Targeting Cholesterol Homeostasis Improves Recovery in Experimental Optic Neuritis
Abstract
:1. Introduction
2. Materials and Methods
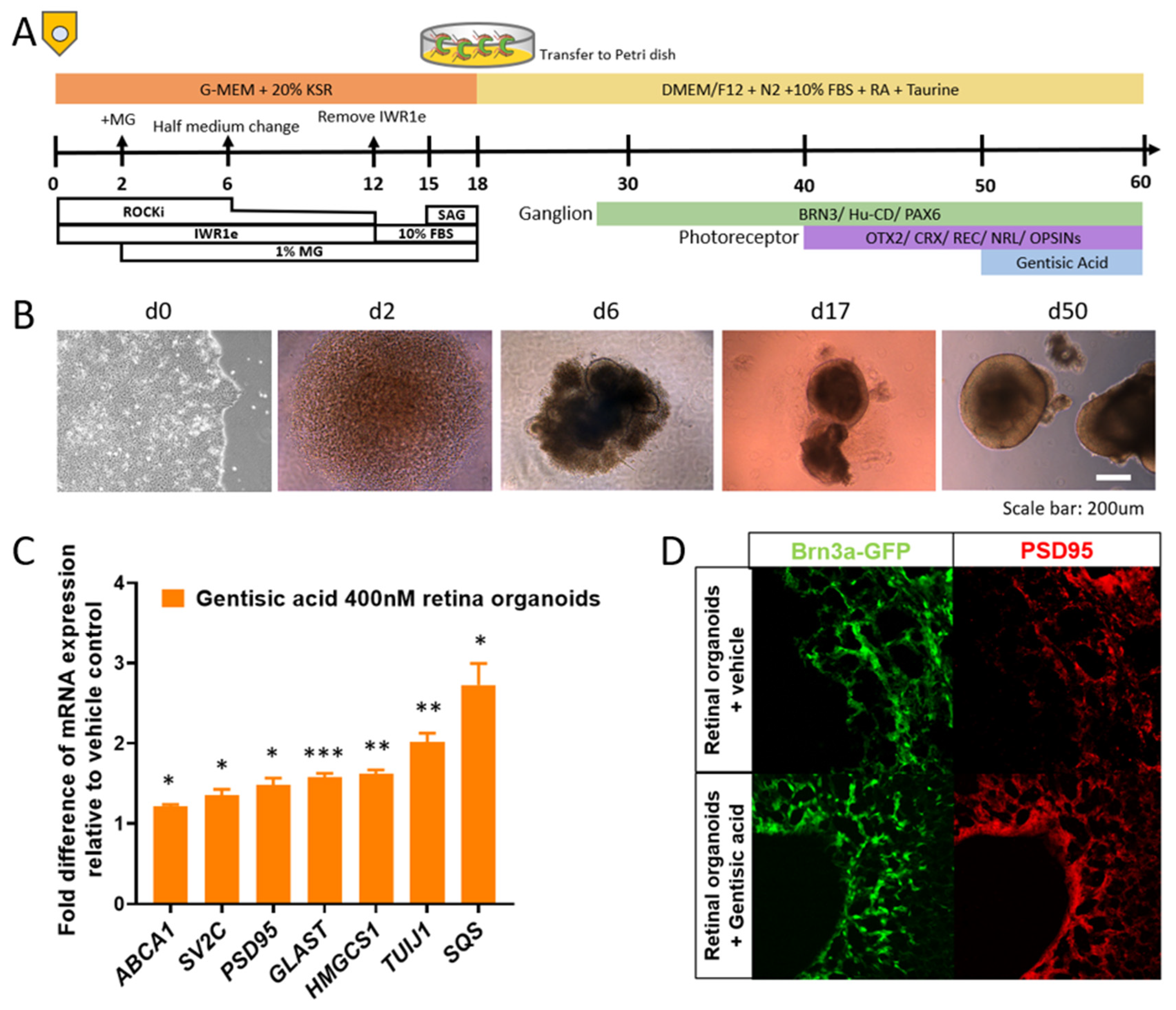
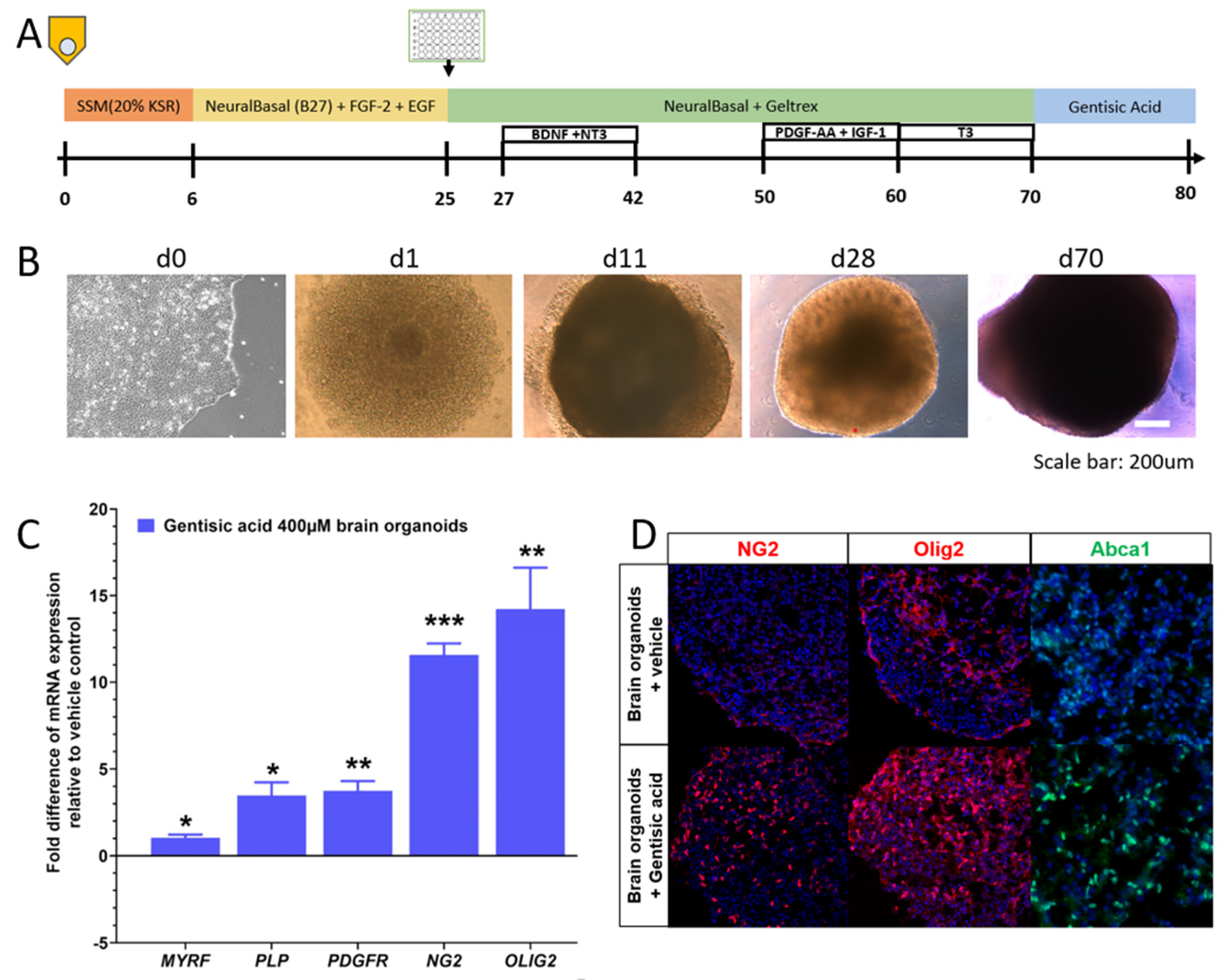
2.1. Human iPSC-Derived Retinal and Brain Organoids
2.2. RT-PCR
2.3. Organoid Immunohistochemistry
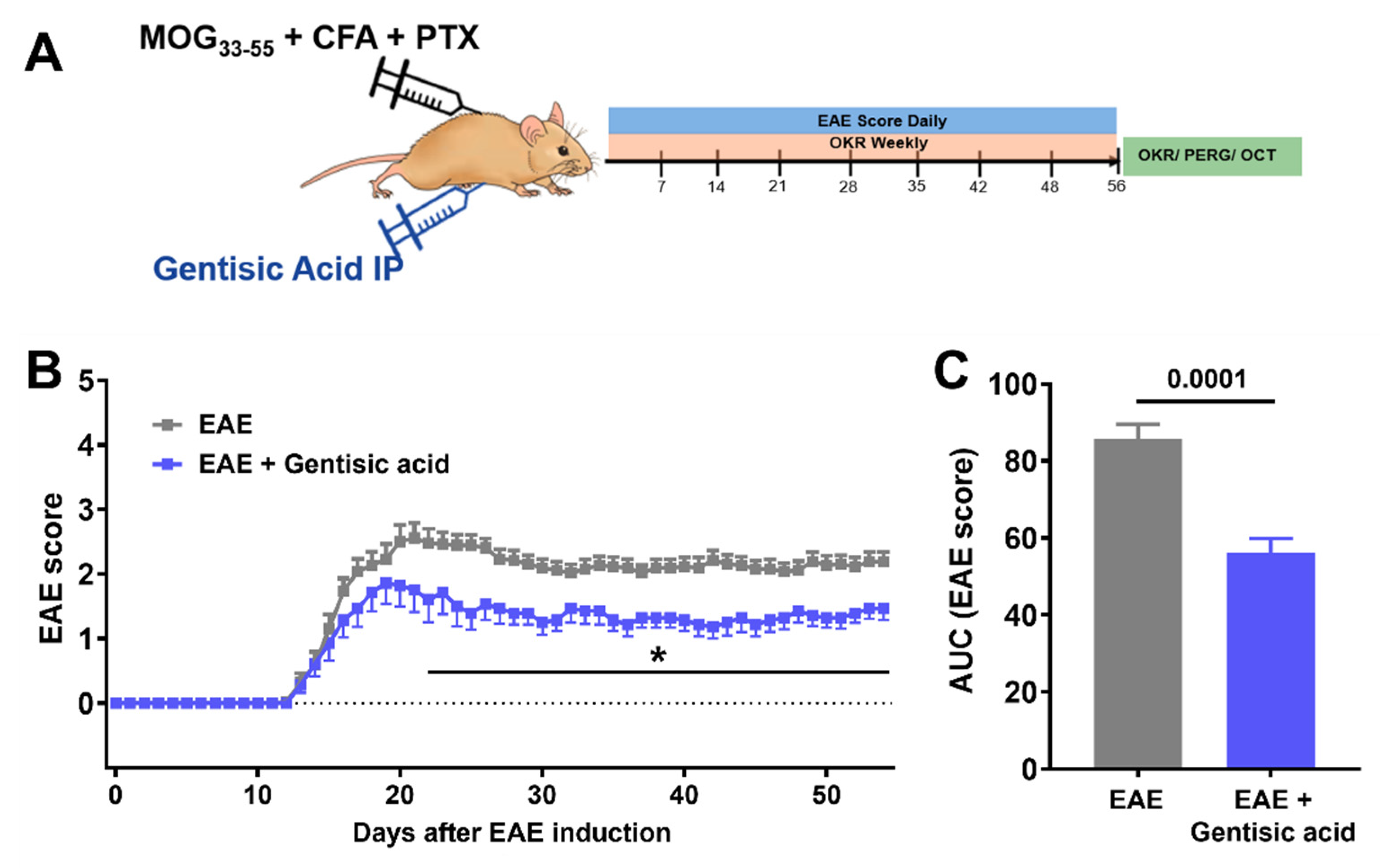
2.4. Animals and Study Design
2.5. EAE-ON Model
2.6. Optokinetic Tracking Response (OKR)
2.7. Pattern Electroretinogram (Pattern ERG)
2.8. Optical Coherence Tomography (OCT)
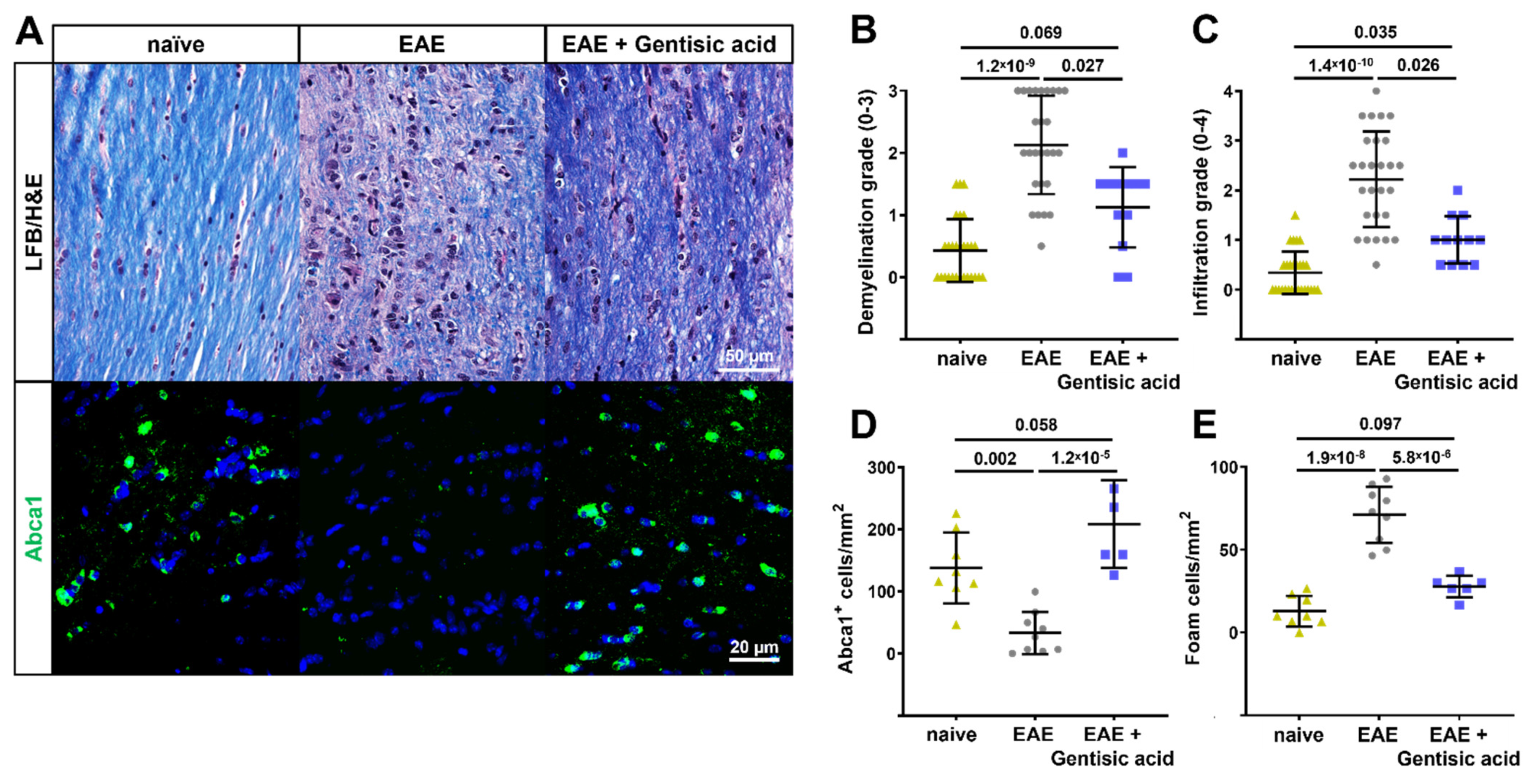
2.9. Optic Nerve Histopathology
2.10. Statistics
3. Results
3.1. Gentisic Acid Accelerates Retinal Cup Maturation
3.2. Gentisic Acid Increases Myelination in Brain Organoids
3.3. Systemic Gentisic Acid Administration Mitigates Motor-Sensory Deficits in EAE Mice
3.4. Gentisic Acid Treatment Improves Visual Structure and Function in EAE Mice
3.5. Gentisic Acid Preserves Myelination, Reduces Inflammation, and Rescues Abca1 Expression in Optic Nerves of EAE Mice
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bennett, J.L. Optic Neuritis. Contin. (Minneapolis, Minn.) 2019, 25, 1236–1264. [Google Scholar] [CrossRef]
- Shams, P.N.; Plant, G.T. Optic neuritis: A review. Int. MS J. 2009, 16, 82–89. [Google Scholar] [PubMed]
- Beck, R.W.; Cleary, P.A.; Anderson, M.M., Jr.; Keltner, J.L.; Shults, W.T.; Kaufman, D.I.; Buckley, E.G.; Corbett, J.J.; Kupersmith, M.J.; Miller, N.R.; et al. A randomized, controlled trial of corticosteroids in the treatment of acute optic neuritis. The Optic Neuritis Study Group. N. Engl. J. Med. 1992, 326, 581–588. [Google Scholar] [CrossRef]
