Parkin Inhibits RANKL-Induced Osteoclastogenesis and Ovariectomy-Induced Bone Loss
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Bone-Marrow-Derived Monocyte Isolation and Osteoclast Differentiation
2.3. Cell Counting Kit-8 Assay
2.4. F-Actin Ring Formation Analysis
2.5. Osteoclastic Bone Resorption Pit Assay
2.6. Quantitative Real-Time Polymerase Chain Reaction
2.7. Western Blot Analysis
2.8. ROS Detection In Vitro
2.9. In Vivo Evaluation
2.10. Statistical Analysis
3. Results
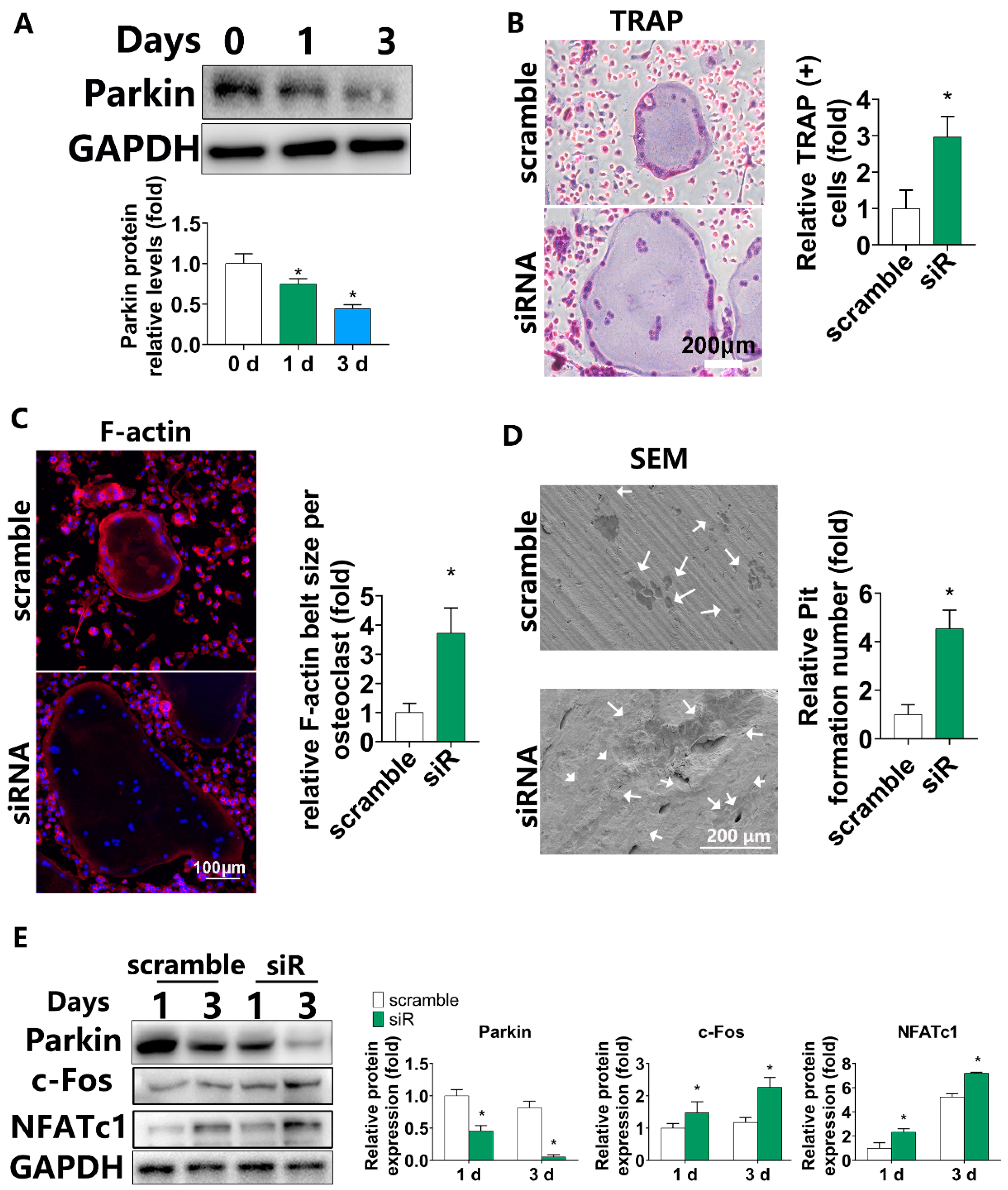
3.1. Parkin Expression Gradually Decreased during Osteoclastic Differentiation and Downregulation of PARKIN by siRNA Increased Osteoclastogenesis in BMMs
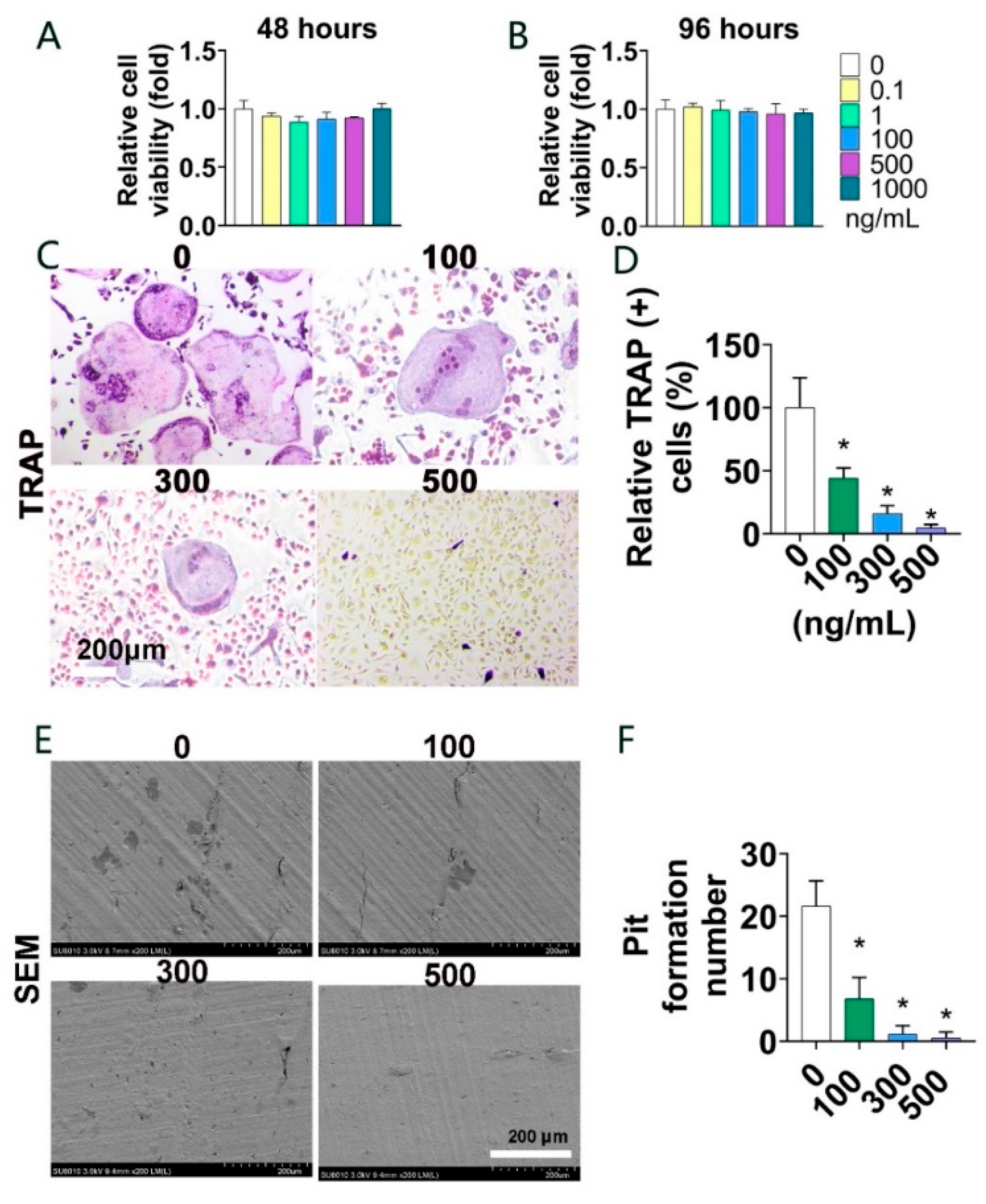
3.2. Parkin Protein Suppresses Osteoclast Formation and Bone Resorption In Vitro
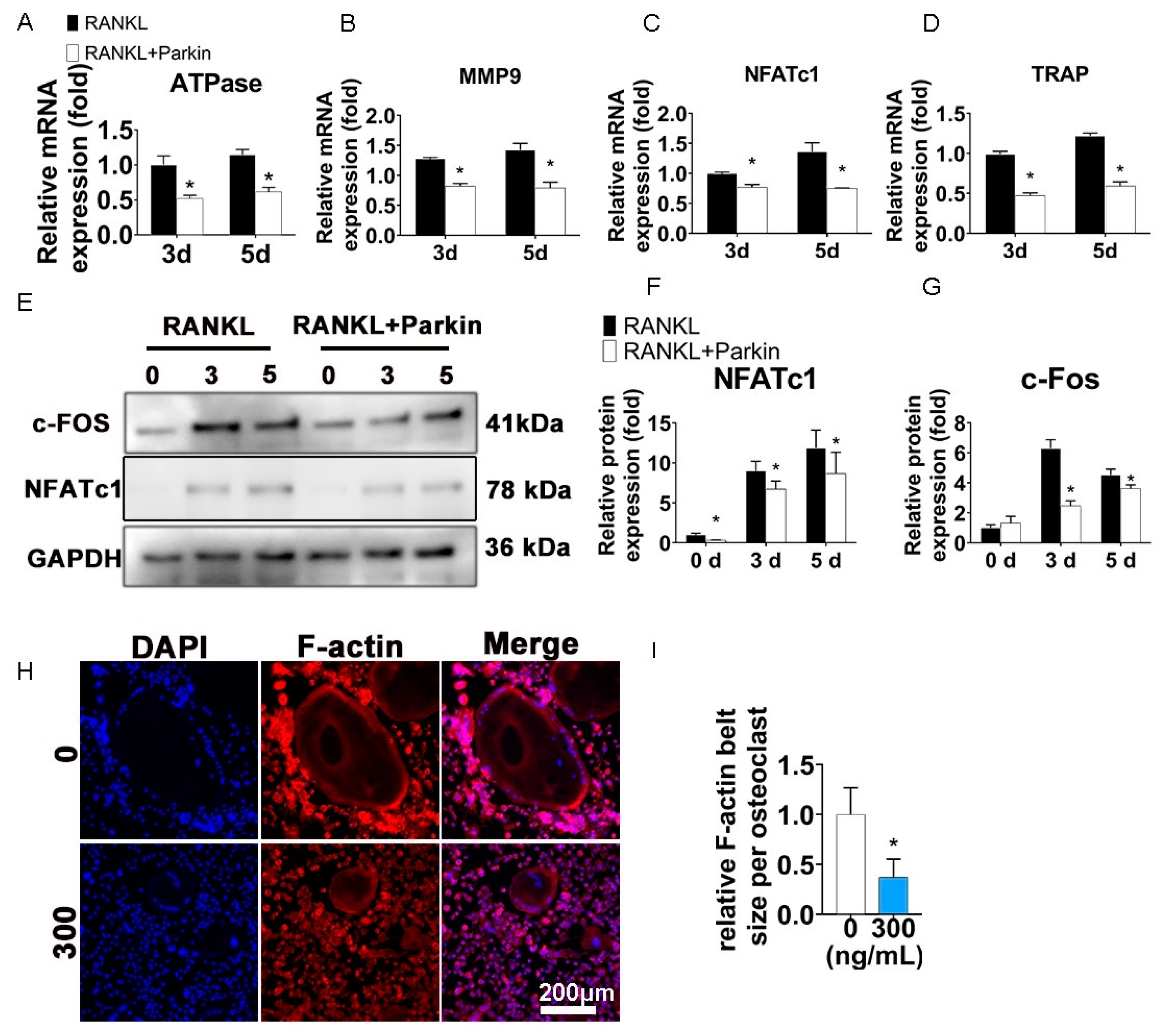
3.3. Parkin Protein Inhibits Osteoclast-Specific Genes and F-Actin Ring Formation
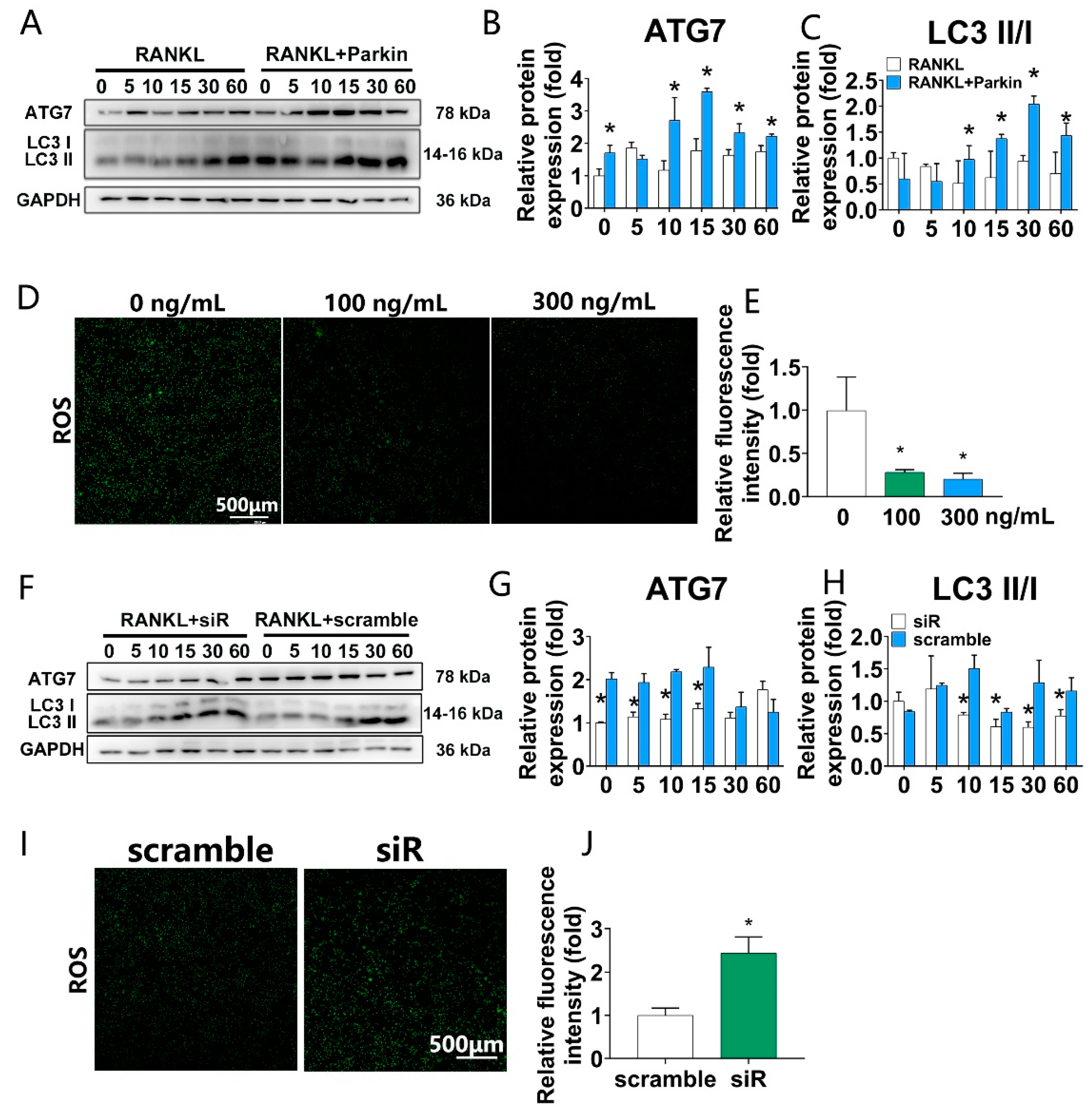
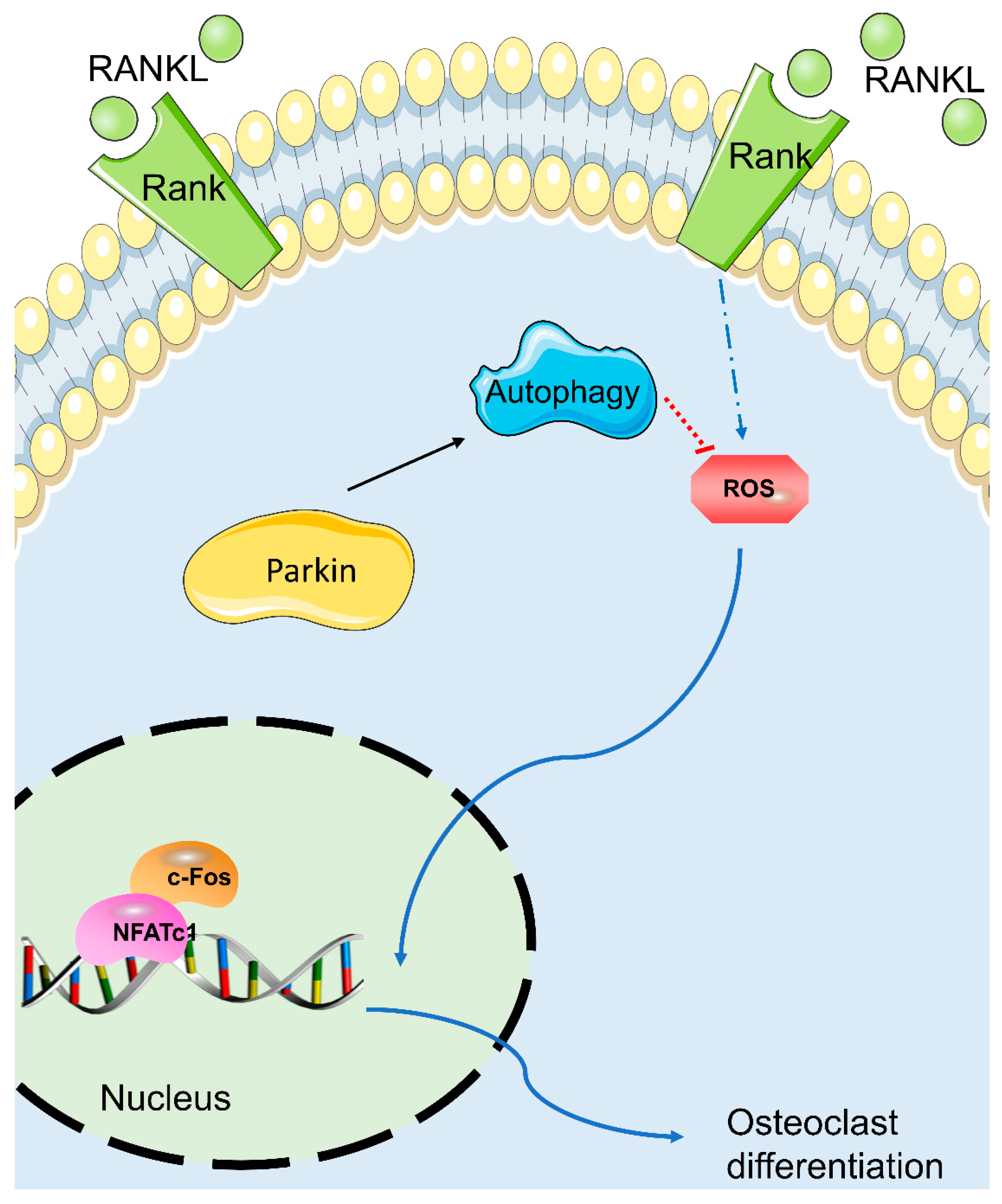
3.4. Parkin Protein Increases Autophagy and Decreases RANKL-Induced ROS Levels, While Parkin Knockdown Decreases Autophagy and Increases RANKL-Induced ROS Levels during Osteoclastogenesis in BMMs
3.5. Parkin Delayed the Progression of OVX-Induced Osteoporosis In Vivo
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Osteoporosis: Overlooked in men for too long. Lancet Diabetes Endocrinol. 2021, 9, 1. [CrossRef]
- Lu, T.; Forgetta, V.; Keller-Baruch, J.; Nethander, M.; Bennett, D.; Forest, M.; Bhatnagar, S.; Walters, R.G.; Lin, K.; Chen, Z.; et al. Improved prediction of fracture risk leveraging a genome-wide polygenic risk score. Genome Med. 2021, 13, 16. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Jang, Y.-J.; Lee, H.; Kim, E.; Kim, Y.; Yoo, T.-K.; Hyun, T.K.; Park, J.-I.; Yi, S.-J.; Kim, K. Transcriptome Analysis Reveals That Abeliophyllum distichum Nakai Extract Inhibits RANKL-Mediated Osteoclastogenensis Mainly Through Suppressing Nfatc1 Expression. Biology 2020, 9, 212. [Google Scholar] [CrossRef] [PubMed]
- Seirafi, M.; Kozlov, G.; Gehring, K. Parkin structure and function. FEBS J. 2015, 282, 2076–2088. [Google Scholar] [CrossRef] [PubMed]
- Maraschi, A.; Ciammola, A.; Folci, A.; Sassone, F.; Ronzitti, G.; Cappelletti, G.; Silani, V.; Sato, S.; Hattori, N.; Mazzanti, M.; et al. Parkin regulates kainate receptors by interacting with the GluK2 subunit. Nat. Commun. 2014, 5, 5182. [Google Scholar] [CrossRef]
- Panicker, N.; Dawson, V.L.; Dawson, T.M. Activation mechanisms of the E3 ubiquitin ligase parkin. Biochem. J. 2017, 474, 3075–3086. [Google Scholar] [CrossRef]
- Chen, C.C.W.; Erlich, A.T.; Crilly, M.J.; Hood, D.A. Parkin is required for exercise-induced mitophagy in muscle: Impact of aging. Am. J. Physiol. Metab. 2018, 315, E404–E415. [Google Scholar] [CrossRef]
- Marycz, K.; Kornicka, K.; Marędziak, M.; Golonka, P.; Nicpoń, J. Equine metabolic syndrome impairs adipose stem cells osteogenic differentiation by predominance of autophagy over selective mitophagy. J. Cell Mol. Med. 2016, 20, 2384–2404. [Google Scholar] [CrossRef]
- Wang, S.; Deng, Z.; Ma, Y.; Jin, J.; Qi, F.; Li, S.; Liu, C.; Lyu, F.-J.; Zheng, Q. The Role of Autophagy and Mitophagy in Bone Metabolic Disorders. Int. J. Biol. Sci. 2020, 16, 2675–2691. [Google Scholar] [CrossRef]
- Narendra, D.; Tanaka, A.; Suen, D.-F.; Youle, R.J. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J. Cell Biol. 2008, 183, 795–803. [Google Scholar] [CrossRef]
