Application of Metal–Organic Framework in Diagnosis and Treatment of Diabetes
Abstract
:1. Introduction
2. The Synthesis of MOFs
2.1. Hydrothermal/Solvothermal Synthesis
2.2. Room-Temperature Synthesis
2.3. Microwave-assisted Synthesis
2.4. Ultrasound-assisted Synthesis
2.5. Mechanochemical Synthesis
2.6. Microfluidic Synthesis
2.7. Biomineralization Synthesis
3. Application of MOFs in Diabetes Diagnosis
3.1. Glucose Sensor
3.2. Acetone and Isopropanol Sensors
3.3. Other Sensors
4. Application of MOFs in Regulating Blood Glucose
4.1. Oral Insulin Delivery
4.2. Wound Intelligent Insulin Delivery
5. Distinguishing between Normal Wounds and Diabetic Wounds
5.1. Normal Wounds
5.2. Diabetic Wound Healing
6. Application of MOFs in Wound Healing
6.1. MOF-Based Antibacterial Material
6.1.1. Physical Contact
6.1.2. Oxidative Stress
6.1.3. Photothermal Effect
6.1.4. Metal Ions or Ligands
6.2. MOF-Based Provascular Materials
6.2.1. Drugs Act in Coordination with Metal Ions
6.2.2. NO
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Colonna, M. Skin function for human CD1a-reactive T cells. Nat. Immunol. 2010, 11, 1079–1080. [Google Scholar] [CrossRef] [PubMed]
- Diabetes. Available online: https://www.who.int/health-topics/diabetes#tab=tab_1. (accessed on 2 August 2022).
- Liang, Y.; Li, M.; Yang, Y.; Qiao, L.; Xu, H.; Guo, B. pH/Glucose Dual Responsive Metformin Release Hydrogel Dressings with Adhesion and Self-Healing via Dual-Dynamic Bonding for Athletic Diabetic Foot Wound Healing. ACS Nano 2022, 16, 3194–3207. [Google Scholar] [CrossRef] [PubMed]
- Martí-Carvajal, A.; Gluud, C.; Nicola, S.; Simancas-Racines, D.; Reveiz, L.; Oliva, P.; Cedeño-Taborda, J. Growth factors for treating diabetic foot ulcers. Cochrane Database Syst. Rev. 2015, CD008548. [Google Scholar] [CrossRef]
- Freund, R.; Zaremba, O.; Arnauts, G.; Ameloot, R.; Skorupskii, G.; Dincă, M.; Bavykina, A.; Gascon, J.; Ejsmont, A.; Goscianska, J.; et al. The Current Status of MOF and COF Applications. Angew. Chem. 2021, 60, 23975–24001. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Su, F.; Ma, M.; Lv, Y. Metal-organic frameworks for the sorption of acetone and isopropanol in exhaled breath of diabetics prior to quantitation by gas chromatography. Mikrochim. Acta 2019, 186, 588. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Lu, Q.; Guo, X.; Wang, S.; Gao, Y.; Wang, L. Au nanoparticles deposited on ultrathin two-dimensional covalent organic framework nanosheets for in vitro and intracellular sensing. Nanoscale 2020, 12, 7776–7781. [Google Scholar] [CrossRef]
- Sava Gallis, D.; Rohwer, L.; Rodriguez, M.; Barnhart-Dailey, M.; Butler, K.; Luk, T.; Timlin, J.; Chapman, K. Multifunctional, Tunable Metal-Organic Framework Materials Platform for Bioimaging Applications. ACS Appl. Mater. Interfaces 2017, 9, 22268–22277. [Google Scholar] [CrossRef]
- Li, Z.; Wang, L.; Qin, L.; Lai, C.; Wang, Z.; Zhou, M.; Xiao, L.; Liu, S.; Zhang, M. Recent advances in the application of water-stable metal-organic frameworks: Adsorption and photocatalytic reduction of heavy metal in water. Chemosphere 2021, 285, 131432. [Google Scholar] [CrossRef]
- Ghaffar, I.; Imran, M.; Perveen, S.; Kanwal, T.; Saifullah, S.; Bertino, M.; Ehrhardt, C.; Yadavalli, V.; Shah, M. Synthesis of chitosan coated metal organic frameworks (MOFs) for increasing vancomycin bactericidal potentials against resistant S. aureus strain. Mater. Sci. Engineering. C Mater. Biol. Appl. 2019, 105, 110111. [Google Scholar] [CrossRef]
- Beg, S.; Rahman, M.; Jain, A.; Saini, S.; Midoux, P.; Pichon, C.; Ahmad, F.; Akhter, S. Nanoporous metal organic frameworks as hybrid polymer-metal composites for drug delivery and biomedical applications. Drug Discov. Today 2017, 22, 625–637. [Google Scholar] [CrossRef]
- Yao, X.; Zhu, G.; Zhu, P.; Ma, J.; Chen, W.; Liu, Z.; Kong, T. Omniphobic ZIF-8@Hydrogel Membrane by Microfluidic-Emulsion-Templating Method for Wound Healing. Adv. Funct. Mater. 2020, 30, 1909389. [Google Scholar] [CrossRef]
- Cong, Y.; Huang, S.; Mei, Y.; Li, T. Metal-Organic Frameworks-Derived Self-Supported Carbon-Based Composites for Electrocatalytic Water Splitting. Chemistry 2021, 27, 15866–15888. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Tao, K.; Han, L. Self-supported metal-organic framework-based nanostructures as binder-free electrodes for supercapacitors. Nanoscale 2022, 14, 2155–2166. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wu, B.; Li, M.; Huang, Y.; Li, L. Heterostructures Made of Upconversion Nanoparticles and Metal-Organic Frameworks for Biomedical Applications. Adv. Sci. 2022, 9, e2103911. [Google Scholar] [CrossRef]
- Li, J.; Liu, X.; Tan, L.; Cui, Z.; Yang, X.; Liang, Y.; Li, Z.; Zhu, S.; Zheng, Y.; Yeung, K.; et al. Zinc-doped Prussian blue enhances photothermal clearance of Staphylococcus aureus and promotes tissue repair in infected wounds. Nat. Commun. 2019, 10, 4490. [Google Scholar] [CrossRef]
- Liang, Q.; Li, W.; Xie, L.; He, Y.; Qiu, B.; Zeng, H.; Zhou, S.; Zeng, J.; Liu, T.; Yan, M.; et al. General Synergistic Capture-Bonding Superassembly of Atomically Dispersed Catalysts on Micropore-Vacancy Frameworks. Nano Lett. 2022, 22, 2889–2897. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, W.; Wu, Q.; Fu, C.; Ren, X.; Lv, K.; Ma, T.; Chen, X.; Tan, L.; Meng, X. Lanthanide europium MOF nanocomposite as the theranostic nanoplatform for microwave thermo-chemotherapy and fluorescence imaging. J. Nanobiotechnology 2022, 20, 133. [Google Scholar] [CrossRef]
- Lee, Y.R.; Kim, J.; Ahn, W.S. Synthesis of metal-organic frameworks: A mini review. Korean J. Chem. Eng. 2013, 30, 1667–1680. [Google Scholar] [CrossRef]
- Safaei, M.; Foroughi, M.M.; Ebrahimpoor, N.; Jahani, S.; Khatami, M. A review on metal-organic frameworks: Synthesis and Applications. TrAC Trends Anal. Chem. 2019, 118, 401–425. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, X.; Ge, J. Antioxidative Composites Based on Multienzyme Systems Encapsulated in Metal-Organic Frameworks. ACS Appl. Mater. Interfaces 2021, 13, 46431–46439. [Google Scholar] [CrossRef]
- Mumtaz, N.; Javaid, A.; Imran, M.; Latif, S.; Hussain, N.; Nawaz, S.; Bilal, M. Nanoengineered metal-organic framework for adsorptive and photocatalytic mitigation of pharmaceuticals and pesticide from wastewater. Environ. Pollut. 2022, 308, 119690. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Kim, J.; Park, C.; Kim, H.; Kim, M.; Park, H.; Kim, I.; Ko, J.; Pak, K.; Choi, S.; et al. Large-Area Synthesis of Ultrathin, Flexible, and Transparent Conductive Metal-Organic Framework Thin Films via a Microfluidic-Based Solution Shearing Process. Adv. Mater. 2022, 34, e2107696. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Koo, W.; Kim, H.; Park, C.; Lee, T.; Hutomo, C.; Choi, S.; Kim, D.; Kim, I.; Park, S. Large-area synthesis of nanoscopic catalyst-decorated conductive MOF film using microfluidic-based solution shearing. Nat. Commun. 2021, 12, 4294. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.; Ricco, R.; Doherty, C.; Styles, M.; Bell, S.; Kirby, N.; Mudie, S.; Haylock, D.; Hill, A.; Doonan, C.; et al. Biomimetic mineralization of metal-organic frameworks as protective coatings for biomacromolecules. Nat. Commun. 2015, 6, 7240. [Google Scholar] [CrossRef]
- Singh, R.; White, J.; de Vries, M.; Beddome, G.; Dai, M.; Bean, A.; Mulet, X.; Layton, D.; Doherty, C. Biomimetic metal-organic frameworks as protective scaffolds for live-virus encapsulation and vaccine stabilization. Acta Biomater. 2022, 142, 320–331. [Google Scholar] [CrossRef]
- Li, R.; Han, X.; Liu, Q.; Qian, A.; Zhu, F.; Hu, J.; Fan, J.; Shen, H.; Liu, J.; Pu, X.; et al. BDCEnhancing Hydrogen Adsorption Capacity of Metal Organic Frameworks M()TED through Constructing a Bimetallic Structure. ACS Omega 2022, 7, 20081–20091. [Google Scholar] [CrossRef]
- Zeng, R.; He, T.; Lu, L.; Li, K.; Luo, Z.; Cai, K. Ultra-thin metal-organic framework nanosheets for chemo-photodynamic synergistic therapy. J. Mater. Chem. B 2021, 9, 4143–4153. [Google Scholar] [CrossRef]
