Characteristics of Collagen Changes in Small Intestine Anastomoses Induced by High-Frequency Electric Field Welding
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Animal Tissue
2.2. Experimental Settings
2.3. Burst Pressure Measurements
2.4. Orientation Analysis of Tissue Collagen Fibers
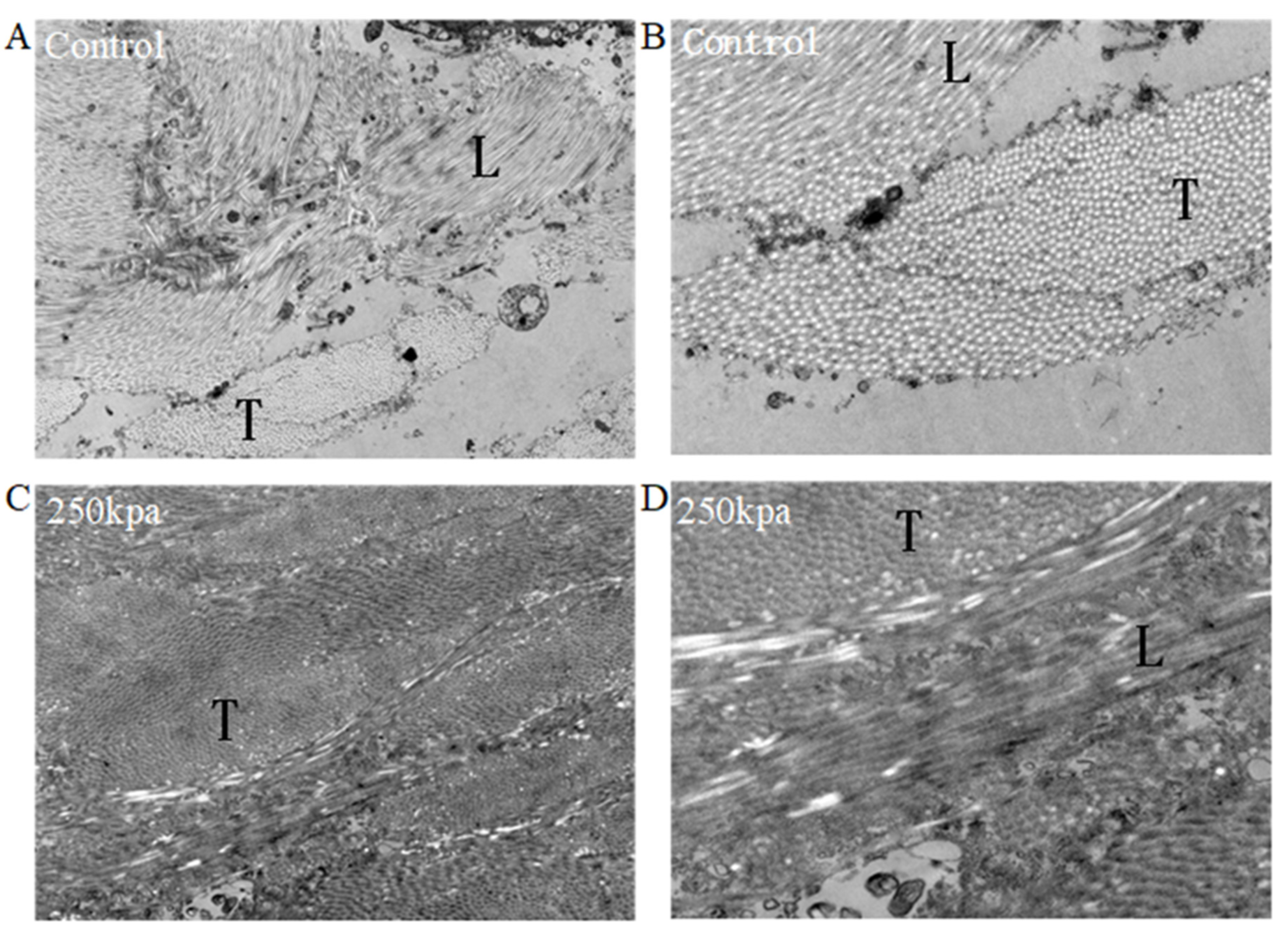
2.5. Transmission Electron Microscopy (TEM)
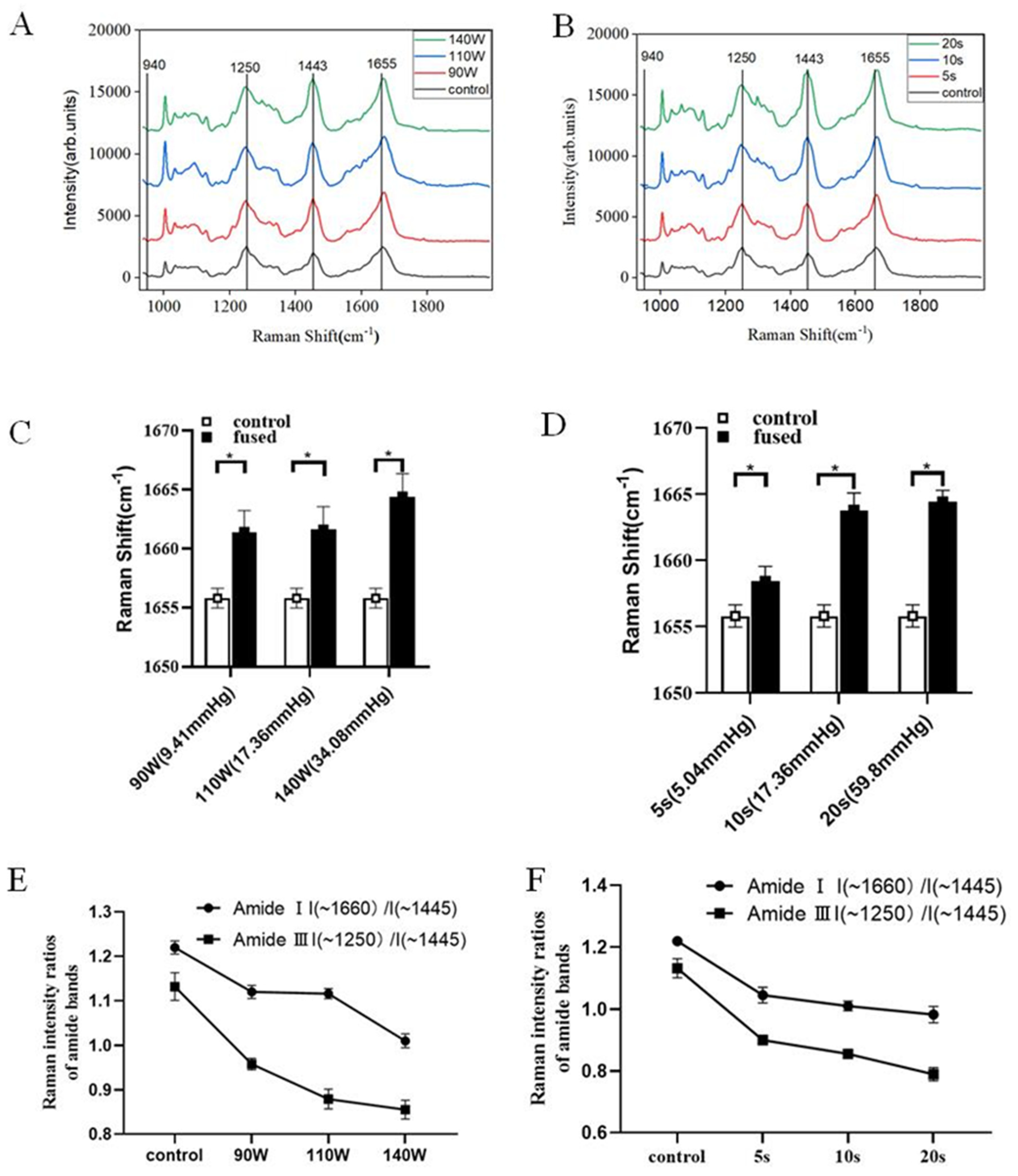
2.6. Raman Micro-Spectroscopy
2.7. Statistical Analysis
3. Results
3.1. Orientation Analysis Results
3.2. Transmission Electron Microscopy Results
3.3. Raman Micro-Spectroscopy Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pan, H.; Leung, K.K.C.; Ng, E.K.W. Tissue fusion technology versus suture and staple in porcine bowel anastomosis: An in vivo study. Braz. J. Med. Biol. Res. 2020, 53, e9305. [Google Scholar] [CrossRef]
- Zhao, L.; Song, C.; Wang, Z.; Zhou, Y.; Li, X.; Zhu, W.; Cuschieri, A. Novel concave-convex electrode for colonic anastomoses by radiofrequency thermo-fusion. Surg. Endosc. 2015, 29, 1809–1816. [Google Scholar] [CrossRef]
- Umanets, N.; Pasyechnikova, N.V.; Naumenko, V.A.; Henrich, P.B. High-frequency electric welding: A novel method for improved immediate chorioretinal adhesion in vitreoretinal surgery. Graefes Arch. Clin. Exp. Ophthalmol. 2014, 252, 1697–1703. [Google Scholar] [CrossRef] [PubMed]
- Newcomb, W.L.; Hope, W.W.; Schmelzer, T.M.; Heath, J.J.; Norton, H.J.; Lincourt, A.E.; Heniford, B.T.; Iannitti, D.A. Comparison of blood vessel sealing among new electrosurgical and ultrasonic devices. Surg. Endosc. 2009, 23, 90–96. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, K.; Xiang, X.; Zhu, C.; Wang, H. The impedance analysis of small intestine fusion by pulse source. Open Life Sci. 2020, 15, 808–818. [Google Scholar] [CrossRef]
