Hydrophobic Rose Bengal Derivatives Exhibit Submicromolar-to-Subnanomolar Activity against Enveloped Viruses
Abstract
:1. Introduction
2. Materials and Methods
2.1. General
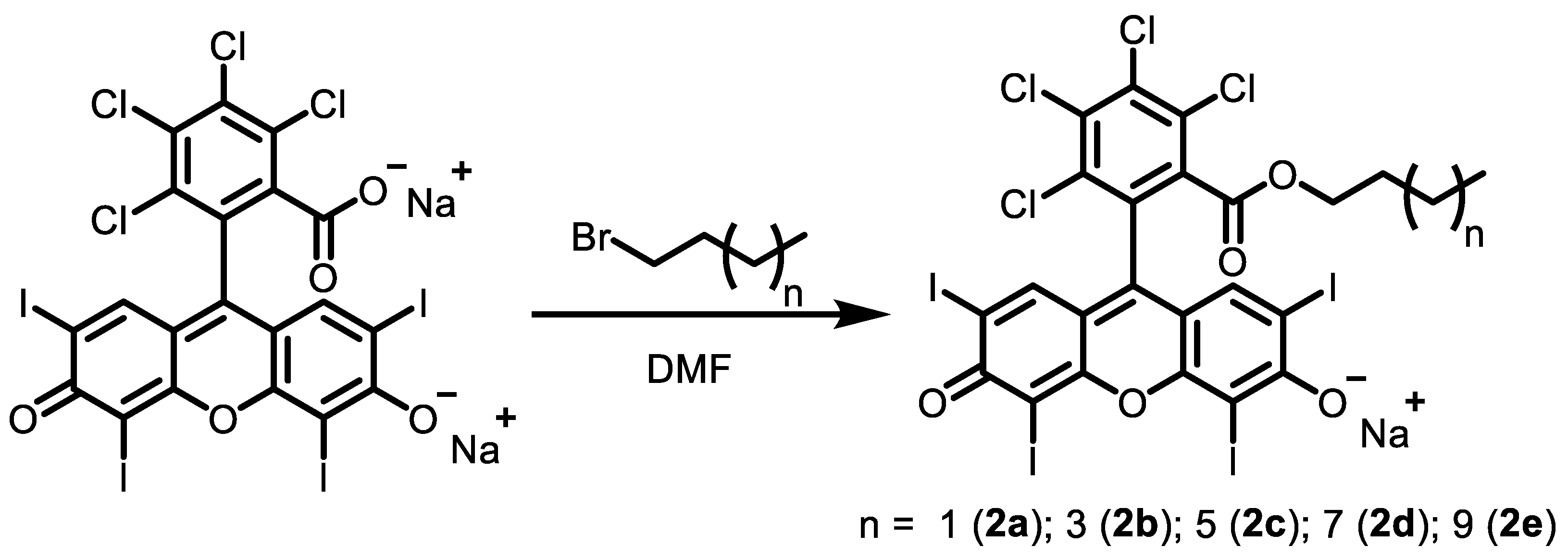
2.2. Synthesis
2.2.1. Rose Bengal N-Butyl Ester, Monosodium Salt 2a
2.2.2. Rose Bengal N-Hexyl Ester, Monosodium Salt 2b
2.2.3. Rose Bengal N-Octyl Ester, Monosodium Salt 2c
2.2.4. Rose Bengal N-Decyl Ester, Monosodium Salt 2d
2.2.5. Rose Bengal N-Dodecyl Ester, Monosodium Salt 2e
2.3. Assessment of 1O2 Generation
2.4. Solubility
2.5. Biological Activity
2.5.1. Cells and Viruses
2.5.2. Cell Viability Assay (for Vero Cells)
2.5.3. Cell Viability Assay (for RD and MT-4 Cells)
2.5.4. Virus-Induced Cytopathic Effect Inhibition Test (SARS-CoV-2, CHIKV)
2.5.5. Cytopathic Effect Inhibition Test (HIV)
2.5.6. Cytopathic Effect Inhibition Test (CVA16, E13 and PV1)
3. Results and Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nguyen, T.H.T.; Guedj, J.; Anglaret, X.; Laouénan, C.; Madelain, V.; Taburet, A.-M.; Baize, S.; Sissoko, D.; Pastorino, B.; Rodallec, A.; et al. Favipiravir pharmacokinetics in Ebola-Infected patients of the JIKI trial reveals concentrations lower than targeted. PLoS Negl. Trop. Dis. 2017, 11, e0005389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agrawal, U.; Raju, R.; Udwadia, Z.F. Favipiravir: A new and emerging antiviral option in COVID-19. Med. J. Armed Forces India 2020, 76, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.E.; Maidhof, A.; Taschner, H.; Zahn, R.K. Virazole (1-β-d-ribofuranosyl-1,2,4-triazole-3-carboxamide; A cytostatic agent. Biochem. Pharmacol. 1977, 26, 1071–1075. [Google Scholar] [CrossRef]
- Jorgensen, S.C.; Kebriaei, R.; Dresser, L.D. Remdesivir: Review of Pharmacology, Pre-clinical Data, and Emerging Clinical Experience for COVID-19. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2020, 40, 659–671. [Google Scholar] [CrossRef]
- Johnson, E.M.; Doyle, J.D.; Wetzel, J.D.; McClung, R.P.; Katunuma, N.; Chappell, J.D.; Washington, M.K.; Dermody, T.S. Genetic and Pharmacologic Alteration of Cathepsin Expression Influences Reovirus Pathogenesis. J. Virol. 2009, 83, 9630–9640. [Google Scholar] [CrossRef] [Green Version]
- Satoh, S.; Mori, K.; Onomura, D.; Ueda, Y.; Dansako, H.; Honda, M.; Kaneko, S.; Ikeda, M.; Kato, N. Ribavirin suppresses hepatic lipogenesis through inosine monophosphate dehydrogenase inhibition: Involvement of adenosine monophosphate-activated protein kinase-related kinases and retinoid X receptor α. Hepatol. Commun. 2017, 1, 550–563. [Google Scholar] [CrossRef]