- Couzon, N.; Ferreira, M.; Duval, S.; El-Achari, A.; Campagne, C.; Loiseau, T.; Volkringer, C. Microwave-Assisted Synthesis of Porous Composites MOF-Textile for the Protection against Chemical and Nuclear Hazards. ACS Appl. Mater. Interfaces 2022, 14, 21497–21508. [Google Scholar] [CrossRef]
- Lu, J.; Jiang, Y.; Xiong, T.; Yu, P.; Jiang, W.; Mao, L. Light-Regulated Nanofluidic Ionic Diodes with Heterogeneous Channels Stemming from Asymmetric Growth of Metal-Organic Frameworks. Anal. Chem. 2022, 94, 4328–4334. [Google Scholar] [CrossRef]
- Jacobsen, J.; Wegner, L.; Reinsch, H.; Stock, N. Ce-MIL-140: Expanding the synthesis routes for cerium(iv) metal-organic frameworks. Dalton Trans. 2020, 49, 11396–11402. [Google Scholar] [CrossRef]
- Matemb Ma Ntep, T.; Gramm, V.; Ruschewitz, U.; Janiak, C. Acetylenedicarboxylate as a linker in the engineering of coordination polymers and metal-organic frameworks: Challenges and potential. Chem. Commun. 2022, 58, 8900–8933. [Google Scholar]
- Wang, Z.; Miao, R.; He, L.; Guan, Q.; Shi, Y. Green synthesis of MIL-100(Fe) derivatives and revealing their structure-activity relationship for 2,4-dichlorophenol photodegradation. Chemosphere 2022, 291, 132950. [Google Scholar] [CrossRef] [PubMed]
- Razavi, S.; Morsali, A. Ultrasonic-Assisted Linker Exchange (USALE): A Novel Post-Synthesis Method for Controlling the Functionality, Porosity, and Morphology of MOFs. Chemistry 2019, 25, 10876–10885. [Google Scholar] [CrossRef]
- Mirzaie, A.; Musabeygi, T.; Afzalinia, A. Sonochemical synthesis of magnetic responsive FeO@TMU-17-NH composite as sorbent for highly efficient ultrasonic-assisted denitrogenation of fossil fuel. Ultrason. Sonochemistry 2017, 38, 664–671. [Google Scholar] [CrossRef]
- Rautenberg, M.; Gernhard, M.; Radnik, J.; Witt, J.; Roth, C.; Emmerling, F. Mechanochemical Synthesis of Fluorine-Containing Co-Doped Zeolitic Imidazolate Frameworks for Producing Electrocatalysts. Front. Chem. 2022, 10, 840758. [Google Scholar]
- Zhang, H.; Hu, X.; Li, T.; Zhang, Y.; Xu, H.; Sun, Y.; Gu, X.; Gu, C.; Luo, J.; Gao, B. MIL series of metal organic frameworks (MOFs) as novel adsorbents for heavy metals in water: A review. J. Hazard. Mater. 2022, 429, 128271. [Google Scholar] [CrossRef]
- Bailey, T.; Pinto, M.; Hondow, N.; Wu, K. Continuous microfluidic synthesis of zirconium-based UiO-67 using a coiled flow invertor reactor. MethodsX 2021, 8, 101246. [Google Scholar] [CrossRef]
- Tanaka, Y.; Yamada, S.; Tanaka, D. Continuous Fluidic Techniques for the Precise Synthesis of Metal-Organic Frameworks. Chem. Plus Chem. 2021, 86, 650–661. [Google Scholar]
- He, M.; Yu, P.; Hu, Y.; Zhang, J.; He, M.; Nie, C.; Chu, X. Erythrocyte-Membrane-Enveloped Biomineralized Metal-Organic Framework Nanoparticles Enable Intravenous Glucose-Responsive Insulin Delivery. ACS Appl. Mater. Interfaces 2021, 13, 19648–19659. [Google Scholar] [PubMed]
- Yao, S.; Wang, Y.; Chi, J.; Yu, Y.; Zhao, Y.; Luo, Y.; Wang, Y. Porous MOF Microneedle Array Patch with Photothermal Responsive Nitric Oxide Delivery for Wound Healing. Adv. Sci. 2022, 9, e2103449. [Google Scholar]
- Zhang, H.; Xu, Z.; Mao, Y.; Zhang, Y.; Li, Y.; Lao, J.; Wang, L. Integrating Porphyrinic Metal-Organic Frameworks in Nanofibrous Carrier for Photodynamic Antimicrobial Application. Polymers 2021, 13, 3942. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, S.; Kalimuthu, R.; Kanagaraj, T.; Kulandaivelu, R.; Nagappan, R.; Pragasan, L.; Ponnusamy, V. Microwave-assisted green synthesis of multi-functional carbon quantum dots as efficient fluorescence sensor for ultra-trace level monitoring of ammonia in environmental water. Environ. Res. 2022, 206, 112589. [Google Scholar] [CrossRef]
- Al Sharabati, M.; Sabouni, R.; Husseini, G. Biomedical Applications of Metal-Organic Frameworks for Disease Diagnosis and Drug Delivery: A Review. Nanomaterials 2022, 12, 277. [Google Scholar] [CrossRef] [PubMed]
- Vinoth, V.; Wu, J.; Asiri, A.; Anandan, S. Sonochemical synthesis of silver nanoparticles anchored reduced graphene oxide nanosheets for selective and sensitive detection of glutathione. Ultrason. Sonochemistry 2017, 39, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Ahn, Y.; Kwak, S. Facile Sonochemical Synthesis of Flexible Fe-Based Metal-Organic Frameworks and Their Efficient Removal of Organic Contaminants from Aqueous Solutions. ACS Omega 2022, 7, 23213–23222. [Google Scholar] [CrossRef]
- Bui, T.; Nguyen, D.; Hua, S.; Chun, H.; Kim, Y. Sonochemical Preparation of a Magnet-Responsive FeO@ZIF-8 Adsorbent for Efficient Cu Removal. Nanomaterials 2022, 12, 753. [Google Scholar] [CrossRef]
- Jin, C.; Chen, Z.; Shi, S.; Li, Y.; Liu, S.; Wang, S.; Wang, H.; Chen, C. Green and Large-Scale Preparation of Chiral Metal-Organic Frameworks via Mechanochemistry. Inorg. Chem. 2022, 61, 12190–12196. [Google Scholar] [CrossRef] [PubMed]
- Rautenberg, M.; Bhattacharya, B.; Das, C.; Emmerling, F. Mechanochemical Synthesis of Phosphonate-Based Proton Conducting Metal-Organic Frameworks. Inorg. Chem. 2022, 61, 10801–10809. [Google Scholar] [CrossRef]
- Lv, M.; Zhou, W.; Tavakoli, H.; Bautista, C.; Xia, J.; Wang, Z.; Li, X. Aptamer-functionalized metal-organic frameworks (MOFs) for biosensing. Biosens. Bioelectron. 2021, 176, 112947. [Google Scholar] [CrossRef]
- Rohra, N.; Gaikwad, G.; Dandekar, P.; Jain, R. Microfluidic Synthesis of a Bioactive Metal-Organic Framework for Glucose-Responsive Insulin Delivery. ACS Appl. Mater. Interfaces 2022, 14, 8251–8265. [Google Scholar] [CrossRef]
- Shi, L.; Wu, J.; Qiao, X.; Ha, Y.; Li, Y.; Peng, C.; Wu, R. In Situ Biomimetic Mineralization on ZIF-8 for Smart Drug Delivery. ACS Biomater. Sci. Eng. 2020, 6, 4595–4603. [Google Scholar] [CrossRef] [PubMed]
- Sha, M.; Maurya, M.; Shafath, S.; Cabibihan, J.; Al-Ali, A.; Malik, R.; Sadasivuni, K. Breath Analysis for the In Vivo Detection of Diabetic Ketoacidosis. ACS Omega 2022, 7, 4257–4266. [Google Scholar] [CrossRef]
- Zhou, T.; Dong, W.; Qiu, Y.; Chen, S.; Wang, X.; Xie, C.; Zeng, D. Selectivity of a ZnO@ZIF-71@PDMS Nanorod Array Gas Sensor Enhanced by Coating a Polymer Selective Separation Membrane. ACS Appl. Mater. Interfaces 2021, 13, 54589–54596. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, D.; Li, T.; Zhang, J.; Yu, L. Green light-driven acetone gas sensor based on electrospinned CdS nanospheres/CoO nanofibers hybrid for the detection of exhaled diabetes biomarker. J. Colloid Interface Sci. 2022, 606, 261–271. [Google Scholar] [CrossRef]
- Gutiérrez, M.; Möslein, A.; Tan, J. Facile and Fast Transformation of Nonluminescent to Highly Luminescent Metal-Organic Frameworks: Acetone Sensing for Diabetes Diagnosis and Lead Capture from Polluted Water. ACS Appl. Mater. Interfaces 2021, 13, 7801–7811. [Google Scholar] [CrossRef]
- Ahmadi, A.; Khoshfetrat, S.; Kabiri, S.; Dorraji, P.; Larijani, B.; Omidfar, K. Electrochemiluminescence paper-based screen-printed electrode for HbA1c detection using two-dimensional zirconium metal-organic framework/FeO nanosheet composites decorated with Au nanoclusters. Mikrochim. Acta 2021, 188, 296. [Google Scholar] [CrossRef]
- Sakthivel, R.; Lin, L.Y.; Duann, Y.F.; Chen, H.H.; Su, C.C.; Liu, X.K.; He, J.H.; Chung, R.J. MOF-Derived Cu-BTC Nanowire-Embedded 2D Leaf-like Structured ZIF Composite-Based Aptamer Sensors for Real-Time In Vivo Insulin Monitoring. ACS Appl. Mater. Interfaces 2022, 14, 28639–28650. [Google Scholar] [CrossRef]
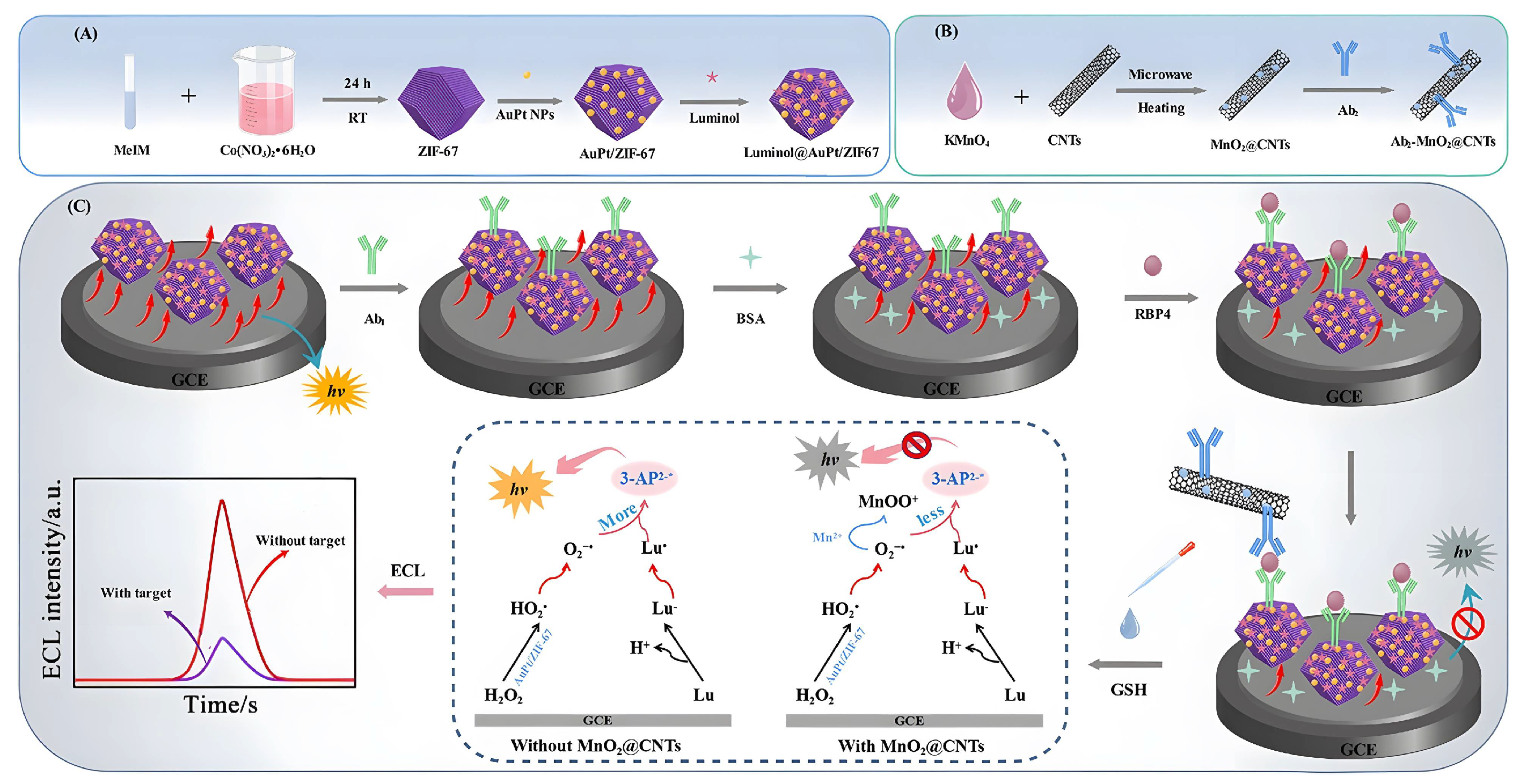
- Gong, W.; Yang, S.; Zhang, F.; Tian, F.; Chen, J.; Yin, Z.; Ding, S.; Yang, W.; Luo, R. A dual-quenched ECL immunosensor for ultrasensitive detection of retinol binding protein 4 based on luminol@AuPt/ZIF-67 and MnO@CNTs. J. Nanobiotechnology 2021, 19, 272. [Google Scholar] [CrossRef]
