Use of Olive and Sunflower Oil Hydrogel Emulsions as Pork Fat Replacers in Goat Meat Burgers: Fat Reduction and Effects in Lipidic Quality
Abstract
:1. Introduction
2. Materials and Methods
2.1. Burger Formulations and Production
2.2. Raw Burger Technological Quality Traits and Composition Analyses
2.3. Cooked Burger Analyses
2.4. Statistical Analysis
3. Results and Discussion
3.1. Raw Burgers Quality Traits and Composition
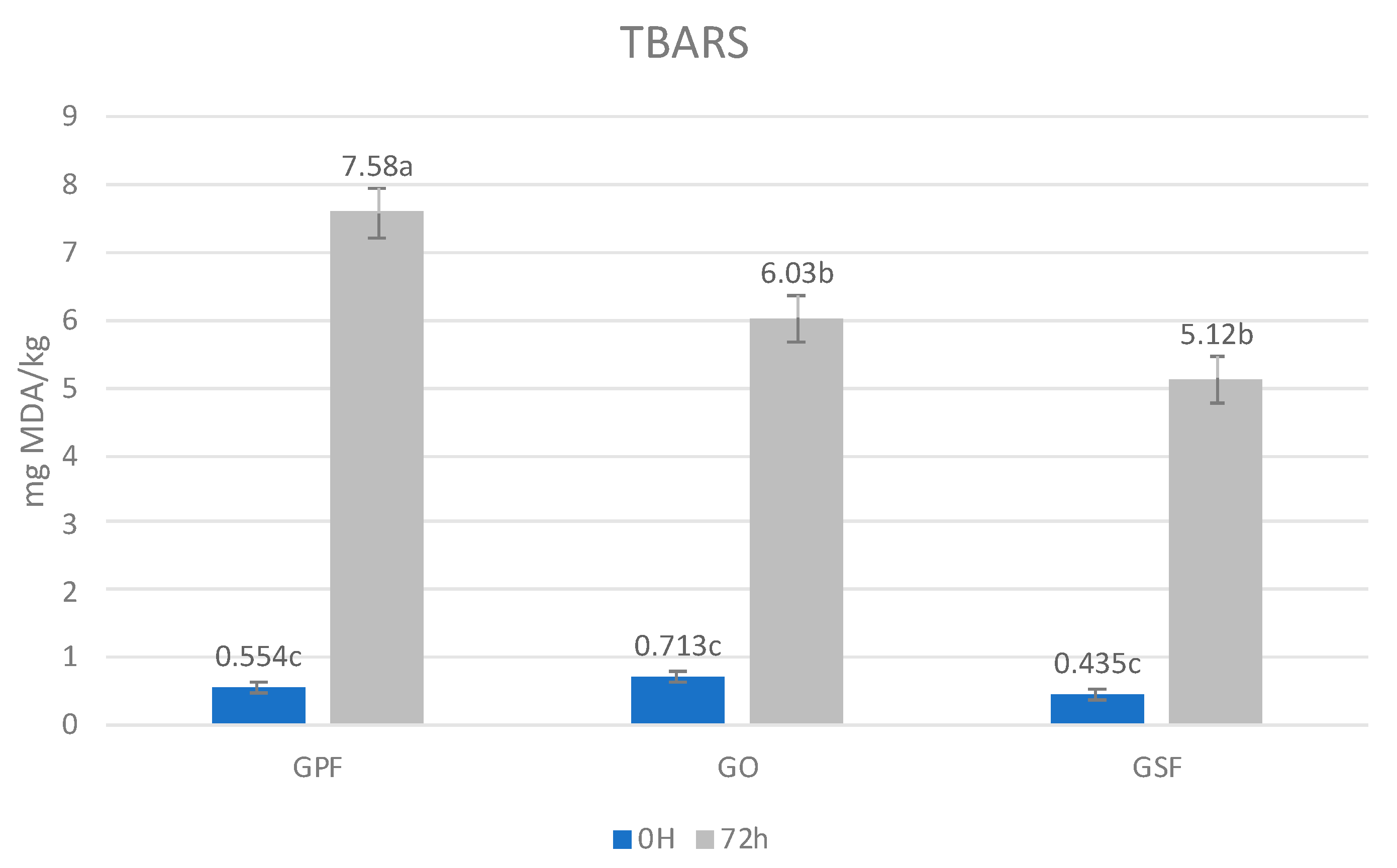
3.2. Cooked Burgers Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McCarthy, M.; Collins, A.; Flaherty, S.J.; McCarthy, S.N. Healthy eating habit: A role for goals, identity, and self-control? Psychol. Mark. 2017, 34, 772–785. [Google Scholar] [CrossRef]
- Buttriss, J.L. Food reformulation: The challenges to the food industry. Proc. Nutr. Soc. 2012, 72, 61–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domínguez, R.; Pateiro, M.; Munekata, P.E.S.; Campagnol, P.C.B.; Lorenzo, J.M. Influence of partial pork backfat replacement by fish oil on nutritional and technological properties of liver pâté. Eur. J. Lipid Sci. Technol. 2016, 119, 1600178. [Google Scholar] [CrossRef]
- Wang, X.; Xie, Y.; Li, X.; Liu, Y.; Yan, W. Effects of partial replacement of pork back fat by a camellia oil gel on certain quality characteristics of a cooked style Harbin sausage. Meat Sci. 2018, 146, 154–159. [Google Scholar] [CrossRef]
- Franco, D.; Martins, A.; López-Pedrouso, M.; Purriños, L.; Cerqueira, M.; Vicente, A.; Pastrana, L.; Zapata, C.; Lorenzo, J. Strategy towards Replacing Pork Backfat with a Linseed Oleogel in Frankfurter Sausages and Its Evaluation on Physicochemical, Nutritional, and Sensory Characteristics. Foods 2019, 8, 366. [Google Scholar] [CrossRef] [Green Version]
- de Souza Paglarini, C.; de Figueiredo Furtado, G.; Honório, A.R.; Mokarzel, L.; da Silva Vidal, V.A.; Ribeiro, A.P.B.; Cunha, R.L.; Pollonio, M.A.R. Functional emulsion gels as pork back fat replacers in Bologna sausage. Food Struct. 2019, 20, 100105. [Google Scholar] [CrossRef]
- Teixeira, A.; Ferreira, I.; Pereira, E.; Vasconcelos, L.; Leite, A.; Rodrigues, S. Physicochemical composition and sen-sory quality of goat meat burgers. Eff. Fat Source. Foods 2021, 10, 1824. [Google Scholar]
- Kausar, T.; Kausar, M.; Khan, S.; Haque, S.; Azad, Z. Optimum Additive Composition to Minimize Fat in Functional Goat Meat Nuggets: A Healthy Red Meat Functional Food. Processes 2021, 9, 475. [Google Scholar] [CrossRef]
- Abuelfatah, K.M.; Ibrahim, M.F.; Babiker, S.A. Fat Content and Fatty acid Composition of Goat Meat at Retail Outlets in Khartoum state, Sudan. J. Vet. Med. Anim. Prod. 2017, 8, 106–111. [Google Scholar]
- Badar, I.H.; Liu, H.; Chen, Q.; Xia, X.; Kong, B. Future trends of processed meat products concerning perceived healthiness: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4739–4778. [Google Scholar] [CrossRef]
