The Credible Role of Curcumin in Oxidative Stress-Mediated Mitochondrial Dysfunction in Mammals
Abstract
:1. Introduction
2. Antioxidant Mechanism of Curcumin
3. The Physiological and Molecular role of Curcumin in Reducing Oxidative Stress and Preventing Mitochondrial Dysfunction
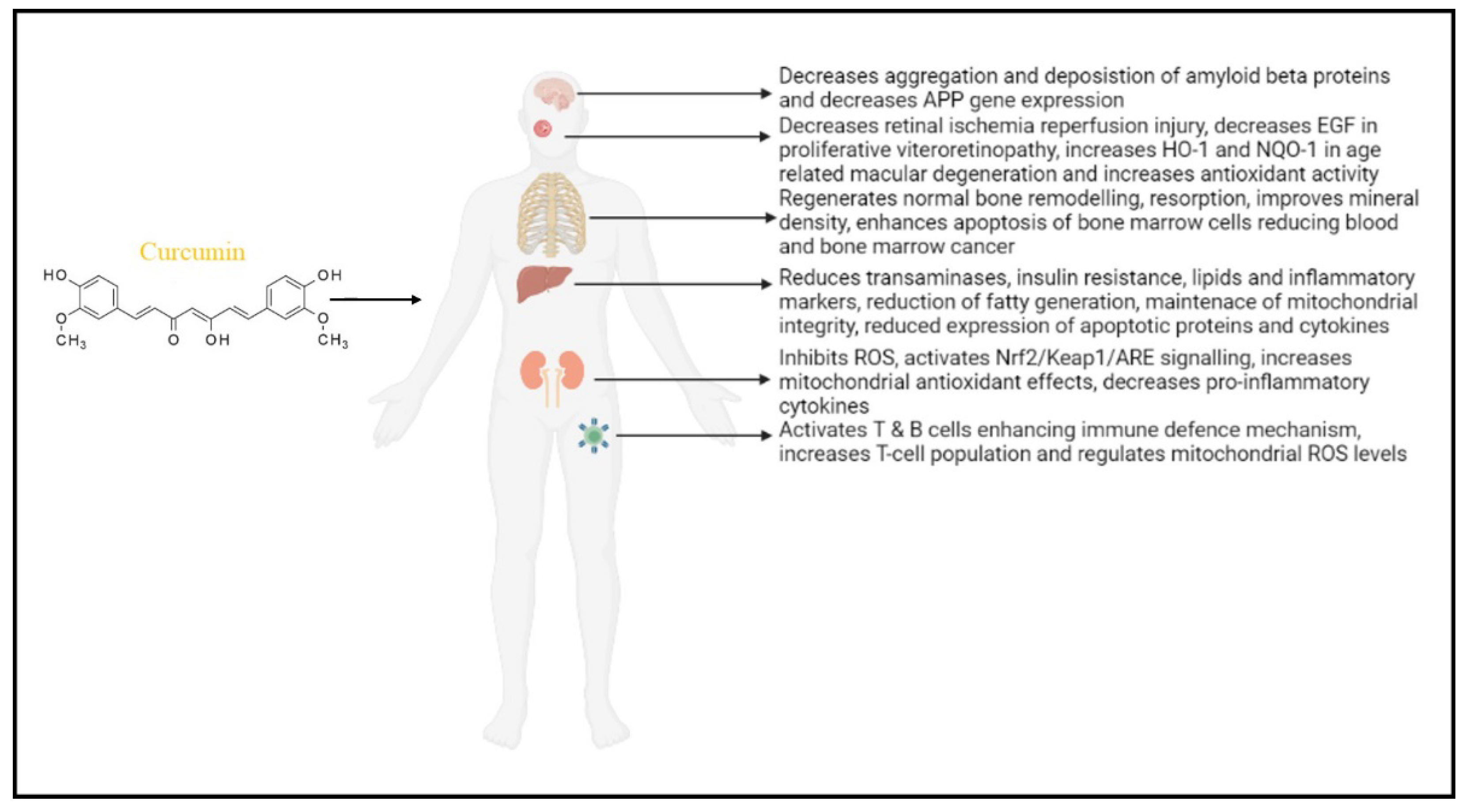
3.1. The Effect of Curcumin in Neurodegenerative Diseases
3.2. The Effect of Curcumin in Liver Function (Alcoholic Fatty Liver and Obesity)
3.3. The Effect of Curcumin in Renal Function
3.4. Effect of Curcumin in Eyes (Retina)
3.5. Effect of Curcumin on the Skeletal System
3.6. Effect of Curcumin on the Lymphatic System
3.7. Effect of Curcumin on Psychiatric Disorders
4. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Duchen, M.R. Mitochondria and Calcium: From Cell Signalling to Cell Death. J. Physiol. 2000, 529, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Andersson, S.G.E.; Zomorodipour, A.; Andersson, J.O.; Sicheritz-Pontén, T.; Alsmark, U.C.M.; Podowski, R.M.; Näslund, A.K.; Eriksson, A.-S.; Winkler, H.H.; Kurland, C.G. The Genome Sequence of Rickettsia Prowazekii and the Origin of Mitochondria. Nature 1998, 396, 133–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, S.; Bankier, A.T.; Barrell, B.G.; de Bruijn, M.H.L.; Coulson, A.R.; Drouin, J.; Eperon, I.C.; Nierlich, D.P.; Roe, B.A.; Sanger, F.; et al. Sequence and Organization of the Human Mitochondrial Genome. Nature 1981, 290, 457–465. [Google Scholar] [CrossRef]
- Santorelli, F.M.; Shanske, S.; Macaya, A.; DeVivo, D.C.; DiMauro, S. The Mutation at Nt 8993 of Mitochondrial DNA Is a Common Cause of Leigh’s Syndrome. Ann. Neurol. 1993, 34, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.P.; Köhler, M.; Graff, C.; Oldfors, A.; Magnuson, M.A.; Berggren, P.-O.; Larsson, N.-G. Impaired Insulin Secretion and β-Cell Loss in Tissue-Specific Knockout Mice with Mitochondrial Diabetes. Nat. Genet. 2000, 26, 336–340. [Google Scholar] [CrossRef]
- Swerdlow, R.H. Brain Aging, Alzheimer’s Disease, and Mitochondria. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2011, 1812, 1630–1639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Twig, G.; Shirihai, O.S. The Interplay Between Mitochondrial Dynamics and Mitophagy. Antioxid. Redox Signal. 2011, 14, 1939–1951. [Google Scholar] [CrossRef] [Green Version]
- Bonora, M.; Patergnani, S.; Rimessi, A.; de Marchi, E.; Suski, J.M.; Bononi, A.; Giorgi, C.; Marchi, S.; Missiroli, S.; Poletti, F.; et al. ATP Synthesis and Storage. Purinergic Signal. 2012, 8, 343–357. [Google Scholar] [CrossRef] [Green Version]
- Tarasov, A.I.; Griffiths, E.J.; Rutter, G.A. Regulation of ATP Production by Mitochondrial Ca2+. Cell Calcium 2012, 52, 28–35. [Google Scholar] [CrossRef] [Green Version]
- Ferrer, M.; Sureda, A.; Mestre, A.; Tur, J.; Pons, A. The Double Edge of Reactive Oxygen Species as Damaging and Signaling Molecules in HL60 Cell Culture. Cell. Physiol. Biochem. 2010, 25, 241–252. [Google Scholar] [CrossRef]
- Sato, H.; Shibata, M.; Shimizu, T.; Shibata, S.; Toriumi, H.; Ebine, T.; Kuroi, T.; Iwashita, T.; Funakubo, M.; Kayama, Y.; et al. Differential Cellular Localization of Antioxidant Enzymes in the Trigeminal Ganglion. Neuroscience 2013, 248, 345–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarro-Yepes, J.; Zavala-Flores, L.; Anandhan, A.; Wang, F.; Skotak, M.; Chandra, N.; Li, M.; Pappa, A.; Martinez-Fong, D.; del Razo, L.M.; et al. Antioxidant Gene Therapy against Neuronal Cell Death. Pharmacol. Ther. 2014, 142, 206–230. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.Q.; Kosten, T.R.; Zhang, X.Y. Free Radicals, Antioxidant Defense Systems, and Schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2013, 46, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Andreyev, A.Y.; Kushnareva, Y.E.; Starkov, A.A. Mitochondrial Metabolism of Reactive Oxygen Species. Biochemistry 2005, 70, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Balaban, R.S.; Nemoto, S.; Finkel, T. Mitochondria, Oxidants, and Aging. Cell 2005, 120, 483–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, P.K. Antimycin-Insensitive Oxidation of Succinate and Reduced Nicotinamide-Adenine Dinucleotide in Electron-Transport Particles I. PH Dependency and Hydrogen Peroxide Formation. Biochim. Biophys. Acta (BBA) Enzymol. Biol. Oxid. 1966, 122, 157–166. [Google Scholar] [CrossRef]