- Yola, M.L.; Atar, N. Novel voltammetric tumor necrosis factor-alpha (TNF-alpha) immunosensor based on gold nanoparticles involved in thiol-functionalized multi-walled carbon nanotubes and bimetallic Ni/Cu-MOFs. Anal. Bioanal. Chem. 2021, 413, 2481–2492. [Google Scholar] [CrossRef]
- Li, M.; Chi, X.; Wang, Y.; Setrerrahmane, S.; Xie, W.; Xu, H. Trends in insulin resistance: Insights into mechanisms and therapeutic strategy. Signal Transduct. Target. Ther. 2022, 7, 216. [Google Scholar] [CrossRef]
- Friesen, M.; Khalil, A.; Barrasa, M.; Jeppesen, J.; Mooney, D.; Jaenisch, R. Development of a physiological insulin resistance model in human stem cell-derived adipocytes. Sci. Adv. 2022, 8, eabn7298. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Wu, H.; Bao, Y.; Wang, C.; Lu, J.; Zhu, J.; Xiang, K. Association of serum retinol-binding protein 4 and visceral adiposity in Chinese subjects with and without type 2 diabetes. J. Clin. Endocrinol. Metab. 2007, 92, 3224–3229. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wei, L.; Bao, Y.; Lu, J.; Huang, P.; Liu, Y.; Jia, W.; Xiang, K. Fenofibrate reduces serum retinol-binding protein-4 by suppressing its expression in adipose tissue. American journal of physiology. Endocrinol. Metab. 2009, 296, E628–E634. [Google Scholar]
- Su, D.D.; Wang, T.H.; Li, A.X.; Ma, Y.; Liu, X.M.; Wang, C.G.; Jia, X.T.; Sun, P.; Liu, F.M.; Yan, X.; et al. Metal-Organic Frameworks Nanoarchitectures Boost Catalytic Activity for the Construction of Sensitive Immunosensor. Adv. Funct. Materials. 2022, 2204130. [Google Scholar] [CrossRef]
- Gorle, D.B.; Ponnada, S.; Kiai, M.S.; Nair, K.K.; Nowduri, A.; Swart, H.C.; Ang, E.H.; Nanda, K.K. Review on recent progress in metal-organic framework-based materials for fabricating electrochemical glucose sensors. J. Mater. Chem. B 2021, 9, 7927–7954. [Google Scholar] [CrossRef]
- Wang, B.; Luo, Y.; Gao, L.; Liu, B.; Duan, G. High-performance field-effect transistor glucose biosensors based on bimetallic Ni/Cu metal-organic frameworks. Biosens. Bioelectron. 2021, 171, 112736. [Google Scholar] [CrossRef]
- Zheng, W.Q.; Liu, Y.Y.; Yang, P.P.; Chen, Y.Y.; Tao, J.; Hu, J.Q.; Zhao, P. Carbon nanohorns enhanced electrochemical properties of Cu-based metal organic framework for ultrasensitive serum glucose sensing. J. Electroanal. Chem. 2020, 862, 114018. [Google Scholar] [CrossRef]
- Adeel, M.; Asif, K.; Rahman, M.M.; Daniele, S.; Canzonieri, V.; Rizzolio, F. Glucose Detection Devices and Methods Based on Metal-Organic Frameworks and Related Materials. Adv. Funct. Mater. 2021, 31, 2106023. [Google Scholar] [CrossRef]
- Jo, Y.M.; Lim, K.; Choi, H.J.; Yoon, J.W.; Kim, S.Y.; Lee, J.H. 2D metal-organic framework derived co-loading of Co3O4 and PdO nanocatalysts on In2O3 hollow spheres for tailored design of high-performance breath acetone sensors. Sens. Actuators B-Chem. 2020, 325, 128821. [Google Scholar] [CrossRef]
- Chang, X.; Li, X.F.; Qiao, X.R.; Li, K.; Xiong, Y.; Li, X.; Guo, T.C.; Zhu, L.; Xue, Q.Z. Metal-organic frameworks derived ZnO@MoS(2)nanosheets core/shell heterojunctions for ppb-level acetone detection: Ultra-fast response and recovery. Sens. Actuators B-Chem. 2020, 304, 127430. [Google Scholar] [CrossRef]
- Zhu, S.; Xu, L.; Yang, S.; Zhou, X.; Chen, X.; Dong, B.; Bai, X.; Lu, G.; Song, H. Cobalt-doped ZnO nanoparticles derived from zeolite imidazole frameworks: Synthesis, characterization, and application for the detection of an exhaled diabetes biomarker. J. Colloid Interface Sci. 2020, 569, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yan, B. A point-of-care diagnostics logic detector based on glucose oxidase immobilized lanthanide functionalized metal-organic frameworks. Nanoscale 2019, 11, 22946–22953. [Google Scholar] [CrossRef] [PubMed]
- Adeel, M.; Canzonieri, V.; Daniele, S.; Vomiero, A.; Rizzolio, F.; Rahman, M. 2D metal azolate framework as nanozyme for amperometric detection of glucose at physiological pH and alkaline medium. Mikrochim. Acta 2021, 188, 77. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Xu, M.; Gu, Z. Copper-based two-dimensional metal-organic framework nanosheets as horseradish peroxidase mimics for glucose fluorescence sensing. Anal. Chim. Acta 2019, 1079, 164–170. [Google Scholar] [CrossRef]
- Yang, F.; Yang, L.; Xu, L.; Guo, W.; Pan, L.; Zhang, C.; Xu, S.; Zhang, N.; Yang, L.; Jiang, C. 3D-printed smartphone-based device for fluorimetric diagnosis of ketosis by acetone-responsive dye marker and red emissive carbon dots. Mikrochim. Acta 2021, 188, 306. [Google Scholar] [CrossRef] [PubMed]
- Diskin, A.; Spanel, P.; Smith, D. Time variation of ammonia, acetone, isoprene and ethanol in breath: A quantitative SIFT-MS study over 30 days. Physiol. Meas. 2003, 24, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Guo, H.; Zhao, B.; Cao, J. The molecular mechanisms of interactions between bioactive peptides and angiotensin-converting enzyme. Bioorganic Med. Chem. Lett. 2011, 21, 3898–3904. [Google Scholar] [CrossRef]
- Davis, P.; Dal Cortivo, L.; Maturo, J. Endogenous isopropanol: Forensic and biochemical implications. J. Anal. Toxicol. 1984, 8, 209–212. [Google Scholar] [CrossRef]
- Jiang, C.; Sun, M.; Wang, Z.; Chen, Z.; Zhao, X.; Yuan, Y.; Li, Y.; Wang, C. A Portable Real-Time Ringdown Breath Acetone Analyzer: Toward Potential Diabetic Screening and Management. Sensors 2016, 16, 1199. [Google Scholar] [CrossRef]
- Turner, C.; Walton, C.; Hoashi, S.; Evans, M. Breath acetone concentration decreases with blood glucose concentration in type I diabetes mellitus patients during hypoglycaemic clamps. J. Breath Res. 2009, 3, 046004. [Google Scholar] [CrossRef] [PubMed]
- Perelló-Roig, R.; Verd, J.; Bota, S.; Soberats, B.; Costa, A.; Segura, J. CMOS-MEMS VOC sensors functionalized via inkjet polymer deposition for high-sensitivity acetone detection. Lab A Chip 2021, 21, 3307–3315. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar, F.; Hajati, S.; Ghaedi, M.; Dashtian, K.; Naderi, H.; Toth, J. Highly selective MXene/VO/CuWO-based ultra-sensitive room temperature ammonia sensor. J. Hazard. Mater. 2021, 416, 126196. [Google Scholar] [CrossRef] [PubMed]
- Hee Cho, C.; Choe, Y.; Chae, S.; Il Lee, T. Highly sensitive breath sensor based on sonochemically synthesized cobalt-doped zinc oxide spherical beads. Ultrason. Sonochemistry 2022, 84, 105956. [Google Scholar] [CrossRef] [PubMed]
- Shahmoradi, A.; Hosseini, A.; Akbarinejad, A.; Alizadeh, N. Noninvasive Detection of Ammonia in the Breath of Hemodialysis Patients Using a Highly Sensitive Ammonia Sensor Based on a Polypyrrole/Sulfonated Graphene Nanocomposite. Anal. Chem. 2021, 93, 6706–6714. [Google Scholar] [CrossRef]
- Zhou, T.; Chen, S.; Wang, X.; Xie, C.; Zeng, D. Catalytic Activation of Cobalt Doping Sites in ZIF-71-Coated ZnO Nanorod Arrays for Enhancing Gas-Sensing Performance to Acetone. ACS Appl. Mater. Interfaces 2020, 12, 48948–48956. [Google Scholar] [CrossRef]
- Chen, J.; Shao, Z.; Zhao, Y.; Xue, X.; Song, H.; Wu, Z.; Cui, S.; Zhang, L.; Huang, C.; Mi, L.; et al. Metal-Ion Coupling in Metal-Organic Framework Materials Regulating the Output Performance of a Triboelectric Nanogenerator. Inorg. Chem. 2022, 61, 2490–2498. [Google Scholar] [CrossRef]
- Begum, S.; Hassan, Z.; Bräse, S.; Wöll, C.; Tsotsalas, M. Metal-Organic Framework-Templated Biomaterials: Recent Progress in Synthesis, Functionalization, and Applications. Acc. Chem. Res. 2019, 52, 1598–1610. [Google Scholar]
- Jonckheere, D.; Coutino-Gonzalez, E.; Baekelant, W.; Bueken, B.; Reinsch, H.; Stassen, I.; Fenwick, O.; Richard, F.; Samorì, P.; Ameloot, R.; et al. Silver-induced reconstruction of an adeninate-based metal-organic framework for encapsulation of luminescent adenine-stabilized silver clusters. J. Mater. Chem. C 2016, 4, 4259–4268. [Google Scholar] [CrossRef]
- Jia, R.; Tan, G.; Chen, Y.; Zuo, L.; Li, B.; Wang, L. Cu Ion Doping Enhances the Water Stability of Luminescent Metal-Organic Framework, Realizing the Detection of Fe and Antibiotics in Aqueous Solutions. Front. Chem. 2022, 10, 860232. [Google Scholar] [CrossRef]
