Grape Pulp Fiber as Possible Fining Agents for Red Wine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Obtention of Pulp Fiber
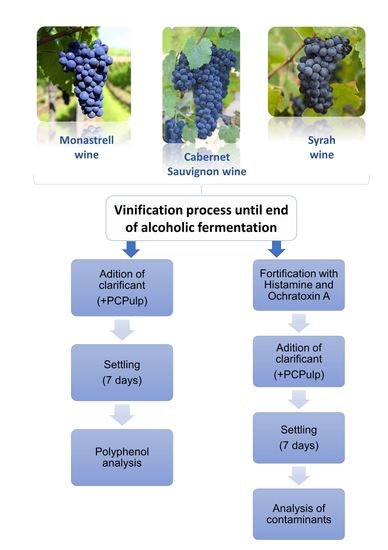
2.2. Winemaking and Finning
2.3. Chromatic Analysis
2.4. Tannin Analysis by Phloroglucinolysis
2.5. Analysis of Ochratoxin A by HPLC
2.6. Analysis of Biogenic Amines by HPLC
2.7. Statistical Data Treatment
3. Results
3.1. Pulp Fiber Composition
3.2. Chromatic Characteristics of the Wines
3.3. HPLC Analysis of the Wine Tannin Concentration and Composition as Affected by Fining
3.4. Removal of OTA and Histamine by Fining
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vidal, S.; Francis, L.; Noble, A.; Kwiatkowski, M.; Cheynier, V.; Waters, E. Taste and mouth-feel properties of different types of tannin-like polyphenolic compounds and anthocyanins in wine. Anal. Chim. Acta 2004, 513, 57–65. [Google Scholar] [CrossRef]
- Sarni-Manchado, P.; Deleris, A.; Avallone, S.; Cheynier, V.; Moutounet, M. Analysis and characterization of wine condensed tannins precipitated by proteins used as fining agent in enology. Am. J. Enol. Vitic. 1999, 50, 81–86. [Google Scholar]
- Yokotsuka, K.; Singleton, V.L. Interactive precipitation between phenolic fractions and peptides in wine-like model solutions: Turbidity, particle size, and residual content as influenced by pH, temperature, and peptide concentration. Am. J. Enol. Vitic. 1995, 46, 329–338. [Google Scholar]
- Versari, A.; Barbanti, D.; Potentini, G.; Parpinello, G.P.; Galassi, S. Preliminary study on the interaction of gelatin-red wine. Ital. J. Food Sci. 1999, 11, 231–239. [Google Scholar]
- Maury, C.; Sarni-Manchado, P.; Lefebvre, S.; Cheynier, V.; Moutounet, M. Influence of fining with different molecular weight gelatins on proanthocyanidin composition and perception of wines. Am. J. Enol. Vitic. 2001, 52, 140–145. [Google Scholar]
- Marchal, R.; Marchal-Delahaut, L.; Lallement, A.; Jeandet, P. Wheat gluten used as a clarifying agent of red wines. J. Agric. Food Chem. 2002, 50, 177–184. [Google Scholar] [CrossRef]
- Renard, C.M.; Baron, A.; Guyot, S.; Drilleau, J.F. Interactions between apple cell walls and native apple polyphenols: Quantification and some consequences. Int. J. Biol. Macromol. 2001, 29, 115–125. [Google Scholar] [CrossRef]
- Padayachee, A.; Netzel, G.; Netzel, M.; Day, L.; Zabaras, D.; Mikkelsen, D.; Gidley, M. Binding of polyphenols to plant cell wall analogues. Part I. Anthocyanins. Food Chem. 2012, 134, 155–161. [Google Scholar] [CrossRef]
- Bindon, K.A.; Li, S.; Kassara, S.; Smith, P.A. Retention of proanthocyanidin in wine-like solution is conferred by a dynamic interaction between soluble and insoluble grape cell wall components. J. Agric. Food Chem. 2016, 64, 8406–8419. [Google Scholar] [CrossRef]
- Castro-López, L.; Gómez-Plaza, E.; Ortega-Regules, A.; Lozada, D.; Bautista-Ortín, A.B. Role of cell wall deconstructing enzymes in the proanthocyanidin-cell wall adsorption-desorption phenomena. Food Chem. 2016, 196, 526–532. [Google Scholar] [CrossRef]
- Osete-Alcaraz, A.; Gómez-Plaza, E.; Martínez-Pérez, P.; Weiller, F.; Schückel, J.; Willats, W.G.; Bautista-Ortín, A.B. The impact of carbohydrate-active enzymes on mediating cell wall polysaccharide-tannin interactions in a wine-like matrix. Food Res. Int. 2020, 129, 108889. [Google Scholar] [CrossRef] [PubMed]
