Diverse and Composite Roles of miRNA in Non-Neuronal Cells and Neuronal Synapses in Alzheimer’s Disease
Abstract
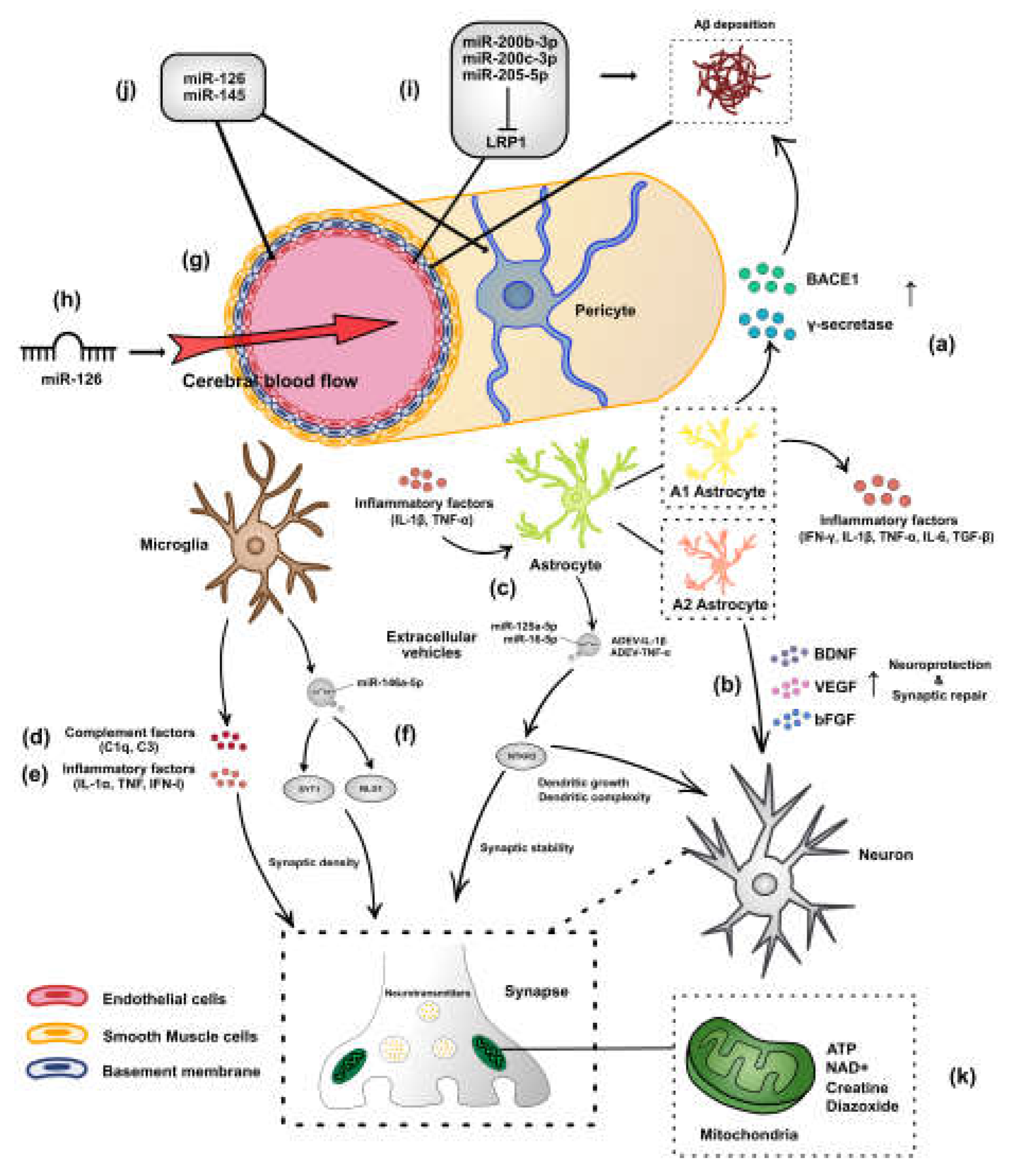
:1. Introduction
2. Role of miRNAs in Regulating Astrocytes and Microglia in AD
3. Role of miRNAs in Regulating the Cerebrovascular System in AD
4. miRNAs Are Associated with AD-Related Synaptic Impairment
4.1. miRNAs Involved in Aβ-Mediated AD Synaptic Dysfunction
4.2. Regulation of Synaptic Function by miRNA-Mediated Tauopathies
5. Mitochondria as a Potential Therapeutic Target for AD-Related Cognitive Disorders
6. miRNAs as Potential Therapeutics or Biomarkers of AD
7. Emerging Imaging Techniques for AD Research and Potential Applications in Diagnosis
7.1. Two-Photon Microscopy
7.2. Optical Coherence Tomography (OCT)
7.3. Surface Plasmon Resonance (SPR)
8. Outstanding Challenges for the Future
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutvágner, G.; Zamore, P.D. A MicroRNA in a Multiple-Turnover RNAi Enzyme Complex. Science 2002, 297, 2056–2060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, L.; Han, M. GW182 Family Proteins Are Crucial for MicroRNA-Mediated Gene Silencing. Trends Cell Biol. 2007, 17, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Target Recognition and Regulatory Functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elkayam, E.; Faehnle, C.R.; Morales, M.; Sun, J.; Li, H.; Joshua-Tor, L. Multivalent Recruitment of Human Argonaute by GW182. Mol. Cell 2017, 67, 646–658.e3. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhang, X.; Cai, Z.; Zhou, J.; Cao, R.; Zhao, Y.; Chen, Z.; Wang, D.; Ruan, W.; Zhao, Q.; et al. A Novel Class of MicroRNA-Recognition Elements That Function Only within Open Reading Frames. Nat. Struct. Mol. Biol. 2018, 25, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Xiao, M.S.; Li, Z.; Shan, G.; Huang, C. Defining an Evolutionarily Conserved Role of GW182 in Circular RNA Degradation. Cell Discov. 2019, 5, 45. [Google Scholar] [CrossRef] [Green Version]
- Oksanen, M.; Lehtonen, S.; Jaronen, M.; Goldsteins, G.; Hämäläinen, R.H.; Koistinaho, J. Astrocyte Alterations in Neurodegenerative Pathologies and Their Modeling in Human Induced Pluripotent Stem Cell Platforms. Cell. Mol. Life Sci. 2019, 76, 2739–2760. [Google Scholar] [CrossRef] [Green Version]
- Frost, G.R.; Li, Y.M. The Role of Astrocytes in Amyloid Production and Alzheimer’s Disease. Open Biol. 2017, 7, 170228. [Google Scholar] [CrossRef] [Green Version]
- Chiareli, R.A.; Carvalho, G.A.; Marques, B.L.; Mota, L.S.; Oliveira-Lima, O.C.; Gomes, R.M.; Birbrair, A.; Gomez, R.S.; Simão, F.; Klempin, F.; et al. The Role of Astrocytes in the Neurorepair Process. Front. Cell Dev. Biol. 2021, 9, 1304. [Google Scholar] [CrossRef]
- Gosselin, R.-D.; Meylan, P.; Decosterd, I. Extracellular Microvesicles from Astrocytes Contain Functional Glutamate Transporters: Regulation by Protein Kinase C and Cell Activation. Front. Cell. Neurosci. 2013, 7, 251. [Google Scholar] [CrossRef] [Green Version]
- Hajj, G.N.M.; Arantes, C.P.; Dias, M.V.S.; Roffé, M.; Costa-Silva, B.; Lopes, M.H.; Porto-Carreiro, I.; Rabachini, T.; Lima, F.R.; Beraldo, F.H.; et al. The Unconventional Secretion of Stress-Inducible Protein 1 by a Heterogeneous Population of Extracellular Vesicles. Cell. Mol. Life Sci. 2013, 70, 3211–3227. [Google Scholar] [CrossRef]
- Cocucci, E.; Meldolesi, J. Ectosomes and Exosomes: Shedding the Confusion between Extracellular Vesicles. Trends Cell Biol. 2015, 25, 364–372. [Google Scholar] [CrossRef]
- Luarte, A.; Henzi, R.; Fernández, A.; Gaete, D.; Cisternas, P.; Pizarro, M.; Batiz, L.F.; Villalobos, I.; Masalleras, M.; Vergara, R.; et al. Astrocyte-Derived Small Extracellular Vesicles Regulate Dendritic Complexity through MiR-26a-5p Activity. Cells 2020, 9, 930. [Google Scholar] [CrossRef] [Green Version]
- Chaudhuri, A.D.; Dastgheyb, R.M.; Yoo, S.W.; Trout, A.; Talbot, C.C.; Hao, H.; Witwer, K.W.; Haughey, N.J. TNFα and IL-1β Modify the MiRNA Cargo of Astrocyte Shed Extracellular Vesicles to Regulate Neurotrophic Signaling in Neurons. Cell Death Dis. 2018, 9, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Eikelenboom, P.; Veerhuis, R. The Role of Complement and Activated Microglia in the Pathogenesis of Alzheimer’s Disease. Neurobiol. Aging 1996, 17, 673–680. [Google Scholar] [CrossRef]
- Leng, F.; Edison, P. Neuroinflammation and Microglial Activation in Alzheimer Disease: Where Do We Go from Here? Nat. Rev. Neurol. 2020, 17, 157–172. [Google Scholar] [CrossRef]
- Schafer, D.P.; Lehrman, E.K.; Kautzman, A.G.; Koyama, R.; Mardinly, A.R.; Yamasaki, R.; Ransohoff, R.M.; Greenberg, M.E.; Barres, B.A.; Stevens, B. Microglia Sculpt Postnatal Neural Circuits in an Activity and Complement-Dependent Manner. Neuron 2012, 74, 691–705. [Google Scholar] [CrossRef] [Green Version]
