Bio-Multifunctional Sponges Containing Alginate/Chitosan/Sargassum Polysaccharides Promote the Healing of Full-Thickness Wounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval of the Study Protocol
2.2. Materials
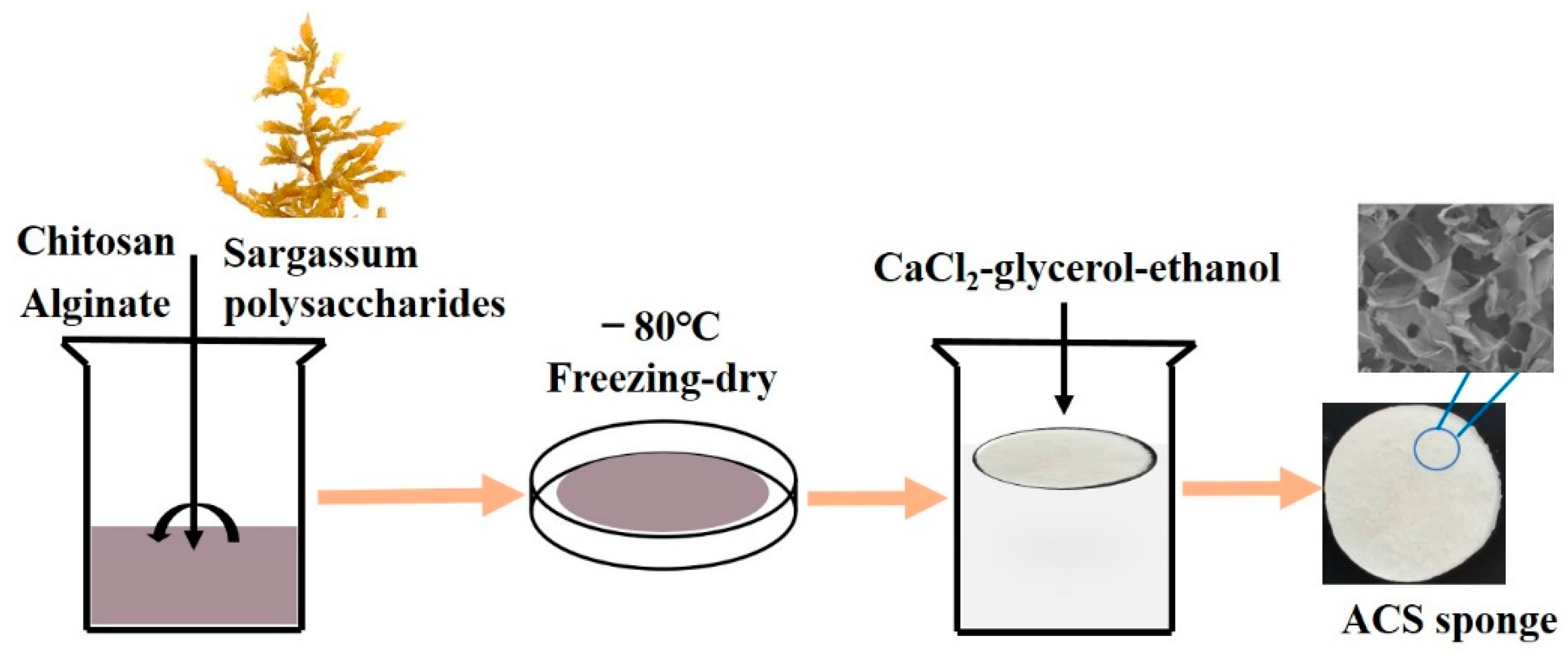
2.3. Preparation of Alginate/Chitosan (AC) and Alginate/Chitosan/SP (ACS) Sponges
2.4. Fourier-Transform Infrared (FTIR) Spectroscopy
2.5. Morphology of AC and ACS Sponges
2.6. Porosity of AC Sponges and ACS Sponges
2.7. Water Vapor Transmission Rate (WVTR)
2.8. Water Absorption Ability
2.9. In Vitro Blood-Clotting Study
2.10. Cytotoxicity
2.11. Antibacterial Activity
2.12. WH In Vivo
2.13. Histology
2.14. Statistical Analyses
3. Results
3.1. Preparation and Characterization of AC Sponges and ACS Sponges
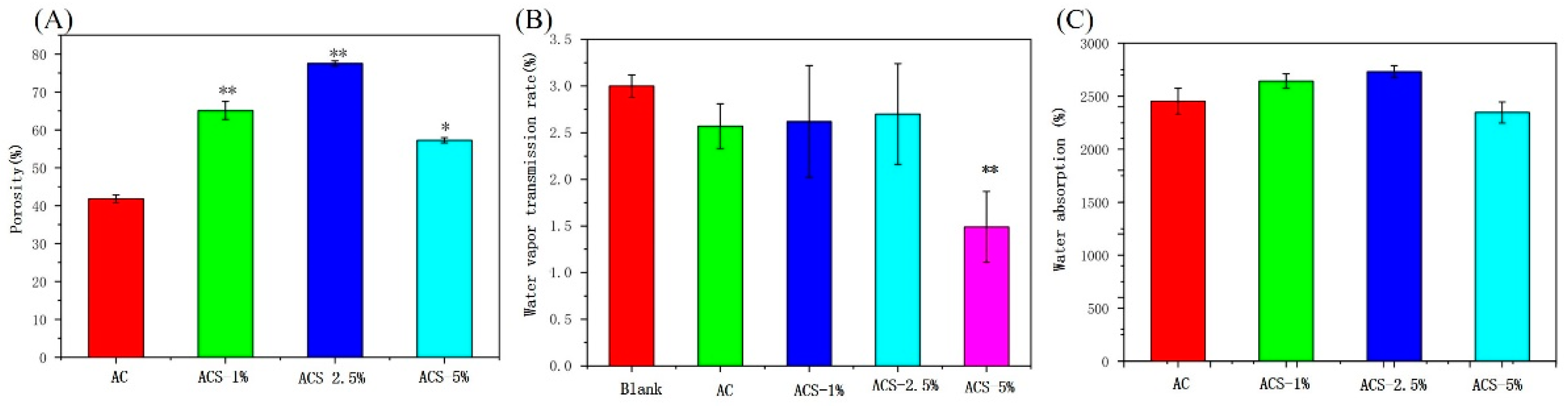
3.2. Porosity, WVTR and Water Absorbability Capacity of AC Sponges and ACS Sponges
3.3. Hemostatic Performance of AC Sponges and ACS Sponges
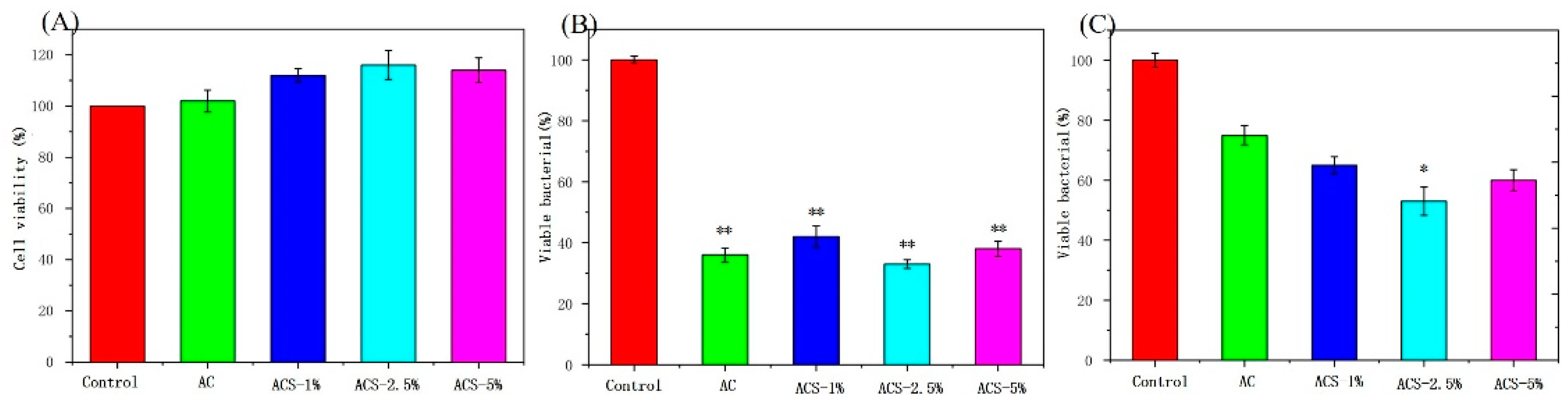
3.4. Biocompatibility and Antibacterial Ability of AC Sponges and ACS Sponges
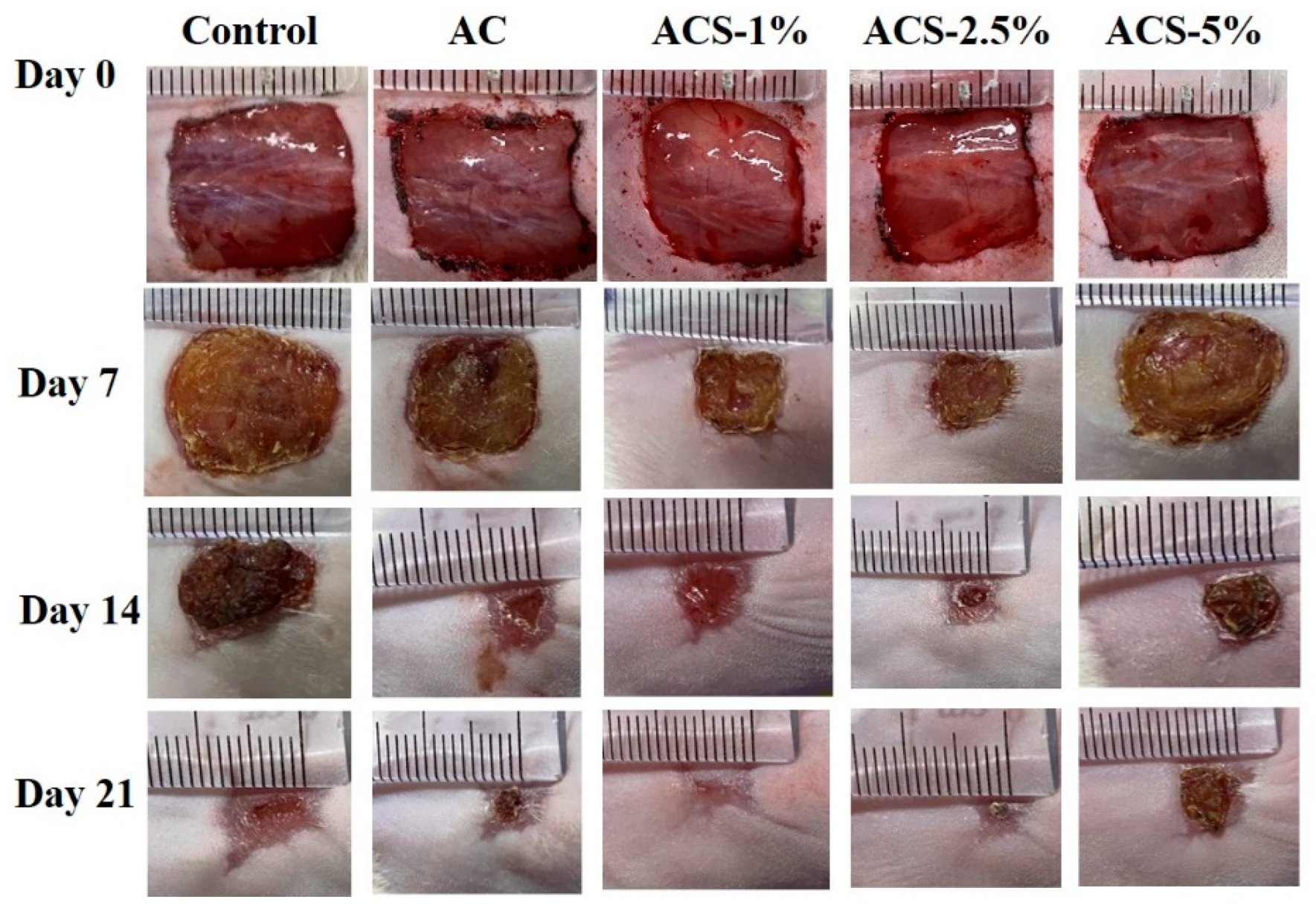
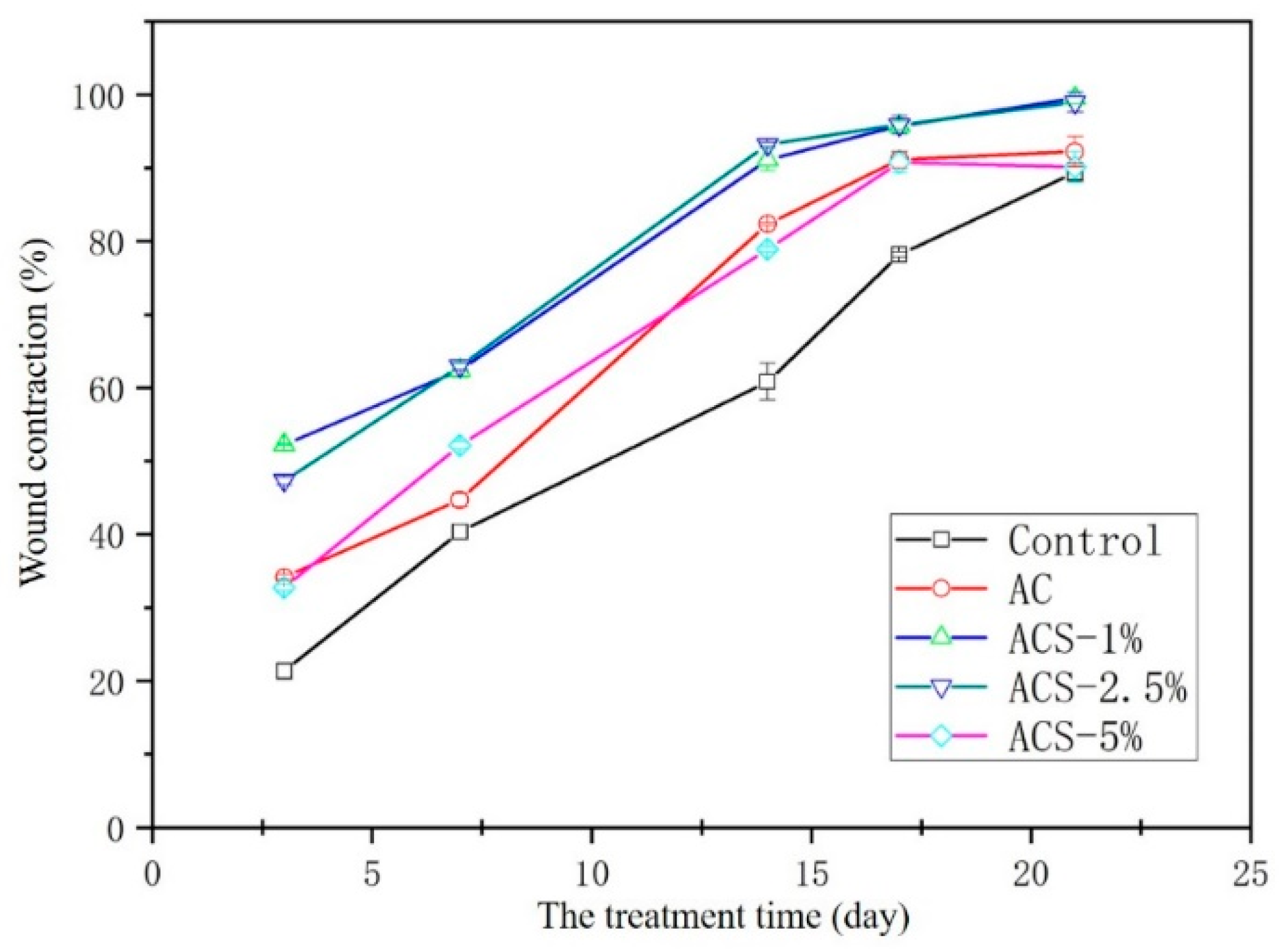
3.5. Healing of Full-Thickness Skin Wounds In Vivo
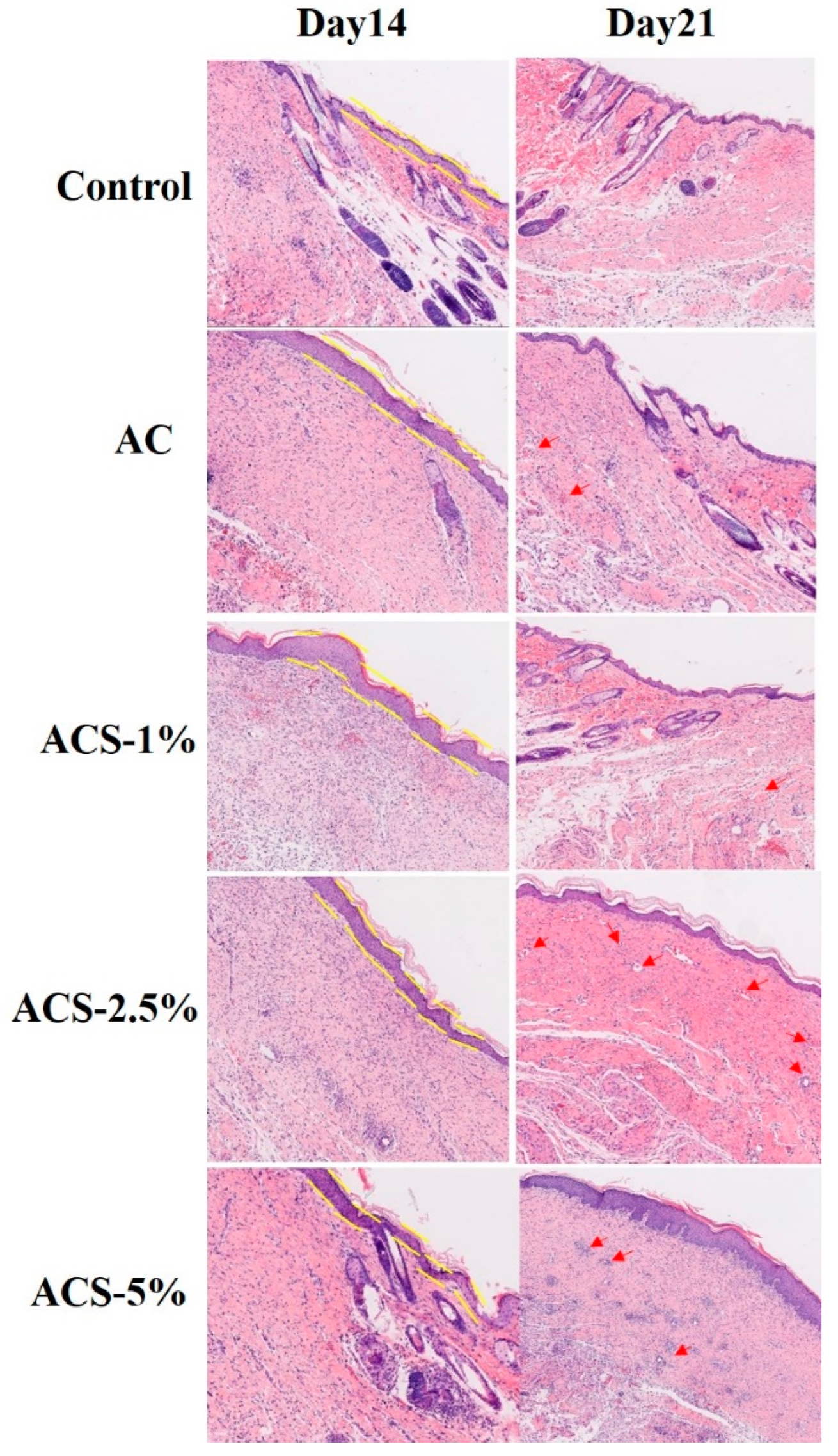
3.6. Histomorphology
