Use of an Acellular Assay to Study Interactions between Actinides and Biological or Synthetic Ligands
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Actinide Compounds
2.3. Preparation of Agarose Gels and Incubation Media
2.4. Collection of Fluids and Activity Measurement
2.5. Data Processing
3. Results
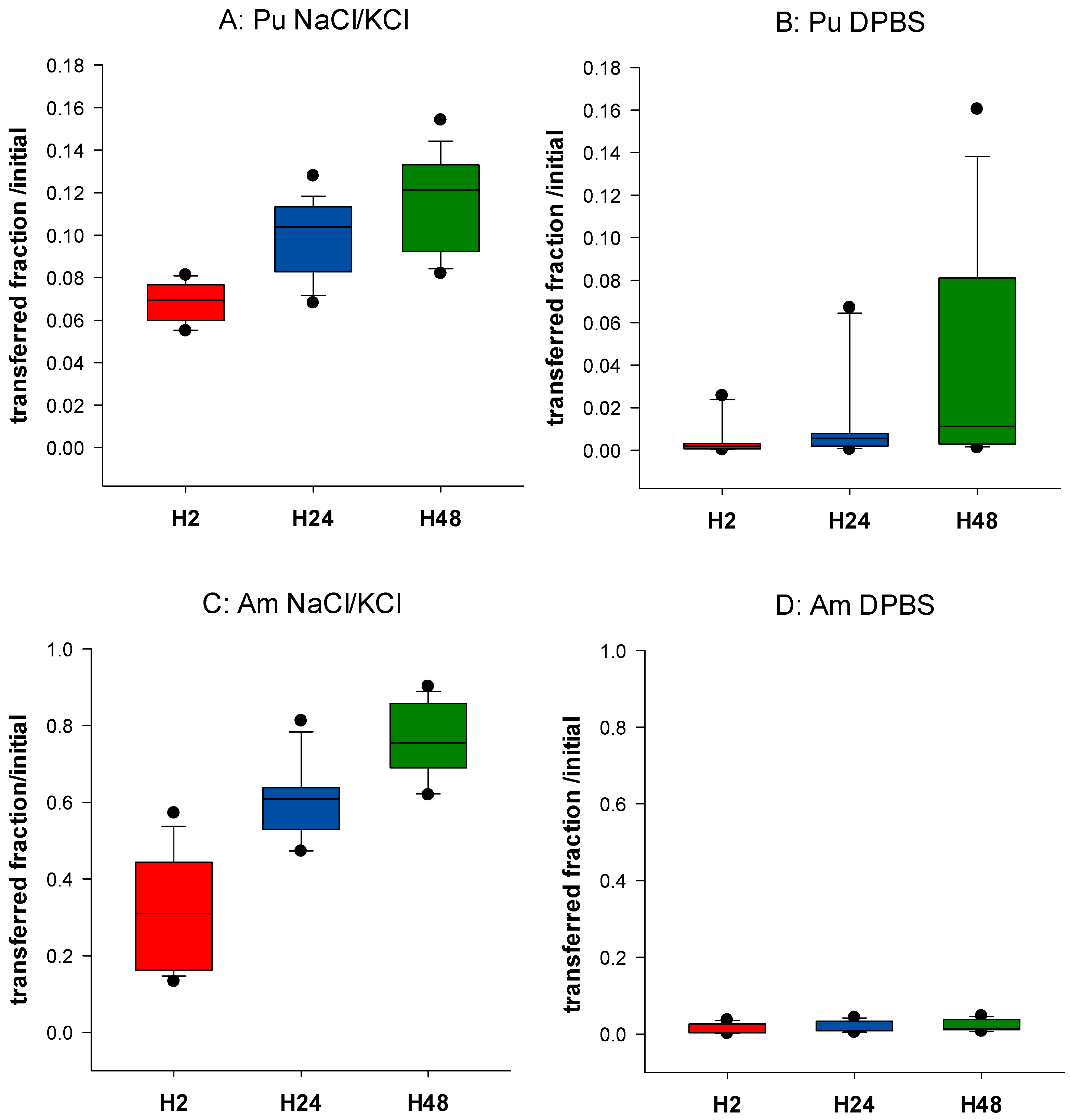
3.1. Influence of Phosphate in the Static Phase on Pu and Am Transfer
3.2. Influence of Various Ligands Added in the Dynamic Phase on the Transfer of Pu/Am
3.2.1. Transfer of Pu/Am in the Presence of a Synthetic Ligand (DTPA) According to the Composition of the Static Phase
3.2.2. The Presence of Bioligand (Tf) in the Dynamic Phase Influences the Transfer of Pu in the Absence of Phosphate
3.2.3. Influence of Bioligands (ApoTf, ferritin) or Synthetic Ligands (DTPA) on Pu/Am Transfer in the Presence of Phosphate
3.3. Behavior of Actinide/Protein Complexes: Transfer from Static to Dynamic Phase
3.3.1. In the Absence of Bioligands in the Dynamic Phase
3.3.2. In the Presence of Ligands in the Dynamic Phase
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ansoborlo, E.; Prat, O.; Moisy, P.; Den Auwer, C.; Guilbaud, P.; Carriere, M.; Gouget, B.; Duffield, J.; Doizi, D.; Vercouter, T.; et al. Actinide speciation in relation to biological processes. Biochimie 2006, 88, 1605–1618. [Google Scholar] [CrossRef] [PubMed]
- ICRP. Occupational Intakes of Radionuclides: Part 4. ICRP Publication 141. Ann. ICRP 2019, 48, 279–365. [Google Scholar]
- ICRP. Occupational Intakes of Radionuclides: Part 1. ICRP Publication 130. Ann. ICRP 2015, 44, 57–93. [Google Scholar]
- Griffiths, N.M.; Van der Meeren, A.; Gremy, O. Comparison of Local and Systemic DTPA Treatment Efficacy According to Actinide Physicochemical Properties Following Lung or Wound Contamination in the Rat. Front. Pharmacol. 2021, 12, 635792. [Google Scholar] [CrossRef]
- Baybarz, R.D. Dissociation constants of the transplutonium element chelates of diethylenetriaminepentaacetic acid (DTPA) and the application of DTPA chelates to solvent extraction separations of transplutonium elements from the lanthanide elements. J. Inorg. Nucl. Chem. 1965, 27, 1831–1839. [Google Scholar] [CrossRef]
- Gremy, O.; Tsapis, N.; Chau, Q.; Renault, D.; Abram, M.C.; Van der Meeren, A. Preferential decorporation of americium by pulmonary administration of DTPA dry powder after inhalation of aged PuO2 containing americium in rats. Radiat. Res. 2010, 174, 637–644. [Google Scholar] [CrossRef]
- Wernli, C.; Eikenberg, J. Twenty-year follow-up of a Pu/Am inhalation case. Radiat. Prot. Dosim. 2007, 125, 506–512. [Google Scholar] [CrossRef][Green Version]
- Paquet, F.; Chazel, V.; Houpert, P.; Guilmette, R.; Muggenburg, B. Efficacy of 3,4,3-LI(1,2-HOPO) for decorporation of Pu, Am and U from rats injected intramuscularly with high-fired particles of MOX. Radiat. Prot. Dosim. 2003, 105, 521–525. [Google Scholar] [CrossRef]