- Furuta, Y.; Takahashi, K.; Shiraki, K.; Sakamoto, K.; Smee, D.F.; Barnard, D.L.; Gowen, B.B.; Julander, J.G.; Morrey, J.D. T-705 (favipiravir) and related compounds: Novel broad-spectrum inhibitors of RNA viral infections. Antivir. Res. 2009, 82, 95–102. [Google Scholar] [CrossRef]
- Te, H.S.; Randall, G.; Jensen, D.M. Mechanism of action of ribavirin in the treatment of chronic hepatitis C. Gastroenterol. Hepatol. 2007, 3, 218–225. [Google Scholar]
- Mariewskaya, K.; Tyurin, A.; Chistov, A.; Korshun, V.; Alferova, V.; Ustinov, A. Photosensitizing Antivirals. Molecules 2021, 26, 3971. [Google Scholar] [CrossRef]
- Kunstek, H.; Vreken, F.; Keita, A.; Hamblin, M.R.; Dumarçay, F.; Varbanov, M. Aspects of Antiviral Strategies Based on Different Phototherapy Approaches: Hit by the Light. Pharmaceuticals 2022, 15, 858. [Google Scholar] [CrossRef]
- Proskurin, G.V.; Orlov, A.A.; Brylev, V.; Kozlovskaya, L.I.; Chistov, A.A.; Karganova, G.G.; Palyulin, V.A.; Osolodkin, D.I.; Korshun, V.A.; Aralov, A.V. 3′-O-Substituted 5-(perylen-3-ylethynyl)-2′-deoxyuridines as tick-borne encephalitis virus reproduction inhibitors. Eur. J. Med. Chem. 2018, 155, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Colpitts, C.C.; Ustinov, A.V.; Epand, R.F.; Epand, R.M.; Korshun, V.A.; Schang, L.M. 5-(Perylen-3-yl)Ethynyl-arabino-Uridine (aUY11), an Arabino-Based Rigid Amphipathic Fusion Inhibitor, Targets Virion Envelope Lipids To Inhibit Fusion of Influenza Virus, Hepatitis C Virus, and Other Enveloped Viruses. J. Virol. 2013, 87, 3640–3654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aralov, A.V.; Proskurin, G.V.; Orlov, A.A.; Kozlovskaya, L.I.; Chistov, A.A.; Kutyakov, S.V.; Karganova, G.G.; Palyulin, V.A.; Osolodkin, D.I.; Korshun, V.A. Perylenyltriazoles inhibit reproduction of enveloped viruses. Eur. J. Med. Chem. 2017, 138, 293–299. [Google Scholar] [CrossRef] [PubMed]
- St.Vincent, M.R.; Colpitts, C.C.; Ustinov, A.V.; Muqadas, M.; Joyce, M.A.; Barsby, N.L.; Epand, R.F.; Epand, R.M.; Khramyshev, S.A.; Valueva, O.A.; et al. Rigid amphipathic fusion inhibitors, small molecule antiviral compounds against enveloped viruses. Proc. Natl. Acad. Sci. USA 2010, 107, 17339–17344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slesarchuk, N.A.; Khvatov, E.V.; Chistov, A.A.; Proskurin, G.V.; Nikitin, T.D.; Lazarevich, A.I.; Ulanovskaya, A.A.; Ulashchik, E.A.; Orlov, A.; Jegorov, A.V.; et al. Simplistic perylene-related compounds as inhibitors of tick-borne encephalitis virus reproduction. Bioorganic Med. Chem. Lett. 2020, 30, 127100. [Google Scholar] [CrossRef] [PubMed]