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, L.; Mauro, T.M.; Dang, E.; Man, G.; Zhang, J.; Lee, D.; Wang, G.; Feingold, K.R.; Elias, P.M.; Man, M.Q. Epidermal dysfunction leads to an age-associated increase in levels of serum inflammatory cytokines. J. Investig. Dermatol. 2017, 137, 1277–1285. [Google Scholar] [CrossRef] [PubMed]
- Venus, M.; Waterman, J.; McNab, I. Basic physiology of the skin. Surgery 2010, 29, 471–474. [Google Scholar]
- Rammanathan, G.; Singaravelu, S.; Raja, M.D.; Nagiah, N.; Padmapriya, P.; Ruban, K.; Kaveri, K.; Naarajan, T.S.; Sivagnanam, U.T.; Perumal, P.T. Fabrication and characterization of a collagen coated electrospun poly (3-hydroxybutyric acid)-gelatin nanofibrous scaffold as a soft bio-mimetic material for skin tissue engineering applications. RSC Adv. 2016, 6, 7914–7922. [Google Scholar] [CrossRef]
- Tobin, D.J. Biochemistry of human skin-our brain on the outside. Chem. Soc. Rev. 2006, 35, 52–67. [Google Scholar] [CrossRef]
- Sadanori, A. Wound repair and regeneration: Mechanisms, signaling. Int. J. Mol.Sci. 2019, 20, 6328. [Google Scholar]
- Jeschke, M.G.; Rehou, S.; McCann, M.R.; Sharhrokhi, S. Allogeneic mesenchymal stem cells for treatment of severe burn injury. Stem Cell Res. Ther. 2019, 10, 337. [Google Scholar] [CrossRef]