- Loschen, G.; Azzi, A.; Richter, C.; Flohé, L. Superoxide Radicals as Precursors of Mitochondrial Hydrogen Peroxide. FEBS Lett. 1974, 42, 68–72. [Google Scholar] [CrossRef] [Green Version]
- Weisiger, R.A.; Fridovich, I. Superoxide Dismutase. J. Biol. Chem. 1973, 248, 3582–3592. [Google Scholar] [CrossRef]
- Anglevo, P.R.; Abramov, A.Y. Role of mitochondrial ROS in the brain: From physiology to neurodegeneration. FEBS Lett. 2018, 592, 692–702. [Google Scholar] [CrossRef]
- Martínez-Morúa, A.; Soto-Urquieta, M.G.; Franco-Robles, E.; Zúñiga-Trujillo, I.; Campos-Cervantes, A.; Pérez-Vázquez, V.; Ramírez-Emiliano, J. Curcumin Decreases Oxidative Stress in Mitochondria Isolated from Liver and Kidneys of High-Fat Diet-Induced Obese Mice. J. Asian Nat. Prod. Res. 2013, 15, 905–915. [Google Scholar] [CrossRef]
- Halliwell, B. How to Characterize an Antioxidant: An Update. Biochem. Soc. Symp. 1995, 61, 73–101. [Google Scholar] [CrossRef] [PubMed]
- Levine, M. Criteria and Recommendations for Vitamin C Intake. JAMA 1999, 281, 1415. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Noguchi, N.; Niki, E. Comparative Study on Dynamics of Antioxidative Action of α-Tocopheryl Hydroquinone, Ubiquinol, and α-Tocopherol against Lipid Peroxidation. Free Radic. Biol. Med. 1999, 27, 334–346. [Google Scholar] [CrossRef]
- Priyadarsini, K. The Chemistry of Curcumin: From Extraction to Therapeutic Agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic Roles of Curcumin: Lessons Learned from Clinical Trials. AAPS J. 2013, 15, 195–218. [Google Scholar] [CrossRef] [Green Version]
- Vera-Ramirez, L.; Pérez-Lopez, P.; Varela-Lopez, A.; Ramirez-Tortosa, M.; Battino, M.; Quiles, J.L. Curcumin and Liver Disease. BioFactors 2013, 39, 88–100. [Google Scholar] [CrossRef]
- Reddy, R.C.; Vatsala, P.G.; Keshamouni, V.G.; Padmanaban, G.; Rangarajan, P.N. Curcumin for Malaria Therapy. Biochem. Biophys. Res. Commun. 2005, 326, 472–474. [Google Scholar] [CrossRef]
- Lestari, M.L.A.D.; Indrayanto, G. Curcumin. Profiles Drug Subst. Excip. Relat. Methodol. 2014, 39, 113–204. [Google Scholar]
- Sahebkar, A.; Serban, M.-C.; Ursoniu, S.; Banach, M. Effect of Curcuminoids on Oxidative Stress: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Funct. Foods 2015, 18, 898–909. [Google Scholar] [CrossRef]
- Banach, M.; Serban, C.; Aronow, W.S.; Rysz, J.; Dragan, S.; Lerma, E.V.; Apetrii, M.; Covic, A. Lipid, Blood Pressure and Kidney Update 2013. Int. Urol. Nephrol. 2014, 46, 947–961. [Google Scholar] [CrossRef] [Green Version]
- Marchiani, A.; Rozzo, C.; Fadda, A.; Delogu, G.; Ruzza, P. Curcumin and Curcumin-like Molecules: From Spice to Drugs. Curr. Med. Chem. 2013, 21, 204–222. [Google Scholar] [CrossRef] [PubMed]
- Menon, V.P.; Sudheer, A.R. Antioxidant and anti-inflammatory properties of curcumin. In The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease; Springer: Boston, MA, USA, 2007; Volume 595, pp. 105–125. [Google Scholar] [CrossRef]
- Lin, Y.G.; Kunnumakkara, A.B.; Nair, A.; Merritt, W.M.; Han, L.Y.; Armaiz-Pena, G.N.; Kamat, A.A.; Spannuth, W.A.; Gershenson, D.M.; Lutgendorf, S.K.; et al. Curcumin Inhibits Tumor Growth and Angiogenesis in Ovarian Carcinoma by Targeting the Nuclear Factor-ΚB Pathway. Clin. Cancer Res. 2007, 13, 3423–3430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Priyadarsini, K.I.; Maity, D.K.; Naik, G.H.; Kumar, M.S.; Unnikrishnan, M.K.; Satav, J.G.; Mohan, H. Role of Phenolic O-H and Methylene Hydrogen on the Free Radical Reactions and Antioxidant Activity of Curcumin. Free Radic. Biol. Med. 2003, 35, 475–484. [Google Scholar] [CrossRef]
- Bimonte, S.; Barbieri, A.; Palma, G.; Rea, D.; Luciano, A.; D’Aiuto, M.; Arra, C.; Izzo, F. Dissecting the Role of Curcumin in Tumour Growth and Angiogenesis in Mouse Model of Human Breast Cancer. BioMed Res. Int. 2015, 2015, 878134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karthikeyan, A.; Senthil, N.; Min, T. Nanocurcumin: A promising candidate for therapeutic applications. Front. Pharmacol. 2020, 11, 487. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, A.; Young, K.N.; Moniruzzaman, M.; Beyene, A.M.; Do, K.; Kalaiselvi, S.; Min, T. Curcumin and its modified formulations on inflammatory bowel disease (IBD): The story so far and future outlook. Pharmaceutics 2021, 13, 484. [Google Scholar] [CrossRef] [PubMed]
