High-Intensity Exercise Training Improves Basal Platelet Prostacyclin Sensitivity and Potentiates the Response to Dual Anti-Platelet Therapy in Postmenopausal Women
Abstract
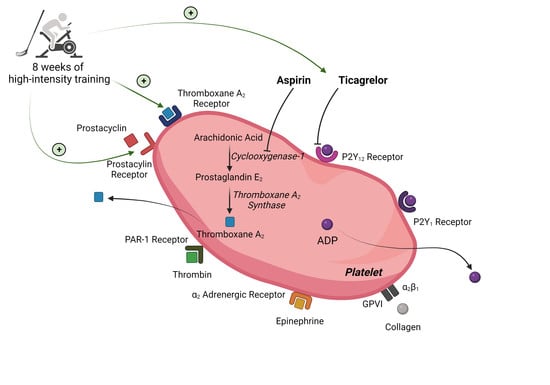
1. Introduction
2. Materials and Methods
2.1. Recruitment of Subjects
2.2. Study Design
2.3. Health Screening Day
2.4. Exercise Training
2.5. Body Composition Days
2.6. Experimental Days
2.7. Basal Platelet Reactivity Assay
2.8. Prostacyclin Sensitivity Assay
2.9. Platelet Isolation
2.10. Western Blotting of Isolated Platelets
2.11. Plasma Thromboxane B2 and 6-Keto Prostaglandin F1α Assays
2.12. Statistical Analyses
3. Results
3.1. Participant Characteristics
3.2. Exercise Training
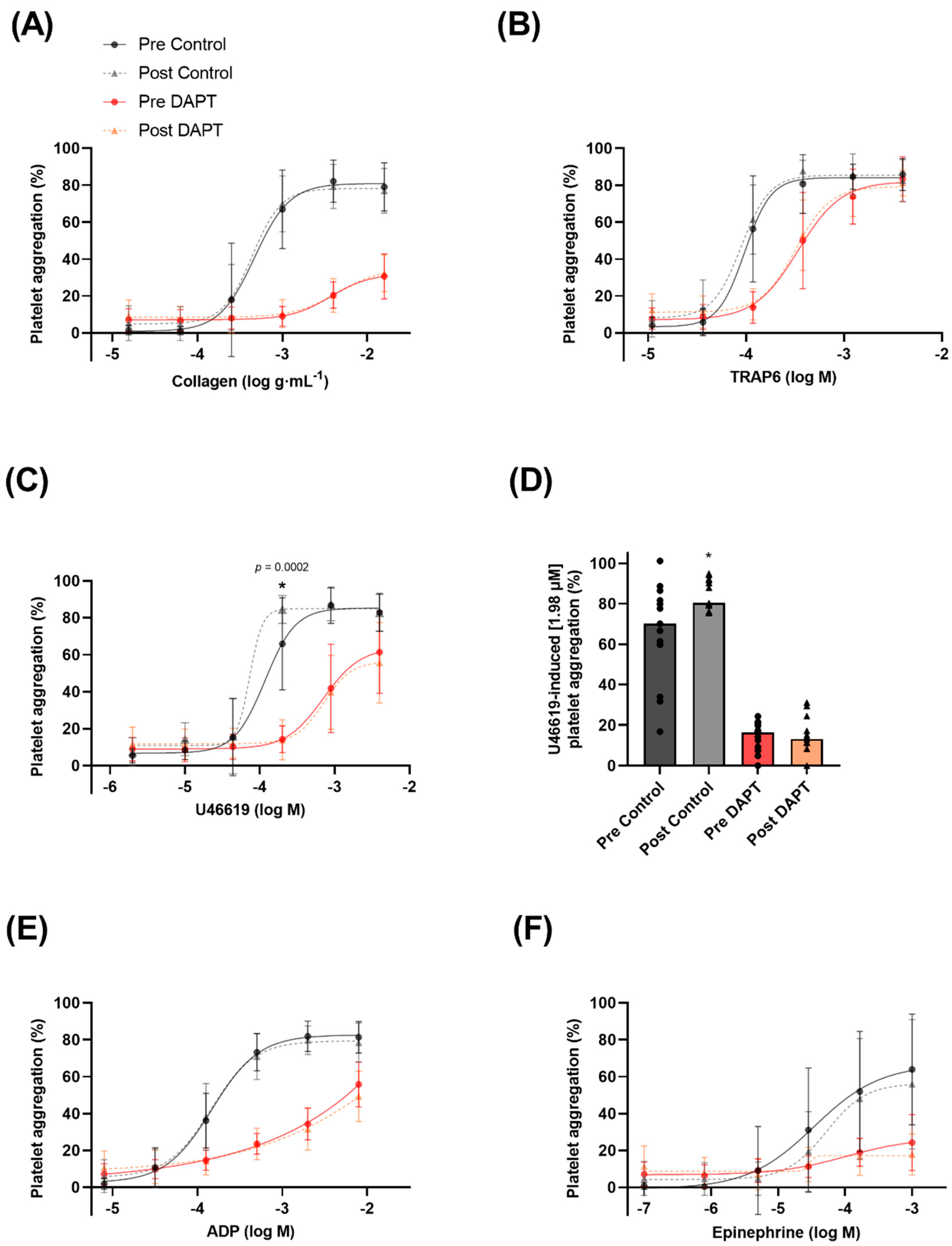
3.3. Exercise Training and Basal Platelet Reactivity
3.4. Exercise Training, Dual Anti-Platelet Therapy, and Basal Platelet Reactivity
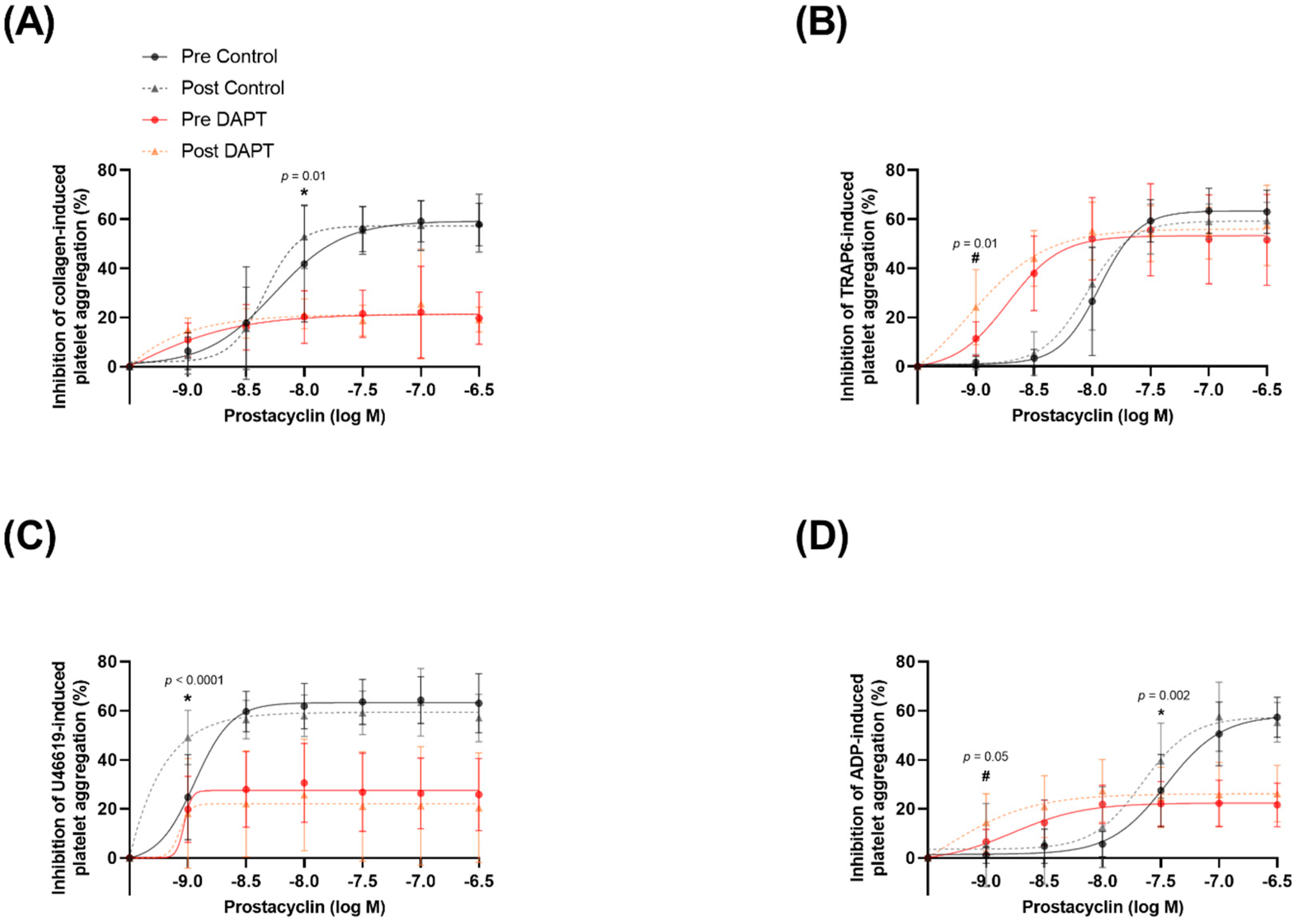
3.5. Exercise Training and Basal Platelet Prostacyclin Sensitivity
3.6. Exercise Training and Prostacyclin Sensitivity in the Presence of DAPT
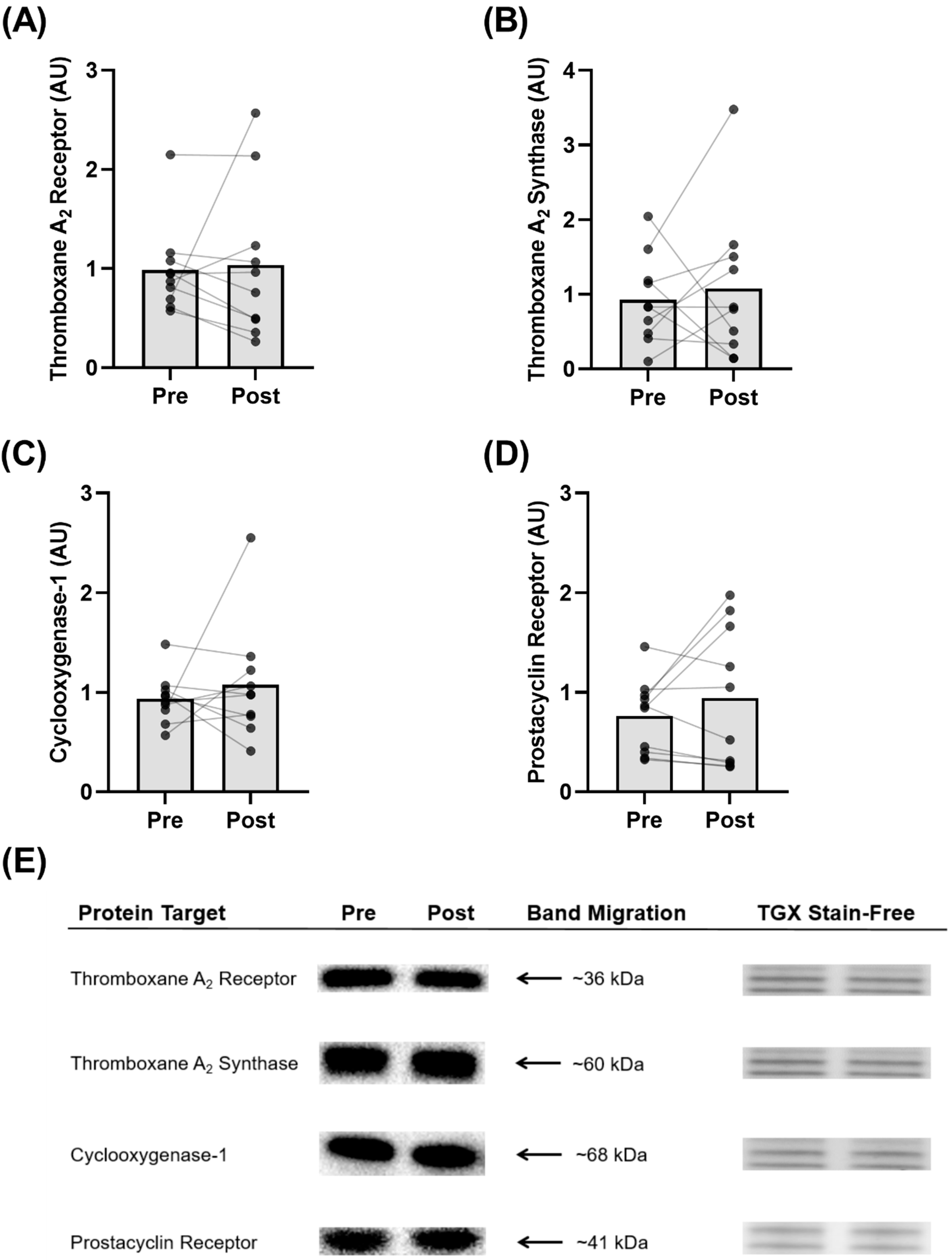
3.7. Protein Expression in Isolated Platelets
3.8. Plasma 6-Keto PGF1α and Thromboxane B2 Levels
4. Discussion
4.1. Exercise Training Improves Basal Prostacyclin Sensitivity
4.2. Exercise Training Potentiates the Effects of DAPT on Prostacyclin Sensitivity
4.3. Exercise Training Increases Basal Platelet Sensitivity to Thromboxane-Induced Aggregation
4.4. Exercise Training Does Not Influence Basal Platelet Reactivity with DAPT
4.5. Study Limitations
5. Conclusions
6. Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 6-Keto PGF1α | 6-Keto Prostaglandin F1α |
| ACS | Acute Coronary Syndrome |
| ADP | Adenosine 5′-Diphosphate |
| BMI | Body Mass Index |
| COVID-19 | Novel Coronavirus |
| DAPT | Dual Anti-Platelet Therapy |
| DMSO | Dimethyl Sulfoxide |