- Reichwald, J.; Danner, S.; Wiederhold, K.H.; Staufenbiel, M. Expression of Complement System Components during Aging and Amyloid Deposition in APP Transgenic Mice. J. Neuroinflamm. 2009, 6, 35. [Google Scholar] [CrossRef] [Green Version]
- Peng, L.; Bestard-Lorigados, I.; Song, W. The Synapse as a Treatment Avenue for Alzheimer’s Disease. Mol. Psychiatry 2022, 27, 2940–2949. [Google Scholar] [CrossRef]
- Ye, Y.; Xu, H.; Su, X.; He, X. Role of MicroRNA in Governing Synaptic Plasticity. Neural Plast. 2016, 2016, 4959523. [Google Scholar] [CrossRef] [Green Version]
- Shi, Q.; Chowdhury, S.; Ma, R.; Le, K.X.; Hong, S.; Caldarone, B.J.; Stevens, B.; Lemere, C.A. Complement C3 Deficiency Protects against Neurodegeneration in Aged Plaque-Rich APP/PS1 Mice. Sci. Transl. Med. 2017, 9, eaaf6295. [Google Scholar] [CrossRef] [Green Version]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.S.; Peterson, T.C.; et al. Neurotoxic Reactive Astrocytes Are Induced by Activated Microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef] [Green Version]
- Lall, D.; Lorenzini, I.; Mota, T.A.; Bell, S.; Mahan, T.E.; Ulrich, J.D.; Davtyan, H.; Rexach, J.E.; Muhammad, A.K.M.G.; Shelest, O.; et al. C9orf72 Deficiency Promotes Microglial-Mediated Synaptic Loss in Aging and Amyloid Accumulation. Neuron 2021, 109, 2275–2291.e8. [Google Scholar] [CrossRef]
- Shi, Y.; Holtzman, D.M. Interplay between Innate Immunity and Alzheimer Disease: APOE and TREM2 in the Spotlight. Nat. Rev. Immunol. 2018, 18, 759–772. [Google Scholar] [CrossRef]
- Prada, I.; Gabrielli, M.; Turola, E.; Iorio, A.; D’Arrigo, G.; Parolisi, R.; de Luca, M.; Pacifici, M.; Bastoni, M.; Lombardi, M.; et al. Glia-to-Neuron Transfer of MiRNAs via Extracellular Vesicles: A New Mechanism Underlying Inflammation-Induced Synaptic Alterations. Acta Neuropathol. 2018, 135, 529–550. [Google Scholar] [CrossRef] [Green Version]
- Liang, C.; Zou, T.; Zhang, M.; Fan, W.; Zhang, T.; Jiang, Y.; Cai, Y.; Chen, F.; Chen, X.; Sun, Y.; et al. MicroRNA-146a Switches Microglial Phenotypes to Resist the Pathological Processes and Cognitive Degradation of Alzheimer’s Disease. Theranostics 2021, 11, 4103. [Google Scholar] [CrossRef]
- Love, S.; Miners, J.S. Cerebrovascular Disease in Ageing and Alzheimer’s Disease. Acta Neuropathol. 2015, 131, 645–658. [Google Scholar] [CrossRef] [Green Version]
- Schilling, S.; DeStefano, A.L.; Sachdev, P.S.; Choi, S.H.; Mather, K.A.; DeCarli, C.D.; Wen, W.; Høgh, P.; Raz, N.; Au, R.; et al. APOE Genotype and MRI Markers of Cerebrovascular Disease. Neurology 2013, 81, 292–300. [Google Scholar] [CrossRef] [Green Version]
- Meng, X.F.; Yu, J.T.; Wang, H.F.; Tan, M.S.; Wang, C.; Tan, C.C.; Tan, L. Midlife Vascular Risk Factors and the Risk of Alzheimer’s Disease: A Systematic Review and Meta-Analysis. J. Alzheimer’s Dis. 2014, 42, 1295–1310. [Google Scholar] [CrossRef]
- Chuang, Y.F.; An, Y.; Bilgel, M.; Wong, D.F.; Troncoso, J.C.; O’Brien, R.J.; Breitner, J.C.; Ferruci, L.; Resnick, S.M.; Thambisetty, M. Midlife Adiposity Predicts Earlier Onset of Alzheimer’s Dementia, Neuropathology and Presymptomatic Cerebral Amyloid Accumulation. Mol. Psychiatry 2015, 21, 910–915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Wang, Y.J.; Zhang, M.; Xu, Z.Q.; Gao, C.Y.; Fang, C.Q.; Yan, J.C.; Zhou, H.D. Vascular Risk Factors Promote Conversion from Mild Cognitive Impairment to Alzheimer Disease. Neurology 2011, 76, 1485–1491. [Google Scholar] [CrossRef] [PubMed]
- De Bruijn, R.F.A.G.; Ikram, M.A. Cardiovascular Risk Factors and Future Risk of Alzheimer’s Disease. BMC Med. 2014, 12, 130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mentis, A.F.A.; Dardiotis, E.; Chrousos, G.P. Apolipoprotein E4 and Meningeal Lymphatics in Alzheimer Disease: A Conceptual Framework. Mol. Psychiatry 2020, 26, 1075–1097. [Google Scholar] [CrossRef]
- Rasmussen, M.K.; Mestre, H.; Nedergaard, M. The Glymphatic Pathway in Neurological Disorders. Lancet Neurol. 2018, 17, 1016–1024. [Google Scholar] [CrossRef] [Green Version]
- Zhang, E.T.; Richards, H.K.; Kida, S.; Weller, R.O. Directional and Compartmentalised Drainage of Interstitial Fluid and Cerebrospinal Fluid from the Rat Brain. Acta Neuropathol. 1992, 83, 233–239. [Google Scholar] [CrossRef]
- Weller, R.O.; Massey, A.; Newman, T.A.; Hutchings, M.; Kuo, Y.M.; Roher, A.E. Cerebral Amyloid Angiopathy: Amyloid β Accumulates in Putative Interstitial Fluid Drainage Pathways in Alzheimer’s Disease. Am. J. Pathol. 1998, 153, 725–733. [Google Scholar] [CrossRef]
- Gate, D.; Saligrama, N.; Leventhal, O.; Yang, A.C.; Unger, M.S.; Middeldorp, J.; Chen, K.; Lehallier, B.; Channappa, D.; de Los Santos, M.B.; et al. Clonally Expanded CD8 T Cells Patrol the Cerebrospinal Fluid in Alzheimer’s Disease. Nature 2020, 577, 399–404. [Google Scholar] [CrossRef]
