Radiobiological Aspects of FLASH Radiotherapy
Abstract
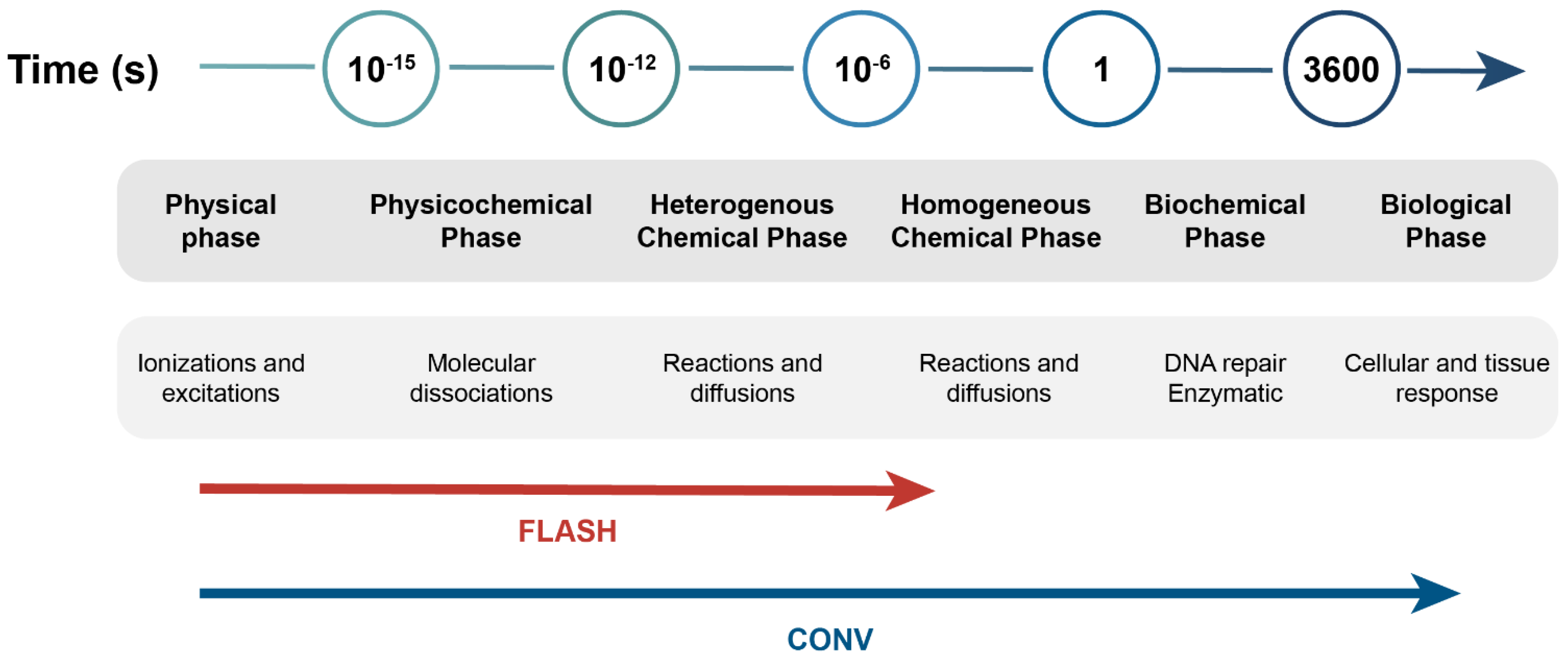
:1. Introduction
2. Biological Mechanisms
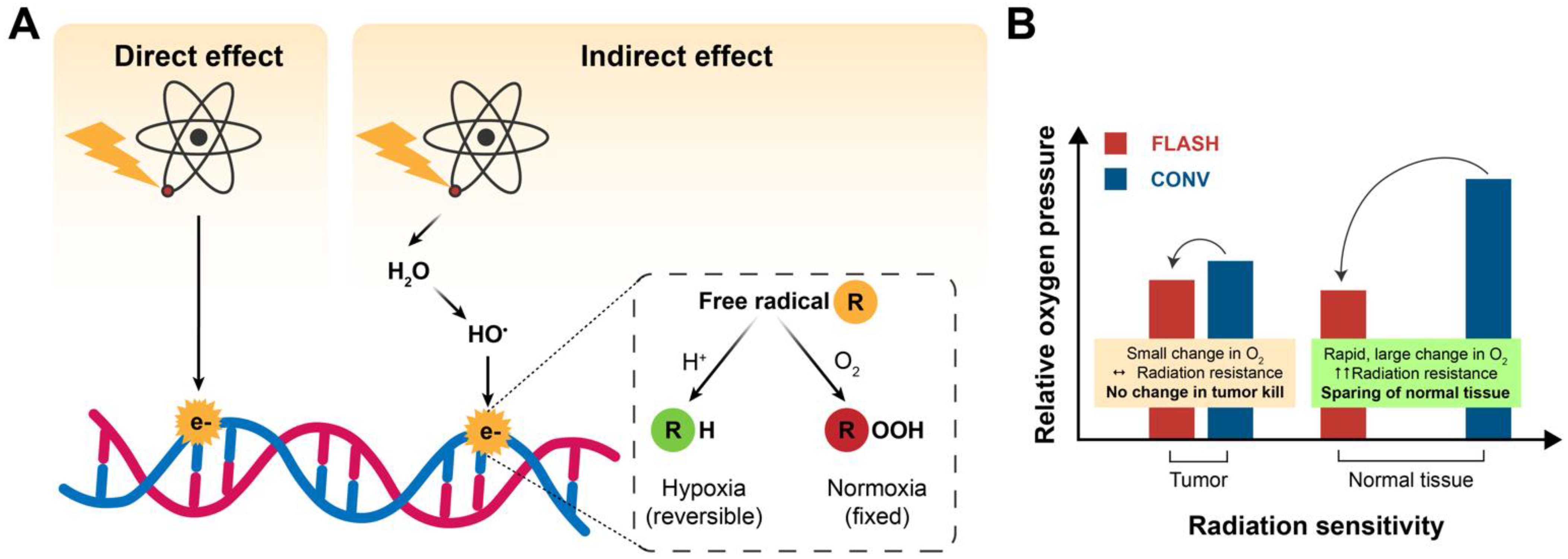
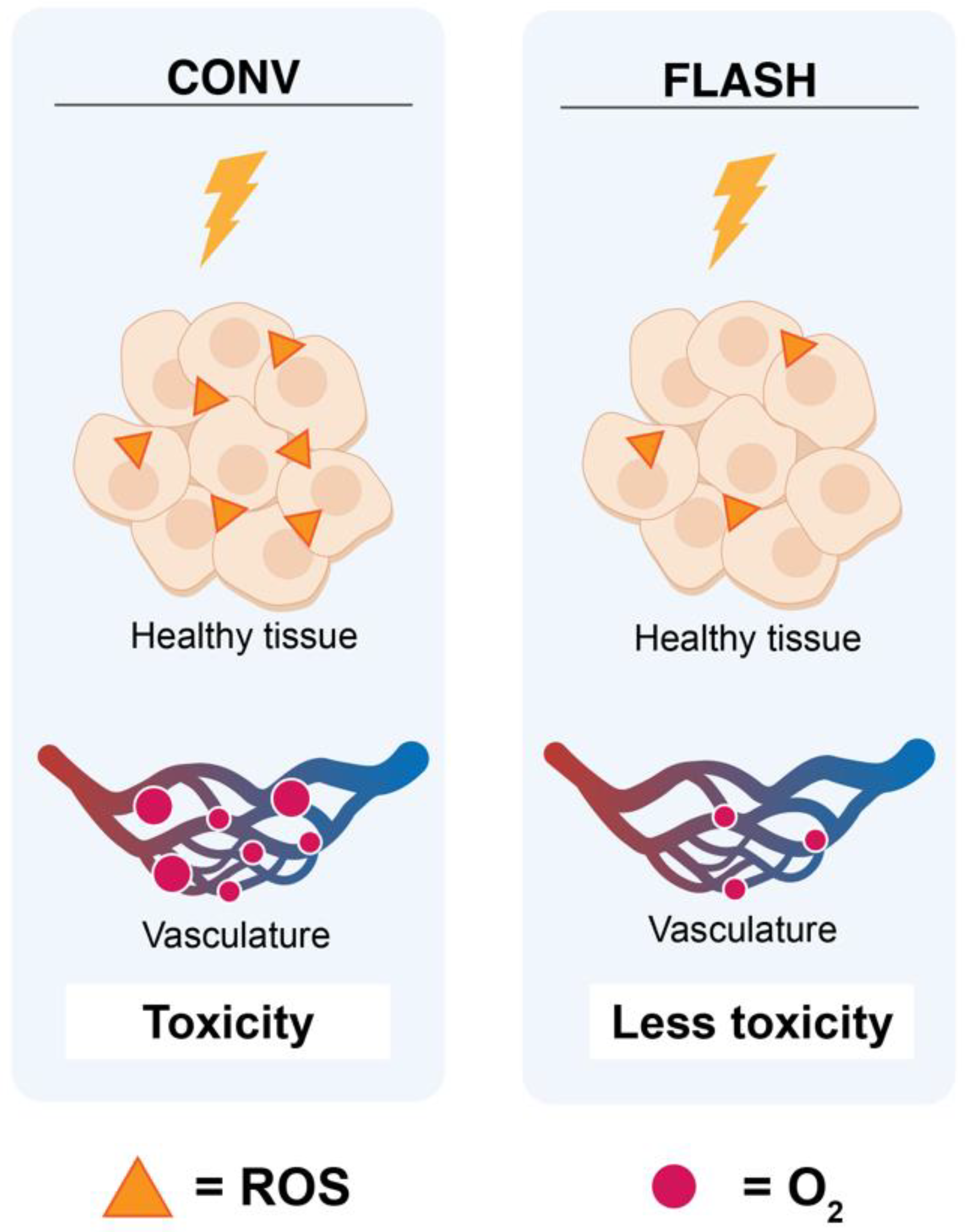
2.1. Oxygen Depletion/ROS
2.2. DNA Damage
2.3. Immune Response
3. In Vivo Studies
3.1. Mice Brain
3.2. Mice Abdomen
3.3. Mice Lungs
3.4. Anti-Tumor Efficacy in Mice
3.5. In Vivo Mice Studies with Negative Results for FLASH
3.6. Zebrafish
| Animal Model (Area/Tumor) | Mean Dose Rate (Gy/s) | Radiation Dose (Gy) | FLASH Source | FLASH-Induced | Tumor Control | FLASH Effect | Reference | |
|---|---|---|---|---|---|---|---|---|
| Acute Effects | Late Effects | |||||||
| Murine models | ||||||||
| Mice (brain) | 35 | NS | Electrons | Increased lymphocyte depletion | Worse overall survival | NS | No | [53] |
| Mice (spleen) | Gastrointestinal mucosal toxicity | |||||||
| Mice (partial body) | 37–41 | NS | Photons | Gastrointestinal toxicity Low body weight Neurological toxicity Clinical symptoms Inflammation | Growth impairment Pulmonary destruction | NS | No | [54] |
| Mice (xenograft human lungs) | 40 | 8 | Electrons | Protection from apoptosis | Decreased lung fibrosis | Equal | Yes | [18] |
| Mice (lung carcinoma) | 40 | 18 | Protons | Increased lymphocyte recruitment | NS | Improved | Yes | [32] |
| Mice (brain) | 40 | 8 | Electrons | NS | Neurocognitive effects | NS | Yes | [4] |
| Mice (focal abdomen) | 63 | 12/18 | Protons | Less intestinal damage | Decreased intestinal fibrosis | Equal to CONV RT | Yes | [49] |
| Mice (subcutaneous pancreatic tumor) | 63 | 12/18 | ||||||
| Mice (leg) | 65–92 | 31.2–53.5 | Protons | Skin toxicity | NS | NS | Yes | [56] |
| Mice (subcutaneous glioblastoma) | 66 | 8 Gy × 2 | Electrons | NS | NS | Yes | Yes | [48] |
| Mice (intracranial glioblastoma) | 74 | 12.5 Gy × 2 | Electrons | NS | NS | Yes | Yes | |
| Mice (whole abdomen) | 94 | 15 | Protons | Increased proliferating cells per crypt | Reduced intestinal fibrosis | NS | Yes | [49] |
| Mice (lymphoblastic leukemia and normal hematopoiesis) | 200 | 4 | Electrons | NS | Decrease in leukemic cells Difference in genetic factors Preservation of hematopoietic/ progenitor cells | Improved | Yes | [52] |
| Mice (whole brain) | 200–300 | 30 | Electrons | No loss of dendrites Decreased neuroinflammation | Protection from neurocognitive effects | NS | Yes | [14] |
| Mice (ovarian cancer) | 216 | 14–16 | Electrons | Body weight Hematopoietic toxicity DNA damage Apoptosis | Better overall survival Similar mucosal damage Sparing of intestinal function Sparing of epithelial integrity | NS | Yes | [50] |
| Mice (whole body) | 276–319 | NS | Photons | Gastrointestinal toxicity Body weight Neurological toxicity Clinical symptoms Inflammation | Growth impairment Pulmonary destruction | NS | No | [54] |
| Mice (subcutaneous lung carcinoma) | 352 | 15 | Electrons | No tumor vascular collapse Increased ROS levels Increased immune cell infiltration | NS | NS | NS | [45] |
| Mice (orthotopic glioblastoma) | 1.9 × 106 | 3.5 Gy × 4 | Electrons | No neurocognitive effects | Tumor control Overall survival | Equal | No | [46] |
| 2.5 × 106 | 25 Gy | Yes | Yes | |||||
| 3.9 × 106 | 7 Gy × 2 | Equal | Yes | |||||
| 5.6 × 106 | 10 Gy × 3 | Equal | Yes | |||||