- Osete-Alcaraz, A.; Gómez-Plaza, E.; Martínez-Pérez, P.; Weiller, F.; Schückel, J.; Willats, W.G.; Bautista-Ortín, A.B. The influence of hydrolytic enzymes on tannin adsorption-desorption onto grape cell walls in a wine-like matrix. Molecules 2021, 26, 770. [Google Scholar] [CrossRef] [PubMed]
- Renard, C.M.; Watrelot, A.A.; Le Bourvellec, C. Interactions between polyphenols and polysaccharides: Mechanisms and consequences in food processing and digestion. Trends Food Sci. Technol. 2017, 60, 43–51. [Google Scholar] [CrossRef]
- Medina-Plaza, C.; Beaver, J.W.; Miller, K.V.; Lerno, L.; Dokoozlian, N.; Ponangi, R.; Oberholster, A. Cell wall–anthocyanin interactions during red wine fermentation-like conditions. Am. J. Enol. Vitic. 2020, 71, 149–156. [Google Scholar] [CrossRef]
- Le Bourvellec, C.; Bouchet, B.; Renard, C.M.G.C. Non-covalent interaction between procyanidins and apple cell wall material. Part II: Quantification and impact of cell wall drying. Biochim. Biophys. Acta Gen. Subj. 2005, 1725, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, F.J.; Rocha, S.M.; Coimbra, M.A. Study of the retention capacity of anthocyanins by wine polymeric material. Food Chem. 2012, 134, 957–963. [Google Scholar] [CrossRef]
- Bautista-Ortín, A.B.; Cano-Lechuga, M.; Ruiz-García, Y.; Gómez-Plaza, E. Interactions between grape skin cell wall material and commercial enological tannins. Practical implications. Food Chem. 2014, 152, 558–565. [Google Scholar] [CrossRef]
- Bautista-Ortín, A.B.; Busse-Valverde, N.; Fernández-Fernández, J.I.; Gómez-Plaza, E.; Gil-Muñoz, R. The extraction kinetics of anthocyanins and proanthocyanidins from grape to wine in three different varieties. OENO ONE 2016, 50, 781. [Google Scholar] [CrossRef]
- Jiménez-Martínez, M.D.; Gómez-Plaza, E.; Molero, N.; Bautista-Ortín, A.B. Fining of red wines with pomace cell wall material: Effect on wine phenolic composition. Food Bioproc. Technol. 2017, 10, 1531–1539. [Google Scholar] [CrossRef]
- García-Moruno, E.; Sanlorenzo, C.; Boccaccino, B.; Di Stefano, R. Treatment with yeast to reduce the concentration of ochratoxin A in red wine. Am. J. Enol. Vitic. 2005, 56, 73–76. [Google Scholar]
- Grazioli, B.; Fumi, M.D.; Silva, A. The role of processing on ochratoxin A content in Italian must and wine: A study on naturally contaminated grapes. Int. J. Food Microbiol. 2006, 111, S93–S96. [Google Scholar] [CrossRef]
- Anli, R.E.; Vural, N.; Bayram, M. Removal of ochratoxin A (OTA) from naturally contaminated wines during the vinification process. J. Ins. Brew. 2011, 117, 456–461. [Google Scholar] [CrossRef]
- Jiménez-Martínez, M.D.; Gil-Muñoz, R.; Gómez-Plaza, E.; Bautista-Ortín, A.B. Performance of purified grape pomace as a fining agent to reduce the levels of some contaminants from wine. Food Addit. Contam. 2018, 35, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.; Galli, R.; Grazioli, B.; Fumi, M.D. Metodi di riduzione di residui di ocratossina A nei vini. Ind. Delle Bevan. 2003, 32, 467–472. [Google Scholar]
