Androgen Signaling in Uterine Diseases: New Insights and New Targets
Abstract
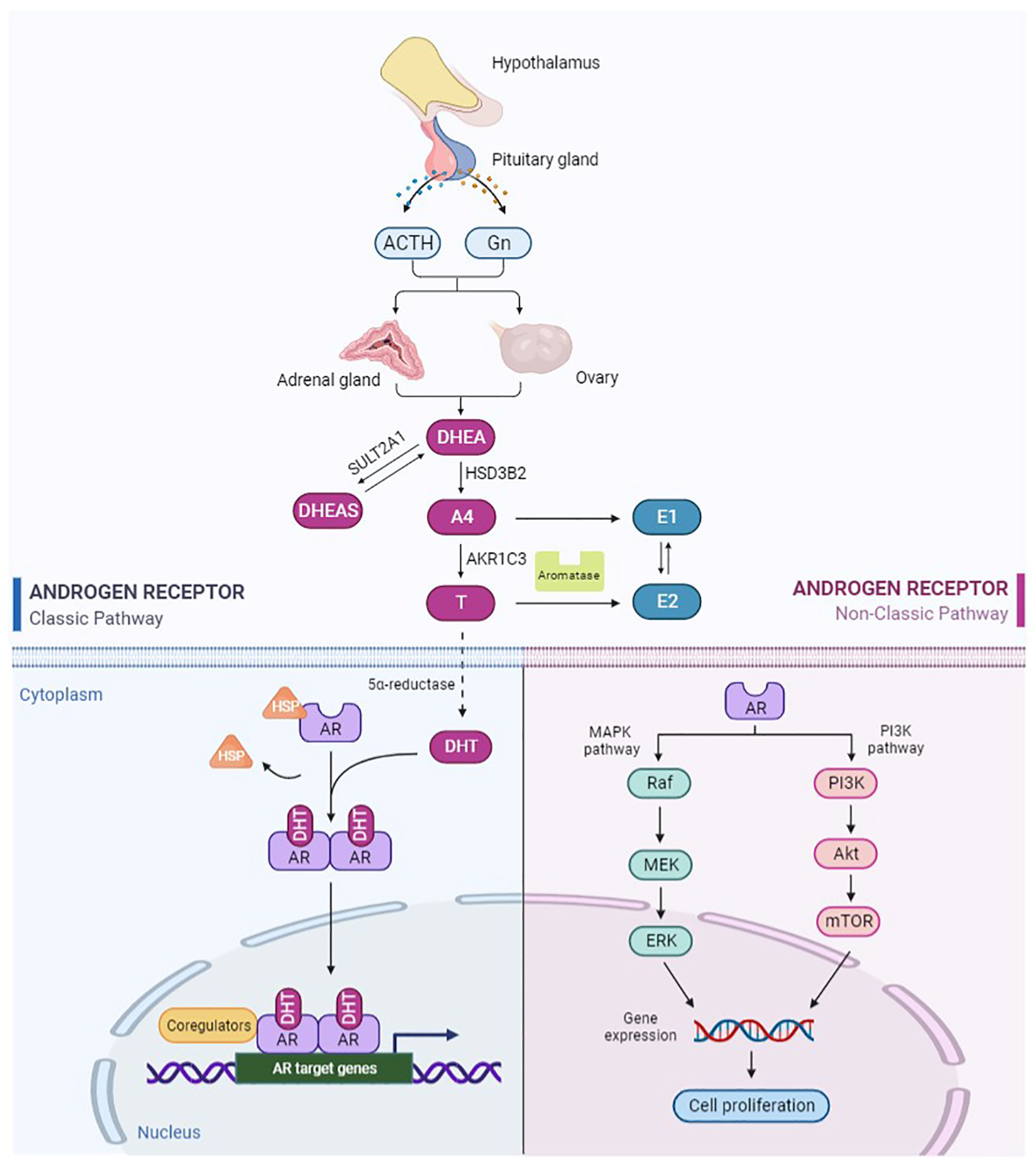
:1. Introduction
2. Androgen and Androgen Receptor
2.1. Metabolism and Biological Activity of Androgen
2.2. Structure and Function of AR
3. Androgen Signaling in Endometriosis
4. Androgen Signaling in Uterine Fibroids
5. Androgen Signaling in Endometrial Polyps
6. Androgen Signaling in Endometrial Hyperplasia
7. Androgen Signaling in Endometrial Cancer
7.1. Dual Effects of Androgens
7.2. AR Expression in EC
8. Androgen Signaling in Infertility Associated with Endometrial Dysfunction
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Allen, N.E.; Key, T.J.; Dossus, L.; Rinaldi, S.; Cust, A.; Lukanova, A.; Peeters, P.H.; Onland-Moret, N.C.; Lahmann, P.H.; Berrino, F.; et al. Endogenous sex hormones and endometrial cancer risk in women in the European Prospective Investigation into Cancer and Nutrition (EPIC). Endocr. -Relat. Cancer 2008, 15, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Burger, H.G. Androgen production in women. Fertil. Steril. 2002, 4, S3–S5. [Google Scholar] [CrossRef]
- Simitsidellis, I.; Saunders, P.T.K.; Gibson, D.A. Androgens and endometrium: New insights and new targets. Mol. Cell. Endocrinol. 2018, 465, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Labrie, F.; Martel, C.; Bélanger, A.; Pelletier, G. Androgens in women are essentially made from DHEA in each peripheral tissue according to intracrinology. J. Steroid Biochem. Mol. Biol. 2017, 168, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Labrie, F.; Simard, J.; Luu-The, V.; Bélanger, A.; Pelletier, G. Structure, function and tissue-specific gene expression of 3β-hydroxysteroid dehydrogenase/5-ene-4-ene isomerase enzymes in classical and peripheral intracrine steroidogenic tissues. J. Steroid Biochem. Mol. Biol. 1992, 43, 805–826. [Google Scholar] [CrossRef]
- Nakamura, Y.; Hornsby, P.J.; Casson, P.; Morimoto, R.; Satoh, F.; Xing, Y.; Kennedy, M.R.; Sasano, H.; Rainey, W.E. Type 5 17beta-hydroxysteroid dehydrogenase (AKR1C3) contributes to testosterone production in the adrenal reticularis. J. Clin. Endocrinol. Metab. 2009, 94, 2192–2198. [Google Scholar] [CrossRef] [Green Version]
- Sinnesael, M.; Claessens, F.; Boonen, S.; Vanderschueren, D. Novel insights in the regulation and mechanism of androgen action on bone. Curr. Opin. Endocrinol. Diabetes Obes. 2013, 20, 240–244. [Google Scholar] [CrossRef]
- Bulun, S.E.; Lin, Z.; Imir, G.; Amin, S.; Demura, M.; Yilmaz, B.; Martin, R.; Utsunomiya, H.; Thung, S.; Gurates, B.; et al. Regulation of aromatase expression in estrogen-responsive breast and uterine disease: From bench to treatment. Pharmacol. Rev. 2005, 57, 359–383. [Google Scholar] [CrossRef] [Green Version]
- Schweizer, M.T.; Yu, E.Y. Persistent androgen receptor addiction in castration-resistant prostate cancer. J. Hematol. Oncol. 2015, 8, 128. [Google Scholar] [CrossRef] [Green Version]
- Apparao, K.B.; Lovely, L.P.; Gui, Y.; Lininger, R.A.; Lessey, B.A. Elevated endometrial androgen receptor expression in women with polycystic ovarian syndrome. Biol. Reprod. 2002, 66, 297–304. [Google Scholar] [CrossRef]
- Mertens, H.J.; Heineman, M.J.; Theunissen, P.H.; de Jong, F.H.; Evers, J.L. Androgen, estrogen and progesterone receptor expression in the human uterus during the menstrual cycle. Eur. J. Obstet. Gynecol. Reprod. Biol. 2001, 98, 58–65. [Google Scholar] [CrossRef]
- Lallous, N.; Dalal, K.; Cherkasov, A.; Rennie, P.S. Targeting alternative sites on the androgen receptor to treat castration-resistant prostate cancer. Int. J. Mol. Sci. 2013, 14, 12496–12519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koryakina, Y.; Ta, H.Q.; Gioeli, D. Androgen receptor phosphorylation: Biological context and functional consequences. Endocr. -Relat. Cancer 2014, 21, T131–T145. [Google Scholar] [CrossRef] [PubMed]
- Zamagni, A.; Cortesi, M.; Zanoni, M.; Tesei, A. Non-nuclear AR Signaling in Prostate Cancer. Front. Chem. 2019, 7, 651. [Google Scholar] [CrossRef] [PubMed]