- Beck, R.W.; Gal, R.L.; Bhatti, M.T.; Brodsky, M.C.; Buckley, E.G.; Chrousos, G.A.; Corbett, J.; Eggenberger, E.; Goodwin, J.A.; Katz, B.; et al. Visual function more than 10 years after optic neuritis: Experience of the optic neuritis treatment trial. Am. J. Ophthalmol. 2004, 137, 77–83. [Google Scholar] [CrossRef] [PubMed]
- De Lott, L.B.; Bennett, J.L.; Costello, F. The changing landscape of optic neuritis: A narrative review. J. Neurol. 2022, 269, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Sabadia, S.B.; Nolan, R.C.; Galetta, K.M.; Narayana, K.M.; Wilson, J.A.; Calabresi, P.A.; Frohman, E.M.; Galetta, S.L.; Balcer, L.J. 20/40 or Better Visual Acuity After Optic Neuritis: Not as Good as We Once Thought? J. Neuroophthalmol. 2016, 36, 369–376. [Google Scholar] [CrossRef]
- Petzold, A.; Balcer, L.J.; Calabresi, P.A.; Costello, F.; Frohman, T.C.; Frohman, E.M.; Martinez-Lapiscina, E.H.; Green, A.J.; Kardon, R.; Outteryck, O.; et al. Retinal layer segmentation in multiple sclerosis: A systematic review and meta-analysis. Lancet Neurol. 2017, 16, 797–812. [Google Scholar] [CrossRef] [Green Version]
- Vabanesi, M.; Pisa, M.; Guerrieri, S.; Moiola, L.; Radaelli, M.; Medaglini, S.; Martinelli, V.; Comi, G.; Leocani, L. In vivo structural and functional assessment of optic nerve damage in neuromyelitis optica spectrum disorders and multiple sclerosis. Sci. Rep. 2019, 9, 10371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arneth, B. Contributions of T cells in multiple sclerosis: What do we currently know? J. Neurol. 2021, 268, 4587–4593. [Google Scholar] [CrossRef] [PubMed]
- Bar-Or, A.; Li, R. Cellular immunology of relapsing multiple sclerosis: Interactions, checks, and balances. Lancet Neurol. 2021, 20, 470–483. [Google Scholar] [CrossRef]