- Ma, J.; Shu, T.; Sun, Y.; Zhou, X.; Ren, C.; Su, L.; Zhang, X. Luminescent Covalent Organic Frameworks for Biosensing and Bioimaging Applications. Small 2022, 18, e2103516. [Google Scholar] [CrossRef]
- Hasan, Z.; Jhung, S. Removal of hazardous organics from water using metal-organic frameworks (MOFs): Plausible mechanisms for selective adsorptions. J. Hazard. Mater. 2015, 283, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Reaven, G. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes 1988, 37, 1595–1607. [Google Scholar] [CrossRef]
- Mo, R.; Jiang, T.; Di, J.; Tai, W.; Gu, Z. Emerging micro- and nanotechnology based synthetic approaches for insulin delivery. Chem. Soc. Rev. 2014, 43, 3595–3629. [Google Scholar] [CrossRef] [PubMed]
- Hua, S. Advances in Oral Drug Delivery for Regional Targeting in the Gastrointestinal Tract - Influence of Physiological, Pathophysiological and Pharmaceutical Factors. Front. Pharmacol. 2020, 11, 524. [Google Scholar] [CrossRef]
- Duan, Y.; Ye, F.; Huang, Y.; Qin, Y.; He, C.; Zhao, S. One-pot synthesis of a metal-organic framework-based drug carrier for intelligent glucose-responsive insulin delivery. Chem. Commun. 2018, 54, 5377–5380. [Google Scholar] [CrossRef]
- Rojas, S.; Hidalgo, T.; Luo, Z.; Ávila, D.; Laromaine, A.; Horcajada, P. Ex VivoPushing the Limits on the Intestinal Crossing of Metal-Organic Frameworks: An and Detailed Study. ACS Nano 2022, 16, 5830–5838. [Google Scholar] [CrossRef]
- Archibald, D.D.; Mann, S. Template mineralization of self-assembled anisotropic lipid microstructures. Nature 1993, 364, 430–433. [Google Scholar] [CrossRef]
- Wang, C.; Sudlow, G.; Wang, Z.; Cao, S.; Jiang, Q.; Neiner, A.; Morrissey, J.; Kharasch, E.; Achilefu, S.; Singamaneni, S. Metal-Organic Framework Encapsulation Preserves the Bioactivity of Protein Therapeutics. Adv. Healthc. Mater. 2018, 7, e1800950. [Google Scholar] [CrossRef]
- Wang, S.; Chen, Y.; Wang, S.; Li, P.; Mirkin, C.; Farha, O. DNA-Functionalized Metal-Organic Framework Nanoparticles for Intracellular Delivery of Proteins. J. Am. Chem. Soc. 2019, 141, 2215–2219. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, L.; Cao, Y.; Yu, S.; He, C.; Chen, X. A Nanocomposite Vehicle Based on Metal-Organic Framework Nanoparticle Incorporated Biodegradable Microspheres for Enhanced Oral Insulin Delivery. ACS Appl. Mater. Interfaces 2020, 12, 22581–22592. [Google Scholar] [CrossRef]
- Chen, Y.; Li, P.; Modica, J.; Drout, R.; Farha, O. Acid-Resistant Mesoporous Metal-Organic Framework toward Oral Insulin Delivery: Protein Encapsulation, Protection, and Release. J. Am. Chem. Soc. 2018, 140, 5678–5681. [Google Scholar] [CrossRef]
- Zou, J.; Wei, G.; Xiong, C.; Yu, Y.; Li, S.; Hu, L.; Ma, S.; Tian, J. Efficient oral insulin delivery enabled by transferrin-coated acid-resistant metal-organic framework nanoparticles. Sci. Adv. 2022, 8, eabm4677. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Luo, G.; Vázquez-González, M.; Cazelles, R.; Sohn, Y.; Nechushtai, R.; Mandel, Y.; Willner, I. Glucose-Responsive Metal-Organic-Framework Nanoparticles Act as “Smart” Sense-and-Treat Carriers. ACS Nano 2018, 12, 7538–7545. [Google Scholar] [CrossRef] [PubMed]
- Tsourdi, E.; Barthel, A.; Rietzsch, H.; Reichel, A.; Bornstein, S. Current aspects in the pathophysiology and treatment of chronic wounds in diabetes mellitus. BioMed Res. Int. 2013, 2013, 385641. [Google Scholar] [CrossRef] [PubMed]
- Yazarlu, O.; Iranshahi, M.; Kashani, H.; Reshadat, S.; Habtemariam, S.; Iranshahy, M.; Hasanpour, M. Perspective on the application of medicinal plants and natural products in wound healing: A mechanistic review. Pharmacol. Res. 2021, 174, 105841. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Hu, J.; Idlett-Ali, S.; Zhang, L.; Caples, K.; Peddibhotla, S.; Reeves, M.; Zgheib, C.; Malany, S.; Liechty, K. Discovery of Small Molecule Activators of Chemokine Receptor CXCR4 That Improve Diabetic Wound Healing. Int. J. Mol. Sci. 2022, 23, 2196. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.; Ji, X.; Zhang, X.; Feng, C.; Tang, Z.; Kong, N.; Xie, A.; Wang, J.; Sui, X.; Deng, L.; et al. In situ sprayed NIR-responsive, analgesic black phosphorus-based gel for diabetic ulcer treatment. Proc. Natl. Acad. Sci. USA 2020, 117, 28667–28677. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Zheng, P.; Wang, H.; Kang, L.; Wu, H.; Fu, X. VEGF alleviates lower limb ischemia in diabetic mice by altering muscle fiber types. Exp. Ther. Med. 2022, 23, 251. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Zhang, X.; Liu, Y.; Fan, L.; Gan, J.; Liu, W.; Zhao, Y.; Sun, L. Polydopamine Decorated Microneedles with Fe-MSC-Derived Nanovesicles Encapsulation for Wound Healing. Adv. Sci. 2022, 9, e2103317. [Google Scholar] [CrossRef]
- Ding, Q.; Sun, T.; Su, W.; Jing, X.; Ye, B.; Su, Y.; Zeng, L.; Qu, Y.; Yang, X.; Wu, Y.; et al. Bioinspired Multifunctional Black Phosphorus Hydrogel with Antibacterial and Antioxidant Properties: A Stepwise Countermeasure for Diabetic Skin Wound Healing. Adv. Healthc. Mater. 2022, 11, e2102791. [Google Scholar] [CrossRef]
- Dubovoy, V.; Ganti, A.; Zhang, T.; Al-Tameemi, H.; Cerezo, J.D.; Boyd, J.M.; Asefa, T. One-Pot Hydrothermal Synthesis of Benzalkonium-Templated Mesostructured Silica Antibacterial Agents. J. Am. Chem. Soc. 2018, 140, 13534–13537. [Google Scholar] [CrossRef] [PubMed]
- Manzano, M.; Vallet-Regí, M. Mesoporous Silica Nanoparticles for Drug Delivery. Adv. Funct. Mater. 2020, 30, 1902634. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, S.; Xu, Y.; Shi, X.; Zhang, M.; Huang, Y.; Liang, Y.; Chen, Y.; Ji, W.; Kim, J.R.; et al. Engineering of hollow polymeric nanosphere-supported imidazolium-based ionic liquids with enhanced antimicrobial activities. Nano Res. 2022, 15, 5556–5568. [Google Scholar] [CrossRef]
- Tang, Y.; Varyambath, A.; Ding, Y.; Chen, B.; Huang, X.; Zhang, Y.; Yu, D.-G.; Kim, I.; Song, W. Porous organic polymers for drug delivery: Hierarchical pore structures, variable morphologies, and biological properties. Biomater. Sci. 2022. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Wang, P.; Zhang, A.; Qin, Z.; Li, Y.; Xianyu, Y.; Zhang, H. Covalent Organic Framework-Incorporated Nanofibrous Membrane as an Intelligent Platform for Wound Dressing. ACS Appl. Mater. Interfaces 2022, 14, 8680–8692. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, L.; Liu, H.; Pan, Y.; Li, C.; Xie, Z.; Jing, X. Ionic Covalent-Organic Framework Nanozyme as Effective Cascade Catalyst against Bacterial Wound Infection. Small 2021, 17, e2100756. [Google Scholar] [CrossRef]
- Tan, L.; Tan, B. Hypercrosslinked porous polymer materials: Design, synthesis, and applications. Chem. Soc. Rev. 2017, 46, 3322–3356. [Google Scholar] [CrossRef]
- Husna, A.; Hossain, I.; Jeong, I.; Kim, T. Mixed Matrix Membranes for Efficient CO Separation Using an Engineered UiO-66 MOF in a Pebax Polymer. Polymers 2022, 14, 655. [Google Scholar] [CrossRef]
- Xi, E.; Zhao, Y.; Xie, Y.; Gao, N.; Bian, Z.; Zhu, G. Biological Application of Porous Aromatic Frameworks: State of the Art and Opportunities. J. Phys. Chem. Lett. 2021, 12, 11050–11060. [Google Scholar] [CrossRef]
- Zhang, W.; Zuo, H.; Cheng, Z.; Shi, Y.; Guo, Z.; Meng, N.; Thomas, A.; Liao, Y. Macroscale Conjugated Microporous Polymers: Controlling Versatile Functionalities Over Several Dimensions. Adv. Mater. 2022, 34, e2104952. [Google Scholar] [CrossRef]
- Zhang, Y.; Kim, I.; Lu, Y.; Xu, Y.; Yu, D.-G.; Song, W. Intelligent poly(l-histidine)-based nanovehicles for controlled drug delivery. J. Control. Release 2022, 349, 963–982. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Song, W.; Lu, Y.; Xu, Y.; Wang, C.; Yu, D.-G.; Kim, I. Recent Advances in Poly(α-L-glutamic acid)-Based Nanomaterials for Drug Delivery. Biomolecules 2022, 12, 636. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Wong, R.; Anisa, A.; Ma, S. Recent development of metal-organic framework nanocomposites for biomedical applications. Biomaterials 2022, 281, 121322. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cai, J.; Liu, D.; Liu, S.; Lei, D.; Zheng, L.; Wei, Q.; Gao, M. Zinc-based metal organic framework with antibacterial and anti-inflammatory properties for promoting wound healing. Regen. Biomater. 2022, 9, rbac019. [Google Scholar] [CrossRef]