- Iadecola, C.; Gottesman, R.F. Cerebrovascular Alterations in Alzheimer Disease Incidental or Pathogenic? Circ. Res. 2018, 123, 406–408. [Google Scholar] [CrossRef]
- Safaiyan, S.; Kannaiyan, N.; Snaidero, N.; Brioschi, S.; Biber, K.; Yona, S.; Edinger, A.L.; Jung, S.; Rossner, M.J.; Simons, M. Age-Related Myelin Degradation Burdens the Clearance Function of Microglia during Aging. Nat. Neurosci. 2016, 19, 995–998. [Google Scholar] [CrossRef]
- Yang, A.C.; Vest, R.T.; Kern, F.; Lee, D.P.; Agam, M.; Maat, C.A.; Losada, P.M.; Chen, M.B.; Schaum, N.; Khoury, N.; et al. A Human Brain Vascular Atlas Reveals Diverse Mediators of Alzheimer’s Risk. Nature 2022, 603, 885–892. [Google Scholar] [CrossRef]
- Gustavsson, A.M.; van Westen, D.; Stomrud, E.; Engström, G.; Nägga, K.; Hansson, O. Midlife Atherosclerosis and Development of Alzheimer or Vascular Dementia. Ann. Neurol. 2020, 87, 52–62. [Google Scholar] [CrossRef] [Green Version]
- Yu, P.; Venkat, P.; Chopp, M.; Zacharek, A.; Shen, Y.; Ning, R.; Liang, L.; Li, W.; Zhang, L.; Landschoot-Ward, J.; et al. Role of MicroRNA-126 in Vascular Cognitive Impairment in Mice. J. Cereb. Blood Flow Metab. 2019, 39, 2497–2511. [Google Scholar] [CrossRef]
- Zheng, H.Z.; Jiang, W.; Zhao, X.F.; Du, J.; Liu, P.G.; Chang, L.D.; Li, W.B.; Hu, H.T.; Shi, X.M. Electroacupuncture Induces Acute Changes in Cerebral Cortical MiRNA Profile, Improves Cerebral Blood Flow and Alleviates Neurological Deficits in a Rat Model of Stroke. Neural Regen. Res. 2016, 11, 1940. [Google Scholar] [CrossRef]
- Chum, P.P.; Hakim, M.A.; Behringer, E.J. Cerebrovascular MicroRNA Expression Profile During Early Development of Alzheimer’s Disease in a Mouse Model. J. Alzheimer’s Dis. 2022, 85, 91–113. [Google Scholar] [CrossRef]
- Hsu, H.W.; Rodriguez-Ortiz, C.J.; Lim, S.L.; Zumkehr, J.; Kilian, J.G.; Vidal, J.; Kitazawa, M. Copper-Induced Upregulation of MicroRNAs Directs the Suppression of Endothelial LRP1 in Alzheimer’s Disease Model. Toxicol. Sci. 2019, 170, 144–156. [Google Scholar] [CrossRef]
- Storck, S.E.; Meister, S.; Nahrath, J.; Meißner, J.N.; Schubert, N.; di Spiezio, A.; Baches, S.; Vandenbroucke, R.E.; Bouter, Y.; Prikulis, I.; et al. Endothelial LRP1 Transports Amyloid-Β1–42 across the Blood–brain barrier. J. Clin. Investig. 2016, 126, 123–136. [Google Scholar] [CrossRef] [Green Version]
- Deane, R.; Wu, Z.; Zlokovic, B.V. RAGE (Yin) versus LRP (Yang) Balance Regulates Alzheimer Amyloid β-Peptide Clearance through Transport across the Blood–brain barrier. Stroke 2004, 35, 2628–2631. [Google Scholar] [CrossRef] [Green Version]
- Bell, R.D.; Sagare, A.P.; Friedman, A.E.; Bedi, G.S.; Holtzman, D.M.; Deane, R.; Zlokovic, B.V. Transport Pathways for Clearance of Human Alzheimer’s Amyloid β-Peptide and Apolipoproteins E and J in the Mouse Central Nervous System. J. Cereb. Blood Flow Metab. 2007, 27, 909–918. [Google Scholar] [CrossRef] [Green Version]
- Fu, L.; Jiang, G.; Weng, H.; Dick, G.M.; Chang, Y.; Kassab, G.S. Cerebrovascular MiRNAs Correlate with the Clearance of Aβ through Perivascular Route in Younger 3xTg-AD Mice. Brain Pathol. 2020, 30, 92–105. [Google Scholar] [CrossRef]
- Rodriguez-Ortiz, C.J.; Prieto, G.A.; Martini, A.C.; Forner, S.; Trujillo-Estrada, L.; LaFerla, F.M.; Baglietto-Vargas, D.; Cotman, C.W.; Kitazawa, M. MiR-181a Negatively Modulates Synaptic Plasticity in Hippocampal Cultures and Its Inhibition Rescues Memory Deficits in a Mouse Model of Alzheimer’s Disease. Aging Cell 2020, 19, e13118. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.F.; Jing, X.; Ma, H.X.; Yuan, R.R.; Dong, Q.; Dong, J.L.; Han, X.F.; Chen, Z.Y.; Li, X.Z.; Wang, Y. MiR-181a Participates in Contextual Fear Memory Formation Via Activating MTOR Signaling Pathway. Cerebral Cortex 2018, 28, 3309–3321. [Google Scholar] [CrossRef] [Green Version]
- Southam, K.A.; Stennard, F.; Pavez, C.; Small, D.H. Knockout of Amyloid β Protein Precursor (APP) Expression Alters Synaptogenesis, Neurite Branching and Axonal Morphology of Hippocampal Neurons. Neurochem. Res. 2019, 44, 1346–1355. [Google Scholar] [CrossRef]
- Wang, R.; Chopra, N.; Nho, K.; Maloney, B.; Obukhov, A.G.; Nelson, P.T.; Counts, S.E.; Lahiri, D.K. Human MicroRNA (MiR-20b-5p) Modulates Alzheimer’s Disease Pathways and Neuronal Function, and a Specific Polymorphism Close to the MIR20B Gene Influences Alzheimer’s Biomarkers. Mol. Psychiatry 2022, 27, 1256–1273. [Google Scholar] [CrossRef]
- Wu, Q.; Yuan, X.; Bai, J.; Han, R.; Li, Z.; Zhang, H.; Xiu, R. MicroRNA-181a Protects against Pericyte Apoptosis via Directly Targeting FOXO1: Implication for Ameliorated Cognitive Deficits in APP/PS1 Mice. Aging 2019, 11, 6120. [Google Scholar] [CrossRef]
- Liu, X.; Feng, Z.; Du, L.; Huang, Y.; Ge, J.; Deng, Y.; Mei, Z. The Potential Role of MicroRNA-124 in Cerebral Ischemia Injury. Int. J. Mol. Sci. 2019, 21, 120. [Google Scholar] [CrossRef] [Green Version]