- Domínguez, R.; Munekata, P.E.; Pateiro, M.; López-Fernández, O.; Lorenzo, J.M. Immobilization of oils using hydrogels as strategy to replace animal fats and improve the healthiness of meat products. Curr. Opin. Food Sci. 2020, 37, 135–144. [Google Scholar] [CrossRef]
- Foggiaro, D.; Domínguez, R.; Pateiro, M.; Cittadini, A.; Munekata, P.E.S.; Campagnol, P.C.B.; Fraqueza, M.J.; De Palo, P.; Lorenzo, J.M. Use of Healthy Emulsion Hydrogels to Improve the Quality of Pork Burgers. Foods 2022, 11, 596. [Google Scholar] [CrossRef] [PubMed]
- Mandolesi, S.; Naspetti, S.; Arsenos, G.; Caramelle-Holtz, E.; Latvala, T.; Martin-Collado, D.; Orsini, S.; Ozturk, E.; Zanoli, R. Motivations and Barriers for Sheep and Goat Meat Consumption in Europe: A Means–End Chain Study. Animals 2020, 10, 1105. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.; Fernandes, A.; Pereira, E. Fat content reduction and lipid profile improvement in Portuguese fermented sausages alheira. Heliyon 2020, 6, e05306. [Google Scholar] [CrossRef]
- Pophiwa, P.; Webb, E.C.; Frylinck, L. A review of factors affecting goat meat quality and mitigating strategies. Small Rumin. Res. 2019, 183, 106035. [Google Scholar] [CrossRef]
- Cunha, L.C.M.; Monteiro, M.L.G.; Lorenzo, J.M.; Munekata, P.E.S.; Muchenje, V.; de Carvalho, F.A.L.; Conte-Junior, C.A. Natural antioxidants in processing and storage stability of sheep and goat meat products. Food Res. Int. 2018, 111, 379–390. [Google Scholar] [CrossRef]
- Teixeira, A.; Silva, S.; Guedes, C.; Rodrigues, S. Sheep and Goat Meat Processed Products Quality: A Review. Foods 2020, 9, 960. [Google Scholar] [CrossRef]
- Stajić, S.; Pisinov, B.; Tomasevic, I.; Djekic, I.; Čolović, D.; Ivanović, S.; Živković, D. Use of culled goat meat in frankfurter production–Effect on sensory quality and technological properties. Int. J. Food Sci. Technol. 2020, 55, 1032–1045. [Google Scholar] [CrossRef]
- Feiner, G. Meat Products Handbook: Practical Science and Technology; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Stajic, S.; Pisinov, B. Goat meat products. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2021; Volume 854, p. 012092. [Google Scholar]
- Leite, A.; Rodrigues, S.; Pereira, E.; Paulos, K.; Oliveira, A.F.; Lorenzo, J.M.; Teixeira, A. Physicochemical properties, fatty acid profile and sensory characteristics of sheep and goat meat sausages manufactured with different pork fat levels. Meat Sci. 2015, 105, 114–120. [Google Scholar] [CrossRef]
- Malekian, F.; Khachaturyan, M.; Gebrelul, S.; Henson, J. Nutritional characteristics and consumer acceptability of sausages with different combinations of goat and beef meats. Funct. Foods Health Dis. 2016, 6, 42. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, A.; Almeida, S.; Pereira, E.; Mangachaia, F.; Rodrigues, S. Physicochemical characteristics of sheep and goat pâtés. differences between fat sources and proportions. Heliyon 2019, 5, e02119. [Google Scholar] [CrossRef]
