Mechanisms of Peritoneal Mesothelial Cells in Peritoneal Adhesion
Abstract
1. Introduction
2. Characteristics and Functions of PMCs
2.1. Anatomical Features of PMCs
2.2. Pathophysiological Function of PMCs
3. Key Steps of PA Formation
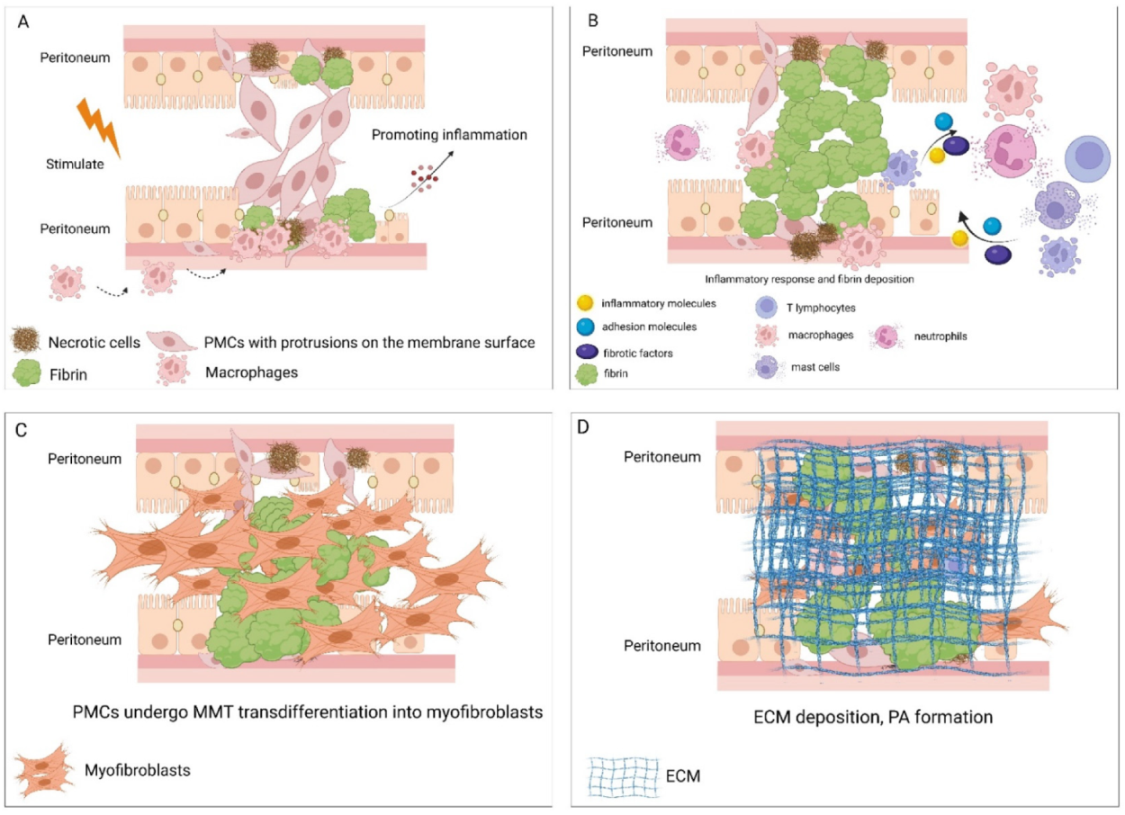
4. The Mechanism of PMCs in Promoting PAs
4.1. Damage to PMCs Initiates PA Formation
4.2. Dysfunction of PMCs Leads to Excessive Fibrin Deposition
4.3. PMCs Regulate the Inflammatory Process of PA Formation
4.4. PMCs Develop MMT and Promote Peritoneal Fibrosis
5. Prevention and Treatment Strategies for PAs from the Perspective of PMCs
5.1. Protection and Reconstitution of PMCs
5.2. Preventing MMT in PMCs
5.3. Application of PA-resistant Biomaterials
6. Summary and Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ferns, G.A.; Hassanian, S.M.; Arjmand, M.-H. Hyperglycaemia and the risk of post-surgical adhesion. Arch. Physiol. Biochem. 2020, 1–7. [Google Scholar] [CrossRef]
- Tsai, J.M.; Sinha, R.; Seita, J.; Fernhoff, N.; Christ, S.; Koopmans, T.; Krampitz, G.W.; McKenna, K.M.; Xing, L.; Sandholzer, M.; et al. Surgical adhesions in mice are derived from mesothelial cells and can be targeted by antibodies against mesothelial markers. Sci. Transl. Med. 2018, 10, eaan6735. [Google Scholar] [CrossRef] [PubMed]
- Herrick, S.; Wilm, B. Post-Surgical Peritoneal Scarring and Key Molecular Mechanisms. Biomolecules 2021, 11, 692. [Google Scholar] [CrossRef] [PubMed]
- Harlow, C.R.; Wu, X.; Van Deemter, M.; Gardiner, F.; Poland, C.; Green, R.; Sarvi, S.; Brown, P.; Kadler, K.E.; Lu, Y.; et al. Targeting lysyl oxidase reduces peritoneal fibrosis. PLoS ONE 2017, 12, e0183013. [Google Scholar] [CrossRef]
- Kalra, A.; Wehrle, C.J.; Tuma, F. Anatomy, Abdomen and Pelvis, Peritoneum; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Kastelein, A.W.; Vos, L.M.; de Jong, K.H.; van Baal, J.O.; Nieuwland, R.; van Noorden, C.J.; Roovers, J.-P.W.; Lok, C.A. Embryology, anatomy, physiology and pathophysiology of the peritoneum and the peritoneal vasculature. Semin. Cell Dev. Biol. 2019, 92, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Bermo, M.S.; Koppula, B.; Kumar, M.; Leblond, A.; Matesan, M.C. The Peritoneum: What Nuclear Radiologists Need to Know. Semin. Nucl. Med. 2020, 50, 405–418. [Google Scholar] [CrossRef] [PubMed]
- Mi, Y.; Yang, F.; Bloomquist, C.; Xia, Y.; Sun, B.; Qi, Y.; Wagner, K.; Montgomery, S.A.; Zhang, T.; Wang, A.Z. Biologically Targeted Photo-Crosslinkable Nanopatch to Prevent Postsurgical Peritoneal Adhesion. Adv. Sci. 2019, 6, 1900809. [Google Scholar] [CrossRef]
- Wallwiener, M.; Brucker, S.; Hierlemann, H.; Brochhausen, C.; Solomayer, E.; Wallwiener, C. Innovative barriers for peritoneal adhesion prevention: Liquid or solid? A rat uterine horn model. Fertil. Steril. 2006, 86, 1266–1276. [Google Scholar] [CrossRef]
- Huang, N.-C.; Teng, K.-W.; Huang, N.-C.; Kang, L.-Y.; Fu, K.-Y.; Hsieh, P.-S.; Dai, L.-G.; Dai, N.-T. Evaluation of Polycaprolactone/Gelatin/Chitosan Electrospun Membrane for Peritoneal Adhesion Reduction. Ann. Plast. Surg. 2020, 84, S116–S122. [Google Scholar] [CrossRef]
- Tang, J.; Xiang, Z.; Bernards, M.T.; Chen, S. Peritoneal adhesions: Occurrence, prevention and experimental models. Acta Biomater. 2020, 116, 84–104. [Google Scholar] [CrossRef]
- Krielen, P.; Stommel, M.W.J.; Pargmae, P.; Bouvy, N.D.; Bakkum, E.A.; Ellis, H.; Parker, M.C.; Griffiths, E.A.; van Goor, H.; Broek, R.P.G.T. Adhesion-related readmissions after open and laparoscopic surgery: A retrospective cohort study (SCAR update). Lancet 2020, 395, 33–41. [Google Scholar] [CrossRef]
- Kang, D.-H. Loosening of the mesothelial barrier as an early therapeutic target to preserve peritoneal function in peritoneal dialysis. Kidney Res. Clin. Pract. 2020, 39, 136–144. [Google Scholar] [CrossRef]
- Wynn, T.A.; Ramalingam, T.R. Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat. Med. 2012, 18, 1028–1040. [Google Scholar] [CrossRef]
- Koopmans, T.; Rinkevich, Y. Mesothelial to mesenchyme transition as a major developmental and pathological player in trunk organs and their cavities. Commun. Biol. 2018, 1, 170. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Ren, S.; Macarak, E.; Rosenbloom, J. Pathobiological mechanisms of peritoneal adhesions: The mesenchymal transition of rat peritoneal mesothelial cells induced by TGF-β1 and IL-6 requires activation of Erk1/2 and Smad2 linker region phosphorylation. Matrix Biol. 2016, 51, 55–64. [Google Scholar] [CrossRef]