- Jakubczyk, K.; Druzga, A.; Katarzyna, J.; Skonieczna-Zydecka, K. Antioxidant potential of curcumin-A meta-analysis of randomized clinical trials. Antioxidants 2020, 9, 1092. [Google Scholar] [CrossRef]
- Shen, L.; Ji, H.F. The pharmacology of curcumin: Is it the degradation products? Trends Mol. Med. 2012, 18, 138–144. [Google Scholar] [CrossRef]
- Del Peado-Audelo, M.L.; Caballero-Floran, I.H.; Meza-Toledo, J.A.; Mendoza-Munoz, N.; Gonzalez-Torres, M.; Floran, B.; Cortes, H.; Levya-Gomez, G. Formulations of curcumin nanoparticles for brain diseases. Biomolecules 2019, 9, 56. [Google Scholar] [CrossRef] [Green Version]
- Huang, M.T. Antioxidant and antitumorigenic properties of curcumin. In Food Factors for Cancer Revention; Ohigashi, H., Osawa, T., Terao, J., Watanabe, S., Yoshikawa, T., Eds.; Springer: Tokyo, Japan, 1997; pp. 249–252. [Google Scholar] [CrossRef]
- Abo-Salem, O.M.; Harisa, G.I.; Ali, T.M.; El-Sayed, E.S.M.; Abou-Elnour, F.M. Curcumin ameliorates streptozotocin-induced heart injury in rats. J. Biochem. Mol. Toxicol. 2014, 28, 263–270. [Google Scholar] [CrossRef]
- Oyetayo, B.O.; Abolaji, A.O.; Fasae, K.D.; Aderibigbe, A. Ameliorative role of diets fortified with curcumin in a Drosophila melanogaster model of aluminium chloride-induced neurotoxicity. J. Funct. Foods 2020, 71, 104035. [Google Scholar] [CrossRef]
- Lin, X.; Bai, D.; Wei, Z.; Zhang, Y.; Huang, Y.; Deng, H.; Huang, X. Curcumin attenuates oxidative stress in RAW264.7 cells by increasing the activity of antioxidant enzymes and activating the Nrf2-Keap1 pathway. PLoS ONE 2019, 14, e0216711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salehi, B.; Calina, D.; Docea, A.O.; Koirala, N.; Aryal, S.; Lombardo, D.; Pasqua, L.; Taheri, Y.; Marina Salgado Castillo, C.; Martorell, M.; et al. Curcumin’s Nanomedicine Formulations for Therapeutic Application in Neurological Diseases. J. Clin. Med. 2020, 9, 430. [Google Scholar] [CrossRef] [Green Version]
- Dugger, B.N.; Dickson, D.W. Pathology of Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2017, 9, a028035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wojda, U.; Salinska, E.; Kuznicki, J. Calcium Ions in Neuronal Degeneration. IUBMB Life 2008, 60, 575–590. [Google Scholar] [CrossRef] [PubMed]
- Kasote, D.M.; Hegde, M.V.; Katyare, S.S. Mitochondrial Dysfunction in Psychiatric and Neurological Diseases: Cause(s), Consequence(s), and Implications of Antioxidant Therapy. BioFactors 2013, 39, 392–406. [Google Scholar] [CrossRef] [PubMed]
- Waseem, M.; Parvez, S. Neuroprotective Activities of Curcumin and Quercetin with Potential Relevance to Mitochondrial Dysfunction Induced by Oxaliplatin. Protoplasma 2016, 253, 417–430. [Google Scholar] [CrossRef]
- Gemmell, E.; Bosomworth, H.; Allan, L.; Hall, R.; Khundakar, A.; Oakley, A.E.; Deramecourt, V.; Polvikoski, T.M.; O’Brien, J.T.; Kalaria, R.N. Hippocampal Neuronal Atrophy and Cognitive Function in Delayed Poststroke and Aging-Related Dementias. Stroke 2012, 43, 808–814. [Google Scholar] [CrossRef] [Green Version]
- Ferreiro, E.; Baldeiras, I.; Ferreira, I.L.; Costa, R.O.; Rego, A.C.; Pereira, C.F.; Oliveira, C.R. Mitochondrial- and Endoplasmic Reticulum-Associated Oxidative Stress in Alzheimer’s Disease: From Pathogenesis to Biomarkers. Int. J. Cell Biol. 2012, 2012, 735206. [Google Scholar] [CrossRef] [Green Version]
- Giasson, B.I.; Ischiropoulos, H.; Lee, V.M.-Y.; Trojanowski, J.Q. The Relationship between Oxidative/Nitrative Stress and Pathological Inclusions in Alzheimer’s and Parkinson’s Diseases1,2 11Guest Editors: Mark A. Smith and George Perry 22This Article Is Part of a Series of Reviews on “Causes and Consequences of Oxidative Stress in Alzheimer’s Disease.” The Full List of Papers May Be Found on the Homepage of the Journal. Free Radic. Biol. Med. 2002, 32, 1264–1275. [Google Scholar] [CrossRef]
- Andersen, J.K. Oxidative Stress in Neurodegeneration: Cause or Consequence? Nat. Med. 2004, 10, S18–S25. [Google Scholar] [CrossRef] [PubMed]
