Pulmonary Thrombosis Promotes Tumorigenesis via Myeloid Hypoxia-Inducible Factors
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Cell Culture
2.3. Mouse Model of Thrombosis-Associated Tumorigenesis
2.4. Histology and Immunostaining
2.5. Image Capture and Analysis
2.6. Protein Arrays
2.7. Migration and Proliferation Assays
2.8. Statistical Analysis
3. Results
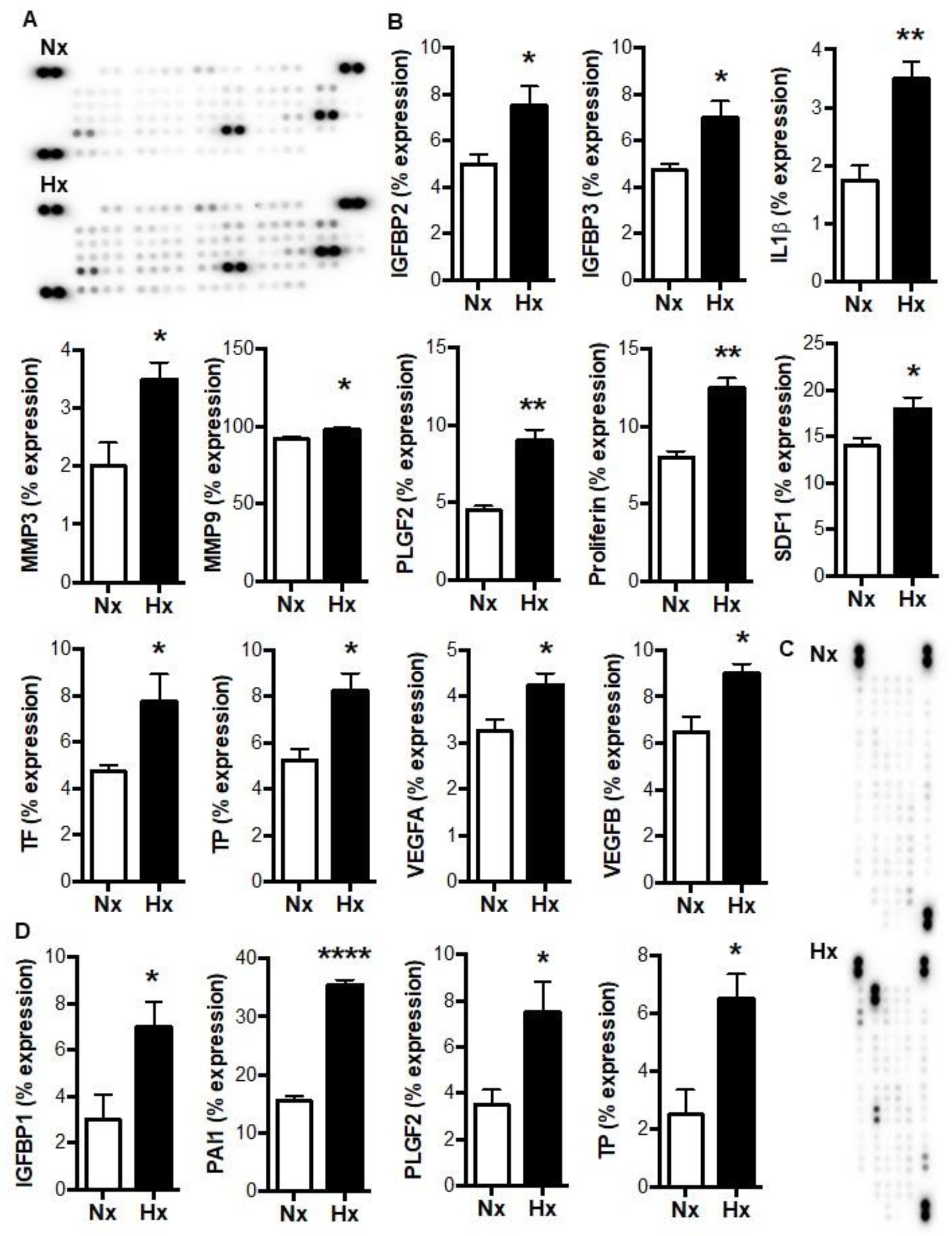
3.1. Hypoxia Increases the Release of Tumorigenic Factors from Myeloid Cells in a HIF-Dependent Manner
3.2. Paracrine Regulation of Lung Cancer Cell Migration and Proliferation by Myeloid HIFs
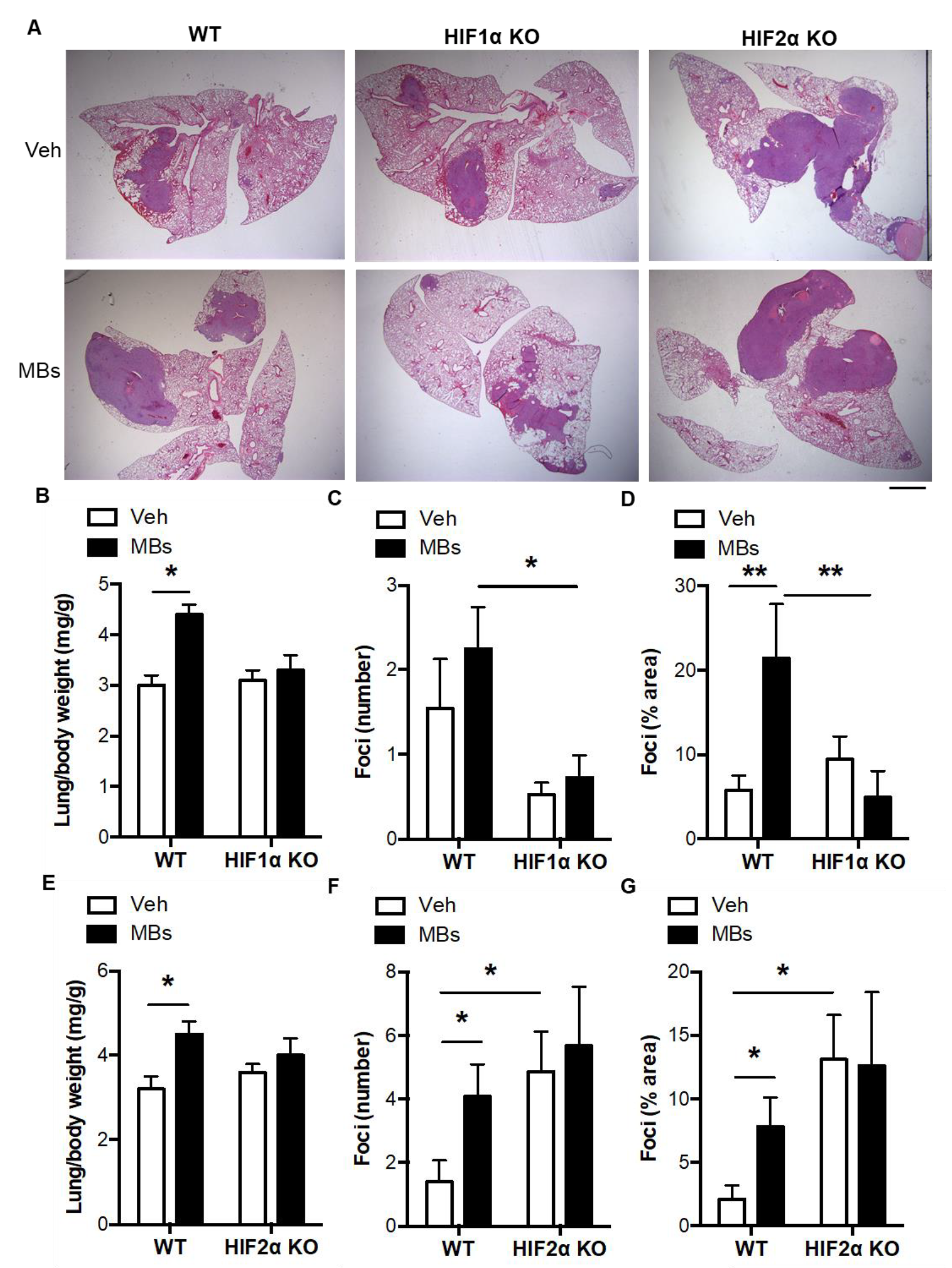
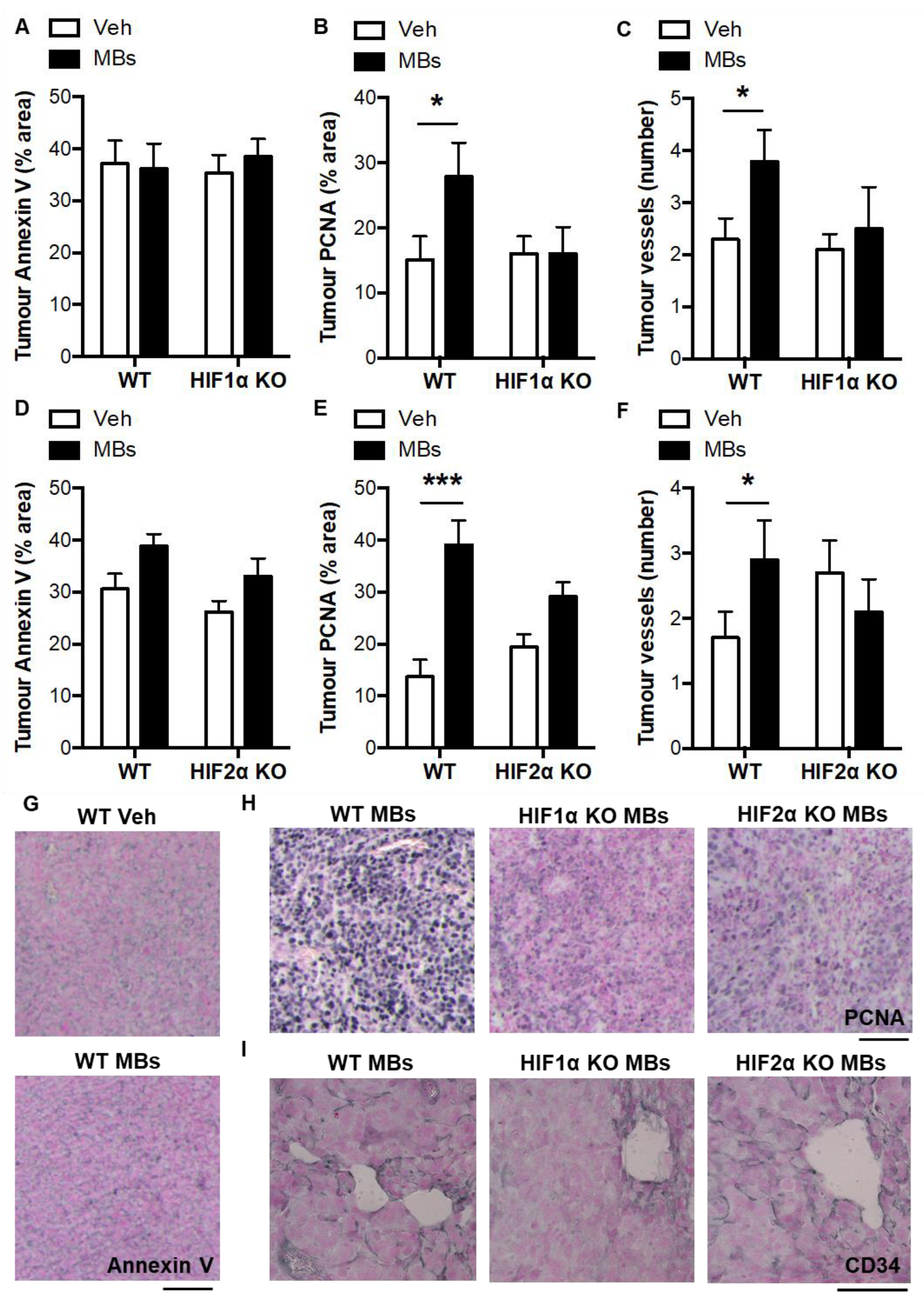
3.3. Pulmonary Microvascular Occlusion Increases Pulmonary Tumorigenesis via Myeloid HIFs
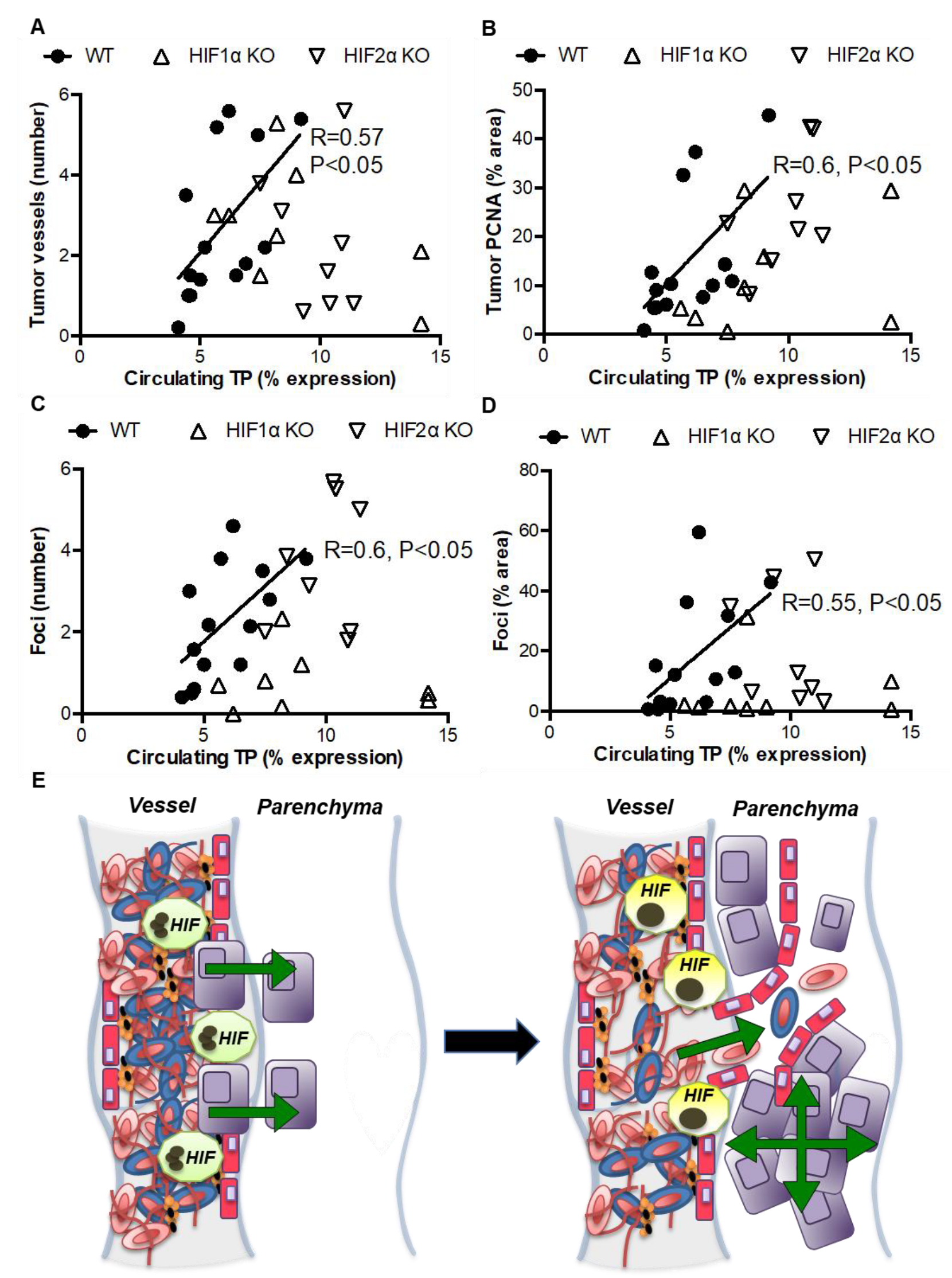
3.4. Indirect Evidence for Paracrine Regulation of Thrombosis-Induced Tumorigenesis by Myeloid HIF-Induced TP
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Douketis, J.D.; Gu, C.; Piccioli, A.; Ghirarduzzi, A.; Pengo, V.; Prandoni, P. The Long-Term Risk of Cancer in Patients with a First Episode of Venous Thromboembolism. J. Thromb. Haemost. 2009, 7, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, H.T.; Svaerke, C.; Farkas, D.K.; Christiansen, C.F.; Pedersen, L.; Lash, T.L.; Prandoni, P.; Baron, J.A. Superficial and Deep Venous Thrombosis, Pulmonary Embolism and Subsequent Risk of Cancer. Eur. J. Cancer 2012, 48, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Blom, J.W.; Doggen, C.J.; Osanto, S.; Rosendaal, F.R. Malignancies, Prothrombotic Mutations, and the Risk of Venous Thrombosis. JAMA J. Am. Med. Assoc. 2005, 293, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Belting, M. Glycosaminoglycans in Cancer Treatment. Thromb. Res. 2014, 133 (Suppl. S2), S95–S101. [Google Scholar] [CrossRef]