- Wolf, M.C.; Freiberg, A.N.; Zhang, T.; Akyol-Ataman, Z.; Grock, A.; Hong, P.W.; Li, J.; Watson, N.F.; Fang, A.Q.; Aguilar, H.C.; et al. A broad-spectrum antiviral targeting entry of enveloped viruses. Proc. Natl. Acad. Sci. USA 2010, 107, 3157–3162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vigant, F.; Lee, J.; Hollmann, A.; Tanner, L.B.; Ataman, Z.A.; Yun, T.; Shui, G.; Aguilar, H.C.; Zhang, D.; Meriwether, D.; et al. A Mechanistic Paradigm for Broad-Spectrum Antivirals that Target Virus-Cell Fusion. PLoS Pathog. 2013, 9, e1003297. [Google Scholar] [CrossRef]
- Vigant, F.; Hollmann, A.; Lee, J.; Santos, N.C.; Jung, M.E.; Lee, B. The Rigid Amphipathic Fusion Inhibitor dUY11 Acts through Photosensitization of Viruses. J. Virol. 2014, 88, 1849–1853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, L.; Wang, M.-D.; Ming, S.-L.; Li, G.-L.; Yu, P.-W.; Qi, Y.-L.; Jiang, D.-W.; Yang, G.-Y.; Wang, J.; Chu, B.-B. An effective inactivant based on singlet oxygen-mediated lipid oxidation implicates a new paradigm for broad-spectrum antivirals. Redox Biol. 2020, 36, 101601. [Google Scholar] [CrossRef]
- Moghaddam, A.; Olszewska, W.; Wang, B.; Tregoning, J.S.; Helson, R.; Sattentau, Q.J.; Openshaw, P.J.M. A potential molecular mechanism for hypersensitivity caused by formalin-inactivated vaccines. Nat. Med. 2006, 12, 905–907. [Google Scholar] [CrossRef]
- Blackburn, N.; Besselaar, T. A study of the effect of chemical inactivants on the epitopes of Rift Valley fever virus glycoproteins using monoclonal antibodies. J. Virol. Methods 1991, 33, 367–374. [Google Scholar] [CrossRef]
- Swenberg, J.A.; Moeller, B.C.; Lu, K.; Rager, J.E.; Fry, R.C.; Starr, T.B. Formaldehyde Carcinogenicity Research: 30 Years and Counting for Mode of Action, Epidemiology, and Cancer Risk Assessment. Toxicol. Pathol. 2012, 41, 181–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bahnemann, H.G. Inactivation of viral antigens for vaccine preparation with particular reference to the application of binary ethylenimine. Vaccine 1990, 8, 299–303. [Google Scholar] [CrossRef]
- Roat, M.I.; Romanowski, E.; Araullo-Cruz, T.; Gordon, Y.J. The Antiviral Effects of Rose Bengal and Fluorescein. Arch. Ophthalmol. 1987, 105, 1415–1417. [Google Scholar] [CrossRef] [PubMed]
- Brooks, S.E.; Kaza, V.; Nakamura, T.; Trousdale, M.D. Photoinactivation of Herpes Simplex Virus by Rose Bengal and Fluorescein: In Vitro and in Vivo Studies. Cornea 1994, 13, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, N.R.; Lenard, J. Antiretroviral activities of hypericin and rose bengal: Photodynamic effects on Friend leukemia virus infection of mice. Antivir. Res. 1993, 21, 119–127. [Google Scholar] [CrossRef]