- ICRP. The Metabolism of Plutonium and Related Elements; Pergamon Press: Oxford, UK, 1986; Volume Publication 48. [Google Scholar]
- Mewhinney, J.A.; Craig, D.K. Studies of americium in laboratory animals. In Actinides in Man and Animals; Wrenn, M.E., Ed.; RD Press: Salt Lake City, UT, USA, 1981. [Google Scholar]
- Okabayashi, H. Differential movement of plutonium and americium in lungs of rats following the inhalation of submicron plutonium nitrate aerosol. J. Radiat. Res. 1980, 21, 111–117. [Google Scholar] [CrossRef]
- Turner, G.A.; Taylor, D.M. The transport of plutonium, americium and curium in the blood of rats. Phys. Med. Biol. 1968, 13, 535–546. [Google Scholar] [CrossRef]
- Taylor, D.M. The bioinorganic chemistry of actinides in blood. J. Alloys Compd. 1998, 271–273, 6–10. [Google Scholar] [CrossRef]
- Taylor, D.M. The biodistribution and toxicity of plutonium, americium and neptunium. Sci. Total Environ. 1989, 83, 217–225. [Google Scholar] [CrossRef]
- Drouet, G.; Devilliers, K.; Van der Meeren, A. In vitro evidence of the influence of complexation of Pu and Am on uptake by human lung epithelial cells Calu-3. Toxicol. In Vitro 2022, 79, 105279. [Google Scholar] [CrossRef]
- McInroy, J.F.; Kathren, R.L.; Voelz, G.L.; Swint, M.J. U.S. Transuranium Registry report on the 239Pu distribution in a human body. Health Phys. 1991, 60, 307–333. [Google Scholar] [CrossRef]
- Nenot, J.C.; Masse, R.; Morin, M.; Lafuma, J. An experimental comparative study of the behaviour of 237Np, 238Pu, 239Pu, 241Am and 242Cm in bone. Health Phys. 1972, 22, 657–665. [Google Scholar] [CrossRef]
- Herring, G.M.; Vaughan, J.; Williamson, M. Preliminary report on the site of localization and possible binding agent for yttrium, americium and plutonium in cortical bone. Health Phys. 1962, 8, 717–724. [Google Scholar] [CrossRef]
- Ellender, M.; Haines, J.W.; Harrison, J.D. The distribution and retention of plutonium, americium and uranium in CBA/H mice. Hum. Exp. Toxicol. 1995, 14, 38–48. [Google Scholar] [CrossRef]
- Stover, B.J.; Bruenger, F.W.; Stevens, W. Association of americium with ferritin in the canine liver. Radiat. Res. 1970, 43, 173–186. [Google Scholar] [CrossRef]
- Bruenger, F.W.; Stover, B.J.; Stevens, W.; Atherton, D.R. Exchange of 239PuIV between transferrin and ferritin in vitro. Health Phys. 1969, 16, 339–340. [Google Scholar] [CrossRef]
- Boocock, G.; Popplewell, D.S. Distribution of plutonium in serum proteins following intravenous injection into rats. Nature 1965, 208, 282–283. [Google Scholar] [CrossRef]
- Jensen, M.P.; Gorman-Lewis, D.; Aryal, B.; Paunesku, T.; Vogt, S.; Rickert, P.G.; Seifert, S.; Lai, B.; Woloschak, G.E.; Soderholm, L. An iron-dependent and transferrin-mediated cellular uptake pathway for plutonium. Nat. Chem. Biol. 2011, 7, 560–565. [Google Scholar] [CrossRef]
