Double-Edged Sword Effect of Pyroptosis: The Role of Caspase-1/-4/-5/-11 in Different Levels of Apical Periodontitis
Abstract
:1. Introduction
2. Material and Methods
2.1. Clinical Specimens
2.2. Label-Free Proteomics
2.3. Establishment of the Experimental Apical Periodontitis (EAP) Rat Model
2.4. Micro-CT Analysis
2.5. Hematoxylin-Eosin Staining (HE) and Immunofluorescence
2.6. TUNEL Staining
2.7. Cell Culture
2.8. Lactic Dehydrogenase (LDH) Release Assay
2.9. Propidium Iodide (PI) Staining
2.10. Western Blot
2.11. Data Analysis
3. Results
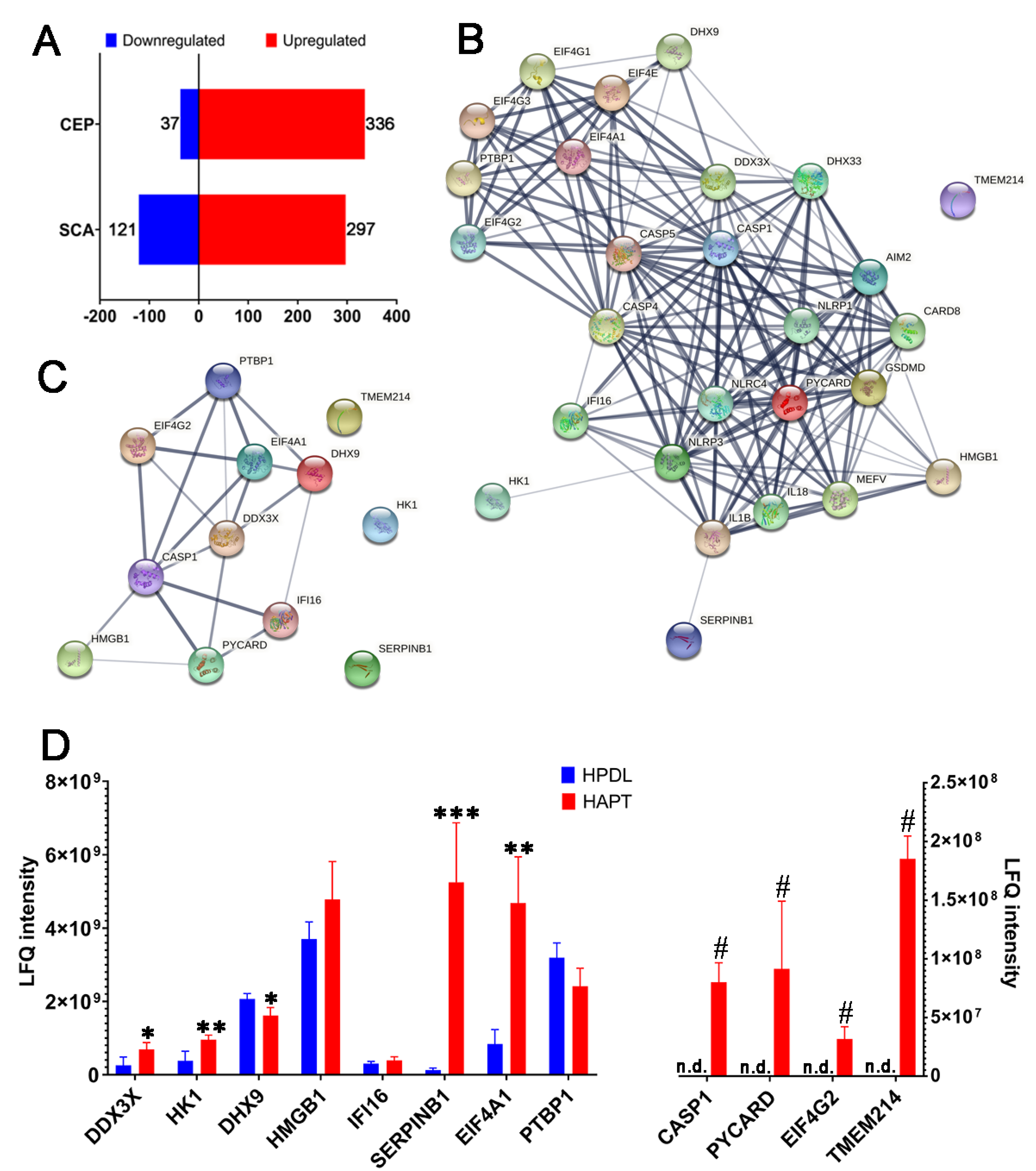
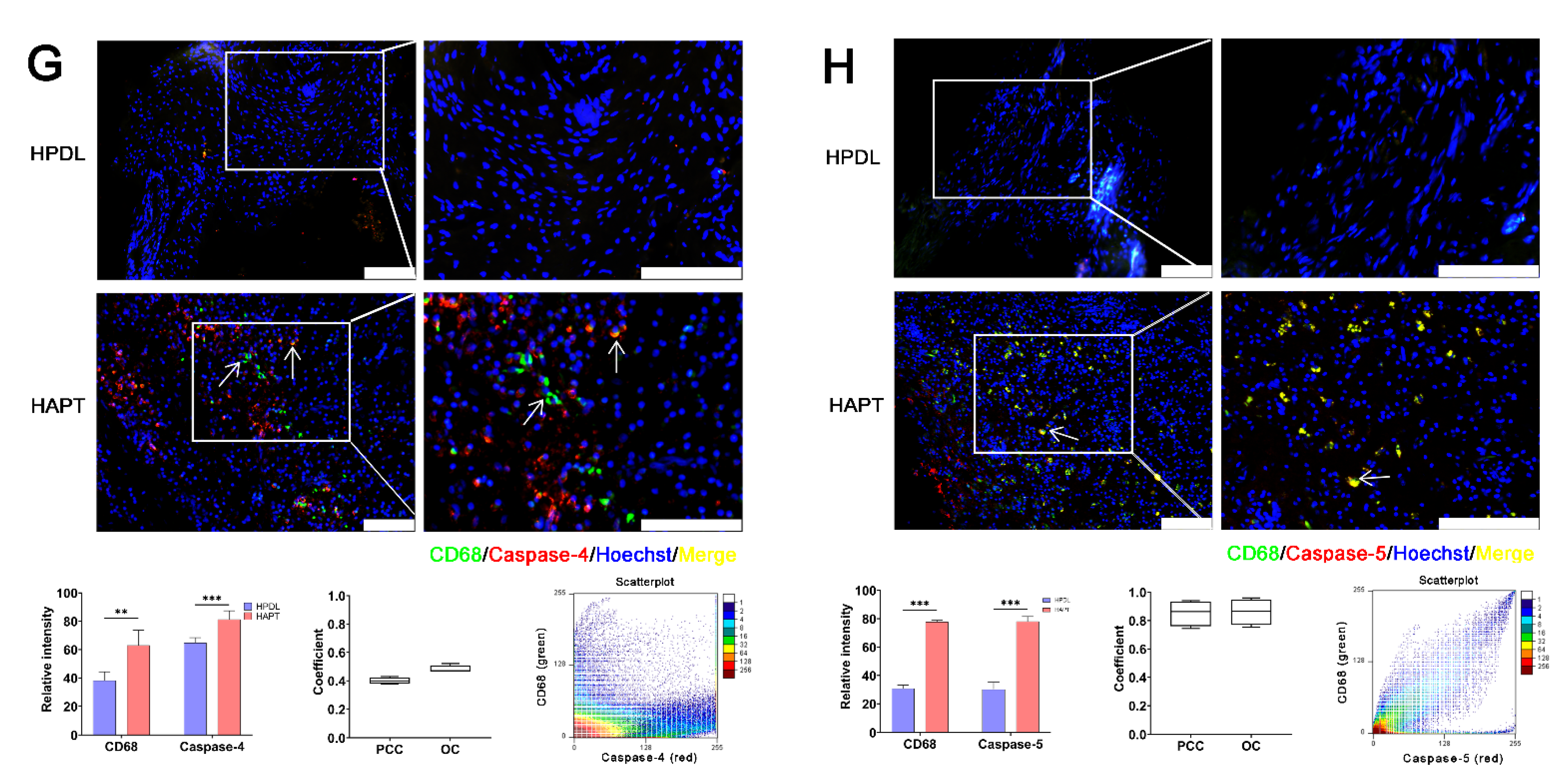
3.1. Caspase-1 and Its Related Proteins Were Only Detected in Human Chronic AP
3.2. Caspase-1/-4/-5 Differentially Mediated Canonical and Noncanonical Pyroptosis in Human AP
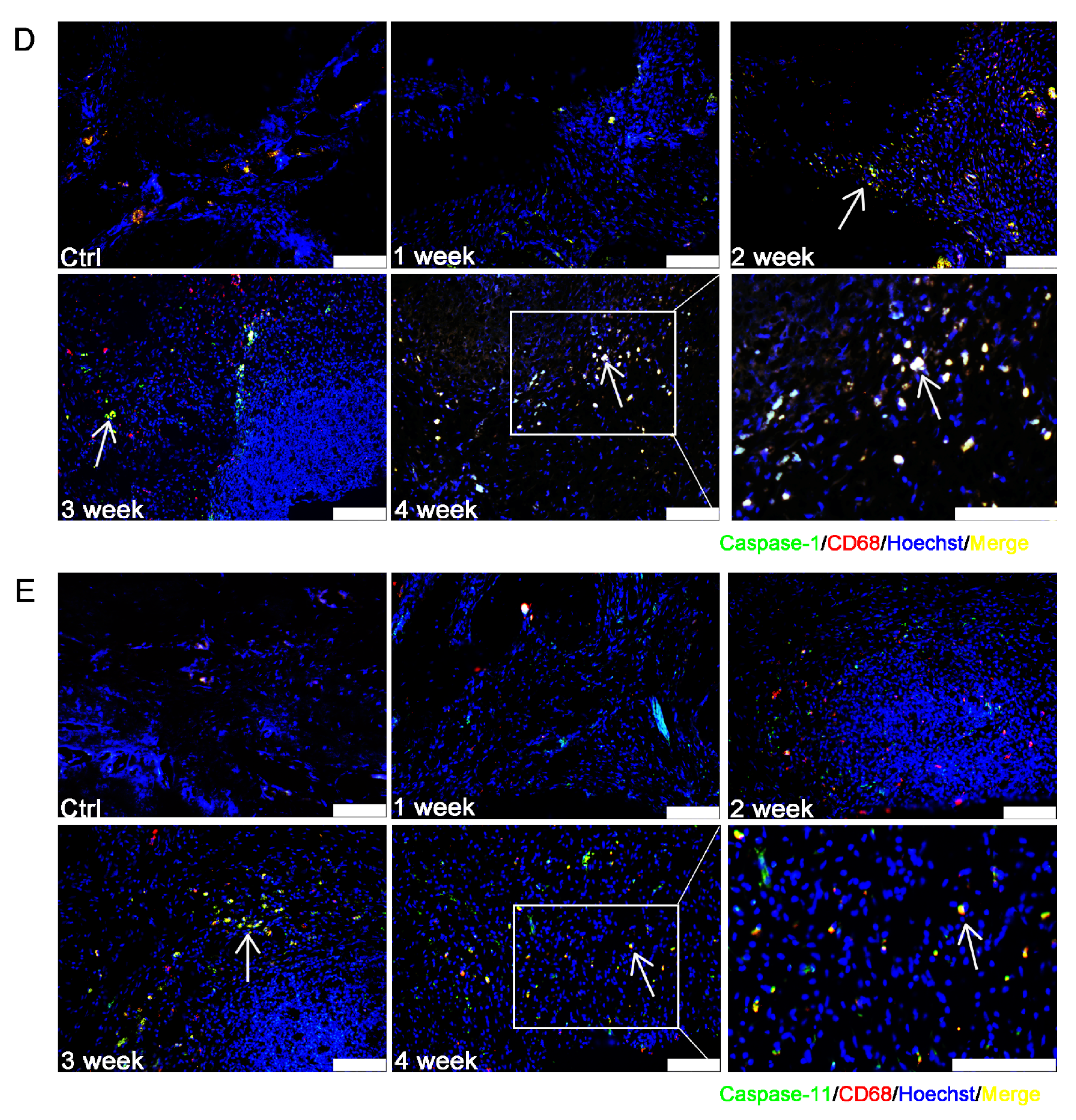
3.3. Caspase-1/-11-Mediated Pyroptosis Contributed to the Progression of EAP
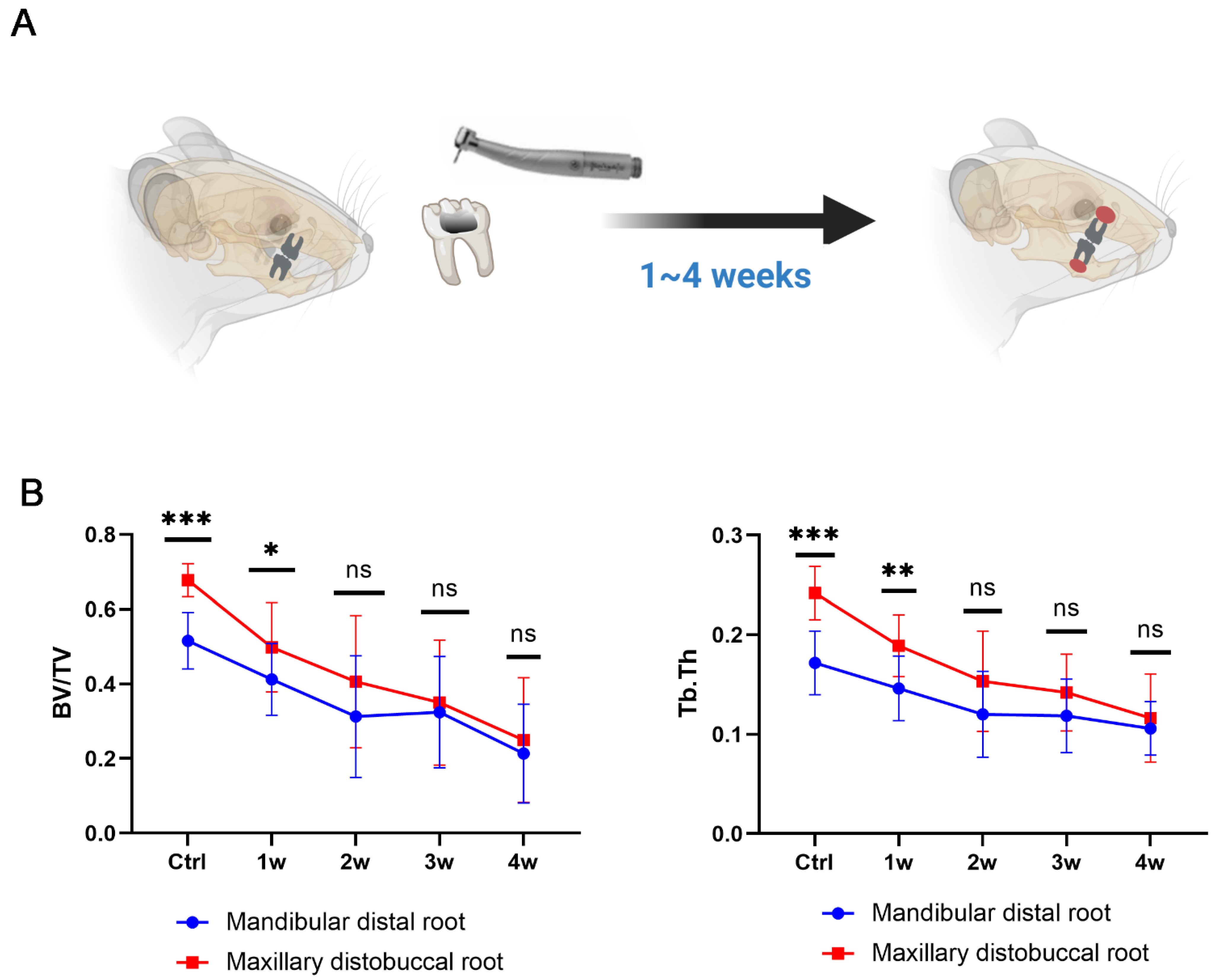
3.4. Establishment of a Dual EAP Model with Both Smaller (in Mandible) and Larger (in Maxilla) Bone Lesions
3.5. Pyroptosis Is a Double-Edged Sword in Inducing Bone Loss in Periapical Inflammation
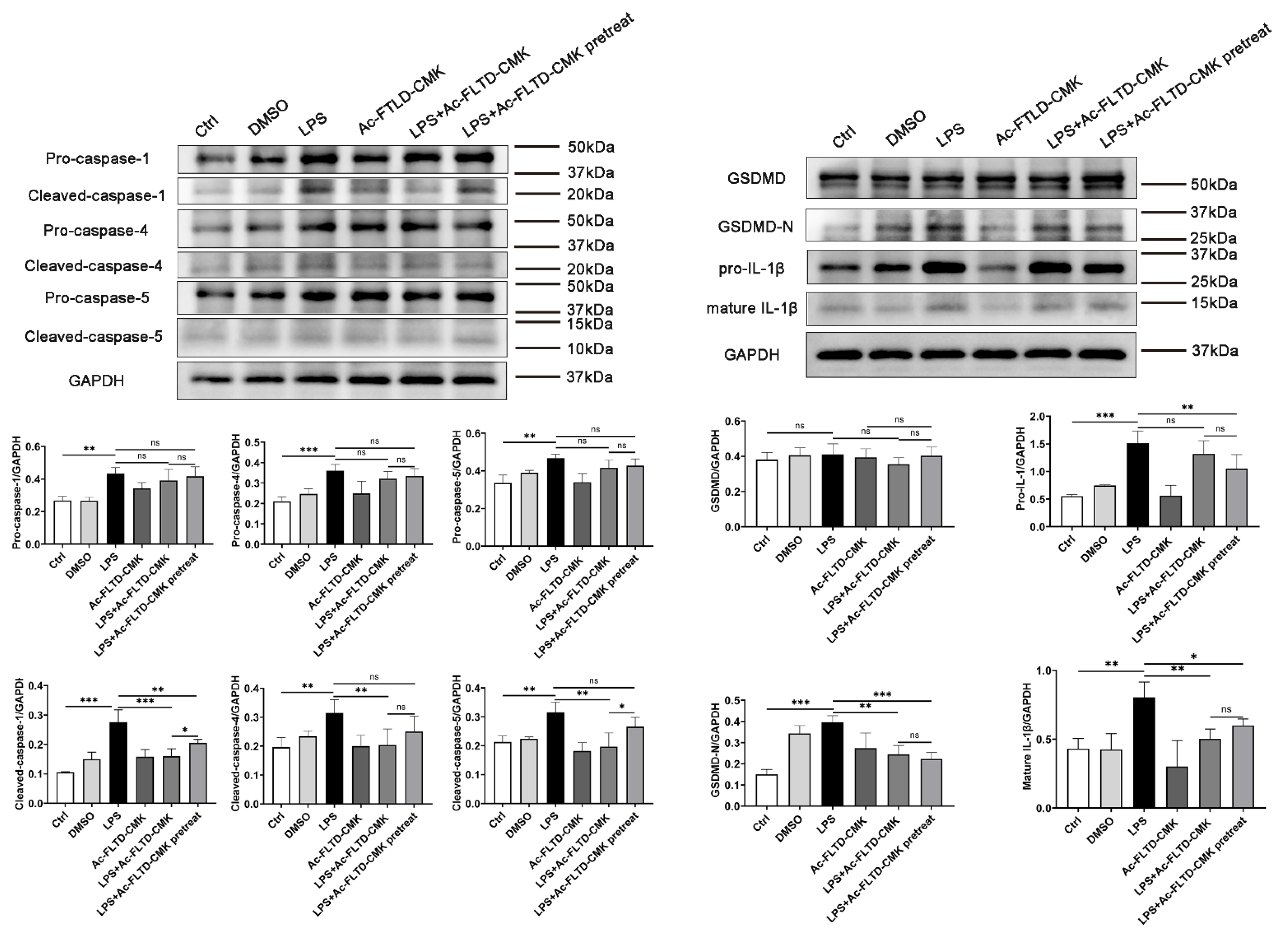
3.6. LPS Induced Pyroptosis in THP-1-Derived Macrophages. Inhibition of Pyroptosis Showed a Double-Edged Sword Effect in the In Vitro Models
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Segura-Egea, J.J.; Martin-Gonzalez, J.; Castellanos-Cosano, L. Endodontic medicine: Connections between apical periodontitis and systemic diseases. Int. Endod. J. 2015, 48, 933–951. [Google Scholar] [CrossRef] [PubMed]
- Braz-Silva, P.H.; Bergamini, M.L.; Mardegan, A.P.; De Rosa, C.S.; Hasseus, B.; Jonasson, P. Inflammatory profile of chronic apical periodontitis: A literature review. Acta Odontol. Scand 2019, 77, 173–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouillaguet, S.; Manoil, D.; Girard, M.; Louis, J.; Gaia, N.; Leo, S.; Schrenzel, J.; Lazarevic, V. Root Microbiota in Primary and Secondary Apical Periodontitis. Front. Microbiol. 