. J. Ethnopharmacol. 2015, 164, 120–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, M.; Carvalho, A.; Rocha, C.; Vilegas, W.; Silva, M.; Gouvêa, C. Ethanolic extract of Mimosa caesalpiniifolia leaves: Chemical characterization and cytotoxic effect on human breast cancer MCF-7 cell line. S. Afr. J. Bot. 2014, 93, 64–69. [Google Scholar] [CrossRef] [Green Version]
- Jain, R.; Arora, R.; Jain, S. Saponins from the Roots of Mimosa hamata Willd. ChemInform 1997, 28, 61–64. [Google Scholar] [CrossRef]
- Jain, R.; Arora, R.; Alam, S.; Jain, S. Pharmacological evaluation of Mimosa hamata roots. Fitoterapia 1997, 68, 377–378. [Google Scholar]
- Jain, R.; Jain, S. Studies on Antimicrobial and Antioxidant Potentials of Triterpenoidal Saponins from Mimosa Hamata Willd. Int. J. Pharm. Phytopharm. Res. 2015, 4, 337–339. [Google Scholar]
- Chiou, C.-T.; Shen, C.-C.; Tsai, T.-H.; Chen, Y.-J.; Lin, L.-C. Meroterpenoids and Chalcone-Lignoids from the Roots of Mimosa diplotricha. J. Nat. Prod. 2016, 79, 2439–2445. [Google Scholar] [CrossRef]
- Camargo, L.M.D.M.; Férézou, J.-P.; Tinoco, L.W.; Kaiser, C.R.; Costa, S.S. Flavonoids from Mimosa xanthocentra (Leguminosae: Mimosoideae) and molecular modeling studies for isovitexin-2″-O-α-l-rhamnopyranoside rotamers. Phytochem. Lett. 2012, 5, 427–431. [Google Scholar] [CrossRef]
- Pachter, I.J.; Zacharias, D.E.; Ribeiro, O. Indole alkaloids of Acer saccharinum (the Silver Maple), Dictyoloma incanescens, Piptadenia colubrina, and Mimosa hostilis. J. Org. Chem. 1959, 24, 1285–1287. [Google Scholar] [CrossRef]
- Chrestani, F.; Sierakowski, M.R.; de Andrade Uchoa, D.E.; Nozawa, C.; Sassaki, G.L.; Gorin, P.A.J.; Ono, L. In vitro antiherpetic and antirotaviral activities of a sulfate prepared from Mimosa scabrella galactomannan. Int. J. Biol. Macromol. 2009, 45, 453–457. [Google Scholar] [CrossRef]
- Gupta, M.; Arias, T.; Etheart, J.; Hatfield, G. The occurrence of tryptamine and N-methyltryptamine in Mimosa somnians. J. Nat. Prod. 1979, 42, 234–236. [Google Scholar] [CrossRef]
- Thompson, J.F.; Morris, C.J.; Smith, I.K. New naturally occurring amino acids. Annu. Rev. Biochem. 1969, 38, 137–158. [Google Scholar] [CrossRef]
- Chukwu, O.J.; Ahuchaogu, A.A.; Ukaogo, P.; Obike, A.; Echeme, J.B.O. Antifungal Activity of Mimosa pudica, Isolation and NMR Characterization of Bioactive Components. Asian J. Chem. Sci. 2017, 2, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Tsurumi, S.; Asahi, Y. Identification of jasmonic acid in Mimosa pudica and its inhibitory effect on auxin-and light-induced opening of the pulvinules. Physiol. Plant. 1985, 64, 207–211. [Google Scholar] [CrossRef]
- Yuan, K.; Lü, J.; Yin, M. Chemical constituents of C-glycosylflavones from Mimosa pudica. Yao Xue Xue Bao Acta Pharm. Sin. 2006, 41, 435–438. [Google Scholar]