| DTT | Dithiothreitol |
| DXA | Dual Energy X-ray Absorptiometry |
| EC50 | Half Maximal Effective Concentration |
| ECG | Electrocardiogram |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| HEPES | 4-(2-Hydroxyethyl)-1-Piperazineethanesulfonic Acid |
| HR | Heart Rate |
| HRmax | Maximum Heart Rate |
| IPAQ | International Physical Activity Questionnaire |
| KCl | Potassium Chloride |
| MgCl2 | Magnesium Chloride |
| mmHg | Millimeters of Mercury |
| NaCl | Sodium Chloride |
| NaHCO3 | Sodium Bicarbonate |
| NO | Nitric Oxide |
| PBS | Phosphate-Buffered Saline |
| PPP | Platelet-Poor Plasma |
| PRP | Platelet-Rich Plasma |
| RBC | Red Blood Cells |
| SDS | Sodium Dodecyl Sulfate |
| TRAP-6 | Thrombin Receptor Activator Peptide 6 |
| V̇O2max | Maximal Oxygen Uptake |
| W | Watts |
| Wmax | Watt Maximum |
| WBC | White Blood Cells |
References
- Alkarithi, G.; Duval, C.; Shi, Y.; Macrae, F.L.; Ariëns, R.A.S. Thrombus Structural Composition in Cardiovascular Disease. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 2370–2383. [Google Scholar] [CrossRef]
- Engbers, M.J.; Van Hylckama Vlieg, A.; Rosendaal, F.R. Venous Thrombosis in the Elderly: Incidence, Risk Factors and Risk Groups. J. Thromb. Haemost. 2010, 8, 2105–2112. [Google Scholar] [CrossRef]
- Troy, G.C. An Overview of Hemostasis. Vet. Clin. North Am. Small Anim. Pract. 1988, 18, 5–20. [Google Scholar] [CrossRef]
- Bucciarelli, P.; Mannucci, P.M. The Hemostatic System through Aging and Menopause. Climacteric 2009, 12 (Suppl. 1), 47–51. [Google Scholar] [CrossRef] [PubMed]
- Kurachi, K.; Kurachi, S. Genetic Mechanisms of Age Regulation of Blood Coagulation. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 902–906. [Google Scholar] [CrossRef] [PubMed]
- Le Blanc, J.; Lordkipanidzé, M. Platelet Function in Aging. Front. Cardiovasc. Med. 2019, 6, 109. [Google Scholar] [CrossRef] [PubMed]