2018, 9, 2374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, W.; Ma, T.; Ye, M.; Li, Z.; Liu, Y.; Hao, P. Microbiota in the apical root canal system of tooth with apical periodontitis. BMC Genom. 2019, 20, 189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes, B.P.F.D.; Herrera, D.R. Etiologic role of root canal infection in apical periodontitis and its relationship with clinical symptomatology. Braz. Oral Res. 2018, 32, 82–110. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.; Sun, F.; Li, X.; Zhou, Y.; Yin, S.; Zhou, X. Porphyromonas endodontalis lipopolysaccharides induce RANKL by mouse osteoblast in a way different from that of Escherichia coli lipopolysaccharide. J. Endod. 2011, 37, 1653–1658. [Google Scholar] [CrossRef]
- Marton, I.J.; Kiss, C. Overlapping protective and destructive regulatory pathways in apical periodontitis. J. Endod. 2014, 40, 155–163. [Google Scholar] [CrossRef]
- Wei, L.; Xu, M.; Xiong, H. An update of knowledge on the regulatory role of Treg cells in apical periodontitis. Oral Dis. 2021, 27, 1356–1365. [Google Scholar] [CrossRef]
- Shen, Z.; Silva, R.M. MicroRNAs: Emerging players in apical periodontitis. J. Appl. Oral Sci. 2021, 29, e20201058. [Google Scholar] [CrossRef]
- Shi, J.; Gao, W.; Shao, F. Pyroptosis: Gasdermin-Mediated Programmed Necrotic Cell Death. Trends Biochem. Sci. 2017, 42, 245–254. [Google Scholar] [CrossRef]
- Yu, C.; Zhang, C.; Kuang, Z.; Zheng, Q. The Role of NLRP3 Inflammasome Activities in Bone Diseases and Vascular Calcification. Inflammation 2021, 44, 434–449. [Google Scholar] [CrossRef] [PubMed]
- Man, S.M.; Karki, R.; Kanneganti, T.D. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol. Rev. 2017, 277, 61–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miao, E.A.; Leaf, I.A.; Treuting, P.M.; Mao, D.P.; Dors, M.; Sarkar, A.; Warren, S.E.; Wewers, M.D.; Aderem, A. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat. Immunol. 2010, 11, 1136–1142. [Google Scholar] [CrossRef] [Green Version]