- Murray, T.J. Diagnosis and treatment of multiple sclerosis. BMJ 2006, 332, 525–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Derfuss, T.; Mehling, M.; Papadopoulou, A.; Bar-Or, A.; Cohen, J.A.; Kappos, L. Advances in oral immunomodulating therapies in relapsing multiple sclerosis. Lancet Neurol. 2020, 19, 336–347. [Google Scholar] [CrossRef]
- Fogarty, E.; Schmitz, S.; Tubridy, N.; Walsh, C.; Barry, M. Comparative efficacy of disease-modifying therapies for patients with relapsing remitting multiple sclerosis: Systematic review and network meta-analysis. Mult. Scler. Relat. Disord. 2016, 9, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Tselis, A.; Perumal, J.; Caon, C.; Hreha, S.; Ching, W.; Din, M.; Van Stavern, G.; Khan, O. Treatment of corticosteroid refractory optic neuritis in multiple sclerosis patients with intravenous immunoglobulin. Eur. J. Neurol. 2008, 15, 1163–1167. [Google Scholar] [CrossRef]
- Ruprecht, K.; Klinker, E.; Dintelmann, T.; Rieckmann, P.; Gold, R. Plasma exchange for severe optic neuritis: Treatment of 10 patients. Neurology 2004, 63, 1081–1083. [Google Scholar] [CrossRef] [PubMed]
- Gugliandolo, A.; Bramanti, P.; Mazzon, E. Mesenchymal Stem Cells in Multiple Sclerosis: Recent Evidence from Pre-Clinical to Clinical Studies. Int. J. Mol. Sci. 2020, 21, 8662. [Google Scholar] [CrossRef]
- Florou, D.; Katsara, M.; Feehan, J.; Dardiotis, E.; Apostolopoulos, V. Anti-CD20 Agents for Multiple Sclerosis: Spotlight on Ocrelizumab and Ofatumumab. Brain Sci. 2020, 10, 758. [Google Scholar] [CrossRef]
- Lavrnja, I.; Smiljanic, K.; Savic, D.; Mladenovic-Djordjevic, A.; Tesovic, K.; Kanazir, S.; Pekovic, S. Expression profiles of cholesterol metabolism-related genes are altered during development of experimental autoimmune encephalomyelitis in the rat spinal cord. Sci. Rep. 2017, 7, 2702. [Google Scholar] [CrossRef] [Green Version]
