Localized Perivascular Therapeutic Approaches to Inhibit Venous Neointimal Hyperplasia in Arteriovenous Fistula Access for Hemodialysis Use
Abstract
1. Arteriovenous Fistula Access for Hemodialysis Use
2. Pathophysiology of Venous Neointimal Hyperplasia
2.1. AVF Creation and the Physiology of Maturation
2.2. Pathways That Lead to Venous Neointimal Hyperplasia
3. Current Clinical Strategies to Prevent AVF Failure
4. Localized Perivascular Therapeutic Approaches
4.1. Mechanical Devices
4.2. Targeted Drug Delivery
4.2.1. Sirolimus
4.2.2. Paclitaxel
4.2.3. Simvastatin
4.2.4. 1α,25-Dihydroxyvitamin D3
4.2.5. Vonapanitase
4.2.6. β-Aminopropionitrile
4.2.7. Bevacizumab
4.2.8. 4-Amino-1,8-naphthalimide
4.3. Perivascular Cell-Based Therapeutics
4.3.1. Endothelial Cells
4.3.2. Mesenchymal Stem/Stromal Cells
5. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qarni, B.; Osman, M.A.; Levin, A.; Feehally, J.; Harris, D.; Jindal, K.; Olanrewaju, T.O.; Samimi, A.; Olah, M.E.; Braam, B.; et al. Kidney Care in Low- and Middle-Income Countries. Clin. Nephrol. 2020, 93, S21–S30. [Google Scholar] [CrossRef]
- Lee, T.; Misra, S. New Insights into Dialysis Vascular Access: Molecular Targets in Arteriovenous Fistula and Arteriovenous Graft Failure and Their Potential to Improve Vascular Access Outcomes. Clin. J. Am. Soc. Nephrol. 2016, 11, 1504–1512. [Google Scholar] [CrossRef]
- Lok, C.E.; Huber, T.S.; Lee, T.; Shenoy, S.; Yevzlin, A.S.; Abreo, K.; Allon, M.; Asif, A.; Astor, B.C.; Glickman, M.H.; et al. KDOQI Clinical Practice Guideline for Vascular Access: 2019 Update. Am. J. Kidney Dis. 2020, 75, S1–S164. [Google Scholar] [CrossRef]
- Santoro, D.; Benedetto, F.; Mondello, P.; Pipitò, N.; Barillà, D.; Spinelli, F.; Ricciardi, C.A.; Cernaro, V.; Buemi, M. Vascular Access for Hemodialysis: Current Perspectives. Int. J. Nephrol. Renovasc. Dis. 2014, 7, 281–294. [Google Scholar] [CrossRef]
- Vachharajani, T.J.; Taliercio, J.J.; Anvari, E. New Devices and Technologies for Hemodialysis Vascular Access: A Review. Am. J. Kidney Dis. 2021, 78, 116–124. [Google Scholar] [CrossRef]
- Woodside, K.J.; Repeck, K.J.; Mukhopadhyay, P.; Schaubel, D.E.; Shahinian, V.B.; Saran, R.; Pisoni, R.L. Arteriovenous Vascular Access-Related Procedural Burden among Incident Hemodialysis Patients in the United States. Am. J. Kidney Dis. 2021, 78, 369–379. [Google Scholar] [CrossRef]
- Al-Balas, A.; Lee, T.; Young, C.J.; Kepes, J.A.; Barker-Finkel, J.; Allon, M. The Clinical and Economic Effect of Vascular Access Selection in Patients Initiating Hemodialysis with a Catheter. J. Am. Soc. Nephrol. 2017, 28, 3679–3687. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, T.; Kim, S.J.; Astor, B.; Shafi, T.; Coresh, J.; Powe, N.R. Vascular Access Type, Inflammatory Markers, and Mortality in Incident Hemodialysis Patients: The Choices for Healthy Outcomes in Caring for End-Stage Renal Disease (CHOICE) Study. Am. J. Kidney Dis. 2014, 64, 954–961. [Google Scholar] [CrossRef]
- Nguyen, M.; Thankam, F.G.; Agrawal, D.K. Sterile Inflammation in the Pathogenesis of Maturation Failure of Arteriovenous Fistula. J. Mol. Med. 2021 99:6 2021, 99, 729–741. [Google Scholar] [CrossRef]
- Siddiqui, M.A.; Ashraff, S.; Santos, D.; Rush, R.; Carline, T.; Raza, Z. Predictive Parameters of Arteriovenous Fistula Maturation in Patients with End-Stage Renal Disease. Kidney Res. Clin. Pract. 2018, 37, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Nordyke, R.J.; Reichert, H.; Bylsma, L.C.; Jackson, J.J.; Gage, S.M.; Fryzek, J.; Roy-Chaudhury, P.; Lithgow, T. Costs Attributable to Arteriovenous Fistula and Arteriovenous Graft Placements in Hemodialysis Patients with Medicare Coverage. Am. J. Nephrol. 2019, 50, 320–328. [Google Scholar] [CrossRef]