- Yuan, K.; Lu, J.L.; Jia, A.; Zhu, J.X. Two new C-glycosylflavones from Mimosa pudica. Chin. Chem. Lett. 2007, 18, 1231–1234. [Google Scholar] [CrossRef]
- Misra, A.; Tiwari, H. Constituents of roots of Boerhaavia diffusa. Phytochemistry 1971, 10, 3318–3319. [Google Scholar] [CrossRef]
- Rajalakshmi, K.; Banu, N. Antimicrobial activity of natural chlorophyllin from endangered medicinal plant Mimosa pudica L. Int. J. Pharm. Pharm. Sci. 2016, 8, 387–389. [Google Scholar]
- Josewin, B.; Ramachandrapai, M.; Suseelan, M. A new phenolic ketone from the leaves of Mimosa pudica Linn. Indian J. Chem. 1999, 38B, 251–253. [Google Scholar]
- Kirk, L.F.; Møller, M.V.; Christensen, J.; Stærk, D.; Ekpe, P.; Jaroszewski, J.W. A 5-deoxyflavonol derivative in Mimosa pudica. Biochem. Syst. Ecol. 2003, 31, 103–106. [Google Scholar] [CrossRef] [Green Version]
- Ueda, M.; Yamamura, S. Leaf-opening substance of Mimosa pudica L.; chemical studies on the other leaf movement of mimosa. Tetrahedron Lett. 1999, 40, 353–356. [Google Scholar] [CrossRef]
- Ueda, M.; Yamamura, S. Leaf-closing substance of Mimosa pudica L.; chemical studies on another leaf-movement of mimosa II. Tetrahedron Lett. 1999, 40, 2981–2984. [Google Scholar] [CrossRef]
- Ueda, M.; Yamamura, S. The chemistry of leaf-movement in Mimosa pudica L. Tetrahedron 1999, 55, 10937–10948. [Google Scholar] [CrossRef]
- Pal, M.; Roychaudhury, A.; Pal, A.; Biswas, S. A novel tubulin from Mimosa pudica: Purification and characterization. Eur. J. Biochem. 1990, 192, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Khare, C. Encyclopedia of Indian Medicinal Plants: Rational Western Therapy, Ayurvedic and Other Traditional Usage, Botany; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Zhang, J.; Yuan, K.; Zhou, W.-l.; Zhou, J.; Yang, P. Studies on the active components and antioxidant activities of the extracts of Mimosa pudica Linn. from southern China. Pharmacogn. Mag. 2011, 7, 35. [Google Scholar]
- Kang, J.G.; Shin, S.Y.; Kim, M.J.; Bajpai, V.; Maheshwari, D.K.; Kang, S.C. Isolation and Anti-fungal Activities of 2-Hydroxymethyl-chroman-4-one Produced by Burkholderia sp. MSSP. J. Antibiot. 2004, 57, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Dinda, B.; Ghosh, B.; Arima, S.; Sato, N.; Harigaya, Y. Steroids and terpenoid from Mimosa pudica roots. J. Indian Chem. Soc. 2006, 83, 1044–1046. [Google Scholar] [CrossRef]
- Shu, W.-J.; Ho, J.-C. Two new antimicrobial diterpenoids from the roots of Mimosa pudica. J. Chin. Med. 2013, 24, 223–229. [Google Scholar]
- Chatterjee, A.; Pakrashi, S.C. The Treatise on Indian Medicinal Plants; Publication and Information Directorate: New Delhi, India, 1991; Volume 3. [Google Scholar]