- Berghoff, S.A.; Spieth, L.; Sun, T.; Hosang, L.; Schlaphoff, L.; Depp, C.; Duking, T.; Winchenbach, J.; Neuber, J.; Ewers, D.; et al. Microglia facilitate repair of demyelinated lesions via post-squalene sterol synthesis. Nat. Neurosci. 2021, 24, 47–60. [Google Scholar] [CrossRef]
- Gramlich, O.W.; Brown, A.J.; Godwin, C.R.; Chimenti, M.S.; Boland, L.K.; Ankrum, J.A.; Kardon, R.H. Systemic Mesenchymal Stem Cell Treatment Mitigates Structural and Functional Retinal Ganglion Cell Degeneration in a Mouse Model of Multiple Sclerosis. Transl. Vis. Sci. Technol. 2020, 9, 16. [Google Scholar] [CrossRef]
- Cantuti-Castelvetri, L.; Fitzner, D.; Bosch-Queralt, M.; Weil, M.T.; Su, M.; Sen, P.; Ruhwedel, T.; Mitkovski, M.; Trendelenburg, G.; Lutjohann, D.; et al. Defective cholesterol clearance limits remyelination in the aged central nervous system. Science 2018, 359, 684–688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, G.; Wang, J.; Rasul, A.; Anwar, H.; Imran, A.; Qasim, M.; Zafar, S.; Kamran, S.K.S.; Razzaq, A.; Aziz, N.; et al. Role of cholesterol and sphingolipids in brain development and neurological diseases. Lipids Health Dis. 2019, 18, 26. [Google Scholar] [CrossRef] [Green Version]
- Pikuleva, I.A.; Curcio, C.A. Cholesterol in the retina: The best is yet to come. Prog. Retin. Eye Res. 2014, 41, 64–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sene, A.; Khan, A.A.; Cox, D.; Nakamura, R.E.; Santeford, A.; Kim, B.M.; Sidhu, R.; Onken, M.D.; Harbour, J.W.; Hagbi-Levi, S.; et al. Impaired cholesterol efflux in senescent macrophages promotes age-related macular degeneration. Cell Metab. 