- Fischer, A.; Koopmans, T.; Ramesh, P.; Christ, S.; Strunz, M.; Wannemacher, J.; Aichler, M.; Feuchtinger, A.; Walch, A.; Ansari, M.; et al. Post-surgical adhesions are triggered by calcium-dependent membrane bridges between mesothelial surfaces. Nat. Commun. 2020, 11, 1–15. [Google Scholar] [CrossRef]
- Isaza-Restrepo, A.; Martin-Saavedra, J.S.; Velez-Leal, J.L.; Vargas-Barato, F.; Riveros-Dueñas, R. The Peritoneum: Beyond the Tissue—A Review. Front. Physiol. 2018, 9, 738. [Google Scholar] [CrossRef]
- Li, J.; Guo, T. Role of Peritoneal Mesothelial Cells in the Progression of Peritoneal Metastases. Cancers 2022, 14, 2856. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, N.F.; Inagaki, F.F.; Kokudo, N.; Miyajima, A. Generation of mesothelial progenitor-like cells from mouse-induced pluripotent stem cells. FEBS Lett. 2019, 593, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.Y.; Chau, M.K.; Chan, C.C.; Tai, A.C.; Cheung, K.F.; Chan, T.M.; Yung, S. miR-200c Prevents TGF-β1-Induced Epithelial-to-Mesenchymal Transition and Fibrogenesis in Mesothelial Cells by Targeting ZEB2 and Notch1. Mol. Ther. Nucleic Acids 2019, 17, 78–91. [Google Scholar] [CrossRef]
- Corciulo, S.; Nicoletti, M.C.; Mastrofrancesco, L.; Milano, S.; Mastrodonato, M.; Carmosino, M.; Gerbino, A.; Corciulo, R.; Russo, R.; Svelto, M.; et al. AQP1-Containing Exosomes in Peritoneal Dialysis Effluent As Biomarker of Dialysis Efficiency. Cells 2019, 8, 330. [Google Scholar] [CrossRef]
- Mutsaers, S.E. The mesothelial cell. Int. J. Biochem. Cell Biol. 2004, 36, 9–16. [Google Scholar] [CrossRef]
- van Baal, J.; Van de Vijver, K.; Nieuwland, R.; van Noorden, C.; van Driel, W.; Sturk, A.; Kenter, G.; Rikkert, L.; Lok, C. The histophysiology and pathophysiology of the peritoneum. Tissue Cell 2017, 49, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-B.; Li, M.; Li, J.-C. Recent Advances in the Research of Lymphatic Stomata. Anat. Rec. 2010, 293, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-Y.; Li, J.-C. Ultrastructure and three-dimensional study of the lymphatic stomata in the costal pleura of the rabbit. Microsc. Res. Tech. 2003, 62, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, Z.; Zhou, J.; Yu, S. A study of the three-dimensional organization of the human diaphragmatic lymphatic lacunae and lymphatic drainage units. Ann. Anat. Anat. Anz. 1996, 178, 537–544. [Google Scholar] [CrossRef]
- Mutsaers, S.E. Mesothelial cells: Their structure, function and role in serosal repair. Respirology 2002, 7, 171–191. [Google Scholar] [CrossRef]
- Wilson, R.B. Hypoxia, cytokines and stromal recruitment: Parallels between pathophysiology of encapsulating peritoneal sclerosis, endometriosis and peritoneal metastasis. Pleura Peritoneum 2018, 3, 20180103. [Google Scholar] [CrossRef] [PubMed]
- Namvar, S.; Woolf, A.S.; Zeef, L.A.; Wilm, T.; Wilm, B.; Herrick, S.E. Functional molecules in mesothelial-to-mesenchymal transition revealed by transcriptome analyses. J. Pathol. 2018, 245, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, Y.; Tao, M.; Zhuang, S.; Liu, N. Peritoneal fibrosis and epigenetic modulation. Perit. Dial. Int. J. Int. Soc. Perit. Dial. 2021, 41, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Sun, L.; Xiao, L.; Gou, R.; Fang, Y.; Liang, Y.; Wang, R.; Li, N.; Liu, F.; Tang, L. Aberrant Wnt/Beta-Catenin Pathway Activation in Dialysate-Induced Peritoneal Fibrosis. Front. Pharmacol. 2017, 8, 774. [Google Scholar] [CrossRef] [PubMed]
- Masola, V.; Bonomini, M.; Borrelli, S.; Di Liberato, L.; Vecchi, L.; Onisto, M.; Gambaro, G.; Palumbo, R.; Arduini, A. Fibrosis of Peritoneal Membrane as Target of New Therapies in Peritoneal Dialysis. Int. J. Mol. Sci. 2022, 23, 4831. [Google Scholar] [CrossRef] [PubMed]
- Carmona, R.; Ariza, L.; Cano, E.; Jiménez-Navarro, M.; Muñoz-Chápuli, R. Mesothelial-mesenchymal transitions in embryogenesis. Semin. Cell Dev. Biol. 2019, 92, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Eleftheriadis, T.; Pissas, G.; Antoniadi, G.; Nikolaou, E.; Golfinopoulos, S.; Liakopoulos, V.; Stefanidis, I. Activation of General Control Nonderepressible-2 Kinase Ameliorates Glucotoxicity in Human Peritoneal Mesothelial Cells, Preserves Their Integrity, and Prevents Mesothelial to Mesenchymal Transition. Biomolecules 2019, 9, 832. [Google Scholar] [CrossRef]
- Duan, C.; Han, J.; Zhang, C.; Wu, K.; Lin, Y. UA promotes epithelial-mesenchymal transition in peritoneal mesothelial cells. Mol. Med. Rep. 2019, 20, 2396–2402. [Google Scholar] [CrossRef]