- Verma, A.K.; Rajkumar, V.; Kumar, S. Effect of amaranth and quinoa seed flour on rheological and physicochemical properties of goat meat nuggets. J. Food Sci. Technol. 2019, 56, 5027–5035. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, D.K.; Das, A.K.; Banerjee, R.; Pateiro, M.; Nanda, P.K.; Gadekar, Y.P.; Biswas, S.; McClements, D.J.; Lorenzo, J.M. Application of Enoki Mushroom (Flammulina Velutipes) Stem Wastes as Functional Ingredients in Goat Meat Nuggets. Foods 2020, 9, 432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jagtap, N.S.; Wagh, R.V.; Chatli, M.K.; Malav, O.P.; Kumar, P.; Mehta, N. Functional goat meat nuggets fortified with novel bioactive Carica papaya L. and Origanum vulgare extracts and storage stability thereof. Nutr. Food Sci. 2019, 50, 402–414. [Google Scholar] [CrossRef]
- Teixeira, A.; Silva, S.; Rodrigues, S. Advances in Sheep and Goat Meat Products Research. In Advances in Food and Nutrition Research; Toldra, F., Ed.; Academic Press: Cambridge, MA, USA, 2019; Volume 87, pp. 305–370. [Google Scholar] [CrossRef]
- Barros, J.C.; Munekata, P.E.S.; De Carvalho, F.A.L.; Pateiro, M.; Barba, F.J.; Domínguez, R.; Trindade, M.A.; Lorenzo, J.M. Use of Tiger Nut (Cyperus esculentus L.) Oil Emulsion as Animal Fat Replacement in Beef Burgers. Foods 2020, 9, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teixeira, P.R.J. Contributo Para a Caracterização do Azeite Extraído na Área de Influência da Cooperativa dos Olivicultores de Valpaços. Master’s Thesis, Instituto Politécnico de Bragança, Bragança, Portugal, 2015. Available online: http://hdl.handle.net/10198/12726 (accessed on 7 July 2021).
- NP 588, 2008; Portuguese Standard for Meat and Meat Products. Definition and Classification. Portuguese Institute of Quality, Ministry of Economy and Innovation: Caparica, Portugal, 2008.
- NP-ISO-3441, 2008; Determinação do pH (Método de referência); In Portuguese Norm–Meat and Meat Products. Portuguese Institute of Quality, Ministry of Economy and Innovation: Caparica, Portugal, 2008.
- Cunniff, P. Methods of Analysis of AOAC International, 16th ed.; AOAC International: Rockville, MD, USA, 1995; ISBN 9780935584547. [Google Scholar]
- Teixeira, A.; Domínguez, R.; Rey, J.; Aleu, G.; Pateiro, M. Lorenzo J. M. Ph and Color. In Methods to Assess the Quality of Meat Products; Lorenzo, J.M., Domínguez, R., Pateiro, M., Munekata, P.E., Eds.; Humana: New York, NY, USA, 2022; Volume 1, pp. 17–23. [Google Scholar]
- NP-ISO-3356/2009; Determination of Thiobarbituric Acid (TBA) Levels, Spectrophotometer Method. Portuguese Institute of Quality, Ministry of Economy and Innovation: Caparica, Portugal, 2009.
- NP 1614/2002; Determination of Moisture Content. Reference Method (ISO 1442:1197). In Portuguese Norm–Meat and Meat Products. Portuguese Institute of Quality, Ministry of Economy Innovation: Caparica, Portugal, 2002.
- NP-ISO-1615/2002; Determination of Total Ashes. Reference Method. In Portuguese Norm–Meat and Meat Products. Portuguese Institute of Quality, Ministry of Economy and Innovation: Caparica, Portugal, 2002.