- Meade, T.W.; Vickers, M.V.; Thompson, S.G.; Stirling, Y.; Haines, A.P.; Miller, G.J. Epidemiological Characteristics of Platelet Aggregability. Br. Med. J. 1985, 290, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Butkiewicz, A.M.; Kemona, H.; Dymicka-Piekarska, V.; Matowicka-Karna, J. Does menopause affect thrombocytopoiesis and platelet activation? Prz. Lek. 2006, 63, 1291–1293. [Google Scholar]
- Lundberg Slingsby, M.H.; Nyberg, M.; Egelund, J.; Mandrup, C.M.; Frikke-Schmidt, R.; Kirkby, N.S.; Hellsten, Y. Aerobic Exercise Training Lowers Platelet Reactivity and Improves Platelet Sensitivity to Prostacyclin in Pre- and Postmenopausal Women. J. Thromb. Haemost. 2017, 15, 2419–2431. [Google Scholar] [CrossRef] [PubMed]
- Roshan, T.M.; Normah, J.; Rehman, A.; Naing, L. Effect of Menopause on Platelet Activation Markers Determined by Flow Cytometry. Am. J. Hematol. 2005, 80, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Oshima, T.; Matsuura, H.; Kajiyama, G.; Kambe, M. Effect of 17β-Estradiol on Inhibition of Platelet Aggregation In Vitro Is Mediated by an Increase in NO Synthesis. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 961–967. [Google Scholar] [CrossRef][Green Version]
- Sobrino, A.; Oviedo, P.J.; Novella, S.; Laguna-Fernandez, A.; Bueno, C.; García-Pérez, M.A.; Tarín, J.J.; Cano, A.; Hermenegildo, C. Estradiol Selectively Stimulates Endothelial Prostacyclin Production through Estrogen Receptor-α. J. Mol. Endocrinol. 2010, 44, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Collet, J.-P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation. Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.V.; Knowles, R.B.M.; Lundberg, M.H.; Tucker, A.T.; Mohamed, N.A.; Kirkby, N.S.; Armstrong, P.C.J.; Mitchell, J.A.; Warner, T.D. P2Y12 Receptor Blockade Synergizes Strongly with Nitric Oxide and Prostacyclin to Inhibit Platelet Activation. Br. J. Clin. Pharm. 2016, 81, 621–633. [Google Scholar] [CrossRef]