- Yáñez-Mó, M.; Lara-Pezzi, E.; Selgas, R.; Ramírez-Huesca, M.; Domínguez-Jiménez, C.; Jiménez-Heffernan, J.A.; Aguilera, A.; Sánchez-Tomero, J.A.; Bajo, M.A.; Álvarez, V.; et al. Peritoneal Dialysis and Epithelial-to-Mesenchymal Transition of Mesothelial Cells. N. Engl. J. Med. 2003, 348, 403–413. [Google Scholar] [CrossRef]
- Zwicky, S.N.; Stroka, D.; Zindel, J. Sterile Injury Repair and Adhesion Formation at Serosal Surfaces. Front. Immunol. 2021, 12, 684967. [Google Scholar] [CrossRef]
- Ito, T.; Shintani, Y.; Fields, L.; Shiraishi, M.; Podaru, M.; Kainuma, S.; Yamashita, K.; Kobayashi, K.; Perretti, M.; Lewis-McDougall, F.; et al. Cell barrier function of resident peritoneal macrophages in post-operative adhesions. Nat. Commun. 2021, 12, 1–12. [Google Scholar] [CrossRef]
- Zindel, J.; Peiseler, M.; Hossain, M.; Deppermann, C.; Lee, W.Y.; Haenni, B.; Zuber, B.; Deniset, J.F.; Surewaard, B.G.J.; Candinas, D.; et al. Primordial GATA6 macrophages function as extravascular platelets in sterile injury. Science 2021, 371, eabe0595. [Google Scholar] [CrossRef]
- Laukka, M.; Hoppela, E.; Salo, J.; Rantakari, P.; Gronroos, T.J.; Orte, K.; Auvinen, K.; Salmi, M.; Gerke, H.; Thol, K.; et al. Preperitoneal Fat Grafting Inhibits the Formation of Intra-abdominal Adhesions in Mice. J. Gastrointest. Surg. 2019, 24, 2838–2848. [Google Scholar] [CrossRef]
- Tsai, J.M.; Shoham, M.; Fernhoff, N.B.; George, B.M.; Marjon, K.D.; McCracken, M.N.; Kao, K.S.; Sinha, R.; Volkmer, A.K.; Miyanishi, M.; et al. Neutrophil and monocyte kinetics play critical roles in mouse peritoneal adhesion formation. Blood Adv. 2019, 3, 2713–2721. [Google Scholar] [CrossRef] [PubMed]
- Uyama, N.; Tsutsui, H.; Wu, S.; Yasuda, K.; Hatano, E.; Qin, X.-Y.; Kojima, S.; Fujimoto, J. Anti-interleukin-6 receptor antibody treatment ameliorates postoperative adhesion formation. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef]
- Honda, M.; Kadohisa, M.; Yoshii, D.; Komohara, Y.; Hibi, T. Directly recruited GATA6 + peritoneal cavity macrophages contribute to the repair of intestinal serosal injury. Nat. Commun. 2021, 12, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Czepielewski, R.S.; Jarjour, N.N.; Erlich, E.; Esaulova, E.; Saunders, B.T.; Grover, S.; Cleuren, A.C.; Broze, G.J.; Edelson, B.T.; et al. Expression of factor V by resident macrophages boosts host defense in the peritoneal cavity. J. Exp. Med. 2019, 216, 1291–1300. [Google Scholar] [CrossRef] [PubMed]
- Mukai, K.; Tsai, M.; Saito, H.; Galli, S.J. Mast cells as sources of cytokines, chemokines, and growth factors. Immunol. Rev. 2018, 282, 121–150. [Google Scholar] [CrossRef]
- Parikova, A.; Hruba, P.; Krediet, R.; Krejcik, Z.; Stranecky, V.; Striz, I.; Viklicky, O. Long-Term Peritoneal Dialysis Treatment Provokes Activation of Genes Related to Adaptive Immunity. Physiol. Res. 2019, 68, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Lian, Z.; Zhang, B.; Li, Z.; Zeng, L.; Li, W.; Bian, Y. Effect of ligustrazine nanoparticles on Th1/Th2 balance by TLR4/MyD88/NF-κB pathway in rats with postoperative peritoneal adhesion. BMC Surg. 2021, 21, 211. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Xia, X.; Kang, X.; Song, P.; Liu, Z.; Wang, M.; Guan, W.; Liu, S. A review of physiological and cellular mechanisms underlying fibrotic postoperative adhesion. Int. J. Biol. Sci. 2021, 17, 298–306. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, A.; Stellavato, A.; Corsuto, L.; Diana, P.; Filosa, R.; La Gatta, A.; De Rosa, M.; Schiraldi, C. Is molecular size a discriminating factor in hyaluronan interaction with human cells? Carbohydr. Polym. 2017, 157, 21–30. [Google Scholar] [CrossRef]
- Suzuki, T.; Kono, T.; Bochimoto, H.; Hira, Y.; Watanabe, T.; Furukawa, H. An injured tissue affects the opposite intact peritoneum during postoperative adhesion formation. Sci. Rep. 2015, 5, 7668. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Bajo, M.-A.; del Peso, G.; Yu, X.; Selgas, R. Preventing peritoneal membrane fibrosis in peritoneal dialysis patients. Kidney Int. 2016, 90, 515–524. [Google Scholar] [CrossRef]
- Koninckx, P.R.; Gomel, V.; Ussia, A.; Adamyan, L. Role of the peritoneal cavity in the prevention of postoperative adhesions, pain, and fatigue. Fertil. Steril. 2016, 106, 998–1010. [Google Scholar] [CrossRef] [PubMed]