- Yule, L. A Comparison of the Binding of Plutonium and Iron to Transferrin and Citrate. Ph.D. Thesis, University of Wales, Cardiff, UK, 1991. [Google Scholar]
- Sauge-Merle, S.; Lemaire, D.; Evans, R.W.; Berthomieu, C.; Aupiais, J. Revisiting binding of plutonium to transferrin by CE-ICP-MS. Dalton. Trans. 2017, 46, 1389–1396. [Google Scholar] [CrossRef]
- Den Auwer, C.; Llorens, I.; Moisy, P.; Vidaud, C.; Goudard, F.; Barbot, C.; Solari, P.L.; Funke, H. Actinide uptake by transferrin and ferritin metalloproteins. Radiochim. Acta 2005, 93, 699–703. [Google Scholar] [CrossRef]
- Jeanson, A.; Ferrand, M.; Funke, H.; Hennig, C.; Moisy, P.; Solari, P.L.; Vidaud, C.; Den Auwer, C. The role of transferrin in actinide(IV) uptake: Comparison with iron(III). Chemistry 2010, 16, 1378–1387. [Google Scholar] [CrossRef] [PubMed]
- Vidaud, C.; Miccoli, L.; Brulfert, F.; Aupiais, J. Fetuin exhibits a strong affinity for plutonium and may facilitate its accumulation in the skeleton. Sci. Rep. 2019, 9, 17584. [Google Scholar] [CrossRef] [PubMed]
- Zurita, C.; Tsushima, S.; Bresson, C.; Garcia Cortes, M.; Solari, P.L.; Jeanson, A.; Creff, G.; Den Auwer, C. How Does Iron Storage Protein Ferritin Interact with Plutonium (and Thorium)? Chem.–A Eur. J. 2021, 27, 2393–2401. [Google Scholar] [CrossRef] [PubMed]
- Deblonde, G.J.; Mattocks, J.A.; Wang, H.; Gale, E.M.; Kersting, A.B.; Zavarin, M.; Cotruvo, J.A., Jr. Characterization of Americium and Curium Complexes with the Protein Lanmodulin: A Potential Macromolecular Mechanism for Actinide Mobility in the Environment. J. Am. Chem. Soc. 2021, 143, 15769–15783. [Google Scholar] [CrossRef] [PubMed]
- Allred, B.E.; Rupert, P.B.; Gauny, S.S.; An, D.D.; Ralston, C.Y.; Sturzbecher-Hoehne, M.; Strong, R.K.; Abergel, R.J. Siderocalin-mediated recognition, sensitization, and cellular uptake of actinides. Proc. Natl. Acad Sci. USA 2015, 112, 10342–10347. [Google Scholar] [CrossRef]
- Creff, G.; Zurita, C.; Jeanson, A.; Carle, G.; Vidaud, C.; Den Auwer, C. What do we know about actinides-proteins interactions? Radiochim. Acta 2019, 107, 993–1009. [Google Scholar] [CrossRef]
- Duffield, J.R.; Raymond, D.P.; Williams, D.R. Speciation of plutonium in biological fluids. Inorg. Chim. Acta 1987, 140, 369–372. [Google Scholar] [CrossRef]
- Griffiths, N.M.; Coudert, S.; Moureau, A.; Laroche, P.; Angulo, J.F.; Van der Meeren, A. Forecasting the In Vivo Behavior of Radiocontaminants of Unknown Physicochemical Properties Using a Simple In Vitro Test. Health Phys. 2016, 111, 93–99. [Google Scholar] [CrossRef]
- Van der Meeren, A.; Angulo, J.F.; Bohand, S.; Griffiths, N.M. A quick and simple in vitro assay to predict bioavailability of actinides following accidental exposure. Toxicol. In Vitro 2019, 58, 142–149. [Google Scholar] [CrossRef]