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- NP 1612/2002; Determination of Total Nitrogen Content. Reference Method (ISO 937:1978). In Portuguese Norm–Meat and Meat Products. Portuguese Institute of Quality, Ministry of Economy and Innovation: Caparica, Portugal, 2002.
- Hornsey, H.C. The colour of cooked cured pork—Estimation of the Nitric oxide-Haem Pigments. J. Sci. Food Agric. 1956, 7, 534–540. [Google Scholar] [CrossRef]
- NP 1987/2002; Carnes e Produtos Carneos; Determinação do Teor em Hidroxiprolina. (Método de referência). In Portuguese Norm–Meat and Meat Products. Portuguese Institute of Quality, Ministry of Economy and Innovation: Caparica, Portugal, 2002.
- NP 1845/1982; Meat and Meat Products—Determination of Chloride Content—Standard Method. Portuguese Institute of Quality, Ministry of Economy Innovation: Caparica, Portugal, 1982.
- Shehata, A.; Deman, J.; Alexander, J. A Simple and Rapid Method for the Preparation of Methyl Esters of Fats in Milligram Amounts for Gas Chromatography. Can. Inst. Food Technol. J. 1970, 3, 85–89. [Google Scholar] [CrossRef]
- Domínguez, R.; Borrajo, P.; Lorenzo, J.M. The effect of cooking methods on nutritional value of foal meat. J. Food Compos. Anal. 2015, 43, 61–67. [Google Scholar] [CrossRef]
- Department of Health, London, UK. Nutritional Aspects of Cardiovascular Disease. Report of the Cardiovascular Review Group Comittee on Medical Aspects of Food Policy. Rep. Health Soc. Subj. 1994, 46, 1–186. [Google Scholar]
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- Santos-Silva, J.; Bessa, R.J.B.; Santos-Silva, F. Effect of genotype, feeding system and slaughter weight on the quality of light lambs: Fatty and composition of meat. Livest Prod Sci. 2002, 77, 187–194. [Google Scholar] [CrossRef]
- Dreeling, N.; Allen, P.; Butler, F. Effect of Cooking Method on Sensory and Instrumental Texture Attributes of Low-fat Beefburgers. LWT 2000, 33, 234–238. [Google Scholar] [CrossRef]
- Zuorro, A. Water Activity Prediction in Sugar and Polyol Systems Using Theoretical Molecular Descriptors. Int. J. Mol. Sci. 2021, 22, 11044. [Google Scholar] [CrossRef]
- Carballo, D.E.; Andrés, S.; Giráldez, F.J.; Khanjari, A.; Caro, I.; Llamazares, D.; Operta, S.; Mateo, J. The effects of storage and hop extract on aroma and flavour compounds in Balkan-style sausages packed under a CO2-containing anaerobic atmosphere. Heliyon 2020, 6, e05251. [Google Scholar] [CrossRef]
- Serdaroğlu, M.; Nacak, B.; Karabıyıkoğlu, M. Effects of beef fat replacement with gelled emulsion prepared with olive oil on quality parameters of chicken patties. Korean J. Food Sci. Anim. Resour. 2017, 37, 376. [Google Scholar]
- Rodríguez-Carpena, J.G.; Morcuende, D.; Estévez, M. Partial replacement of pork back-fat by vegetable oils in burger patties: Effect on oxidative stability and texture and color changes during cooking and chilled storage. J. Food Sci. 2011, 76, C1025–C1031. [Google Scholar] [CrossRef] [PubMed]
- Nieto, G.; Lorenzo, J.M. Use of olive oil as fat replacer in meat emulsions. Curr. Opin. Food Sci. 2021, 40, 179–186. [Google Scholar] [CrossRef]
- Lu, F.; Kuhnle, G.; Cheng, Q. Vegetable oil as fat replacer inhibits formation of heterocyclic amines and polycyclic aromatic hydrocarbons in reduced fat pork patties. Food Control 2017, 81, 113–125. [Google Scholar] [CrossRef]