- Sliter, D.A.; Martinez, J.; Hao, L.; Chen, X.; Sun, N.; Fischer, T.D.; Burman, J.L.; Li, Y.; Zhang, Z.; Narendra, D.P.; et al. Parkin and PINK1 mitigate STING-induced inflammation. Nature 2018, 561, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Barodia, S.K.; Creed, R.B.; Goldberg, M.S. Parkin and PINK1 functions in oxidative stress and neurodegeneration. Brain Res. Bull. 2017, 133, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Basit, F.; Van Oppen, L.M.P.E.; Schöckel, L.; Bossenbroek, H.M.; Van Emst-de Vries, S.E.; Hermeling, J.C.W.; Grefte, S.; Kopitz, C.; Heroult, M.; Willems, P.H.; et al. Mitochondrial complex I inhibition triggers a mitophagy-dependent ROS increase leading to necroptosis and ferroptosis in melanoma cells. Cell Death Dis. 2017, 8, e2716. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Hou, W.; Chen, M.; Chen, E.; Xue, D.; Ye, C.; Li, W.; Pan, Z. Upregulation of Parkin Accelerates Osteoblastic Differentiation of Bone Marrow-Derived Mesenchymal Stem Cells and Bone Regeneration by Enhancing Autophagy and beta-Catenin Signaling. Front. Cell Dev. Biol. 2020, 8, 576104. [Google Scholar] [CrossRef]
- Lorenzo, J. The many ways of osteoclast activation. J. Clin. Investig. 2017, 127, 2530–2532. [Google Scholar] [CrossRef]
- Kearns, A.E.; Khosla, S.; Kostenuik, P.J. Receptor activator of nuclear factor kappaB ligand and osteoprotegerin regulation of bone remodeling in health and disease. Endocr. Rev. 2008, 29, 155–192. [Google Scholar] [CrossRef]