- Ueda, T.; Mawji, N.R.; Bruchovsky, N.; Sadar, M.D. Ligand-independent activation of the androgen receptor by interleukin-6 and the role of steroid receptor coactivator-1 in prostate cancer cells. J. Biol. Chem. 2002, 277, 38087–38094. [Google Scholar] [CrossRef] [Green Version]
- Bonaccorsi, L.; Marchiani, S.; Ferruzzi, P.; Muratori, M.; Crescioli, C.; Forti, G.; Maggi, M.; Baldi, E. Non-genomic effects of the androgen receptor and vitamin D agonist are involved in suppressing invasive phenotype of prostate cancer cells. Steroids 2006, 71, 304–309. [Google Scholar] [CrossRef]
- Giudice, L.C.; Kao, L.C. Endometriosis. Lancet 2004, 364, 1789–1799. [Google Scholar] [CrossRef]
- Giudice, L.C. Clinical practice. Endometriosis. N. Engl. J. Med. 2010, 362, 2389–2398. [Google Scholar] [CrossRef]
- Huhtinen, K.; Saloniemi-Heinonen, T.; Keski-Rahkonen, P.; Desai, R.; Laajala, D.; Ståhle, M.; Häkkinen, M.R.; Awosanya, M.; Suvitie, P.; Kujari, H.; et al. Intra-tissue steroid profiling indicates differential progesterone and testosterone metabolism in the endometrium and endometriosis lesions. J. Clin. Endocrinol. Metab. 2014, 99, E2188–E2197. [Google Scholar] [CrossRef] [Green Version]
- Carneiro, M.M.; Morsch, D.M.; Camargos, A.F.; Reis, F.M.; Spritzer, P.M. Androgen receptor and 5alpha-reductase are expressed in pelvic endometriosis. BJOG 2008, 115, 113–117. [Google Scholar] [CrossRef]
- Yang, H.; Kang, K.; Cheng, C.; Mamillapalli, R.; Taylor, H.S. Integrative Analysis Reveals Regulatory Programs in Endometriosis. Reprod. Sci. 2015, 22, 1060–1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitsumori, K.; Terai, A.; Oka, H.; Segawa, T.; Ogura, K.; Yoshida, O.; Ogawa, O. Androgen receptor CAG repeat length polymorphism in benign prostatic hyperplasia (BPH): Correlation with adenoma growth. Prostate 1999, 41, 253–257. [Google Scholar] [CrossRef]
- Hsieh, Y.Y.; Chang, C.C.; Tsai, F.J.; Wu, J.Y.; Tsai, C.H.; Tsai, H.D. Androgen receptor trinucleotide polymorphism in endometriosis. Fertil. Steril. 2001, 76, 412–413. [Google Scholar] [CrossRef]
- Vannuccini, S.; Luisi, S.; Tosti, C.; Sorbi, F.; Petraglia, F. Role of medical therapy in the management of uterine adenomyosis. Fertil. Steril. 2018, 109, 398–405. [Google Scholar] [CrossRef] [Green Version]
- Barbieri, R.L. Danazol: Molecular, endocrine, and clinical pharmacology. Prog. Clin. Biol. Res. 1990, 323, 241–252. [Google Scholar]
- Barbieri, R.L. Endometriosis 1990. Current treatment approaches. Drugs 1990, 39, 502–510. [Google Scholar] [CrossRef]
- Selak, V.; Farquhar, C.; Prentice, A.; Singla, A. Danazol for pelvic pain associated with endometriosis. Cochrane Database Syst. Rev. 2007, 4, Cd000068. [Google Scholar]
- Ferrero, S.; Tramalloni, D.; Venturini, P.L.; Remorgida, V. Vaginal danazol for women with rectovaginal endometriosis and pain symptoms persisting after insertion of a levonorgestrel-releasing intrauterine device. Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet. 2011, 113, 116–119. [Google Scholar] [CrossRef]
- Cobellis, L.; Razzi, S.; Fava, A.; Severi, F.M.; Igarashi, M.; Petraglia, F. A danazol-loaded intrauterine device decreases dysmenorrhea, pelvic pain, and dyspareunia associated with endometriosis. Fertil. Steril. 2004, 82, 239–240. [Google Scholar] [CrossRef]
- Farquhar, C.; Brosens, I. Medical and surgical management of adenomyosis. Best Pract. Res. Clin. Obstet. Gynaecol. 2006, 20, 603–616. [Google Scholar] [CrossRef]
- Igarashi, M. A new therapy for pelvic endometriosis and uterine adenomyosis: Local effect of vaginal and intrauterine danazol application. Asia-Ocean. J. Obstet. Gynaecol. 1990, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Shawki, O.A. Danazol loaded intrauterine device (D-IUD): A novel conservative management for uterine adenomyosis. Middle East Fertil. J. 2002, 7, 214–220. [Google Scholar]
- Zhang, X.; Yuan, H.; Deng, L.; Hu, F.; Ma, J.; Lin, J. Evaluation of the efficacy of a danazol-loaded intrauterine contraceptive device on adenomyosis in an ICR mouse model. Hum. Reprod. 2008, 23, 2024–2030. [Google Scholar] [CrossRef] [PubMed]
- Ueki, K.; Kumagai, K.; Yamashita, H.; Li, Z.L.; Ueki, M.; Otsuki, Y. Expression of apoptosis-related proteins in adenomyotic uteri treated with danazol and GnRH agonists. Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet. 2004, 23, 248–258. [Google Scholar] [CrossRef]