2013, 17, 549–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ban, N.; Lee, T.J.; Sene, A.; Choudhary, M.; Lekwuwa, M.; Dong, Z.; Santeford, A.; Lin, J.B.; Malek, G.; Ory, D.S.; et al. Impaired monocyte cholesterol clearance initiates age-related retinal degeneration and vision loss. JCI Insight 2018, 3, e120824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Storti, F.; Klee, K.; Todorova, V.; Steiner, R.; Othman, A.; van der Velde-Visser, S.; Samardzija, M.; Meneau, I.; Barben, M.; Karademir, D.; et al. Impaired ABCA1/ABCG1-mediated lipid efflux in the mouse retinal pigment epithelium (RPE) leads to retinal degeneration. Elife 2019, 8, e45100. [Google Scholar] [CrossRef] [PubMed]
- Ananth, S.; Gnana-Prakasam, J.P.; Bhutia, Y.D.; Veeranan-Karmegam, R.; Martin, P.M.; Smith, S.B.; Ganapathy, V. Regulation of the cholesterol efflux transporters ABCA1 and ABCG1 in retina in hemochromatosis and by the endogenous siderophore 2,5-dihydroxybenzoic acid. Biochim. Biophys. Acta 2014, 1842, 603–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abedi, F.; Razavi, B.M.; Hosseinzadeh, H. A review on gentisic acid as a plant derived phenolic acid and metabolite of aspirin: Comprehensive pharmacology, toxicology, and some pharmaceutical aspects. Phytother. Res. 2020, 34, 729–741. [Google Scholar] [CrossRef] [PubMed]
- Holmes, T.J., Jr.; Vennerstrom, J.L.; John, V. Inhibition of cyclooxygenase mediated by electrochemical oxidation of gentisic acid. J. Biol. Chem. 1985, 260, 14092–14095. [Google Scholar] [CrossRef]
- Nakano, T.; Ando, S.; Takata, N.; Kawada, M.; Muguruma, K.; Sekiguchi, K.; Saito, K.; Yonemura, S.; Eiraku, M.; Sasai, Y. Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell 2012, 10, 771–785. [Google Scholar] [CrossRef]