- Honjo, K.; Munakata, S.; Tashiro, Y.; Salama, Y.; Shimazu, H.; Eiamboonsert, S.; Dhahri, D.; Ichimura, A.; Dan, T.; Miyata, T.; et al. Plasminogen activator inhibitor-1 regulates macrophage-dependent postoperative adhesion by enhancing EGF-HER1 signaling in mice. FASEB J. 2017, 31, 2625–2637. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, S.P.; Shin, J.; Vaughan, J.W.; Chandra, P.K.; Holcomb, J.B.; Atala, A.J. Regenerative Medicine Therapies for Prevention of Abdominal Adhesions: A Scoping Review. J. Surg. Res. 2022, 275, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Rojo, D.; Conget, P. Acellular derivatives of mesenchymal stem cells prevent peritoneal adhesions in an animal model. J. Surg. Res. 2018, 223, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Liu, H.; Zhang, T.; Zhang, M.; Tang, X.; Zhang, Z.; Lu, W.; Yang, S.; Jiang, Z.; Cui, Q.; et al. Extracellular Vesicles Derived from Adipose Mesenchymal Stem Cells Promote Peritoneal Healing by Activating MAPK-ERK1/2 and PI3K-Akt to Alleviate Postoperative Abdominal Adhesion. Stem Cells Int. 2022, 2022, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.-C.; Chou, T.-H.; Huang, J.-W.; Lee, C.-C.; Chen, S.-C. The Small Molecule Inhibitor QLT-0267 Decreases the Production of Fibrin-Induced Inflammatory Cytokines and Prevents Post-Surgical Peritoneal Adhesions. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Chu, Y.; Wang, Y.; Zheng, Z.; Lin, Y.; He, R.; Liu, J.; Yang, X. Proinflammatory Effect of High Glucose Concentrations on HMrSV5 Cells via the Autocrine Effect of HMGB1. Front. Physiol. 2017, 8, 762. [Google Scholar] [CrossRef]
- Terri, M.; Trionfetti, F.; Montaldo, C.; Cordani, M.; Tripodi, M.; Lopez-Cabrera, M.; Strippoli, R. Mechanisms of Peritoneal Fibrosis: Focus on Immune Cells–Peritoneal Stroma Interactions. Front. Immunol. 2021, 12, 607204. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Huang, Y.; Zhou, R.; Wu, K.; Li, W.; Shi, L.; Xia, Z.; Tao, K.; Wang, G.; Wang, G. Regulation and function of IL-22 in peritoneal adhesion formation after abdominal surgery. Wound Repair Regen. 2019, 28, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Balzer, M.S.; Helmke, A.; Ackermann, M.; Casper, J.; Dong, L.; Hiss, M.; Kiyan, Y.; Rong, S.; Timrott, K.; Von Vietinghoff, S.; et al. Protein kinase C beta deficiency increases glucose-mediated peritoneal damage via M1 macrophage polarization and up-regulation of mesothelial protein kinase C alpha. Nephrol. Dial. Transplant. 2019, 34, 947–960. [Google Scholar] [CrossRef]
- Jiang, N.; Zhang, Q.; Chau, M.K.; Yip, M.S.; Lui, S.L.; Liu, S.; Chu, K.M.; Ngan, H.Y.; Chan, T.M.; Yung, S. Anti-fibrotic effect of decorin in peritoneal dialysis and PD-associated peritonitis. eBioMedicine 2020, 52, 102661. [Google Scholar] [CrossRef] [PubMed]
- Katz, S.; Zsiros, V.; Dóczi, N.; Kiss, A.L. Inflammation-Induced Epithelial-to-Mesenchymal Transition and GM-CSF Treatment Stimulate Mesenteric Mesothelial Cells to Transdifferentiate into Macrophages. Inflammation 2018, 41, 1825–1834. [Google Scholar] [CrossRef] [PubMed]
- Katz, S.; Zsiros, V.; Kiss, A.L. Under inflammatory stimuli mesenteric mesothelial cells transdifferentiate into macrophages and produce pro-inflammatory cytokine IL-6. Agents Actions 2019, 68, 525–528. [Google Scholar] [CrossRef]
- Catar, R.A.; Bartosova, M.; Kawka, E.; Chen, L.; Marinovic, I.; Zhang, C.; Zhao, H.; Wu, D.; Zickler, D.; Stadnik, H.; et al. Angiogenic Role of Mesothelium-Derived Chemokine CXCL1 During Unfavorable Peritoneal Tissue Remodeling in Patients Receiving Peritoneal Dialysis as Renal Replacement Therapy. Front. Immunol. 2022, 13, 821681. [Google Scholar] [CrossRef]
- Da, J.; Yang, Y.; Dong, R.; Shen, Y.; Zha, Y. Therapeutic effect of 1,25(OH)2-VitaminD3 on fibrosis and angiogenesis of peritoneum induced by chlorhexidine. Biomed. Pharmacother. 2020, 129, 110431. [Google Scholar] [CrossRef]
- Yu, M.; Shi, J.; Sheng, M. Exosomes: The New Mediator of Peritoneal Membrane Function. Kidney Blood Press. Res. 2018, 43, 1010–1022. [Google Scholar] [CrossRef]
- Yang, X.; Yan, H.; Jiang, N.; Yu, Z.; Yuan, J.; Ni, Z.; Fang, W. IL-6trans-signaling drives a STAT3-dependent pathway that leads to structural alterations of the peritoneal membrane. Am. J. Physiol. Physiol. 2020, 318, F338–F353. [Google Scholar] [CrossRef] [PubMed]