- Kayagaki, N.; Warming, S.; Lamkanfi, M.; Vande Walle, L.; Louie, S.; Dong, J.; Newton, K.; Qu, Y.; Liu, J.F.; Heldens, S.; et al. Non-canonical inflammasome activation targets caspase-11. Nature 2011, 479, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhao, Y.; Shao, F. Non-canonical activation of inflammatory caspases by cytosolic LPS in innate immunity. Curr. Opin. Immunol. 2015, 32, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Bergsbaken, T.; Fink, S.L.; Cookson, B.T. Pyroptosis: Host cell death and inflammation. Nat Rev Microbiol 2009, 7, 99–109. [Google Scholar] [CrossRef] [Green Version]
- Fink, S.L.; Cookson, B.T. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell Microbiol. 2006, 8, 1812–1825. [Google Scholar] [CrossRef]
- Takahashi, K. Microbiological, pathological, inflammatory, immunological and molecular biological aspects of periradicular disease. Int. Endod. J. 1998, 31, 311–325. [Google Scholar] [CrossRef]
- Cheng, R.; Wu, Z.; Li, M.; Shao, M.; Hu, T. Interleukin-1beta is a potential therapeutic target for periodontitis: A narrative review. Int. J. Oral Sci. 2020, 12, 2. [Google Scholar] [CrossRef] [Green Version]
- Strowig, T.; Henao-Mejia, J.; Elinav, E.; Flavell, R. Inflammasomes in health and disease. Nature 2012, 481, 278–286. [Google Scholar] [CrossRef]
- Lin, Z.; Altaf, N.; Li, C.; Chen, M.; Pan, L.H.; Wang, D.; Xie, L.P.; Zheng, Y.; Fu, H.L.; Han, Y.; et al. Hydrogen sulfide attenuates oxidative stress-induced NLRP3 inflammasome activation via S-sulfhydrating c-Jun at Cys269 in macrophages. BBA-Mol. Basis Dis. 2018, 1864, 2890–2900. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.B.; Sun, H.Y.; Luo, Z.L.; Cheng, L.; Duan, X.M.; Ren, J.D. Plasma-derived exosomes contribute to pancreatitis-associated lung injury by triggering NLRP3-dependent pyroptosis in alveolar macrophages. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165685. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Schlittenbauer, T.; Moebius, P.; Buttner-Herold, M.; Ries, J.; Preidl, R.; Geppert, C.I.; Neukam, F.W.; Wehrhan, F. Macrophage polarization differs between apical granulomas, radicular cysts, and dentigerous cysts. Clin. Oral Investig. 