- Cheng, L.; Cring, M.R.; Wadkins, D.A.; Kuehn, M.H. Absence of Connexin 43 results in smaller retinas and arrested, depolarized retinal progenitor cells in human retinal organoids. Stem Cells 2022, 40, 592–604. [Google Scholar] [CrossRef]
- Madhavan, M.; Nevin, Z.S.; Shick, H.E.; Garrison, E.; Clarkson-Paredes, C.; Karl, M.; Clayton, B.L.L.; Factor, D.C.; Allan, K.C.; Barbar, L.; et al. Induction of myelinating oligodendrocytes in human cortical spheroids. Nat. Methods 2018, 15, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Nafees, S.; Ahmad, S.T.; Arjumand, W.; Rashid, S.; Ali, N.; Sultana, S. Modulatory effects of gentisic acid against genotoxicity and hepatotoxicity induced by cyclophosphamide in Swiss albino mice. J. Pharm. Pharm. 2012, 64, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Bittner, S.; Afzali, A.M.; Wiendl, H.; Meuth, S.G. Myelin oligodendrocyte glycoprotein (MOG35-55) induced experimental autoimmune encephalomyelitis (EAE) in C57BL/6 mice. J. Vis. Exp. JoVE 2014, 86, e51275. [Google Scholar] [CrossRef] [Green Version]
- Prusky, G.T.; Alam, N.M.; Beekman, S.; Douglas, R.M. Rapid quantification of adult and developing mouse spatial vision using a virtual optomotor system. Investig. Ophthalmol. Vis. Sci. 2004, 45, 4611–4616. [Google Scholar] [CrossRef] [Green Version]
- Zeng, H.; Dumitrescu, A.V.; Wadkins, D.; Elwood, B.W.; Gramlich, O.W.; Kuehn, M.H. Systemic Treatment with Pioglitazone Reverses Vision Loss in Preclinical Glaucoma Models. Biomolecules 2022, 12, 281. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.H.; Bohorquez, J.; Toft-Nielsen, J.; Ozdamar, O.; Porciatti, V. Robust mouse pattern electroretinograms derived simultaneously from each eye using a common snout electrode. Investig. Ophthalmol. Vis. Sci. 2014, 55, 2469–2475. [Google Scholar] [CrossRef] [PubMed]