- Lenard, J.; Rabson, A.; Vanderoef, R. Photodynamic inactivation of infectivity of human immunodeficiency virus and other enveloped viruses using hypericin and rose bengal: Inhibition of fusion and syncytia formation. Proc. Natl. Acad. Sci. USA 1993, 90, 158–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demartis, S.; Obinu, A.; Gavini, E.; Giunchedi, P.; Rassu, G. Nanotechnology-based rose Bengal: A broad-spectrum biomedical tool. Dye. Pigment. 2021, 188, 109236. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Carmagnola, I.; Chiono, V.; Gentile, P.; Fracchia, L.; Ceresa, C.; Georgiev, G.; Ciardelli, G. Surface modification of poly(dimethylsiloxane) by two-step plasma treatment for further grafting with chitosan–Rose Bengal photosensitizer. Surf. Coat. Technol. 2013, 223, 92–97. [Google Scholar] [CrossRef]
- Tegos, G.P.; Hamblin, M.R. Phenothiazinium Antimicrobial Photosensitizers Are Substrates of Bacterial Multidrug Resistance Pumps. Antimicrob. Agents Chemother. 2006, 50, 196–203. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.-J.; Zhou, X.-B.; Wang, A.-L.; Zheng, B.-Y.; Yeh, C.-K.; Huang, J.-D. Synthesis and biological characterization of novel rose bengal derivatives with improved amphiphilicity for sono-photodynamic therapy. Eur. J. Med. Chem. 2018, 145, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Ludvíková, L.; Friš, P.; Heger, D.; Šebej, P.; Wirz, J.; Klán, P. Photochemistry of rose bengal in water and acetonitrile: A comprehensive kinetic analysis. Phys. Chem. Chem. Phys. 2016, 18, 16266–16273. [Google Scholar] [CrossRef] [PubMed]
- Kozlovskaya, L.I.; Volok, V.P.; Shtro, A.A.; Nikolaeva, Y.V.; Chistov, A.A.; Matyugina, E.S.; Belyaev, E.S.; Jegorov, A.V.; Snoeck, R.; Korshun, V.A.; et al. Phenoxazine nucleoside derivatives with a multiple activity against RNA and DNA viruses. Eur. J. Med. Chem. 2021, 220, 113467. [Google Scholar] [CrossRef] [PubMed]
- Sheahan, T.P.; Sims, A.C.; Zhou, S.; Graham, R.L.; Pruijssers, A.J.; Agostini, M.L.; Leist, S.R.; Schäfer, A.; Dinnon, K.H., 3rd; Stevens, L.J.; et al. An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice. Sci. Transl. Med. 2020, 12, eabb5883. [Google Scholar] [CrossRef] [Green Version]
- Kärber, G. Beitrag zur kollektiven Behandlung pharmakologischer Reihenversuche. Arch. Exp. Pathol. Pharmakol. 1931, 162, 480–483. [Google Scholar] [CrossRef]
- Ryazantsev, D.Y.; Myshkin, M.Y.; Alferova, V.A.; Tsvetkov, V.B.; Shustova, E.Y.; Kamzeeva, P.N.; Kovalets, P.V.; Zaitseva, E.R.; Baleeva, N.S.; Zatsepin, T.S.; et al. Probing GFP Chromophore Analogs as Anti-HIV Agents Targeting LTR-III G-Quadruplex. Biomolecules 2021, 11, 1409. [Google Scholar] [CrossRef]