- Bejaoui, H.; Mathieu, F.; Taillandier, P.; Lebrihi, A. Ochratoxin A removal in synthetic and natural grape juices by selected oenological Saccharomyces strains. J. Appl. Microbiol. 2004, 97, 1038–1044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Košmerl, T.; Šućur, S.; Prosen, H. Biogenic amines in red wine: The impact of technological processing of grape and wine. Acta Agric. Slov. 2013, 101, 1854–1941. [Google Scholar] [CrossRef]
- Landete, J.M.; Ferrer, S.; Polo, L.; Pardo, I. Biogenic amines in wines from three Spanish regions. J. Agric. Food Chem. 2005, 53, 1119–1124. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Prats-Moya, M.S. Free amino acids and biogenic amines in Alicante Monastrell wines. Food Chem. 2012, 135, 1511–1519. [Google Scholar] [CrossRef]
- Bindon, K.A.; Bacic, A.; Kennedy, J.A. Tissue-specific and developmental modifications of grape cell walls influence the adsorption of proanthocyanidins. J. Agric. Food Chem. 2012, 60, 9249–9260. [Google Scholar] [CrossRef] [PubMed]
- Osete-Alcaraz, A.; Bautista-Ortín, A.B.; Ortega-Regules, A.; Gómez-Plaza, E. Elimination of suspended cell wall material in musts improves the phenolic content and color of red wines. Am. J. Enol. Vitic. 2019, 70, 201–204. [Google Scholar] [CrossRef]
- Apolinar-Valiente, R.; Romero-Cascales, I.; Gómez-Plaza, E.; López-Roca, J.M.; Ros-García, J.M. Cell wall compounds of red grapes skins and their grape marcs from three different winemaking techniques. Food Chem. 2015, 187, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Apolinar-Valiente, R.; Gómez-Plaza, E.; Terrier, N.; Doco, T.; Ros-García, J.M. The composition of cell walls from grape skin in Vitis vinifera intraspecific hybrids. J. Sci. Food Agric. 2017, 97, 4029–4035. [Google Scholar] [CrossRef] [PubMed]
- Ho, P.; Silva, M.D.C.M.; Hogg, T.A. Changes in colour and phenolic composition during the early stages of maturation of port in wood, stainless steel and glass. J. Sci. Food Agric. 2001, 81, 1269–1280. [Google Scholar] [CrossRef] [Green Version]
- Smith, P.A. Precipitation of tannin with methyl cellulose allows tannin quantification in grape and wine samples. Technol. Rev. AWRI 2005, 158, 3–7. [Google Scholar]
- Osete-Alcaraz, A.; Bautista-Ortín, A.B.; Ortega-Regules, A.E.; Gómez-Plaza, E. Combined use of pectolytic enzymes and ultrasounds for improving the extraction of phenolic compounds during vinification. Food Bioproc. Technol. 2019, 12, 1330–1339. [Google Scholar] [CrossRef]
- Castellari, M.; Fabbri, S.; Fabiani, A.; Amati, A.; Galassi, S. Comparison of different immunoaffinity clean-up procedures for high-performance liquid chromatographic analysis of ochratoxin A in wines. J. Chromatogr. A 2000, 888, 129–136. [Google Scholar] [CrossRef]
- Gómez-Alonso, S.; Hermosín-Gutiérrez, I.; García-Romero, E. Simultaneous HPLC analysis of biogenic amines, amino acids, and ammonium ion as aminoenone derivatives in wine and beer samples. J. Agric. Food Chem. 2007, 55, 608–613. [Google Scholar] [CrossRef]
- Springer, L.F.; Sherwood, R.W.; Sacks, G.L. Pathogenesis-related proteins limit the retention of condensed tannin additions to red wines. J. Agric. Food Chem. 2016, 64, 1309–1317. [Google Scholar] [CrossRef]
- Springer, L.F.; Chen, L.A.; Stahlecker, A.C.; Cousins, P.; Sacks, G.L. Relationship of soluble grape-derived proteins to condensed tannin extractability during red wine fermentation. J. Agric. Food Chem. 2016, 64, 8191–8199. [Google Scholar] [CrossRef]