- Sun, M.; Hou, X.; Ren, G.; Zhang, Y.; Cheng, H. Dynamic Changes in MiR-124 Levels in Patients with Acute Cerebral Infarction. Int. J. Neurosci. 2019, 129, 649–653. [Google Scholar] [CrossRef]
- Chen, S.-H.; Sun, H.; Zhang, Y.M.; Xu, H.; Yang, Y.; Wang, F.M. Effects of Acupuncture at Baihui (GV 20) and Zusanli (ST 36) on Peripheral Serum Expression of MicroRNA 124, Laminin and Integrin Β1 in Rats with Cerebral Ischemia Reperfusion Injury. Chin. J. Integr. Med. 2015, 22, 49–55. [Google Scholar] [CrossRef]
- Doeppner, T.R.; Doehring, M.; Bretschneider, E.; Zechariah, A.; Kaltwasser, B.; Müller, B.; Koch, J.C.; Bähr, M.; Hermann, D.M.; Michel, U. MicroRNA-124 Protects against Focal Cerebral Ischemia via Mechanisms Involving Usp14-Dependent REST Degradation. Acta Neuropathol. 2013, 126, 251–265. [Google Scholar] [CrossRef]
- Saraiva, C.; Talhada, D.; Rai, A.; Ferreira, R.; Ferreira, L.; Bernardino, L.; Ruscher, K. MicroRNA-124-Loaded Nanoparticles Increase Survival and Neuronal Differentiation of Neural Stem Cells in Vitro but Do Not Contribute to Stroke Outcome in Vivo. PLoS ONE 2018, 13, e0193609. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, J.; Shao, Y.; Wan, D. Catalpol May Improve Axonal Growth via Regulating MiR-124 Regulated PI3K/AKT/MTOR Pathway in Neurons after Ischemia. Ann. Transl. Med. 2019, 7, 306. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, D.; Huang, H.Z.; Wang, Z.H.; Hou, T.Y.; Yang, X.; Pang, P.; Wei, N.; Zhou, Y.F.; Dupras, M.J.; et al. A Novel MicroRNA-124/PTPN1 Signal Pathway Mediates Synaptic and Memory Deficits in Alzheimer’s Disease. Biol. Psychiatry 2018, 83, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.N.; Li, W.F.; Li, F.; Zhang, Z.; Dai, Y.D.; Xu, A.L.; Qi, C.; Gao, J.M.; Gao, J. Resveratrol Improves Learning and Memory in Normally Aged Mice through MicroRNA-CREB Pathway. Biochem. Biophys. Res. Commun. 2013, 435, 597–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, K.; Hu, F.; Zhou, Y.; Zhang, J.; Zheng, J.; Lai, C.; Xiong, W.; Cui, K.; Hu, Y.Z.; Han, Z.T.; et al. MiR-135a-5p Mediates Memory and Synaptic Impairments via the Rock2/Adducin1 Signaling Pathway in a Mouse Model of Alzheimer’s Disease. Nat. Commun. 2021, 12, 1903. [Google Scholar] [CrossRef]
- Lu, Y.; Tan, L.; Wang, X. Circular HDAC9/MicroRNA-138/Sirtuin-1 Pathway Mediates Synaptic and Amyloid Precursor Protein Processing Deficits in Alzheimer’s Disease. Neurosci. Bull. 2019, 35, 877–888. [Google Scholar] [CrossRef]
- Wingo, T.S.; Yang, J.; Fan, W.; Min Canon, S.; Gerasimov, E.S.; Lori, A.; Logsdon, B.; Yao, B.; Seyfried, N.T.; Lah, J.J.; et al. Brain MicroRNAs Associated with Late-Life Depressive Symptoms Are Additionally, Associated with Cognitive Trajectory and Dementia. NPJ Genom. Med. 2020, 5, 6. [Google Scholar] [CrossRef] [Green Version]
- Hosseinian, S.; Arefian, E.; Rakhsh-Khorshid, H.; Eivani, M.; Rezayof, A.; Pezeshk, H.; Marashi, S.A. A Meta-Analysis of Gene Expression Data Highlights Synaptic Dysfunction in the Hippocampus of Brains with Alzheimer’s Disease. Sci. Rep. 2020, 10, 8384. [Google Scholar] [CrossRef]
- Wei, Z.; Meng, X.; el Fatimy, R.; Sun, B.; Mai, D.; Zhang, J.; Arora, R.; Zeng, A.; Xu, P.; Qu, S.; et al. Environmental Enrichment Prevents Aβ Oligomer-Induced Synaptic Dysfunction through Mirna-132 and Hdac3 Signaling Pathways. Neurobiol. Dis 2020, 134, 104617. [Google Scholar] [CrossRef]
- Shi, L.; Zhang, R.; Li, T.; Han, X.; Yuan, N.; Jiang, L.; Zhou, H.; Xu, S. Decreased MiR-132 Plays a Crucial Role in Diabetic Encephalopathy by Regulating the GSK-3β/Tau Pathway. Aging 2021, 13, 4590. [Google Scholar] [CrossRef]
- Min, S.W.; Sohn, P.D.; Li, Y.; Devidze, N.; Johnson, J.R.; Krogan, N.J.; Masliah, E.; Mok, S.A.; Gestwicki, J.E.; Gan, L. SIRT1 Deacetylates Tau and Reduces Pathogenic Tau Spread in a Mouse Model of Tauopathy. J. Neurosci. 2018, 38, 3680–3688. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Hu, M.; Zhang, J.; Teng, Z.Q.; Chen, C. A Novel Mechanism of Synaptic and Cognitive Impairments Mediated via MicroRNA-30b in Alzheimer’s Disease. EBioMedicine 2019, 39, 409–421. [Google Scholar] [CrossRef] [Green Version]
- Shi, Z.; Zhang, K.; Zhou, H.; Jiang, L.; Xie, B.; Wang, R.; Xia, W.; Yin, Y.; Gao, Z.; Cui, D.; et al. Increased MiR-34c Mediates Synaptic Deficits by Targeting Synaptotagmin 1 through ROS-JNK-P53 Pathway in Alzheimer’s Disease. Aging Cell 2020, 19, e13125. [Google Scholar] [CrossRef] [Green Version]
- Penzes, P.; Cahill, M.E.; Jones, K.A.; Vanleeuwen, J.E.; Woolfrey, K.M. Dendritic Spine Pathology in Neuropsychiatric Disorders. Nat. Neurosci. 2011, 14, 285–293. [Google Scholar] [CrossRef] [Green Version]
- Scheff, S.W.; Price, D.A.; Schmitt, F.A.; Dekosky, S.T.; Mufson, E.J. Synaptic Alterations in CA1 in Mild Alzheimer Disease and Mild Cognitive Impairment. Neurology 2007, 68, 1501–1508. [Google Scholar] [CrossRef]