- Kirkby, N.S.; Lundberg, M.H.; Chan, M.V.; Vojnovic, I.; Solomon, A.B.; Emerson, M.; Mitchell, J.A.; Warner, T.D. Blockade of the Purinergic P2Y12 Receptor Greatly Increases the Platelet Inhibitory Actions of Nitric Oxide. Proc. Natl. Acad. Sci. USA 2013, 110, 15782–15787. [Google Scholar] [CrossRef]
- Warner, T.D.; Armstrong, P.C.; Chan, M.V.; Knowles, R.B. The Importance of Endothelium-Derived Mediators to the Efficacy of Dual Anti-Platelet Therapy. Expert Rev. Hematol. 2016, 9, 223–225. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Candelaria, D.; Randall, S.; Ladak, L.; Gallagher, R. Health-Related Quality of Life and Exercise-Based Cardiac Rehabilitation in Contemporary Acute Coronary Syndrome Patients: A Systematic Review and Meta-Analysis. Qual. Life Res. 2020, 29, 579–592. [Google Scholar] [CrossRef] [PubMed]
- Green, D.J.; Smith, K.J. Effects of Exercise on Vascular Function, Structure, and Health in Humans. Cold Spring Harb. Perspect. Med. 2018, 8, a029819. [Google Scholar] [CrossRef]
- Braga, V.A.V.N.; Couto, G.K.; Lazzarin, M.C.; Rossoni, L.V.; Medeiros, A. Aerobic Exercise Training Prevents the Onset of Endothelial Dysfunction via Increased Nitric Oxide Bioavailability and Reduced Reactive Oxygen Species in an Experimental Model of Menopause. PLoS ONE 2015, 10, e0125388. [Google Scholar] [CrossRef] [PubMed]
- Seals, D.R.; Nagy, E.E.; Moreau, K.L. Aerobic Exercise Training and Vascular Function with Ageing in Healthy Men and Women. J. Physiol. 2019, 597, 4901–4914. [Google Scholar] [CrossRef]
- Zaros, P.R.; Pires, C.E.R.; Bacci, M.; Moraes, C.; Zanesco, A. Effect of 6-Months of Physical Exercise on the Nitrate/Nitrite Levels in Hypertensive Postmenopausal Women. BMC Women’s Health 2009, 9, 17. [Google Scholar] [CrossRef]