- Gremy, O.; Griffiths, N.; Miccoli, L.; Van der Meeren, A. From in vivo to in vitro models to assess bioavailability properties of plutonium compounds. In Proceedings of the 12th International Conference on the Health Effects of Incorporated Radionuclides (HEIR 2018), Paris, France, 7 May 2019; p. 02007. [Google Scholar]
- MacGillivray, R.T.; Moore, S.A.; Chen, J.; Anderson, B.F.; Baker, H.; Luo, Y.; Bewley, M.; Smith, C.A.; Murphy, M.E.; Wang, Y.; et al. Two high resolution crystal structures of the recombinant N-lobe of human transferrin reveal a structural change implicated in iron release. Biochemistry 1998, 37, 7919–7928. [Google Scholar] [CrossRef]
- Arosio, P.; Adelman, T.G.; Drysdale, J.W. On ferritin heterogeneity. Further evidence for heteropolymers. J. Biol. Chem. 1978, 253, 4451–4458. [Google Scholar] [CrossRef]
- Pan, Y.H.; Sader, K.; Powell, J.J.; Bleloch, A.; Gass, M.; Trinick, J.; Warley, A.; Li, A.; Brydson, R.; Brown, A. 3D morphology of the human hepatic ferritin mineral core: New evidence for a subunit structure revealed by single particle analysis of HAADF-STEM images. J. Struct. Biol. 2009, 166, 22–31. [Google Scholar] [CrossRef]
- Bevington, A.; Mundy, K.I.; Yates, A.J.; Kanis, J.A.; Russell, R.G.; Taylor, D.J.; Rajagopalan, B.; Radda, G.K. A study of intracellular orthophosphate concentration in human muscle and erythrocytes by 31P nuclear magnetic resonance spectroscopy and selective chemical assay. Clin. Sci. 1986, 71, 729–735. [Google Scholar] [CrossRef]
- Tukey, J.W. Box-and-Whisker Plots. In Exploratory Data Analysis; Addison-Wesley: Reading, MA, USA, 1977. [Google Scholar]
- Gomme, P.T.; McCann, K.B.; Bertolini, J. Transferrin: Structure, function and potential therapeutic actions. Drug Discov. Today 2005, 10, 267–273. [Google Scholar] [CrossRef]
- Walters, G.O.; Miller, F.M.; Worwood, M. Serum ferritin concentration and iron stores in normal subjects. J. Clin. Pathol. 1973, 26, 770–772. [Google Scholar] [CrossRef]
- Harrison, J.D.; Stather, J.W. The assessment of doses and effects from intakes of radioactive particles. J. Anat. 1996, 189, 521–530. [Google Scholar]
- Li, S.; Pang, X.; Zhao, J.; Zhang, Q.; Shan, Y. Evaluating the single-molecule interactions between targeted peptides and the receptors on living cell membrane. Nanoscale 2021, 13, 17318–17324. [Google Scholar] [CrossRef]
- Marcuello, C.; Frempong, G.A.; Balsera, M.; Medina, M.; Lostao, A. Atomic Force Microscopy to Elicit Conformational Transitions of Ferredoxin-Dependent Flavin Thioredoxin Reductases. Antioxidants 2021, 10, 1437. [Google Scholar] [CrossRef]
- Neck, V.; Altmaier, M.; Fanghänel, T. Solubility of plutonium hydroxides/hydrous oxides under reducing conditions and in the presence of oxygen. Comptes Rendus Chim. 2007, 10, 959–977. [Google Scholar] [CrossRef]