- Ockerman, H.W. Quality Control of Post-Mortem Muscle Tissue; Department of Animal Sciences, Ohio State University: Columbus, OH, USA, 1985. [Google Scholar]
- Hoa, V.-B.; Seol, K.-H.; Seo, H.-W.; Seong, P.-N.; Kang, S.-M.; Kim, Y.-S.; Moon, S.-S.; Kim, J.-H.; Cho, S.-H. Meat quality characteristics of pork bellies in relation to fat level. Anim. Biosci. 2021, 34, 1663–1673. [Google Scholar] [CrossRef] [PubMed]
- Barros, J.C.; Munekata, P.E.; de Carvalho, F.A.L.; Domínguez, R.; Trindade, M.A.; Pateiro, M.; Lorenzo, J.M. Healthy beef burgers: Effect of animal fat replacement by algal and wheat germ oil emulsions. Meat Sci. 2020, 173, 108396. [Google Scholar] [CrossRef]
- Pintado, T.; Herrero, A.; Jiménez-Colmenero, F.; Cavalheiro, C.P.; Ruiz-Capillas, C. Chia and oat emulsion gels as new animal fat replacers and healthy bioactive sources in fresh sausage formulation. Meat Sci. 2018, 135, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Poyato, C.; Astiasarán, I.; Barriuso, B.; Ansorena, D. A new polyunsaturated gelled emulsion as replacer of pork back-fat in burger patties: Effect on lipid composition, oxidative stability and sensory acceptability. LWT 2015, 62, 1069–1075. [Google Scholar] [CrossRef]
- Özer, C.O.; Çelegen, Ş. Evaluation of quality and emulsion stability of a fat-reduced beef burger prepared with an olive oil oleogel-based emulsion. J. Food Process. Preserv. 2020, 45, 14547. [Google Scholar] [CrossRef]
- Kumar, Y. Development of Low-Fat/Reduced-Fat Processed Meat Products using Fat Replacers and Analogues. Food Rev. Int. 2019, 37, 296–312. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; Pintado, T.; Jiménez-Colmenero, F.; Cofrades, S. Assessment of a healthy oil combination structured in ethyl cellulose and beeswax oleogels as animal fat replacers in low-fat, PUFA-enriched pork burgers. Food Bioprocess Technol. 2019, 12, 1068–1081. [Google Scholar] [CrossRef] [Green Version]
- Heck, R.T.; Saldaña, E.; Lorenzo, J.M.; Correa, L.P.; Fagundes, M.B.; Cichoski, A.J.; de Menezes, C.R.; Wagner, R.; Campagnol, P.C.B. Hydrogelled emulsion from chia and linseed oils: A promising strategy to produce low-fat burgers with a healthier lipid profile. Meat Sci. 2019, 156, 174–182. [Google Scholar] [CrossRef]
- Cittadini, A.; Domínguez, R.; Munekata, P.E.S.; Pateiro, M.; Sarriés, M.V.; Lorenzo, J.M. Use of oil mixture emulsion hydrogels as partial animal fat replacers in dry-fermented foal sausages. Food Res. Int. 2022, 161, 111881. [Google Scholar] [CrossRef]
- Cittadini, A.; Munekata, P.E.S.; Pateiro, M.; Sarriés, M.V.; Domínguez, R.; Lorenzo, J.M. Physicochemical composition and nutritional properties of foal burgers enhanced with healthy oil emulsion hydrogels. Int. J. Food Sci. Technol. 2021, 56, 6182–6191. [Google Scholar] [CrossRef]
- de Carvalho, F.A.L.; Munekata, P.E.; de Oliveira, A.L.; Pateiro, M.; Domínguez, R.; Trindade, M.A.; Lorenzo, J.M. Turmeric (Curcuma longa L.) extract on oxidative stability, physicochemical and sensory properties of fresh lamb sausage with fat replacement by tiger nut (Cyperus esculentus L.) oil. Food Res. Int. 2020, 136, 109487. [Google Scholar] [CrossRef]