- Lustbader, J.W.; Cirilli, M.; Lin, C.; Xu, H.W.; Takuma, K.; Wang, N.; Caspersen, C.; Chen, X.; Pollak, S.; Chaney, M.; et al. ABAD Directly Links Aß to Mitochondrial Toxicity in Alzheimer’s Disease. Science 2004, 304, 448–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dumont, M.; Ho, D.J.; Calingasan, N.Y.; Xu, H.; Gibson, G.; Beal, M.F. Mitochondrial Dihydrolipoyl Succinyltransferase Deficiency Accelerates Amyloid Pathology and Memory Deficit in a Transgenic Mouse Model of Amyloid Deposition. Free Radic. Biol. Med. 2009, 47, 1019–1027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, P.H.; Manczak, M.; Yin, X.; Grady, M.C.; Mitchell, A.; Kandimalla, R.; Kuruva, C.S. Protective Effects of a Natural Product, Curcumin, against Amyloid β Induced Mitochondrial and Synaptic Toxicities in Alzheimer’s Disease. J. Investig. Med. 2016, 64, 1220–1234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lakshman, R.; Shah, R.; Reyes-Gordillo, K.; Varatharajalu, R. Synergy between NAFLD and AFLD and Potential Biomarkers. Clin. Res. Hepatol. Gastroenterol. 2015, 39, S29–S34. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.M.; deVera, M.E.; Fontes, P.; Shaikh, O.; Ahmad, J. Outcome After Liver Transplantation for NASH Cirrhosis. Am. J. Transplant. 2009, 9, 782–793. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.P.; Pitts, A.; Younossi, Z.M. Increased Overall Mortality and Liver-Related Mortality in Non-Alcoholic Fatty Liver Disease. J. Hepatol. 2008, 49, 608–612. [Google Scholar] [CrossRef]
- Lefkowitch, J.H. Morphology of Alcoholic Liver Disease. Clin. Liver Dis. 2005, 9, 37–53. [Google Scholar] [CrossRef]
- Siegel, A.B.; Zhu, A.X. Metabolic Syndrome and Hepatocellular Carcinoma. Cancer 2009, 115, 5651–5661. [Google Scholar] [CrossRef] [Green Version]
- Rao, M.S.; Reddy, J.K. PPAR? In the Pathogenesis of Fatty Liver Disease. Hepatology 2004, 40, 783–786. [Google Scholar] [CrossRef]
- Feldstein, A.E.; Canbay, A.; Guicciardi, M.E.; Higuchi, H.; Bronk, S.F.; Gores, G.J. Diet Associated Hepatic Steatosis Sensitizes to Fas Mediated Liver Injury in Mice. J. Hepatol. 2003, 39, 978–983. [Google Scholar] [CrossRef]
- Rafiee, P.; Binion, D.G.; Wellner, M.; Behmaram, B.; Floer, M.; Mitton, E.; Nie, L.; Zhang, Z.; Otterson, M.F. Modulatory Effect of Curcumin on Survival of Irradiated Human Intestinal Microvascular Endothelial Cells: Role of Akt/MTOR and NF-ΚB. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 298, G865–G877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Connell, M.A.; Rushworth, S.A. Curcumin: Potential for Hepatic Fibrosis Therapy? Br. J. Pharmacol. 2008, 153, 403–405. [Google Scholar] [CrossRef] [PubMed]
- Kuo, J.-J.; Chang, H.-H.; Tsai, T.-H.; Lee, T.-Y. Curcumin Ameliorates Mitochondrial Dysfunction Associated with Inhibition of Gluconeogenesis in Free Fatty Acid-Mediated Hepatic Lipoapoptosis. Int. J. Mol. Med. 2012, 30, 643–649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guzik, T.J.; West, N.E.J.; Pillai, R.; Taggart, D.P.; Channon, K.M. Nitric Oxide Modulates Superoxide Release and Peroxynitrite Formation in Human Blood Vessels. Hypertension 2002, 39, 1088–1094. [Google Scholar] [CrossRef] [Green Version]
- Saavedra-Molina, A.; Ramírez-Emiliano, J.; Clemente-Guerrero, M.; Pérez-Vázquez, V.; Aguilera-Aguirre, L.; González-Hernández, J.C. Mitochondrial Nitric Oxide Inhibits ATP Synthesis Effect of Free Calcium in Rat Heart. Amino Acids 2003, 24, 95–102. [Google Scholar] [CrossRef]
- Weisberg, S.P.; Leibel, R.; Tortoriello, D.V. Dietary Curcumin Significantly Improves Obesity-Associated Inflammation and Diabetes in Mouse Models of Diabesity. Endocrinology 2008, 149, 3549–3558. [Google Scholar] [CrossRef] [Green Version]
- Razmaria, A.A. Chronic Kidney Disease. JAMA 2016, 315, 2248. [Google Scholar] [CrossRef]
- Chen, T.K.; Knicely, D.H.; Grams, M.E. Chronic Kidney Disease Diagnosis and Management. JAMA 2019, 322, 1294. [Google Scholar] [CrossRef]
- Webster, A.C.; Nagler, E.V.; Morton, R.L.; Masson, P. Chronic Kidney Disease. Lancet 2017, 389, 1238–1252. [Google Scholar] [CrossRef]
- Ábrigo, J.; Elorza, A.A.; Riedel, C.A.; Vilos, C.; Simon, F.; Cabrera, D.; Estrada, L.; Cabello-Verrugio, C. Role of Oxidative Stress as Key Regulator of Muscle Wasting during Cachexia. Oxidative Med. Cell. Longev. 2018, 2018, 2063179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moorthi, R.N.; Avin, K.G. Clinical Relevance of Sarcopenia in Chronic Kidney Disease. Curr. Opin. Nephrol. Hypertens. 2017, 26, 219–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leitner, L.M.; Wilson, R.J.; Yan, Z.; Gödecke, A. Reactive Oxygen Species/Nitric Oxide Mediated Inter-Organ Communication in Skeletal Muscle Wasting Diseases. Antioxid. Redox Signal. 2017, 26, 700–717. [Google Scholar] [CrossRef]