- Yadava, R.; Yadav, S. A novel bufadienolide from the seeds of Mimosa pudica Linn. Asian J. Chem. 2001, 13, 1157–1160. [Google Scholar]
- Zaware, B.; Chaudhari, S.; Shinde, M. An overview of Mimosa pudica Linn. Chem. Pharmacol. Profile. Res. J. Pharm. Biol. Chem. Sci. 2014, 5, 754–761. [Google Scholar]
- Yeung, A.W.K.; Tzvetkov, N.T.; Georgieva, M.G.; Ognyanov, I.V.; Kordos, K.; Jóźwik, A.; Kühl, T.; Perry, G.; Petralia, M.C.; Mazzon, E.; et al. Reactive Oxygen Species and Their Impact in Neurodegenerative Diseases: Literature Landscape Analysis. Antioxid. Redox Signal. 2020, 34, 402–420. [Google Scholar] [CrossRef]
- Sueishi, Y.; Nii, R. A comparative study of the antioxidant profiles of olive fruit and leaf extracts against five reactive oxygen species as measured with a multiple free-radical scavenging method. J. Food Sci. 2020, 85, 2737–2744. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K.; Rathi, R.B. Evaluation of Anticancer Action of Martynia annua Linn Root Extract on Different Human Cancer Cell Lines. J. Pharm. Res. Int. 2021, 33, 96–109. [Google Scholar] [CrossRef]
- Khan, T.; Ali, M.; Khan, A.; Nisar, P.; Jan, S.A.; Afridi, S.; Shinwari, Z.K. Anticancer plants: A review of the active phytochemicals, applications in animal models, and regulatory aspects. Biomolecules 2020, 10, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
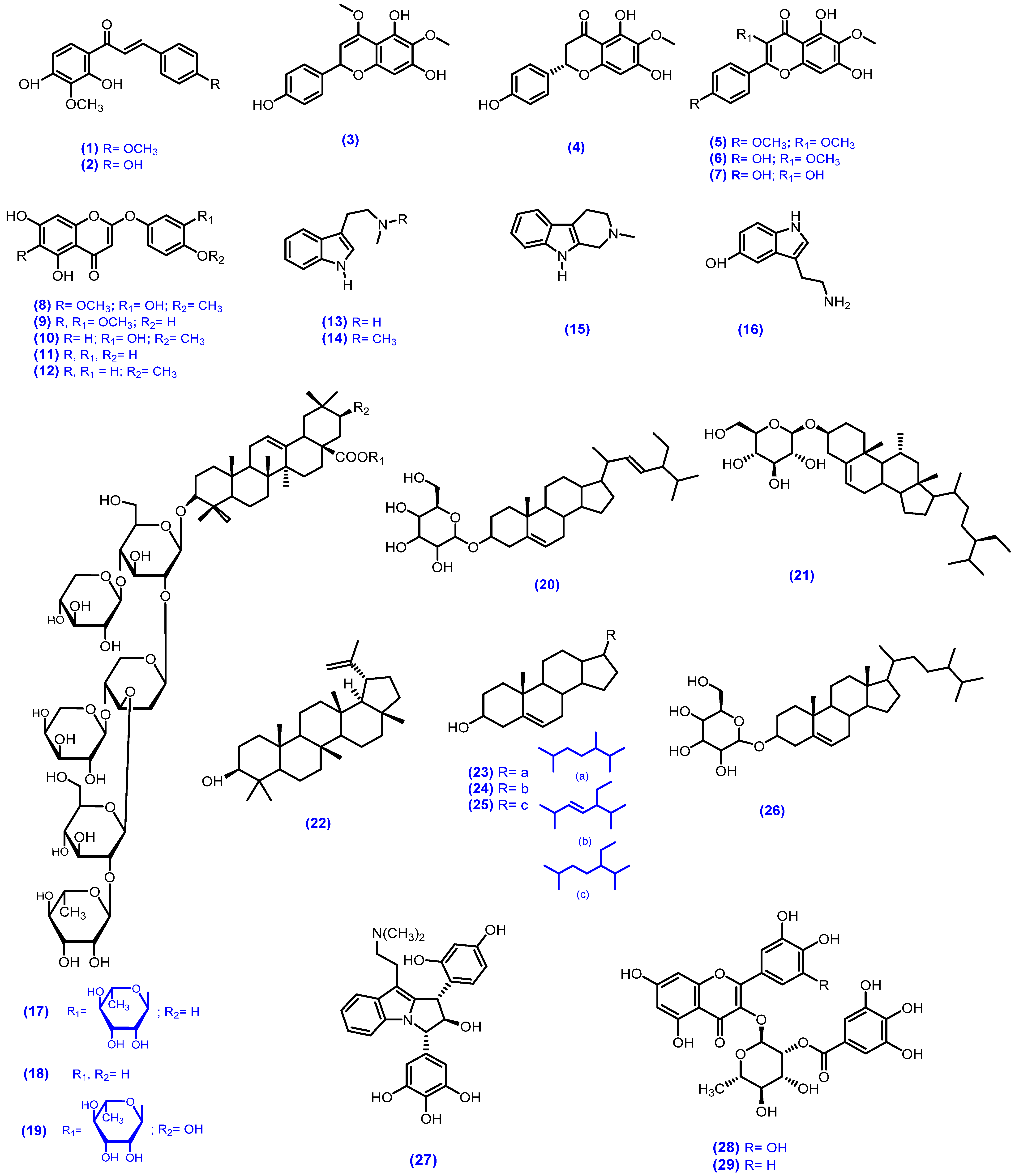





| Plant | Plant Part/Extract | Phytochemicals | References |
|---|---|---|---|
| M. tenuiflora | Whole plant | condensed tannins, procyanidin, prodelphinidins | [82] |
| leaves, stem | total phenols, total tannins and condensed tannins | [83] | |
| M. pudica | Leaves/EtOH extract | alkaloids, flavonois, saponins and triterpenes | [84] |
| Whole plant/EtOH extract | alkaloids, flavonoids, steroids, saponins, phenols, tannins, cyanogenic glycosides and anthocyanins | [85] | |
| Leaves/aq. extract | flavonoids, alkaloids, proteins, steroids, tannins, saponins and terpenoids | [24] | |
| Leaves/MeOH extract | alkaloids, glycosides, terpenoids and amino acids | [86] | |
| Aerial parts | alkaloids, saponins, flavonoids, terpenoids and coumarins | [29] | |
| Roots/EtOH extract | proteins, sterols, tannins, terpenoids, alkaloids, flavonoids and phenolic compounds | [34] | |
| Whole plant/aq. extract | alkaloids, flavonoids and tannins | [87] | |
| Leaves and roots/EtOH, MeOH, PE and ACE extracts | flavonoids, alkaloids, terpenoids, carbohydrates, saponins, amino acids, phenols, tannins, proteins and steroids | [88] | |
| Leaves/ACE, EtOH and aq. extracts | tannin, terpenoids, phlobatannins, steroids, saponin and glycoside | [89] | |
| Whole plant/MeOH extract | flavonoids alkaloids, saponins, terpenoids, phenols, glycosides, tannins, and coumarins | [26] | |