- Ortega-Regules, A.; Ros-García, J.M.; Bautista-Ortín, A.B.; López-Roca, J.M.; Gómez-Plaza, E. Differences in morphology and composition of skin and pulp cell walls from grapes (Vitis vinifera L.): Technological implications. Eur. Food Res. Technol. 2008, 227, 223–231. [Google Scholar] [CrossRef]
- Osete-Alcaraz, A.; Bautista-Ortín, A.B.; Pérez-Porras, P.; Gómez-Plaza, E. The Application of Ultrasound and Enzymes Could Be Promising Tools for Recovering Polyphenols during the Aging on Lees Process in Red Winemaking. Foods 2021, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- Le Bourvellec, C.; Bouchet, B.; Renard, C.M.G.C. Non-covalent interaction between procyanidins and apple cell wall material. Part III: Study on model polysaccharides. Biochim. Biophys. Acta Gen. Subj. 2005, 1725, 10–18. [Google Scholar] [CrossRef]
- Castillo-Sánchez, J.J.; Mejuto, J.C.; Garrido, J.; García-Falcón, S. Influence of wine-making protocol and fining agents on the evolution of the anthocyanin content, colour and general organoleptic quality of Vinhão wines. Food Chem. 2006, 97, 130–136. [Google Scholar] [CrossRef]
- Castillo-Sánchez, J.X.; García-Falcón, M.S.; Garrido, J.; Martínez-Carballo, E.; Martins-Dias, L.R.; Mejuto, X.C. Phenolic compounds and colour stability of Vinhao wines: Influence of wine-making protocol and fining agents. Food Chem. 2008, 106, 18–26. [Google Scholar] [CrossRef]
- Guadalupe, Z.; Ayestarán, B. Effect of commercial mannoprotein addition on polysaccharide, polyphenolic, and color composition in red wines. J. Agric. Food Chem. 2008, 56, 9022–9029. [Google Scholar] [CrossRef]
- Jiménez-Martínez, M.D.; Bautista-Ortín, A.B.; Gil-Muñoz, R.; Gómez-Plaza, E. Fining with purified grape pomace. Effect of dose, contact time and varietal origin on the final wine phenolic composition. Food Chem. 2019, 271, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Bindon, K.A.; Smith, P.A.; Kennedy, J.A. Interaction between grape-derived proanthocyanidins and cell wall material. 1. Effect on proanthocyanidin composition and molecular mass. J. Agric. Food Chem. 2010, 58, 2520–2528. [Google Scholar] [CrossRef]
- Le Bourvellec, C.; Guyot, S.; Renard, C.M.G.C. Non-covalent interaction between procyanidins and apple cell wall material: Part I. Effect of some environmental parameters. Biochim. Biophys. Acta Gen. Subj. 2004, 1672, 192–202. [Google Scholar] [CrossRef]
- Iturmendi, N.; Durán, D.; Marín-Arroyo, M.R. Fining of red wines with gluten or yeast extract protein. Int. J. Food Sci. 2010, 45, 200–207. [Google Scholar] [CrossRef]
- Castellari, M.; Versari, A.; Fabiani, A.; Parpinello, G.P.; Galassi, S. Removal of ochratoxin A in red wines by means of adsorption treatments with commercial fining agents. J. Agric. Food Chem. 2001, 49, 3917–3921. [Google Scholar] [CrossRef]
- Mayer, K.; Pause, G. Bentonitbehandlung von wein: Einfluss auf den gehalt an biogenen aminen. Schweiz. Z. Obs. Und Weinbau. 1978, 114, 544–547. [Google Scholar]
- Amghouz, Z.; Ancín-Azpilicueta, C.; Burusco, K.K.; García, J.R.; Khainakov, S.A.; Luquin, A.; Garrido, J.J. Biogenic amines in wine: Individual and competitive adsorption on a modified zirconium phosphate. Microporous Mesoporous Mater. 2014, 197, 130–139. [Google Scholar] [CrossRef]