| 5.6 × 106 | 10 | Yes | Yes | |||||
| 7.8 × 106 | 14 | Impaired neurocognitive effects | Equal | No | ||||
| Juvenile mice (whole brain) | 4.4 × 106 | 8 | Electrons | Attenuated memory-impaired functions Preservation of growth hormones | Recovered impaired memory updating Preserved neurogenesis Minimized anxiety-like behaviors | NS | Yes | [47] |
| Mice (whole abdomen) | 2–6 × 106 | 7.5–20 | Electrons | Increased crypt survival Reduced change in gut microbiome | NS | NS | Yes | [51] |
| Mice (whole brain) | NS | 10 | X-rays | Reduced astrogliosis | Protection from neurocognitive effects | NS | Yes | [13] |
| Mice (lungs) | NS | NS | Electrons | Less DNA damage Minimized induction of pro-inflammatory genes | Less senescence Decreased fibrosis | NS | Yes | [12] |
| Fishes | ||||||||
| Zebrafish | 40 | 8 | Electrons | NS | Neurocognitive effects | NS | Yes | [4] |
| Zebrafish embryo | 100 | NS | Protons | NS | No difference in malformation Reduced pericardial edema No difference in survival | NS | Yes | [55] |
| Zebrafish embryo | 177; 287; 2.5 × 105 | 32 | Electrons | Reduced morphological alterations | NS | NS | Yes | [57] |
| 300 | 30 | Protons | ||||||
| Zebrafish embryo | 1 × 105 | 26 | Electrons | Reduced morphological alterations | NS | NS | NS | [58] |
| Large animals | ||||||||
| Mini pigs | 150 | 31 | Electrons | Depilation | Erythema Ulceration Hyperkeratosis Skin contracture | NS | [59] | |
| Mini pig (skin) | 160 | 31–41 | Electrons | Preserved hair follicles | Decreased fibrosis No permanent late toxicities | NS | Yes | [16] |
| Cat (nasal planum) | 300–400 | 41 | Electrons | Permanent depilation | ||||
| Cat (nasal planum) | 1500 | 30 | Electrons | 1 observation: Moist desquamation | Mucosal breakdown Bone necrosis | NS | Yes | [59] |
| Dog (leg) | 61–128 | 4–12 | Protons | Decreased TGF-β levels | NS | Yes | Yes | [60] |
| Dog | 400–500 | 8 or 12 | Electrons | Alopecia Desquamation Leukotricia Mild erythema | NS | Yes | Yes | [61] |
3.7. Larger Animals
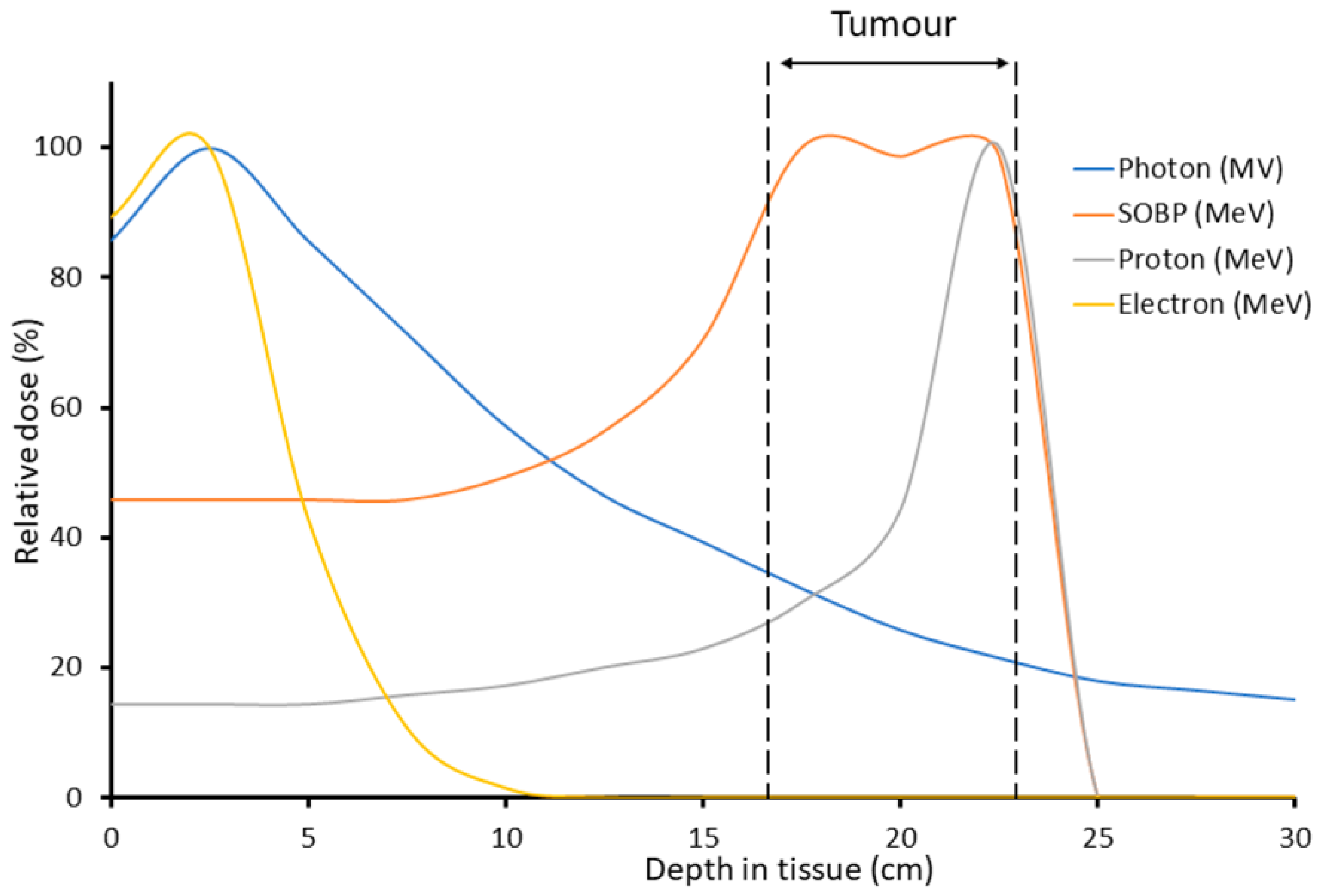
4. Towards the Clinic
4.1. The First Human Study
4.2. Devices for Clinical FLASH RT
4.3. Clinical Translation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yap, M.L.; Zubizarreta, E.; Bray, F.; Ferlay, J.; Barton, M. Global Access to Radiotherapy Services: Have We Made Progress during the Past Decade? J. Glob. Oncol. 2016, 2, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.R.; Parsons, J.L. FLASH Radiotherapy: Current Knowledge and Future Insights Using Proton-Beam Therapy. Int. J. Mol. Sci. 2020, 21, 6492. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G. Mechanisms Underlying FLASH Radiotherapy, a Novel Way to Enlarge the Differential Responses to Ionizing Radiation between Normal and Tumor Tissues. Radiat. Med. Prot. 2020, 1, 35–40. [Google Scholar] [CrossRef]
- Montay-Gruel, P.; Acharya, M.M.; Petersson, K.; Alikhani, L.; Yakkala, C.; Allen, B.D.; Ollivier, J.; Petit, B.; Jorge, P.G.; Syage, A.R.; et al. Long-Term Neurocognitive Benefits of FLASH Radiotherapy Driven by Reduced Reactive Oxygen Species. Proc. Natl. Acad. Sci. USA 2019, 116, 10943–10951. [Google Scholar] [CrossRef] [PubMed]
