Periodontal Regenerative Therapy Using rhFGF-2 and Deproteinized Bovine Bone Mineral versus rhFGF-2 Alone: 4-Year Extended Follow-Up of a Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Clinical and Radiographic Examinations
2.4. Patient Reported Outcome (PRO) Measure
2.5. Surgical Interventions
2.6. Postsurgical Care
2.7. Statistical Analysis
3. Results
3.1. Participants
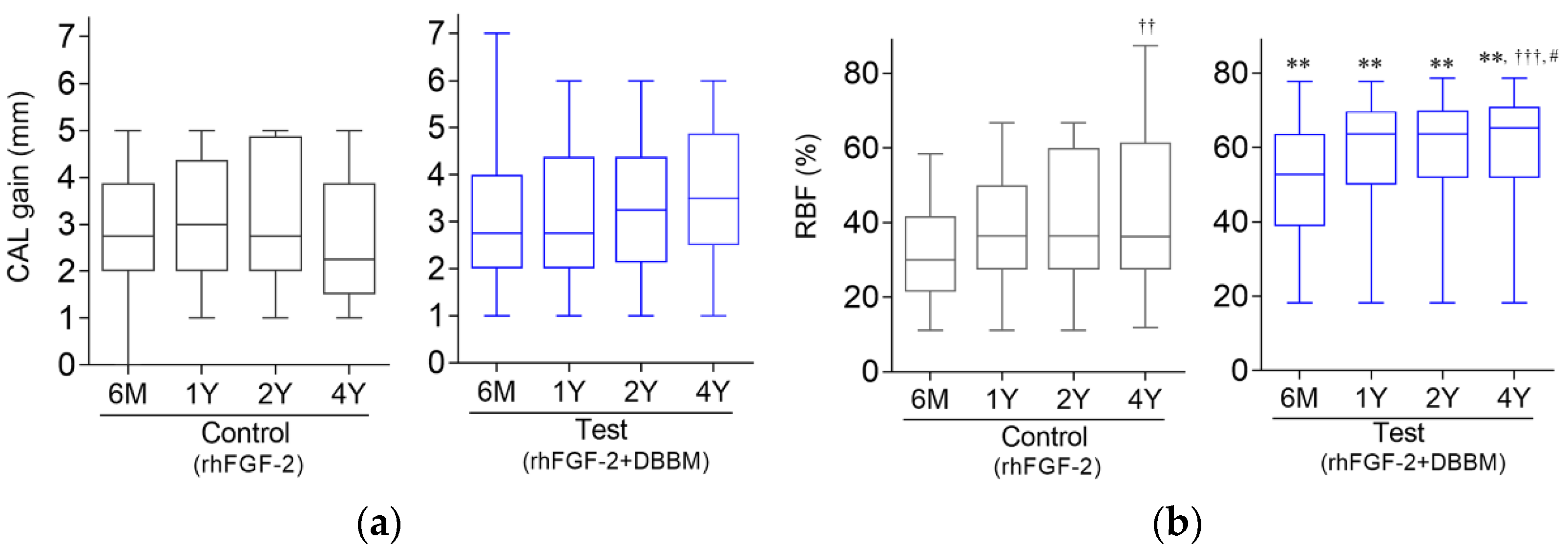
3.2. Clinical Outcomes
3.3. Relationship between Baseline Variables and CAL Gain at 4 Years
3.4. Radiographic Outcome
3.5. CAL Gain and RBF in Different Defect Configurations
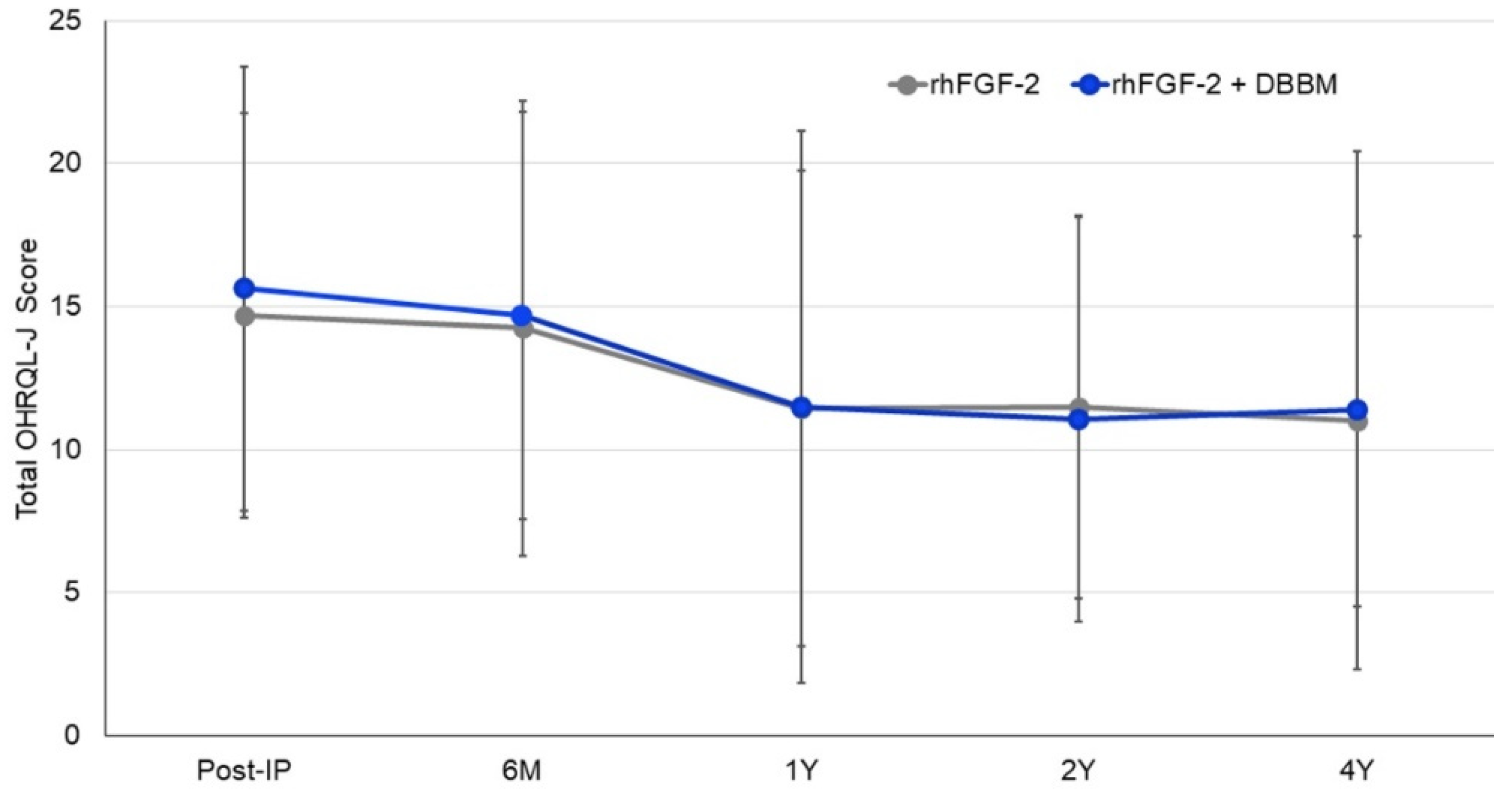
3.6. OHRQL-J Scores
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bartold, P.M.; Gronthos, S.; Ivanovski, S.; Fisher, A.; Hutmacher, D.W. Tissue engineered periodontal products. J. Periodontal Res. 2015, 51, 1–15. [Google Scholar] [CrossRef]
- Murakami, S.; Takayama, S.; Ikezawa, K.; Sltimabukuro, Y.; Kitamura, M.; Nozaki, T.; Terashima, A.; Asano, T.; Okada, H. Regeneration of periodontal tissues by basic fibroblast growth factor. J. Periodontal Res. 1999, 34, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Murakami, S. Periodontal tissue regeneration by signaling molecule(s): What role does basic fibroblast growth factor (FGF-2) have in periodontal therapy? Periodontology 2000 2011, 56, 188–208. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, M.; Akamatsu, M.; Kawanami, M.; Furuichi, Y.; Fujii, T.; Mori, M.; Kunimatsu, K.; Shimauchi, H.; Ogata, Y.; Yamamoto, M.; et al. Randomized placebo-controlled and controlled non-inferiority phase III trials comparing trafermin, a recombinant human fibroblast growth factor 2, and enamel matrix derivative in periodontal regeneration in intrabony defects. J. Bone Miner. Res. 2016, 31, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, M.; Akamatsu, M.; Machigashira, M.; Hara, Y.; Sakagami, R.; Hirofuji, T.; Hamachi, T.; Maeda, K.; Yokota, M.; Kido, J.; et al. FGF-2sStimulates periodontal regeneration: Results of a multi-center randomized clinical trial. J. Dent. Res. 2011, 90, 35–40. [Google Scholar] [CrossRef]