- Mannino, M.; Vassanelli, G.; Triulzi, G. Trattamenti al vino per ridurre il contenuto in ammine biogene e loro quantificazione. VVQ Vigne Vini Qual. 2006, 33, 72–76. [Google Scholar]
- Kállay, M.; Bódy-Szalkai, M. Ammine biogene nei vini ungheresi. Riv. Vitic. E Enol. 1996, 3, 29–38. [Google Scholar]
- Grossmann, M.; Smit, I.; Loehnertz, O.; Ansorge, A. Biogenic amines and grapes: Effect of microbes and fining agents. Bull. De L'oiv 2007, 80, 245–250. [Google Scholar]
| Samples | P | PC | CS | NCS | UA |
|---|---|---|---|---|---|
| Pulp fiber | 53.29 ± 1.84 | 18.76 ± 1.30 | 25.61 ± 0.67 | 16.11 ± 3.40 | 3.55 ± 0.64 |
| Samples | CI | TA | TPI | PolA | MCPT |
|---|---|---|---|---|---|
| Monastrell | |||||
| Control | 13.7 ± 0.0 b | 606.7 ± 4.1 b | 57.8 ± 0.4 b | 31.7 ± 0.1 b | 1560.2 ± 6.5 b |
| +Pulp Fiber | 11.7 ± 0.0 a | 532.3 ± 0.9 a | 51.2 ± 0.4 a | 27.9 ± 0.1 a | 1273.6 ± 39.3 a |
| Syrah | |||||
| Control | 20.4 ± 0.3 b | 763.8 ± 5.3 b | 54.9 ± 0.6 b | 66.2 ± 0.2 b | 1269.9 ± 54.1 b |
| +Pulp Fiber | 17.5 ± 0.0 a | 677.7 ± 4.5 a | 50.0 ± 1.0 a | 57.5 ± 0.2 a | 1088.6 ± 32.9 a |
| Cabernet Sauvignon | |||||
| Control | 21.2 ± 0.1 b | 635.8 ± 5.2 b | 53.7 ± 0.4 b | 96.9 ± 0.7 b | 1324.4 ± 48.5 b |
| +Pulp Fiber | 17.5 ± 0.3 a | 556.0 ± 0.5 a | 46.5 ± 0.3 a | 81.3 ± 0.7 a | 1019.8 ± 48.6 a |
| Samples | TT Phloro | mDP | EGC | ECG |
|---|---|---|---|---|
| Monastrell | ||||
| Control | 1015.5 ± 20.4 b | 6.9 ± 0.2 a | 612.3 ± 24.1 b | 87.2 ± 7.0 b |
| +Pulp Fiber | 850.3 ± 12.4 a | 7.1 ± 0.1 a | 485.9 ± 6.5 a | 75.1 ± 1.1 a |
| Syrah | ||||
| Control | 393.5 ± 7.9 b | 6.4 ± 0.3 a | 281.0 ± 6.8 b | 80.7 ± 2.0 b |
| +Pulp Fiber | 323.5 ± 9.9 a | 6.1 ± 0.2 a | 225.5 ± 6.4 a | 72.9 ± 2.0 a |
| Cabernet Sauvignon | ||||
| Control | 452.6 ± 48.3 a | 6.0 ± 0.1 a | 448.2 ± 52.1 a | 62.5 ± 4.8 a |
| +Pulp Fiber | 443.5 ± 50.0 a | 6.7 ± 0.5 a | 387.6 ± 28.9 a | 61.7 ± 4.0 a |
| Samples | Histamine | OTA |
|---|---|---|
| Monastrell | ||
| Control | 65.6 ± 1.2 b | 7.9 ± 1.1 b |
| +Pulp Fiber | 60.4 ± 1.0 a | 4.0 ± 0.3 a |
| Syrah | ||
| Control | 65.2 ± 1.5 a | 8.3 ± 0.1 b |
| +Pulp Fiber | 63.0 ± 0.9 a | 4.0 ± 0.2 a |
| Cabernet Sauvignon | ||
| Control | 66.6 ± 1.6 b | 9.0 ± 0.6 b |
| +Pulp Fiber | 61.2 ± 0.3 a | 3.8 ± 0.1 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osete-Alcaraz, A.; Osete-Alcaraz, L.; Ortega-Regules, A.E.; Bautista-Ortín, A.B.; Gómez-Plaza, E. Grape Pulp Fiber as Possible Fining Agents for Red Wine. Biomolecules 2022, 12, 1519. https://doi.org/10.3390/biom12101519
Osete-Alcaraz A, Osete-Alcaraz L, Ortega-Regules AE, Bautista-Ortín AB, Gómez-Plaza E. Grape Pulp Fiber as Possible Fining Agents for Red Wine. Biomolecules. 2022; 12(10):1519. https://doi.org/10.3390/biom12101519
Chicago/Turabian StyleOsete-Alcaraz, Andrea, Lucía Osete-Alcaraz, Ana Eugenia Ortega-Regules, Ana Belen Bautista-Ortín, and Encarna Gómez-Plaza. 2022. "Grape Pulp Fiber as Possible Fining Agents for Red Wine" Biomolecules 12, no. 10: 1519. https://doi.org/10.3390/biom12101519
APA StyleOsete-Alcaraz, A., Osete-Alcaraz, L., Ortega-Regules, A. E., Bautista-Ortín, A. B., & Gómez-Plaza, E. (2022). Grape Pulp Fiber as Possible Fining Agents for Red Wine. Biomolecules, 12(10), 1519. https://doi.org/10.3390/biom12101519