- Langer, F.; Amirkhosravi, A.; Ingersoll, S.B.; Walker, J.M.; Spath, B.; Eifrig, B.C.; Francis, J.L. Experimental Metastasis and Primary Tumor Growth in Mice with Hemophilia A. J. Thromb. Haemost. 2006, 4, 1056–1062. [Google Scholar] [CrossRef]
- Varki, A. Trousseau’s Syndrome: Multiple Definitions and Multiple Mechanisms. Blood J. Am. Soc. Hematol. 2007, 110, 1723–1729. [Google Scholar] [CrossRef]
- Wenzel, J.; Zeisig, R.; Haider, W.; Habedank, S.; Fichtner, I. Inhibition of Pulmonary Metastasis in a Human Mt3 Breast Cancer Xenograft Model by Dual Liposomes Preventing Intravasal Fibrin Clot Formation. Breast Cancer Res. Treat. 2010, 121, 13–22. [Google Scholar] [CrossRef][Green Version]
- Semenza, G.L. Targeting Hif-1 for Cancer Therapy. Nat. Rev. Cancer 2003, 3, 721–732. [Google Scholar] [CrossRef]
- Evans, C.E.; Humphries, J.; Mattock, K.; Waltham, M.; Wadoodi, A.; Saha, P.; Modarai, B.; Maxwell, P.J.; Smith, A. Hypoxia and Upregulation of Hypoxia-Inducible Factor 1{Alpha} Stimulate Venous Thrombus Recanalization. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2443–2451. [Google Scholar] [CrossRef]
- Semenza, G.L. Oxygen Sensing, Homeostasis, and Disease. N. Engl. J. Med. 2011, 365, 537–547. [Google Scholar] [CrossRef]
- Evans, C.E.; Branco-Price, C.; Johnson, R.S. Hif-Mediated Endothelial Response During Cancer Progression. Int. J. Hematol. 2012, 95, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Palazon, A.; Goldrath, A.W.; Nizet, V.; Johnson, R.S. Hif Transcription Factors, Inflammation, and Immunity. Immunity 2014, 41, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.E.; Wadoodi, A.; Humphries, J.; Lu, X.; Grover, S.P.; Saha, P.; Smith, A. Local Accumulation of Hypoxia-Inducible Factor 2 Alpha During Venous Thrombus Resolution. Thromb. Res. 2014, 134, 757–760. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.E.; Palazon, A.; Sim, J.; Tyrakis, P.A.; Prodger, A.; Lu, X.; Chan, S.; Bendahl, P.-O.; Belting, M.; Von Euler, L.; et al. Modelling Pulmonary Microthrombosis Coupled to Metastasis: Distinct Effects of Thrombogenesis on Tumorigenesis. Biol. Open 2017, 6, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.E.; Humphries, J.; Mattock, K.; Saha, P.; Smith, A. Hif1 Signalling Regulates Venous Thrombus Resolution. Thromb. Res. 2012, 130, 971–973. [Google Scholar] [CrossRef]
- Gupta, N.; Zhao, Y.Y.; Evans, C.E. The Stimulation of Thrombosis by Hypoxia. Thromb. Res. 2019, 181, 77–83. [Google Scholar] [CrossRef]
- Gupta, N.; Sahu, A.; Prabhakar, A.; Chatterjee, T.; Tyagi, T.; Kumari, B.; Khan, N.; Nair, V.; Bajaj, N.; Sharma, M. Activation of Nlrp3 Inflammasome Complex Potentiates Venous Thrombosis in Response to Hypoxia. Proc. Natl. Acad. Sci. USA 2017, 114, 4763–4768. [Google Scholar] [CrossRef]
- Evans, C.E.; Humphries, J.; Waltham, M.; Saha, P.; Mattock, K.; Patel, A.; Ahmad, A.; Wadoodi, A.; Modarai, B.; Burnand, K.; et al. Upregulation of Hypoxia-Inducible Factor 1 Alpha in Local Vein Wall Is Associated with Enhanced Venous Thrombus Resolution. Thromb Res. 2011, 128, 346–351. [Google Scholar] [CrossRef]
- Rohwer, N.; Jumpertz, S.; Erdem, M.; Egners, A.; Warzecha, K.T.; Fragoulis, A.; Kühl, A.A.; Kramann, R.; Neuss, S.; Rudolph, I.; et al. Non-Canonical Hif-1 Stabilization Contributes to Intestinal Tumorigenesis. Oncogene 2019, 38, 5670–5685. [Google Scholar] [CrossRef]
- Cramer, T.; Yamanishi, Y.; Clausen, B.E.; Forster, I.; Pawlinski, R.; Mackman, N.; Haase, V.H.; Jaenisch, R.; Corr, M.; Nizet, V.; et al. Hif-1alpha Is Essential for Myeloid Cell-Mediated Inflammation. Cell 2003, 112, 645–657. [Google Scholar] [CrossRef]