- Vozenin, M.-C.; Hendry, J.H.; Limoli, C.L. Biological Benefits of Ultra-High Dose Rate FLASH Radiotherapy: Sleeping Beauty Awoken. Clin. Oncol. 2019, 31, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Hornsey, S.; Alper, T. Unexpected Dose-Rate Effect in the Killing of Mice by Radiation. Nature 1966, 210, 212–213. [Google Scholar] [CrossRef]
- Hornsey, S.; Bewley, D.K. Hypoxia in Mouse Intestine Induced by Electron Irradiation at High Dose-Rates. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1971, 19, 479–483. [Google Scholar] [CrossRef]
- Field, S.B.; Bewley, D.K. Effects of Dose-Rate on the Radiation Response of Rat Skin. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1974, 26, 259–267. [Google Scholar] [CrossRef]
- Hendry, J.H.; Moore, J.V.; Hodgson, B.W.; Keene, J.P. The Constant Low Oxygen Concentration in All the Target Cells for Mouse Tail Radionecrosis. J. Radiat. Res. 1982, 92, 172–181. [Google Scholar] [CrossRef]
- Wilson, P.; Jones, B.; Yokoi, T.; Hill, M.; Vojnovic, B. Revisiting the Ultra-High Dose Rate Effect: Implications for Charged Particle Radiotherapy Using Protons and Light Ions. Br. J. Radiol. 2012, 85, e933–e939. [Google Scholar] [CrossRef] [Green Version]
- Desouky, O.; Ding, N.; Zhou, G. Targeted and Non-Targeted Effects of Ionizing Radiation. J. Radiat. Res. Appl. Sci. 2015, 8, 247–254. [Google Scholar] [CrossRef]
- Fouillade, C.; Curras-Alonso, S.; Giuranno, L.; Quelennec, E.; Heinrich, S.; Bonnet-Boissinot, S.; Beddok, A.; Leboucher, S.; Karakurt, H.U.; Bohec, M.; et al. FLASH Irradiation Spares Lung Progenitor Cells and Limits the Incidence of Radio-Induced Senescence. Clin. Cancer Res. 2020, 26, 1497–1506. [Google Scholar] [CrossRef] [PubMed]
- Montay-Gruel, P.; Bouchet, A.; Jaccard, M.; Patin, D.; Serduc, R.; Aim, W.; Petersson, K.; Petit, B.; Bailat, C.; Bourhis, J.; et al. X-rays Can Trigger the FLASH Effect: Ultra-High Dose-Rate Synchrotron Light Source Prevents Normal Brain Injury after Whole Brain Irradiation in Mice. Radiother. Oncol. 2018, 129, 582–588. [Google Scholar] [CrossRef]
- Simmons, D.A.; Lartey, F.M.; Schüler, E.; Rafat, M.; King, G.; Kim, A.; Ko, R.; Semaan, S.; Gonzalez, S.; Jenkins, M.; et al. Reduced Cognitive Deficits after FLASH Irradiation of Whole Mouse Brain Are Associated with Less Hippocampal Dendritic Spine Loss and Neuroinflammation. Radiother. Oncol. 2019, 139, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Loo, B.W.; Schuler, E.; Lartey, F.M.; Rafat, M.; King, G.J.; Trovati, S.; Koong, A.C.; Maxim, P.G. (P003) Delivery of Ultra-Rapid Flash Radiation Therapy and Demonstration of Normal Tissue Sparing After Abdominal Irradiation of Mice. Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, E16. [Google Scholar] [CrossRef]
- Vozenin, M.-C.; De Fornel, P.; Petersson, K.; Favaudon, V.; Jaccard, M.; Germond, J.-F.; Petit, B.; Burki, M.; Ferrand, G.; Patin, D.; et al. The Advantage of FLASH Radiotherapy Confirmed in Mini-Pig and Cat-Cancer Patients. Clin. Cancer Res. 2019, 25, 35–42. [Google Scholar] [CrossRef]
- Bourhis, J.; Sozzi, W.J.; Jorge, P.G.; Gaide, O.; Bailat, C.; Duclos, F.; Patin, D.; Ozsahin, M.; Bochud, F.; Germond, J.-F.; et al. Treatment of a First Patient with FLASH-Radiotherapy. Radiother. Oncol. 2019, 139, 18–22. [Google Scholar] [CrossRef]