- Pan, M.; Ouyang, Y.; Song, Y.; Si, L.; Jiang, M.; Yu, X.; Xu, L.; Willner, I. Au -Functionalized UiO-67 Metal-Organic Framework Nanoparticles: O and •OH Generating Nanozymes and Their Antibacterial Functions. Small 2022, 18, e2200548. [Google Scholar] [CrossRef]
- Yin, M.; Wu, J.; Deng, M.; Wang, P.; Ji, G.; Wang, M.; Zhou, C.; Blum, N.; Zhang, W.; Shi, H.; et al. Multifunctional Magnesium Organic Framework-Based Microneedle Patch for Accelerating Diabetic Wound Healing. ACS Nano 2021, 15, 17842–17853. [Google Scholar] [CrossRef]
- Yang, J.; Yang, Y.-W. Metal–Organic Frameworks for Biomedical Applications. Small 2020, 16, 1906846. [Google Scholar] [CrossRef]
- Kim, S.; Kwon, Y.; Kim, D.; Park, H.; Cho, Y.; Nam, S.; Park, Y. A Review on Polymer Precursors of Carbon Molecular Sieve Membranes for Olefin/Paraffin Separation. Membranes 2021, 11, 482. [Google Scholar] [CrossRef]
- Tian, Y.; Zhu, G. Porous Aromatic Frameworks (PAFs). Chem. Rev. 2020, 120, 8934–8986. [Google Scholar] [CrossRef]
- Luo, S.; Almatrafi, E.; Tang, L.; Song, B.; Zhou, C.; Zeng, Y.; Zeng, G.; Liu, Z. Processable Conjugated Microporous Polymer Gels and Monoliths: Fundamentals and Versatile Applications. ACS Appl. Mater. Interfaces 2022. [Google Scholar] [CrossRef]
- Ragothaman, K.; Elmarsafi, T.; Mobaraki, A.; Zarick, C.; Evans, K.; Steinberg, J.; Attinger, C.; Kim, P. Evaluation of Polymerase Chain Reaction in the Identification and Quantification of Clinically Relevant Bacterial Species in Lower Extremity Wound Infections. J. Foot Ankle Surg. Off. Publ. Am. Coll. Foot Ankle Surg. 2022, 61, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Gardner, S.; Frantz, R. Wound bioburden and infection-related complications in diabetic foot ulcers. Biol. Res. Nurs. 2008, 10, 44–53. [Google Scholar] [CrossRef]
- Hu, F.; Xia, S.; He, Y.; Huang, Z.; Ke, H.; Liao, J. Reactive organic radical-doped Ag(I)-based coordination compounds for highly efficient antibacterial wound therapy. Colloids Surfaces. B Biointerfaces 2022, 213, 112425. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Xu, Q.; Sun, H.; Liu, W.; Chen, Y.; Xia, X.; Wang, C. Plasmonic Nanozymes: Localized Surface Plasmonic Resonance Regulates Reaction Kinetics and Antibacterial Performance. J. Phys. Chem. Lett. 2022, 13, 312–323. [Google Scholar] [CrossRef]
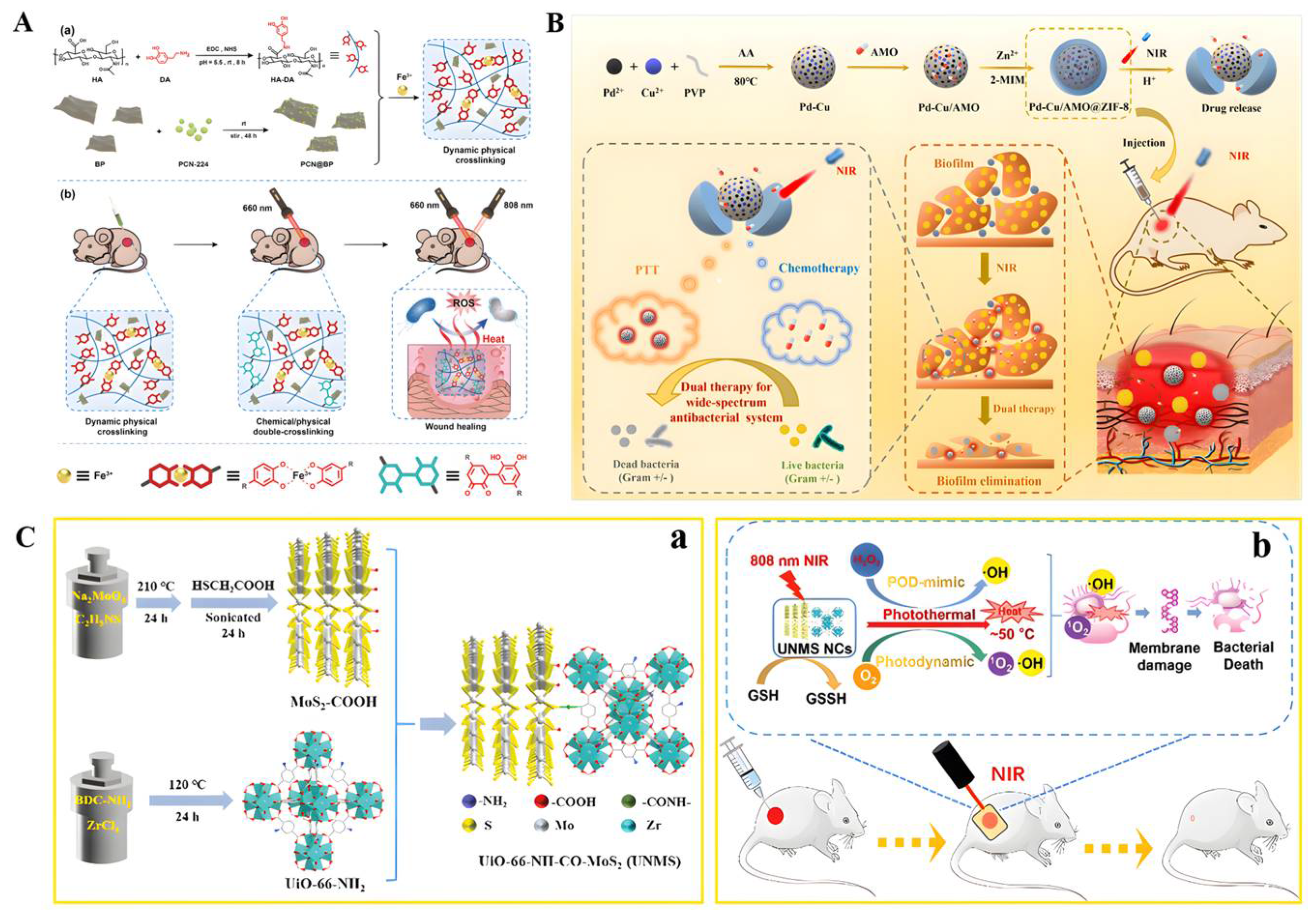
- Li, Q.; Liu, K.; Jiang, T.; Ren, S.; Kang, Y.; Li, W.; Yao, H.; Yang, X.; Dai, H.; Chen, Z. Injectable and self-healing chitosan-based hydrogel with MOF-loaded α-lipoic acid promotes diabetic wound healing. Mater. Sci. Engineering. C Mater. Biol. Appl. 2021, 131, 112519. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Zhu, Y.; Huddleston, S.; Li, P.; Xiao, B.; Farha, O.K.; Ameer, G.A. Copper Metal–Organic Framework Nanoparticles Stabilized with Folic Acid Improve Wound Healing in Diabetes. ACS Nano 2018, 12, 1023–1032. [Google Scholar] [CrossRef]
- Nie, X.; Wu, S.; Mensah, A.; Wang, Q.; Huang, F.; Wei, Q. FRET as a novel strategy to enhance the singlet oxygen generation of porphyrinic MOF decorated self-disinfecting fabrics. Chem. Eng. J. 2020, 395, 125012. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, X.; Yang, K.; Li, Q.; Wan, R.; Hu, G.; Ye, J.; Zhang, Y.; He, J.; Gu, H.; et al. Peroxidase- and UV-triggered oxidase mimetic activities of the UiO-66-NH2/chitosan composite membrane for antibacterial properties. Biomater. Sci. 2021, 9, 2647–2657. [Google Scholar] [CrossRef]
- Karakeçili, A.; Topuz, B.; Korpayev, S.; Erdek, M. Metal-organic frameworks for on-demand pH controlled delivery of vancomycin from chitosan scaffolds. Mater. Sci. Eng. C 2019, 105, 110098. [Google Scholar] [CrossRef]
- Mao, D.; Hu, F.; Kenry; Ji, S.; Wu, W.; Ding, D.; Kong, D.; Liu, B. Metal–Organic-Framework-Assisted In Vivo Bacterial Metabolic Labeling and Precise Antibacterial Therapy. Adv. Mater. 2018, 30, 1706831. [Google Scholar] [CrossRef]
- Melaiye, A.; Sun, Z.; Hindi, K.; Milsted, A.; Ely, D.; Reneker, D.; Tessier, C.; Youngs, W. Silver(I)-imidazole cyclophane gem-diol complexes encapsulated by electrospun tecophilic nanofibers: Formation of nanosilver particles and antimicrobial activity. J. Am. Chem. Soc. 2005, 127, 2285–2291. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Yoon, E.; Tak, Y.; Choi, E.; Song, J. Synthesis of highly antibacterial nanocrystalline trivalent silver polydiguanide. J. Am. Chem. Soc. 2009, 131, 16147–16155. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Ghosh, A. Development and evaluation of silver sulfadiazine loaded microsponge based gel for partial thickness (second degree) burn wounds. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2017, 96, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wu, L.; Li, B.; Zhao, Y.; Qu, P. Highly water-dispersible silver sulfadiazine decorated with polyvinyl pyrrolidone and its antibacterial activities. Mater. Sci. Engineering. C Mater. Biol. Appl. 2016, 60, 54–59. [Google Scholar] [CrossRef]
- Richards, R.; Taylor, R.; Zhu, Z. Mechanism for synergism between sulphonamides and trimethoprim clarified. J. Pharm. Pharmacol. 1996, 48, 981–984. [Google Scholar] [CrossRef]
- Dellera, E.; Bonferoni, M.; Sandri, G.; Rossi, S.; Ferrari, F.; Del Fante, C.; Perotti, C.; Grisoli, P.; Caramella, C. Development of chitosan oleate ionic micelles loaded with silver sulfadiazine to be associated with platelet lysate for application in wound healing. Eur. J. Pharm. Biopharm. Off. J. Arb. Fur Pharm. VerFahrenstechnik E. V 2014, 88, 643–650. [Google Scholar] [CrossRef]
- Luo, T.; Shakya, S.; Mittal, P.; Ren, X.; Guo, T.; Bello, M.; Wu, L.; Li, H.; Zhu, W.; Regmi, B.; et al. Co-delivery of superfine nano-silver and solubilized sulfadiazine for enhanced antibacterial functions. Int. J. Pharm. 2020, 584, 119407. [Google Scholar] [CrossRef]
- Javanbakht, S.; Nabi, M.; Shadi, M.; Amini, M.; Shaabani, A. Carboxymethyl cellulose/tetracycline@UiO-66 nanocomposite hydrogel films as a potential antibacterial wound dressing. Int. J. Biol. Macromol. 2021, 188, 811–819. [Google Scholar] [CrossRef]
- Li, C.; Chen, C.; Zhao, J.; Tan, M.; Zhai, S.; Wei, Y.; Wang, L.; Dai, T. Electrospun Fibrous Membrane Containing a Cyclodextrin Covalent Organic Framework with Antibacterial Properties for Accelerating Wound Healing. ACS Biomater. Sci. Eng. 2021, 7, 3898–3907. [Google Scholar] [CrossRef]
- Zhu, J.; Qiu, W.; Yao, C.; Wang, C.; Wu, D.; Pradeep, S.; Yu, J.; Dai, Z. Water-stable zirconium-based metal-organic frameworks armed polyvinyl alcohol nanofibrous membrane with enhanced antibacterial therapy for wound healing. J. Colloid Interface Sci. 2021, 603, 243–251. [Google Scholar] [CrossRef]
- Gliozzi, M.; Scicchitano, M.; Bosco, F.; Musolino, V.; Carresi, C.; Scarano, F.; Maiuolo, J.; Nucera, S.; Maretta, A.; Paone, S.; et al. Modulation of Nitric Oxide Synthases by Oxidized LDLs: Role in Vascular Inflammation and Atherosclerosis Development. Int. J. Mol. Sci. 2019, 20, 3294. [Google Scholar] [CrossRef] [PubMed]
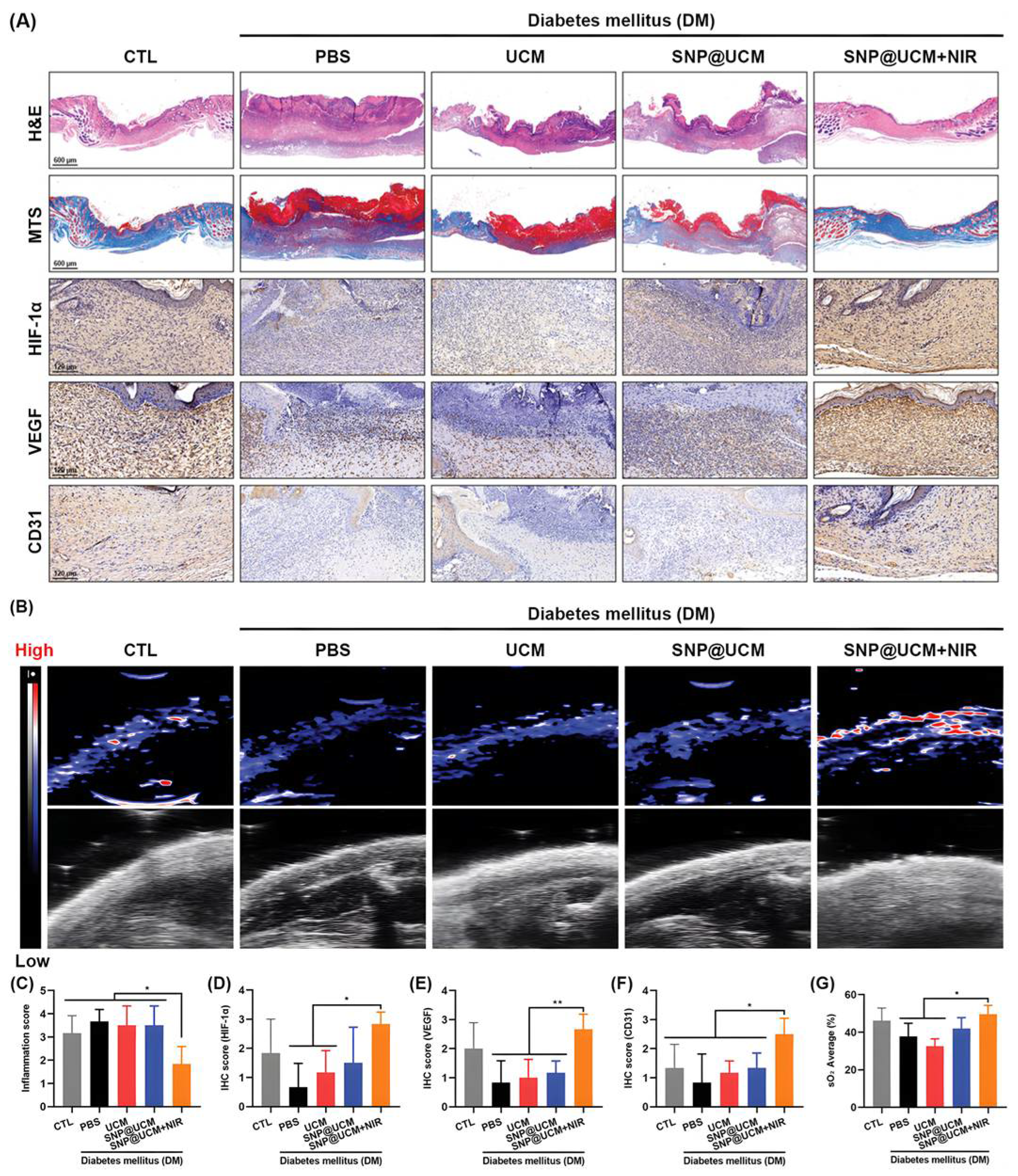
- Yang, Y.; Huang, K.; Wang, M.; Wang, Q.; Chang, H.; Liang, Y.; Wang, Q.; Zhao, J.; Tang, T.; Yang, S. Ubiquitination Flow Repressors: Enhancing Wound Healing of Infectious Diabetic Ulcers through Stabilization of Polyubiquitinated Hypoxia-Inducible Factor-1α by Theranostic Nitric Oxide Nanogenerators. Adv. Mater. 2021, 33, e2103593. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.; Boulton, A.; Bus, S. Diabetic Foot Ulcers and Their Recurrence. New Engl. J. Med. 2017, 376, 2367–2375. [Google Scholar] [CrossRef]