- Doedens, A.L.; Stockmann, C.; Rubinstein, M.P.; Liao, D.; Zhang, N.; DeNardo, D.G.; Coussens, L.M.; Karin, M.; Goldrath, A.W.; Johnson, R.S. Macrophage Expression of Hypoxia-Inducible Factor-1 Alpha Suppresses T-Cell Function and Promotes Tumor Progression. Cancer Res. 2010, 70, 7465–7475. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.A.; Elks, P.M.; Marriott, H.M.; Eamsamarng, S.; Higgins, K.R.; Lewis, A.; Williams, L.; Parmar, S.; Shaw, G.; McGrath, E.E.; et al. Hypoxia-Inducible Factor 2alpha Regulates Key Neutrophil Functions in Humans, Mice, and Zebrafish. Blood J. Am. Soc. Hematol. 2014, 123, 366–376. [Google Scholar] [CrossRef]
- Branco-Price, C.; Zhang, N.; Schnelle, M.; Evans, C.; Katschinski, D.M.; Liao, D.; Ellies, L.; Johnson, R.S. Endothelial Cell Hif-1alpha and Hif-2alpha Differentially Regulate Metastatic Success. Cancer Cell 2012, 21, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.E.; Grover, S.P.; Saha, P.; Humphries, J.; Kim, J.W.; Modarai, B.; Smith, A. Suppression of Angiogenic Response in Local Vein Wall Is Associated with Reduced Thrombus Resolution. Thromb. Res. 2014, 134, 682–685. [Google Scholar] [CrossRef]
- Evans, C.E.; Grover, S.P.; Humphries, J.; Saha, P.; Patel, A.P.; Patel, A.S.; Lyons, O.T.; Waltham, M.; Modarai, B.; Smith, A. Antiangiogenic Therapy Inhibits Venous Thrombus Resolution. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 565–570. [Google Scholar] [CrossRef]
- Saha, P.; Andia, M.E.; Modarai, B.; Blume, U.; Humphries, J.; Patel, A.S.; Phinikaridou, A.; Evans, C.E.; Mattock, K.; Grover, S.P. Magnetic Resonance T1 Relaxation Time of Venous Thrombus Is Determined by Iron Processing and Predicts Susceptibility to Lysis. Circulation 2013, 128, 729–736. [Google Scholar] [CrossRef]