- Favaudon, V.; Caplier, L.; Monceau, V.; Pouzoulet, F.; Sayarath, M.; Fouillade, C.; Poupon, M.-F.; Brito, I.; Hupé, P.; Bourhis, J.; et al. Ultrahigh Dose-Rate FLASH Irradiation Increases the Differential Response between Normal and Tumor Tissue in Mice. Sci. Transl. Med. 2014, 6, 245ra93. [Google Scholar] [CrossRef]
- Montay-Gruel, P.; Petersson, K.; Jaccard, M.; Boivin, G.; Germond, J.-F.; Petit, B.; Doenlen, R.; Favaudon, V.; Bochud, F.; Bailat, C.; et al. Irradiation in a Flash: Unique Sparing of Memory in Mice after Whole Brain Irradiation with Dose Rates above 100 Gy/s. Radiother. Oncol. 2017, 124, 365–369. [Google Scholar] [CrossRef]
- Lempart, M.; Blad, B.; Adrian, G.; Bäck, S.; Knöös, T.; Ceberg, C.; Petersson, K. Modifying a Clinical Linear Accelerator for Delivery of Ultra-High Dose Rate Irradiation. Radiother. Oncol. 2019, 139, 40–45. [Google Scholar] [CrossRef]
- Schüler, E.; Trovati, S.; King, G.; Lartey, F.; Rafat, M.; Villegas, M.; Praxel, A.J.; Loo, B.W.; Maxim, P.G. Experimental Platform for Ultra-High Dose Rate FLASH Irradiation of Small Animals Using a Clinical Linear Accelerator. Int. J. Radiat. Oncol. Biol. Phys. 2017, 97, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Carter, R.J.; Nickson, C.M.; Thompson, J.M.; Kacperek, A.; Hill, M.A.; Parsons, J.L. Complex DNA Damage Induced by High Linear Energy Transfer Alpha-Particles and Protons Triggers a Specific Cellular DNA Damage Response. Int. J. Radiat. Oncol. Biol. Phys. 2018, 100, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Adrian, G.; Konradsson, E.; Lempart, M.; Bäck, S.; Ceberg, C.; Petersson, K. The FLASH Effect Depends on Oxygen Concentration. Br. J. Radiol. 2020, 93, 20190702. [Google Scholar] [CrossRef] [PubMed]
- Pratx, G.; Kapp, D.S. A Computational Model of Radiolytic Oxygen Depletion during FLASH Irradiation and Its Effect on the Oxygen Enhancement Ratio. Phys. Med. Biol. 2019, 64, 185005. [Google Scholar] [CrossRef] [PubMed]
- Spitz, D.R.; Buettner, G.R.; Petronek, M.S.; St-Aubin, J.J.; Flynn, R.T.; Waldron, T.J.; Limoli, C.L. An Integrated Physico-Chemical Approach for Explaining the Differential Impact of FLASH versus Conventional Dose Rate Irradiation on Cancer and Normal Tissue Responses. Radiother. Oncol. 2019, 139, 23–27. [Google Scholar] [CrossRef]
- Buonanno, M.; Grilj, V.; Brenner, D.J. Biological Effects in Normal Cells Exposed to FLASH Dose Rate Protons. Radiother. Oncol. 2019, 139, 51–55. [Google Scholar] [CrossRef]
- Ling, C.C.; Michaels, H.B.; Epp, E.R.; Peterson, E.C. Oxygen Diffusion into Mammalian Cells Following Ultrahigh Dose Rate Irradiation and Lifetime Estimates of Oxygen-Sensitive Species. Radiat. Res. 1978, 76, 522–532. [Google Scholar] [CrossRef]
- Scharpfenecker, M.; Kruse, J.J.C.M.; Sprong, D.; Russell, N.S.; ten Dijke, P.; Stewart, F.A. Ionizing Radiation Shifts the PAI-1/ID-1 Balance and Activates Notch Signaling in Endothelial Cells. Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 506–513. [Google Scholar] [CrossRef]
- Farrés, J.; Martín-Caballero, J.; Martínez, C.; Lozano, J.J.; Llacuna, L.; Ampurdanés, C.; Ruiz-Herguido, C.; Dantzer, F.; Schreiber, V.; Villunger, A.; et al. Parp-2 Is Required to Maintain Hematopoiesis Following Sublethal γ-Irradiation in Mice. Blood 2013, 122, 44. [Google Scholar] [CrossRef]