| Leaves and stem/aq. extract | saponins, alkaloids, flavonoids, tannins and phenols | [90,91] | |
| Leaves and stem/aq. extract | magnesium, phosphorus, calcium, nitrogen and potassium | [91] | |
| Leaves | neoxanthin, viola xanthin, lutein, lycopene, carotenes, tocopherol, total carotenoids and total vitamins | [92] | |
| Whole plant/EtOH extrac | alkaloids, flavonoids, tannins, phenolics and steroids | [93] | |
| M. pigra | Roots/EtOH extract | tannins, phlobatannins, flavonoids, triterpenes and saponins | [94,95] |
| Leaves/MeOH and aq. extracts | flavonoids, quinones, saponins, sterols and tannins | [96] | |
| M. hamata | Whole plant/EtOH and MeOH extracts | flavonoids, alkaloids, phytosterols, glycosides, tannins, phenolic compounds, saponins and carbohydrates, | [97] |
| M. invisa | Leaves/aq. extract | flavones, glycosides, saponins alkaloids and tannins | [98] |
| M. albida | Whole plant/aq. extract | total phenolic contents | [99] |
| M. scabrella bentham | Honeydew honeys | lignin-derived aldehydes, coumarins, phenolic acids and flavonoids | [100] |
| M. rubicaulis | Stem/MeOH extract | flavonoids, tannins, triterpenes and carbohydrates | [101] |
| Species | Extract | Parts | Classification | Compounds | Modal/Assay | Responses along with Critical Assessment | Ref. |
|---|---|---|---|---|---|---|---|
| M. tenuiflora | DCM-Hex -MeOH | Stem bark | Chalcones | kukulkan A (1), kukulkan B (2) | [103] | ||
| Hex, ACE, MeOH | Leaves and flowers | Flavonoids | 6-methoxy-4-O-metylnaringenin (3), 6-methoxynaringenin (4), santin (5), 4,5,7-trihydroxy-3,6-dimethoxy flavone (6), 6-methoxykaempferol (7), tenuiflorin A (8), tenuiflorin B (9), tenuiflorin C (10), 6-demethoxycapillarisin (11), 6-demethoxy-4-O-methyl capillarisin (12) | In vitro/Antiprotozoal assays/E. histolytica, G. lamblia | (IC50 μg/mL) against E. histolytica and G. lamblia (3) = 72.7 μg/mL and 82.9 μg/mL; (4) = 69.7 μg/mL and 75.3 μg/mL; (5) = 76.4 μg/mL and 84.1 μg/mL; (6) = 41.1 μg/mL and 108.6 μg/mL; (7) = 69.8 μg/mL and 77.1 μg/mL; (8) = 80.7 μg/mL and 91.8 μg/mL; (9) = 71.6 μg/mL and 77.8 μg/mL; (10) = 82.8 μg/mL and 92.8 μg/mL; (11) = 89.9 μg/mL and 100.9 μg/mL; (12) = 78.7 μg/mL and 86.6 μg/mL; positive control; emetinec = 2.2 μg/mL and 0.8 μg/mL; Metronidazolec = 0.23 μg/mL and 1.22 μg/mL respectively. Overall good activity | [104,105] | |
| MeOH, Crude Alkaloid Extracts | Leaves and seeds | Indole alkaloid | N-methyltryptamine (13), N,N-dimethyltryptamine (14), 2-methyltetrahydro-β-carboline (15) | [106] | |||
| Hex; ACE: MeOH | Trunk bark, root bark | Indole alkaloid | N, N-dimethyltryptamine (14), 5-hydroxy-tryptamine (16) | [107] | |||
| MeOH | Stem bark | Terpenoids saponins | mimonoside A (17), mimonoside B (18), mimonoside C (19) | [13,108,109] | |||