2018, 22, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Bertasso, A.S.; Leon, J.E.; Silva, R.A.B.; Silva, L.A.B.; de Queiroz, A.M.; Pucinelli, C.M.; Romualdo, P.C.; Nelson, P. Immunophenotypic quantification of M1 and M2 macrophage polarization in radicular cysts of primary and permanent teeth. Int. Endod. J. 2020, 53, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Jiang, M.; Qi, L.; Wu, Y.; Song, D.; Gan, J.; Li, Y.; Bai, Y. Pyroptosis, a new bridge to tumor immunity. Cancer Sci. 2021, 112, 3979–3994. [Google Scholar] [CrossRef]
- Yu, P.; Zhang, X.; Liu, N.; Tang, L.; Peng, C.; Chen, X. Pyroptosis: Mechanisms and diseases. Signal Transduct. Target. Ther. 2021, 6, 128. [Google Scholar] [CrossRef]
- Cheng, R.; Feng, Y.; Zhang, R.; Liu, W.; Lei, L.; Hu, T. The extent of pyroptosis varies in different stages of apical periodontitis. Biochim. Biophys. Acta 2018, 1864, 226–237. [Google Scholar] [CrossRef]
- Miao, N.J.; Xie, H.Y.; Xu, D.; Yin, J.Y.; Wang, Y.Z.; Wang, B.; Yin, F.; Zhou, Z.L.; Cheng, Q.; Chen, P.P.; et al. Caspase-11 promotes renal fibrosis by stimulating IL-1beta maturation via activating caspase-1. Acta Pharmacol. Sin. 2019, 40, 790–800. [Google Scholar] [CrossRef]
- Zhang, R.; Wu, Z.; Li, M.; Yang, J.; Cheng, R.; Hu, T. Canonical and noncanonical pyroptosis are both activated in periodontal inflammation and bone resorption. J. Periodontal. Res. 2022. [Google Scholar] [CrossRef]
- Yin, J.; Xu, J.; Cheng, R.; Shao, M.; Qin, Y.; Yang, H.; Hu, T. Role of connexin 43 in odontoblastic differentiation and structural maintenance in pulp damage repair. Int. J. Oral Sci. 2021, 13, 1. [Google Scholar] [CrossRef]
- Xu, R.; Guo, D.; Zhou, X.; Sun, J.; Zhou, Y.; Fan, Y.; Zhou, X.; Wan, M.; Du, W.; Zheng, L. Disturbed bone remodelling activity varies in different stages of experimental, gradually progressive apical periodontitis in rats. Int. J. Oral Sci. 2019, 11, 27. [Google Scholar] [CrossRef] [Green Version]