- Saito, A.; Bizenjima, T.; Takeuchi, T.; Suzuki, E.; Sato, M.; Yoshikawa, K.; Kitamura, Y.; Matsugami, D.; Aoki, H.; Kita, D.; et al. Treatment of intrabony periodontal defects using rh FGF -2 in combination with deproteinized bovine bone mineral or rh FGF -2 alone: A 6-month randomized controlled trial. J. Clin. Periodontol. 2019, 46, 332–341. [Google Scholar] [CrossRef]
- Japanese Society of Periodontology. JSP Clinical Practice Guidelines for the Periodontal Treatment 2022; Japanese Society of Periodontology: Tokyo, Japan, 2022; p. 60, (In Japanese). Available online: https://www.perio.jp/publication/upload_file/guideline_perio_2022.pdf (accessed on 1 October 2022).
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Berglundh, T.; Sculean, A.; Tonetti, M.S. Treatment of stage I–III periodontitis—The EFP S3 level clinical practice guideline. J. Clin. Periodontol. 2020, 47, 4–60. [Google Scholar] [CrossRef]
- Matarasso, M.; Iorio- Siciliano, V.; Blasi, A.; Ramaglia, L.; Salvi, G.E.; Sculean, A. Enamel matrix derivative and bone grafts for periodontal regeneration of intrabony defects. A systematic review and meta-analysis. Clin. Oral Investig. 2015, 19, 1581–1593. [Google Scholar] [CrossRef]
- Hoffmann, T.; Al-Machot, E.; Meyle, J.; Jervøe-Storm, P.-M.; Jepsen, S. Three-year results following regenerative periodontal surgery of advanced intrabony defects with enamel matrix derivative alone or combined with a synthetic bone graft. Clin. Oral Investig. 2015, 20, 357–364. [Google Scholar] [CrossRef]
- Pietruska, M.; Pietruski, J.; Nagy, K.; Brecx, M.; Arweiler, N.B.; Sculean, A. Four-year results following treatment of intrabony periodontal defects with an enamel matrix derivative alone or combined with a biphasic calcium phosphate. Clin. Oral Investig. 2011, 16, 1191–1197. [Google Scholar] [CrossRef]
- Chen, F.-M.; Zhang, J.; Zhang, M.; An, Y.; Chen, F.; Wu, Z.-F. A review on endogenous regenerative technology in periodontal regenerative medicine. Biomaterials 2010, 31, 7892–7927. [Google Scholar] [CrossRef]