- Guilmette, R.A.; Lindhorst, P.S.; Hanlon, L.L. Interaction of Pu and Am with bone mineral in vitro. Radiat. Prot. Dosim. 1998, 79, 453–458. [Google Scholar] [CrossRef]
- Poudel, D.; Avtandilashvili, M.; Bertelli, L.; Klumpp, J.A.; Tolmachev, S.Y. Long-term Retention of Plutonium in the Respiratory Tracts of Two Acutely-exposed Workers: Estimation of Bound Fraction. Health Phys. 2021, 120, 258–270. [Google Scholar] [CrossRef]
- Birchall, A.; Puncher, M.; Hodgson, A.; Tolmachev, S.Y. The Importance and Quantification of Plutonium Binding in Human Lungs. Health Phys. 2019, 117, 133–142. [Google Scholar] [CrossRef]
- Muller, B.; von Wichert, P. Bronchoalveolar lavage proteins. Klin. Wochenschr. 1985, 63, 781–787. [Google Scholar] [CrossRef]
- Planas-Bohne, F.; Rau, W. Comparison of the binding of 59Fe- and 239Pu-transferrin to rat liver cell membranes. Hum. Exp. Toxicol. 1990, 9, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Bruenger, F.W.; Stevens, W.; Stover, B.J. Americium-241 in the blood: In vivo and in vitro observations. Radiat. Res. 1969, 37, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.M. Interactions between transuranium elements and the components of cells and tissues. Health Phys. 1972, 22, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.M.; Seidel, A.; Planas-Bohne, F.; Schuppler, U.; Neu-Müller, M.; Wirth, R.E. Biochemical studies of the interactions of plutonium, neptunium and proactinium with blood and liver cell proteins. Inoganica Chim. Acta 1987, 140, 361–363. [Google Scholar] [CrossRef]
- Zurita, C.; Tsushima, S.; Solari, P.L.; Jeanson, A.; Creff, G.; Den Auwer, C. Interaction of Th(IV), Pu(IV) and Fe(III) with ferritin protein: How similar? J. Synchrotron Radiat. 2022, 29 Pt 1, 45–52. [Google Scholar] [CrossRef]
- Gremy, O.; Laurent, D.; Coudert, S.; Griffiths, N.M.; Miccoli, L. Decorporation of Pu/Am Actinides by Chelation Therapy: New Arguments in Favor of an Intracellular Component of DTPA Action. Radiat. Res. 2016, 185, 568–579. [Google Scholar] [CrossRef]
- Taylor, D.M.; Chipperfield, A.R.; James, A.C. The effects of tetracycline on the deposition of plutonium and related elements, in rat bone. Health Phys. 1971, 21, 197–204. [Google Scholar] [CrossRef]




| Dynamic Phase | Static Phase Pu | Static Phase Am | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NaCl | DPBS | NaCl | DPBS | |||||||||
| H2 | H24 | H48 | H2 | H24 | H48 | H2 | H24 | H48 | H2 | H24 | H48 | |
| NaCl/KCl DTPA 50 µM | 4.5 | 54.5 | 37.1 | - | - | - | 1.8 | 1.5 | 1.3 | - | - | - |
| DPBS DTPA 50 µM | - | - | - | 5.5 | 11.0 | 11.6 | - | - | - | 13.2 | 34.2 | 34.9 |
| Dynamic Phase | Static Phase Pu | Static Phase Am | |||||
|---|---|---|---|---|---|---|---|
| H2 | H24 | H48 | H2 | H24 | H48 | ||