- Schwabenland, M.; Bruck, W.; Priller, J.; Stadelmann, C.; Lassmann, H.; Prinz, M. Analyzing microglial phenotypes across neuropathologies: A practical guide. Acta Neuropathol. 2021, 142, 923–936. [Google Scholar] [CrossRef] [PubMed]
- Porciatti, V.; Ventura, L.M. Retinal ganglion cell functional plasticity and optic neuropathy: A comprehensive model. J. Neuroophthalmol. 2012, 32, 354–358. [Google Scholar] [CrossRef]
- Gautier, H.O.; Evans, K.A.; Volbracht, K.; James, R.; Sitnikov, S.; Lundgaard, I.; James, F.; Lao-Peregrin, C.; Reynolds, R.; Franklin, R.J.; et al. Neuronal activity regulates remyelination via glutamate signalling to oligodendrocyte progenitors. Nat. Commun. 2015, 6, 8518. [Google Scholar] [CrossRef] [Green Version]
- Smith, M.J.; Pharm, M. Some new drugs in the treatment of rheumatic fever. Postgrad. Med. J. 1952, 28, 179–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, M.J.; Choi, W.; Yoo, S.H.; Nam, S.W.; Shin, P.G.; Kim, K.K.; Kim, G.D. Modulation of Inflammatory Pathways and Adipogenesis by the Action of Gentisic Acid in RAW 264.7 and 3T3-L1 Cell Lines. J. Microbiol. Biotechnol. 2021, 31, 1079–1087. [Google Scholar] [CrossRef]
- Bove, R.M.; Green, A.J. Remyelinating Pharmacotherapies in Multiple Sclerosis. Neurotherapeutics 2017, 14, 894–904. [Google Scholar] [CrossRef]

| Target | Forward Sequence | Reverse Sequence |
|---|---|---|
| ABCA1 | 5′-GCTACCCACCCTATGAACAAC-3′ | 5′-AGATAATCCCCTGAACCCAAG-3′ |
| SV2C | 5′-GCTCTGCATGTTCTGGATGA-3′ | 5′-GATGACAAACACACGCCAAC-3′ |
| PSD95 (DLG4) | 5′-ATACCGCTACCAAGATGAAGAC-3′ | 5′-TCACCTGCAACTCATATCCTG-3′ |
| GLAST (SLC1A3) | 5′-GAGGATGTTACAGATGCTGGTC-3′ | 5′-TAATAGACTACAGCTCGCATTCC-3′ |
