Importance of TGFβ in Cancer and Nematode Infection and Their Interaction—Opinion
Abstract
:1. Introduction
Many Faces of TGFβ
2. TGFβ Signalling in Helminths
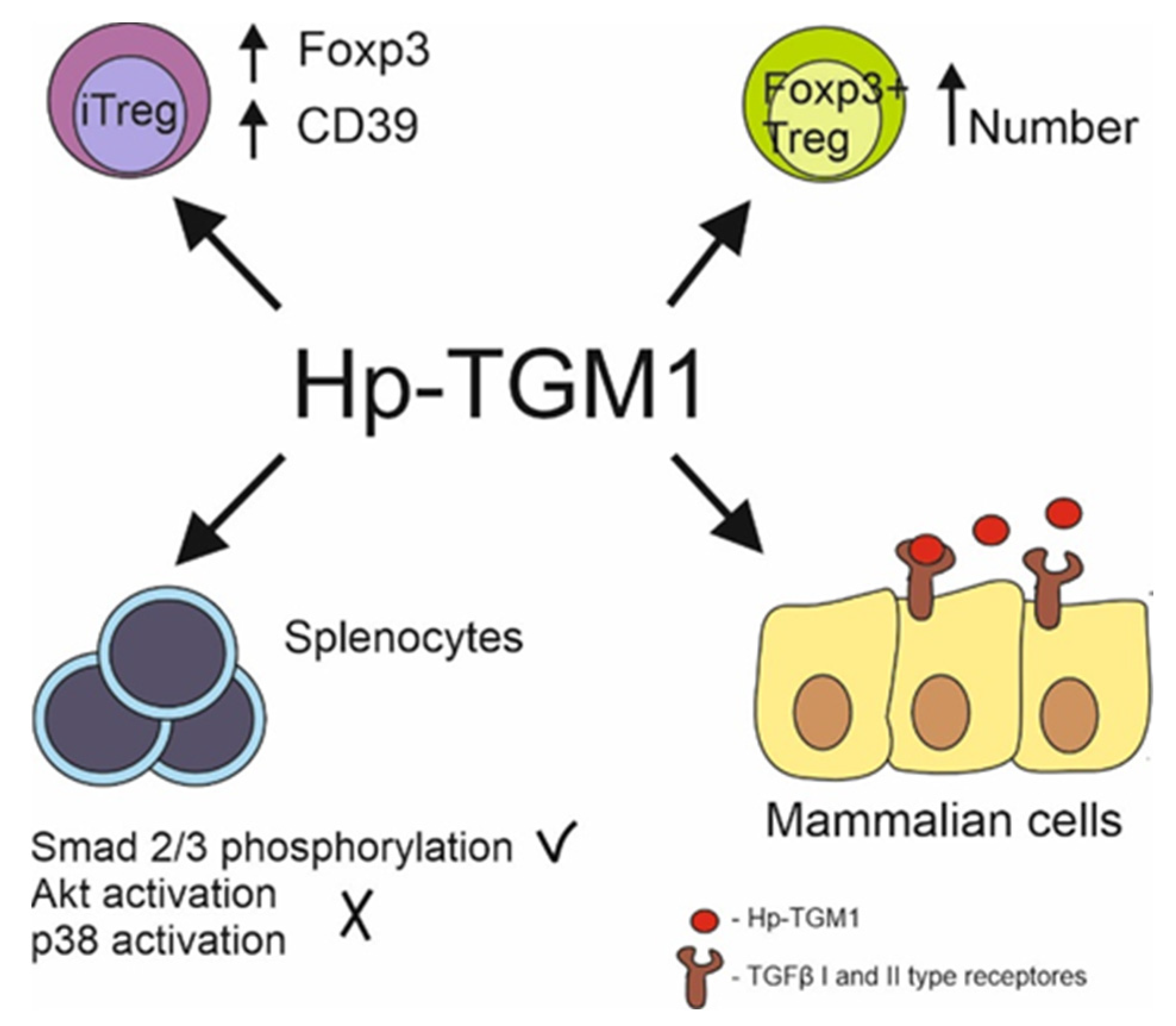
Nematode TGFβ Mimic
3. Potential Influence of Nematode Infection on Tumour Development
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Derynck, R.; Akhurst, R.J. Differentiation plasticity regulated by TGF-beta family proteins in development and disease. Nat. Cell Biol. 2007, 9, 1000–1004. [Google Scholar] [CrossRef] [PubMed]
- Massagué, J. TGFbeta in Cancer. Cell 2008, 134, 215–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, M.; Neil, J.R.; Schiemann, W.P. Transforming growth factor-β and the hallmarks of cancer. Cell Signal. 2011, 23, 951–962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Derynck, R.; Muthusamy, B.P.; Saeteurn, K.Y. Signaling pathway cooperation in TGF-β-induced epithelial-mesenchymal transition. Curr. Opin. Cell Biol. 2014, 31, 56–66. [Google Scholar] [CrossRef] [Green Version]
- Morikawa, M.; Derynck, R.; Miyazono, K. TGF-β and the TGF-β Family: Context-Dependent Roles in Cell and Tissue Physiology. Cold Spring Harb. Perspect. Biol. 2016, 8, a021873. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Ding, W.; Neiman, J.; Mulder, K.M. Requirement of Smad3 and CREB-1 in mediating transforming growth factor-beta (TGF beta) induction of TGF beta 3 secretion. J. Biol. Chem. 2006, 281, 29479–29490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinck, A.P.; Archer, S.J.; Qian, S.W.; Roberts, A.B.; Sporn, M.B.; Weatherbee, J.A.; Tsang, M.L.-S.; Lucas, R.; Zhang, B.-L.; Wenker, J.; et al. Transforming Growth Factor β1: Three-Dimensional Structure in Solution and Comparison with the X-ray Structure of Transforming Growth Factor β2. Biochemistry 1996, 35, 8517–8534. [Google Scholar] [CrossRef]
- Sehnal, D.; Bittrich, S.; Deshpande, M.; Svobodová, R.; Berka, K.; Bazgier, V.; Ve-lankar, S.; Burley, S.K.; Koča, J.; Rose, A.S. Mol* Viewer: Modern web app for 3D visualization and analysis of large biomolecular structures. Nucleic Acids Res. 2021, 49, 431–437. [Google Scholar] [CrossRef]
- Ten Dijke, P.; Goumans, M.J.; Itoh, F.; Itoh, S. Regulation of cell proliferation by Smad proteins. J. Cell Physiol. 2002, 191, 1–16. [Google Scholar] [CrossRef]
- Itoh, S.; Ten Dijke, P. Negative regulation of TGF-beta receptor/Smad signal transduction. Curr. Opin. Cell Biol. 2007, 19, 176–184. [Google Scholar] [CrossRef]
- Hata, A.; Chen, Y.G. TGF-β signaling from receptors to smads. Cold Spring Harb. Perspect. Biol. 2016, 8, a022061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, B.; Chen, Y.G. Regulation of TGF-β Signal Transduction. Scientifica 2014, 2014, 874065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, F.; Weigel, K.J.; Zhou, H.; Wang, X.J. Paradoxical roles of TGFβ signaling in suppressing and promoting squamous cell carcinoma. Acta Biochim. Biophys. Sin. 2018, 50, 98–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, H.I.; Kiyono, K.; Miyazono, K. Regulation of autophagy by transforming growth factor-β (TGF-β) signaling. Autophagy 2010, 6, 645–647. [Google Scholar] [CrossRef] [Green Version]
- Gratchev, A. TGF-β signalling in tumour associated macrophages. Immunobiology 2017, 222, 75–81. [Google Scholar] [CrossRef]
- Derynck, R.; Zhang, Y.E. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 2003, 425, 577–584. [Google Scholar] [CrossRef]
- Zhang, Y.E. Non-Smad pathways in TGF-beta signaling. Cell Res. 2009, 19, 128–139. [Google Scholar] [CrossRef] [Green Version]
- Gratchev, A.; Kzhyshkowska, J.; Kannookadan, S.; Ochsenreiter, M.; Popova, A.; Yu, X.; Mamidi, S.; Stonehouse-Usselmann, E.; Muller-Molinet, I.; Gooi, L.; et al. Activation of a TGF-beta-specific multistep gene expression program in mature macrophages requires glucocorticoid-mediated surface expression of TGF-beta receptor II. J. Immunol. 2008, 15, 6553–6565. [Google Scholar] [CrossRef] [Green Version]