- Mecca, A.P.; O’Dell, R.S.; Sharp, E.S.; Banks, E.R.; Bartlett, H.H.; Zhao, W.; Lipior, S.; Diepenbrock, N.G.; Chen, M.K.; Naganawa, M.; et al. Synaptic Density and Cognitive Performance in Alzheimer’s Disease: A PET Imaging Study with [11C]UCB-J. Alzheimer’s Dement. 2021, 1–10. [Google Scholar] [CrossRef]
- Harris, K.M.; Jensen, F.E.; Tsao, B. Three-Dimensional Structure of Dendritic Spines and Synapses in Rat Hippocampus (CA1) at Postnatal Day 15 and Adult Ages: Implications for the Maturation of Synaptic Physiology and Long-Term Potentiation [Published Erratum Appears in J Neurosci 1992 Aug;12(8):Following Table of Contents]. J. Neurosci. 1992, 12, 2685–2705. [Google Scholar] [CrossRef] [Green Version]
- Androuin, A.; Potier, B.; Nägerl, U.V.; Cattaert, D.; Danglot, L.; Thierry, M.; Youssef, I.; Triller, A.; Duyckaerts, C.; el Hachimi, K.H.; et al. Evidence for Altered Dendritic Spine Compartmentalization in Alzheimer’s Disease and Functional Effects in a Mouse Model. Acta Neuropathol. 2018, 135, 839–854. [Google Scholar] [CrossRef]
- Sarkar, S.; Jun, S.; Rellick, S.; Quintana, D.D.; Cavendish, J.Z.; Simpkins, J.W. Expression of MicroRNA-34a in Alzheimer’s Disease Brain Targets Genes Linked to Synaptic Plasticity, Energy Metabolism, and Resting State Network Activity. Brain Res. 2016, 1646, 139–151. [Google Scholar] [CrossRef] [Green Version]
- Bazrgar, M.; Khodabakhsh, P.; Prudencio, M.; Mohagheghi, F.; Ahmadiani, A. The Role of MicroRNA-34 Family in Alzheimer’s Disease: A Potential Molecular Link between Neurodegeneration and Metabolic Disorders. Pharmacol. Res. 2021, 172, 105805. [Google Scholar] [CrossRef]
- Moradifard, S.; Hoseinbeyki, M.; Ganji, S.M.; Minuchehr, Z. Analysis of MicroRNA and Gene Expression Profiles in Alzheimer’s Disease: A Meta-Analysis Approach. Sci. Rep. 2018, 8, 4767. [Google Scholar] [CrossRef]
- Salta, E.; Sierksma, A.; Eynden, E.V.; de Strooper, B. MiR-132 Loss de-Represses ITPKB and Aggravates Amyloid and TAU Pathology in Alzheimer’s Brain. EMBO Mol. Med. 2016, 8, 1005–1018. [Google Scholar] [CrossRef]
- Shaked, I.; Meerson, A.; Wolf, Y.; Avni, R.; Greenberg, D.; Gilboa-Geffen, A.; Soreq, H. MicroRNA-132 Potentiates Cholinergic Anti-Inflammatory Signaling by Targeting Acetylcholinesterase. Immunity 2009, 31, 965–973. [Google Scholar] [CrossRef] [Green Version]
- O’Neill, L.A.J. Boosting the Brain’s Ability to Block Inflammation via MicroRNA-132. Immunity 2009, 31, 854–855. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Chen, P.; Wang, X.; Yao, J.; Zhuang, S. MiR-34a Deficiency in APP/PS1 Mice Promotes Cognitive Function by Increasing Synaptic Plasticity via AMPA and NMDA Receptors. Neurosci. Lett. 2018, 670, 94–104. [Google Scholar] [CrossRef]
- Ghetti, B.; Oblak, A.L.; Boeve, B.F.; Johnson, K.A.; Dickerson, B.C.; Goedert, M. Invited Review: Frontotemporal Dementia Caused by Microtubule-Associated Protein Tau Gene (MAPT) Mutations: A Chameleon for Neuropathology and Neuroimaging. Neuropathol. Appl. Neurobiol. 2015, 41, 24–46. [Google Scholar] [CrossRef] [Green Version]
- Guo, T.; Noble, W.; Hanger, D.P. Roles of Tau Protein in Health and Disease. Acta Neuropathol. 2017, 133, 665–704. [Google Scholar] [CrossRef] [Green Version]
- Tapia-Rojas, C.; Cabezas-Opazo, F.; Deaton, C.A.; Vergara, E.H.; Johnson, G.V.W.; Quintanilla, R.A. It’s All about Tau. Prog. Neurobiol. 2019, 175, 54–76. [Google Scholar] [CrossRef]
- Shin, R.W.; Iwaki, T.; Kitamoto, T.; Sato, Y.; Tateishi, J. Massive Accumulation of Modified Tau and Severe Depletion of Normal Tau Characterize the Cerebral Cortex and White Matter of Alzheimer’s Disease. Demonstration Using the Hydrated Autoclaving Method. Am. J. Pathol. 1992, 140, 937. [Google Scholar]
- Dugger, B.N.; Dickson, D.W. Pathology of Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2017, 9, a028035. [Google Scholar] [CrossRef] [Green Version]
- Bilen, J.; Liu, N.; Burnett, B.G.; Pittman, R.N.; Bonini, N.M. MicroRNA Pathways Modulate Polyglutamine-Induced Neurodegeneration. Mol. Cell 2006, 24, 157–163. [Google Scholar] [CrossRef]
- Smith, P.Y.; Delay, C.; Girard, J.; Papon, M.A.L.; Planel, E.; Sergeant, N.; Buée, L.; Hébert, S.S. MicroRNA-132 Loss Is Associated with Tau Exon 10 Inclusion in Progressive Supranuclear Palsy. Hum. Mol. Genet. 2011, 20, 4016–4024. [Google Scholar] [CrossRef] [PubMed]
- Bazrgar, M.; Khodabakhsh, P.; Mohagheghi, F.; Prudencio, M.; Ahmadiani, A. Brain MicroRNAs Dysregulation: Implication for Missplicing and Abnormal Post-Translational Modifications of Tau Protein in Alzheimer’s Disease and Related Tauopathies. Pharmacol. Res. 2020, 155, 104729. [Google Scholar] [CrossRef] [PubMed]