- Luisi, S.; Razzi, S.; Lazzeri, L.; Bocchi, C.; Severi, F.M.; Petraglia, F. Efficacy of vaginal danazol treatment in women with menorrhagia during fertile age. Fertil. Steril. 2009, 92, 1351–1354. [Google Scholar] [CrossRef] [PubMed]
- Takebayashi, T.; Fujino, Y.; Umesaki, N.; Ogita, S. Danazol suspension injected into the uterine cervix of patients with adenomyosis and myoma. Prelim. Study Gynecol. Obstet. Investig. 1995, 39, 207–211. [Google Scholar] [CrossRef]
- Friend, D.R. Drug delivery for the treatment of endometriosis and uterine fibroids. Drug Deliv. Transl. Res. 2017, 7, 829–839. [Google Scholar] [CrossRef]
- Moravek, M.B.; Yin, P.; Ono, M.; Coon, J.S.t.; Dyson, M.T.; Navarro, A.; Marsh, E.E.; Chakravarti, D.; Kim, J.J.; Wei, J.J.; et al. Ovarian steroids, stem cells and uterine leiomyoma: Therapeutic implications. Hum. Reprod. Update 2015, 21, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Taylor, D.K.; Leppert, P.C. Treatment for Uterine Fibroids: Searching for Effective Drug Therapies. Drug Discov. Today Ther. Strateg. 2012, 9, e41–e49. [Google Scholar] [CrossRef] [Green Version]
- Ke, L.Q.; Yang, K.; Li, J.; Li, C.M. Danazol for uterine fibroids. Cochrane Database Syst. Rev. 2009, 2009, Cd007692. [Google Scholar]
- Wong, J.Y.; Gold, E.B.; Johnson, W.O.; Lee, J.S. Circulating Sex Hormones and Risk of Uterine Fibroids: Study of Women’s Health Across the Nation (SWAN). J. Clin. Endocrinol. Metab. 2016, 101, 123–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bulun, S.E.; Imir, G.; Utsunomiya, H.; Thung, S.; Gurates, B.; Tamura, M.; Lin, Z. Aromatase in endometriosis and uterine leiomyomata. J. Steroid Biochem. Mol. Biol. 2005, 95, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Moroni, R.; Vieira, C.; Ferriani, R.; Candido-Dos-Reis, F.; Brito, L. Pharmacological treatment of uterine fibroids. Ann. Med. Health Sci. Res. 2014, 4, S185–S192. [Google Scholar] [CrossRef] [PubMed]
- Horie, K.; Takakura, K.; Imai, K.; Liao, S.; Mori, T. Immunohistochemical localization of androgen receptor in the human endometrium, decidua, placenta and pathological conditions of the endometrium. Hum. Reprod. 1992, 7, 1461–1466. [Google Scholar] [CrossRef] [PubMed]
- Lan, M.; Li, H.; Bao, L.; Li, M.; Lye, S.; Dong, X. In Vivo Evidence of the Androgen Receptor in Association With Myometrial Cell Proliferation and Apoptosis. Reprod. Sci. 2016, 23, 264–271. [Google Scholar] [CrossRef]
- Gao, Z.; Matsuo, H.; Wang, Y.; Nakago, S.; Maruo, T. Up-regulation by IGF-I of proliferating cell nuclear antigen and Bcl-2 protein expression in human uterine leiomyoma cells. J. Clin. Endocrinol. Metab. 2001, 86, 5593–5599. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, Y.; Xie, N.; Shynlova, O.; Challis, J.R.; Slater, D.; Lye, S.; Dong, X. Proliferative action of the androgen receptor in human uterine myometrial cells--a key regulator for myometrium phenotype programming. J. Clin. Endocrinol. Metab. 2013, 98, 218–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Li, Y.; Morin, D.; Plymate, S.; Lye, S.; Dong, X. The androgen receptor mediates antiapoptotic function in myometrial cells. Cell Death Dis. 2014, 5, e1338. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, Y.Y.; Chang, C.C.; Tsai, F.J.; Lin, C.C.; Yeh, L.S.; Peng, C.T. Androgen receptor trinucleotide polymorphism in leiomyoma. J. Assist. Reprod. Genet. 2004, 21, 453–457. [Google Scholar] [CrossRef] [Green Version]
- Shaik, N.A.; Govindan, S.; Kodati, V.; Rao, K.P.; Hasan, Q. Polymorphic (CAG)n repeats in the androgen receptor gene: A risk marker for endometriosis and uterine leiomyomas. Hematol. Oncol. Stem Cell Ther. 2009, 2, 289–293. [Google Scholar] [CrossRef] [Green Version]
- Rosa, F.E.; Rde, A.C.; Ambrosio, E.P.; Cirilo, P.D.R.; Pontes, A.; Rainho, C.A.; Rogatto, S.R. Polymorphisms of CYP17A1, CYP19, and androgen in Brazilian women with uterine leiomyomas. Clin. Chem. Lab. Med. 2008, 46, 814–823. [Google Scholar] [CrossRef] [PubMed]