- Thomas, S. A review of the physical. Biological and clinical properties of a bacterial cellulose wound dressing. J. Wound Care 2008, 17, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Laurienzo, P. Marine polysaccharides in pharmaceutical applications: An overview. Mar. Drugs 2010, 8, 2435–2465. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, X.; Hu, T.; Chen, B.; Yin, Z.; Ma, P.X.; Guo, B. Adhesive hemostatic conducting injectable composite hydrogels with sustained drug release and photothermal antibacterial activity to promote full-thickness skinregeneration during wound healing. Small 2019, 15, 1900046. [Google Scholar] [CrossRef]
- Tao, G.; Cai, R.; Wang, Y.; Liu, L.; Zuo, H.; Zhao, P.; Umar, A.; Mao, C.; Xia, Q.; He, H. Bioinspired design of AgNPs embedded silk sericin-based sponges for efficiently combating bacteria and promoting wound healing. Mater. Des. 2019, 180, 107940. [Google Scholar] [CrossRef]
- Shefa, A.A.; Sultana, T.; Park, M.K.; Lee, S.Y.; Gwon, J.G.; Lee, B.T. Curcumin incorporation into an oxidized cellulose nanofiber-polyvinyl alcohol hydrogel system promotes wound healing. Mater. Des. 2020, 186, 108313. [Google Scholar] [CrossRef]
- Hao, Y.; Zhao, W.; Zhang, L.; Zeng, X.; Sun, Z.; Zhang, D.; Shen, P.; Li, Z.; Han, Y.; Li, P.; et al. Bio-multifunctional alginate/chitosan/fucoidan sponges with enhanced angiogenesis and hair follicle regeneration for promoting full-thickness wound healing. Mater. Des. 2020, 193, 108863. [Google Scholar] [CrossRef]