- Estévez, M.; Morcuende, D.; Ventanas, A.S.; Cava, R. Analysis of Volatiles in Meat from Iberian Pigs and Lean Pigs after Refrigeration and Cooking by Using SPME-GC-MS. J. Agric. Food Chem. 2003, 51, 3429–3435. [Google Scholar] [CrossRef] [PubMed]
- Resconi, V.C.; Escudero, A.; Campo, M.M. The Development of Aromas in Ruminant Meat. Molecules 2013, 18, 6748–6781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bassam, S.M.; Noleto-Dias, C.; Farag, M.A. Dissecting grilled red and white meat flavor: Its characteristics, production mechanisms, influencing factors and chemical hazards. Food Chem. 2021, 371, 131139. [Google Scholar] [CrossRef] [PubMed]
- Madruga, M.S.; Elmore, J.S.; Dodson, A.T.; Mottram, D.S. Volatile flavour profile of goat meat extracted by three widely used techniques. Food Chem. 2009, 115, 1081–1087. [Google Scholar] [CrossRef]
- Wettasinghe, M.; Vasanthan, T.; Temelli, F.; Swallow, K. Volatile flavour composition of cooked by-product blends of chicken, beef and pork: A quantitative GC–MS investigation. Food Res. Int. 2001, 34, 149–158. [Google Scholar] [CrossRef]
- Shahidi, F.; Pegg, R.B. Hexanal as an indicator of meat flavor deterioration. J. Food Lipids 1994, 1, 177–186. [Google Scholar] [CrossRef]
- Rodríguez-Carpena, J.; Morcuende, D.; Estévez, M. Avocado, sunflower and olive oils as replacers of pork back-fat in burger patties: Effect on lipid composition, oxidative stability and quality traits. Meat Sci. 2012, 90, 106–115. [Google Scholar] [CrossRef]
- Mahungu, S.M.; Hansen, S.L.; Artz, W.E. Volatile compounds in heated oleic acid-esterified propoxylated glycerol. J. Am. Oil Chem. Soc. 1998, 75, 683–690. [Google Scholar] [CrossRef]


| Ingredients (%) | GPF | GO | GSF |
|---|---|---|---|
| Goat meat | 87.9 | 87.9 | 87.9 |
| Pork fat | 4 | - | - |
| Olive oil hydrogel | - | 4 | - |
| Sunflower oil hydrogel | - | - | 4 |
| NaCl | 1.1 | 1.1 | 1.1 |
| H2O | 7 | 7 | 7 |
| Parameter | GPF | GO | GSF | SEM | SIG | |||
|---|---|---|---|---|---|---|---|---|
| pH | 5.91 | b | 5.89 | a | 5.92 | b | 0.009 | 0.017 |
| aw | 0.982 | a | 0.968 | a, b | 0.967 | b | 0.005 | 0.024 |
| L* | 45.45 | 46.28 | 44.55 | 0.628 | 0.196 | |||
| a* | 18.64 | 19.86 | 18.23 | 0.811 | 0.362 | |||
| b* | 15.41 | b | 16.81 | a | 15.03 | b | 0.251 | 0.007 |
| h* | 39.63 | 40.27 | 39.51 | 0.816 | 0.766 | |||
| C* | 24.19 | 26.02 | 23.63 | 0.775 | 0.140 | |||
| TBARS (mg MDA/kg) | 0.474 | 0.386 | 0.511 | 0.069 | 0.431 | |||
| Moisture (%) | 73.57 | 76.71 | 75.61 | 1.243 | 0.216 | |||
| Ashes (%) | 1.93 | 1.96 | 2.03 | 0.078 | 0.572 | |||
| Fat (%) | 5.46 | a | 3.79 | b | 3.26 | b | 0.069 | 0.030 |
| Protein (%) | 18.10 | 17.82 | 17.68 | 0.265 | 0.647 | |||
| Pigments (%) | 2.86 | 3.18 | 2.88 | 0.199 | 0.707 | |||
| Collagen (%) | 0.34 | a | 0.29 | a, b | 0.21 | b | 0.038 | 0.019 |
| Chlorides (%) | 1.15 | 1.10 | 1.29 | 0.069 | 0.176 | |||
| Fatty Acids (%) | GPF | GO | GSF | SEM | SIG | |||
|---|---|---|---|---|---|---|---|---|
| C14:0 | 2.016 | 2.104 | 2.669 | 0.501 | 0.568 | |||
| C14:1 | 0.094 | 0.121 | 0.118 | 0.040 | 0.754 | |||
| C15:0 | 0.202 | 0.285 | 0.256 | 0.031 | 0.169 | |||
| C16:0 | 23.762 | a | 19.529 | b | 17.911 | a | 0.683 | <0.0001 |
| C16:1n-7 | 2.171 | 1.899 | 1.904 | 0.336 | 0.784 | |||
| C17:1n-7 | 0.427 | 0.506 | 0.359 | 0.056 | 0.199 | |||