- de Charette, M.; Houot, R. Hide or defend, the two strategies of lymphoma immune evasion: Potential implications for immunotherapy. Haematologica 2018, 103, 1256–1268. [Google Scholar] [CrossRef]
- Dunn, G.P.; Bruce, A.T.; Ikeda, H.; Old, L.J.; Schreiber, R.D. Cancer immunoediting: From immuno- surveillance to tumor escape. Nat. Immunol. 2002, 3, 991–998. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 4, 646–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Folmont, A.; Bourhis, J.-H.; Chouaib, S.; Terry, S. Multifaceted Role of the Transforming Growth Factor β on Effector T Cells and the Implication for CAR-T Cell Therapy. Immuno 2021, 1, 160–173. [Google Scholar] [CrossRef]
- Kushwah, R.; Wu, J.; Oliver, J.R.; Jiang, G.; Zhang, J.; Siminovitch, K.A.; Hu, J. Uptake of apoptotic DC converts immature DC into tolerogenic DC that induce differentiation of Foxp3+ Treg. Eur. J. Immunol. 2010, 40, 1022–1035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, P.; Lee, Y.; Liu, W.; Krausz, T.; Chong, A.; Schreiber, H.; Fu, Y.X. Intratumor depletion of CD4+ cells unmasks tumor immunogenicity leading to the rejection of late-stage tumors. J. Exp. Med. 2005, 201, 779–791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soares, K.C.; Rucki, A.A.; Kim, V.; Foley, K.; Solt, S.; Wolfgang, C.L.; Jaffee, E.M.; Zheng, L. TGF-β blockade depletes T regulatory cells from metastatic pancreatic tumors in a vaccine dependent manner. Oncotarget 2015, 6, 43005–43015. [Google Scholar] [CrossRef] [Green Version]
- Park, B.V.; Freeman, Z.T.; Ghasemzadeh, A.; Chattergoon, M.A.; Rutebemberwa, A.; Steigner, J.; Winter, M.E.; Huynh, T.V.; Sebald, S.M.; Lee, S.J.; et al. TGFβ1-Mediated SMAD3 Enhances PD-1 Expression on Antigen-Specific T Cells in Cancer. Cancer Discov. 2016, 6, 1366–1381. [Google Scholar] [CrossRef] [Green Version]
- Thomas, D.A.; Massagué, J. TGF-beta directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell 2005, 8, 369–380. [Google Scholar] [CrossRef] [Green Version]
- Fionda, C.; Malgarini, G.; Soriani, A.; Zingoni, A.; Cecere, F.; Iannitto, M.L.; Ricciardi, M.R.; Federico, V.; Petrucci, M.T.; Santoni, A.; et al. Inhibition of glycogen synthase kinase-3 increases NKG2D ligand MICA expression and sensitivity to NK cell-mediated cytotoxicity in multiple myeloma cells: Role of STAT3. J. Immunol. 2013, 190, 6662–6672. [Google Scholar] [CrossRef] [Green Version]
- Laouar, Y.; Sutterwala, F.S.; Gorelik, L.; Flavell, R.A. Transforming growth factor-β controls T helper type 1 cell development through regulation of natural killer cell interferon-γ. Nat. Immunol. 2005, 6, 600–607. [Google Scholar] [CrossRef]
- Batlle, E.; Massagué, J. Transforming Growth Factor-β signaling in immunity and cancer. Immunity 2019, 50, 924–940. [Google Scholar] [CrossRef]
- Uribe-Querol, E.; Rosales, C. Neutrophils in Cancer: Two Sides of the Same Coin. J. Immunol. Res. 2015, 2015, 983698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakir, B.; Chiarella, A.M.; Pitarresi, J.R.; Rustgi, A.K. EMT, MET, Plasticity, and Tumor Metastasis. Trends Cell Biol. 2020, 30, 764–776. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, S.; Doi, R.; Toyoda, E.; Tsuji, S.; Wada, M.; Koizumi, M.; Tulachan, S.S.; Ito, D.; Kami, K.; Mori, T.; et al. N-cadherin expression and epithelial-mesenchymal transition in pancreatic carcinoma. Clin. Cancer Res. 2004, 10, 4125–4133. [Google Scholar] [CrossRef] [Green Version]
- Usman, S.; Waseem, N.H.; Nguyen, T.K.N.; Mohsin, S.; Jamal, A.; Teh, M.T.; Waseem, A. Vimentin ss at the heart of Epithelial Mesenchymal Transition (EMT) mediated metastasis. Cancers 2021, 13, 4985. [Google Scholar] [CrossRef] [PubMed]
- Katsuno, Y.; Lamouille, S.; Derynck, R. TGF-β signaling and epithelial-mesenchymal transition in cancer progression. Curr. Opin. Oncol. 2013, 25, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Brunen, D.; Willems, S.; Kellner, U.; Midgley, R.; Simon, I.; Bernards, R. TGF-β: An emerging player in drug resistance. Cell Cycle 2013, 12, 2960–2968. [Google Scholar] [CrossRef] [Green Version]