- Santini, M.; Fiorelli, A.; Messina, G.; Laperuta, P.; Mazzella, A.; Accardo, M. Use of the LigaSure device and the Stapler for closure of the small bowel: A comparative ex vivo study. Surg. Today 2013, 43, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Winter, H.; Holmer, C.; Buhr, H.J.; Lindner, G.; Lauster, R.; Kraft, M.; Ritz, J.P. Pilot study of bipolar radiofrequency-induced anastomotic thermofusion-exploration of therapy parameters ex vivo. Int. J. Colorectal Dis. 2010, 25, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, X.; Madeniyeti, N.; Qiu, J.; Zhu, C.; Yin, L.; Liu, K. Temperature Distribution of Vessel Tissue by High Frequency Electric Welding with Combination Optical Measure and Simulation. Biosensors 2022, 12, 209. [Google Scholar] [CrossRef]
- Zhao, L.; Zhuo, C.; Song, C.; Li, X.; Zhou, Y.; Shi, D. Histological characteristics of collagen denaturation and injuries in bipolar radiofrequency-induced colonic anastomoses. Pathol. Res. Pract. 2015, 211, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Foschi, D.; Cellerino, P.; Corsi, F.; Taidelli, T.; Morandi, E.; Rizzi, A.; Trabucchi, E. The mechanisms of blood vessel closure in humans by the application of ultrasonic energy. Surg. Endosc. 2002, 16, 814–819. [Google Scholar] [CrossRef] [PubMed]
- Kramer, E.A.; Rentschler, M.E. Energy-Based Tissue Fusion for Sutureless Closure: Applications, Mechanisms, and Potential for Functional Recovery. Annu. Rev. Biomed. Eng. 2018, 20, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Tu, L.; Zhou, Y.; Song, C.; Li, Y.; Chen, L.; Xue, Y. Preliminary study of a control algorithm for radio-frequency-induced intestinal tissue fusion. Int. J. Hyperth. 2019, 36, 1297–1306. [Google Scholar] [CrossRef]