- Matoori, S.; Veves, A.; Mooney, D. Advanced bandages for diabetic wound healing. Sci. Transl. Med. 2021, 13, eabe4839. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, F.; Canaud, B.; Eckardt, K.; Stenvinkel, P.; Wanner, C.; Zoccali, C. Oxidative stress in end-stage renal disease: An emerging threat to patient outcome. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc.-Eur. Ren. Assoc. 2003, 18, 1272–1280. [Google Scholar] [CrossRef]
- Liakopoulos, V.; Roumeliotis, S.; Bozikas, A.; Eleftheriadis, T.; Dounousi, E. Antioxidant Supplementation in Renal Replacement Therapy Patients: Is There Evidence? Oxidative Med. Cell. Longev. 2019, 2019, 9109473. [Google Scholar] [CrossRef] [Green Version]
- Xia, S.; Weng, T.; Jin, R.; Yang, M.; Yu, M.; Zhang, W.; Wang, X.; Han, C. Curcumin-incorporated 3D bioprinting gelatin methacryloyl hydrogel reduces reactive oxygen species-induced adipose-derived stem cell apoptosis and improves implanting survival in diabetic wounds. Burn. Trauma 2022, 10, tkac001. [Google Scholar] [CrossRef]
- Xu, Z.; Deng, B.; Wang, X.; Yu, J.; Xu, Z.; Liu, P.; Liu, C.; Cai, Y.; Wang, F.; Zong, R.; et al. Nanofiber-mediated sequential photothermal antibacteria and macrophage polarization for healing MRSA-infected diabetic wounds. J. Nanobiotechnology 2021, 19, 404. [Google Scholar] [CrossRef]
- Yang, P.; Zhang, J.; Xiang, S.; Jin, Z.; Zhu, F.; Wang, T.; Duan, G.; Liu, X.; Gu, Z.; Li, Y. Green Nanoparticle Scavengers against Oxidative Stress. ACS Appl. Mater. Interfaces 2021, 13, 39126–39134. [Google Scholar] [CrossRef]
- Poole, K.; Nelson, C.; Joshi, R.; Martin, J.; Gupta, M.; Haws, S.; Kavanaugh, T.; Skala, M.; Duvall, C. ROS-responsive microspheres for on demand antioxidant therapy in a model of diabetic peripheral arterial disease. Biomaterials 2015, 41, 166–175. [Google Scholar] [CrossRef]
- Dang, T.; Thai, A.; Cohen, J.; Slosberg, J.; Siniakowicz, K.; Doloff, J.; Ma, M.; Hollister-Lock, J.; Tang, K.; Gu, Z.; et al. Enhanced function of immuno-isolated islets in diabetes therapy by co-encapsulation with an anti-inflammatory drug. Biomaterials 2013, 34, 5792–5801. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, W.; Meng, F.; Zhou, Z.; Zhao, Z.; Mei, Z. Analgesic and neuroprotective effects of Baimai Ointment on diabetic peripheral neuropathy. J. Ethnopharmacol. 2022, 292, 115122. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Sheng, J.; Yang, R. Encapsulation of curcumin in CD-MOFs: Promoting its incorporation into water-based products and consumption. Food Funct. 2021, 12, 10795–10805. [Google Scholar] [CrossRef]
- Chen, Y.; Tai, K.; Ma, P.; Su, J.; Dong, W.; Gao, Y.; Mao, L.; Liu, J.; Yuan, F. Novel γ-cyclodextrin-metal-organic frameworks for encapsulation of curcumin with improved loading capacity, physicochemical stability and controlled release properties. Food Chem. 2021, 347, 128978. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; He, L.; Zhang, P.; Zhang, J.; Mei, X.; Wang, D.; Zhang, Y.; Ren, X.; Chen, Z. Encapsulation of green tea polyphenol nanospheres in PVA/alginate hydrogel for promoting wound healing of diabetic rats by regulating PI3K/AKT pathway. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110686. [Google Scholar] [CrossRef]
- Song, H.; Li, X.; He, Y.; Peng, Y.; Pan, J.; Niu, X.; Zhao, H.; Lan, M. Colorimetric evaluation of the hydroxyl radical scavenging ability of antioxidants using carbon-confined CoO as a highly active peroxidase mimic. Mikrochim. Acta 2019, 186, 354. [Google Scholar] [CrossRef]
- Tian, Y.; Li, Y.; Liu, J.; Lin, Y.; Jiao, J.; Chen, B.; Wang, W.; Wu, S.; Li, C. Photothermal therapy with regulated Nrf2/NF-κB signaling pathway for treating bacteria-induced periodontitis. Bioact. Mater. 2022, 9, 428–445. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Ye, Z.; Zhang, M.; Song, Q.; Chu, X.; Gao, S.; Zhang, Q.; Jiang, C.; Zhou, N.; Yao, C.; et al. Light-Activated Biodegradable Covalent Organic Framework-Integrated Heterojunction for Photodynamic, Photothermal, and Gaseous Therapy of Chronic Wound Infection. ACS Appl. Mater. Interfaces 2021, 13, 42396–42410. [Google Scholar] [CrossRef]
- Abdelhamid, H. Zeolitic Imidazolate Frameworks (ZIF-8) for Biomedical Applications: A Review. Curr. Med. Chem. 2021, 28, 7023–7075. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, X.; Zhao, X.; Chen, L.; Yan, X. Near-Infrared Photothermal/Photodynamic-in-One Agents Integrated with a Guanidinium-Based Covalent Organic Framework for Intelligent Targeted Imaging-Guided Precision Chemo/PTT/PDT Sterilization. ACS Appl. Mater. Interfaces 2021, 13, 27895–27903. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; He, P.; Wang, Y.; Bai, H.; Wang, S.; Xu, J.; Zhang, X. Supramolecular Radical Anions Triggered by Bacteria In Situ for Selective Photothermal Therapy. Angew. Chem. 2017, 56, 16239–16242. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Li, H.; Wang, B.; Ye, Z.; Lei, W.; Jia, F.; Jin, Q.; Ren, K.; Ji, J. Surface-Adaptive Gold Nanoparticles with Effective Adherence and Enhanced Photothermal Ablation of Methicillin-Resistant Staphylococcus aureus Biofilm. ACS Nano 2017, 11, 9330–9339. [Google Scholar] [CrossRef]
- Wang, X.; Su, K.; Tan, L.; Liu, X.; Cui, Z.; Jing, D.; Yang, X.; Liang, Y.; Li, Z.; Zhu, S.; et al. Rapid and Highly Effective Noninvasive Disinfection by Hybrid Ag/CS@MnO Nanosheets Using Near-Infrared Light. ACS Appl. Mater. Interfaces 2019, 11, 15014–15027. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Li, B.; He, H.; Zhou, W.; Chen, B.; Qian, G. Metal-Organic Frameworks as Platforms for Functional Materials. Acc. Chem. Res. 2016, 49, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wu, X.; He, C.; Huang, J.; Yin, S.; Zhou, M.; Ma, L.; Zhao, W.; Qiu, L.; Cheng, C.; et al. Metal-Organic Framework/Ag-Based Hybrid Nanoagents for Rapid and Synergistic Bacterial Eradication. ACS Appl. Mater. Interfaces 2020, 12, 13698–13708. [Google Scholar] [CrossRef] [PubMed]
- Jagadeesh, R.V.; Murugesan, K.; Alshammari, A.S.; Neumann, H.; Pohl, M.M.; Radnik, J.; Beller, M. MOF-derived cobalt nanoparticles catalyze a general synthesis of amines. Science 2017, 358, 326–332. [Google Scholar] [CrossRef] [Green Version]
- Lustig, W.; Mukherjee, S.; Rudd, N.; Desai, A.; Li, J.; Ghosh, S. Metal-organic frameworks: Functional luminescent and photonic materials for sensing applications. Chem. Soc. Rev. 2017, 46, 3242–3285. [Google Scholar]
- Cui, Y.; Zhang, J.; He, H.; Qian, G. Photonic functional metal-organic frameworks. Chem. Soc. Rev. 2018, 47, 5740–5785. [Google Scholar] [CrossRef]
- Liao, Z.Y.; Xia, Y.M.; Zuo, J.M.; Wang, T.; Hu, D.T.; Li, M.Z.; Shao, N.N.; Chen, D.; Song, K.X.; Yu, X.; et al. Metal-Organic Framework Modified MoS Nanozyme for Synergetic Combating Drug-Resistant Bacterial Infections via Photothermal Effect and Photodynamic Modulated Peroxidase-Mimic Activity. Adv. Healthc. Mater. 2022, 11, e2101698. [Google Scholar] [CrossRef]
- Xiao, Y.; Xu, M.; Lv, N.; Cheng, C.; Huang, P.; Li, J.; Hu, Y.; Sun, M. Dual stimuli-responsive metal-organic framework-based nanosystem for synergistic photothermal/pharmacological antibacterial therapy. Acta Biomater. 2021, 122, 291–305. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, H.; Li, P.; Liu, W.; Zhang, Y.; Dong, A. Dual-Light-Triggered In Situ Structure and Function Regulation of Injectable Hydrogels for High-Efficient Anti-Infective Wound Therapy. Adv. Healthc. Mater. 2022, 11, e2101722. [Google Scholar] [CrossRef] [PubMed]