- Cahill, R.A.; Wang, J.H.; Redmond, H.P. Enteric bacteria and their antigens may stimulate postoperative peritoneal adhesion formation. Surgery 2007, 141, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Ditzig, Z.; Wilson, C.M.; Salas, J.; Serve, K.M. Plasminogen Binding and Activation at the Mesothelial Cell Surface Promotes Invasion through a Collagen Matrix. Int. J. Mol. Sci. 2022, 23, 5984. [Google Scholar] [CrossRef] [PubMed]
- Strippoli, R.; Sandoval, P.; Moreno-Vicente, R.; Rossi, L.; Battistelli, C.; Terri, M.; Pascual-Antón, L.; Loureiro, M.; Matteini, F.; Calvo, E.; et al. Caveolin1 and YAP drive mechanically induced mesothelial to mesenchymal transition and fibrosis. Cell Death Dis. 2020, 11, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Rossi, L.; Battistelli, C.; De Turris, V.; Noce, V.; Zwergel, C.; Valente, S.; Moioli, A.; Manzione, A.; Palladino, M.; Bordoni, V.; et al. HDAC1 inhibition by MS-275 in mesothelial cells limits cellular invasion and promotes MMT reversal. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef]
- Ma, C.; Tarnuzzer, R.W.; Chegini, N. Expression of matrix metalloproteinases and tissue inhibitor of matrix metalloproteinases in mesothelial cells and their regulation by transforming growth factor-β1. Wound Repair Regen. 1999, 7, 477–485. [Google Scholar] [CrossRef]
- Karki, S.; Surolia, R.; Hock, T.D.; Guroji, P.; Zolak, J.S.; Duggal, R.; Ye, T.; Thannickal, V.J.; Antony, V.B. Wilms’ tumor 1 (Wt1) regulates pleural mesothelial cell plasticity and transition into myofibroblasts in idiopathic pulmonary fibrosis. FASEB J. 2014, 28, 1122–1131. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Asahina, K. Mesothelial cells give rise to hepatic stellate cells and myofibroblasts via mesothelial–mesenchymal transition in liver injury. Proc. Natl. Acad. Sci. USA 2013, 110, 2324–2329. [Google Scholar] [CrossRef]
- Deb, A.; Ubil, E. Cardiac fibroblast in development and wound healing. J. Mol. Cell. Cardiol. 2014, 70, 47–55. [Google Scholar] [CrossRef]
- Ruiz-Villalba, A.; Simón, A.M.; Pogontke, C.; Castillo, M.I.; Abizanda, G.; Pelacho, B.; Sanchez, R.; Segovia, J.C.; Prosper, F.; Pérez-Pomares, J.M. Interacting Resident Epicardium-Derived Fibroblasts and Recruited Bone Marrow Cells Form Myocardial Infarction Scar. J. Am. Coll. Cardiol. 2015, 65, 2057–2066. [Google Scholar] [CrossRef]
- Sandoval, P.; Jiménez-Heffernan, J.A.; Guerra-Azcona, G.; Pérez-Lozano, M.L.; Rynne-Vidal, A.; Albar-Vizcaíno, P.; Gil-Vera, F.; Martín, P.; Coronado, M.J.; Barcena, C.; et al. Mesothelial-to-mesenchymal transition in the pathogenesis of post-surgical peritoneal adhesions. J. Pathol. 2016, 239, 48–59. [Google Scholar] [CrossRef]
- Kang, S.H.; Kim, S.W.; Kim, K.J.; Cho, K.H.; Park, J.W.; Kim, C.-D.; Do, J.Y. Effects of tranilast on the epithelial-to-mesenchymal transition in peritoneal mesothelial cells. Kidney Res. Clin. Pr. 2019, 38, 472–480. [Google Scholar] [CrossRef]
- Jin, G.; Su, Y.; Dong, Q.; Zhao, X.; Zhang, L.; Yan, X. Arctigenin alleviates TGF-β1-induced epithelial-mesenchymal transition and PAI-1 expression via AMPK/NF-κB pathway in peritoneal mesothelial cells. Biochem. Biophys. Res. Commun. 2019, 520, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Zindel, J.; Mittner, J.; Bayer, J.; April-Monn, S.L.; Kohler, A.; Nusse, Y.; Dosch, M.; Büchi, I.; Sanchez-Taltavull, D.; Dawson, H.; et al. Intraperitoneal microbial contamination drives post-surgical peritoneal adhesions by mesothelial EGFR-signaling. Nat. Commun. 2021, 12, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Masola, V.; Granata, S.; Bellin, G.; Gambaro, G.; Onisto, M.; Rugiu, C.; Lupo, A.; Zaza, G. Specific heparanase inhibition reverses glucose-induced mesothelial-to-mesenchymal transition. Nephrol. Dial. Transplant. 2017, 32, 1145–1154. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lua, I.; Li, Y.; Pappoe, L.S.; Asahina, K. Myofibroblastic Conversion and Regeneration of Mesothelial Cells in Peritoneal and Liver Fibrosis. Am. J. Pathol. 2015, 185, 3258–3273. [Google Scholar] [CrossRef]
- Miyake, T.; Sakai, N.; Tamai, A.; Sato, K.; Kamikawa, Y.; Miyagawa, T.; Ogura, H.; Yamamura, Y.; Oshima, M.; Nakagawa, S.; et al. Trehalose ameliorates peritoneal fibrosis by promoting Snail degradation and inhibiting mesothelial-to-mesenchymal transition in mesothelial cells. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef]