- Venkat Ramanan, S.; Prabhu, R.A.; Rao, I.R.; Chawla, A.; Shenoy, S.V.; Nagaraju, S.P.; Bhojaraja, M.V. Outcomes and Predictors of Failure of Arteriovenous Fistulae for Hemodialysis. Int. Urol. Nephrol. 2022, 54, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Bartolomeo, K.; Vachharajani, T.J.; Shingarev, R. The Sisyphean Task of Getting the Arteriovenous Fistula to Mature. Kidney360 2021, 2, 1873. [Google Scholar] [CrossRef] [PubMed]
- Robbin, M.L.; Greene, T.; Allon, M.; Dember, L.M.; Imrey, P.B.; Cheung, A.K.; Himmelfarb, J.; Huber, T.S.; Kaufman, J.S.; Radeva, M.K.; et al. Prediction of Arteriovenous Fistula Clinical Maturation from Postoperative Ultrasound Measurements: Findings from the Hemodialysis Fistula Maturation Study. J. Am. Soc. Nephrol. 2018, 29, 2735–2744. [Google Scholar] [CrossRef]
- Bashar, K.; Conlon, P.J.; Kheirelseid, E.A.H.; Aherne, T.; Walsh, S.R.; Leahy, A. Arteriovenous Fistula in Dialysis Patients: Factors Implicated in Early and Late AVF Maturation Failure. Surgeon 2016, 14, 294–300. [Google Scholar] [CrossRef]
- Remuzzi, A.; Bozzetto, M. Biological and Physical Factors Involved in the Maturation of Arteriovenous Fistula for Hemodialysis. Cardiovasc. Eng. Technol. 2017, 8, 273–279. [Google Scholar] [CrossRef]
- Lu, D.Y.; Chen, E.Y.; Wong, D.J.; Yamamoto, K.; Protack, C.D.; Williams, W.T.; Assi, R.; Hall, M.R.; Sadaghianloo, N.; Dardik, A. Vein Graft Adaptation and Fistula Maturation in the Arterial Environment. J. Surg. Res. 2014, 188, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Hou, G.; Hou, Y.; Sun, X.; Yin, N.; Feng, G.; Yan, Y.; Guangyi, L. Venous Distensibility Is More Important than Venous Diameter in Primary Survival of Autogenous Radiocephalic Arteriovenous Fistulas. J. Vasc. Access 2020, 21, 963–968. [Google Scholar] [CrossRef]
- Caplice, N.M.; Wang, S.; Tracz, M.; Croatt, A.J.; Grande, J.P.; Katusic, Z.S.; Nath, K.A. Neoangiogenesis and the Presence of Progenitor Cells in the Venous Limb of an Arteriovenous Fistula in the Rat. Am. J. Physiol. Ren. Physiol. 2007, 293, 470–475. [Google Scholar] [CrossRef]
- Bai, H.; Wei, S.; Xie, B.; Wang, Z.; Li, M.; Qiao, Z.; Sun, P.; Wang, W. Endothelial Nitric Oxide Synthase (ENOS) Mediates Neointimal Thickness in Arteriovenous Fistulae with Different Anastomotic Angles in Rats. J. Vasc. Access. 2022, 23, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Pike, D.; Shiu, Y.T.; Cho, Y.F.; Le, H.; Somarathna, M.; Isayeva, T.; Guo, L.; Symons, J.D.; Kevil, C.G.; Totenhagen, J.; et al. The Effect of Endothelial Nitric Oxide Synthase on the Hemodynamics and Wall Mechanics in Murine Arteriovenous Fistulas. Sci. Rep. 2019, 9, 4299. [Google Scholar] [CrossRef]
- Somarathna, M.; Hwang, P.T.; Millican, R.C.; Alexander, G.C.; Isayeva-Waldrop, T.; Sherwood, J.A.; Brott, B.C.; Falzon, I.; Northrup, H.; Shiu, Y.T.; et al. Nitric Oxide Releasing Nanomatrix Gel Treatment Inhibits Venous Intimal Hyperplasia and Improves Vascular Remodeling in a Rodent Arteriovenous Fistula. Biomaterials 2022, 280, 121254. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Khalil, R.A. Matrix Metalloproteinases, Vascular Remodeling, and Vascular Disease. Adv. Pharmacol. 2018, 81, 241–330. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.S.; Shen, Q.; Pitts, R.L.; Guo, M.; Wu, M.H.; Sun, S.C.; Yuan, S.Y. Serum Metalloproteinases MMP-2, MMP-9, and Metalloproteinase Tissue Inhibitors in Patients Are Associated with Arteriovenous Fistula Maturation. J. Vasc. Surg. 2011, 54, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.C.; Chen, P.Y.; Ko, T.M.; Huang, P.H.; Ma, H.; Tarng, D.C. Mmp-9 Deletion Attenuates Arteriovenous Fistula Neointima through Reduced Perioperative Vascular Inflammation. Int. J. Mol. Sci. 2021, 22, 5448. [Google Scholar] [CrossRef] [PubMed]