| MeOH | Stem bark | Steroids saponins | stigmasterol-3-O-β-D-glucopyranosyl (20), β-sitosterol 3-O-β-D-glucopyranosyl (21), lupeol (22), campesterols (23), stigmasterol (24), β-sitosterol (25), campesterol-3-O-β-D-glucopyranosyl (26) | [108] | |||
| MeOH | Root bark, stem bark | phytoindole Alkaloid | yuremamine (27) | [110,111] | |||
| M. pigra | DEE and EtOAc fraction | Leaves | Acylated flavonol glycosides | myricetin (2-O-galloyl)- 3-O-α-L-rhamnopyranoside (28), quercetin (2-O-galloyl)- 3-O-α-L-rhamnopyranoside (29), myricetin 3-O-α-L-rhamnopyranoside (30), quercetin 3-O-α-L-rhamnopyranoside (31), quercetin 3-O-β-L-arabinopyranoside (32) | [112] | ||
| BuOH | Stem bark | Triterpene glycosides | Z/E-methoxycinnamic (33,34), E-cinnamic acid (35) | [113] | |||
| Hydro-MeOH extract | Leaves | Tryptophan, amino acid and phenols | tryptophan (36), myricitrin (37), quercitrin (38), quercetin 3-O-pentose (39), quercetin 3-O-hexose (40), kampferol 3-O-desoxyhexose (41) | [114] | |||
| M. caesalpiniifolia | EtOH extract | Inflorescence | gallic acid (42), methylgallate (43), 5-hydroxy-4,7-dimethoxy-flavone (44), quercetin (38), quercetin-O-hexoside (45), vicenin-2 (46), rutin (47) | [115] | |||
| EtOH extract | leaves | Phenols and flavonids | catechin (48), 2,3 dihydroquercetagetin (49), procyanidin (50) | [116] | |||
| M. hamata | MeOH extract | Roots | Triterpenoidal Saponins | mimonoside A (17), mimonoside B (18), mimonoside C (19), saponin A; (3-O-L rhamnopyran osyl -D-glucopyranosylmorolic acid) (51), saponin B; (3-O-L-Arabinosyl-D-glucosylmorolic acid) (52) | In vitro/Antimicrobial activity agar well diffusion method/against B. subtilis, E. coli, E. aerogenes, S. aureus, P. aeruginosa, A. flavus, K. pneumonia, A. niger, C. albicans, R. bataticola | Good activity against Gram -ve bacteria and fungi Marginal activity toward Gram +ve bacteria | [117,118,119] |
| Antioxidant activity/DPPH free radical scavenging assay | Compounds exhibited IC50; (17) = 0.45 μg/mL; (18) = 0.55 μg/mL; (19) = 0.60 μg/mL; (51) = 0.085 μg/mL; (52) = 0.10 μg/mL; Standard Quercetin = 0.06 μg/mL. Overall good activity | [119] | |||||
| ACE | Flowers, leaves | 4-ethylgallic acid (53), gallic acid (42) | [7,60] | ||||
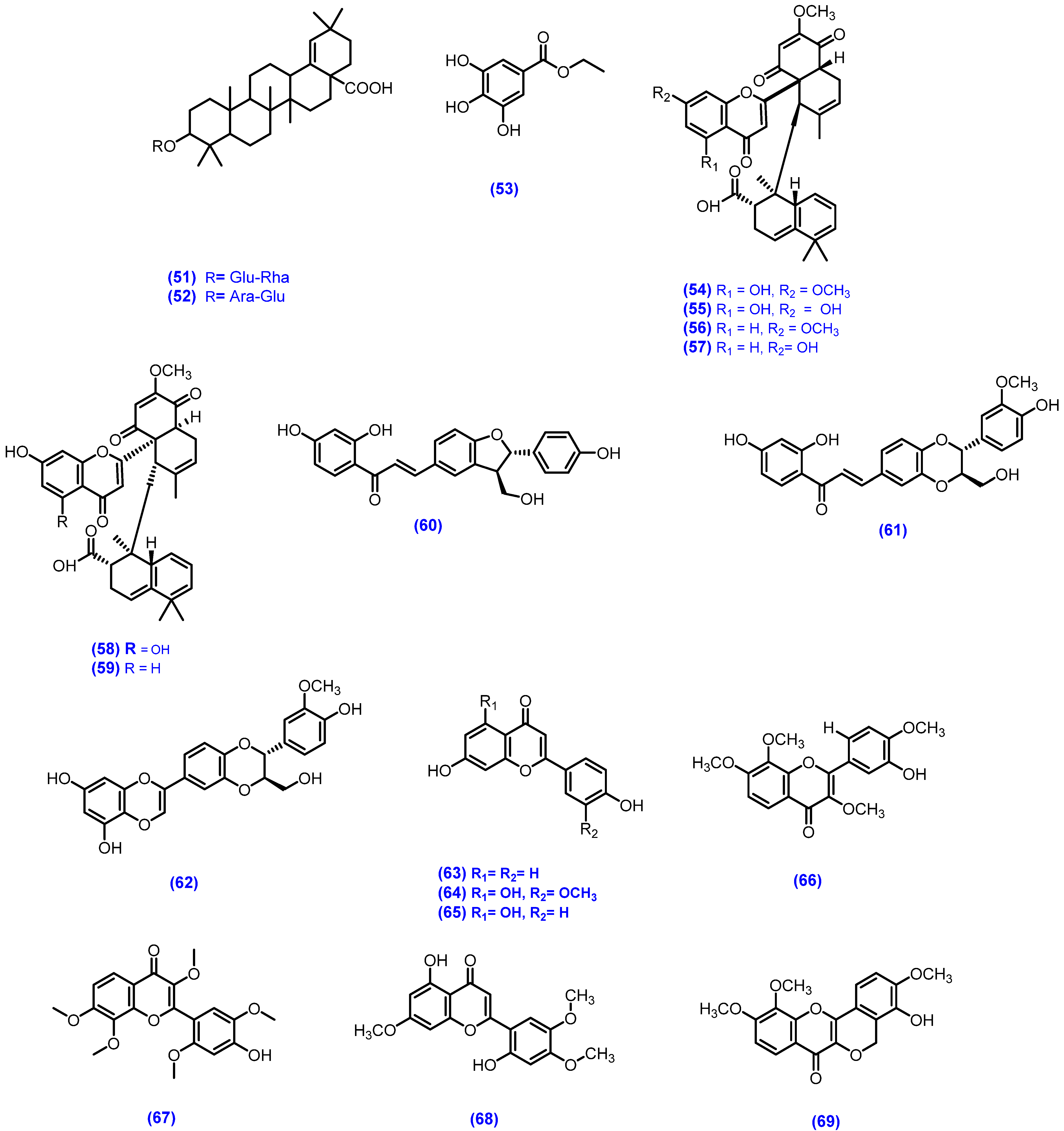
| M. diplotricha | CHCl3 | Root | Meroterpenoids, chalcone-lignoids | diplomeroterpenoid A (54), diplomeroterpenoid B (55), diplomeroterpenoid C (56), diplomeroterpenoid D (57), diplomeroterpenoid E (58), diplomeroterpenoid F (59), diploflavolin A (60), diploflavolin B (61), hydnocarpin (62), 7,4-dihydroxyflavone (63), chrysoeriol (64), apigenin (65), diplotrin B (66), 2-hydroxy-3,7,4′,8,5′-pentamethoxyflavone (67), hernancorizin (68), diplotasin D (69), 7-hydroxy-8-methoxychromone (70), (+)-syringaresinol (71), 4-hydroxy-3,5-dimethoxybenzoic acid (72), β-sitosterol (25), β-sitosterol glucoside (73) | Antiproliferative activity against human hepatoblastoma HepG2 cells/SRB assay | Compound 54 showed antiproliferative activity GI50 = 8.6 μM) while Compounds 55–60 ≥ 10 μM. Marginal activity | [120] |