- Han, S.M.; Ryu, H.-M.; Suh, J.; Lee, K.-J.; Choi, S.-Y.; Choi, S.; Kim, Y.-L.; Huh, J.Y.; Ha, H. Network-based integrated analysis of omics data reveal novel players of TGF-β1-induced EMT in human peritoneal mesothelial cells. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Loureiro, J.; Aguilera, A.; Selgas, R.; Sandoval, P.; Albar-Vizcaíno, P.; Pérez-Lozano, M.L.; Ruiz-Carpio, V.; Majano, P.L.; Lamas, S.; Rodríguez-Pascual, F.; et al. Blocking TGF-β1 Protects the Peritoneal Membrane from Dialysate-Induced Damage. J. Am. Soc. Nephrol. 2011, 22, 1682–1695. [Google Scholar] [CrossRef]
- Helmke, A.; Nordlohne, J.; Balzer, M.S.; Dong, L.; Rong, S.; Hiss, M.; Shushakova, N.; Haller, H.; von Vietinghoff, S. CX3CL1–CX3CR1 interaction mediates macrophage-mesothelial cross talk and promotes peritoneal fibrosis. Kidney Int. 2019, 95, 1405–1417. [Google Scholar] [CrossRef]
- Kim, K.K.; Sheppard, D.; Chapman, H.A. TGF-β1 Signaling and Tissue Fibrosis. Cold Spring Harb. Perspect. Biol. 2018, 10, a022293. [Google Scholar] [CrossRef]
- Heo, J.-Y.; Do, J.-Y.; Lho, Y.; Kim, A.-Y.; Kim, S.-W.; Kang, S.-H. TGF-β1 Receptor Inhibitor SB525334 Attenuates the Epithelial to Mesenchymal Transition of Peritoneal Mesothelial Cells via the TGF-β1 Signaling Pathway. Biomedicines 2021, 9, 839. [Google Scholar] [CrossRef]
- Yang, D.; Fu, W.; Li, L.; Xia, X.; Liao, Q.; Yue, R.; Chen, H.; Chen, X.; An, S.; Zeng, C.; et al. Therapeutic effect of a novel Wnt pathway inhibitor on cardiac regeneration after myocardial infarction. Clin. Sci. 2017, 131, 2919–2932. [Google Scholar] [CrossRef] [PubMed]
- Akcora, B.Ö.; Storm, G.; Bansal, R. Inhibition of canonical WNT signaling pathway by β-catenin/CBP inhibitor ICG-001 ameliorates liver fibrosis in vivo through suppression of stromal CXCL12. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 804–818. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, C.J.; Liu, Y. Wnt Signaling in Kidney Development and Disease. Organogenesis 2018, 153, 181–207. [Google Scholar] [CrossRef]
- Fan, Y.; Zhao, X.; Ma, J.; Yang, L. LncRNA GAS5 Competitively Combined With miR-21 Regulates PTEN and Influences EMT of Peritoneal Mesothelial Cells via Wnt/β-Catenin Signaling Pathway. Front. Physiol. 2021, 12, 654951. [Google Scholar] [CrossRef]
- Wang, Y.; He, G.; Wang, F.; Zhang, C.; Ge, Z.; Zheng, X.; Deng, H.; Yuan, C.; Zhou, B.; Tao, X.; et al. Aspirin inhibits adipogenesis of tendon stem cells and lipids accumulation in rat injury tendon through regulating PTEN/PI3K/AKT signalling. J. Cell. Mol. Med. 2019, 23, 7535–7544. [Google Scholar] [CrossRef]
- He, J.; Peng, H.; Wang, M.; Liu, Y.; Guo, X.; Wang, B.; Dai, L.; Cheng, X.; Meng, Z.; Yuan, L.; et al. Isoliquiritigenin inhibits TGF-β1-induced fibrogenesis through activating autophagy via PI3K/AKT/mTOR pathway in MRC-5 cells. Acta Biochim. et Biophys. Sin. 2020, 52, 810–820. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, X.; Xu, Y.; Shen, Z.; Cheng, H.; Cheng, F.; Liu, X.; Wang, R. RhoA/Rho-kinase triggers epithelial-mesenchymal transition in mesothelial cells and contributes to the pathogenesis of dialysis-related peritoneal fibrosis. Oncotarget 2018, 9, 14397–14412. [Google Scholar] [CrossRef]
- Cano Sanchez, M.; Lancel, S.; Boulanger, E.; Neviere, R. Targeting Oxidative Stress and Mitochondrial Dysfunction in the Treatment of Impaired Wound Healing: A Systematic Review. Antioxidants 2018, 7, 98. [Google Scholar] [CrossRef]
- Raa, S.T.; Tol, M.P.V.D.; Sluiter, W.; Hofland, L.J.; van Eijck, C.H.; Jeekel, H. The Role of Neutrophils and Oxygen Free Radicals in Post-Operative Adhesions. J. Surg. Res. 2006, 136, 45–52. [Google Scholar] [CrossRef]
- Yang, H.-L.; Thiyagarajan, V.; Shen, P.-C.; Mathew, D.C.; Lin, K.-Y.; Liao, J.-W.; Hseu, Y.-C. Anti-EMT properties of CoQ0 attributed to PI3K/AKT/NFKB/MMP-9 signaling pathway through ROS-mediated apoptosis. J. Exp. Clin. Cancer Res. 2019, 38, 1–21. [Google Scholar] [CrossRef]
- Chatterjee, R.; Chatterjee, J. ROS and oncogenesis with special reference to EMT and stemness. Eur. J. Cell Biol. 2020, 99, 151073. [Google Scholar] [CrossRef] [PubMed]
- Hara, K.; Hamada, C.; Wakabayashi, K.; Kanda, R.; Kaneko, K.; Horikoshi, S.; Tomino, Y.; Suzuki, Y. Scavenging of reactive oxygen species by astaxanthin inhibits epithelial–mesenchymal transition in high glucose-stimulated mesothelial cells. PLoS ONE 2017, 12, e0184332. [Google Scholar] [CrossRef]