- Jiang, W.; Fu, Y.; Yang, F.; Liu, T.; Zheng, W.; Zeng, L.; Chen, T. Gracilaria lemaneiformis polysaccharide as integrin-targeting surface decorator of selenium nanoparticles to achieve enhanced anticancer efficacy. ACS Appl. Mater. Interfaces 2014, 6, 13738–13748. [Google Scholar] [CrossRef] [PubMed]
- Ferro, V.; Dredge, K.; Liu, L.; Hammond, E.; Bytheway, I.; Li, C.; Johnstone, K.; Karoli, T.; Davis, K.; Copeman, E.; et al. PI-88 and novel heparin sulfate mimetics inhibit angiogenesis. Semin. Thromb. Hemost. 2007, 33, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Suzuki-Hirano, A.; Shimogori, T. The role of Fgf8 in telncephalic and diencephalic patterning. Semin. Cell Dev. Biol. 2009, 20, 719–725. [Google Scholar] [CrossRef]
- Lu, Y.; Mei, S.; Wang, P.; Ouyang, P.; Liao, X.; Ye, H.; Wu, K.; Ma, X. Protective effects of Gracilaria lemaneiformis extract against ultraviolet B-induced damage in HaCaT cells. Pharmacogn. Mag. 2020, 16, 510. [Google Scholar]
- Ouyang, Q.; Li, Y.; Mei, S.; Zhang, Q.; Li, X.; Luo, H.; Zhu, Y.; Wu, K. Protective effects of GLHP from Gracilaria lemaneiformis against UVB-induced photodamage in human immortalized keratinocytes cells and BALB/c mice. Exp. Gerontol. 2021, 155, 111550. [Google Scholar] [CrossRef]