- Wang, S.; Ma, Q.; Xie, Z.; Shen, Y.; Zheng, B.; Jiang, C.; Yuan, P.; An, Q.; Fan, S.; Jie, Z. An Antioxidant Sesquiterpene Inhibits Osteoclastogenesis Via Blocking IPMK/TRAF6 and Counteracts OVX-Induced Osteoporosis in Mice. J. Bone Miner. Res. 2021, 36, 1850–1865. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, C.; Wang, G.; Sun, Y.; Deng, Z.; Chen, L.; Chen, K.; Tickner, J.; Kenny, J.; Song, D.; et al. Loureirin B suppresses RANKL-induced osteoclastogenesis and ovariectomized osteoporosis via attenuating NFATc1 and ROS activities. Heranostics 2019, 9, 4648–4662. [Google Scholar] [CrossRef]
- Goettsch, C.; Babelova, A.; Trummer, O.; Erben, R.G.; Rauner, M.; Rammelt, S.; Weissmann, N.; Weinberger, V.; Benkhoff, S.; Kampschulte, M.; et al. NADPH oxidase 4 limits bone mass by promoting osteoclastogenesis. J. Clin. Investig. 2013, 123, 4731–4738. [Google Scholar] [CrossRef]
- Lee, N.K.; Choi, Y.G.; Baik, J.Y.; Han, S.Y.; Jeong, D.-W.; Bae, Y.S.; Kim, N.; Lee, S.Y. A crucial role for reactive oxygen species in RANKL-induced osteoclast differentiation. Blood 2005, 106, 852–859. [Google Scholar] [CrossRef]
- Lean, J.M.; Davies, J.T.; Fuller, K.; Jagger, C.J.; Kirstein, B.; Partington, G.A.; Urry, Z.L.; Chambers, T.J. A crucial role for thiol antioxidants in estrogen-deficiency bone loss. J. Clin. Investig. 2003, 112, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Li, S.; Jiang, N.; Shao, X.; Zhang, M.; Jin, H.; Zhen, Z.; Jianxiao, S.; Yijun, Z.; Wenyan, Z. PINK1-parkin pathway of mitophagy protects against contrast-induced acute kidney injury via decreasing mitochondrial ROS and NLRP3 inflammasome activation. Redox Biol. 2019, 26, 101254. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.Y.; Khan, N.M.; Ahmad, I.; Haqqi, T.M. Parkin clearance of dysfunctional mitochondria regulates ROS levels and increases survival of human chondrocytes. Osteoarthr. Cartil. 