- Ramil-Gómez, O.; Rodríguez-Carmona, A.; Fernández-Rodríguez, J.; Pérez-Fontán, M.; Ferreiro-Hermida, T.; López-Pardo, M.; Pérez-López, T.; López-Armada, M. Mitochondrial Dysfunction Plays a Relevant Role in Pathophysiology of Peritoneal Membrane Damage Induced by Peritoneal Dialysis. Antioxidants 2021, 10, 447. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.-S.; Ko, J.; Kim, D.-A.; Ryu, E.-S.; Ryu, H.-M.; Park, S.-H.; Kim, Y.-L.; Oh, E.-S.; Kang, D.-H. Metformin ameliorates the Phenotype Transition of Peritoneal Mesothelial Cells and Peritoneal Fibrosis via a modulation of Oxidative Stress. Sci. Rep. 2017, 7, 5690. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.; Kang, H.-J.; Kim, D.-A.; Ryu, E.-S.; Yu, M.; Lee, H.; Lee, H.K.; Ryu, H.-M.; Park, S.-H.; Kim, Y.-L.; et al. Paricalcitol attenuates TGF-β1–induced phenotype transition of human peritoneal mesothelial cells (HPMCs) via modulation of oxidative stress and NLRP3 inflammasome. FASEB J. 2019, 33, 3035–3050. [Google Scholar] [CrossRef]
- Roumeliotis, S.; Dounousi, E.; Salmas, M.; Eleftheriadis, T.; Liakopoulos, V. Unfavorable Effects of Peritoneal Dialysis Solutions on the Peritoneal Membrane: The Role of Oxidative Stress. Biomolecules 2020, 10, 768. [Google Scholar] [CrossRef]
- Casalena, G.; Daehn, I.; Bottinger, E. Transforming Growth Factor-β, Bioenergetics, and Mitochondria in Renal Disease. Semin. Nephrol. 2012, 32, 295–303. [Google Scholar] [CrossRef]
- Nakamoto, H.; Imai, H.; Fukushima, R.; Ishida, Y.; Yamanouchi, Y.; Suzuki, H. Role of the renin-angiotensin system in the pathogenesis of peritoneal fibrosis. Perit. Dial. Int. J. Int. Soc. Perit. Dial. 2008, 28, S83–S87. [Google Scholar] [CrossRef]
- Bian, Y.; Yang, L.; Zhang, B.; Li, W.; Wang, S.; Jiang, S.; Chen, X.; Li, W.; Zeng, L. LincRNA Cox-2 Regulates Lipopolysaccharide-Induced Inflammatory Response of Human Peritoneal Mesothelial Cells via Modulating miR-21/NF-κB Axis. Mediat. Inflamm. 2019, 2019, 1–11. [Google Scholar] [CrossRef]
- Bender, T.O.; Böhm, M.; Kratochwill, K.; Lederhuber, H.; Endemann, M.; Bidmon, B.; Aufricht, C. HSP-Mediated Cytoprotection of Mesothelial Cells in Experimental Acute Peritoneal Dialysis. Perit. Dial. Int. J. Int. Soc. Perit. Dial. 2010, 30, 294–299. [Google Scholar] [CrossRef]
- Ferrantelli, E.; Liappas, G.; Vila Cuenca, M.; Keuning, E.D.; Foster, T.L.; Vervloet, M.G.; Lopéz-Cabrera, M.; Beelen, R.H. The dipeptide alanyl-glutamine ameliorates peritoneal fibrosis and attenuates IL-17 dependent pathways during peritoneal dialysis. Kidney Int. 2016, 89, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Li, S.; Wang, Y.; Shen, J.; Zhou, Y.; Li, H.; Yu, X.; Mao, H. Acetylation of HMGB1 by JNK1 Signaling Promotes LPS-Induced Peritoneal Mesothelial Cells Apoptosis. BioMed Res. Int. 2018, 2018, 1–12. [Google Scholar] [CrossRef]
- Wang, N.; Li, Q.; Zhang, L.; Lin, H.; Hu, J.; Li, D.; Shi, S.; Cui, S.; Zhou, J.; Ji, J.; et al. Mesenchymal Stem Cells Attenuate Peritoneal Injury through Secretion of TSG-6. PLoS ONE 2012, 7, e43768. [Google Scholar] [CrossRef]
- Cil, A.T.B.; Aydogdu, I.O. Effect of Fat Graftıng on Postoperatıve Intraabdomınal Adhesions on a Rat Model. Arch. Med Res. 2018, 49, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Bresson, L.; Leblanc, E.; Lemaire, A.S.; Okitsu, T.; Chai, F. Autologous peritoneal grafts permit rapid reperitonealization and prevent postoperative abdominal adhesions in an experimental rat study. Surgery 2017, 162, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Kao, H.-H.; Kuo, C.-Y.; Chen, K.-S.; Chen, J.-P. Preparation of Gelatin and Gelatin/Hyaluronic Acid Cryogel Scaffolds for the 3D Culture of Mesothelial Cells and Mesothelium Tissue Regeneration. Int. J. Mol. Sci. 2019, 20, 4527. [Google Scholar] [CrossRef] [PubMed]
- Rynne-Vidal, A.; Au-Yeung, C.L.; Jiménez-Heffernan, J.A.; Perez-Lozano, M.-L.; Cremades-Jimeno, L.; Bárcena, C.; Cristobal, I.; Fernández-Chacón, C.; Yeung, T.L.; Mok, S.C.; et al. Mesothelial-to-mesenchymal transition as a possible therapeutic target in peritoneal metastasis of ovarian cancer. J. Pathol. 2017, 242, 140–151. [Google Scholar] [CrossRef]
- Sandoval, P.; Jiménez-Heffernan, J.A.; Rynne-Vidal, A.; Perez-Lozano, M.-L.; Gilsanz, A.; Ruiz-Carpio, V.; Reyes, R.; García-Bordas, J.; Stamatakis, K.; Dotor, J.; et al. Carcinoma-associated fibroblasts derive from mesothelial cells via mesothelial-to-mesenchymal transition in peritoneal metastasis. J. Pathol. 2013, 231, 517–531. [Google Scholar] [CrossRef] [PubMed]
- Rynne-Vidal, A.; Jiménez-Heffernan, J.; Fernández-Chacón, C.; López-Cabrera, M.; Sandoval, P. The Mesothelial Origin of Carcinoma Associated-Fibroblasts in Peritoneal Metastasis. Cancers 2015, 7, 1994–2011. [Google Scholar] [CrossRef] [PubMed]