- Arya, S.; Hadjievangelou, N.; Lei, S.; Kudo, H.; Goldin, R.D.; Darzi, A.W.; Elson, D.S.; Hanna, G.B. Radiofrequency-induced small bowel thermofusion: An ex vivo study of intestinal seal adequacy using mechanical and imaging modalities. Surg. Endosc. 2013, 27, 3485–3496. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Cheng, C.H. Effective elastic moduli of misoriented short-fiber composites. Int. J. Solids Struct. 1996, 33, 2519–2539. [Google Scholar] [CrossRef]
- Martin, R.B.; Lau, S.T.; Mathews, P.V.; Gibson, V.A.; Stover, S.M. Collagen fiber organization is related to mechanical properties and remodeling in equine bone. A comparison of two methods. J. Biomech. 1996, 29, 1515–1521. [Google Scholar] [CrossRef]
- Tian, L.; Wang, Z.; Liu, Y.; Eickhoff, J.C.; Eliceiri, K.W.; Chesler, N.C. Validation of an arterial constitutive model accounting for collagen content and crosslinking. Acta Biomater. 2016, 31, 276–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Keikhosravi, A.; Mehta, G.S.; Drifka, C.R.; Eliceiri, K.W. Methods for Quantifying Fibrillar Collagen Alignment. Methods Mol. Biol. 2017, 1627, 429–451. [Google Scholar] [CrossRef] [PubMed]
- George, J.; Thomas, J. Raman spectroscopy of protein and nucleic acid assemblies. Annu. Rev. Biophys. Biomol. Struct. 1999, 28, 1–27. [Google Scholar] [CrossRef] [Green Version]
- Han, S.; Cai, Z.; Ning, X.; He, L.; Chen, J.; Huang, Z.; Zhou, H.; Huang, D.; Zhang, P.; Li, Z. Comparison of a New High-Frequency Electric Welding System for Intestinal Closure with Hand-Sewn In Vivo Pig Model. J. Laparoendosc. Adv. Surg. Tech. A 2015, 25, 662–667. [Google Scholar] [CrossRef]