- Sadaghianloo, N.; Contenti, J.; Dardik, A.; Mazure, N.M. Role of Hypoxia and Metabolism in the Development of Neointimal Hyperplasia in Arteriovenous Fistulas. Int. J. Mol. Sci. 2019, 20, 5387. [Google Scholar] [CrossRef] [PubMed]
- Allon, M.; Litovsky, S.; Young, C.J.; Deierhoi, M.H.; Goodman, J.; Hanaway, M.; Lockhart, M.E.; Robbin, M.L. Medial Fibrosis, Vascular Calcification, Intimal Hyperplasia, and Arteriovenous Fistula Maturation. Am. J. Kidney Dis. 2011, 58, 437–443. [Google Scholar] [CrossRef]
- Castier, Y.; Brandes, R.P.; Leseche, G.; Tedgui, A.; Lehoux, S. P47phox-Dependent NADPH Oxidase Regulates Flow-Induced Vascular Remodeling. Circ. Res. 2005, 97, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.K.; Imrey, P.B.; Alpers, C.E.; Robbin, M.L.; Radeva, M.; Larive, B.; Shiu, Y.T.; Allon, M.; Dember, L.M.; Greene, T.; et al. Intimal Hyperplasia, Stenosis, and Arteriovenous Fistula Maturation Failure in the Hemodialysis Fistula Maturation Study. J. Am. Soc. Nephrol. 2017, 28, 3005–3013. [Google Scholar] [CrossRef]
- Simone, S.; Loverre, A.; Cariello, M.; Divella, C.; Castellano, G.; Gesualdo, L.; Pertosa, G.; Grandaliano, G. Arteriovenous Fistula Stenosis in Hemodialysis Patients Is Characterized by an Increased Adventitial Fibrosis. J. Nephrol. 2014, 27, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Roy-Chaudhury, P.; Arend, L.; Zhang, J.; Krishnamoorthy, M.; Wang, Y.; Banerjee, R.; Samaha, A.; Munda, R. Neointimal Hyperplasia in Early Arteriovenous Fistula Failure. Am. J. Kidney Dis. 2007, 50, 782–790. [Google Scholar] [CrossRef]
- Brahmbhatt, A.; Remuzzi, A.; Franzoni, M.; Misra, S. The Molecular Mechanisms of Hemodialysis Vascular Access Failure. Kidney Int. 2016, 89, 303. [Google Scholar] [CrossRef]
- Franzoni, M.; Cattaneo, I.; Longaretti, L.; Figliuzzi, M.; Ene-Iordache, B.; Remuzzi, A. Endothelial Cell Activation by Hemodynamic Shear Stress Derived from Arteriovenous Fistula for Hemodialysis Access. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H49–H59. [Google Scholar] [CrossRef]
- Cai, Y.; Nagel, D.J.; Zhou, Q.; Cygnar, K.D.; Zhao, H.; Li, F.; Pi, X.; Knight, P.A.; Yan, C. Role of CAMP-Phosphodiesterase 1C Signaling in Regulating Growth Factor Receptor Stability, Vascular Smooth Muscle Cell Growth, Migration, and Neointimal Hyperplasia. Circ. Res. 2015, 116, 1120–1132. [Google Scholar] [CrossRef]
- Liang, M.; Wang, Y.; Liang, A.; Mitch, W.E.; Roy-Chaudhury, P.; Han, G.; Cheng, J. Migration of Smooth Muscle Cells from the Arterial Anastomosis of Arteriovenous Fistulas Requires Notch Activation to Form Neointima. Kidney Int. 2015, 88, 490–502. [Google Scholar] [CrossRef]
- Ashino, T.; Yamamoto, M.; Yoshida, T.; Numazawa, S. Redox-Sensitive Transcription Factor Nrf2 Regulates Vascular Smooth Muscle Cell Migration and Neointimal Hyperplasia. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 760–768. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, Y.; Zhang, S.; Lin, Y.; Yang, J.; Zhang, C. A Necessary Role of MiR-221 and MiR-222 in Vascular Smooth Muscle Cell Proliferation and Neointimal Hyperplasia. Circ. Res. 2009, 104, 476–486. [Google Scholar] [CrossRef]
- Zhao, J.; Jourd’heuil, F.L.; Xue, M.; Conti, D.; Lopez-Soler, R.I.; Ginnan, R.; Asif, A.; Singer, H.A.; Jourd’heuil, D.; Long, X. Dual Function for Mature Vascular Smooth Muscle Cells During Arteriovenous Fistula Remodeling. J. Am. Heart Assoc. 2017, 6, e004891. [Google Scholar] [CrossRef]
- Janardhanan, R.; Yang, B.; Vohra, P.; Roy, B.; Withers, S.; Bhattacharya, S.; Mandrekar, J.; Kong, H.; Leof, E.B.; Mukhopadhyay, D.; et al. Simvastatin Reduces Venous Stenosis Formation in a Murine Hemodialysis Vascular Access Model. Kidney Int. 2013, 84, 338–352. [Google Scholar] [CrossRef]
- Yang, B.; Janardhanan, R.; Vohra, P.; Greene, E.L.; Bhattacharya, S.; Withers, S.; Roy, B.; Nieves Torres, E.C.; Mandrekar, J.; Leof, E.B.; et al. Adventitial Transduction of Lentivirus-ShRNA-VEGF-A in Arteriovenous Fistula Reduces Venous Stenosis Formation. Kidney Int. 2014, 85, 289–306. [Google Scholar] [CrossRef]