- Zenchenko, A.A.; Oslovsky, V.E.; Varizhuk, I.V.; Karpova, E.V.; Osolodkin, D.I.; Kozlovskaya, L.I.; Ishmukhametov, A.A.; Drenichev, M.S. Cytotoxicity reduction by O-nicotinoylation of antiviral 6-benzylaminopurine ribonucleosides. Toxicol. Vitr. 2022, 82, 105355. [Google Scholar] [CrossRef]
- Jodlbauer, A.; Von Tappeiner, H. Die Beteiligung Des Sauerstoffs Bei Der Wirkung Fluorescierender Stoffe. Dtsch. Arch. Klin. Med. 1905, 82, 520–546. [Google Scholar]
- Lamberts, J.J.M.; Schumacher, D.R.; Neckers, D.C. Novel rose bengal derivatives: Synthesis and quantum yield studies. J. Am. Chem. Soc. 1984, 106, 5879–5883. [Google Scholar] [CrossRef]
- Sugita, N.; Kawabata, K.-I.; Sasaki, K.; Sakata, A.I.; Umemura, S.-I. Synthesis of Amphiphilic Derivatives of Rose Bengal and Their Tumor Accumulation. Bioconjugate Chem. 2007, 18, 866–873. [Google Scholar] [CrossRef]
- Pereira, P.C.D.S.; Costa, P.F.D.A.; Pellosi, D.S.; Calori, I.R.; Vilsinski, B.H.; Estevão, B.M.; Hioka, N.; Caetano, W. Photophysical properties and interaction studies of Rose Bengal derivatives with biomimetic systems based in micellar aqueous solutions. J. Mol. Liq. 2017, 230, 674–685. [Google Scholar] [CrossRef]
- Mitsuya, H.; Weinhold, K.J.; Furman, P.A.; Clair, M.H.S.; Lehrman, S.N.; Gallo, R.C.; Bolognesi, D.; Barry, D.W.; Broder, S. 3’-Azido-3’-deoxythymidine (BW A509U): An antiviral agent that inhibits the infectivity and cytopathic effect of human T-lymphotropic virus type III/lymphadenopathy-associated virus in vitro. Proc. Natl. Acad. Sci. USA 1985, 82, 7096–7100. [Google Scholar] [CrossRef] [PubMed]
- Fischl, M.A.; Richman, D.D.; Grieco, M.H.; Gottlieb, M.S.; Volberding, P.A.; Laskin, O.L.; Leedom, J.M.; Groopman, J.E.; Mildvan, D.; Schooley, R.T.; et al. The efficacy of azidothymidine (AZT) in the treatment of patients with AIDS and AIDS-related complex. A double-blind, placebo-controlled trial. N. Engl. J. Med. 1987, 317, 185–191. [Google Scholar] [CrossRef]
- Zhou, S.; Hill, C.S.; Sarkar, S.; Tse, L.V.; Woodburn, B.M.D.; Schinazi, R.F.; Sheahan, T.P.; Baric, R.S.; Heise, M.T.; Swanstrom, R. β-d-N4-hydroxycytidine Inhibits SARS-CoV-2 Through Lethal Mutagenesis But Is Also Mutagenic To Mammalian Cells. J. Infect. Dis. 2021, 224, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Valneva Successfully Completes Pivotal Phase 3 Trial of Single-Shot Chikungunya Vaccine Candidate. Available online: https://valneva.com/press-release/valneva-successfully-completes-pivotal-phase-3-trial-of-single-shot-chikungunya-vaccine-candidate/ (accessed on 18 October 2022).