- Wu, H.; Kanatous, S.B.; Thurmond, F.A.; Gallardo, T.; Isotani, E.; Bassel-Duby, R.; Williams, R.S. Regulation of Mitochondrial Biogenesis in Skeletal Muscle by CaMK. Science 2002, 296, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Chen, J.; Liu, X.; Zheng, P.; Song, G.; Yi, T.; Li, S. RETRACTED ARTICLE: A Chinese Herbal Formula, Jian-Pi-Yi-Shen Decoction, Improves Muscle Atrophy via Regulating Mitochondrial Quality Control Process in 5/6 Nephrectomised Rats. Sci. Rep. 2017, 7, 9253. [Google Scholar] [CrossRef] [Green Version]
- Aparicio-Trejo, O.E.; Tapia, E.; Molina-Jijón, E.; Medina-Campos, O.N.; Macías-Ruvalcaba, N.A.; León-Contreras, J.C.; Hernández-Pando, R.; García-Arroyo, F.E.; Cristóbal, M.; Sánchez-Lozada, L.G.; et al. Curcumin Prevents Mitochondrial Dynamics Disturbances in Early 5/6 Nephrectomy: Relation to Oxidative Stress and Mitochondrial Bioenergetics. BioFactors 2017, 43, 293–310. [Google Scholar] [CrossRef]
- Correa, F.; Buelna-Chontal, M.; Hernández-Reséndiz, S.; García-Niño, W.R.; Roldán, F.J.; Soto, V.; Silva-Palacios, A.; Amador, A.; Pedraza-Chaverrí, J.; Tapia, E.; et al. Curcumin Maintains Cardiac and Mitochondrial Function in Chronic Kidney Disease. Free Radic. Biol. Med. 2013, 61, 119–129. [Google Scholar] [CrossRef]
- Trujillo, J.; Granados-Castro, L.F.; Zazueta, C.; Andérica-Romero, A.C.; Chirino, Y.I.; Pedraza-Chaverrí, J. Mitochondria as a Target in the Therapeutic Properties of Curcumin. Arch. Pharm. 2014, 347, 873–884. [Google Scholar] [CrossRef]
- González-Salazar, A.; Molina-Jijón, E.; Correa, F.; Zarco-Márquez, G.; Calderón-Oliver, M.; Tapia, E.; Zazueta, C.; Pedraza-Chaverri, J. Curcumin Protects from Cardiac Reperfusion Damage by Attenuation of Oxidant Stress and Mitochondrial Dysfunction. Cardiovasc. Toxicol. 2011, 11, 357–364. [Google Scholar] [CrossRef]
- Quiros, Y.; Vicente-Vicente, L.; Morales, A.I.; Lopez-Novoa, J.M.; Lopez-Hernandez, F.J. An Integrative Overview on the Mechanisms Underlying the Renal Tubular Cytotoxicity of Gentamicin. Toxicol. Sci. 2011, 119, 245–256. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Wong, H.S.; Leung, H.Y.; Leong, P.K.; Chan, W.M.; Chen, N.; Ko, K.M. An Ursolic Acid-Enriched Extract of Cynomorium Songaricum Protects against Carbon Tetrachloride Hepatotoxicity and Gentamicin Nephrotoxicity in Rats Possibly through a Mitochondrial Pathway: A Comparison with Ursolic Acid. J. Funct. Foods 2014, 7, 330–341. [Google Scholar] [CrossRef]
- Negrette-Guzmán, M.; Huerta-Yepez, S.; Medina-Campos, O.N.; Zatarain-Barrón, Z.L.; Hernández-Pando, R.; Torres, I.; Tapia, E.; Pedraza-Chaverri, J. Sulforaphane Attenuates Gentamicin-Induced Nephrotoxicity: Role of Mitochondrial Protection. Evid. Based Complement. Altern. Med. 2013, 2013, 135314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manikandan, R.; Beulaja, M.; Thiagarajan, R.; Priyadarsini, A.; Saravanan, R.; Arumugam, M. Ameliorative Effects of Curcumin against Renal Injuries Mediated by Inducible Nitric Oxide Synthase and Nuclear Factor Kappa B during Gentamicin-Induced Toxicity in Wistar Rats. Eur. J. Pharmacol. 2011, 670, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.H.; Al-Wabel, N.; Mahmoud, O.; Mousa, H.M.; Hashad, M. Curcumin Has a Palliative Action on Gentamicin-Induced Nephrotoxicity in Rats. Fundam. Clin. Pharmacol. 2005, 19, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.S.; Massey, H.D.; Krieg, R.; Fazelbhoy, Z.A.; Ghosh, S.; Sica, D.A.; Fakhry, I.; Gehr, T.W.B. Curcumin Ameliorates Renal Failure in 5/6 Nephrectomized Rats: Role of Inflammation. Am. J. Physiol. Ren. Physiol. 2009, 296, F1146–F1157. [Google Scholar] [CrossRef] [Green Version]
- Tapia, E.; Soto, V.; Ortiz-Vega, K.M.; Zarco-Márquez, G.; Molina-Jijón, E.; Cristóbal-García, M.; Santamaría, J.; García-Niño, W.R.; Correa, F.; Zazueta, C.; et al. Curcumin Induces Nrf2 Nuclear Translocation and Prevents Glomerular Hypertension, Hyperfiltration, Oxidant Stress, and the Decrease in Antioxidant Enzymes in 5/6 Nephrectomized Rats. Oxidative Med. Cell. Longev. 2012, 2012, 269039. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Deeb, D.; Jiang, H.; Liu, Y.B.; Dulchavsky, S.A.; Gautam, S.C. Curcumin differentially sensitizes malignant glioma cells to TRAIL/Apo2L-mediated apoptosis through activation of procaspases and release of cytochrome c from mitochondria. J. Exp. Ther. Oncol. 2005, 5, 39–48. [Google Scholar]