- Misra, S.; Fu, A.A.; Misra, K.D.; Shergill, U.M.; Leof, E.B.; Mukhopadhyay, D. Hypoxia-Induced Phenotypic Switch of Fibroblasts to Myofibroblasts through a Matrix Metalloproteinase 2/Tissue Inhibitor of Metalloproteinase–Mediated Pathway: Implications for Venous Neointimal Hyperplasia in Hemodialysis Access. J. Vasc. Interv. Radiol. 2010, 21, 896–902. [Google Scholar] [CrossRef][Green Version]
- Sadaghianloo, N.; Yamamoto, K.; Bai, H.; Tsuneki, M.; Protack, C.D.; Hall, M.R.; Declemy, S.; Hassen-Khodja, R.; Madri, J.; Dardik, A. Increased Oxidative Stress and Hypoxia Inducible Factor-1 Expression during Arteriovenous Fistula Maturation. Ann. Vasc. Surg. 2017, 41, 225–234. [Google Scholar] [CrossRef]
- Li, Z.; Li, M.; Li, X.; Zhang, M.; Zhao, Y.; Ren, W.; Cheng, J.; Wang, X. Hyperbaric Oxygen Inhibits Venous Neointimal Hyperplasia Following Arteriovenous Fistulization. Int. J. Mol. Med. 2017, 39, 1299–1306. [Google Scholar] [CrossRef]
- Wan, J.; Lata, C.; Santilli, A.; Green, D.; Roy, S.; Santilli, S. Supplemental Oxygen Reverses Hypoxia-Induced Smooth Muscle Cell Proliferation by Modulating HIF-Alpha and VEGF Levels in a Rabbit Arteriovenous Fistula Model. Ann. Vasc. Surg. 2014, 28, 725–736. [Google Scholar] [CrossRef]
- Wish, J.B.; Moe, S.M. Moving Beyond the Assumed: Improving Fistula Success Rates. J. Am. Soc. Nephrol. 2017, 28, 2827. [Google Scholar] [CrossRef]
- D’Ayala, M.; Smith, R.M.; Martone, C.; Briggs, W.; Deitch, J.S.; Wise, L. The Effect of Systemic Anticoagulation in Patients Undergoing Angioaccess Surgery. Ann. Vasc. Surg. 2008, 22, 11–15. [Google Scholar] [CrossRef]
- Irish, A.B.; Viecelli, A.K.; Hawley, C.M.; Hooi, L.S.; Pascoe, E.M.; Paul-Brent, P.A.; Badve, S.V.; Mori, T.A.; Cass, A.; Kerr, P.G.; et al. Effect of Fish Oil Supplementation and Aspirin Use on Arteriovenous Fistula Failure in Patients Requiring Hemodialysis: A Randomized Clinical Trial. JAMA Intern. Med. 2017, 177, 184–193. [Google Scholar] [CrossRef]
- Dember, L.M.; Beck, G.J.; Allon, M.; Delmez, J.A.; Dixon, B.S.; Greenberg, A.; Himmelfarb, J.; Vazquez, M.A.; Gassman, J.J.; Greene, T.; et al. Effect of Clopidogrel on Early Failure of Arteriovenous Fistulas for Hemodialysis: A Randomized Controlled Trial. JAMA 2008, 299, 2164–2171. [Google Scholar] [CrossRef]
- Mohamed, I.; Kamarizan, M.F.A.; da Silva, A. Medical Adjuvant Treatment to Increase Patency of Arteriovenous Fistulae and Grafts. Cochrane Database Syst. Rev. 2021, 7, CD002786. [Google Scholar] [CrossRef]
- Karydis, N.; Bevis, P.; Beckitt, T.; Silverberg, D.; Halak, M.; Calder, F. An Implanted Blood Vessel Support Device for Arteriovenous Fistulas: A Randomized Controlled Trial. Am. J. Kidney Dis. 2020, 75, 45–53. [Google Scholar] [CrossRef]
- Paulson, W.D.; Kipshidze, N.; Kipiani, K.; Beridze, N.; DeVita, M.V.; Shenoy, S.; Iyer, S.S. Safety and Efficacy of Local Periadventitial Delivery of Sirolimus for Improving Hemodialysis Graft Patency: First Human Experience with a Sirolimus-Eluting Collagen Membrane (Coll-R). Nephrology Dialysis Transplantation 2012, 27, 1219–1224. [Google Scholar] [CrossRef]
- Clair, D.; Moritz, M.; Burgess, J.; Illig, K.; Moldovan, S.; Parden, J.; Ross, J.; Iyer, S.; Health, P. Arteriovenous Fistula Outcomes After Local Vascular Delivery of a Sirolimus Formulation. J. Vasc. Surg. 2019, 70, e37–e38. [Google Scholar] [CrossRef]
- Zhao, C.; Zuckerman, S.T.; Cai, C.; Kilari, S.; Singh, A.; Simeon, M.; von Recum, H.A.; Korley, J.N.; Misra, S. Periadventitial Delivery of Simvastatin-Loaded Microparticles Attenuate Venous Neointimal Hyperplasia Associated with Arteriovenous Fistula. J. Am. Heart Assoc. 2020, 9, e018418. [Google Scholar] [CrossRef]