- Zeitvogel, F.; Schmid, G.; Hao, L.; Ingino, P.; Obst, M. ScatterJ: An ImageJ plugin for the evaluation of analytical microscopy datasets. J. Microsc. 2016, 261, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Dunn, K.W.; Kamocka, M.M.; McDonald, J.H. A practical guide to evaluating colocalization in biological microscopy. Am. J. Physiol. Cell Physiol. 2011, 300, C723–C742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, P.; Li, Q.; Su, S.; Dong, W.; Zong, S.; Ma, Q.; Yang, X.; Zuo, D.; Zheng, S.; Meng, X.; et al. Interleukin 37 Suppresses M1 Macrophage Polarization Through Inhibition of the Notch1 and Nuclear Factor Kappa B Pathways. Front. Cell Dev. Biol. 2020, 8, 56. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, Z.; Wang, C.; Yang, R.; Rathkey, J.K.; Pinkard, O.W.; Shi, W.; Chen, Y.; Dubyak, G.R.; Abbott, D.W.; et al. Mechanism of gasdermin D recognition by inflammatory caspases and their inhibition by a gasdermin D-derived peptide inhibitor. Proc. Natl. Acad. Sci. USA 2018, 115, 6792–6797. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Fu, A.; Yossifon, G. Active Particle Based Selective Transport and Release of Cell Organelles and Mechanical Probing of a Single Nucleus. Small 2020, 16, e1906682. [Google Scholar] [CrossRef]
- Chen, S.; Lei, H.; Luo, Y.; Jiang, S.; Zhang, M.; Lv, H.; Cai, Z.; Huang, X. Micro-CT analysis of chronic apical periodontitis induced by several specific pathogens. Int. Endod. J. 2019, 52, 1028–1039. [Google Scholar] [CrossRef]
- Sun, Z.; Nyanzu, M.; Yang, S.; Zhu, X.; Wang, K.; Ru, J.; Yu, E.; Zhang, H.; Wang, Z.; Shen, J.; et al. VX765 Attenuates Pyroptosis and HMGB1/TLR4/NF-kappaB Pathways to Improve Functional Outcomes in TBI Mice. Oxid. Med. Cell. Longev. 2020, 2020, 7879629. [Google Scholar] [CrossRef] [Green Version]
- Vandevska-Radunovic, V.; Kristiansen, A.B.; Heyeraas, K.J.; Kvinnsland, S. Changes in blood circulation in teeth and supporting tissues incident to experimental tooth movement. Eur. J. Orthod. 1994, 16, 361–369. [Google Scholar] [CrossRef]