- Glück, S.; Guey, B.; Gulen, M.F.; Wolter, K.; Kang, T.-W.; Schmacke, N.A.; Bridgeman, A.; Rehwinkel, J.; Zender, L.; Ablasser, A. Innate Immune Sensing of Cytosolic Chromatin Fragments through CGAS Promotes Senescence. Nat. Cell Biol. 2017, 19, 1061–1070. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, Q.; Zhang, F.; Meng, F.; Liu, S.; Zhou, R.; Wu, Q.; Li, X.; Shen, L.; Huang, J.; et al. HER2 Recruits AKT1 to Disrupt STING Signalling and Suppress Antiviral Defence and Antitumour Immunity. Nat. Cell Biol. 2019, 21, 1027–1040. [Google Scholar] [CrossRef] [PubMed]
- Rama, N.; Saha, T.; Shukla, S.; Goda, C.; Milewski, D.; Mascia, A.E.; Vatner, R.E.; Sengupta, D.; Katsis, A.; Abel, E.; et al. Improved Tumor Control Through T-Cell Infiltration Modulated by Ultra-High Dose Rate Proton FLASH Using a Clinical Pencil Beam Scanning Proton System. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, S164–S165. [Google Scholar] [CrossRef]
- Kepp, O.; Senovilla, L.; Vitale, I.; Vacchelli, E.; Adjemian, S.; Agostinis, P.; Apetoh, L.; Aranda, F.; Barnaba, V.; Bloy, N.; et al. Consensus Guidelines for the Detection of Immunogenic Cell Death. OncoImmunology 2014, 3, e955691. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.D.; Hammond, E.M.; Higgins, G.S.; Petersson, K. Ultra-High Dose Rate (FLASH) Radiotherapy: Silver Bullet or Fool’s Gold? Front. Oncol. 2020, 9, 1563. [Google Scholar] [CrossRef] [PubMed]
- Grimes, D.R.; Partridge, M. A Mechanistic Investigation of the Oxygen Fixation Hypothesis and Oxygen Enhancement Ratio. Biomed. Phys. Eng. Express 2015, 1, 045209. [Google Scholar] [CrossRef] [PubMed]
- Spitz, D.R.; Buettner, G.R.; Limoli, C.L. Response to Letter Regarding “An Integrated Physico-Chemical Approach for Explaining the Differential Impact of FLASH versus Conventional Dose Rate Irradiation on Cancer and Normal Tissue Responses”. Radiother. Oncol. 2019, 139, 64–65. [Google Scholar] [CrossRef] [PubMed]
- Tinganelli, W.; Sokol, O.; Quartieri, M.; Puspitasari, A.; Dokic, I.; Abdollahi, A.; Durante, M.; Haberer, T.; Debus, J.; Boscolo, D.; et al. Ultra-High Dose Rate (FLASH) Carbon Ion Irradiation: Dosimetry and First Cell Experiments. Int. J. Radiat. Oncol. Biol. Phys. 2022, 112, 1012–1022. [Google Scholar] [CrossRef]
- Khan, S.; Bassenne, M.; Wang, J.; Manjappa, R.; Melemenidis, S.; Breitkreutz, D.Y.; Maxim, P.G.; Xing, L.; Loo, B.W.; Pratx, G. Multicellular Spheroids as In Vitro Models of Oxygen Depletion during FLASH Irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 833–844. [Google Scholar] [CrossRef]
- Cao, X.; Zhang, R.; Esipova, T.V.; Allu, S.R.; Ashraf, R.; Rahman, M.; Gunn, J.R.; Bruza, P.; Gladstone, D.J.; Williams, B.B.; et al. Quantification of Oxygen Depletion during FLASH Irradiation In Vitro and In Vivo. Int. J. Radiat. Oncol. Biol. Phys. 2021, 111, 240–248. [Google Scholar] [CrossRef]
- Jansen, J.; Knoll, J.; Beyreuther, E.; Pawelke, J.; Skuza, R.; Hanley, R.; Brons, S.; Pagliari, F.; Seco, J. Does FLASH Deplete Oxygen? Experimental Evaluation for Photons, Protons, and Carbon Ions. Med. Phys. 2021, 48, 3982–3990. [Google Scholar] [CrossRef]
- Favaudon, V.; Labarbe, R.; Limoli, C.L. Model Studies of the Role of Oxygen in the FLASH Effect. Med. Phys. 2022, 49, 2068–2081. [Google Scholar] [CrossRef] [PubMed]