2018, 26, 1087–1097. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, J.; Dai, B.; Wang, X.; Guo, Q.; Qin, L. Targeting autophagy in osteoporosis: From pathophysiology to potential therapy. Ageing Res. Rev. 2020, 62, 101098. [Google Scholar] [CrossRef]
- Montaseri, A.; Giampietri, C.; Rossi, M.; Riccioli, A.; Del Fattore, A.; Filippini, A. The Role of Autophagy in Osteoclast Differentiation and Bone Resorption Function. Biomolecules 2020, 10, 1398. [Google Scholar] [CrossRef]
- Li, J.-Y.; Tawfeek, H.; Bedi, B.; Yang, X.; Adams, J.; Gao, K.Y.; Zayzafoon, M.; Weitzmann, M.N.; Pacifici, R. Ovariectomy disregulates osteoblast and osteoclast formation through the T-cell receptor CD40 ligand. Proc. Natl. Acad. Sci. USA 2010, 108, 768–773. [Google Scholar] [CrossRef]
- Ni, S.; Yuan, Y.; Qian, Z.; Zhong, Z.; Lv, T.; Kuang, Y.; Yu, B. Hypoxia inhibits RANKL-induced ferritinophagy and protects osteoclasts from ferroptosis. Free Radic. Biol. Med. 2021, 169, 271–282. [Google Scholar] [CrossRef]
- Ye, C.; Hou, W.; Chen, M.; Lu, J.; Chen, E.; Tang, L.; Hang, K.; Ding, Q.; Li, Y.; Zhang, W.; et al. IGFBP7 acts as a negative regulator of RANKL-induced osteoclastogenesis and oestrogen deficiency-induced bone loss. Cell Prolif. 2019, 53, e12752. [Google Scholar] [CrossRef]
- Jin, S.; Zhang, Q.Y.; Kang, X.M.; Wang, J.X.; Zhao, W.H. Daidzein induces MCF-7 breast cancer cell apoptosis via the mitochondrial pathway. Ann. Oncol. 2010, 21, 263–268. [Google Scholar] [CrossRef]