| C18:0 | 12.093 | a | 9.613 | a,b | 9.334 | b | 0.935 | 0.022 |
| 9t-C18:1 | 1.164 | 1.396 | 1.285 | 0.197 | 0.691 | |||
| C18:1n-9 | 46.417 | b | 53.632 | a | 38.872 | c | 0.877 | <0.0001 |
| 9t,12t-C18:2 | 0.114 | 0.150 | 0.143 | 0.028 | 0.563 | |||
| C18:2n-6 | 7.643 | b | 6.921 | b | 24.070 | a | 1.150 | <0.0001 |
| C20:0 | 0.152 | 0.176 | 0.088 | 0.032 | 0.143 | |||
| C18:3n-6 | 0.024 | a | 0.017 | a,b | 0.004 | b | 0.007 | 0.015 |
| C20:1n-9 | 0.689 | a | 0.195 | b | 0.080 | b | 0.041 | <0.0001 |
| C18:3n-3 | 0.430 | a | 0.526 | a | 0.238 | b | 0.041 | 0.001 |
| C21:0 | 0.354 | 0.498 | 0.468 | 0.113 | 0.974 | |||
| C20:2n-6 | 0.291 | a | 0.040 | b | 0.010 | b | 0.018 | <0.0001 |
| C22:0 | 0.079 | b | 0.074 | b | 0.256 | b | 0.038 | 0.004 |
| C20:3n-6 | 0.076 | a | 0.042 | a,b | 0.029 | b | 0.014 | 0.008 |
| C20:3n-3 | 0.068 | a, b | 0.167 | a | 0.029 | b | 0.041 | 0.025 |
| C20:4n-6 | 0.851 | 1.093 | 0.916 | 0.087 | 0.144 | |||
| C24:0 | ND | b | 0.014 | a,b | 0.058 | a | 0.017 | 0.045 |
| C24:1n-9 | 0.099 | a | 0.089 | a | 0.040 | b | 0.016 | 0.049 |
| C22:6n-3 | 0.046 | b | 0.082 | a,b | 0.116 | a | 0.022 | 0.031 |
| ∑SFA | 39.28 | a | 33.00 | b | 31.68 | b | 0.883 | <0.0001 |
| ∑MUFA | 51.12 | b | 57.90 | a | 42.70 | c | 1.109 | <0.0001 |
| ∑PUFA | 9.59 | b | 9.10 | b | 25.62 | a | 1.170 | <0.0001 |
| ∑UFA/∑SFA | 1.55 | b | 2.04 | a | 2.17 | a | 0.079 | <0.0001 |
| ∑n-6 | 8.89 | b | 8.11 | b | 25.03 | a | 1.153 | <0.0001 |
| ∑n-3 | 0.59 | b | 0.83 | a | 0.52 | b | 0.078 | 0.027 |
| ∑n-6/∑n-3 | 15.96 | b | 10.81 | b | 50.48 | a | 2.914 | <0.0001 |
| AI | 0.53 | 0.42 | 0.42 | 0.041 | 0.652 | |||
| TI | 1.19 | a | 0.89 | b | 0.85 | b | 0.040 | <0.0001 |
| h/H | 2.15 | b | 2.95 | a | 3.25 | a | 0.213 | 0.004 |
| Parameter | GPF | GO | GSF | SEM | SIG | |||
|---|---|---|---|---|---|---|---|---|
| WL (%) | 64.30 | b | 64.60 | b | 70.75 | a | 0.961 | 0.029 |
| Shrinkage (%) | 27.26 | 22.71 | 22.75 | 1.505 | 0.398 | |||
| TI (%) | 20.22 | 27.59 | 30.99 | 4.882 | 0.191 | |||
| Max Force (N) | 101.80 | 113.33 | 108.83 | 4.129 | 0.283 | |||
| Cohesivity | 0.337 | b | 0.366 | a | 0.373 | a | 0.005 | 0.030 |
| Elasticity | 0.640 | 0.591 | 0.587 | 0.022 | 0.314 | |||
| Chewiness | 22.19 | 24.52 | 23.81 | 0.962 | 0.346 | |||
| Compound | GPF | GO | GSF | SEM | SIG | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0H | 72 h | 0H | 72 h | 0H | 72 h | FS | T | FS × T | ||||||||
| Pentane | 477.0 | 778.6 | 500.6 | 476.4 | 858.2 | 1336.8 | 309.30 | 0.200 | 0.356 | 0.725 | ||||||
| Hexane | 50.8 | a | 20.65 | b | 46.5 | a | 28.05 | b | 51.8 | a | 36.6 | b | 9.44 | 0.654 | 0.033 | 0.720 |
| Heptane | 158.3 | 47.8 | 162.2 | 103.5 | 245.8 | 125.0 | 67.98 | 0.511 | 0.132 | 0.889 | ||||||
| Octane | 405 | c | 138.6 | c | 1458.4 | a | 475.0 | c | 935.6 | b | 433.0 | c | 125.52 | 0.004 | 0.001 | 0.071 |
| Octene | 8.9 | 25.6 | 20.4 | 9.3 | 22.4 | 17.6 | 9.23 | 0.860 | 0.097 | 0.353 | ||||||
| Heptane, 2,2,4,6,6-pentamethyl | 98.2 | 166.2 | 143.2 | 77.9 | 76.2 | 81.1 | 50.10 | 0.589 | 0.952 | 0.460 | ||||||
| Sum of hydrocarbons | 1198.8 | 1177.4 | 2331.2 | 1170.2 | 2189.8 | 2030.1 | 465.36 | 0.217 | 0.284 | 0.458 | ||||||
| Ethanal | 18.5 | b, c | 25.3 | a, b | 12.8 | c | 29.8 | a | 14.2 | c | 26.8 | a, b | 2.42 | 0.854 | 0.001 | 0.183 |