- Lundberg Slingsby, M.H.; Gliemann, L.; Thrane, M.; Rytter, N.; Egelund, J.; Chan, M.V.; Armstrong, P.C.; Warner, T.D.; Hellsten, Y. Platelet Responses to Pharmacological and Physiological Interventions in Middle-Aged Men with Different Habitual Physical Activity Levels. Acta Physiol. 2018, 223, e13028. [Google Scholar] [CrossRef] [PubMed]
- Pelliccia, F.; Gragnano, F.; Pasceri, V.; Marazzi, G.; Cacciotti, L.; Placanica, A.; Niccoli, G.; Palmerini, T.; Speciale, G.; Granatelli, A.; et al. Gender-Related Differences in Changes of Estimated Bleeding Risk in Patients on Dual Antiplatelet Therapy: The RE-SCORE Multicenter Prospective Registry. Platelets 2022, 1–9. [Google Scholar] [CrossRef]
- Pradhan, A.; Vishwakarma, P.; Sethi, R. Changing Scenario in Management of Acute Coronary Syndromes in Females—Evidence from Recent Studies. Indian J. Cardiovasc. Dis. Women WINCARS 2018, 3, 245–250. [Google Scholar] [CrossRef]
- Becker, D.M.; Segal, J.; Vaidya, D.; Yanek, L.R.; Herrera-Galeano, J.E.; Bray, P.F.; Moy, T.F.; Becker, L.C.; Faraday, N. Sex Differences in Platelet Reactivity and Response to Low-Dose Aspirin Therapy. JAMA 2006, 295, 1420–1427. [Google Scholar] [CrossRef] [PubMed]
- Ranucci, M.; Aloisio, T.; Di Dedda, U.; Menicanti, L.; de Vincentiis, C.; Baryshnikova, E. Gender-Based Differences in Platelet Function and Platelet Reactivity to P2Y12 Inhibitors. PLoS ONE 2019, 14, e0225771. [Google Scholar] [CrossRef]
- Lordkipanidzé, M.; Lowe, G.C.; Kirkby, N.S.; Chan, M.V.; Lundberg, M.H.; Morgan, N.V.; Bem, D.; Nisar, S.P.; Leo, V.C.; Jones, M.L.; et al. Characterization of Multiple Platelet Activation Pathways in Patients with Bleeding as a High-Throughput Screening Option: Use of 96-Well Optimul Assay. Blood 2014, 123, e11–e22. [Google Scholar] [CrossRef]