- Pan, H.; Lam, P.K.; Tong, S.W.; Leung, K.K.; Teoh, A.Y.; Ng, E.K. Mesenchymal Stem Cells Combined with Tissue Fusion Technology Promoted Wound Healing in Porcine Bowel Anastomosis. Stem. Cells Int. 2020, 2020, 5142797. [Google Scholar] [CrossRef] [PubMed]
- Zelickson, B.D.; Kist, D.; Bernstein, E.; Brown, D.B.; Ksenzenko, S.; Burns, J.; Kilmer, S.; Mehregan, D.; Pope, K. Histological and ultrastructural evaluation of the effects of a radiofrequency-based nonablative dermal remodeling device - A pilot study. Arch. Dermatol. 2004, 140, 204–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salameh, J.R.; Schwartz, J.H.; Hildebrandt, D.A. Can LigaSure seal and divide the small bowel? Am. J. Surg. 2006, 191, 791–793. [Google Scholar] [CrossRef]
- Shields, C.A.; Schechter, D.A.; Tetzlaff, P.; Baily, A.L.; Dycus, S.; Cosgriff, N. Method for creating ideal tissue fusion in soft-tissue structures using radio frequency (RF) energy. Surg. Technol. Int. 2004, 13, 49–55. [Google Scholar] [PubMed]
- Tarnowski, C.P.; Stewart, S.; Holder, K.; Campbell-Clark, L.; Thoma, R.J.; Adams, A.K.; Moore, M.A. Effects of treatment protocols and subcutaneous implantation on bovine pericardium: A Raman spectroscopy study. J. Biomed. Opt. 2003, 8, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Yan, X.; Pang, X.; Liu, S. Temperature-dependent Raman spectra of collagen and DNA. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2004, 60, 557–561. [Google Scholar] [CrossRef]
- Carden, A.; Rajachar, R.M.; Morris, M.D.; Kohn, D.H. Ultrastructural changes accompanying the mechanical deformation of bone tissue: A Raman imaging study. Calcif. Tissue Int. 2003, 72, 166–175. [Google Scholar] [CrossRef] [PubMed]