- Mao, Q.; Wang, Y.; Yao, X.; Bian, L.; Wu, X.; Xu, M.; Liang, Z. Coxsackievirus A16: Epidemiology, Diagnosis, and Vaccine. Hum. Vaccines Immunother. 2013, 10, 360–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wells, A.I.; Coyne, C.B. Enteroviruses: A Gut-Wrenching Game of Entry, Detection, and Evasion. Viruses 2019, 11, 460. [Google Scholar] [CrossRef] [Green Version]
- Fatima, K.; Masood, N.; Luqman, S. Quenching of singlet oxygen by natural and synthetic antioxidants and assessment of electronic UV/Visible absorption spectra for alleviating or enhancing the efficacy of photodynamic therapy. Biomed. Res. Ther. 2016, 3, 8. [Google Scholar] [CrossRef]
- Stockert, J.C.; Herkovits, J. Photodynamic toxicity and its prevention by antioxidative agents in Bufo arenarum embryos. Toxicology 2003, 192, 211–218. [Google Scholar] [CrossRef]
- Mendes, B.; Kassumeh, S.; Aguirre-Soto, A.; Pei, Q.; Heyne, B.; Kochevar, I.E. Influence of Rose Bengal Dimerization on Photosensitization. Photochem. Photobiol. 2021, 97, 718–726. [Google Scholar] [CrossRef]
- Kasha, M. Energy Transfer Mechanisms and the Molecular Exciton Model for Molecular Aggregates. Radiat. Res. 1963, 20, 55. [Google Scholar] [CrossRef]
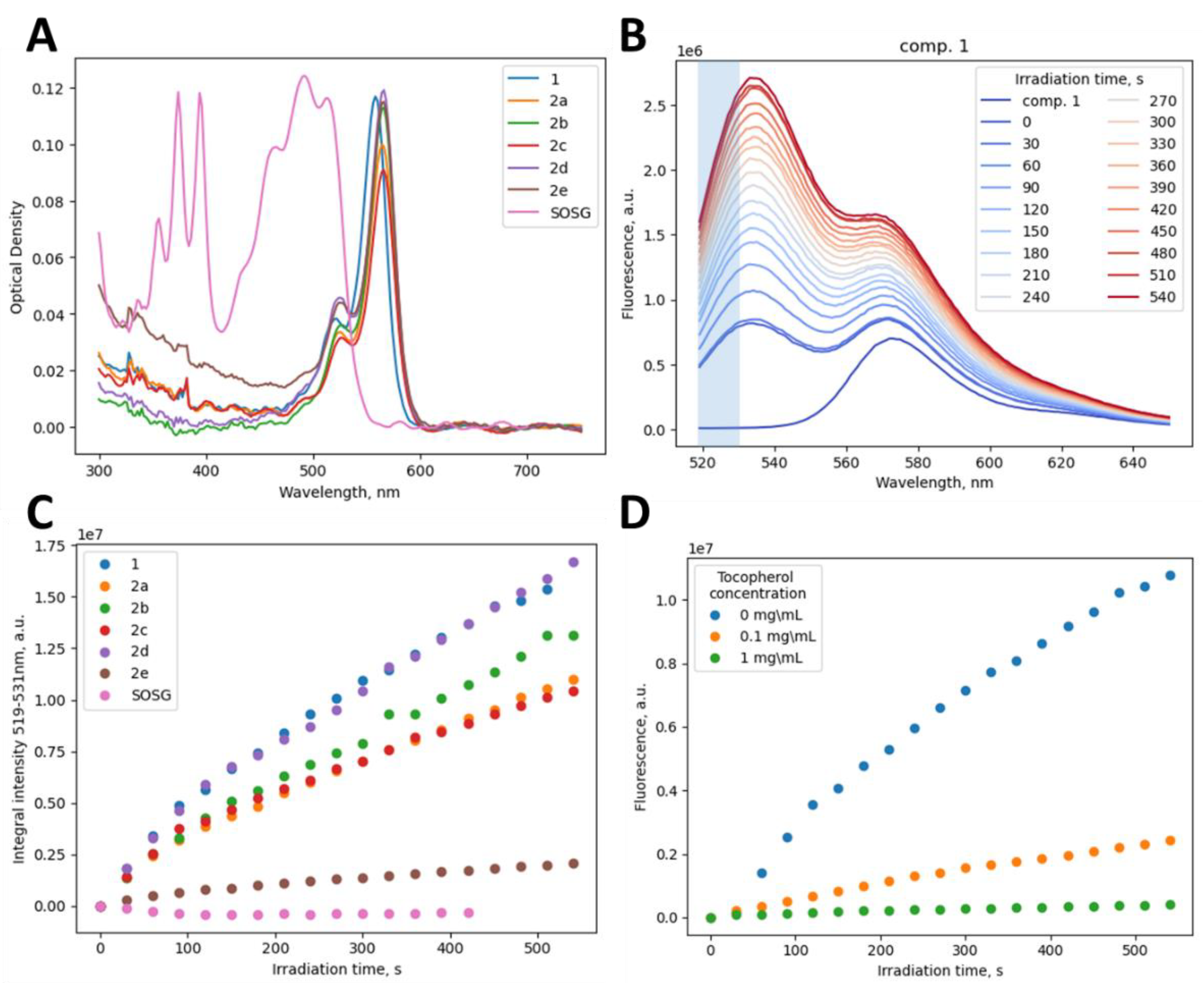
| Cmpd | EC50, µM (M ± SD) a | CC50, µM (M ± SD) b | SI c | |||||
|---|---|---|---|---|---|---|---|---|
| SARS-CoV-2 | CHIKV | HIV | Vero Cells | MT-4 Cells | SARS-CoV-2 | CHIKV | HIV | |
| 1 | 0.5 ± 0.2 | 0.11 ± 0.05 | 18 ± 7 | 43 ± 10 | >50 | 91 | 391 | >3 |
| 2a | 0.05 ± 0.02 | 0.004 ± 0.002 | 6.3 ± 2.6 | 8 ± 1 | 15 ± 4 | 169 | 1860 | 2.4 |
| 2b | 0.4 ± 0.3 | 0.0007 ± 0.0003 | 4.4 ± 1.8 | 60 ± 15 | 15 ± 4 | 146 | 90,909 | 3.4 |
| 2c | 5.3 ± 1.3 | 0.0002 ± 0.0001 | 8.8 ± 3.7 | 53 ± 25 | 42 ± 10 | 10 | 230,435 | 4.8 |
| 2d | 6.6 ± 3.1 | 0.002 ± 0.001 | 8.8 ± 2.6 | 60 ± 15 | 42 ± 10 | 9 | 28,571 | 4.8 |
| 2e | 43 ± 10 | 0.038 ± 0.030 | >50 | >100 | >50 | >2 | >2632 | n/d |