- Leadbeater, P.D.M.; Kirkby, N.S.; Thomas, S.; Dhanji, A.-R.; TUCKER, A.T.; Milne, G.L.; Mitchell, J.A.; Warner, T.D. Aspirin Has Little Additional Anti-Platelet Effect in Healthy Volunteers Receiving Prasugrel. J. Thromb. Haemost. 2011, 9, 2050–2056. [Google Scholar] [CrossRef]
- Winter, M.; Schneeweiss, T.; Cremer, R.; Biesinger, B.; Hengstenberg, C.; Prüller, F.; Wallner, M.; Kolesnik, E.; von Lewinski, D.; Lang, I.M.; et al. Platelet Reactivity Patterns in Patients Treated with Dual Antiplatelet Therapy. Eur. J. Clin. Investig. 2019, 49, e13102. [Google Scholar] [CrossRef]
- Russo, I.; Traversa, M.; Bonomo, K.; De Salve, A.; Mattiello, L.; Del Mese, P.; Doronzo, G.; Cavalot, F.; Trovati, M.; Anfossi, G. In Central Obesity, Weight Loss Restores Platelet Sensitivity to Nitric Oxide and Prostacyclin. Obesity 2010, 18, 788–797. [Google Scholar] [CrossRef]
- Gliemann, L.; Hellsten, Y. The Exercise Timing Hypothesis: Can Exercise Training Compensate for the Reduction in Blood Vessel Function after Menopause If Timed Right? J. Physiol. 2019, 597, 4915–4925. [Google Scholar] [CrossRef] [PubMed]
- Gliemann, L.; Tamariz-Ellemann, A.; Baasch-Skytte, T.; Ehlers, T.S.; Gunnarsson, T.P. Increased Prostacyclin Formation after High-Intensity Interval Training in Late Postmenopausal Women. Eur. J. Appl. Physiol. 2020, 120, 1711–1720. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.H.; Nyberg, M.; Bangsbo, J.; Saltin, B.; Hellsten, Y. Exercise Training Alters the Balance between Vasoactive Compounds in Skeletal Muscle of Individuals with Essential Hypertension. Hypertension 2011, 58, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Gan, X.-D.; Wei, B.-Z.; Fang, D.; Fang, Q.; Li, K.-Y.; Ding, S.-L.-Y.; Peng, S.; Wan, J. Efficacy and Safety Analysis of New P2Y12 Inhibitors versus Clopidogrel in Patients with Percutaneous Coronary Intervention: A Meta-Analysis. Curr. Med. Res. Opin. 2015, 31, 2313–2323. [Google Scholar] [CrossRef]