- Laksanalamai, P.; Maeder, D.; Robb, F. Regulation and mechanism of action of the small heat shock protein from the hyperthermophilic archaeon Pyrococcus furiosus. J. Bacteriol. 2001, 183, 5198–5202. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Peng, Y.; Zhou, Y.; Zhang, S.; Tan, J.; Li, H.; He, D.; Deng, L. Pd-Cu nanoalloy for dual stimuli-responsive chemo-photothermal therapy against pathogenic biofilm bacteria. Acta Biomater. 2022, 137, 276–289. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Luo, Y.; Liu, X.; Cui, Z.; Zheng, Y.; Liang, Y.; Li, Z.; Zhu, S.; Lei, J.; Feng, X.; et al. The enhanced photocatalytic sterilization of MOF-Based nanohybrid for rapid and portable therapy of bacteria-infected open wounds. Bioact. Mater. 2022, 13, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Ezraty, B.; Gennaris, A.; Barras, F.; Collet, J. Oxidative stress, protein damage and repair in bacteria. Nat. Rev. Microbiol. 2017, 15, 385–396. [Google Scholar] [CrossRef]
- Yin, W.; Yu, J.; Lv, F.; Yan, L.; Zheng, L.; Gu, Z.; Zhao, Y. Functionalized Nano-MoS with Peroxidase Catalytic and Near-Infrared Photothermal Activities for Safe and Synergetic Wound Antibacterial Applications. ACS Nano 2016, 10, 11000–11011. [Google Scholar] [CrossRef]
- Hu, W.; Younis, M.; Zhou, Y.; Wang, C.; Xia, X. In Situ Fabrication of Ultrasmall Gold Nanoparticles/2D MOFs Hybrid as Nanozyme for Antibacterial Therapy. Small 2020, 16, e2000553. [Google Scholar] [CrossRef]
- Shakir, A.; Abate, D.; Tebeje, F.; Weledegebreal, F. Magnitude of Surgical Site Infections, Bacterial Etiologies, Associated Factors and Antimicrobial Susceptibility Patterns of Isolates Among Post-Operative Patients in Harari Region Public Hospitals, Harar, Eastern Ethiopia. Infect. Drug Resist. 2021, 14, 4629–4639. [Google Scholar] [CrossRef]
- Moaness, M.; Mabrouk, M.; Ahmed, M.; Das, D.; Beherei, H. Novel zinc-silver nanocages for drug delivery and wound healing: Preparation, characterization and antimicrobial activities. Int. J. Pharm. 2022, 616, 121559. [Google Scholar] [CrossRef]
- Haseena; Khan, A.; Ghaffar, I.; Baty, R.; Abdel-Daim, M.; Habib, S.; Kanwal, T.; Shah, M. Synthesis of Ribose-Coated Copper-Based Metal-Organic Framework for Enhanced Antibacterial Potential of Chloramphenicol against Multi-Drug Resistant Bacteria. Antibiotics 2021, 10, 1469. [Google Scholar] [CrossRef]
- Haseena; Khan, A.; Aslam, F.; Kanwal, T.; Shah, M.; Khalil, A.; Shah, S.; Alshammari, E.; El-Masry, E.; Batiha, G.; et al. Helicobacter pyloriEnhanced Antibacterial Potential of Amoxicillin against Mediated by Lactobionic Acid Coated Zn-MOFs. AntiBiotics 2021, 10, 1071. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Jiang, W.; Huo, S.; Li, S.; Xu, Y.; Ding, S.; Zhou, J.; Liu, H.; Lv, W.; Wang, Y. Nano-metal-organic-frameworks for treating HO-Secreting bacteria alleviate pulmonary injury and prevent systemic sepsis. Biomaterials 2021, 279, 121237. [Google Scholar] [CrossRef] [PubMed]
- Min, T.; Sun, X.; Zhou, L.; Du, H.; Zhu, Z.; Wen, Y. Electrospun pullulan/PVA nanofibers integrated with thymol-loaded porphyrin metal-organic framework for antibacterial food packaging. Carbohydr. Polym. 2021, 270, 118391. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, Z.; Abdi, J.; Vossoughi, M.; Alemzadeh, I. Ag-doped magnetic metal organic framework as a novel nanostructured material for highly efficient antibacterial activity. Environ. Res. 2020, 188, 109555. [Google Scholar] [CrossRef]
- Tan, L.; Yuan, G.; Wang, P.; Feng, S.; Tong, Y.; Wang, C. pH-responsive Ag-Phy@ZIF-8 nanoparticles modified by hyaluronate for efficient synergistic bacteria disinfection. Int. J. Biol. Macromol. 2022, 206, 605–613. [Google Scholar] [CrossRef]
- Peng, L.; Yang, X.; Wang, S.; Chan, Y.; Chen, Y.; Yang, Z.; Mao, Y.; Li, L.; Yang, W.; Deng, Y. Bimetal metal-organic framework domino micro-reactor for synergistic antibacterial starvation/chemodynamic therapy and robust wound healing. Nanoscale 2022, 14, 2052–2064. [Google Scholar] [CrossRef]
- Ma, Y.; Xu, H.; Sun, B.; Du, S.; Cui, S.; Zhang, L.; Ding, N.; Yang, D. pH-Responsive Oxygen and Hydrogen Peroxide Self-Supplying Nanosystem for Photodynamic and Chemodynamic Therapy of Wound Infection. ACS Appl. Mater. Interfaces 2021, 13, 59720–59730. [Google Scholar] [CrossRef]
- Fatima, S.; Ambreen, S.; Mathew, A.; Elwakiel, A.; Gupta, A.; Singh, K.; Krishnan, S.; Rana, R.; Khawaja, H.; Gupta, D.; et al. ER-Stress and Senescence Coordinately Promote Endothelial Barrier Dysfunction in Diabetes-Induced Atherosclerosis. Nutrients 2022, 14, 2786. [Google Scholar] [CrossRef]
- Wu, C.; Long, L.; Zhang, Y.; Xu, Y.; Lu, Y.; Yang, Z.; Guo, Y.; Zhang, J.; Hu, X.; Wang, Y. Injectable conductive and angiogenic hydrogels for chronic diabetic wound treatment. J. Control. Release Off. J. Control. Release Soc. 2022, 344, 249–260. [Google Scholar] [CrossRef]
- Wang, T.; Zhou, Z.; Liu, J.; Hou, X.; Zhou, Z.; Dai, Y.; Hou, Z.; Chen, F.; Zheng, L. Donut-like MOFs of copper/nicotinic acid and composite hydrogels with superior bioactivity for rh-bFGF delivering and skin wound healing. J. Nanobiotechnology 2021, 19, 275. [Google Scholar] [CrossRef]
- Gérard, C.; Bordeleau, L.; Barralet, J.; Doillon, C. The stimulation of angiogenesis and collagen deposition by copper. Biomaterials 2010, 31, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Chen, S.; Yi, J.; Zhang, H.; Ameer, G. A Cooperative Copper Metal-Organic Framework-Hydrogel System Improves Wound Healing in Diabetes. Adv. Funct. Mater. 2017, 27, 1604872. [Google Scholar] [CrossRef]
- Sen, C.; Khanna, S.; Venojarvi, M.; Trikha, P.; Ellison, E.; Hunt, T.; Roy, S. Copper-induced vascular endothelial growth factor expression and wound healing. American journal of physiology. Heart Circ. Physiol. 2002, 282, H1821–H1827. [Google Scholar] [CrossRef] [PubMed]
- Philips, N.; Hwang, H.; Chauhan, S.; Leonardi, D.; Gonzalez, S. Stimulation of cell proliferation and expression of matrixmetalloproteinase-1 and interluekin-8 genes in dermal fibroblasts by copper. Connect. Tissue Res. 2010, 51, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Kargozar, S.; Mozafari, M.; Ghodrat, S.; Fiume, E.; Baino, F. Copper-containing bioactive glasses and glass-ceramics: From tissue regeneration to cancer therapeutic strategies. Mater. Sci. Engineering. C Mater. Biol. Appl. 2021, 121, 111741. [Google Scholar] [CrossRef]
- Tummanapalli, S.; Kuppusamy, R.; Yeo, J.; Kumar, N.; New, E.; Willcox, M. The role of nitric oxide in ocular surface physiology and pathophysiology. Ocul. Surf. 2021, 21, 37–51. [Google Scholar] [CrossRef]
- Su, C.; Li, W.; Tsao, L.; Wang, L.; Hsu, Y.; Wang, W.; Liao, M.; Lee, C.; Yeh, C. Enhancing Microcirculation on Multitriggering Manner Facilitates Angiogenesis and Collagen Deposition on Wound Healing by Photoreleased NO from Hemin-Derivatized Colloids. ACS Nano 2019, 13, 4290–4301. [Google Scholar] [CrossRef]
- Moncada, S.; Palmer, R.; Higgs, E. Biosynthesis of nitric oxide from L-arginine. A pathway for the regulation of cell function and communication. Biochem. Pharmacol. 1989, 38, 1709–1715. [Google Scholar] [CrossRef]
- Schäffer, M.; Tantry, U.; Efron, P.; Ahrendt, G.; Thornton, F.; Barbul, A. Diabetes-impaired healing and reduced wound nitric oxide synthesis: A possible pathophysiologic correlation. Surgery 1997, 121, 513–519. [Google Scholar] [CrossRef]
- Alharbi, M.; Zhang, C.; Lu, C.; Milovanova, T.; Yi, L.; Ryu, J.; Jiao, H.; Dong, G.; O’Connor, J.; Graves, D. FOXO1 Deletion Reverses the Effect of Diabetic-Induced Impaired Fracture Healing. Diabetes 2018, 67, 2682–2694. [Google Scholar] [CrossRef]
- Kämpfer, H.; Pfeilschifter, J.; Frank, S. Expression and activity of arginase isoenzymes during normal and diabetes-impaired skin repair. J. Investig. Dermatol. 2003, 121, 1544–1551. [Google Scholar] [CrossRef] [PubMed]
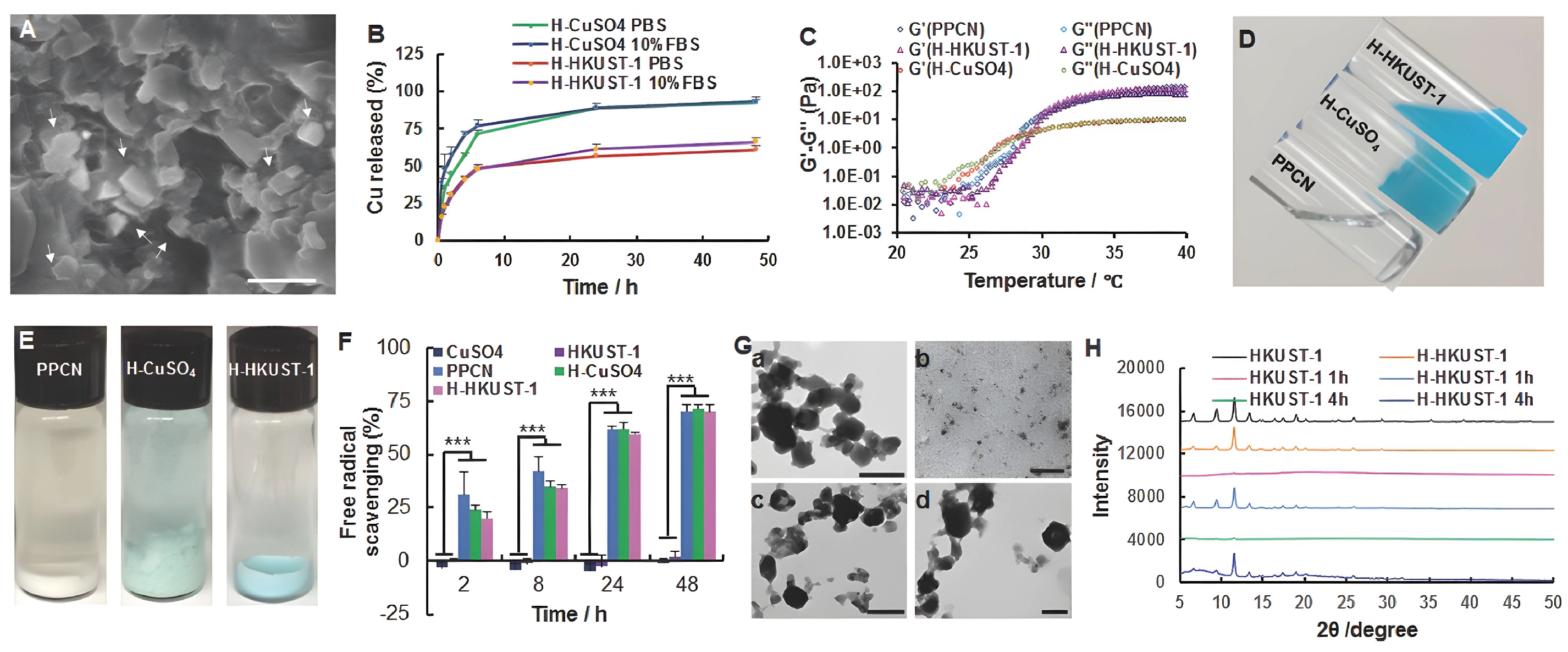
- Zhang, P.; Li, Y.; Tang, Y.; Shen, H.; Li, J.; Yi, Z.; Ke, Q.; Xu, H. Copper-Based Metal-Organic Framework as a Controllable Nitric Oxide-Releasing Vehicle for Enhanced Diabetic Wound Healing. ACS Appl. Mater. Interfaces 2020, 12, 18319–18331. [Google Scholar] [CrossRef] [PubMed]