- Dhakal, H.P.; Nesland, J.M.; Forsund, M.; Trope, C.G.; Holm, R. Primary Tumor Vascularity, Hif-1alpha and Vegf Expression in Vulvar Squamous Cell Carcinomas: Their Relationships with Clinicopathological Characteristics and Prognostic Impact. BMC Cancer 2013, 13, 506. [Google Scholar] [CrossRef]
- Nagaoka, H.; Iino, Y.; Takei, H.; Morishita, Y. Platelet-Derived Endothelial Cell Growth Factor/Thymidine Phosphorylase Expression in Macrophages Correlates with Tumor Angiogenesis and Prognosis in Invasive Breast Cancer. Int. J. Oncol. 1998, 13, 449–454. [Google Scholar] [CrossRef]
- Nijaguna, M.B.; Patil, V.; Urbach, S.; Shwetha, S.D.; Sravani, K.; Hegde, A.S.; Chandramouli, B.A.; Arivazhagan, A.A.; Marin, P.; Santosh, V.; et al. Glioblastoma-Derived Macrophage Colony-Stimulating Factor (Mcsf) Induces Microglial Release of Insulin-Like Growth Factor-Binding Protein 1 (Igfbp1) to Promote Angiogenesis. J. Biol. Chem. 2015, 290, 23401–23415. [Google Scholar] [CrossRef]
- Giatromanolaki, A.; Sivridis, E.; Maltezos, E.; Athanassou, N.; Papazoglou, D.; Gatter, K.C.; Harris, A.L.; Koukourakis, M. Upregulated Hypoxia Inducible Factor-1alpha and -2alpha Pathway in Rheumatoid Arthritis and Osteoarthritis. Arthritis Res. Ther. 2003, 5, R193–R201. [Google Scholar] [CrossRef]
- Tannahill, G.M.; Curtis, A.M.; Adamik, J.; Palsson-McDermott, E.M.; McGettrick, A.F.; Goel, G.; Frezza, C.; Bernard, N.J.; Kelly, B.; Foley, N.H.; et al. Succinate Is an Inflammatory Signal That Induces Il-1beta through Hif-1alpha. Nature 2013, 496, 238–242. [Google Scholar] [CrossRef]