- Brahmbhatt, A.; NievesTorres, E.; Yang, B.; Edwards, W.D.; Chaudhury, P.R.; Lee, M.K.; Kong, H.; Mukhopadhyay, D.; Kumar, R.; Misra, S. The Role of Iex-1 in the Pathogenesis of Venous Neointimal Hyperplasia Associated with Hemodialysis Arteriovenous Fistula. PLoS ONE 2014, 9, e102542. [Google Scholar] [CrossRef]
- Singh, A.K.; Cai, C.; Kilari, S.; Zhao, C.; Simeon, M.L.; Takahashi, E.; Edelman, E.R.; Kong, H.; Macedo, T.; Singh, R.J.; et al. 1α,25-Dihydroxyvitamin D3 Encapsulated in Nanoparticles Prevents Venous Neointimal Hyperplasia and Stenosis in Porcine Arteriovenous Fistulas. J. Am. Soc. Nephrol. 2021, 32, 866. [Google Scholar] [CrossRef]
- Bleyer, A.J.; Scavo, V.A.; Wilson, S.E.; Browne, B.J.; Ferris, B.L.; Ozaki, C.K.; Lee, T.; Peden, E.K.; Dixon, B.S.; Mishler, R.; et al. A Randomized Trial of Vonapanitase (PATENCY-1) to Promote Radiocephalic Fistula Patency and Use for Hemodialysis. J. Vasc. Surg. 2019, 69, 507–515. [Google Scholar] [CrossRef]
- Peden, E.K.; Lucas, J.F.; Browne, B.J.; Settle, S.M.; Scavo, V.A.; Bleyer, A.J.; Ozaki, C.K.; Teruya, T.H.; Wilson, S.E.; Mishler, R.E.; et al. PATENCY-2 Trial of Vonapanitase to Promote Radiocephalic Fistula Use for Hemodialysis and Secondary Patency. J. Vasc. Access. 2022, 23, 265–274. [Google Scholar] [CrossRef]
- Hernandez, D.R.; Applewhite, B.; Martinez, L.; Laurito, T.; Tabbara, M.; Rojas, M.G.; Wei, Y.; Selman, G.; Knysheva, M.; Velazquez, O.C.; et al. Inhibition of Lysyl Oxidase with β-Aminopropionitrile Improves Venous Adaptation after Arteriovenous Fistula Creation. Kidney360 2021, 2, 270–278. [Google Scholar] [CrossRef]
- Shiu, Y.T.; He, Y.; Tey, J.C.S.; Knysheva, M.; Anderson, B.; Kauser, K. Natural Vascular Scaffolding Treatment Promotes Outward Remodeling During Arteriovenous Fistula Development in Rats. Front. Bioeng. Biotechnol. 2021, 9, 84. [Google Scholar] [CrossRef]
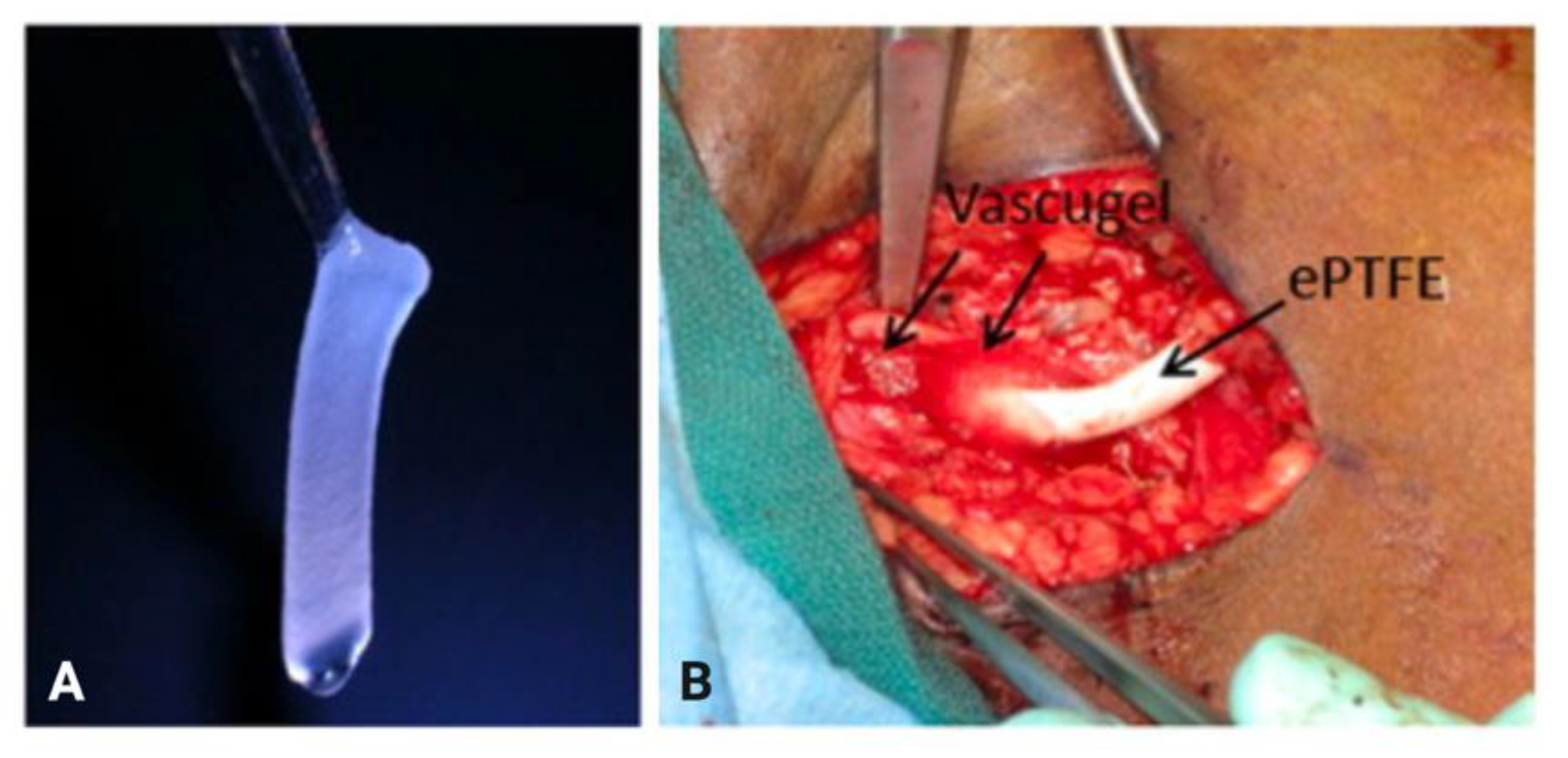
- Conte, M.S.; Nugent, H.M.; Gaccione, P.; Guleria, I.; Roy-Chaudhury, P.; Lawson, J.H. Multicenter Phase I/II Trial of the Safety of Allogeneic Endothelial Cell Implants after the Creation of Arteriovenous Access for Hemodialysis Use: The V-HEALTH Study. J. Vasc. Surg. 2009, 50, 1359–1368. [Google Scholar] [CrossRef][Green Version]
- Yang, B.; Brahmbhatt, A.; Torres, E.N.; Thielen, B.; McCall, D.L.; Engel, S.; Bansal, A.; Pandey, M.K.; Dietz, A.B.; Leof, E.B.; et al. Tracking and Therapeutic Value of Human Adipose Tissue–Derived Mesenchymal Stem Cell Transplantation in Reducing Venous Neointimal Hyperplasia Associated with Arteriovenous Fistula. Radiology 2015, 279, 513–522. [Google Scholar] [CrossRef]
- DeVita, M.V.; Khine, S.K.; Shivarov, H. Novel Approaches to Arteriovenous Access Creation, Maturation, Suitability, and Durability for Dialysis. Kidney Int. Rep. 2020, 5, 769–778. [Google Scholar] [CrossRef]
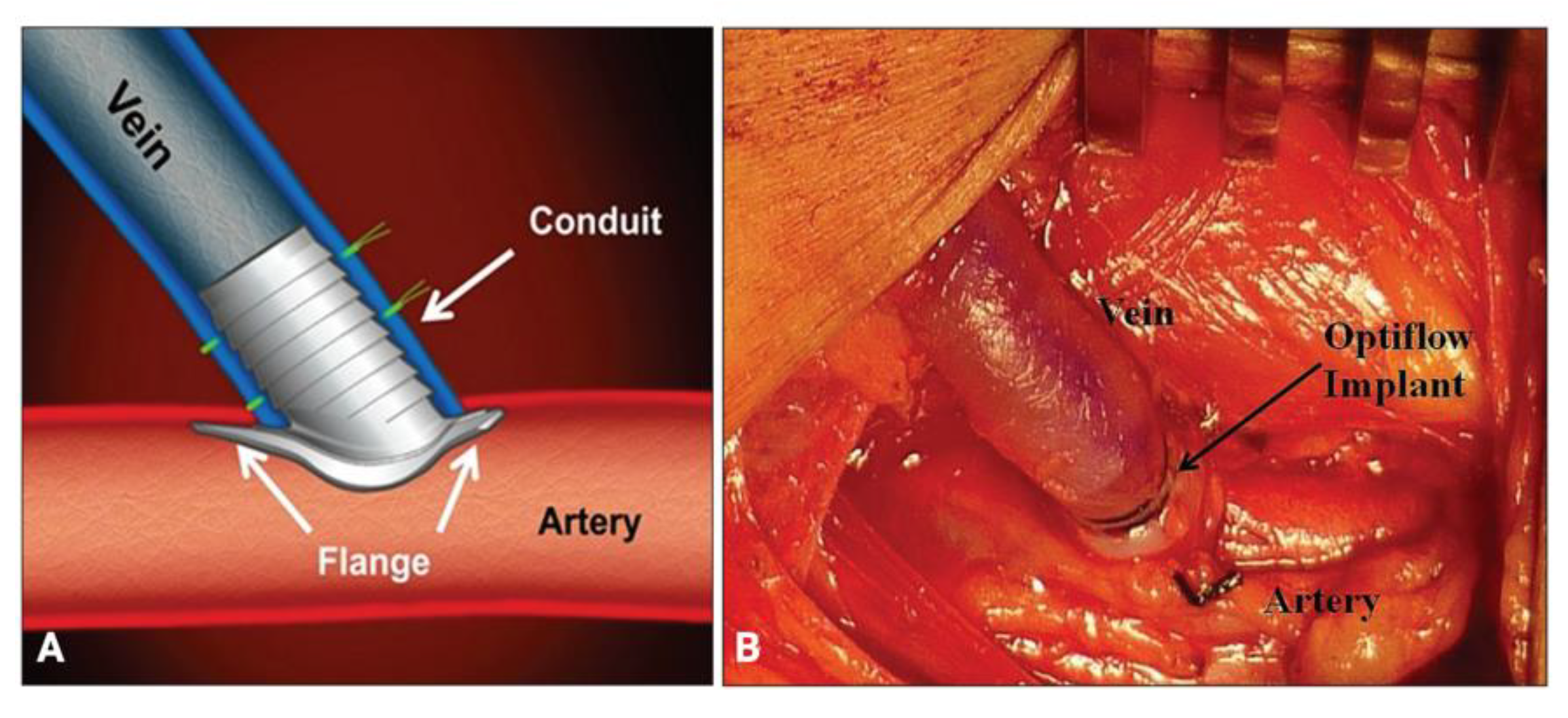
- Chemla, E.; Tavakoli, A.; Nikam, M.; Mitra, S.; Malete, T.; Evans, J.; Roy-Chaudhury, P. Arteriovenous Fistula Creation Using the OptiflowTM Vascular Anastomotic Connector: The OPEN (Optiflow PatEncy and MaturatioN) Study. J. Vasc. Access 2014, 15, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Moses, J.W.; Leon, M.B.; Popma, J.J.; Fitzgerald, P.J.; Holmes, D.R.; O’Shaughnessy, C.; Caputo, R.P.; Kereiakes, D.J.; Williams, D.O.; Teirstein, P.S.; et al. Sirolimus-Eluting Stents versus Standard Stents in Patients with Stenosis in a Native Coronary Artery. N. Engl. J. Med. 2003, 349, 1315–1323. [Google Scholar] [CrossRef]
- Miller, P.E.; Carlton, D.; Deierhoi, M.H.; Redden, D.T.; Allon, M. Natural History of Arteriovenous Grafts in Hemodialysis Patients. Am. J. Kidney Dis. 2000, 36, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Cinat, M.E.; Hopkins, J.; Wilson, S.E. A Prospective Evaluation of PTFE Graft Patency and Surveillance Techniques in Hemodialysis Access. Ann. Vasc. Surg. 1999, 13, 191–198. [Google Scholar] [CrossRef]