- Labarbe, R.; Hotoiu, L.; Barbier, J.; Favaudon, V. A Physicochemical Model of Reaction Kinetics Supports Peroxyl Radical Recombination as the Main Determinant of the FLASH Effect. Radiother. Oncol. 2020, 153, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Asaithamby, A.; Hu, B.; Delgado, O.; Ding, L.-H.; Story, M.D.; Minna, J.D.; Shay, J.W.; Chen, D.J. Irreparable Complex DNA Double-Strand Breaks Induce Chromosome Breakage in Organotypic Three-Dimensional Human Lung Epithelial Cell Culture. Nucleic Acids Res. 2011, 39, 5474. [Google Scholar] [CrossRef] [PubMed]
- Bourhis, J.; Montay-Gruel, P.; Jorge, P.G.; Bailat, C.; Petit, B.; Ollivier, J.; Jeanneret-Sozzi, W.; Ozsahin, M.; Bochud, F.; Moeckli, R.; et al. Clinical Translation of FLASH Radiotherapy: Why and How? Radiother. Oncol. 2019, 139, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-E.; Gwak, S.-H.; Hong, B.-J.; Oh, J.-M.; Choi, H.-S.; Kim, M.S.; Oh, D.; Lartey, F.M.; Rafat, M.; Schüler, E.; et al. Effects of Ultra-High Doserate FLASH Irradiation on the Tumor Microenvironment in Lewis Lung Carcinoma: Role of Myosin Light Chain. Int. J. Radiat. Oncol. Biol. Phys. 2021, 109, 1440–1453. [Google Scholar] [CrossRef]
- Montay-Gruel, P.; Acharya, M.M.; Gonçalves Jorge, P.; Petit, B.; Petridis, I.G.; Fuchs, P.; Leavitt, R.; Petersson, K.; Gondré, M.; Ollivier, J.; et al. Hypofractionated FLASH-RT as an Effective Treatment against Glioblastoma That Reduces Neurocognitive Side Effects in Mice. Clin. Cancer Res. 2021, 27, 775–784. [Google Scholar] [CrossRef]
- Alaghband, Y.; Cheeks, S.N.; Allen, B.D.; Montay-Gruel, P.; Doan, N.-L.; Petit, B.; Jorge, P.G.; Giedzinski, E.; Acharya, M.M.; Vozenin, M.-C.; et al. Neuroprotection of Radiosensitive Juvenile Mice by Ultra-High Dose Rate FLASH Irradiation. Cancers 2020, 12, 1671. [Google Scholar] [CrossRef]
- Liljedahl, E.; Konradsson, E.; Gustafsson, E.; Jonsson, K.F.; Olofsson, J.K.; Ceberg, C.; Redebrandt, H.N. Long-Term Anti-Tumor Effects Following Both Conventional Radiotherapy and FLASH in Fully Immunocompetent Animals with Glioblastoma. Sci. Rep. 2022, 12, 12285. [Google Scholar] [CrossRef]
- Diffenderfer, E.S.; Verginadis, I.I.; Kim, M.M.; Shoniyozov, K.; Velalopoulou, A.; Goia, D.; Putt, M.; Hagan, S.; Avery, S.; Teo, K.; et al. Design, Implementation, and in Vivo Validation of a Novel Proton FLASH Radiation Therapy System. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 440–448. [Google Scholar] [CrossRef]
- Levy, K.; Natarajan, S.; Wang, J.; Chow, S.; Eggold, J.T.; Loo, P.E.; Manjappa, R.; Melemenidis, S.; Lartey, F.M.; Schüler, E.; et al. Abdominal FLASH Irradiation Reduces Radiation-Induced Gastrointestinal Toxicity for the Treatment of Ovarian Cancer in Mice. Sci. Rep. 2020, 10, 21600. [Google Scholar] [CrossRef]
- Ruan, J.-L.; Lee, C.; Wouters, S.; Tullis, I.D.C.; Verslegers, M.; Mysara, M.; Then, C.K.; Smart, S.C.; Hill, M.A.; Muschel, R.J.; et al. Irradiation at Ultra-High (FLASH) Dose Rates Reduces Acute Normal Tissue Toxicity in the Mouse Gastrointestinal System. Int. J. Radiat. Oncol. Biol. Phys. 2021, 111, 1250–1261. [Google Scholar] [CrossRef] [PubMed]