| EtOH | Whole plant | 5-deoxy flavones, flavonoids, flavonolignans and triterpenoids | diplotrin A (74), diplotrin B (66), diplotrin C (75), diplotasin D (69), 5-methoxyhydnocarpin-D (76), 7,3′,4′-trihydroxy-3,8-dimethoxyflavone (77), 2-hydroxy-3,7,8,4,5 pentamethoxy flavone (67), hernancorizin (68), 5,3′-di-O-methylluteolin (78), betulinic acid (79), luteolin (80), quercetin (38), quercetin-3-O-xylopyranoside (81), myricetin-3-O-xylopyranoside (82), quercetin-3-O-arabino furanoside (83), myricetin-3-O-arabino furanoside (84) | In vitro/Cytotoxic activity/A549, AGS, HT-29, and PC3 human cancer cell line/SRB assay | Against all cell lines GI50 (66) = 2.7 μM, 1.7 μM, 7.5 μM, and 20.8 μM, (76) 20.3 μM, 24.8 μM, 4.1 μM, and 2.3 μM, respectively. Excellent activity. | [58] | |
| M. xanthocentra | EtOAc, BuOH | Aerial parts | flavones | isovitexin-2-O- α rhamnopyranoside (85), vitexin-2-O-α–L rhamnopyranoside (86), quercetin-3-O xylopyranoside (81), quercetin-3-O-arabino furanoside (83) | [121] | ||
| M. hostilis | EtOAc | Roots | Indole Alkaloid | N,N-dimethyltryptamine (14) | [122] | ||
| M.artemisiana | n-Hex, MeOH | Leaves and branches | Flavonoids, flavonolignans, glycosylated steroid, triterpene, steroids, indole carboxylate | quercetina-3-O-raminosídeo (87), miricetina-3-O-raminoside (88), Euphaline, 3,5,4-trihydroxy-6,7-dimethoxy flavone (89), flavonolignana(90,91), sitosterol-3-O-β-D glycopyranoside (21), lupeol (22), steroids sitostenone (92), stigmastenone (93), campestenone (94), campesterol (23), stigmasterol (24), sitosterol (25), methyl indole-3-carboxilate (95), indole-3-carboxaldehyde (96) | [25] | ||
| M. invisa | DCM/MeOH | Aerial parts | Fatty aldol ester | 17-O-triacontanoylheptadecanal (97) and β-sitosterol (25), α-amyrine (98), lupeol (22), 4-O-methylepinumisoflavone (99), alpinumisoflavone (100), betulinic acid (79), sitosterol 3-O-β-D-glucopyranoside (21) and epirobinetinidol (101) | Antimicrobial activity/E. coli, E. aerogenes, S. aureus, P. aeruginosa, K. pneumonia, S. typhi, C. albicans/XTT colorimetric assay | Compound (97) and (101) were most active against K. pneumonia MIC = 64 mg/mL. Overall good activity | [28] |
| M. scabrella | Polysaccharide | Seeds | Sulfated galactomannan (BRS) (102) | in vitro/antiviral activity against Herpes simplex virus 1 (HSV-1)/plaque reduction method | At concentration 20 μg/mL IC50 lesser than 2.5 µg/mL was observed. Marginal activity. Excellent activity. | [123] | |