- Huang, Y.C.; Liu, T.J. Mobilization of mesenchymal stem cells by stromal cell-derived factor-1 released from chitosan/tripolyphosphate/fucoidan nanoparticles. Acta Biomater. 2012, 8, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.; Xu, H.; Zhong, Y.; Wu, Q.; Liu, Z. Surface modification of patterned electrospun nanofibrous films via the adhesion of DOPA-bFGF and DOPA-ponericin G1 for skin wound healing. Mater. Des. 2020, 188, 108432. [Google Scholar] [CrossRef]
- Ouyang, Q.; Hou, T.; Li, C.; Hu, Z.; Liang, L.; Li, S.; Zhong, Q.; Li, P. Construction of a composite sponge containing tilapia peptides and chitosan with improved hemostatic performance. Int. J. Biol. Macromol. 2019, 139, 719–729. [Google Scholar] [CrossRef]
- Don, T.M.; King, C.F.; Chiu, W.Y.; Peng, C.A. Preparation and characterization of chitosan-g-poly(vinyl alcohol)/poly(vinyl alcohol) blends used for the evaluation of blood-contacting compatibility. Carbohydr. Polym. 2006, 63, 331–339. [Google Scholar] [CrossRef]
- Brugnerotto, J.; Lizardi, J.; Goycoolea, F.M.; Argüelles-Monal, W.; Desbrières, J.; Rinaudo, M. An infrared investigation in relation with chitin and chitosan characterization. Polymer 2001, 42, 3569–3580. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, J.; Zhou, J.; Yang, G.; Du, Y. Blend membranes from carboxymethylated chitosan/alginate in aqueous solution. J. Appl. Polym. Sci. 2000, 77, 610–616. [Google Scholar] [CrossRef]