- Stavropoulos, A.; Karring, T. Guided tissue regeneration combined with a deproteinized bovine bone mineral (Bio-Oss®) in the treatment of intrabony periodontal defects: 6-year results from a randomized-controlled clinical trial. J. Clin. Periodontol. 2010, 37, 200–210. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Cortellini, P.; Lang, N.P.; Suvan, J.E.; Adriaens, P.; Dubravec, D.; Fonzar, A.; Fourmousis, I.; Rasperini, G.; Rossi, R.; et al. Clinical outcomes following treatment of human intrabony defects with GTR/bone replacement material or access flap alone. A multicenter randomized controlled clinical trial. J. Clin. Periodontol. 2004, 31, 770–776. [Google Scholar] [CrossRef]
- Sculean, A.; Nikolidakis, D.; Schwarz, F. Regeneration of periodontal tissues: Combinations of barrier membranes and grafting material—Biological foundation and preclinical evidence: A systematic review. J. Clin. Periodontol. 2008, 35, 106–116. [Google Scholar] [CrossRef]
- Irokawa, D.; Okubo, N.; Nikaido, M.; Shimizu, H.; Konobu, N.; Matsui, T.; Fujita, T.; Goto, H.; Takeuchi, T.; Ishii, Y.; et al. Periodontal regenerative therapy of intrabony defects using deproteinized bovine bone mineral in combination with collagen barrier membrane: A multicenter prospective case-series study. Int. J. Periodontics Restor. Dent. 2017, 37, 393–401. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Irokawa, D.; Takeuchi, T.; Noda, K.; Goto, H.; Egawa, M.; Tomita, S.; Sugito, H.; Nikaido, M.; Saito, A. Clinical outcome of periodontal regenerative therapy using collagen membrane and deproteinized bovine bone mineral: A 2.5-year follow-up study. BMC Res. Notes 2017, 10, 102. [Google Scholar] [CrossRef] [PubMed]
- Zucchelli, G.; Amore, C.; Montebugnoli, L.; De Sanctis, M. Enamel matrix protines bovine porous bone mineral in the treatment of intrabony defects: Comparative controlled clinical trial. J. Periodontol. 2003, 74, 1725–1735. [Google Scholar] [CrossRef] [PubMed]
- Aoki, H.; Bizenjima, T.; Seshima, F.; Sato, M.; Irokawa, D.; Yoshikawa, K.; Yoshida, W.; Imamura, K.; Matsugami, D.; Kitamura, Y.; et al. Periodontal surgery using rhFGF-2 with deproteinized bovine bone mineral or rhFGF-2 alone: 2-year follow-up of a randomized controlled trial. J. Clin. Periodontol. 2020, 48, 92–100. [Google Scholar] [CrossRef]