- Mares-Perlman, J.A.; Klein, R.; Klein, B.E.; Greger, J.L.; Brady, W.E.; Palta, M.; Ritter, L.L. Association of zinc and antioxidant nutrients with age-related maculopathy. Arch. Ophthalmol. 1996, 114, 991–997. [Google Scholar] [CrossRef]
- Kowluru, R.A.; Kanwar, M. Effects of curcumin on retinal oxidative stress and inflammation in diabetes. Nutr. Metab. 2007, 4, 8. [Google Scholar] [CrossRef] [Green Version]
- Vasireddy, V.; Chavali, V.R.; Joseph, V.T.; Kadam, R.; Lin, J.H.; Jamison, J.A.; Ayyagari, R. Rescue of photoreceptor degeneration by curcumin in transgenic rats with P23H rhodopsin mutation. PLoS ONE 2011, 6, e21193. [Google Scholar] [CrossRef] [Green Version]
- Mandal, M.N.A.; Patlolla, J.M.; Zheng, L.; Agbaga, M.P.; Tran, J.T.A.; Wicker, L.; Anderson, R.E. Curcumin protects retinal cells from light-and oxidant stress-induced cell death. Free Radic. Biol. Med. 2009, 46, 672–679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Premanand, C.; Rema, M.; Sameer, M.Z.; Sujatha, M.; Balasubramanyam, M. Effect of curcumin on proliferation of human retinal endothelial cells under in vitro conditions. Investig. Ophthalmol. Vis. Sci. 2006, 47, 2179–2184. [Google Scholar] [CrossRef] [PubMed]
- Strauss, O. The retinal pigment epithelium in visual function. Physiol. Rev. 2005, 85, 845–881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinlaor, S.; Yongvanit, P.; Prakobwong, S.; Kaewsamut, B.; Khoontawad, J.; Pinlaor, P.; Hiraku, Y. Curcumin reduces oxidative and nitrative DNA damage through balancing of oxidant–antioxidant status in hamsters infected with Opisthorchis viverrini. Mol. Nutr. Food Res. 2009, 53, 1316–1328. [Google Scholar] [CrossRef] [PubMed]
- Alex, A.F.; Spitznas, M.; Tittel, A.P.; Kurts, C.; Eter, N. Inhibitory effect of epigallocatechin gallate (EGCG), resveratrol, and curcumin on proliferation of human retinal pigment epithelial cells in vitro. Curr. Eye Res. 2010, 35, 1021–1033. [Google Scholar] [CrossRef]
- Roth, F.; Bindewald, A.; Holz, F.G. Keypathophysiologic pathways in age-related macular disease. Graefe’s Arch. Clin. Exp. Ophthalmol. 2004, 242, 710–716. [Google Scholar] [CrossRef]
- Molina-Jijón, E.; Tapia, E.; Zazueta, C.; El Hafidi, M.; Zatarain-Barrón, Z.L.; Hernández-Pando, R.; Pedraza-Chaverri, J. Curcumin prevents Cr (VI)-induced renal oxidant damage by a mitochondrial pathway. Free Radic. Biol. Med. 2011, 51, 1543–1557. [Google Scholar] [CrossRef]
- Flynn, D.L.; Rafferty, M.F.; Boctor, A.M. Inhibition of 5-hydroxy-eicosatetraenoic acid (5-HETE) formation in intact human neutrophils by naturally-occurring diarylheptanoids: Inhibitory activities of curcuminoids and yakuchinones. Prostaglandins Leukot. Med. 1986, 22, 357–360. [Google Scholar] [CrossRef]
- Terruzzi, I.; Montesano, A.; Senesi, P.; Villa, I.; Ferraretto, A.; Bottani, M.; Rubinacci, A. L-Carnitine reduces oxidative stress and promotes cells differentiation and bone matrix proteins expression in human osteoblast-like cells. BioMed Res. Int. 2019, 2019, 5678548. [Google Scholar] [CrossRef]
- Domazetovic, V.; Marcucci, G.; Iantomasi, T.; Brandi, M.L.; Vincenzini, M.T. Oxidative stress in bone remodeling: Role of antioxidants. Clin. Cases Miner. Bone Metab. 2017, 14, 209. [Google Scholar] [CrossRef]
- Peddada, K.V.; Peddada, K.V.; Shukla, S.K.; Mishra, A.; Verma, V. Role of curcumin in common musculoskeletal disorders: A review of current laboratory, translational, and clinical data. Orthop. Surg. 2015, 7, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Rohanizadeh, R.; Deng, Y.; Verron, E. Therapeutic actions of curcumin in bone disorders. BoneKEy Rep. 2016, 5, 793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jagetia, G.C.; Aggarwal, B.B. “Spicing up” of the immune system by curcumin. J. Clin. Immunol. 2007, 27, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Da, W.; Zhang, J.; Zhang, R.; Zhu, J. Curcumin inhibits the lymphangiogenesis of gastric cancer cells by inhibition of HMGB1/VEGF-D signaling. Int. J. Immunopathol. Pharmacol. 2019, 33, 2058738419861600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattacharyya, S.; Md Sakib Hossain, D.; Mohanty, S.; Sankar Sen, G.; Chattopadhyay, S.; Banerjee, S.; Chakraborty, J.; Das, K.; Sarkar, D.; Das, T.; et al. Curcumin reverses T cell-mediated adaptive immune dysfunctions in tumor-bearing hosts. Cell Mol. Immunol. 2010, 7, 306–315. [Google Scholar] [CrossRef] [Green Version]