- El Fatimy, R.; Li, S.; Chen, Z.; Mushannen, T.; Gongala, S.; Wei, Z.; Balu, D.T.; Rabinovsky, R.; Cantlon, A.; Elkhal, A.; et al. MicroRNA-132 Provides Neuroprotection for Tauopathies via Multiple Signaling Pathways. Acta Neuropathol. 2018, 136, 537–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Bian, Z. Alzheimer’s Disease and MicroRNA-132: A Widespread Pathological Factor and Potential Therapeutic Target. Front. Neurosci. 2021, 15, 687973. [Google Scholar] [CrossRef]
- Wishart, T.M.; Rooney, T.M.; Lamont, D.J.; Wright, A.K.; Morton, A.J.; Jackson, M.; Freeman, M.R.; Gillingwater, T.H. Combining Comparative Proteomics and Molecular Genetics Uncovers Regulators of Synaptic and Axonal Stability and Degeneration In Vivo. PLoS Genet. 2012, 8, e1002936. [Google Scholar] [CrossRef]
- Zhou, Z.; Meng, Y.; Asrar, S.; Todorovski, Z.; Jia, Z. A Critical Role of Rho-Kinase ROCK2 in the Regulation of Spine and Synaptic Function. Neuropharmacology 2009, 56, 81–89. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Wang, S.; Que, R.; Zhao, W.; An, L. Exploration of the Molecular Mechanism for Lipoprotein Lipase Expression Variations in SH-SY5Y Cells Exposed to Different Doses of Amyloid-Beta Protein. Front. Aging Neurosci. 2020, 12, 132. [Google Scholar] [CrossRef]
- Liu, D.; Tang, H.; Li, X.Y.; Deng, M.F.; Wei, N.; Wang, X.; Zhou, Y.F.; Wang, D.Q.; Fu, P.; Wang, J.Z.; et al. Targeting the HDAC2/HNF-4A/MiR-101b/AMPK Pathway Rescues Tauopathy and Dendritic Abnormalities in Alzheimer’s Disease. Mol. Ther. 2017, 25, 752–764. [Google Scholar] [CrossRef] [Green Version]
- Jeong, H.; Shin, S.; Lee, J.S.; Lee, S.H.; Baik, J.H.; Lim, S.; Kim, Y.K. Pan-HDAC Inhibitors Promote Tau Aggregation by Increasing the Level of Acetylated Tau. Int. J. Mol. Sci. 2019, 20, 4283. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Li, N.; Shi, F.X.; Xu, W.Q.; Cao, Y.; Lei, Y.; Wang, J.Z.; Tian, Q.; Zhou, X.W. Upregulation of AMPK Ameliorates Alzheimer’s Disease-Like Tau Pathology and Memory Impairment. Mol. Neurobiol. 2020, 57, 3349–3361. [Google Scholar] [CrossRef]
- Radhakrishnan, B.; Alwin Prem Anand, A. Role of MiRNA-9 in Brain Development. J. Exp. Neurosci. 2016, 10, 101–120. [Google Scholar] [CrossRef]
- Giusti, S.A.; Vogl, A.M.; Brockmann, M.M.; Vercelli, C.A.; Rein, M.L.; Trümbach, D.; Wurst, W.; Cazalla, D.; Stein, V.; Deussing, J.M.; et al. MicroRNA-9 Controls Dendritic Development by Targeting REST. Elife 2014, 3, e02755. [Google Scholar] [CrossRef]
- Elibol, B.; Kilic, U. High Levels of SIRT1 Expression as a Protective Mechanism against Disease-Related Conditions. Front. Endocrinol. 2018, 9, 614. [Google Scholar] [CrossRef]
- Subramanian, M.; Hyeon, S.J.; Das, T.; Suh, Y.S.; Kim, Y.K.; Lee, J.S.; Song, E.J.; Ryu, H.; Yu, K. UBE4B, a MicroRNA-9 Target Gene, Promotes Autophagy-Mediated Tau Degradation. Nat. Commun. 2021, 12, 1–15. [Google Scholar] [CrossRef]
- Boscher, E.; Goupil, C.; Petry, S.; Keraudren, R.; Loiselle, A.; Planel, E.; Hébert, S.S. MicroRNA-138 Overexpression Alters Aβ42 Levels and Behavior in Wildtype Mice. Front. Neurosci. 2021, 14, 1445. [Google Scholar] [CrossRef]
- Kann, O.; Kovács, R. Mitochondria and Neuronal Activity. Am. J. Physiol. Cell Physiol. 2007, 292, 641–657. [Google Scholar] [CrossRef] [Green Version]
- LaManna, J.C.; Harik, S.I. Regional Comparisons of Brain Glucose Influx. Brain Res. 1985, 326, 299–305. [Google Scholar] [CrossRef]
- Kapogiannis, D.; Mattson, M.P. Disrupted Energy Metabolism and Neuronal Circuit Dysfunction in Cognitive Impairment and Alzheimer’s Disease. Lancet Neurol. 2011, 10, 187–198. [Google Scholar] [CrossRef] [Green Version]
- Goldberg, J.; Currais, A.; Prior, M.; Fischer, W.; Chiruta, C.; Ratliff, E.; Daugherty, D.; Dargusch, R.; Finley, K.; Esparza-Moltó, P.B.; et al. The Mitochondrial ATP Synthase Is a Shared Drug Target for Aging and Dementia. Aging Cell 2018, 17, e12715. [Google Scholar] [CrossRef]
- Fang, E.F. Mitophagy and NAD+ Inhibit Alzheimer Disease. Autophagy 2019, 15, 1112–1114. [Google Scholar] [CrossRef] [Green Version]
- Marques, E.P.; Wyse, A.T.S. Creatine as a Neuroprotector: An Actor That Can Play Many Parts. Neurotox. Res. 2019, 36, 411–423. [Google Scholar] [CrossRef]
- Guan, L.; Ji, Y.Q.; Liu, J.; Kong, M.; Sun, Z.W.; Shen, X.Q.; Ren, C.; Yu, G.P.; Ba, M.W. Diazoxide Induces Endoplasmic Reticulum Stress-Related Neuroprotection Mediated by P38 MAPK against Aβ25-35 Insults. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 6133–6138. [Google Scholar] [CrossRef]
- Pickett, E.K.; Rose, J.; McCrory, C.; McKenzie, C.A.; King, D.; Smith, C.; Gillingwater, T.H.; Henstridge, C.M.; Spires-Jones, T.L. Region-Specific Depletion of Synaptic Mitochondria in the Brains of Patients with Alzheimer’s Disease. Acta Neuropathol. 2018, 136, 747–757. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Hemachandra Reddy, P. A New Discovery of MicroRNA-455-3p in Alzheimer’s Disease. J. Alzheimer’s Dis. 2019, 72, S117–S130. [Google Scholar] [CrossRef]