- Munro, M.G. Uterine polyps, adenomyosis, leiomyomas, and endometrial receptivity. Fertil. Steril. 2019, 111, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.K.; Kor, C.T.; Chen, C.P.; Chen, H.T.; Yang, P.T.; Tsai, C.D.; Huang, C.H. Increased Risk of Venous Thromboembolism in Women with Uterine Leiomyoma: A Nationwide, Population-Based Case-Control Study. Acta Cardiol. Sin. 2018, 34, 66–76. [Google Scholar] [PubMed]
- Ishihara, H.; Kitawaki, J.; Kado, N.; Koshiba, H.; Fushiki, S.; Honjo, H. Gonadotropin-releasing hormone agonist and danazol normalize aromatase cytochrome P450 expression in eutopic endometrium from women with endometriosis, adenomyosis, or leiomyomas. Fertil. Steril. 2003, 79 (Suppl. S1), 735–742. [Google Scholar] [CrossRef]
- Xuan, J.; Deng, G.; Liu, R.; Chen, X.; Zheng, Y. Analysis of medication data of women with uterine fibroids based on data mining technology. J. Infect. Public Health 2020, 13, 1513–1516. [Google Scholar] [CrossRef]
- De Leo, V.; la Marca, A.; Morgante, G. Short-term treatment of uterine fibromyomas with danazol. Gynecol. Obstet. Investig. 1999, 47, 258–262. [Google Scholar] [CrossRef]
- De Leo, V.; Morgante, G.; Lanzetta, D.; D’Antona, D.; Bertieri, R.S. Danazol administration after gonadotrophin-releasing hormone analogue reduces rebound of uterine myomas. Hum. Reprod. 1997, 12, 357–360. [Google Scholar] [CrossRef] [Green Version]
- la Marca, A.; Musacchio, M.C.; Morgante, G.; Petraglia, F.; de Leo, V. Hemodynamic effect of danazol therapy in women with uterine leiomyomata. Fertil. Steril. 2003, 79, 1240–1242. [Google Scholar] [CrossRef]
- Lethaby, A.; Suckling, J.; Barlow, D.; Farquhar, C.M.; Jepson, R.G.; Roberts, H. Hormone replacement therapy in postmenopausal women: Endometrial hyperplasia and irregular bleeding. Cochrane Database Syst. Rev. 2004, Cd000402. [Google Scholar] [CrossRef]
- Dreisler, E.; Sorensen, S.S.; Ibsen, P.H.; Lose, G. Prevalence of endometrial polyps and abnormal uterine bleeding in a Danish population aged 20–74 years. Ultrasound Obstet. Gynecol. 2009, 33, 102–108. [Google Scholar] [CrossRef]
- Maia, H., Jr.; Maltez, A.; Fahel, P.; Coutinho, E. Hysteroscopic and immunohistochemical findings in type I and type II endometrial carcinomas. J. Am. Assoc. Gynecol. Laparosc. 2001, 8, 222–230. [Google Scholar] [CrossRef]
- Cohen, I. Endometrial pathologies associated with postmenopausal tamoxifen treatment. Gynecol. Oncol. 2004, 94, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Chalas, E.; Costantino, J.P.; Wickerham, D.L.; Wolmark, N.; Lewis, G.C.; Bergman, C.; Runowicz, C.D. Benign gynecologic conditions among participants in the Breast Cancer Prevention Trial. Am. J. Obstet. Gynecol. 2005, 192, 1230–1237. [Google Scholar] [CrossRef] [PubMed]
- Taylor, L.J.; Jackson, T.L.; Reid, J.G.; Duffy, S.R. The differential expression of oestrogen receptors, progesterone receptors, Bcl-2 and Ki67 in endometrial polyps. BJOG 2003, 110, 794–798. [Google Scholar] [CrossRef] [PubMed]
- Brody, S.; Carlström, K.; von Uexküll, A.K.; Lagrelius, A.; Lunell, N.O.; Rosenborg, L. Peripheral hormone levels and the endometrial condition in postmenopausal women. Acta Obstet. Gynecol. Scand. 1983, 62, 525–529. [Google Scholar] [CrossRef]
- Maia, H., Jr.; Pimentel, K.; Silva, T.M.; Freitas, L.A.; Zausner, B.; Athayde, C.; Coutinho, E.M. Aromatase and cyclooxygenase-2 expression in endometrial polyps during the menstrual cycle. Gynecol. Endocrinol. 2006, 22, 219–224. [Google Scholar] [CrossRef]
- Filho, A.M.; Barbosa, I.C.; Maia, H., Jr.; Genes, C.C.; Coutinho, E.M. Effects of subdermal implants of estradiol and testosterone on the endometrium of postmenopausal women. Gynecol. Endocrinol. 2007, 23, 511–517. [Google Scholar] [CrossRef]
- Horn, L.C.; Schnurrbusch, U.; Bilek, K.; Hentschel, B.; Einenkel, J. Risk of progression in complex and atypical endometrial hyperplasia: Clinicopathologic analysis in cases with and without progestogen treatment. Int. J. Gynecol. Cancer 2004, 14, 348–353. [Google Scholar] [CrossRef]
- Nees, L.K.; Heublein, S.; Steinmacher, S.; Juhasz-Böss, I.; Brucker, S.; Tempfer, C.B.; Wallwiener, M. Endometrial hyperplasia as a risk factor of endometrial cancer. Arch. Gynecol. Obstet. 2022, 306, 407–421. [Google Scholar] [CrossRef]