- Lan, Y.; Zhang, D.; Xu, C.; Hance, K.W.; Marelli, B.; Qi, J.; Yu, H.; Qin, G.; Sircar, A.; Hernández, V.M.; et al. Enhanced preclinical antitumor activity of M7824, a bifunctional fusion protein simultaneously targeting PD-L1 and TGF-β. Sci. Transl. Med. 2018, 17, eaan5488. [Google Scholar] [CrossRef] [Green Version]
- Dodagatta-Marri, E.; Meyer, D.S.; Reeves, M.Q.; Paniagua, R.; To, M.D.; Binnewies, M.; Broz, M.L.; Mori, H.; Wu, D.; Adoumie, M.; et al. α-PD-1 therapy elevates Treg/Th balance and increases tumor cell pSmad3 that are both targeted by α-TGFβ antibody to promote durable rejection and immunity in squamous cell carcinomas. J. Immunother. Cancer 2019, 47, 62. [Google Scholar] [CrossRef]
- Huminiecki, L.; Goldovsky, L.; Freilich, S.; Moustakas, A.; Ouzounis, C.; Heldin, C.H. Emergence, development and diversification of the TGF-beta signalling pathway within the animal kingdom. BMC Evol. Biol. 2009, 9, 28. [Google Scholar] [CrossRef] [Green Version]
- Massagué, J. TGF β signalling in context. Nat. Rev. Mol. Cell. Biol. 2012, 13, 616–630. [Google Scholar] [CrossRef] [Green Version]
- Savage-Dunn, C.; Padgett, R.W. The TGF-β Family in Caenorhabditis elegans. Cold Spring Harb. Perspect. Biol. 2017, 9, a022178. [Google Scholar] [CrossRef] [Green Version]
- Viney, M.E.; Thompson, F.J.; Crook, M. TGF-β and the evolution of nematode parasitism. Int. J. Parasitol. 2005, 35, 1473–1475. [Google Scholar] [CrossRef] [PubMed]
- Crook, M. The dauer hypothesis and the evolution of parasitism: 20 years on and still going strong. Int. J. Parasitol. 2014, 44, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vlaar, L.E.; Bertran, A.; Rahimi, M.; Dong, L.; Kammenga, J.E.; Helder, J.; Goverse, A.; Bouwmeester, H.J. On the role of dauer in the adaptation of nematodes to a parasitic lifestyle. Parasit. Vectors. 2021, 14, 554. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, K.; Ray, P.; Okkema, P.G. CEH-28 acti-vatesdbl-1expression and TGF-bsignaling in the C. elegans M4 neuron. Dev. Biol. 2014, 390, 149–159. [Google Scholar] [CrossRef] [Green Version]
- Luo, S.; Shaw, W.M.; Ashraf, J.; Murphy, C.T. TGF-beta Sma/Mab signaling mutations uncouple reproductive aging from somatic aging. PLoS Genet. 2009, 5, e1000789. [Google Scholar] [CrossRef] [Green Version]
- Gilabert, A.; Curran, D.M.; Harvey, S.C.; Wasmuth, J.D. Expanding the view on the evolution of the nematode dauer signalling pathways: Refinement through gene gain and pathway co-option. BMC Genomics 2016, 17, 476. [Google Scholar] [CrossRef] [Green Version]
- Blaxter, M.; Koutsovoulos, G. The evolution of parasitism in Nematoda. Parasitology 2015, 142, S26–S39. [Google Scholar] [CrossRef] [Green Version]
- Viney, M.E. How did parasitic worms evolve? Bioessays 2009, 31, 496–499. [Google Scholar] [CrossRef]
- Gomez-Escobar, N.; Lewis, E.; Maizels, R.M. A novel member of the transforming growth factor-β (TGF-β) superfamily from the filarial nematodes Brugia malayi and B. pahangi. Exp. Parasitol. 1998, 88, 200–209. [Google Scholar] [CrossRef]
- Gomez-Escobar, N.; Gregory, W.F.; Maizels, R.M. Identification of tgh-2, a filarial nematode homolog of Caenorhabditis elegans daf-7 and human transforming growth factor beta, expressed in microfilarial and adult stages of Brugia malayi. Infect. Immun. 2000, 68, 6402–6410. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Escobar, N.; van den Biggelaar, A.; Maizels, R. A member of the TGF-b receptor gene family in the parasitic nematode Brugia pahangi. Gene 1997, 199, 101–109. [Google Scholar] [CrossRef]
- Dissous, C.; Khayath, N.; Vicogne, J.; Capron, M. Growth factor receptors in helminth parasites: Signalling and host-parasite relationships. FEBS Lett. 2006, 580, 2968–2975. [Google Scholar] [CrossRef] [Green Version]