| DPBS + ApoTf (50 µM) | Fold increase/no ligand | 308.3 | 518.4 | 437.2 | 56.0 | 80.4 | 98.3 |
| Cumulative transfer (%) | 14.6 | 67.8 | 86.3 | 16.3 | 55.2 | 92.4 | |
| DPBS + ferritin (10 µM) | Fold increase/no ligand | 7.1 | 28.7 | 31.1 | 43.8 | 66.8 | 64.5 |
| Cumulative transfer (%) | 0.3 | 3.7 | 6.1 | 12.8 | 45.8 | 60.7 | |
| DPBS + DTPA (50 µM) | Fold increase/no ligand | 16.5 | 314.8 | 357.8 | 171.9 | 121.9 | 100.7 |
| Cumulative transfer (%) | 0.8 | 41.1 | 70.6 | 50.2 | 83.7 | 94.6 | |
| Dynamic Phase | Static Phase Pu | Static Phase Am | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pu-ApoTf | Pu-Ferritin | Am-ApoTf | Am-Ferritin | |||||||||
| DPBS | H2 | H24 | H48 | H2 | H24 | H48 | H2 | H24 | H48 | H2 | H24 | H48 |
| 1.68 | 2.85 | 2.49 | 1.28 | 2.07 | 2.22 | 1.61 | 1.38 | 1.34 | 26.3 | 24.6 | 22.4 | |
| Dynamic Phase | Static Phase Pu | Static Phase Am | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pu-Tf | Pu-Ferritin | Am-Tf | Am-Ferritin | ||||||||||
| H2 | H24 | H48 | H2 | H24 | H48 | H2 | H24 | H48 | H2 | H24 | H48 | ||
| DPBS + 50 µM ApoTf | Fold increase/ no ligand | 215.4 | 188.1 | 180.9 | - | - | - | 55.5 | 70.1 | 67.1 | - | - | - |
| Cumulative transfer (%) | 17.2 | 70.34 | 88.8 | - | - | - | 26.2 | 73.4 | 91.2 | - | - | - | |
| DPBS + 10 µM ferritin | Fold increase/ no ligand | - | - | - | 6.1 | 12.9 | 13.5 | - | - | - | 2.3 | 3.0 | 3.2 |
| Cumulative transfer (%) | - | - | - | 0.5 | 4.1 | 6.9 | - | - | - | 17.6 | 51.2 | 66.8 | |
| DPBS + 50 µM DTPA | Fold increase/ no ligand | 38.9 | 132 | 150.8 | 30.2 | 148.2 | 142.7 | 100.6 | 77.8 | 69.1 | 6.5 | 4.9 | 4.5 |
| Cumulative transfer (%) | 3.1 | 49.3 | 74.1 | 2.4 | 46.7 | 72.85 | 47.4 | 81.5 | 93.9 | 49.6 | 82.5 | 94.4 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van der Meeren, A.; Berthomieu, C.; Moureau, A.; Defrance, M.; Griffiths, N.M. Use of an Acellular Assay to Study Interactions between Actinides and Biological or Synthetic Ligands. Biomolecules 2022, 12, 1553. https://doi.org/10.3390/biom12111553
Van der Meeren A, Berthomieu C, Moureau A, Defrance M, Griffiths NM. Use of an Acellular Assay to Study Interactions between Actinides and Biological or Synthetic Ligands. Biomolecules. 2022; 12(11):1553. https://doi.org/10.3390/biom12111553
Chicago/Turabian StyleVan der Meeren, Anne, Catherine Berthomieu, Agnès Moureau, Martine Defrance, and Nina M. Griffiths. 2022. "Use of an Acellular Assay to Study Interactions between Actinides and Biological or Synthetic Ligands" Biomolecules 12, no. 11: 1553. https://doi.org/10.3390/biom12111553
APA StyleVan der Meeren, A., Berthomieu, C., Moureau, A., Defrance, M., & Griffiths, N. M. (2022). Use of an Acellular Assay to Study Interactions between Actinides and Biological or Synthetic Ligands. Biomolecules, 12(11), 1553. https://doi.org/10.3390/biom12111553