- Su, C.; Zhao, H.; Yang, H.; Chen, R. Stearic acid-modified starch/chitosan composite sponge with asymmetric and gradient wettability for wound dressing. ACS Appl. Bio. Mater. 2019, 2, 171–181. [Google Scholar] [CrossRef]
- Doulabi, A.H.; Mirzadeh, H.; Imani, M.; Samadi, N. Chitosan/polyethylene glycol fumarate blend film: Physical and antibacterial properties. Carbohydr. Polym. 2013, 92, 48–56. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Mohanty, M.; Umashankar, P.R.; Jayakrishnan, A. Evaluation of an in situ forming hydrogel wound dressing based on oxidized alginate and gelatin. Biomaterials 2005, 26, 6335–6342. [Google Scholar] [CrossRef]
- Goh, M.; Hwang, Y.; Tae, G. Epidermal growth factor loaded heparin-based hydrogel sheet for skin wound healing. Carbohydr. Polym. 2016, 147, 251–260. [Google Scholar] [CrossRef]
- Ouyang, Q.; Kong, S.; Huang, Y.; Ju, X.; Li, S.; Li, P.; Luo, H. Preparation of nano-hydroxyapatite/chitosan/tilapia skin peptides hydrogels and its burn wound treatment. Int. J. Biol. Macromol. 2021, 181, 369–377. [Google Scholar]