- Cattaneo, M.; Lecchi, A. Inhibition of the Platelet P2Y12 Receptor for Adenosine Diphosphate Potentiates the Antiplatelet Effect of Prostacyclin. J. Thromb. Haemost. 2007, 5, 577–582. [Google Scholar] [CrossRef]
- Singla, A.; Bliden, K.P.; Jeong, Y.-H.; Abadilla, K.; Antonino, M.J.; Muse, W.C.; Mathew, D.P.; Bailon, O.; Tantry, U.S.; Gurbel, P.A. Platelet Reactivity and Thrombogenicity in Postmenopausal Women. Menopause 2013, 20, 57–63. [Google Scholar] [CrossRef]
- Medvedev, I.N.; Karpov, V.Y.; Eremin, M.V.; Boldov, A.S.; Shalupin, V.I.; Voronova, N.N.; Malyshev, A.V. The Functional Characteristics of the Organism of Physically Inactive Students Who Have Started Regular Physical Training. J. Biochem. Technol. 2021, 12, 33–37. [Google Scholar] [CrossRef]
- Madla, C.M.; Gavins, F.K.H.; Merchant, H.A.; Orlu, M.; Murdan, S.; Basit, A.W. Let’s Talk about Sex: Differences in Drug Therapy in Males and Females. Adv. Drug Deliv. Rev. 2021, 175, 113804. [Google Scholar] [CrossRef]
- Liu, K.A.; Mager, N.A.D. Women’s Involvement in Clinical Trials: Historical Perspective and Future Implications. Pharm. Pract. 2016, 14, 708. [Google Scholar] [CrossRef]



| Participant Characteristics | |
|---|---|
| Age (years) | 61 ± 5 |
| Years After Menopause | 10 ± 6 |
| Height (cm) | 166 ± 5 |
| Estradiol (nmol·L−1) | 0.09 ± 0.00 |
| Progesterone (IU·L−1) | 0.6 ± 0.0 |
| Testosterone (nmol·L−1) | 0.4 ± 0.1 |
| LH (IU·L−1) | 36.0 ± 15.2 |
| FSH (IU·L−1) | 67.2 ± 25.6 |
| Pre-Training | Post-Training | Statistical Significance | |
|---|---|---|---|
| Body Composition Parameters | |||
| Body Mass (kg) | 72.2 ± 9.0 | 72.2 ± 8.6 | p = 0.92 |
| Lean Body Mass (kg) | 40.9 ± 3.9 | 41.5 ± 3.5 | p = 0.01 * |
| Fat Mass (kg) | 28.8 ± 5.8 | 28.3 ± 5.7 | p = 0.16 |
| Lean Mass (%) | 60.2 ± 4.4 | 61.1 ± 4.1 | p = 0.02 * |
| Fat Mass (%) | 39.8 ± 4.4 | 38.9 ± 4.1 | p = 0.02 * |
| Hematological Parameters | |||