- Armitage, G.C. Development of a classification system for periodontal diseases and conditions. Ann. Periodontol. 1999, 4, 1–6. [Google Scholar] [CrossRef]
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89, S173–S182. [Google Scholar] [CrossRef]
- Japanese Society of Periodontology. JSP Clinical Practice Guidelines for the Periodontal Treatment 2015; Japanese Society of Periodontology: Tokyo, Japan, 2015; p. 26. Available online: https://www.perio.jp/publication/upload_file/guideline_perio_plan2015_en.pdf (accessed on 20 December 2016).
- Seshima, F.; Aoki, H.; Takeuchi, T.; Suzuki, E.; Irokawa, D.; Makino-Oi, A.; Sugito, H.; Tomita, S.; Saito, A. Periodontal regenerative therapy with enamel matrix derivative in the treatment of intrabony defects: A prospective 2-year study. BMC Res. Notes 2017, 10, 256. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.; Hosaka, Y.; Kikuchi, M.; Akamatsu, M.; Fukaya, C.; Matsumoto, S.; Ueshima, F.; Hayakawa, H.; Fujinami, K.; Nakagawa, T. Effect of initial periodontal therapy on oral health–related quality of life in patients with periodontitis in Japan. J. Periodontol. 2010, 81, 1001–1009. [Google Scholar] [CrossRef]
- Saito, A.; Ota, K.; Hosaka, Y.; Akamatsu, M.; Hayakawa, H.; Fukaya, C.; Ida, A.; Fujinami, K.; Sugito, H.; Nakagawa, T. Potential impact of surgical periodontal therapy on oral health-related quality of life in patients with periodontitis: A pilot study. J. Clin. Periodontol. 2011, 38, 1115–1121. [Google Scholar] [CrossRef]
- Davies, G.; Jordan, S.; Brooks, C.J.; Thayer, D.; Storey, M.; Morgan, G.; Allen, S.; Garaiova, I.; Plummer, S.; Gravenor, M. Long term extension of a randomised controlled trial of probiotics using electronic health records. Sci. Rep. 2018, 8, 7668. [Google Scholar] [CrossRef]