- Dai, M.; Zheng, X.; Xu, X.; Kong, X.; Li, X.; Guo, G.; Luo, F.; Zhao, X.; Wei, Y.Q.; Qian, Z. Chitosan-alginate sponge: Preparation and application in curcumin delivery for dermal wound healing in rat. J. Biomed. Biotechnol. 2009, 2009, 595126. [Google Scholar] [CrossRef]
- Meng, X.; Tian, F.; Yang, J.; He, C.N.; Xing, N.; Li, F. Chitosan and alginate polyelectrolyte complex membranes and their properties for wound dressing application. J. Mater. Sci. Mater. Med. 2010, 21, 1751–1759. [Google Scholar] [CrossRef]
- Li, M.; Li, H.; Li, X.; Zhu, H.; Xu, Z.; Liu, L.; Ma, J.; Zhang, M. A bioinspired alginate-gum Arabic hydrogel with micro-/nanoscale structures for controlled drug release in chronic wound healing. ACS Appl. Mater. Interfaces 2017, 9, 22160–22175. [Google Scholar] [CrossRef] [PubMed]
- Margolis, G.; Polyak, B.; Cohen, S. Magnetic induction of multiscale anisotropy in macroporous alginate scaffolds. Nano Lett. 2018, 18, 7314–7322. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Shan, X.; Zhao, X.; Zha, X.; Chen, H.; Wang, X.; Cai, J.; Wang, C.; Li, X.; Hao, G.; et al. Spongy bilayer dressing composed of chitosan-Ag nanoparticles and chitosan-Bletilla striata polysaccharide for wound healing applications. Carbohydr. Polym. 2017, 157, 1538–1547. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.S.; Fu, X.B.; Wang, P. Effects of guaichuang paste on the healing of surface ulcers in experimental animals. J. Exter. Ther. Tradit. Chin. Med. 2007, 6, 5–6. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quan, W.; Li, P.; Wei, J.; Jiang, Y.; Liang, Y.; Zhang, W.; Chen, Q.; Wu, K.; Luo, H.; Ouyang, Q. Bio-Multifunctional Sponges Containing Alginate/Chitosan/Sargassum Polysaccharides Promote the Healing of Full-Thickness Wounds. Biomolecules 2022, 12, 1601. https://doi.org/10.3390/biom12111601
Quan W, Li P, Wei J, Jiang Y, Liang Y, Zhang W, Chen Q, Wu K, Luo H, Ouyang Q. Bio-Multifunctional Sponges Containing Alginate/Chitosan/Sargassum Polysaccharides Promote the Healing of Full-Thickness Wounds. Biomolecules. 2022; 12(11):1601. https://doi.org/10.3390/biom12111601
Chicago/Turabian StyleQuan, Weiyan, Puwang Li, Jinsong Wei, Yuwei Jiang, Yingye Liang, Weilin Zhang, Qizhou Chen, Kefeng Wu, Hui Luo, and Qianqian Ouyang. 2022. "Bio-Multifunctional Sponges Containing Alginate/Chitosan/Sargassum Polysaccharides Promote the Healing of Full-Thickness Wounds" Biomolecules 12, no. 11: 1601. https://doi.org/10.3390/biom12111601
APA StyleQuan, W., Li, P., Wei, J., Jiang, Y., Liang, Y., Zhang, W., Chen, Q., Wu, K., Luo, H., & Ouyang, Q. (2022). Bio-Multifunctional Sponges Containing Alginate/Chitosan/Sargassum Polysaccharides Promote the Healing of Full-Thickness Wounds. Biomolecules, 12(11), 1601. https://doi.org/10.3390/biom12111601