- Gowda, P.; Reddy, P.H.; Kumar, S. Deregulated Mitochondrial MicroRNAs in Alzheimer’s Disease: Focus on Synapse and Mitochondria. Ageing Res. Rev. 2022, 73, 101529. [Google Scholar] [CrossRef]
- Adams, D.; Gonzalez-Duarte, A.; O’Riordan, W.D.; Yang, C.-C.; Ueda, M.; Kristen, A.V.; Tournev, I.; Schmidt, H.H.; Coelho, T.; Berk, J.L.; et al. Patisiran, an RNAi Therapeutic, for Hereditary Transthyretin Amyloidosis. N. Engl. J. Med. 2018, 379, 11–21. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, D.Y.; Huang, L. In Vivo Delivery of MiRNAs for Cancer Therapy: Challenges and Strategies. Adv. Drug Deliv. Rev. 2015, 81, 128–141. [Google Scholar] [CrossRef] [Green Version]
- De Almeida, S.S.T.; Horst, C.H.; Soto-Sánchez, C.; Fernandez, E.; de Almeida, R.T. Delivery of MiRNA-Targeted Oligonucleotides in the Rat Striatum by Magnetofection with Neuromag®. Molecules 2018, 23, 1825. [Google Scholar] [CrossRef] [Green Version]
- Lim, L.P.; Lau, N.C.; Garrett-Engele, P.; Grimson, A.; Schelter, J.M.; Castle, J.; Bartel, D.P.; Linsley, P.S.; Johnson, J.M. Microarray Analysis Shows That Some MicroRNAs Downregulate Large Numbers of Target MRNAs. Nature 2005, 433, 769–773. [Google Scholar] [CrossRef]
- Liufu, Z.; Zhao, Y.; Guo, L.; Miao, G.; Xiao, J.; Lyu, Y.; Chen, Y.; Shi, S.; Tang, T.; Wu, C.I. Redundant and Incoherent Regulations of Multiple Phenotypes Suggest MicroRNAs’ Role in Stability Control. Genome Res. 2017, 27, 1665–1673. [Google Scholar] [CrossRef] [Green Version]
- Gabr, M.T.; Brogi, S. MicroRNA-Based Multitarget Approach for Alzheimer’s Disease: Discovery of the First-In-Class Dual Inhibitor of Acetylcholinesterase and MicroRNA-15b Biogenesis. J. Med. Chem. 2020, 63, 9695–9704. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Doecke, J.D.; Sharples, R.A.; Villemagne, V.L.; Fowler, C.J.; Rembach, A.; Martins, R.N.; Rowe, C.C.; Macaulay, S.L.; Masters, C.L.; et al. Prognostic Serum MiRNA Biomarkers Associated with Alzheimer’s Disease Shows Concordance with Neuropsychological and Neuroimaging Assessment. Mol. Psychiatry 2014, 20, 1188–1196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, W.; Du, S.; Shi, W.; Liu, Y.; Hu, Y.; Xie, Z.; Yao, X.; Liu, Z.; Ma, W.; Xu, L.; et al. Inhibition of Rac1-Dependent Forgetting Alleviates Memory Deficits in Animal Models of Alzheimer’s Disease. Protein Cell 2019, 10, 745–759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borin, M.; Saraceno, C.; Catania, M.; Lorenzetto, E.; Pontelli, V.; Paterlini, A.; Fostinelli, S.; Avesani, A.; di Fede, G.; Zanusso, G.; et al. Rac1 Activation Links Tau Hyperphosphorylation and Aß Dysmetabolism in Alzheimer’s Disease. Acta Neuropathol. Commun. 2018, 6, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Noguchi, J.; Nagaoka, A.; Watanabe, S.; Ellis-Davies, G.C.R.; Kitamura, K.; Kano, M.; Matsuzaki, M.; Kasai, H. In Vivo Two-Photon Uncaging of Glutamate Revealing the Structure–Function Relationships of Dendritic Spines in the Neocortex of Adult Mice. J. Physiol. 2011, 589, 2447–2457. [Google Scholar] [CrossRef]
- Jo, S.; Yarishkin, O.; Hwang, Y.J.; Chun, Y.E.; Park, M.; Woo, D.H.; Bae, J.Y.; Kim, T.; Lee, J.; Chun, H.; et al. GABA from Reactive Astrocytes Impairs Memory in Mouse Models of Alzheimer’s Disease. Nat. Med. 2014, 20, 886–896. [Google Scholar] [CrossRef]
- Corkrum, M.; Covelo, A.; Lines, J.; Bellocchio, L.; Pisansky, M.; Loke, K.; Quintana, R.; Rothwell, P.E.; Lujan, R.; Marsicano, G.; et al. Dopamine-Evoked Synaptic Regulation in the Nucleus Accumbens Requires Astrocyte Activity. Neuron 2020, 105, 1036–1047.e5. [Google Scholar] [CrossRef] [Green Version]
- Heo, C.H.; Kim, K.H.; Kim, H.J.; Baik, S.H.; Song, H.; Kim, Y.S.; Lee, J.; Mook-Jung, I.; Kim, H.M. A Two- Photon Fluorescent Probe for Amyloid-β Plaques in Living Mice. Chem. Commun. 2013, 49, 1303–1305. [Google Scholar] [CrossRef]
- Verwilst, P.; Kim, H.R.; Seo, J.; Sohn, N.W.; Cha, S.Y.; Kim, Y.; Maeng, S.; Shin, J.W.; Kwak, J.H.; Kang, C.; et al. Rational Design of in Vivo Tau Tangle-Selective Near-Infrared Fluorophores: Expanding the BODIPY Universe. J. Am. Chem. Soc. 2017, 139, 13393–13403. [Google Scholar] [CrossRef]
- Savelieff, M.G.; Detoma, A.S.; Derrick, J.S.; Lim, M.H. The Ongoing Search for Small Molecules to Study Metal-Associated Amyloid-β Species in Alzheimers Disease. Acc. Chem. Res. 2014, 47, 2475–2482. [Google Scholar] [CrossRef]
- Kim, D.; Baik, S.H.; Kang, S.; Cho, S.W.; Bae, J.; Cha, M.Y.; Sailor, M.J.; Mook-Jung, I.; Ahn, K.H. Close Correlation of Monoamine Oxidase Activity with Progress of Alzheimer’s Disease in Mice, Observed by in Vivo Two-Photon Imaging. ACS Cent. Sci. 2016, 2, 967–975. [Google Scholar] [CrossRef]
- Wu, W.; Zheng, T.; Tian, Y. An Enzyme-Free Amplification Strategy Based on Two-Photon Fluorescent Carbon Dots for Monitoring MiR-9 in Live Neurons and Brain Tissues of Alzheimer’s Disease Mice. Chem. Commun. 2020, 56, 8083–8086. [Google Scholar] [CrossRef]