- Hou, W.; Ye, C.; Chen, M.; Gao, W.; Xie, X.; Wu, J.; Zhang, K.; Zhang, W.; Zheng, Y.; Cai, X. Excavating bioactivities of nanozyme to remodel microenvironment for protecting chondrocytes and delaying osteoarthritis. Bioact. Mater. 2021, 6, 2439–2451. [Google Scholar] [CrossRef]
- Kim, H.; Wrann, C.D.; Jedrychowski, M.; Vidoni, S.; Kitase, Y.; Nagano, K.; Brooks, D.; Alexander, R.; Kaitlyn, G.; Laura, M.; et al. Irisin Mediates Effects on Bone and Fat via alphaV Integrin Receptors. Cell 2018, 175, 1756–1768.e17. [Google Scholar] [CrossRef] [PubMed]
- Garbe, A.I.; Roscher, A.; Schüler, C.; Lutter, A.-H.; Glösmann, M.; Bernhardt, R.; Chopin, M.; Hempel, U.; Hofbauer, L.C.; Rammelt, S.; et al. Regulation of bone mass and osteoclast function depend on the F-actin modulator SWAP-70. J. Bone Miner. Res. 2012, 27, 2085–2096. [Google Scholar] [CrossRef] [PubMed]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xiao, Z.; Quarles, L.D.; Li, W. Osteoporosis: Mechanism, Molecular Target and Current Status on Drug Development. Curr. Med. Chem. 2021, 28, 1489–1507. [Google Scholar] [CrossRef]
- Callaway, D.A.; Jiang, J.X. Reactive oxygen species and oxidative stress in osteoclastogenesis, skeletal aging and bone diseases. J. Bone Miner. Metab. 2015, 33, 359–370. [Google Scholar] [CrossRef]
- Henriksen, K.; Bollerslev, J.; Everts, V.; Karsdal, M.A. Osteoclast activity and subtypes as a function of physiology and pathology--implications for future treatments of osteoporosis. Endocr. Rev. 2011, 32, 31–63. [Google Scholar] [CrossRef]
- Seo, W.; Lee, S.; Tran, P.T.; Ngo, T.Q.-M.; Kim, O.; Le, T.H.; Dang, N.H.; Hwangbo, C.; Min, B.S.; Lee, J.-H. 3-Hydroxyolean-12-en-27-oic Acids Inhibit RANKL-Induced Osteoclastogenesis in Vitro and Inflammation-Induced Bone Loss in Vivo. Int. J. Mol. Sci. 2020, 21, 5240. [Google Scholar] [CrossRef]