- Chanput, W.; Mes, J.J.; Wichers, H.J. THP-1 cell line: An in vitro cell model for immune modulation approach. Int. Immunopharmacol. 2014, 23, 37–45. [Google Scholar] [CrossRef]
- Evavold, C.L.; Ruan, J.; Tan, Y.; Xia, S.; Wu, H.; Kagan, J.C. The Pore-Forming Protein Gasdermin D Regulates Interleukin-1 Secretion from Living Macrophages. Immunity 2018, 48, 35–44.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filiou, M.D.; Martins-de-Souza, D.; Guest, P.C.; Bahn, S.; Turck, C.W. To label or not to label: Applications of quantitative proteomics in neuroscience research. Proteomics 2012, 12, 736–747. [Google Scholar] [CrossRef]
- Neilson, K.A.; Ali, N.A.; Muralidharan, S.; Mirzaei, M.; Mariani, M.; Assadourian, G.; Lee, A.; van Sluyter, S.C.; Haynes, P.A. Less label, more free: Approaches in label-free quantitative mass spectrometry. Proteomics 2011, 11, 535–553. [Google Scholar] [CrossRef] [PubMed]
- Downs, K.P.; Nguyen, H.; Dorfleutner, A.; Stehlik, C. An overview of the non-canonical inflammasome. Mol. Asp. Med. 2020, 76, 100924. [Google Scholar] [CrossRef]
- Kobayashi, T.; Ogawa, M.; Sanada, T.; Mimuro, H.; Kim, M.; Ashida, H.; Akakura, R.; Yoshida, M.; Kawalec, M.; Reichhart, J.M.; et al. The Shigella OspC3 effector inhibits caspase-4, antagonizes inflammatory cell death, and promotes epithelial infection. Cell Host Microbe 2013, 13, 570–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pallett, M.A.; Crepin, V.F.; Serafini, N.; Habibzay, M.; Kotik, O.; Sanchez-Garrido, J.; Di Santo, J.P.; Shenoy, A.R.; Berger, C.N.; Frankel, G. Bacterial virulence factor inhibits caspase-4/11 activation in intestinal epithelial cells. Mucosal Immunol. 2017, 10, 602–612. [Google Scholar] [CrossRef] [Green Version]
- Marton, I.J.; Kiss, C. Protective and destructive immune reactions in apical periodontitis. Oral Microbiol. Immun. 2000, 15, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Kayagaki, N.; Stowe, I.B.; Lee, B.L.; O’Rourke, K.; Anderson, K.; Warming, S.; Cuellar, T.; Haley, B.; Roose-Girma, M.; Phung, Q.T.; et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 2015, 526, 666–671. [Google Scholar] [CrossRef]