| in vitro/Cytotoxic activity/in Vero and MA-104 cells/MTT assay | At the concentrations ≥39 µg/mL BRS reduced by 15% the viability of Vero cells (CC50 = 454 µg/mL) At the concentrations 625 µg/mL BRS reduced by 24% the viability of MA-104 cells (CC50 > 625 µg/mL). Marginal activity | ||||||
| M. somniam | MeOH | Whole plant | Alkaloid | tryptamine (103), N-methyl tryptamine (13) | [124] | ||
| M. pudica | EtOAc fration | Whole plant | Flavonoid | 2-(2′,6′-dimethyl-3′,4′,5′-alkyl or hydroxy alkyl substituted phenyl)-3-oxy-(alkyl or hydoxy alkyl) 5,7-dihydroxy-chromen-4-one (104–107) | In vitro/Cytotoxic activity/MTT assay/human lung adenocarcinoma cell line (A549) & human erythroleukemic cell line (K562) | (IC50) of against A549 = 76.67 µg/mL and K562 = 287.63 µg/mL, while positive control Doxorubicin A549 = 2.76 µg/mL and K562 = 4.72 µg/mL. Marginal activity | [50] |
| HyOH extract | Whole plant | Amino acid | L-mimosine (108) | Antioxidant effect/DPPH radical scavenging activity | Compound at 250 μg/mL (IC50 = 233.06 μM). Good activity | [34,125] | |
| In vitro/Cytotoxic activity/daudi cell line/MTT assay | Compound 108, (IC50 = 86.61 μM). Excellent activity | ||||||
| CHCl3 extracts | Whole plant | Triterpenoid | triterpenoid glycoside (109) | [126] | |||
| CHCl3 extracts | Whole plant | - | jasmonic acid (110), abscisic acid (111), | [127] | |||
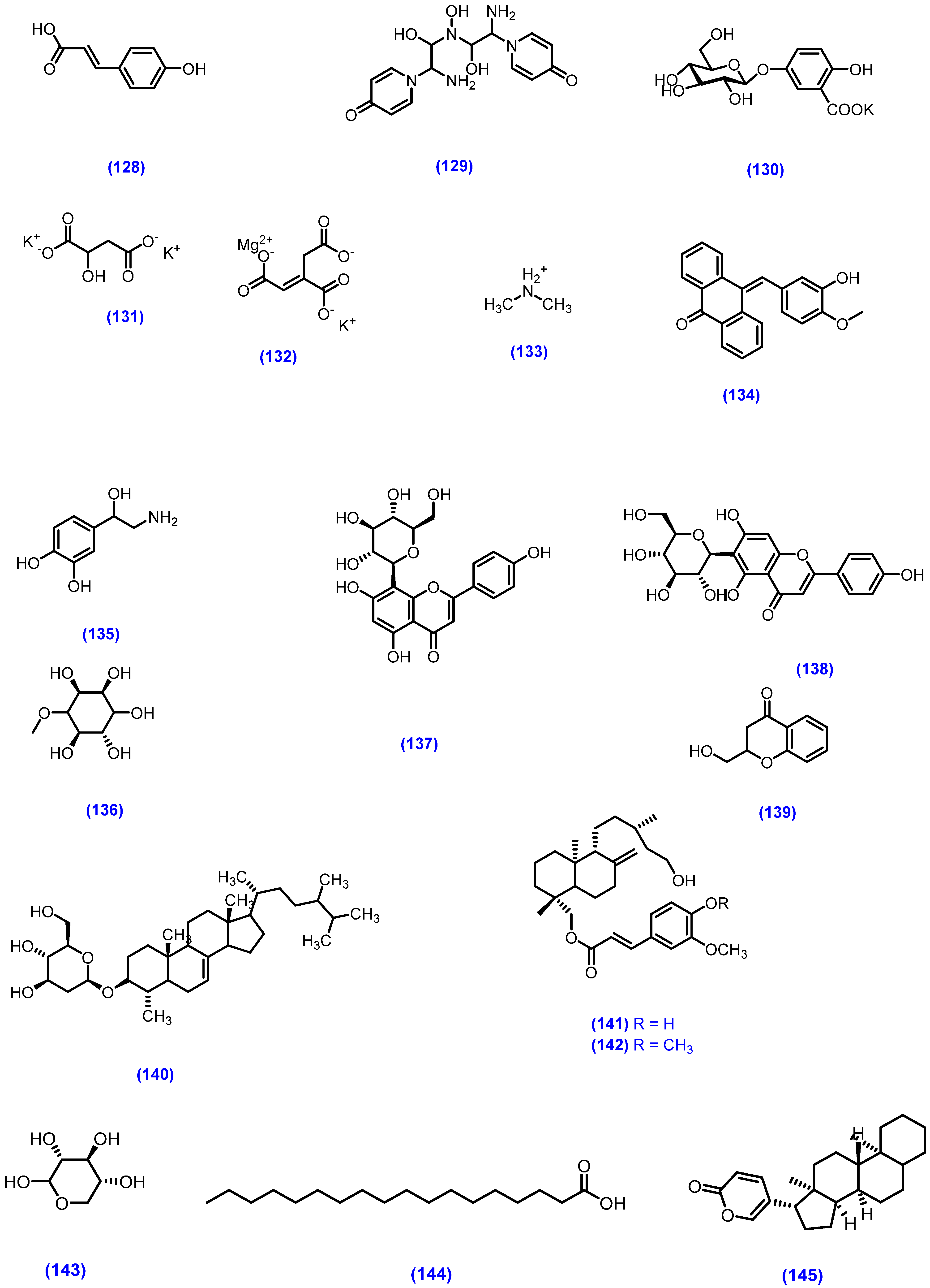
| EtOH | Whole plant | Flavonoids | 5,7,3′,4′-tetrahydroxy-6-C-[β-D-apiose-(1→4)]-β-D-glucopyranosyl flavones (112), 7,8,3′,4′-tetrahydroxy-6-C-[α-L-rhamnopyranosyl-(1→2)]-β-D-glucopyranosyl flavone (113), 5,7,4′-trihydroxyl-8-C-β-D-glucopyranosyl flavones (114), mimosinamine (115), mimosinic acid (116), Tyrosin (117) | [128] | |||
| EtOH | Whole plant | Flavonoids | 5,7,3′,4′-tetrahydroxy-6-C-[β-D-apiose-(1→4)]-β-D-glucopyranosyl flavones (112), 6,7,3′,4′-tetrahydroxy-8-C-[α-L-rhamnopyranosyl-(1→2)]-β-D-glucopyranosyl flavone (118) | [129] | |||
| Arial parts | Flavonoids | isoquercitrin (119), avicularin (120), apigenin-7-O-D-glucoside (121), cassiaoccidentalin B (122), orientin (123), isoorientin (124) | [130] | ||||
| MeOH | Leaves | chlorophyllin (125) | Antimicrobial activity/P. aeruginosa, E. coli, S. aureus, K. pneomoniae, C. albicans/well diffusion method | Zone of inhibition at 25 μg/mL conc. P. aeruginosa = 12 mm, E. coli = 8 mm, S. aureus = 14 mm, K. pneomoniae = 13 mm, C. albican = 9 mm. At 100 μg/mL conc. P. aeruginosa = 18 mm, E. coli = 13 mm, S. aureus = 19 mm, K. pneomoniae = 18 mm, C. albicans = 13 mm. The streptomycin sulphate and nystatin (standred) at 10 μg/mL showed maximum inhibition 18 mm–19 mm. Good activity | [131] | ||
| EtOAc-benzene (1:9) | Leaves | Phenolic ketone | 4-(24′-methoxy-24′-methyl-1′-oxo-5′-n-propyl-tetracosanyl)- phenol (126) | [132] | |||