- Korten, S.; Büttner, D.W.; Schmetz, C.; Hoerauf, A.; Mand, S.; Brattig, N. The nematode parasite Onchocerca volvulus generates the transforming growth factor-beta (TGF-beta). Parasitol. Res. 2009, 105, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Freitas, T.C.; Arasu, P. Cloning and characterisation of genes encoding two transforming growth factor-beta-like ligands from the hookworm, Ancylostoma caninum. Int. J. Parasitol. 2005, 35, 1477–1487. [Google Scholar] [CrossRef]
- Arasu, P. In vitro reactivation of Ancylostoma caninum tissue-arrested third-stage larvae by transforming growth factor-beta. J. Parasitol. 2001, 87, 733–738. [Google Scholar] [CrossRef]
- He, L.; Gasser, R.B.; Korhonen, P.K.; Di, W.; Li, F.; Zhang, H.; Li, F.; Zhou, Y.; Fang, R.; Zhao, J.; et al. A TGF-β type I receptor-like molecule with a key functional role in Haemonchus contortus development. Int. J. Parasitol. 2008, 48, 1023–1033. [Google Scholar] [CrossRef]
- He, L.; Gasser, R.B.; Li, T.; Di, W.; Li, F.; Zhang, H.; Zhou, C.; Fang, R.; Hu, M.A. TGF-β type II receptor that associates with developmental transition in Haemonchus contortus in vitro. PLoS Negl. Trop. Dis. 2019, 13, e0007913. [Google Scholar] [CrossRef] [Green Version]
- Colak, S.; Ten Dijke, P. Targeting TGF-β signaling in cancer. Trends Cancer 2017, 3, 56–71. [Google Scholar] [CrossRef]
- Di, W.; Liu, L.; Zhang, T.; Li, F.; He, L.; Wang, C.; Ahmad, A.A.; Hassan, M.; Fang, R.; Hu, M. A DAF-3 co-Smad molecule functions in Haemonchus contortus development. Parasit. Vectors 2019, 12, 609. [Google Scholar] [CrossRef]
- He, L.; Liu, H.; Zhang, B.Y.; Li, F.F.; Di, W.D.; Wang, C.Q.; Zhou, C.X.; Liu, L.; Li, T.T.; Zhang, T.; et al. A daf-7-related TGF-β ligand (Hc-tgh-2) shows important regulations on the development of Haemonchus contortus. Parasit. Vectors 2020, 13, 326. [Google Scholar] [CrossRef] [PubMed]
- McSorley, H.J.; Grainger, J.R.; Harcus, Y.; Murray, J.; Nisbet, A.J.; Knox, D.P.; Maizels, R.M. daf-7-related TGF-beta homologues from Trichostrongyloid nematodes show contrasting life-cycle expression patterns. Parasitology 2010, 137, 159–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, S.J.; Shoemaker, C.B.; Pearce, E.J. A divergent member of the transforming growth factor beta receptor family from Schistosoma mansoni is expressed on the parasite surface membrane. J. Biol. Chem. 1998, 273, 11234–11240. [Google Scholar] [CrossRef] [Green Version]
- Forrester, S.G.; Warfel, P.W.; Pearce, E.J. Tegumental expression of a novel type II receptor serine/threonine kinase (SmRK2) in Schistosoma mansoni. Mol. Biochem. Parasitol. 2004, 136, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Beall, M.J.; McGonigle, S.; Pearce, E.J. Functional conservation of Schistosoma mansoni Smads in TGF-beta signaling. Mol. Biochem. Parasitol. 2000, 111, 131–142. [Google Scholar] [CrossRef]
- Osman, A.; Niles, E.G.; LoVerde, P.T. Identification and characterization of a Smad2 homologue from Schistosoma mansoni, a transforming growth factor-beta signal transducer. J. Biol. Chem. 2001, 276, 10072–10082. [Google Scholar] [CrossRef] [Green Version]
- Carlo, J.M.; Osman, A.; Niles, E.G.; Wu, W.; Fantappie, M.R.; Oliveira, F.M.; LoVerde, P.T. Identification and characterization of an R-Smad ortholog (SmSmad1B) from Schistosoma mansoni. Febs J. 2007, 274, 4075–4093. [Google Scholar] [CrossRef]
- Freitas, T.C.; Jung, E.; Pearce, E.J. TGF-beta signaling controls embryo development in the parasitic flatworm Schistosoma mansoni. PLoS Pathog. 2007, 3, e52. [Google Scholar] [CrossRef] [Green Version]
- Freitas, T.C.; Jung, E.; Pearce, E.J. A bone morphogenetic protein homologue in the parasitic flatworm, Schistosoma mansoni. Int. J. Parasitol. 2009, 39, 281–287. [Google Scholar] [CrossRef] [Green Version]
- Loverde, P.T.; Osman, A.; Hinck, A. Schistosoma mansoni: TGF-beta signaling pathways. Exp. Parasitol. 2007, 117, 304–317. [Google Scholar] [CrossRef]