- Gordillo, C.H.; Sandoval, P.; Muñoz-Hernández, P.; Pascual-Antón, L.; López-Cabrera, M.; Jiménez-Heffernan, J.A. Mesothelial-to-Mesenchymal Transition Contributes to the Generation of Carcinoma-Associated Fibroblasts in Locally Advanced Primary Colorectal Carcinomas. Cancers 2020, 12, 499. [Google Scholar] [CrossRef]
- Guo, R.; Hao, G.; Bao, Y.; Xiao, J.; Zhan, X.; Shi, X.; Luo, L.; Zhou, J.; Chen, Q.; Wei, X. MiR-200a negatively regulates TGF-β1-induced epithelial-mesenchymal transition of peritoneal mesothelial cells by targeting ZEB1/2 expression. Am. J. Physiol. Physiol. 2018, 314, F1087–F1095. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, N.; Tian, D. MiR-30b is involved in methylglyoxal-induced epithelial-mesenchymal transition of peritoneal mesothelial cells in rats. Cell. Mol. Biol. Lett. 2014, 19, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Yang, M.; Lan, H.; Yu, X. miR-30a Negatively Regulates TGF-β1–Induced Epithelial-Mesenchymal Transition and Peritoneal Fibrosis by Targeting Snai1. Am. J. Pathol. 2013, 183, 808–819. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Huang, Q.; Li, P.; Wang, Y.; Zheng, C.; Lei, X.; Li, S.; Gong, W.; Yin, B.; Luo, C.; et al. MicroRNA-145 promotes the epithelial-mesenchymal transition in peritoneal dialysis-associated fibrosis by suppressing fibroblast growth factor 10. J. Biol. Chem. 2019, 294, 15052–15067. [Google Scholar] [CrossRef]
- Yang, L.; Fan, Y.; Zhang, X.; Gao, L.; Ma, J. Role of miRNA-21/PTEN on the high glucose-induced EMT in human mesothelial peritoneal cells. Am. J. Transl. Res. 2018, 10, 2590–2599. [Google Scholar]
- Gao, L.; Fan, Y.; Zhang, X.; Yang, L.; Huang, W.; Hang, T.; Li, M.; Du, S.; Ma, J. Zinc supplementation inhibits the high glucose-induced EMT of peritoneal mesothelial cells by activating the Nrf2 antioxidant pathway. Mol. Med. Rep. 2019, 20, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Lu, Y.; Li, Y.; Gao, L.; Shen, H.; Song, K. Hydrogen sulfide inhibits epithelial-mesenchymal transition in peritoneal mesothelial cells. Sci. Rep. 2018, 8, 1–7. [Google Scholar] [CrossRef]
- Allègre, L.; Le Teuff, I.; Leprince, S.; Warembourg, S.; Taillades, H.; Garric, X.; Letouzey, V.; Huberlant, S. A new bioabsorbable polymer film to prevent peritoneal adhesions validated in a post-surgical animal model. PLoS ONE 2018, 13, e0202285. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-H.; Chen, S.-H.; Mao, S.-H.; Tsai, M.-J.; Chou, P.-Y.; Liao, C.-H.; Chen, J.-P. Injectable thermosensitive hydrogel containing hyaluronic acid and chitosan as a barrier for prevention of postoperative peritoneal adhesion. Carbohydr. Polym. 2017, 173, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Abuzar, S.; Seo, Y.; Han, H.; Jeon, Y.; Park, E.J.; Baik, S.H.; Hwang, S.-J. Oxaliplatin-loaded chemically cross-linked hydrogels for prevention of postoperative abdominal adhesion and colorectal cancer therapy. Int. J. Pharm. 2019, 565, 50–58. [Google Scholar] [CrossRef]
- Foster, D.S.; Marshall, C.D.; Gulati, G.S.; Chinta, M.S.; Nguyen, A.; Salhotra, A.; Jones, R.E.; Burcham, A.; Lerbs, T.; Cui, L.; et al. Elucidating the fundamental fibrotic processes driving abdominal adhesion formation. Nat. Commun. 2020, 11, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.-Y.; Peng, H.-W.; Wei, S.-Y.; Chen, C.-S.; Chen, Y.-C. Dynamically and Spatially Controllable Albumin-Based Hydrogels for the Prevention of Postoperative Adhesion. ACS Biomater. Sci. Eng. 2021, 7, 3293–3305. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-X.; Yuan, F.; Zhang, H.-H.; Liao, N.-N.; Luo, J.-W.; Sun, Y.-L. Evaluation of surgical anti-adhesion products to reduce postsurgical intra-abdominal adhesion formation in a rat model. PLoS ONE 2017, 12, e0172088. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Guo, T.; Li, J. Mechanisms of Peritoneal Mesothelial Cells in Peritoneal Adhesion. Biomolecules 2022, 12, 1498. https://doi.org/10.3390/biom12101498
Wang R, Guo T, Li J. Mechanisms of Peritoneal Mesothelial Cells in Peritoneal Adhesion. Biomolecules. 2022; 12(10):1498. https://doi.org/10.3390/biom12101498
Chicago/Turabian StyleWang, Ruipeng, Tiankang Guo, and Junliang Li. 2022. "Mechanisms of Peritoneal Mesothelial Cells in Peritoneal Adhesion" Biomolecules 12, no. 10: 1498. https://doi.org/10.3390/biom12101498
APA StyleWang, R., Guo, T., & Li, J. (2022). Mechanisms of Peritoneal Mesothelial Cells in Peritoneal Adhesion. Biomolecules, 12(10), 1498. https://doi.org/10.3390/biom12101498