- Sivertsen, H.; Bjorkorf, G.H.; Engedal, K.; Selbaek, G.; Helvik, A.S. Depression and Quality of Life in Older Persons: A Review. Dement. Geriatr. Cogn. Disord. 2015, 40, 311–339. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, L.; Zhang, J. Curcumin in Antidepressant treatments: An overview of potential mechanisms, preclinical/clinical trials and ongoing challenges. Basic Clin. Pharmacol. Toxicol. 2020, 127, 243–253. [Google Scholar] [CrossRef]
- Norat, P.; Soldozy, S.; Sokolowski, J.D.; Gorick, C.M.; Kumar, J.S.; Chae, Y.; Yağmurlu, K.; Prada, F.; Walker, M.; Levitt, M.R.; et al. Mitochondrial Dysfunction in Neurological Disorders: Exploring Mitochondrial Transplantation. NPJ Regen. Med. 2020, 5, 22. [Google Scholar] [CrossRef]
- Mansouri, A.; Gattolliat, C.-H.; Asselah, T. Mitochondrial Dysfunction and Signaling in Chronic Liver Diseases. Gastroenterology 2018, 155, 629–647. [Google Scholar] [CrossRef] [Green Version]
- Cloonan, S.M.; Kim, K.; Esteves, P.; Trian, T.; Barnes, P.J. Mitochondrial Dysfunction in Lung Ageing and Disease. Eur. Respir. Rev. 2020, 29, 200165. [Google Scholar] [CrossRef]
- Schrier, S.A.; Falk, M.J. Mitochondrial Disorders and the Eye. Curr. Opin. Ophthalmol. 2011, 22, 325–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chanséaume, E.; Morio, B. Potential Mechanisms of Muscle Mitochondrial Dysfunction in Aging and Obesity and Cellular Consequences. Int. J. Mol. Sci. 2009, 10, 306–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gouspillou, G.; Hepple, R.T. Editorial: Mitochondria in Skeletal Muscle Health, Aging and Diseases. Front. Physiol. 2016, 7, 446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, M.A.; Volpi, S.; Sims, K.B.; Walter, J.E.; Traggiai, E. Powering the Immune System: Mitochondria in Immune Function and Deficiency. J. Immunol. Res. 2014, 2014, 164309. [Google Scholar] [CrossRef]
- Wang, D.; Yang, Y.; Zou, X.; Zheng, Z.; Zhang, J. Curcumin Ameliorates CKD-Induced Mitochondrial Dysfunction and Oxidative Stress through Inhibiting GSK-3β Activity. J. Nutr. Biochem. 2020, 83, 108404. [Google Scholar] [CrossRef]
- Daverey, A.; Agrawal, S.K. Curcumin Alleviates Oxidative Stress and Mitochondrial Dysfunction in Astrocytes. Neuroscience 2016, 333, 92–103. [Google Scholar] [CrossRef]
- Soto-Urquieta, M.G.; López-Briones, S.; Pérez-Vázquez, V.; Saavedra-Molina, A.; González-Hernández, G.A.; Ramírez-Emiliano, J. Curcumin Restores Mitochondrial Functions and Decreases Lipid Peroxidation in Liver and Kidneys of Diabetic Db/Db Mice. Biol. Res. 2014, 47, 74. [Google Scholar] [CrossRef] [Green Version]
- Sabet, N.S.; Atashbar, S.; Khanlou, E.M.; Kahrizi, F.; Salimi, A. Curcumin Attenuates Bevacizumab-Induced Toxicity via Suppressing Oxidative Stress and Preventing Mitochondrial Dysfunction in Heart Mitochondria. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020, 393, 1447–1457. [Google Scholar] [CrossRef]
- Li, L.; Liu, S.; Zhou, Y.; Zhao, M.; Wang, Y.; Wang, C.; Lou, P.; Huang, R.; Ma, L.; Lu, Y.; et al. Indispensable Role of Mitochondria in Maintaining the Therapeutic Potential of Curcumin in Acute Kidney Injury. J. Cell. Mol. Med. 2021, 25, 9863–9877. [Google Scholar] [CrossRef]

| Organ | Causes of Mitochondrial Dysfunction | Affected Genes/Proteins | Anticipated Disease State | Reference |
|---|---|---|---|---|
| Brain | Excessive accumulation of calcium in the mitochondrial matrix Opening of mitochondrial permeability transition pore Release of cytochrome C leading to activation of apoptosis Dysfunction in fission and fusion activities in mitochondria | Cyclophilin D (Cyp D) Cytochrome C (Cyt C) Mitofusin (Mfn) Dynamin-related protein1 (Drp1) Optic atrophy mitochondrial protein (OPA) | Traumatic brain injury (TBI) Alzheimer’s disease Parkinson’s disease Huntington’s disease Ischemic stroke | [110] |
| Liver | Inner mitochondrial lesions Dynamic alterations in mitochondria Lower levels if respiratory chain complex enzymes Inability to synthesize ATP | Nuclear factor- κB (NF- κB) I kappa B-kinase (IKK-α,β,γ) Stimulation of Interferon genes (STING) TANK binding kinase 1 (TBK1) Interferon regulatory factors (IRF3, IRF7) | Non-alcoholic fatty liver disease Alcoholic fatty liver disease Drug-associated fatty liver disease Hepatitis B Hepatitis C | [111] |