- Palumbo, J.S.; Talmage, K.E.; Massari, J.V.; La Jeunesse, C.M.; Flick, M.J.; Kombrinck, K.W.; Hu, Z.; Barney, K.A.; Degen, J.L. Tumor Cell-Associated Tissue Factor and Circulating Hemostatic Factors Cooperate to Increase Metastatic Potential through Natural Killer Cell-Dependent and-Independent Mechanisms. Blood J. Am. Soc. Hematol. 2007, 110, 133–141. [Google Scholar] [CrossRef]
- Palumbo, J.S.; Talmage, K.E.; Massari, J.V.; La Jeunesse, C.M.; Flick, M.J.; Kombrinck, K.W.; Jirousková, M.; Degen, J.L. Platelets and Fibrin(Ogen) Increase Metastatic Potential by Impeding Natural Killer Cell-Mediated Elimination of Tumor Cells. Blood J. Am. Soc. Hematol. 2005, 105, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Takeda, N.; O’Dea, E.L.; Doedens, A.; Kim, J.W.; Weidemann, A.; Stockmann, C.; Asagiri, M.; Simon, M.C.; Hoffmann, A.; Johnson, R.S. Differential Activation and Antagonistic Function of Hif-{Alpha} Isoforms in Macrophages Are Essential for No Homeostasis. Genes Dev. 2010, 24, 491–501. [Google Scholar] [CrossRef]
- Fujimoto, J.; Sakaguchi, H.; Hirose, R.; Ichigo, S.; Tamaya, T. Expression of Platelet-Derived Endothelial Cell Growth Factor (Pd-Ecgf) and Its Mrna in Uterine Cervical Cancers. Br. J. Cancer 1999, 79, 1249–1254. [Google Scholar] [CrossRef][Green Version]
- Usuki, K.; Norberg, L.; Larsson, E.; Miyazono, K.; Hellman, U.; Wernstedt, C.; Rubin, K.; Heldin, C.H. Localization of Platelet-Derived Endothelial Cell Growth Factor in Human Placenta and Purification of an Alternatively Processed Form. Cell Regul. 1990, 1, 577–584. [Google Scholar] [CrossRef]
- Stewart, M.; Talks, K.; Leek, R.; Turley, H.; Pezzella, F.; Harris, A.; Gatter, K. Expression of Angiogenic Factors and Hypoxia Inducible Factors Hif 1, Hif 2 and Ca Ix in Non-Hodgkin’s Lymphoma. Histopathology 2002, 40, 253–260. [Google Scholar] [CrossRef]
- Perez-Perez, M.J.; Priego, E.M.; Hernandez, A.I.; Camarasa, M.J.; Balzarini, J.; Liekens, S. Thymidine Phosphorylase Inhibitors: Recent Developments and Potential Therapeutic Applications. Mini Rev. Med. Chem. 2005, 5, 1113–1123. [Google Scholar] [CrossRef]