- Beall, M.J.; Pearce, E.J. Human transforming growth factor-beta activates areceptor serine/threonine kinase from the intravascular parasite Schistosoma mansoni. J. Biol. Chem. 2001, 276, 31613–31619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adalid-Peralta, L.; Rosas, G.; Arce-Sillas, A.; Bobes, R.J.; Cárdenas, G.; Hernández, M.; Trejo, C.; Meneses, G.; Hernández, B.; Estrada, K.; et al. Effect of Transforming Growth Factor-β upon Taenia solium and Taenia crassiceps Cysticerci. Sci. Rep. 2017, 7, 12345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zavala-Góngora, R.; Kroner, A.; Bernthaler, P.; Knaus, P.; Brehm, K. A member of the transforming growth factor-beta receptor family from Echinococcus multilocularis is activated by human bone morphogenetic protein 2. Mol. Biochem. Parasitol. 2006, 146, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Zavala-Góngora, R.; Kroner, A.; Wittek, B.; Knaus, P.; Brehm, K. Identification and characterisation of two distinct Smad proteins from the fox-tapeworm Echinococcus multilocularis. Int. J. Parasitol. 2003, 33, 1665–1677. [Google Scholar] [CrossRef]
- Epping, K.; Brehm, K. Echinococcus multilocularis: Molecular characterization of EmSmadE, a novel BR-Smad involved in TGF-β and BMP signaling. Exp. Parasitol. 2011, 129, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, L.; Wang, H.; Pu, H.; Yang, L.; Li, J.; Wang, J.; Lü, G.; Lu, X.; Zhang, W.; et al. Identification and characterization of functional Smad8 and Smad4 homologues from Echinococcus granulosus. Parasitol. Res. 2014, 113, 3745–3757. [Google Scholar] [CrossRef]
- Grainger, J.R.; Smith, K.A.; Hewitson, J.P.; McSorley, H.J.; Harcus, Y.; Filbey, K.J.; Finney, C.A.; Greenwood, E.J.; Knox, D.P.; Wilson, M.S.; et al. Helminth secretions induce de novo T cell Foxp3 expression and regulatory function through the TGF-β pathway. J. Exp. Med. 2010, 207, 2331–2341. [Google Scholar] [CrossRef]
- Doligalska, M.; Rzepecka, J.; Drela, N.; Donskow, K.; Gerwel-Wronka, M. The role of TGF-beta in mice infected with Heligmosomoides polygyrus. Parasite Immunol. 2006, 28, 387–395. [Google Scholar] [CrossRef]
- Reynolds, L.A.; Filbey, K.J.; Maizels, R.M. Immunity to the model intestinal helminth parasite Heligmosomoides polygyrus. Semin. Immunopathol. 2012, 34, 829–846. [Google Scholar] [CrossRef] [Green Version]
- Maizels, R.M.; Smits, H.H.; McSorley, H.J. Modulation of host immunity by helminths: The expanding repertoire of parasite effector molecules. Immunity 2018, 49, 801–818. [Google Scholar] [CrossRef]
- Johnston, C.J.C.; Smyth, D.J.; Kodali, R.B.; White, M.P.J.; Harcus, Y.; Filbey, K.J.; Hewitson, J.P.; Hinck, C.S.; Ivens, A.; Kemter, A.M.; et al. A structurally distinct TGF-β mimic from an intestinal helminth parasite potently induces regulatory T cells. Nat. Commun. 2017, 8, 1741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smyth, D.J.; Harcus, Y.; White, M.P.J.; Gregory, W.F.; Nahler, J.; Stephens, I.; Toke-Bjolgerud, E.; Hewitson, J.P.; Ivens, A.; McSorley, H.J.; et al. TGF-β mimic proteins form an extended gene family in the murine parasite Heligmosomoides polygyrus. Int. J. Parasitol. 2018, 48, 379–385. [Google Scholar] [CrossRef]
- Mukundan, A.; Byeon, C.H.; Hinck, C.S.; Cunningham, K.; Campion, T.; Smyth, D.J.; Maizels, R.M.; Hinck, A.P. Convergent evolution of a parasite-encoded complement control protein-scaffold to mimic binding of mammalian TGF-β to its receptors, TβRI and TβRII. J. Biol. Chem. 2022, 298, 101994. [Google Scholar] [CrossRef] [PubMed]
- Sow, H.S.; Ren, J.; Camps, M.; Ossendorp, F.; Ten Dijke, P. Combined inhibition of TGF-β signaling and the PD-L1 immune checkpoint is differentially effective in tumor models. Cells 2019, 8, 320. [Google Scholar] [CrossRef] [Green Version]
- White, M.P.J.; Smyth, D.J.; Cook, L.; Ziegler, S.F.; Levings, M.K.; Maizels, R.M. The parasite cytokine mimic Hp-TGM potently replicates the regulatory effects of TGF-β on murine CD4+ T cells. Immunol. Cell Biol. 2021, 99, 848–864. [Google Scholar] [CrossRef] [PubMed]