- Ram, S.J.; Nassar, R.; Work, J.; Abreo, K.; Dossabhoy, N.R.; Paulson, W.D. Risk of Hemodialysis Graft Thrombosis: Analysis of Monthly Flow Surveillance. Am. J. Kidney Dis. 2008, 52, 930–938. [Google Scholar] [CrossRef] [PubMed]
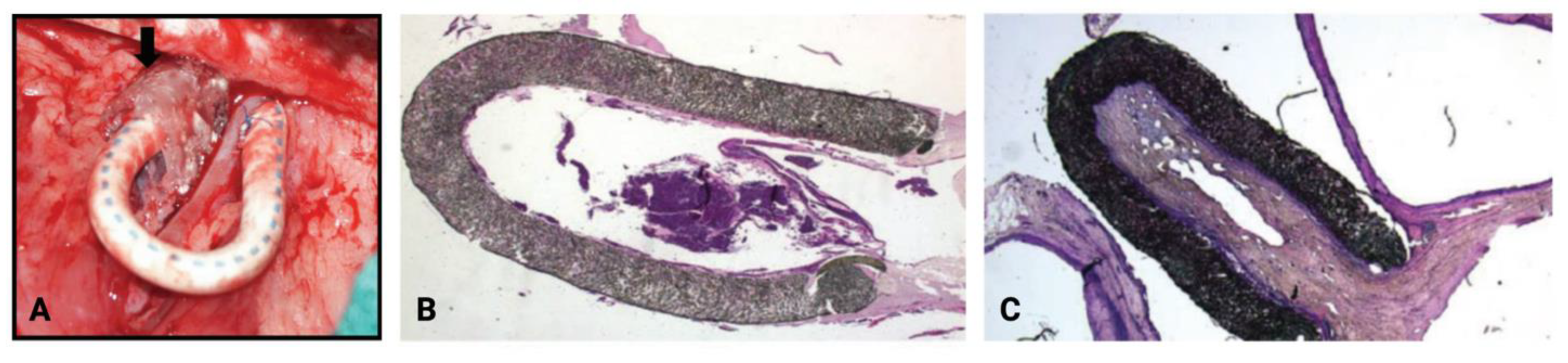
- Kelly, B.; Melhem, M.; Zhang, J.; Kasting, G.; Li, J.; Krishnamoorthy, M.; Heffelfinger, S.; Rudich, S.; Desai, P.; Roy-Chaudhury, P. Perivascular Paclitaxel Wraps Block Arteriovenous Graft Stenosis in a Pig Model. Nephrol. Dial. Transplant. 2006, 21, 2425–2431. [Google Scholar] [CrossRef][Green Version]
- Kohler, T.R.; Toleikis, P.M.; Gravett, D.M.; Avelar, R.L. Inhibition of Neointimal Hyperplasia in a Sheep Model of Dialysis Access Failure with the Bioabsorbable Vascular Wrap Paclitaxel-Eluting Mesh. J. Vasc. Surg. 2007, 45, 1029–1038. [Google Scholar] [CrossRef]
- Ray, K.K.; Cannon, C.P. The Potential Relevance of the Multiple Lipid-Independent (Pleiotropic) Effects of Statins in the Management of Acute Coronary Syndromes. J. Am. Coll. Cardiol. 2005, 46, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lu, H.; Huang, J.; Guan, Y.; Sun, H. Simvastatin Exerts Favourable Effects on Neointimal Formation in a Mouse Model of Vein Graft. Eur. J. Vasc. Endovasc. Surg. 2011, 42, 393–399. [Google Scholar] [CrossRef][Green Version]
- Chang, H.H.; Chang, Y.K.; Lu, C.W.; Huang, C.T.; Chien, C.T.; Hung, K.Y.; Huang, K.C.; Hsu, C.C. Statins Improve Long Term Patency of Arteriovenous Fistula for Hemodialysis. Sci. Rep. 2016, 6, 22197. [Google Scholar] [CrossRef] [PubMed]
- Im, H.J.; Craig, T.A.; Pittelkow, M.R.; Kumar, R. Characterization of a Novel Hexameric Repeat DNA Sequence in the Promoter of the Immediate Early Gene, IEX-1, That Mediates 1alpha,25-Dihydroxyvitamin D(3)-Associated IEX-1 Gene Repression. Oncogene 2002, 21, 3706–3714. [Google Scholar] [CrossRef][Green Version]
- Kawashima, I.; Tani, T.; Mita-Honjo, K.; Shimoda-Takano, K.; Ohmine, T.; Furukawa, H.; Takiguchi, Y. Genomic Organization of the Human Homologue of the Rat Pancreatic Elastase I Gene. DNA Seq. 1992, 2, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Gulati, R.; Lerman, A.; Simari, R.D. Therapeutic Uses of Autologous Endothelial Cells for Vascular Disease. Clin. Sci. 2005, 109, 27–37. [Google Scholar] [CrossRef]
- Gulati, R.; Jevremovic, D.; Peterson, T.E.; Witt, T.A.; Kleppe, L.S.; Mueske, C.S.; Lerman, A.; Vile, R.G.; Simari, R.D. Autologous Culture-Modified Mononuclear Cells Confer Vascular Protection after Arterial Injury. Circulation 2003, 108, 1520–1526. [Google Scholar] [CrossRef]
- Hughes, D.; Fu, A.A.; Puggioni, A.; Glockner, J.F.; Anwer, B.; Mcguire, A.M.; Mukhopadhyay, D.; Misra, S. Adventitial Transplantation of Blood Outgrowth Endothelial Cells in Porcine Haemodialysis Grafts Alleviates Hypoxia and Decreases Neointimal Proliferation through a Matrix Metalloproteinase-9-Mediated Pathway-a Pilot Study. Nephrol. Dial Transplant. 2009, 24, 85–96. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef]
- Prockop, D.J.; Olson, S.D. Clinical Trials with Adult Stem/Progenitor Cells for Tissue Repair: Let’s Not Overlook Some Essential Precautions. Blood 2007, 109, 3147–3151. [Google Scholar] [CrossRef]
- Nagaya, N.; Kangawa, K.; Itoh, T.; Iwase, T.; Murakami, S.; Miyahara, Y.; Fujii, T.; Uematsu, M.; Ohgushi, H.; Yamagishi, M.; et al. Transplantation of Mesenchymal Stem Cells Improves Cardiac Function in a Rat Model of Dilated Cardiomyopathy. Circulation 2005, 112, 1128–1135. [Google Scholar] [CrossRef]
- Ortiz, L.A.; Gambelli, F.; McBride, C.; Gaupp, D.; Baddoo, M.; Kaminski, N.; Phinney, D.G. Mesenchymal Stem Cell Engraftment in Lung Is Enhanced in Response to Bleomycin Exposure and Ameliorates Its Fibrotic Effects. Proc. Natl. Acad. Sci. USA 2003, 100, 8407–8411. [Google Scholar] [CrossRef] [PubMed]