- Jin-no, K.; Tanimizu, M.; Hyodo, I.; Nishikawa, Y.; Hosokawa, Y.; Endo, H.; Doi, T.; Mandai, K.; Ishitsuka, H. Circulating Platelet-Derived Endothelial Cell Growth Factor Increases in Hepatocellular Carcinoma Patients. Cancer 1998, 82, 1260–1267. [Google Scholar] [CrossRef]
- Kabuubi, P.; Loncaster, J.A.; Davidson, S.E.; Hunter, R.D.; Kobylecki, C.; Stratford, I.J.; West, C.M. No Relationship between Thymidine Phosphorylase (Tp, Pd-Ecgf) Expression and Hypoxia in Carcinoma of the Cervix. Br. J. Cancer 2006, 94, 115–120. [Google Scholar] [CrossRef][Green Version]
- Ioachim, E.; Michael, M.; Salmas, M.; Michael, M.M.; Stavropoulos, N.E.; Malamou-Mitsi, V. Hypoxia-Inducible Factors Hif-1alpha and Hif-2alpha Expression in Bladder Cancer and Their Associations with Other Angiogenesis-Related Proteins. Urol Int. 2006, 77, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Leek, R.D.; Talks, K.L.; Pezzella, F.; Turley, H.; Campo, L.; Brown, N.S.; Bicknell, R.; Taylor, M.; Gatter, K.C.; Harris, A.L. Relation of Hypoxia-Inducible Factor-2 Alpha (Hif-2 Alpha) Expression in Tumor-Infiltrative Macrophages to Tumor Angiogenesis and the Oxidative Thymidine Phosphorylase Pathway in Human Breast Cancer. Cancer Res. 2002, 62, 1326–1329. [Google Scholar] [PubMed]
- Sivridis, E.; Giatromanolaki, A.; Gatter, K.C.; Harris, A.L.; Koukourakis, M.I.; Tumor and Angiogenesis Research Group. Association of Hypoxia-Inducible Factors 1alpha and 2alpha with Activated Angiogenic Pathways and Prognosis in Patients with Endometrial Carcinoma. Cancer 2002, 95, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, S.; Nitanda, T.; Furukawa, T.; Sumizawa, T.; Tani, A.; Nishimoto, K.; Akiba, S.; Miyadera, K.; Fukushima, M.; Yamada, Y.; et al. The Effect of a Thymidine Phosphorylase Inhibitor on Angiogenesis and Apoptosis in Tumors. Cancer Res. 1999, 59, 1911–1916. [Google Scholar]