- Chauché, C.; Rasid, O.; Donachie, A.M.; McManus, C.M.; Löser, S.; Campion, T.; Richards, J.; Smyth, D.J.; McSorley, H.J.; Maizels, R.M. Suppression of airway allergic eosinophilia by Hp-TGM, a helminth mimic of TGF-β. Immunology 2022, 167, 197–211. [Google Scholar] [CrossRef]
- Maruszewska-Cheruiyot, M.; Szewczak, L.; Krawczak-Wójcik, K.; Głaczyńska, M.; Donskow-Łysoniewska, K. The production of excretory-secretory molecules from Heligmosomoides polygyrus bakeri fourth stage larvae varies between mixed and single sex cultures. Parasit. Vectors 2021, 14, 106. [Google Scholar] [CrossRef] [PubMed]
- Weaver, H.J.; Hawdon, J.M.; Hoberg, E.P. Soil-transmitted helminthiases: Implications of climate change and human behavior. Trends Parasitol. 2010, 26, 574–581. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Soil-Transmitted Helminth Infections. WHO. Available online: http://www.who.int/mediacentre/factsheets/fs366/en/ (accessed on 21 August 2022).
- Centers for Disease Control and Prevention—Parasites—Soil-Transmitted Helminths. Available online: https://www.cdc.gov/parasites/sth/index.html (accessed on 21 August 2022).
- Raza, A.; Qamar, A.G.; Hayat, K.; Ashraf, S.; Williams, A.R. Anthelmintic resistance and novel control options in equine gastrointestinal nematodes. Parasitology 2019, 146, 425–437. [Google Scholar] [CrossRef]
- Taylor, M.D.; Van Der Werf, N.; Maizels, R.M. T cells in helminth infection: The regulators and the regulated. Trends Immunol. 2012, 33, 181–189. [Google Scholar] [CrossRef]
- Khan, A.R.; Fallon, P.G. Helminth therapies: Translating the unknown unknowns to known knowns. Int. J. Parasitol. 2013, 43, 293–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maizels, R.M. Parasitic helminth infections and the control of human allergic and autoimmune disorders. Clin. Microbiol. Infect. 2016, 22, 481–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smallwood, T.B.; Giacomin, P.R.; Loukas, A.; Mulvenna, J.P.; Clark, R.J.; Miles, J.J. Helminth immunomodulation in autoimmune disease. Front. Immunol. 2017, 8, 453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davidson, A.; Diamond, B. Autoimmune diseases. N. Engl. J. Med. 2001, 345, 340–350. [Google Scholar] [CrossRef]
- Croese, J.; O’neil, J.; Masson, J.; Cooke, S.; Melrose, W.; Pritchard, D.; Speare, R. A proof of concept study establishing Necator americanus in Crohn’s patients and reservoir donors. Gut 2006, 55, 136–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elliott, D.E.; Weinstock, J.; Summers, R.W.; Landry-Wheeler, A.; Silver, N.; Harnett, M.D.; Hanauer, S.B.; Sandborn, W.J. Randomised clinical trial: The safety and tolerability of Trichuris suis ova in patients with Crohn’s disease. Aliment. Pharmacol. Ther. 2013, 38, 255–263. [Google Scholar] [CrossRef]
- Voldsgaard, A.; Bager, P.; Garde, E.; Åkeson, P.; Leffers, A.M.; Madsen, C.G.; Kapel, C.; Roepstorff, A.; Thamsborg, S.M.; Melbye, M. Trichuris suis ova therapy in relapsing multiple sclerosis is safe but without signals of beneficial effect. Mult. Scler. 2015, 21, 1723–1729. [Google Scholar] [CrossRef]
- Fleming, J.; Hernandez, G.; Hartman, L.; Maksimovic, J.; Nace, S.; Lawler, B.; Risa, T.; Cook, T.; Agni, R.; Reichelderfer, M.; et al. Safety and efficacy of helminth treatment in relapsing/remitting multiple sclerosis: Results of the HINT 2 clinical trial. Mult. Scler. 2019, 25, 81–91. [Google Scholar] [CrossRef]
- Axelrad, J.E.; Lichtiger, S.; Yajnik, V. Inflammatory bowel disease and cancer: The role of inflammation, immunosuppression, and cancer treatment. World J. Gastroenterol. 2016, 22, 4794–4801. [Google Scholar] [CrossRef]
- Marrie, R.A.; Reider, N.; Cohen, J.; Stuve, O.; Trojano, M.; Sorensen, P.S.; Reingold, S.C.; Cutter, G. A systematic review of the incidence and prevalence of cancer in multiple sclerosis. Mult. Scler. 2015, 21, 294–304. [Google Scholar] [CrossRef]
- Donskow-Łysoniewska, K.; Bien, J.; Brodaczewska, K.; Krawczak, K.; Doligalska, M. Colitis promotes adaptation of an intestinal nematode: A Heligmosomoides polygyrus mouse model system. PLoS ONE 2013, 8, e78034. [Google Scholar] [CrossRef]