| Positive control | 4.7 ± 2.9 d | 23.6 ± 10.2 d | 0.01 e | >100 | >5 | >21 | >4 | >455 |
| Cmpd | 1O2 Generation | Solubility | ||
|---|---|---|---|---|
| Cgen, μM a | k, ×10−3 1/s b | A, ×10−7 a.u.f c | Csat, μM d | |
| 1 | 1.3 | 1.86 | 3.23 | >50.0 |
| 2a | 1.2 | 1.43 | 2.28 | 3.1 |
| 2b | 1.3 | 0.97 | 4.04 | 1.0 |
| 2c | 1.5 | 2.23 | 1.98 | ND |
| 2d | 1.1 | 1.29 | 3.54 | ND |
| 2e | 2.3 | 2.21 | 0.635 | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubekina, A.A.; Kamzeeva, P.N.; Alferova, V.A.; Shustova, E.Y.; Kolpakova, E.S.; Yakovchuk, E.V.; Karpova, E.V.; Borodulina, M.O.; Belyaev, E.S.; Khrulev, A.A.; et al. Hydrophobic Rose Bengal Derivatives Exhibit Submicromolar-to-Subnanomolar Activity against Enveloped Viruses. Biomolecules 2022, 12, 1609. https://doi.org/10.3390/biom12111609
Rubekina AA, Kamzeeva PN, Alferova VA, Shustova EY, Kolpakova ES, Yakovchuk EV, Karpova EV, Borodulina MO, Belyaev ES, Khrulev AA, et al. Hydrophobic Rose Bengal Derivatives Exhibit Submicromolar-to-Subnanomolar Activity against Enveloped Viruses. Biomolecules. 2022; 12(11):1609. https://doi.org/10.3390/biom12111609
Chicago/Turabian StyleRubekina, Anna A., Polina N. Kamzeeva, Vera A. Alferova, Elena Yu. Shustova, Ekaterina S. Kolpakova, Elizaveta V. Yakovchuk, Evgenia V. Karpova, Maria O. Borodulina, Evgeny S. Belyaev, Alexei A. Khrulev, and et al. 2022. "Hydrophobic Rose Bengal Derivatives Exhibit Submicromolar-to-Subnanomolar Activity against Enveloped Viruses" Biomolecules 12, no. 11: 1609. https://doi.org/10.3390/biom12111609
APA StyleRubekina, A. A., Kamzeeva, P. N., Alferova, V. A., Shustova, E. Y., Kolpakova, E. S., Yakovchuk, E. V., Karpova, E. V., Borodulina, M. O., Belyaev, E. S., Khrulev, A. A., Korshun, V. A., Shirshin, E. A., Kozlovskaya, L. I., & Aralov, A. V. (2022). Hydrophobic Rose Bengal Derivatives Exhibit Submicromolar-to-Subnanomolar Activity against Enveloped Viruses. Biomolecules, 12(11), 1609. https://doi.org/10.3390/biom12111609