- Tao, Z.; Wang, J.; Wen, K.; Yao, R.; Da, W.; Zhou, S.; Meng, Y.; Qiu, S.; Yang, K.; Zhu, Y.; et al. Pyroptosis in Osteoblasts: A Novel Hypothesis Underlying the Pathogenesis of Osteoporosis. Front. Endocrinol. 2020, 11, 548812. [Google Scholar] [CrossRef]
- Li, Y.; Ling, J.; Jiang, Q. Inflammasomes in Alveolar Bone Loss. Front. Immunol. 2021, 12, 691013. [Google Scholar] [CrossRef]
- Sartoretto, S.; Gemini-Piperni, S.; da Silva, R.A.; Calasans, M.D.; Rucci, N.; Pires Dos Santos, T.M.; Lima, I.B.C.; Rossi, A.M.; Alves, G.; Granjeiro, J.M.; et al. Apoptosis-associated speck-like protein containing a caspase-1 recruitment domain (ASC) contributes to osteoblast differentiation and osteogenesis. J. Cell. Physiol. 2019, 234, 4140–4153. [Google Scholar] [CrossRef] [PubMed]
- Kolly, L.; Busso, N.; Palmer, G.; Talabot-Ayer, D.; Chobaz, V.; So, A. Expression and function of the NALP3 inflammasome in rheumatoid synovium. Immunology 2010, 129, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Jun, H.K.; Jung, Y.J.; Choi, B.K. Treponema denticola, Porphyromonas gingivalis, and Tannerella forsythia induce cell death and release of endogenous danger signals. Arch. Oral Biol. 2017, 73, 72–78. [Google Scholar] [CrossRef]
- Wang, Q.A.; Zhou, X.D.; Zheng, Q.H.; Wang, Y.; Tang, L.; Huang, D.M. Distribution of Porphyromonas gingivalis fimA Genotypes in Chronic Apical Periodontitis Associated with Symptoms. J. Endod. 2010, 36, 1790–1795. [Google Scholar] [CrossRef] [PubMed]
- Kitano, T.; Mikami, Y.; Iwase, T.; Asano, M.; Komiyama, K. Loop-mediated isothermal amplification combined with PCR and immunohistochemistry for detecting Porphyromonas gingivalis in periapical periodontitis. J. Oral Sci. 2016, 58, 163–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, L.; Wang, X.; Liu, H.; Jiang, C.; Liao, H.; Xu, S.; Guo, Y.; Cao, Z. CXXC5 Mediates P. gingivalis-suppressed Cementoblast Functions