- Maruszewska-Cheruiyot, M.; Donskow-Łysoniewska, K.; Krawczak, K.; Machcińska, M.; Doligalska, M. Immunomodulatory potential of nematodes against dendritic cells is dependent on intestinal inflammation. Dev. Comp. Immunol. 2021, 115, 103879. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wang, L.; Tang, Y.; Sun, X. Parasite-derived proteins for the treatment of allergies and autoimmune diseases. Front. Microbiol. 2017, 8, 2164. [Google Scholar] [CrossRef] [Green Version]
- Ryan, S.M.; Eichenberger, R.M.; Ruscher, R.; Giacomin, P.R.; Loukas, A. Harnessing helminth-driven immunoregulation in the search for novel therapeutic modalities. PLoS Pathog. 2020, 16, e1008508. [Google Scholar] [CrossRef] [PubMed]
- De Martel, C.; Ferlay, J.; Franceschi, S.; Vignat, J.; Bray, F.; Forman, D.; Plummer, M. Global burden of cancers attributable to infections in 2008: A review and synthetic analysis. Lancet Oncol. 2012, 13, 607–615. [Google Scholar] [CrossRef]
- Herrera, L.A.; Ostrosky-Wegman, P. Do helminths play a role in carcinogenesis? Trends Parasitol. 2001, 17, 172–175. [Google Scholar] [CrossRef]
- Scholte, L.L.S.; Pascoal-Xavier, M.A.; Nahum, L.A. Helminths and cancers from the evolutionary perspective. Front. Med. 2018, 5, 90. [Google Scholar] [CrossRef] [Green Version]
- Huby, F.; Hoste, H.; Mallet, S.; Fournel, S.; Nano, J.L. Effects of the excretory/secretory products of six nematode species, parasites of the digestive tract, on the proliferation of HT29-D4 and HGT-1 cell lines. Epithel. Cell Biol. 1995, 4, 156–162. [Google Scholar]
- Garcia-Perez, J.C.; Rodríguez-Perez, R.; Ballestero, A.; Zuloaga, J.; Fernandez-Puntero, B.; Arias-Díaz, J.; Caballero, M.L. Previous exposure to the fish parasite Anisakis as a potential risk factor for gastric or colon adenocarcinoma. Medicine 2015, 94, e1699. [Google Scholar] [CrossRef] [PubMed]
- Emery, D.L.; Hunt, P.W.; Le Jambre, L.F. Haemonchus contortus: The then and now, and where to from here? Int. J. Parasitol. 2016, 46, 755–769. [Google Scholar] [CrossRef] [Green Version]
- Harnett, W. Secretory products of helminth parasites as immunomodulators. Mol. Biochem. Parasitol. 2014, 195, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Donskow-Łysoniewska, K.; Maruszewska-Cheruiyot, M.; Stear, M. The interaction of host and nematode galectins influences the outcome of gastrointestinal nematode infections. Parasitology 2021, 148, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Cheng, X.; Liu, M.; Wang, X.; Boireau, P. Trichinella spiralis and tumors: Cause, coincidence or treatment? Anticancer Agents Med. Chem. 2018, 18, 1091–1099. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, B.A.; Chetty, A.; Horsnell, W.G.C.; Schäfer, G.; Prince, S.; Smith, K.A. Hookworm exposure decreases human papillomavirus uptake and cervical cancer cell migration through systemic regulation of epithelial-mesenchymal transition marker expression. Sci. Rep. 2018, 8, 11547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Przemeck, S.; Huber, A.; Brown, S.; Pedley, K.C.; Simpson, H.V. Excretory/secretory products of sheep abomasal nematode parasites cause vacuolation and increased neutral red uptake by HeLa cells. Parasitol. Res. 2005, 95, 213–217. [Google Scholar] [CrossRef]
- Lu, Y.; Yang, Y.; Yang, S.; Xia, Q. Immunomodulatory action of excretory-secretory products of Angiostrongylus cantonensis in a mouse tumour model. Parasitol. Res. 2020, 119, 3705–3718. [Google Scholar] [CrossRef]
- Luo, J.; Yu, L.; Xie, G.; Li, D.; Su, M.; Zhao, X.; Du, L. Study on the mitochondrial apoptosis pathways of small cell lung cancer H446 cells induced by Trichinella spiralis muscle larvae ESPs. Parasitology 2017, 144, 793–800. [Google Scholar] [CrossRef]
- Zakeri, A. Helminth-induced apoptosis: A silent strategy for immunosuppression. Parasitology 2017, 144, 1663–1676. [Google Scholar] [CrossRef]