| HMGCS1 | 5′GAAACAGTGACAGACCTGGAG-3′ | 5′-AGCAAGCTTCTGCATTCAAAG-3′ |
| TUJ1 (TUBB3) | 5′-GGCCTTTGGACATCTCTTCAG-3′ | 5′-CCTCCGTGTAGTTGACCCTT-3′ |
| SQS (FDFT1) | 5′-ACAACCTGGTGCGCTTC-3′ | 5′-GATAACAGCTGCGAAACTGC-3′ |
| TBP | 5′-GCTGTTTAACTTCGCTTCCG-3′ | 5′-CAGCAACTTCCTCAATTCCTTG-3′ |
| MBP | 5′-GGCCGGACCCAAGATGAAAA-3′ | 5′-CCCCAGCTAAATCTGCTCAGG-3′ |
| MYRF | 5′-CAGTCCCAGTCAGACCGGA-3′ | 5′-CCCTTCTTACGCATGTTGTTAGC-3′ |
| PLP1 | 5′-ACCTATGCCCTGACCGTTG-3′ | 5′-TGCTGGGGAAGGCAATAGACT-3′ |
| PDGFR | 5′-TTGAAGGCAGGCACATTTACA-3′ | 5′-GCGACAAGGTATAATGGCAGAAT-3′ |
| OLIG2 | 5′-TGGCTTCAAGTCATCCTCGTC-3′ | 5′-ATGGCGATGTTGAGGTCGTG-3′ |
| NG2 (CSPG4) | 5′-AGGACGAAGGAACCCTAGAGT-3′ | 5′-CACAGGCACACTGTTGTGGA-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Godwin, C.R.; Anders, J.J.; Cheng, L.; Elwood, B.W.; Kardon, R.H.; Gramlich, O.W. Targeting Cholesterol Homeostasis Improves Recovery in Experimental Optic Neuritis. Biomolecules 2022, 12, 1437. https://doi.org/10.3390/biom12101437
Godwin CR, Anders JJ, Cheng L, Elwood BW, Kardon RH, Gramlich OW. Targeting Cholesterol Homeostasis Improves Recovery in Experimental Optic Neuritis. Biomolecules. 2022; 12(10):1437. https://doi.org/10.3390/biom12101437
Chicago/Turabian StyleGodwin, Cheyanne R., Jeffrey J. Anders, Lin Cheng, Benjamin W. Elwood, Randy H. Kardon, and Oliver W. Gramlich. 2022. "Targeting Cholesterol Homeostasis Improves Recovery in Experimental Optic Neuritis" Biomolecules 12, no. 10: 1437. https://doi.org/10.3390/biom12101437
APA StyleGodwin, C. R., Anders, J. J., Cheng, L., Elwood, B. W., Kardon, R. H., & Gramlich, O. W. (2022). Targeting Cholesterol Homeostasis Improves Recovery in Experimental Optic Neuritis. Biomolecules, 12(10), 1437. https://doi.org/10.3390/biom12101437