| Lungs | Increased concentration of iron mitochondria Abnormal metabolic activity due to excessive mtROS production Decrease in mitochondrial number and function | Mammalian target of rapamycin (mTOR) Peroxisome proliferator- activated receptor gamma coactivator 1-alpha (PGC-1α) Angiotensin converting enzyme 2 (ACE2) Tumor necrosis factor-α (TNF-α) Interleukin-6 (IL-6) Matrix metalloproteinase 2 (MMP2) Transforming growth factor-β (TGF-β) | Cystic fibrosis Asthma Pneumonia Tuberculosis Lung cancer Chronic obstructive pulmonary disease (COPD) | [112] |
| Eye | Defects in mitochondrial respiratory chain subunit complex I enzymes Deletion of mitochondrial DNA Fragments in mitochondrial network Loss of membrane potential Unproper arrangement cristae structure of optic nerve mitochondria | OPA 1 and 3 Thymidine phosphoryase (TYMP) Adenine nucleoside translocator 1 (ANT1) Twinkle mtDNA helicase (PEO1) DNA polymerase subunit gamma 1 (PLOG1) | Dominant optic atrophy (DOA) Leber Hereditary optic neuropathy (LHOA) Chronic progressive external ophthalmoplegia (CPEO) Pigmentary retinopathy | [113] |
| Skeletal system | Lower levels of mitochondrial enzyme production Decreased ATP production Decline in mitochondrial density Lower protein levels in ATP synthase subunit β Insulin resistance | Cytochrome C oxidase (COX) Forkhead box class-I (FoxO1) PGC-1α NADH dehydrogenase subunit IV (NADH) Protein kinase B (AKT) | Aging Cancer cachexia Disuse-induced muscle atrophy | [114,115] |
| Lymphatic system | Decreased ATP production Lower levels of mitochondrial respiratory chain complex enzymes | Adenylate kinase 2 (AK2) Tafazzin, Phospholipid-Lysophospholipid Transacylase (TAZ) | Severe combined immune deficiency disease (SCID) | [116] |
| Disease | Action of Curcumin | Effects of Curcumin | Animal Model/Cell Type | Reference |
|---|---|---|---|---|
| Chronic kidney disease (CKD)-induced muscle atrophy | Inhibition of GSK-3β activity | Improves muscle function Higher ATP levels Suppressing mitochondrial membrane potential Decreases mitochondrial oxidative stress and increases antioxidant levels | C57BL/6 mice | [117] |
| Neurodegenerative disease | Inhibits GFAP, vimentin and Prdx6 upregulation | Suppresses oxidative stress-induced inflammation Alleviates apoptosis Suppresses mitochondrial fragmentation | Human glioblastoma cell line -A172 Human astrocytes cell line derived from spinal cord- HA-sp | [118] |
| Insulin resistance in non-alcoholic fatty liver disease | Inhibits lipoapoptosis, ROS generation and ATP depletion | Lowers high free fatty acid-induced synthesizes of phosphoenol pyruvate carboxykinase (PEPCK) and glucose-6-phosphate Contributes cell survival Restores mitochondrial membrane potential | Hepatocytes | [66] |
| Hyperglycemia | Inhibits increased oxygen consumption and decreased nitric oxide levels | Decreased state 3 oxygen consumption rate Declines the levels of thiobarbituaric acid-reactive substances | Female and male heterozygote non-diabetic db/+ mice | [119] |
| Heart failure | Acts as an adjuvant therapy | Inhibits mitochondrial impairment Alleviates oxidative stress Decreases mitochondrial membrane potential collapse | Male wistar rats | [120] |
| Alzheimer’s disease | Protects β-amyloid protein | Enhances mitochondrial fusion activity Decreases fission machinery Increased biogenesis and synaptic proteins | SHSY5Y cells | [56] |
| Acute kidney injury | Suppresses NF-κB activation in reducing inflammation and stimulates NRF2/HO-1 signaling reduced mitochondrial dysfunction | Decline in the level of mitochondrial ROS Reduced mitochondrial fragmentation level Enhanced TCA cycle, mitochondrial biogenesis | Human renal proximal tubular epithelial cell (TEC) line—HK2 | [121] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sathyabhama, M.; Priya Dharshini, L.C.; Karthikeyan, A.; Kalaiselvi, S.; Min, T. The Credible Role of Curcumin in Oxidative Stress-Mediated Mitochondrial Dysfunction in Mammals. Biomolecules 2022, 12, 1405. https://doi.org/10.3390/biom12101405
Sathyabhama M, Priya Dharshini LC, Karthikeyan A, Kalaiselvi S, Min T. The Credible Role of Curcumin in Oxidative Stress-Mediated Mitochondrial Dysfunction in Mammals. Biomolecules. 2022; 12(10):1405. https://doi.org/10.3390/biom12101405
Chicago/Turabian StyleSathyabhama, Muthuswamy, Loganathan Chandramani Priya Dharshini, Adhimoolam Karthikeyan, Senthil Kalaiselvi, and Taesun Min. 2022. "The Credible Role of Curcumin in Oxidative Stress-Mediated Mitochondrial Dysfunction in Mammals" Biomolecules 12, no. 10: 1405. https://doi.org/10.3390/biom12101405