- Sculean, A.; Pietruska, M.; Arweiler, N.B.; Auschill, T.M.; Nemcovsky, C. Four-year results of a prospective-controlled clinical study evaluating healing of intra-bony defects following treatment with an enamel matrix protein derivative alone or combined with a bioactive glass. J. Clin. Periodontol. 2007, 34, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Corbella, S.; Alberti, A.; Calciolari, E.; Taschieri, S.; Francetti, L. Enamel matrix derivative for the treatment of partially contained intrabony defects: 12-month results. Aust. Dent. J. 2018, 64, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Lang, N.P.; Cortellini, P.; Suvan, J.E.; Adriaens, P.; Dubravec, D.; Fonzar, A.; Fourmousis, I.; Mayfield, L.; Rossi, R.; et al. Enamel matrix proteins in the regenerative therapy of deep intrabony defects. J. Clin. Periodontol. 2002, 29, 317–325. [Google Scholar] [CrossRef]
- Zucchelli, G.; Bernardi, F.; Montebugnoli, L.; De Sanctis, M. Enamel matrix proteins and guided tissue regeneration with titanium-reinforced expanded polytetrafluoroethylene membranes in the treatment of infrabony defects: A comparative controlled clinical trial. J. Periodontol. 2002, 73, 3–12. [Google Scholar] [CrossRef]
- Trombelli, L.; Farina, R.; Vecchiatini, R.; Maietti, E.; Simonelli, A. A simplified composite outcome measure to assess the effect of periodontal regenerative treatment in intraosseous defects. J. Periodontol. 2019, 91, 723–731. [Google Scholar] [CrossRef]
- Cochran, D.; Oh, T.-J.; Mills, M.; Clem, D.; McClain, P.; Schallhorn, R.; McGuire, M.; Scheyer, E.; Giannobile, W.; Reddy, M.; et al. A Randomized Clinical Trial Evaluating rh-FGF-2/β-TCP in Periodontal Defects. J. Dent. Res. 2016, 95, 523–530. [Google Scholar] [CrossRef]
- Renvert, S.; Garrett, S.; Nilveus, R.; Chamberlain, A.D.H.; Egelberg, J. Healing after treatment of periodontal intraosseous defects. VI. Factors influencing the healing response. J. Clin. Periodontol. 1985, 12, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Cortellini, P.; Tonetti, M.S. Focus on intrabony defects: Guided tissue regeneration. Periodontology 2000 2000, 22, 104–132. [Google Scholar] [CrossRef]