- Zaino, R.; Carinelli, S.; Eng, C.; Kurman, R.; Carcangiu, M.; Herrington, C.; Young, R. Tumours of the uterine corpus. In WHO Classification of Tumours of Female Reproductive Organs; World Health Organization Classification of Tumours; IARC Press: Lyon, France, 2014. [Google Scholar]
- Vitoratos, N.; Gregoriou, O.; Hassiakos, D.; Zourlas, P.A. The role of androgens in the late-premenopausal woman with adenomatous hyperplasia of the endometrium. Int. J. Gynaecol. Obstet. 1991, 34, 157–161. [Google Scholar] [CrossRef]
- Ito, K.; Suzuki, T.; Akahira, J.; Moriya, T.; Kaneko, C.; Utsunomiya, H.; Yaegashi, N.; Okamura, K.; Sasano, H. Expression of androgen receptor and 5alpha-reductases in the human normal endometrium and its disorders. Int. J. Cancer 2002, 99, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Tamaoka, Y.; Orikasa, H.; Sumi, Y.; Sakakura, K.; Kamei, K.; Nagatani, M.; Ezawa, S. Treatment of endometrial hyperplasia with a danazol-releasing intrauterine device: A prospective study. Gynecol. Obstet. Investig. 2004, 58, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Rigano, A.; Sturlese, E.; Rigano, M.; Baviera, G. Endocrine changes in postmenopausal women after high-dose danazol therapy. Panminerva Med. 1999, 41, 139–142. [Google Scholar] [PubMed]
- Niwa, K.; Hashimoto, M.; Morishita, S.; Yokoyama, Y.; Lian, Z.; Tagami, K.; Mori, H.; Tamaya, T. Preventive effects of danazol on endometrial carcinogenesis in mice. Cancer Lett. 2000, 158, 133–139. [Google Scholar] [CrossRef]
- Azziz, R.; Woods, K.S.; Reyna, R.; Key, T.J.; Knochenhauer, E.S.; Yildiz, B.O. The prevalence and features of the polycystic ovary syndrome in an unselected population. J. Clin. Endocrinol. Metab. 2004, 89, 2745–2749. [Google Scholar] [CrossRef] [Green Version]
- Pillay, O.C.; Fong, L.F.T.; Crow, J.C.; Benjamin, E.; Mould, T.; Atiomo, W.; Menon, P.A.; Leonard, A.J.; Hardiman, P. The association between polycystic ovaries and endometrial cancer. Hum. Reprod. 2006, 21, 924–929. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, S.R.; Goyeneche, A.A.; Heber, M.F.; Abruzzese, G.A.; Telleria, C.M.; Motta, A.B. Prenatally androgenized female rats develop uterine hyperplasia when adult. Mol. Cell. Endocrinol. 2020, 499, 110610. [Google Scholar] [CrossRef]
- Pinola, P.; Piltonen, T.T.; Puurunen, J.; Vanky, E.; Sundström-Poromaa, I.; Stener-Victorin, E.; Ruokonen, A.; Puukka, K.; Tapanainen, J.S.; Morin-Papunen, L.C. Androgen Profile Through Life in Women With Polycystic Ovary Syndrome: A Nordic Multicenter Collaboration Study. J. Clin. Endocrinol. Metab. 2015, 100, 3400–3407. [Google Scholar] [CrossRef] [Green Version]
- Plaza-Parrochia, F.; Romero, C.; Valladares, L.; Vega, M. Endometrium and steroids, a pathologic overview. Steroids 2017, 126, 85–91. [Google Scholar] [CrossRef]
- Shang, K.; Jia, X.; Qiao, J.; Kang, J.; Guan, Y. Endometrial abnormality in women with polycystic ovary syndrome. Reprod. Sci. 2012, 19, 674–683. [Google Scholar] [CrossRef]
- Li, X.; Pishdari, B.; Cui, P.; Hu, M.; Yang, H.P.; Guo, Y.R.; Jiang, H.Y.; Feng, Y.; Billig, H.; Shao, R. Regulation of Androgen Receptor Expression Alters AMPK Phosphorylation in the Endometrium: In Vivo and In Vitro Studies in Women with Polycystic Ovary Syndrome. Int. J. Biol. Sci. 2015, 11, 1376–1389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Guo, Y.R.; Lin, J.F.; Feng, Y.; Billig, H.; Shao, R. Combination of Diane-35 and Metformin to Treat Early Endometrial Carcinoma in PCOS Women with Insulin Resistance. J. Cancer 2014, 5, 173–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bokhman, J.V. Two pathogenetic types of endometrial carcinoma. Gynecol. Oncol. 1983, 15, 10–17. [Google Scholar] [CrossRef]
- Wei, J.J.; Paintal, A.; Keh, P. Histologic and immunohistochemical analyses of endometrial carcinomas: Experiences from endometrial biopsies in 358 consultation cases. Arch. Pathol. Lab. Med. 2013, 137, 1574–1583. [Google Scholar] [CrossRef] [PubMed]
- Cree, I.A.; White, V.A.; Indave, B.I.; Lokuhetty, D. Revising the WHO classification: Female genital tract tumours. Histopathology 2020, 76, 151–156. [Google Scholar] [CrossRef]