Partially via MAPK Signaling Network. Int. J. Biol. Sci. 2019, 15, 1685–1695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, W.Z.; Zhou, W.; Wang, H.Z.; Liang, S. Roles of Porphyromonas gingivalis and its virulence factors in periodontitis. Adv. Protein Chem. Str. 2020, 120, 45–84. [Google Scholar] [CrossRef]
- Ran, S.; Liu, B.; Gu, S.; Sun, Z.; Liang, J. Analysis of the expression of NLRP3 and AIM2 in periapical lesions with apical periodontitis and microbial analysis outside the apical segment of teeth. Arch. Oral Biol. 2017, 78, 39–47. [Google Scholar] [CrossRef]
- Tiburcio-Machado, C.S.; Michelon, C.; Zanatta, F.B.; Gomes, M.S.; Marin, J.A.; Bier, C.A. The global prevalence of apical periodontitis: A systematic review and meta-analysis. Int. Endod. J. 2021, 54, 712–735. [Google Scholar] [CrossRef]
- Hussein, H.; Kishen, A. Local Immunomodulatory Effects of Intracanal Medications in Apical Periodontitis. J. Endod. 2022, 48, 430–456. [Google Scholar] [CrossRef]
- Zhang, R.; Yang, H.; Yu, X.; Wang, H.; Hu, T.; Dummer, P.M. Use of CBCT to identify the morphology of maxillary permanent molar teeth in a Chinese subpopulation. Int. Endod. J. 2011, 44, 162–169. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.; Li, M.; Ren, X.; Zhang, R.; He, J.; Cheng, L.; Cheng, R.; Hu, T. Double-Edged Sword Effect of Pyroptosis: The Role of Caspase-1/-4/-5/-11 in Different Levels of Apical Periodontitis. Biomolecules 2022, 12, 1660. https://doi.org/10.3390/biom12111660
Wu Z, Li M, Ren X, Zhang R, He J, Cheng L, Cheng R, Hu T. Double-Edged Sword Effect of Pyroptosis: The Role of Caspase-1/-4/-5/-11 in Different Levels of Apical Periodontitis. Biomolecules. 2022; 12(11):1660. https://doi.org/10.3390/biom12111660
Chicago/Turabian StyleWu, Zhiwu, Mingming Li, Xiaolin Ren, Rui Zhang, Jinfeng He, Li Cheng, Ran Cheng, and Tao Hu. 2022. "Double-Edged Sword Effect of Pyroptosis: The Role of Caspase-1/-4/-5/-11 in Different Levels of Apical Periodontitis" Biomolecules 12, no. 11: 1660. https://doi.org/10.3390/biom12111660