- Soto, R.; Merricks, E.; Bellinger, D.; Nichols, T.; Schoenfisch, M. Influence of diabetes on the foreign body response to nitric oxide-releasing implants. Biomaterials 2018, 157, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Breen, A.; Dockery, P.; O’Brien, T.; Pandit, A. The use of therapeutic gene eNOS delivered via a fibrin scaffold enhances wound healing in a compromised wound model. Biomaterials 2008, 29, 3143–3151. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, K.; Liu, Z.; Xiao, M.; Chen, H.; Goldstein, L.; Buerk, D.; Nedeau, A.; Thom, S.; Velazquez, O. Diabetic impairments in NO-mediated endothelial progenitor cell mobilization and homing are reversed by hyperoxia and SDF-1 alpha. J. Clin. Investig. 2007, 117, 1249–1259. [Google Scholar] [CrossRef]
- Kumar, P.; Anand, B.; Tsang, Y.; Kim, K.; Khullar, S.; Wang, B. Regeneration, degradation, and toxicity effect of MOFs: Opportunities and challenges. Environ. Res. 2019, 176, 108488. [Google Scholar] [CrossRef]
- Ploetz, E.; Engelke, H.; Lchelt, U.; Wuttke, S. The Chemistry of Reticular Framework Nanoparticles: MOF, ZIF, and COF Materials. Adv. Funct. Mater. 2020, 1909062. [Google Scholar] [CrossRef]
- Ettlinger, R.; Lächelt, U.; Gref, R.; Horcajada, P.; Lammers, T.; Serre, C.; Couvreur, P.; Morris, R.; Wuttke, S. Toxicity of metal-organic framework nanoparticles: From essential analyses to potential applications. Chemical Society Reviews 2022, 51, 464–484. [Google Scholar] [CrossRef]
- Sajid, M.; Khan, I. Toxicity of nanoscale metal-organic frameworks in biological systems. Met.-Org. Framew. Biomed. Appl. 2020, 383–395. [Google Scholar]



| Synthetic Methods | Advantages | Disadvantages | Ref. |
|---|---|---|---|
| Hydrothermal/solvothermal synthesis | Generality, simplicity, and low cost. Can large-scale preparation at mild temperatures. | Long reaction time, high temperature requirement | [22,27,28] |
| Microwave-assisted synthesis | Shorten the reaction time to a few hours or even several minutes. No excessive by-products and the high purity and small size of MOFs obtained. | Reaction solvent requirements are limited | [29,30,31] |
| Room-temperature synthesis | Reaction conditions are simple and can be prepared on a large scale. | Limited range of adaptation | [32,33] |
| Ultrasonic-assisted synthesis | Quickly disperse solutes and speed up the reaction process, improve the reaction efficiency. | Hard to obtain a lot of product | [34,35] |
| Mechanochemical synthesis | Safety, environmental protection | Hard to obtain a lot of product | [36,37] |
| Microfluidic synthesis | The effective mixing of organic phase and inorganic phase is realized. | Limited range of adaptation | [38,39] |
| Biomimetic mineralization | Beneficial to enhance the robustness and biocompatibility of biomacromolecules. | Only suitable for biomacromolecules that are similar in size to the pore size of MOFs and are solvent resistant in the preparation of MOFs. | [25,26,40] |
| Porous Materials | Composition | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|
| Metal–organic framework (MOF) | Organic ligands and their coordinated metal ions/ion clusters | Ordered porous structure, biocompatibility, and ease of functional modification. | Targeting and potential biotoxicity. | [124,128] |
| Mesoporous silica nanoparticles (MSN) | Silica (SiO2) | Huge loading capacity, controllable particle size and shape, suitability for easy functionalization, and biocompatibility. | Poor dispersibility and stability, prone to accumulation, and requires modification. A fully reversible lid is required to close the pore access. | [113] |
| Hollow polymeric nanosphere (HPN) | 1,4-Bisbenzenedimethanol (BDM), 1,2-dichloroethane (DCE) | Has uniform hollow spherical shape, sufficient surface area, and excellent physicochemical stability. | The synthesis scale is small, the cost is high, the controllability is poor, and the mechanism research is not in-depth. | [114] |
| Poly (α-L-glutamic acid) (PGA) | L-glutamic acid | Inherent biodegradability, biocompatibility, and ion charging characteristics. | The synthesis and purification procedures are complex, and the scalability and repeatability need to be improved. | [123] |
| Poly (L-histidine) (PLH) | α-Amino acid N-carboxylic anhydride (NCAs) | Positive charge, suitable for PH-triggered targeted drug delivery. | The synthesis process has poor repeatability and multiple molecular weight distributions. Unpredictable drug coupling sites increase the heterogeneity of PLH. | [122] |
| Covalent organic frameworks (COFs) | Light elements (H, C, N, O, B) | Large surface area, high thermal stability, good biocompatibility, and good biodegradability. | The synthesis condition is not mild enough, the preparation cost is high, and the structure is uncontrollable. | [116] |
| Hyper-crosslinked polymers (HCPs) | Light elements (H, C, N, O, B) | High specific surface area, mild synthesis conditions, a wide range of monomer sources, cheap and easy to obtain catalyst. | Modification strategies and synthesis methods need to be improved. | [118] |
| Polymers of intrinsic microporosity (PIMs) | Light elements (H, C, N, O, B) | Highly microporous | The resulting materials are amorphous and have a wide pore size distribution, which is not easy to adjust and control. | [119,129] |
| Porous aromatic frameworks (PAFs) | Light elements (H, C, N, O, B) | High stability, large specific surface area, large pore volume, strong modifiability. | Locally ordered and long-range disordered skeleton structure | [120,130] |
| Conjugated microporous polymers (CMPs) | Light elements (H, C, N, O, B) | Multi-micropore, high surface area, chemical stability, thermal stability, structure adjustable. | Expensive production costs | [131] |
| MOF | MOF Skeleton Components | Antibacterial Composition | Pathogenic Bacteria | References |
|---|---|---|---|---|
| MIL-53 | Fe3+, terephthalic acid, chitosan | Vancomycin | S. aureus | [10] |
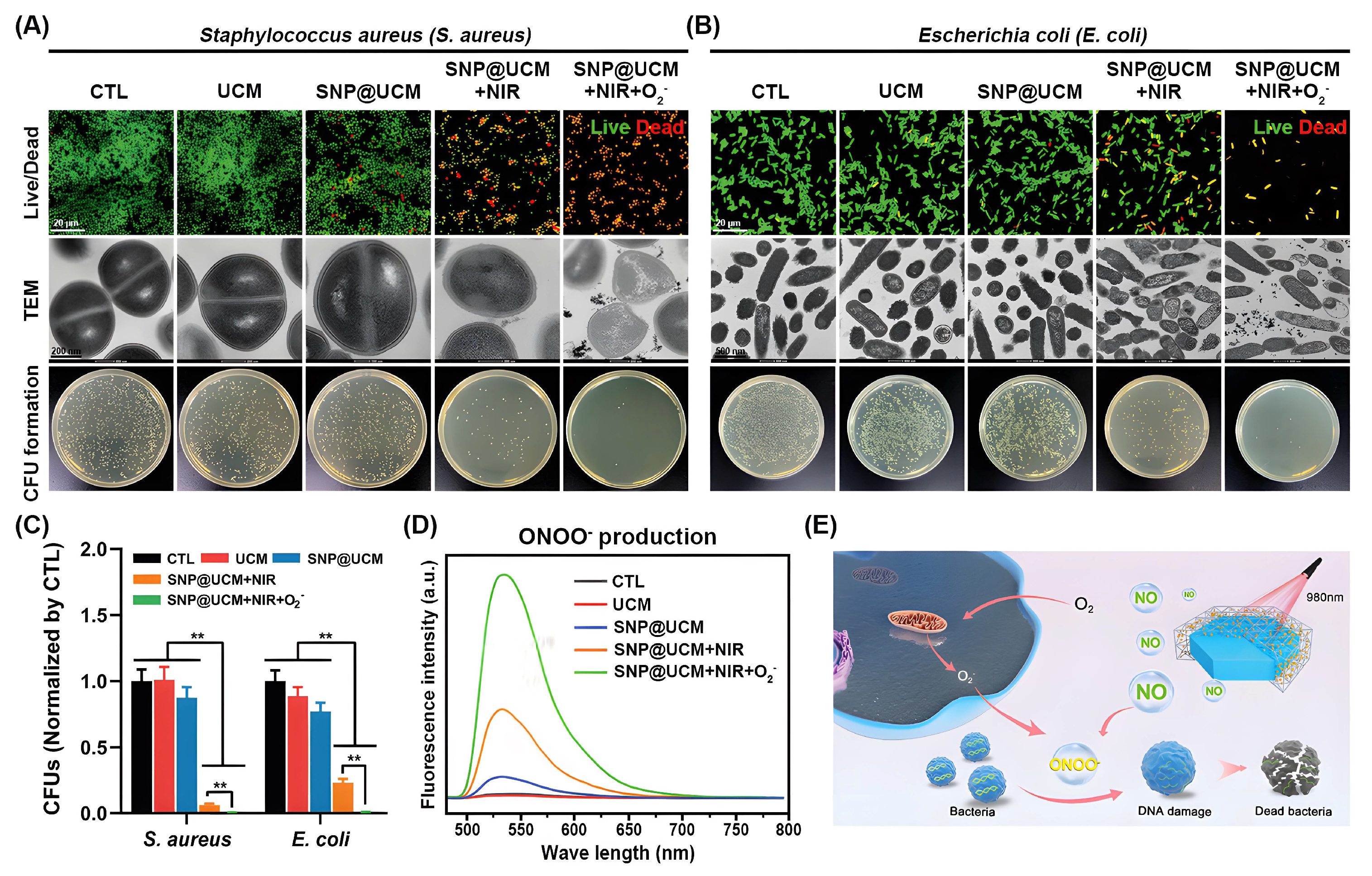
| SNP@UCM | SNP, ssPDA, UCNP | NO, ROS | S. aureus and E. coli | [153] |
| Cu-MOFs | Cu2+, ribose, chloramphenicol | CHL, Cu2+ | E. coli and P. aeruginosa | [191] |
| Zn-MOF | Zn2+, lactobionic acid | Amoxicillin, Zn2+ | H. pylori | [192] |
| nFMs@Amp | Fe3+, pluronic F-127 | •OH | S. pneumoniae | [193] |
| PCN-224 MOFs | Zr4+, pullulan, polyvinyl alcohol | 1O2 | E. coli and S. aureus | [194] |
| LV@UiO-66-NH2@PVA | Nanofibrous membranes, UiO-66-NH2, polyvinyl alcohol | Levofloxacin | E. coli and S. aureus | [151] |
| FSZ-Ag | Ag+, Zn2+, 2-methylimidazole | Ag+, Zn2+ | E. coli and S. aureus | [195] |
| C-Zn/Ag | Ag+, Zn2+, 2-methylimidazole | Ag+, Zn2+ | E. coli and S. aureus | [176] |
| Ag-Phy@ZIF-8@HA | Ag+, Zn2+, Physcion, 2-methylimidazole | Ag+, Physcion | E. coli and S. aureus | [196] |
| BMOF-DMR | Cu2+, Zn2+, 2-methylimidazole | Cu2+, Zn2+ | E. coli and S. aureus | [197] |
| PCN@BP | Zr4+, TCPP, benzoic acid, DMF, BP | ROS | E. coli and S. aureus | [182] |
| CaO2/GQDs@ZIF-67 | Co2+, 2-methylimidazole, CaO2 | •OH | E. coli and S. aureus | [198] |
| Au3+-UiO-67 | Au3+, Zr4+, 2,2′-bipyridine-5,5′-dicarboxy acid | •OH, 1O2 | E. coli and S. aureus | [126] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Q.; Bai, Q.; Zheng, C.; Sun, N.; Liu, J.; Chen, W.; Hu, F.; Lu, T. Application of Metal–Organic Framework in Diagnosis and Treatment of Diabetes. Biomolecules 2022, 12, 1240. https://doi.org/10.3390/biom12091240
Gao Q, Bai Q, Zheng C, Sun N, Liu J, Chen W, Hu F, Lu T. Application of Metal–Organic Framework in Diagnosis and Treatment of Diabetes. Biomolecules. 2022; 12(9):1240. https://doi.org/10.3390/biom12091240
Chicago/Turabian StyleGao, Qian, Que Bai, Caiyun Zheng, Na Sun, Jinxi Liu, Wenting Chen, Fangfang Hu, and Tingli Lu. 2022. "Application of Metal–Organic Framework in Diagnosis and Treatment of Diabetes" Biomolecules 12, no. 9: 1240. https://doi.org/10.3390/biom12091240
APA StyleGao, Q., Bai, Q., Zheng, C., Sun, N., Liu, J., Chen, W., Hu, F., & Lu, T. (2022). Application of Metal–Organic Framework in Diagnosis and Treatment of Diabetes. Biomolecules, 12(9), 1240. https://doi.org/10.3390/biom12091240