- Abdel Aziz, M.T.; Atta, H.M.; Mahfouz, S.; Fouad, H.H.; Roshdy, N.K.; Ahmed, H.H.; Rashed, L.A.; Sabry, D.; Hassouna, A.A.; Hasan, N.M. Therapeutic Potential of Bone Marrow-Derived Mesenchymal Stem Cells on Experimental Liver Fibrosis. Clin. Biochem. 2007, 40, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, E.; Yagi, K.; Kojima, M.; Yagyuu, T.; Ohshima, A.; Sobajima, S.; Tadokoro, M.; Katsube, Y.; Isoda, K.; Kondoh, M.; et al. Multipotent Cells from the Human Third Molar: Feasibility of Cell-Based Therapy for Liver Disease. Differentiation 2008, 76, 495–505. [Google Scholar] [CrossRef]
- Ninichuk, V.; Gross, O.; Segerer, S.; Hoffmann, R.; Radomska, E.; Buchstaller, A.; Huss, R.; Akis, N.; Schlöndorff, D.; Anders, H.J. Multipotent Mesenchymal Stem Cells Reduce Interstitial Fibrosis But Do Not Delay Progression of Chronic Kidney Disease in Collagen4A3-Deficient Mice. Kidney Int. 2006, 70, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I. Why Are MSCs Therapeutic? New Data: New Insight. J. Pathol. 2009, 217, 318–324. [Google Scholar] [CrossRef]
- Hu, X.; Yu, S.P.; Fraser, J.L.; Lu, Z.; Ogle, M.E.; Wang, J.A.; Wei, L. Transplantation of Hypoxia-Preconditioned Mesenchymal Stem Cells Improves Infarcted Heart Function via Enhanced Survival of Implanted Cells and Angiogenesis. J. Thorac. Cardiovasc. Surg. 2008, 135, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudifar, N.; Doran, P.M. Osteogenic Differentiation and Osteochondral Tissue Engineering Using Human Adipose-Derived Stem Cells. Biotechnol. Prog. 2012, 29, 176–185. [Google Scholar] [CrossRef] [PubMed]
- San Valentin, E.M.D.; Barcena, A.J.R.; Klusman, C.; Martin, B.; Melancon, M.P. Nano-Embedded Medical Devices and Delivery Systems in Interventional Radiology. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnology 2022, e1841. [Google Scholar] [CrossRef]
- Yu, D.-G.; Zhu, L.-M.; White, K.; Branford-White, C. Electrospun Nanofiber-Based Drug Delivery Systems. Health N Hav. 2009, 01, 67–75. [Google Scholar] [CrossRef]
- Hu, H.; Patel, S.; Hanisch, J.J.; Santana, J.M.; Hashimoto, T.; Bai, H.; Kudze, T.; Foster, T.R.; Guo, J.; Yatsula, B.; et al. Future Research Directions to Improve Fistula Maturation and Reduce Access Failure. Semin. Vasc. Surg. 2016, 29, 153. [Google Scholar] [CrossRef]
- Liang, D.; Hsiao, B.S.; Chu, B. Functional Electrospun Nanofibrous Scaffolds for Biomedical Applications. Adv. Drug Deliv. Rev. 2007, 59, 1392. [Google Scholar] [CrossRef] [PubMed]
- Sill, T.J.; von Recum, H.A. Electrospinning: Applications in Drug Delivery and Tissue Engineering. Biomaterials 2008, 29, 1989–2006. [Google Scholar] [CrossRef] [PubMed]
- Green, J.J.; Elisseeff, J.H. Mimicking Biological Functionality with Polymers for Biomedical Applications. Nature 2016, 540, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Perez, J.V.D.; Jacobsen, M.C.; Damasco, J.A.; Melancon, A.; Huang, S.Y.; Layman, R.R.; Melancon, M.P. Optimization of the Differentiation and Quantification of High-Z Nanoparticles Incorporated in Medical Devices for CT-Guided Interventions. Med. Phys. 2021, 48, 300–312. [Google Scholar] [CrossRef]




| Approach | Specific Therapy | Development Stage | Results | Reference or ClinicalTrials.gov ID |
|---|---|---|---|---|
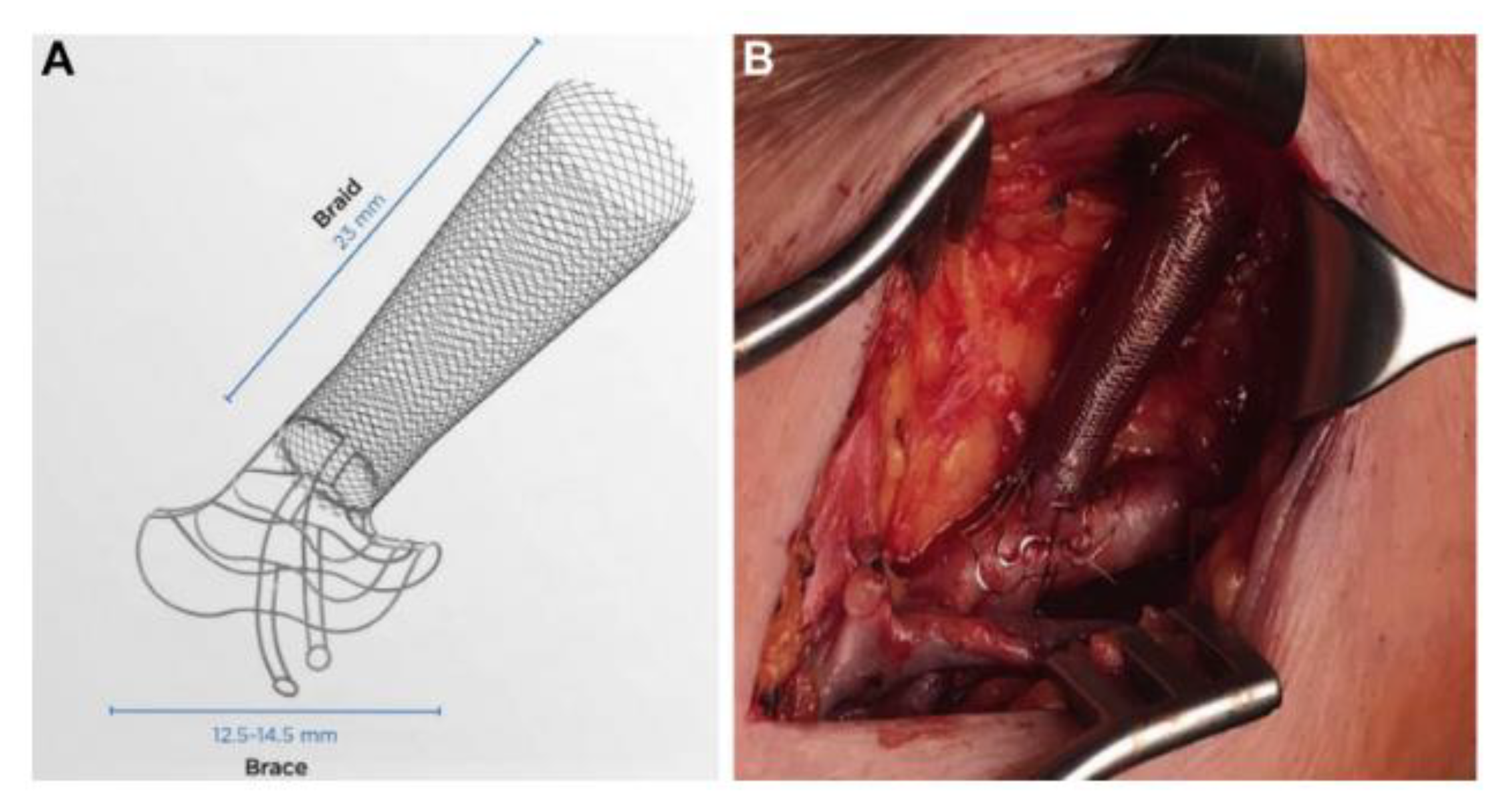
| Mechanical device | VasQ | Clinical (Phase 1/2) | Significantly increased venous luminal diameters at three and six months and increased functional patency at six months following brachiocephalic fistula formation | Karydis et al. (2020) [50] |
| Targeted drug delivery | Sirolimus | Clinical (Phase 1/2) | Local delivery using a collagen membrane produced 12- and 24-month primary unassisted patency rates of 76% and 38%, respectively, and a thrombosis rate of 0.37 events per patient-year | Paulson et al. (2012) [51] |