- Audet-Walsh, E.; Lépine, J.; Grégoire, J.; Plante, M.; Caron, P.; Têtu, B.; Ayotte, P.; Brisson, J.; Villeneuve, L.; Bélanger, A.; et al. Profiling of endogenous estrogens, their precursors, and metabolites in endometrial cancer patients: Association with risk and relationship to clinical characteristics. J. Clin. Endocrinol. Metab. 2011, 96, E330–E339. [Google Scholar] [CrossRef]
- Tanaka, S.; Miki, Y.; Hashimoto, C.; Takagi, K.; Doe, Z.; Li, B.; Yaegashi, N.; Suzuki, T.; Ito, K. The role of 5α-reductase type 1 associated with intratumoral dihydrotestosterone concentrations in human endometrial carcinoma. Mol. Cell. Endocrinol. 2015, 401, 56–64. [Google Scholar] [CrossRef]
- Ruth, K.S.; Day, F.R.; Tyrrell, J.; Thompson, D.J.; Wood, A.R.; Mahajan, A.; Beaumont, R.N.; Wittemans, L.; Martin, S.; Busch, A.S. Using human genetics to understand the disease impacts of testosterone in men and women. Nat. Med. 2020, 26, 252–258. [Google Scholar] [CrossRef]
- Mullee, A.; Dimou, N.; Allen, N.; O’Mara, T.; Gunter, M.J.; Murphy, N. Testosterone, sex hormone-binding globulin, insulin-like growth factor-1 and endometrial cancer risk: Observational and Mendelian randomization analyses. Br. J. Cancer 2021, 125, 1308–1317. [Google Scholar] [CrossRef]
- Siiteri, P.K. Adipose tissue as a source of hormones. Am. J. Clin. Nutr. 1987, 45, 277–282. [Google Scholar] [CrossRef]
- Che, Q.; Liu, B.Y.; Liao, Y.; Zhang, H.J.; Yang, T.T.; He, Y.Y.; Xia, Y.H.; Lu, W.; He, X.Y.; Chen, Z.; et al. Activation of a positive feedback loop involving IL-6 and aromatase promotes intratumoral 17β-estradiol biosynthesis in endometrial carcinoma microenvironment. Int. J. Cancer 2014, 135, 282–294. [Google Scholar] [CrossRef] [PubMed]
- Segawa, T.; Shozu, M.; Murakami, K.; Kasai, T.; Shinohara, K.; Nomura, K.; Ohno, S.; Inoue, M. Aromatase expression in stromal cells of endometrioid endometrial cancer correlates with poor survival. Clin. Cancer Res. 2005, 11, 2188–2194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, M.; Bao, W.; Wang, J.; Yang, T.; He, X.; Liao, Y.; Wan, X. FOXA1 promotes tumor cell proliferation through AR involving the Notch pathway in endometrial cancer. BMC Cancer 2014, 14, 78. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chang, W.C.; Hung, Y.C.; Chang, Y.Y.; Bao, B.Y.; Huang, H.C.; Chung, W.M.; Shyr, C.R.; Ma, W.L. Androgen receptor increases CD133 expression and progenitor-like population that associate with cisplatin resistance in endometrial cancer cell line. Reprod. Sci. 2014, 21, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Tuckerman, E.M.; Okon, M.A.; Li, T.; Laird, S.M. Do androgens have a direct effect on endometrial function? An in vitro study. Fertil. Steril. 2000, 74, 771–779. [Google Scholar] [CrossRef]
- Jamison, P.M.; Altekruse, S.F.; Chang, J.T.; Zahn, J.; Lee, R.; Noone, A.M.; Barroilhet, L. Site-specific factors for cancer of the corpus uteri from SEER registries: Collaborative stage data collection system, version 1 and version 2. Cancer 2014, 120 (Suppl. 23), 3836–3845. [Google Scholar] [CrossRef] [Green Version]
- Lovely, L.P.; Rao, K.B.A.; Gui, Y.; Lessey, B.A. Characterization of androgen receptors in a well-differentiated endometrial adenocarcinoma cell line (Ishikawa). J. Steroid Biochem. Mol. Biol. 2000, 74, 235–241. [Google Scholar] [CrossRef]
- Park, C.; Babayev, S.; Carr, B.R.; Keller, P.W.; Word, R.A.; Bukulmez, O. Androgen regulation of progesterone receptor (PR) expression in endometrium: Implications for endometriosis. Fertil. Steril. 2014, 102, e79–e80. [Google Scholar] [CrossRef]
- Brenner, R.M.; Slayden, O.D. Progesterone receptor antagonists and the endometrial antiproliferative effect. Semin. Reprod. Med. 2005, 23, 74–81. [Google Scholar] [CrossRef]
- Hackenberg, R.; Schulz, K.D. Androgen receptor mediated growth control of breast cancer and endometrial cancer modulated by antiandrogen- and androgen-like steroids. J. Steroid Biochem. Mol. Biol. 1996, 56, 113–117. [Google Scholar] [CrossRef]