| Platelet Count (×103) | 180 ± 31 | 182 ± 28 | p = 0.76 |
| Red Blood Cells (×106) | 5.2 ± 0.9 | 5.1 ± 1.3 | p = 0.65 |
| White Blood Cells (×106) | 4.2 ± 0.3 | 4.4 ± 0.3 | p = 0.06 |
| Health and Fitness Parameters | |||
| V̇O2max (mL∙min−1) | 1895 ± 295 | 1956 ± 298 | p = 0.15 |
| V̇O2max (mL∙kg−1∙min−1) | 26.6 ± 5.0 | 27.4 ± 4.8 | p = 0.14 |
| Total Cholesterol (mmol·L−1) | 6.1 ± 0.7 | 5.7 ± 0.7 | p = 0.07 |
| Body Mass Index (kg∙m−2) | 26.0 ± 2.0 | 26.1 ± 2.0 | p = 0.74 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wickham, K.A.; Nørregaard, L.B.; Lundberg Slingsby, M.H.; Cheung, S.S.; Hellsten, Y. High-Intensity Exercise Training Improves Basal Platelet Prostacyclin Sensitivity and Potentiates the Response to Dual Anti-Platelet Therapy in Postmenopausal Women. Biomolecules 2022, 12, 1501. https://doi.org/10.3390/biom12101501
Wickham KA, Nørregaard LB, Lundberg Slingsby MH, Cheung SS, Hellsten Y. High-Intensity Exercise Training Improves Basal Platelet Prostacyclin Sensitivity and Potentiates the Response to Dual Anti-Platelet Therapy in Postmenopausal Women. Biomolecules. 2022; 12(10):1501. https://doi.org/10.3390/biom12101501
Chicago/Turabian StyleWickham, Kate A., Line B. Nørregaard, Martina H. Lundberg Slingsby, Stephen S. Cheung, and Ylva Hellsten. 2022. "High-Intensity Exercise Training Improves Basal Platelet Prostacyclin Sensitivity and Potentiates the Response to Dual Anti-Platelet Therapy in Postmenopausal Women" Biomolecules 12, no. 10: 1501. https://doi.org/10.3390/biom12101501
APA StyleWickham, K. A., Nørregaard, L. B., Lundberg Slingsby, M. H., Cheung, S. S., & Hellsten, Y. (2022). High-Intensity Exercise Training Improves Basal Platelet Prostacyclin Sensitivity and Potentiates the Response to Dual Anti-Platelet Therapy in Postmenopausal Women. Biomolecules, 12(10), 1501. https://doi.org/10.3390/biom12101501