- Chabi, S.; To, T.H.V.; Leavitt, R.; Poglio, S.; Jorge, P.G.; Jaccard, M.; Petersson, K.; Petit, B.; Roméo, P.-H.; Pflumio, F.; et al. Ultra-High-Dose-Rate FLASH and Conventional-Dose-Rate Irradiation Differentially Affect Human Acute Lymphoblastic Leukemia and Normal Hematopoiesis. Int. J. Radiat. Oncol. Biol. Phys. 2021, 109, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Venkatesulu, B.P.; Sharma, A.; Pollard-Larkin, J.M.; Sadagopan, R.; Symons, J.; Neri, S.; Singh, P.K.; Tailor, R.; Lin, S.H.; Krishnan, S. Ultra High Dose Rate (35 Gy/Sec) Radiation Does Not Spare the Normal Tissue in Cardiac and Splenic Models of Lymphopenia and Gastrointestinal Syndrome. Sci. Rep. 2019, 9, 17180. [Google Scholar] [CrossRef] [PubMed]
- Smyth, L.M.L.; Donoghue, J.F.; Ventura, J.A.; Livingstone, J.; Bailey, T.; Day, L.R.J.; Crosbie, J.C.; Rogers, P.A.W. Comparative Toxicity of Synchrotron and Conventional Radiation Therapy Based on Total and Partial Body Irradiation in a Murine Model. Sci. Rep. 2018, 8, 12044. [Google Scholar] [CrossRef] [PubMed]
- Beyreuther, E.; Brand, M.; Hans, S.; Hideghéty, K.; Karsch, L.; Leßmann, E.; Schürer, M.; Szabó, E.R.; Pawelke, J. Feasibility of Proton FLASH Effect Tested by Zebrafish Embryo Irradiation. Radiother. Oncol. 2019, 139, 46–50. [Google Scholar] [CrossRef]
- Singers Sørensen, B.; Krzysztof Sitarz, M.; Ankjærgaard, C.; Johansen, J.; Andersen, C.E.; Kanouta, E.; Overgaard, C.; Grau, C.; Poulsen, P. In Vivo Validation and Tissue Sparing Factor for Acute Damage of Pencil Beam Scanning Proton FLASH. Radiother. Oncol. 2022, 167, 109–115. [Google Scholar] [CrossRef]
- Karsch, L.; Pawelke, J.; Brand, M.; Hans, S.; Hideghéty, K.; Jansen, J.; Lessmann, E.; Löck, S.; Schürer, M.; Schurig, R.; et al. Beam Pulse Structure and Dose Rate as Determinants for the Flash Effect Observed in Zebrafish Embryo. Radiother. Oncol. 2022, 173, 49–54. [Google Scholar] [CrossRef]
- Pawelke, J.; Brand, M.; Hans, S.; Hideghéty, K.; Karsch, L.; Lessmann, E.; Löck, S.; Schürer, M.; Szabó, E.R.; Beyreuther, E. Electron Dose Rate and Oxygen Depletion Protect Zebrafish Embryos from Radiation Damage. Radiother. Oncol. 2021, 158, 7–12. [Google Scholar] [CrossRef]
- Rohrer Bley, C.; Wolf, F.; Gonçalves Jorge, P.; Grilj, V.; Petridis, I.; Petit, B.; Böhlen, T.T.; Moeckli, R.; Limoli, C.; Bourhis, J.; et al. Dose- and Volume-Limiting Late Toxicity of FLASH Radiotherapy in Cats with Squamous Cell Carcinoma of the Nasal Planum and in Mini Pigs. Clin. Cancer Res. 2022, 28, 3814–3823. [Google Scholar] [CrossRef]
- Velalopoulou, A.; Karagounis, I.V.; Cramer, G.M.; Kim, M.M.; Skoufos, G.; Goia, D.; Hagan, S.; Verginadis, I.I.; Shoniyozov, K.; Chiango, J.; et al. FLASH Proton Radiotherapy Spares Normal Epithelial and Mesenchymal Tissues While Preserving Sarcoma Response. Cancer Res. 2021, 81, 4808–4821. [Google Scholar] [CrossRef]
- Konradsson, E.; Arendt, M.L.; Bastholm Jensen, K.; Børresen, B.; Hansen, A.E.; Bäck, S.; Kristensen, A.T.; Munck af Rosenschöld, P.; Ceberg, C.; Petersson, K. Establishment and Initial Experience of Clinical FLASH Radiotherapy in Canine Cancer Patients. Front. Oncol. 2021, 11, 658004. [Google Scholar] [CrossRef] [PubMed]
- Search of: FLASH Radiotherapy—List Results—ClinicalTrials.Gov. Available online: https://www.clinicaltrials.gov/ct2/results?cond=&term=FLASH+Radiotherapy&cntry=&state=&city=&dist (accessed on 29 August 2022).