- Hackenberg, R.; Beck, S.; Filmer, A.; Nia, A.H.; Kunzmann, R.; Koch, M.; Slater, E.P.; Schulz, K.D. Androgen responsiveness of the new human endometrial cancer cell line MFE-296. Int. J. Cancer 1994, 57, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Sasagawa, S.; Shimizu, Y.; Kami, H.; Takeuchi, T.; Mita, S.; Imada, K.; Kato, S.; Mizuguchi, K. Dienogest is a selective progesterone receptor agonist in transactivation analysis with potent oral endometrial activity due to its efficient pharmacokinetic profile. Steroids 2008, 73, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.X.; Yeap, B.B. Dihydrotestosterone and cancer risk. Curr. Opin. Endocrinol. Diabetes Obes. 2018, 25, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Koivisto, C.S.; Parrish, M.; Bonala, S.B.; Ngoi, S.; Torres, A.; Gallagher, J.; Sanchez-Hodge, R.; Zeinner, V.; Nahhas, G.J.; Liu, B.; et al. Evaluating the efficacy of enzalutamide and the development of resistance in a preclinical mouse model of type-I endometrial carcinoma. Neoplasia 2020, 22, 484–496. [Google Scholar] [CrossRef]
- Mahdi, Z.; Abdulfatah, E.; Pardeshi, V.; Hassan, O.; Schultz, D.; Morris, R.; Cote, M.L.; Elshaikh, M.A.; Bandyopadhyay, S.; Ali-Fehmi, R. The Impact of Androgen Receptor Expression on Endometrial Carcinoma Recurrence and Survival. Int. J. Gynecol. Pathol. 2017, 36, 405–411. [Google Scholar] [CrossRef]
- Kamal, A.M.; Bulmer, J.N.; DeCruze, S.B.; Stringfellow, H.F.; Martin-Hirsch, P.; Hapangama, D.K. Androgen receptors are acquired by healthy postmenopausal endometrial epithelium and their subsequent loss in endometrial cancer is associated with poor survival. Br. J. Cancer 2016, 114, 688–696. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Zhong, X.; Huo, X.; Zhang, J.; Yang, X.; Zhang, Y. The Clinicopathological Significance and Prognostic Value of Androgen Receptor in Endometrial Carcinoma: A Meta-Analysis. Front. Oncol. 2022, 12, 905809. [Google Scholar] [CrossRef]
- Gan, Q.; Crumley, S.; Broaddus, R.R. Molecular Modifiers of Hormone Receptor Action: Decreased Androgen Receptor Expression in Mismatch Repair Deficient Endometrial Endometrioid Adenocarcinoma. Int. J. Gynecol. Pathol. 2019, 38, 44–51. [Google Scholar] [CrossRef]
- Sasaki, M.; Sakuragi, N.; Dahiya, R. The CAG repeats in exon 1 of the androgen receptor gene are significantly longer in endometrial cancer patients. Biochem. Biophys. Res. Commun. 2003, 305, 1105–1108. [Google Scholar] [CrossRef]
- Sasaki, M.; Oh, B.R.; Dharia, A.; Fujimoto, S.; Dahiya, R. Inactivation of the human androgen receptor gene is associated with CpG hypermethylation in uterine endometrial cancer. Mol. Carcinog. 2000, 29, 59–66. [Google Scholar] [CrossRef]
- Murali, R.; Davidson, B.; Fadare, O.; Carlson, J.A.; Crum, C.P.; Gilks, C.B.; Irving, J.A.; Malpica, A.; Matias-Guiu, X.; McCluggage, W.G.; et al. High-grade Endometrial Carcinomas: Morphologic and Immunohistochemical Features, Diagnostic Challenges and Recommendations. Int. J. Gynecol. Pathol. 2019, 38 (Suppl. 1), S40–S63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, J.; Hacking, S.; Chavarria-Bernal, H.D.; Bhuiya, T.A.; Khutti, S. Androgen Receptor Immunohistochemical Expression in Undifferentiated/Dedifferentiated Endometrial Carcinoma. Int. J. Gynecol. Pathol. 2022, 41, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Munoz, J.; Wheler, J.J.; Kurzrock, R. Androgen receptors beyond prostate cancer: An old marker as a new target. Oncotarget 2015, 6, 592–603. [Google Scholar] [CrossRef] [Green Version]
- Mills, A.M.; Gottlieb, C.E.; Wendroth, S.M.; Brenin, C.M.; Atkins, K.A. Pure Apocrine Carcinomas Represent a Clinicopathologically Distinct Androgen Receptor-Positive Subset of Triple-Negative Breast Cancers. Am. J. Surg. Pathol. 2016, 40, 1109–1116. [Google Scholar] [CrossRef]
- Zadeh, S.L.; Duska, L.R.; Mills, A.M. Androgen Receptor Expression in Endometrial Carcinoma. Int. J. Gynecol. Pathol. 2018, 37, 167–173. [Google Scholar] [CrossRef]
- Gibson, D.A.; Simitsidellis, I.; Saunders, P.T. Regulation of androgen action during establishment of pregnancy. J. Mol. Endocrinol. 2016, 57, R35–R47. [Google Scholar] [CrossRef] [Green Version]