- Kim, J.H.; Youn, B.U.; Kim, K.; Moon, J.B.; Lee, J.; Nam, K.-I.I.; Park, Y.-W.; O’Leary, D.D.M.; Kim, N. Lhx2 regulates bone remodeling in mice by modulating RANKL signaling in osteoclasts. Cell Death Differ. 2014, 21, 1613–1621. [Google Scholar] [CrossRef]
- Mizushima, N. The ATG conjugation systems in autophagy. Curr. Opin. Cell Biol. 2019, 63, 1–10. [Google Scholar] [CrossRef]
- Yu, S.; Yu, M.; He, X.; Wen, L.; Bu, Z.; Feng, J. KCNQ1OT1 promotes autophagy by regulating miR-200a/FOXO3/ATG7 pathway in cerebral ischemic stroke. Aging Cell 2019, 18, e12940. [Google Scholar] [CrossRef]
- Laha, D.; Deb, M.; Das, H. KLF2 (kruppel-like factor 2 [lung]) regulates osteoclastogenesis by modulating autophagy. Autophagy 2019, 15, 2063–2075. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.Y.; Beyer, C.; Giessl, A.; Kireva, T.; Scholtysek, C.; Uderhardt, S.; Brian, S.; Oliver, D.; Georg, S.; Jörg, D.; et al. Autophagy regulates TNFalpha-mediated joint destruction in experimental arthritis. Ann. Rheum. Dis. 2013, 72, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Pickrell, A.M.; Youle, R.J. The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson’s disease. Neuron 2015, 85, 257–273. [Google Scholar] [CrossRef] [PubMed]
- Araya, J.; Tsubouchi, K.; Sato, N.; Ito, S.; Minagawa, S.; Hara, H.; Hosaka, Y.; Ichikawa, A.; Saito, N.; Kadota, T.; et al. PRKN-regulated mitophagy and cellular senescence during COPD pathogenesis. Autophagy 2018, 15, 510–526. [Google Scholar] [CrossRef] [PubMed]
- Agidigbi, T.S.; Kim, C. Reactive Oxygen Species in Osteoclast Differentiation and Possible Pharmaceutical Targets of ROS-Mediated Osteoclast Diseases. Int. J. Mol. Sci. 2019, 20, 3576. [Google Scholar] [CrossRef]