| Pentanal | 181.2 | a, b | 477.2 | a | 167.4 | a, b | 416.6 | a, b | 151.9 | b | 407.4 | a, b | 91.45 | 0.857 | 0.012 | 0.962 |
| Hexanal | 4038.8 | d | 11,480.2 | a | 2255.3 | e | 9728.2 | b | 3057.2 | d, e | 7593.2 | c | 338.84 | 0.001 | <0.0001 | 0.007 |
| Heptanal | 260.7 | b, c | 487.5 | a, b | 230.8 | b, c | 690.7 | a | 91.8 | c | 435.0 | a, b | 88.10 | 0.160 | 0.003 | 0.464 |
| Octanal | 5.75 | b | 90.2 | a, b | ND | 140.2 | a | ND | 36.1 | a, b | 33.02 | 0.352 | 0.018 | 0.354 | ||
| Nonanal | ND | 124.8 | b | ND | 257.4 | a | ND | 96.5 | b | 20.29 | 0.016 | <0.0001 | 0.016 | |||
| Sum of aldehydes | 4504.9 | c | 12,685.2 | a | 2666.3 | d | 11,262.8 | a | 3315.0 | c, d | 8595.1 | b | 505.41 | 0.006 | <0.0001 | 0.033 |
| Octanedione + dodecane + 2-pentyl-furan | 183.0 | b | 2512.5 | a,b | 42.7 | b | 3010.8 | a | 41.4 | b | 741.0 | a,b | 748.36 | 0.332 | 0.017 | 0.359 |
| Carbon disulfide | 63.2 | 94.6 | 106.0 | 62.2 | 105.5 | 139.0 | 48.68 | 0.645 | 0.865 | 0.682 | ||||||
| Fatty Acid | F Ratio | Prob > F |
|---|---|---|
| C4:0 | 0.094 | 0.911 |
| C8:0 | 0.451 | 0.647 |
| C10:0 | 2.299 | 0.143 |
| C11:0 | 2.247 | 0.148 |
| C12:0 | 0.658 | 0.536 |
| C14:0 | 0.960 | 0.410 |
| C14:1 | 0.554 | 0.589 |
| C15:0 | 0.620 | 0.554 |
| C15:1 | 0.467 | 0.638 |
| C16:0 | 1.059 | 0.377 |
| C16:1n-7 | 0.939 | 0.418 |
| C17:0 | 0.831 | 0.459 |
| C17:1n-7 | 0.770 | 0.485 |
| C18:0 | 1.551 | 0.252 |
| 9t-C18:1 | 0.556 | 0.588 |
| C18:1n-9 | 91.503 | 0.000 |
| 9t.12t-C18:2 | 0.341 | 0.718 |
| C18:2n-6 | 1.722 | 0.220 |
| C20:0 | 1.080 | 0.370 |
| C18:3n-6 | 1.437 | 0.276 |
| C20:1n-9 | 3.138 | 0.080 |
| C18:3n-3 | 2.825 | 0.099 |
| C21:0 | 0.669 | 0.530 |
| C20:2n-6 | 106.620 | 0.000 |
| C22:0 | 0.773 | 0.483 |
| C20:3n-6 | 1.036 | 0.385 |
| C22:1n-9 | 0.025 | 0.975 |
| C20:3n-3 | 2.011 | 0.176 |
| C23:0 | 3.237 | 0.075 |
| C20:4n-6 | 1.869 | 0.196 |
| C22:2n-6 | 1.755 | 0.214 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, I.; Vasconcelos, L.; Leite, A.; Botella-Martínez, C.; Pereira, E.; Mateo, J.; Kasaiyan, S.; Teixeira, A. Use of Olive and Sunflower Oil Hydrogel Emulsions as Pork Fat Replacers in Goat Meat Burgers: Fat Reduction and Effects in Lipidic Quality. Biomolecules 2022, 12, 1416. https://doi.org/10.3390/biom12101416
Ferreira I, Vasconcelos L, Leite A, Botella-Martínez C, Pereira E, Mateo J, Kasaiyan S, Teixeira A. Use of Olive and Sunflower Oil Hydrogel Emulsions as Pork Fat Replacers in Goat Meat Burgers: Fat Reduction and Effects in Lipidic Quality. Biomolecules. 2022; 12(10):1416. https://doi.org/10.3390/biom12101416
Chicago/Turabian StyleFerreira, Iasmin, Lia Vasconcelos, Ana Leite, Carmen Botella-Martínez, Etelvina Pereira, Javier Mateo, Seyedalireza Kasaiyan, and Alfredo Teixeira. 2022. "Use of Olive and Sunflower Oil Hydrogel Emulsions as Pork Fat Replacers in Goat Meat Burgers: Fat Reduction and Effects in Lipidic Quality" Biomolecules 12, no. 10: 1416. https://doi.org/10.3390/biom12101416
APA StyleFerreira, I., Vasconcelos, L., Leite, A., Botella-Martínez, C., Pereira, E., Mateo, J., Kasaiyan, S., & Teixeira, A. (2022). Use of Olive and Sunflower Oil Hydrogel Emulsions as Pork Fat Replacers in Goat Meat Burgers: Fat Reduction and Effects in Lipidic Quality. Biomolecules, 12(10), 1416. https://doi.org/10.3390/biom12101416