- Bouvard, V.; Baan, R.; Straif, K.; Grosse, Y.; Secretan, B.; El Ghissassi, F.; Benbrahim-Tallaa, L.; Guha, N.; Freeman, C.; Galichet, L.; et al. WHO International Agency for Research on Cancer Monograph Working Group. A review of human carcinogens--Part B: Biological agents. Lancet Oncol. 2009, 10, 321–322. [Google Scholar] [CrossRef]
- Botelho, M.C.; Machado, J.C.; Brindley, P.J.; Correia da Costa, J.M. Targeting molecular signaling pathways of Schistosoma haemotobium infection in bladder cancer. Virulence 2011, 2, 267–279. [Google Scholar] [CrossRef] [Green Version]
- Pastille, E.; Frede, A.; McSorley, H.J.; Gräb, J.; Adamczyk, A.; Kollenda, S.; Hansen, W.; Epple, M.; Buer, J.; Maizels, R.M.; et al. Intestinal helminth infection drives carcinogenesis in colitis-associated colon cancer. PLoS Pathog. 2017, 22, e1006649. [Google Scholar] [CrossRef] [PubMed]
- Freitas, T.C.; Pearce, E.J. Growth factors and chemotactic factors from parasitic helminths: Molecular evidence for roles in hostparasite interactions versus parasite development. Int. J. Parasitol. 2010, 40, 761–773. [Google Scholar] [CrossRef] [PubMed]
- Donskow-Łysoniewska, K.; Krawczak, K.; Machcińska, M.; Brodaczewska, K. Intestinal nematode infection affects metastasis of EL4 lymphoma cells. Arch. Immunol. Ther. Exp. 2020, 68, 39. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, B.A.; Prince, S.; Smith, K.A. Gastrointestinal nematode-derived antigens alter colorectal cancer cell proliferation and migration through regulation of cell cycle and epithelial-mesenchymal transition proteins. Int. J. Mol. Sci. 2020, 21, 7845. [Google Scholar] [CrossRef]

| Process | Helminths | Cancer |
|---|---|---|
| Signalling modification | Increased level of TGFβ and modification of molecules involved in TGFβ signalling pathway levels: - Induction of TGFβ production by host cells; - Modification of host ligands and receptor production; - Secretion of TGFβ signalling molecule homologs and mimics by parasite. | Excessive production of TGFβ by cancer cells under genetic alternations. |
| Growth | Influence on helminth life cycle: - Parasite growth induction; - Helminth development regulation. | Increased tumour growth: - Proliferation; - Autophagy; - Apoptosis. |
| Propagation and spreading | Influence on parasite survival and reproduction: - Decreased resistance to infection. | Increased invasion and metastasis: - Extracellular matrix (ECM) remodelling; - Epithelial–mesenchymal transition (EMT). |
| Immunosuppression | Creation of a milieu conducive to the protection of the helminth against the antiparasitic immune response: - Treg cells generation and expansion; - Th1 and Th2 cell activity suppression. | Creation of immunosuppressive tumour microenvironment: - Expansion of Tregs; - Promotion of tumour-associated macrophages (TAM); - Inhibition of Th1 and cytotoxic T cells, antigen-presenting cells (APC), NK cells, and neutrophile generation and activity. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maruszewska-Cheruiyot, M.; Stear, M.J.; Machcińska, M.; Donskow-Łysoniewska, K. Importance of TGFβ in Cancer and Nematode Infection and Their Interaction—Opinion. Biomolecules 2022, 12, 1572. https://doi.org/10.3390/biom12111572
Maruszewska-Cheruiyot M, Stear MJ, Machcińska M, Donskow-Łysoniewska K. Importance of TGFβ in Cancer and Nematode Infection and Their Interaction—Opinion. Biomolecules. 2022; 12(11):1572. https://doi.org/10.3390/biom12111572
Chicago/Turabian StyleMaruszewska-Cheruiyot, Marta, Michael James Stear, Maja Machcińska, and Katarzyna Donskow-Łysoniewska. 2022. "Importance of TGFβ in Cancer and Nematode Infection and Their Interaction—Opinion" Biomolecules 12, no. 11: 1572. https://doi.org/10.3390/biom12111572
APA StyleMaruszewska-Cheruiyot, M., Stear, M. J., Machcińska, M., & Donskow-Łysoniewska, K. (2022). Importance of TGFβ in Cancer and Nematode Infection and Their Interaction—Opinion. Biomolecules, 12(11), 1572. https://doi.org/10.3390/biom12111572