- Im, E.; Yoo, L.; Hyun, M.; Shin, W.H.; Chung, K.C. Covalent ISG15 conjugation positively regulates the ubiquitin E3 ligase activity of parkin. Open Biol. 2016, 6, 160193. [Google Scholar] [CrossRef]
- Malochet-Guinamand, S.; Durif, F.; Thomas, T. Parkinson’s disease: A risk factor for osteoporosis. Jt. Bone Spine 2015, 82, 406–410. [Google Scholar] [CrossRef]

| Gene | Reverse (5′-3′) | Reverse (3′-5′) |
|---|---|---|
| TRAP | CACTCCCACCCTGAGATTTGT | CCCCAGAGACATGATGAAGTCA |
| NFATc1 | GGAGAGTCCGAGAATCGAGAT | TTGCAGCTAGGAAGTACGTCT |
| ATPase | CAGAGCTGTACTTCAATGTGGAC | AGGTCTCACACTGCACTAGGT |
| MMP-9 | CTGGACAGCCAGACACTAAAG | CTCGCGGCAAGTCTTCAGAG |
| GAPDH | CGACTTCAACAGCAACTCCCACTCTTCC | TGGGTGGTCCAGGGTTTCTTACTCCTT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, W.; Chen, M.; Ye, C.; Chen, E.; Li, W.; Zhang, W. Parkin Inhibits RANKL-Induced Osteoclastogenesis and Ovariectomy-Induced Bone Loss. Biomolecules 2022, 12, 1602. https://doi.org/10.3390/biom12111602
Hou W, Chen M, Ye C, Chen E, Li W, Zhang W. Parkin Inhibits RANKL-Induced Osteoclastogenesis and Ovariectomy-Induced Bone Loss. Biomolecules. 2022; 12(11):1602. https://doi.org/10.3390/biom12111602
Chicago/Turabian StyleHou, Weiduo, Mo Chen, Chenyi Ye, Erman Chen, Weixu Li, and Wei Zhang. 2022. "Parkin Inhibits RANKL-Induced Osteoclastogenesis and Ovariectomy-Induced Bone Loss" Biomolecules 12, no. 11: 1602. https://doi.org/10.3390/biom12111602
APA StyleHou, W., Chen, M., Ye, C., Chen, E., Li, W., & Zhang, W. (2022). Parkin Inhibits RANKL-Induced Osteoclastogenesis and Ovariectomy-Induced Bone Loss. Biomolecules, 12(11), 1602. https://doi.org/10.3390/biom12111602