- Zhang, X.; Tian, Y.; Yuan, P.; Li, Y.; Yaseen, M.A.; Grutzendler, J.; Moore, A.; Ran, C. A Bifunctional Curcumin Analogue for Two-Photon Imaging and Inhibiting Crosslinking of Amyloid Beta in Alzheimer’s Disease. Chem. Commun. 2014, 50, 11550–11553. [Google Scholar] [CrossRef] [Green Version]
- Takasaki, K.; Abbasi-Asl, R.; Waters, J. Superficial Bound of the Depth Limit of Two-Photon Imaging in Mouse Brain. eNeuro 2020, 7, ENEURO.0255-19.2019. [Google Scholar] [CrossRef] [Green Version]
- Gu, L.; Kleiber, S.; Schmid, L.; Nebeling, F.; Chamoun, M.; Steffen, J.; Wagner, J.; Fuhrmann, M. Long-Term In Vivo Imaging of Dendritic Spines in the Hippocampus Reveals Structural Plasticity. J. Neurosci. 2014, 34, 13948–13953. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.J.; Choi, S.C.; Cho, J.Y.; Joo, H.J.; Park, J.H.; Yu, C.W.; Lim, D.S. Pioglitazone Increases Circulating MicroRNA-24 with Decrease in Coronary Neointimal Hyperplasia in Type 2 Diabetic Patients—Optical Coherence Tomography Analysis. Circ. J. 2015, 79, CJ-14-0964. [Google Scholar] [CrossRef] [Green Version]
- Koga, S.; Ikeda, S.; Yoshida, T.; Nakata, T.; Takeno, M.; Koide, Y.; Kawano, H.; Maemura, K.; Georges, J.L.; Hanssen, M.; et al. Decrease in Circulating MicroRNA-24 Is Associated with Increase in Neointimal Hyperplasia: Optical Coherence Tomography Analysis. Eur. Heart J. 2013, 34, P5438. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Sun, Y.; Wu, J.; Tian, J.; Zheng, Y.; Wang, P.; Yu, M.; Zhang, S.; Wang, M.; Dong, H.; et al. GW27-E0063 Application of Optical Coherent Tomography to Evaluate the Stability of Atherosclerotic Plaque in Macrophages Regulated by MicroRNA Let-7b. J. Am. Coll. Cardiol. 2016, 68, C3. [Google Scholar] [CrossRef]
- O’Bryhim, B.E.; Lin, J.B.; van Stavern, G.P.; Apte, R.S. OCT Angiography Findings in Preclinical Alzheimer’s Disease: 3-Year Follow-Up. Ophthalmology 2021, 128, 1489–1491. [Google Scholar] [CrossRef]
- Chan, V.T.T.; Sun, Z.; Tang, S.; Chen, L.J.; Wong, A.; Tham, C.C.; Wong, T.Y.; Chen, C.; Ikram, M.K.; Whitson, H.E.; et al. Spectral-Domain OCT Measurements in Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Ophthalmology 2019, 126, 497–510. [Google Scholar] [CrossRef]
- Van de Kreeke, J.A.; Nguyen, H.T.; Konijnenberg, E.; Tomassen, J.; den Braber, A.; ten Kate, M.; Yaqub, M.; van Berckel, B.; Lammertsma, A.A.; Boomsma, D.I.; et al. Optical Coherence Tomography Angiography in Preclinical Alzheimer’s Disease. Br. J. Ophthalmol. 2020, 104, 157–161. [Google Scholar] [CrossRef]
- Leuba, G.; Saini, K. Pathology of Subcortical Visual Centres in Relation to Cortical Degeneration in Alzheimer’s Disease. Neuropathol. Appl. Neurobiol. 1995, 21, 410–422. [Google Scholar] [CrossRef]
- Song, S.; Lee, J.U.; Jeon, M.J.; Kim, S.; Sim, S.J. Detection of Multiplex Exosomal MiRNAs for Clinically Accurate Diagnosis of Alzheimer’s Disease Using Label-Free Plasmonic Biosensor Based on DNA-Assembled Advanced Plasmonic Architecture. Biosens. Bioelectron. 2022, 199, 113864. [Google Scholar] [CrossRef]
- Jebelli, A.; Oroojalian, F.; Fathi, F.; Mokhtarzadeh, A.; Guardia, M. de la Recent Advances in Surface Plasmon Resonance Biosensors for MicroRNAs Detection. Biosens. Bioelectron. 2020, 169, 112599. [Google Scholar] [CrossRef]
| Phenotype | miRNA | Target Gene | References |
|---|---|---|---|
| Synaptic density | miR-135a-5p | ROCK2 | [64] |
| miR-138 | SIRT1 | [65] | |
| Synaptic transmission | miR-26a-5p | - | [14] |
| miR-135a-5p | ROCK2 | [64] | |
| miR-484 | - | [66] | |
| Synaptic plasticity | miR-484 | - | [66] |
| miR-129 | CAMK2D | [67] | |
| CAMK4 | |||
| PPP3CA | |||
| PRKCB | |||
| miR-181a | GluA2 | [51] | |
| miR-132 | HDAC | [68] | |
| RBFOX1 | [69] | ||
| miR-135a-5p | ROCK2 | [64] | |
| miR-124 | PTPN1 | [62] | |
| Synaptotoxicity | miR-181a | GluA2 | [51] |
| miR-132 | HDAC | [68] | |
| Synaptic loss | miR-146a | NKD2 | [27] |
| miR-9-5p | SIRT1 | [70] | |
| Synaptic integrity | miR-30b | EPHB2 | [71] |
| SIRT1 | |||
| GluA2 | |||
| miR-34c | SYT1 | [72] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Chen, S.-C.; Ip, J.P.K. Diverse and Composite Roles of miRNA in Non-Neuronal Cells and Neuronal Synapses in Alzheimer’s Disease. Biomolecules 2022, 12, 1505. https://doi.org/10.3390/biom12101505
Li X, Chen S-C, Ip JPK. Diverse and Composite Roles of miRNA in Non-Neuronal Cells and Neuronal Synapses in Alzheimer’s Disease. Biomolecules. 2022; 12(10):1505. https://doi.org/10.3390/biom12101505
Chicago/Turabian StyleLi, Xinrong, Shih-Chi Chen, and Jacque Pak Kan Ip. 2022. "Diverse and Composite Roles of miRNA in Non-Neuronal Cells and Neuronal Synapses in Alzheimer’s Disease" Biomolecules 12, no. 10: 1505. https://doi.org/10.3390/biom12101505