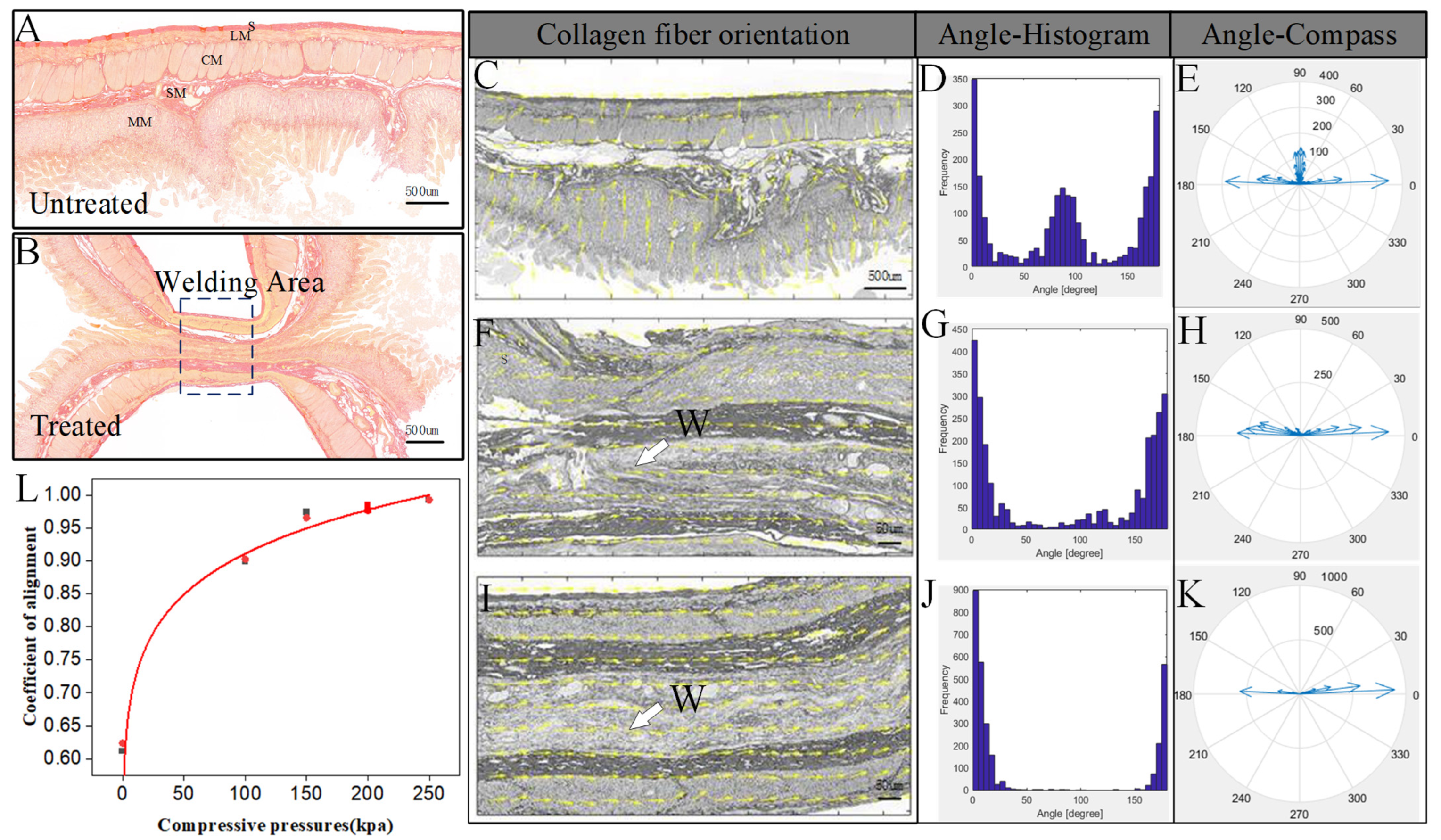

| Compressive Pressure (kPa) | Burst Pressure (Mean ± SD) (mmHg) | Alignment Coefficient of Collagen Fibers |
|---|---|---|
| 0 | 0 | 0.61 ± 0.0086 |
| 100 | 11.30 ± 6.52 | 0.90 ± 0.0022 |
| 150 | 23.40 ± 8.10 | 0.96 ± 0.0062 |
| 200 | 27.20 ± 5.29 | 0.98 ± 0.0054 |
| 250 | 35.05 ± 10.28 | 0.99 ± 0.0002 |
| Sample | Position (~1250 cm−1) | Position (~1655 cm−1) | I (~1250 cm−1)/I (~1445 cm−1) | I (~1660 cm−1)/I (~1445 cm−1) |
|---|---|---|---|---|
| 90 W × 10 s | 1250 | 1655.8 | 1.132 | 1.220 |
| 110 W × 10 s | 1248 | 1661.4 | 0.957 | 1.120 |
| 140 W × 10 s | 1248 | 1661.6 | 0.879 | 1.116 |
| 140 W × 5 s | 1246 | 1664.4 | 0.855 | 1.010 |
| 140 W × 20 s | 1249 | 1658.4 | 0.900 | 1.045 |
| 90 W × 10 s | 1248 | 1665 | 0.789 | 0.982 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, C.; Yin, L.; Xu, J.; Yang, X.; Wang, H.; Xiang, X.; Liu, H.; Liu, K. Characteristics of Collagen Changes in Small Intestine Anastomoses Induced by High-Frequency Electric Field Welding. Biomolecules 2022, 12, 1683. https://doi.org/10.3390/biom12111683
Zhu C, Yin L, Xu J, Yang X, Wang H, Xiang X, Liu H, Liu K. Characteristics of Collagen Changes in Small Intestine Anastomoses Induced by High-Frequency Electric Field Welding. Biomolecules. 2022; 12(11):1683. https://doi.org/10.3390/biom12111683
Chicago/Turabian StyleZhu, Caihui, Li Yin, Jianzhi Xu, Xingjian Yang, Hao Wang, Xiaowei Xiang, Haotian Liu, and Kefu Liu. 2022. "Characteristics of Collagen Changes in Small Intestine Anastomoses Induced by High-Frequency Electric Field Welding" Biomolecules 12, no. 11: 1683. https://doi.org/10.3390/biom12111683