- Rofstad, E.K.; Halsor, E.F. Vascular Endothelial Growth Factor, Interleukin 8, Platelet-Derived Endothelial Cell Growth Factor, and Basic Fibroblast Growth Factor Promote Angiogenesis and Metastasis in Human Melanoma Xenografts. Cancer Res. 2000, 60, 4932–4938. [Google Scholar]
- Bronckaers, A.; Gago, F.; Balzarini, J.; Liekens, S. The Dual Role of Thymidine Phosphorylase in Cancer Development and Chemotherapy. Med. Res. Rev. 2009, 29, 903–953. [Google Scholar] [CrossRef]
- Takao, S.; Akiyama, S.I.; Nakajo, A.; Yoh, H.; Kitazono, M.; Natsugoe, S.; Miyadera, K.; Fukushima, M.; Yamada, Y.; Aikou, T. Suppression of Metastasis by Thymidine Phosphorylase Inhibitor. Cancer Res. 2000, 60, 5345–5348. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, X.; Prodger, A.; Sim, J.; Evans, C.E. Pulmonary Thrombosis Promotes Tumorigenesis via Myeloid Hypoxia-Inducible Factors. Biomolecules 2022, 12, 1354. https://doi.org/10.3390/biom12101354
Lu X, Prodger A, Sim J, Evans CE. Pulmonary Thrombosis Promotes Tumorigenesis via Myeloid Hypoxia-Inducible Factors. Biomolecules. 2022; 12(10):1354. https://doi.org/10.3390/biom12101354
Chicago/Turabian StyleLu, Xiao, Alice Prodger, Jingwei Sim, and Colin E. Evans. 2022. "Pulmonary Thrombosis Promotes Tumorigenesis via Myeloid Hypoxia-Inducible Factors" Biomolecules 12, no. 10: 1354. https://doi.org/10.3390/biom12101354
APA StyleLu, X., Prodger, A., Sim, J., & Evans, C. E. (2022). Pulmonary Thrombosis Promotes Tumorigenesis via Myeloid Hypoxia-Inducible Factors. Biomolecules, 12(10), 1354. https://doi.org/10.3390/biom12101354