- Gellersen, B.; Brosens, J.J. Cyclic decidualization of the human endometrium in reproductive health and failure. Endocr. Rev. 2014, 35, 851–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibson, D.A.; Simitsidellis, I.; Cousins, F.L.; Critchley, H.O.; Saunders, P.T. Intracrine Androgens Enhance Decidualization and Modulate Expression of Human Endometrial Receptivity Genes. Sci. Rep. 2016, 6, 19970. [Google Scholar] [CrossRef] [Green Version]
- Gibson, D.A.; Simitsidellis, I.; Kelepouri, O.; Critchley, H.O.D.; Saunders, P.T.K. Dehydroepiandrosterone enhances decidualization in women of advanced reproductive age. Fertil. Steril. 2018, 109, 728–734.e722. [Google Scholar] [CrossRef] [Green Version]
- Young, S.L. Androgens and endometrium: New lessons from the corpus luteum via the adrenal cortex? Fertil. Steril. 2018, 109, 623–624. [Google Scholar] [CrossRef] [Green Version]
- Diao, H.L.; Su, R.W.; Tan, H.N.; Li, S.J.; Lei, W.; Deng, W.B.; Yang, Z.M. Effects of androgen on embryo implantation in the mouse delayed-implantation model. Fertil. Steril. 2008, 90, 1376–1383. [Google Scholar] [CrossRef]
- Gleicher, N.; Kim, A.; Weghofer, A.; Kushnir, V.A.; Shohat-Tal, A.; Lazzaroni, E.; Lee, H.J.; Barad, D.H. Hypoandrogenism in association with diminished functional ovarian reserve. Hum. Reprod. 2013, 28, 1084–1091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barad, D.; Brill, H.; Gleicher, N. Update on the use of dehydroepiandrosterone supplementation among women with diminished ovarian function. J. Assist. Reprod. Genet. 2007, 24, 629–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kara, M.; Aydin, T.; Aran, T.; Turktekin, N.; Ozdemir, B. Does dehydroepiandrosterone supplementation really affect IVF-ICSI outcome in women with poor ovarian reserve? Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 173, 63–65. [Google Scholar] [CrossRef] [PubMed]
- Burris, T.P.; Solt, L.A.; Wang, Y.; Crumbley, C.; Banerjee, S.; Griffett, K.; Lundasen, T.; Hughes, T.; Kojetin, D.J. Nuclear receptors and their selective pharmacologic modulators. Pharmacol. Rev. 2013, 65, 710–778. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, R.; Coss, C.C.; Dalton, J.T. Development of selective androgen receptor modulators (SARMs). Mol. Cell. Endocrinol. 2018, 465, 134–142. [Google Scholar] [CrossRef]



| Conditions | Study Phase | Sample Size | Interventions | NCT Number |
|---|---|---|---|---|
| Endometrial Cancer | II | 56 | Drug: danazol | NCT00003946 |
| Endometrioid Endometrial Cancer | II | 69 | Drug: Enzalutamide Drug: Carboplatin Drug: Paclitaxel | NCT02684227 |
| Endometriosis | II | 30 | Drug: Vaginal Danazol Drug: Oral Danatrol | NCT03352076 |
| Endometriosis Ovarian Cysts Infertility | IV | 150 | Drug: Danazol | NCT01779232 |
| Endometriosis | II | 66 | Drug: Danazol Once Weekly Drug: Danazol Twice Weekly | NCT00758953 |
| Hypoactive Sexual Desire Disorder | III | 1271 | Drug: Testosterone Transdermal System | NCT00467259 |
| Postmenopausal Women | III | 32 | Drug: tibolone Drug: Tibolone 2.5 mg Drug: CE/MPA | NCT00745108 |
| Postmenopause Osteoporosis | IV | 35 | Drug: Tibolone Drug: Estradiol Drug: Estradiol + MPA | NCT00294463 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, M.; Yu, J.; Huang, Y.; Ma, J.; Xiang, J.; Wang, Y.; Li, L.; Zhang, Z.; Liao, H. Androgen Signaling in Uterine Diseases: New Insights and New Targets. Biomolecules 2022, 12, 1624. https://doi.org/10.3390/biom12111624
Lv M, Yu J, Huang Y, Ma J, Xiang J, Wang Y, Li L, Zhang Z, Liao H. Androgen Signaling in Uterine Diseases: New Insights and New Targets. Biomolecules. 2022; 12(11):1624. https://doi.org/10.3390/biom12111624
Chicago/Turabian StyleLv, Mu, Juanjuan Yu, Yan Huang, Jie Ma, Jun Xiang, Yanqiu Wang, Linxia Li, Zhenbo Zhang, and Hong Liao. 2022. "Androgen Signaling in Uterine Diseases: New Insights and New Targets" Biomolecules 12, no. 11: 1624. https://doi.org/10.3390/biom12111624
APA StyleLv, M., Yu, J., Huang, Y., Ma, J., Xiang, J., Wang, Y., Li, L., Zhang, Z., & Liao, H. (2022). Androgen Signaling in Uterine Diseases: New Insights and New Targets. Biomolecules, 12(11), 1624. https://doi.org/10.3390/biom12111624