| Clinical (Phase 2) | Local delivery using collagen-based technology demonstrated an AVF maturation success rate of 87% | Clair et al. (2019) [52] | ||
| Clinical (Phase 3) | Ongoing trial | NCT05425056 | ||
| Simvastatin | Preclinical (Murine) | Periadventitial delivery using cyclodextrin microparticles significantly reduced the expression of VEGF-A, TGF-β1, and MCP-1 and reduced the average neointimal area and neointimal cell density | Zhao et al. (2020) [53] | |
| 1α,25-dihydroxyvitamin D3 | Preclinical (Murine) | Periadventitial delivery using PLGA and Pluronic F127 hydrogel significantly reduced IER3 gene expression and the average ratio of neointima to media plus adventitia | Brahmbhatt et al. (2014) [54] | |
| Preclinical (Porcine) | Perivascular nanoparticle-based administration significantly reduced IER3 expression on days 3 and 28, increased the average luminal area in the outflow vein, and decreased the average neointimal area | Singh et al. (2021) [55] | ||
| Vonapanitase | Clinical (Phase 1/2) | Topical application onto the anastomotic site did not improve primary patency but increased secondary patency and use for hemodialysis | Bleyer et al. (2019) [56] | |
| Clinical (Phase 1/2) | Topical application produced no significant improvement in AVF outcomes | Peden et al. (2022) [57] | ||
| β-aminopropionitrile | Preclinical (Murine) | Local delivery through an electrospun scaffold significantly decreased wall fibrosis and demonstrated a nonsignificant trend toward increased blood flow on day 21 | Hernandez et al. (2021) [58] | |
| 4-amino-1,8-naphtalamide | Preclinical (Murine) | Local delivery and photoactivation significantly increased the open luminal area and % of the open luminal area of the venous limb of AVFs | Shiu et al. (2021) [59] | |
| Cell-based therapeutics | Endothelial cell | Clinical (Phase 1/2) | Local delivery using gelatin sponges revealed no statistically significant differences in primary or assisted primary patency between the treatment and control groups | Conte et al. (2009) [60] |
| Mesenchymal stem cell | Preclinical (Murine) | Direct injection into the adventitia significantly reduced the expression of MCP-1 and HIF-1α, increased the mean luminal area, and reduced the mean neointimal area and neointimal cell density | Yang et al. (2015) [61] | |
| Clinical (Phase 1/2) | Ongoing trial | NCT02808208 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barcena, A.J.R.; Perez, J.V.D.; Liu, O.; Mu, A.; Heralde, F.M., III; Huang, S.Y.; Melancon, M.P. Localized Perivascular Therapeutic Approaches to Inhibit Venous Neointimal Hyperplasia in Arteriovenous Fistula Access for Hemodialysis Use. Biomolecules 2022, 12, 1367. https://doi.org/10.3390/biom12101367
Barcena AJR, Perez JVD, Liu O, Mu A, Heralde FM III, Huang SY, Melancon MP. Localized Perivascular Therapeutic Approaches to Inhibit Venous Neointimal Hyperplasia in Arteriovenous Fistula Access for Hemodialysis Use. Biomolecules. 2022; 12(10):1367. https://doi.org/10.3390/biom12101367
Chicago/Turabian StyleBarcena, Allan John R., Joy Vanessa D. Perez, Olivia Liu, Amy Mu, Francisco M. Heralde, III, Steven Y. Huang, and Marites P. Melancon. 2022. "Localized Perivascular Therapeutic Approaches to Inhibit Venous Neointimal Hyperplasia in Arteriovenous Fistula Access for Hemodialysis Use" Biomolecules 12, no. 10: 1367. https://doi.org/10.3390/biom12101367
APA StyleBarcena, A. J. R., Perez, J. V. D., Liu, O., Mu, A., Heralde, F. M., III, Huang, S. Y., & Melancon, M. P. (2022). Localized Perivascular Therapeutic Approaches to Inhibit Venous Neointimal Hyperplasia in Arteriovenous Fistula Access for Hemodialysis Use. Biomolecules, 12(10), 1367. https://doi.org/10.3390/biom12101367