| EtOH | Leaves | Flavonoids | 7,3′,4′-triacetoxy-3,8-dimethoxyflavone (127), p-coumaric acid (128), 7,3′,4′-trihydroxy-3,8-dimethoxyflavone (77) | [133] | |||
| MeOH | Leaves | mimopudine (129) | Responsible for leaves opening | [134] | |||
| MeOH | Leaves | potassium 5-O-β-D-glucupyranosylgentisate (130) | Responsible for leaves closing | [135] | |||
| MeOH | Leaves | mimopudine (129), potassium 5-O-β-D-glucupyranosylgentisate (130), potassium L-malate (131), magnesium potassium trans-aconitate (132), dimethyl ammoniumsalt (133) | Responsible for rapid sensitive actions, such as heat and touch. Periodic slow actions, such as nyctinastic actions | [136] | |||
| Fresh leaves | tubulin (134) | [137] | |||||
| EtOH | Leaves | nor-epinephrine (135), d-pinitol (136), β-sitosterol (25) | [138] | ||||
| EtOH | Leaves | 5,7,3′,4′-tetrahydroxy-6-C-[β-D-apiose-(1→4)]-β-D-glycopyranosyl flavone (112), orientin (123), isorientin (124), vitexin (137), isovitexin (138) | [139] | ||||
| Roots | Chroman | 2-hydroxymethyl-chroman-4-one (139) | Antifungal activity/dilution agar plate method/P. ultimum, P. capsici, R. solani, B. cinerea, A. panax and S. sclerotiorum | Compound (139) showed good ED50 value against P. capsici = 35.7 μg/mL, S. sclerotiorum = 52.1 μg/mL, P. ultimum = 54.9 μg/mL. Overall good activity | [140] | ||
| Roots | Sterolglucoside | stigmasterol (24), β-sitosterol (25), betulinic acid (79), 4-a,24-dimethylcholest-7-en-3β-ol-3β-D-glucoside (140) | [141] | ||||
| MeOH | Roots | Diterpenoids | 19-O-trans-feruloyl-labd-8(17)-en-15,19-diol (141), 19-O-[(E)-3′,4′-dimethoxy cinnamoyl]-labd-8(17)-en- 15,19-diol (142) | [142] | |||
| Seeds | Fatty acids | D-xylose (143), D-glucuronic acid 4-O-(3,5-dihydroxybenzoic acid)-b-D-glucuronide (144) | [143] | ||||
| Seeds | Cardiac glycosides | bufadienolide (145) | [144] | ||||
| Stem | Amino acids | mimosine (108) | [145] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizwan, K.; Majeed, I.; Bilal, M.; Rasheed, T.; Shakeel, A.; Iqbal, S. Phytochemistry and Diverse Pharmacology of Genus Mimosa: A Review. Biomolecules 2022, 12, 83. https://doi.org/10.3390/biom12010083
Rizwan K, Majeed I, Bilal M, Rasheed T, Shakeel A, Iqbal S. Phytochemistry and Diverse Pharmacology of Genus Mimosa: A Review. Biomolecules. 2022; 12(1):83. https://doi.org/10.3390/biom12010083
Chicago/Turabian StyleRizwan, Komal, Ismat Majeed, Muhammad Bilal, Tahir Rasheed, Ahmad Shakeel, and Shahid Iqbal. 2022. "Phytochemistry and Diverse Pharmacology of Genus Mimosa: A Review" Biomolecules 12, no. 1: 83. https://doi.org/10.3390/biom12010083
APA StyleRizwan, K., Majeed, I., Bilal, M., Rasheed, T., Shakeel, A., & Iqbal, S. (2022). Phytochemistry and Diverse Pharmacology of Genus Mimosa: A Review. Biomolecules, 12(1), 83. https://doi.org/10.3390/biom12010083