- Esplen, N.; Mendonca, M.S.; Bazalova-Carter, M. Physics and Biology of Ultrahigh Dose-Rate (FLASH) Radiotherapy: A Topical Review. Phys. Med. Biol. 2020, 65, 23TR03. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Gao, F.; Yang, Y.; Wu, D.; Zhang, Y.; Feng, G.; Dai, T.; Du, X. FLASH Radiotherapy: History and Future. Front. Oncol. 2021, 11, 644400. [Google Scholar] [CrossRef] [PubMed]
- Diffenderfer, E.S.; Sørensen, B.S.; Mazal, A.; Carlson, D.J. The Current Status of Preclinical Proton FLASH Radiation and Future Directions. Med. Phys. 2022, 49, 2039–2054. [Google Scholar] [CrossRef]
- de Kruijff, R.M. FLASH Radiotherapy: Ultra-High Dose Rates to Spare Healthy Tissue. Int. J. Radiat. Biol. 2020, 96, 419–423. [Google Scholar] [CrossRef]
- Boucher, S.; Esarey, E.; Geddes, C.G.R.; Johnstone, C.; Kutsaev, S.; Loo, B.W., Jr.; Méot, F.; Nakamura, K.; Nanni, E.; Obst-Huebl, L.; et al. Transformative Technology for FLASH Radiation Therapy: A Snowmass 2021 White Paper. arXiv 2021, arXiv:2203.11047. [Google Scholar]
- Gao, F.; Yang, Y.; Zhu, H.; Wang, J.; Xiao, D.; Zhou, Z.; Dai, T.; Zhang, Y.; Feng, G.; Li, J.; et al. First Demonstration of the FLASH Effect with Ultrahigh Dose Rate High-Energy X-rays. Radiother. Oncol. 2022, 166, 44–50. [Google Scholar] [CrossRef]
- Weber, U.A.; Scifoni, E.; Durante, M. FLASH Radiotherapy with Carbon Ion Beams. Med. Phys. 2022, 49, 1974–1992. [Google Scholar] [CrossRef]
- Gerlach, S.; Schlaefer, A. Robotic Systems in Radiotherapy and Radiosurgery. Curr. Robot. Rep. 2022, 3, 9–19. [Google Scholar] [CrossRef]
- PMB-Alcen Announces the Launch of FLASHKNiFE, the FLASH Radiotherapy System Dedicated to Clinical Trials. Available online: https://www.prnewswire.com/news-releases/pmb-alcen-announces-the-launch-of-flashknife-the-flash-radiotherapy-system-dedicated-to-clinical-trials-301081180.html (accessed on 7 June 2022).
- Moeckli, R.; Gonçalves Jorge, P.; Grilj, V.; Oesterle, R.; Cherbuin, N.; Bourhis, J.; Vozenin, M.-C.; Germond, J.-F.; Bochud, F.; Bailat, C. Commissioning of an Ultra-High Dose Rate Pulsed Electron Beam Medical LINAC for FLASH RT Preclinical Animal Experiments and Future Clinical Human Protocols. Med. Phys. 2021, 48, 3134–3142. [Google Scholar] [CrossRef]
- Felici, G.; Barca, P.; Barone, S.; Bortoli, E.; Borgheresi, R.; De Stefano, S.; Di Francesco, M.; Grasso, L.; Linsalata, S.; Marfisi, D.; et al. Transforming an IORT Linac into a FLASH Research Machine: Procedure and Dosimetric Characterization. Front. Phys. 2020, 8, 374. [Google Scholar] [CrossRef]
- Maxim, P.G.; Tantawi, S.G.; Loo, B.W. PHASER: A Platform for Clinical Translation of FLASH Cancer Radiotherapy. Radiother. Oncol. 2019, 139, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Berg, T.J.; Pietras, A. Radiotherapy-Induced Remodeling of the Tumor Microenvironment by Stromal Cells. Semin. Cancer Biol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Krisnawan, V.E.; Stanley, J.A.; Schwarz, J.K.; DeNardo, D.G. Tumor Microenvironment as a Regulator of Radiation Therapy: New Insights into Stromal-Mediated Radioresistance. Cancers 2020, 12, 2916. [Google Scholar] [CrossRef]
| Factor | Normal Tissue | Tumor | Normal and Tumor |
|---|---|---|---|
| Oxygen depletion hypothesis | |||
| Oxygen [23,24] | Rapid oxygen depletion | Small change in oxygen | - |
| ROS [4,25] | Reduction of ROS | No change of ROS | - |
| Oxygen to hydroperoxide conversion [25] | High removal of hydroperoxides | Slow removal of hydroperoxides | - |
| Capillary oxygen Tension [24] | Higher | Lower | - |
| DNA damage hypothesis | |||
| Yields of DNA damage [26] | Smaller amounts of DSBs | Higher amount of DSBs | - |
| Pattern of DNA Damage [27] | Higher amount of clustered DNA damage will lead to activation of different factors (DNA repair, immune system) | Lower amount of clustered DNA damage will lead to activation of different factors (DNA repair, immune system) | - |
| DNA damage repair pathways [28,29] | Unknown pathway, decreasing ROS and DNA damage | PARP-TGF-β pathway | - |
| Factors induced by DNA damage [30,31] | - | - | Initiation of cGAS-STING pathway is different between tumor and healthy tissue |
| Immune hypothesis | |||
| TGF-β and other immune factors [18,26] | Reduction of TGF-β | Induction of TGF-β | - |
| Immune cells and microenvironment [32] | - | Increase of T-lymphocytes into the tumor microenvironment | - |
| Immunogenic cell death [33] | - | - | Effects of FLASH on immunogenic cell death remain unclear |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hageman, E.; Che, P.-P.; Dahele, M.; Slotman, B.J.; Sminia, P. Radiobiological Aspects of FLASH Radiotherapy. Biomolecules 2022, 12, 1376. https://doi.org/10.3390/biom12101376
Hageman E, Che P-P, Dahele M, Slotman BJ, Sminia P. Radiobiological Aspects of FLASH Radiotherapy. Biomolecules. 2022; 12(10):1376. https://doi.org/10.3390/biom12101376
Chicago/Turabian StyleHageman, Eline, Pei-Pei Che, Max Dahele, Ben J. Slotman, and Peter Sminia. 2022. "Radiobiological Aspects of FLASH Radiotherapy" Biomolecules 12, no. 10: 1376. https://doi.org/10.3390/biom12101376