- Iorio-Siciliano, V.; Andreuccetti, G.; Blasi, A.; Matarasso, M.; Sculean, A.; Salvi, G.E. Clinical outcomes following regenerative therapy of non-contained intrabony defects using a deproteinized bovine bone mineral combined with either enamel matrix derivative or collagen membrane. J. Periodontol. 2014, 85, 1342–1350. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.-S.; Choi, S.-H.; Chai, J.-K.; Cho, K.-S.; Moon, I.-S.; Wikesjö, U.M.; Kim, C.-K. Periodontal repair in surgically created intrabony defects in dogs: Influence of the number of bone walls on healing response. J. Periodontol. 2004, 75, 229–235. [Google Scholar] [CrossRef]
- Murakami, T.; Matsugami, D.; Yoshida, W.; Imamura, K.; Bizenjima, T.; Seshima, F.; Saito, A. Healing of experimental periodontal defects following treatment with fibroblast growth factor-2 and deproteinized bovine bone mineral. Biomolecules 2021, 11, 805. [Google Scholar] [CrossRef] [PubMed]
- Shirakata, Y.; Setoguchi, F.; Sena, K.; Nakamura, T.; Imafuji, T.; Shinohara, Y.; Iwata, M.; Noguchi, K. Comparison of periodontal wound healing/regeneration by recombinant human fibroblast growth factor-2 combined with β-tricalcium phosphate, carbonate apatite, or deproteinized bovine bone mineral in a canine one-wall intra-bony defect model. J. Clin. Periodontol. 2022, 49, 599–608. [Google Scholar] [CrossRef]
- Troiano, G.; Laino, L.; Zhurakivska, K.; Cicciù, M.; Muzio, L.L.; Russo, L.L. Addition of enamel matrix derivatives to bone substitutes for the treatment of intrabony defects: A systematic review, meta-analysis and trial sequential analysis. J. Clin. Periodontol. 2017, 44, 729–738. [Google Scholar] [CrossRef]
- Lee, J.; Jeong, S. Long-term stability of adjunctive use of enamel matrix protein derivative on porcine-derived xenograft for the treatment of one-wall intrabony defects: A 4-year extended follow-up of a randomized controlled trial. J. Periodontol. 2021, 93, 231–238. [Google Scholar] [CrossRef]
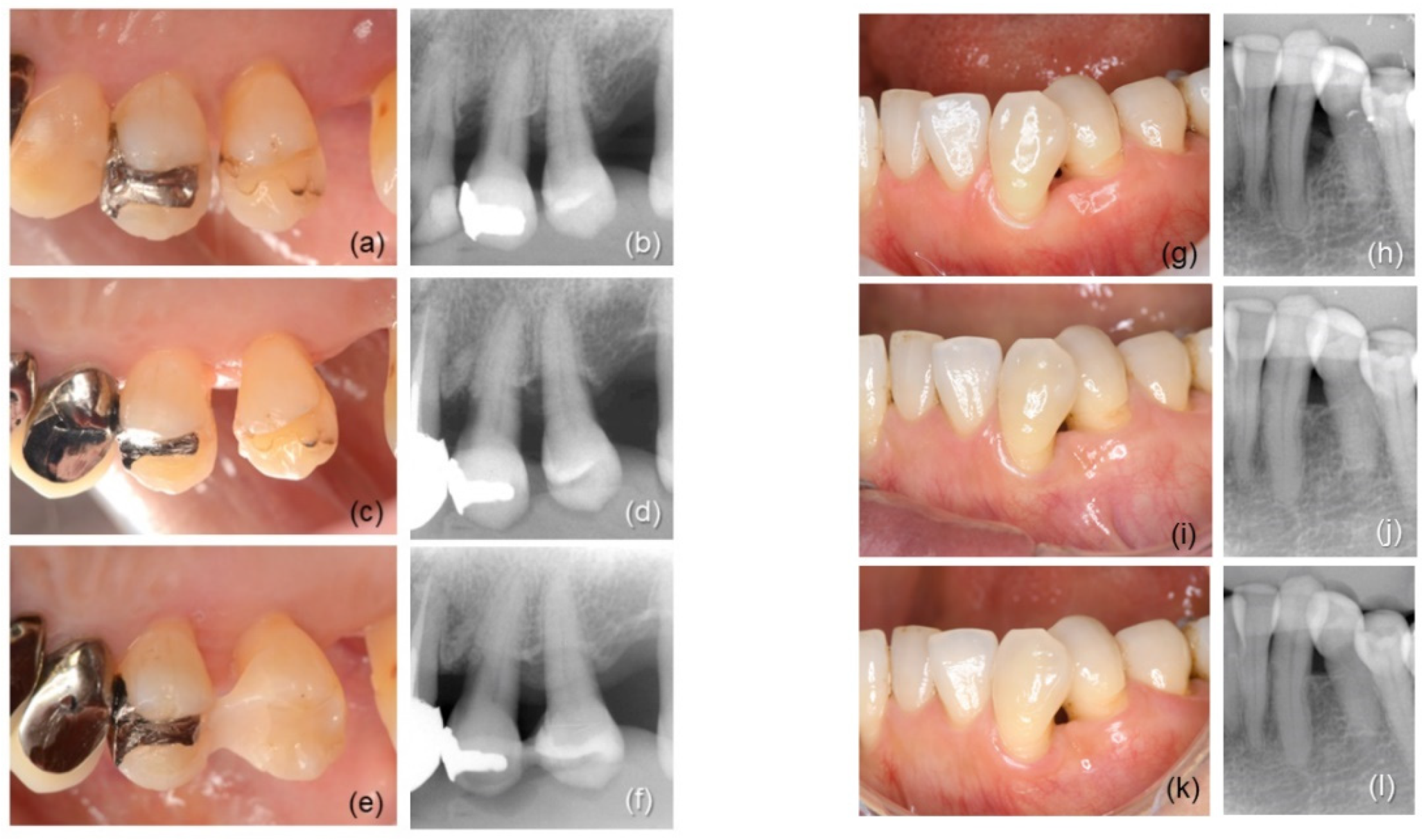
| Intrabony Defect | rhFGF-2 (Control, n = 16) | rhFGF-2 + DBBM (Test, n = 16) |
|---|---|---|
| Position [n (%)] | ||
| Maxilla | 6 (37.5) | 9 (45.0) |
| Mandible | 10 (62.5) | 7 (55.0) |
| Anterior teeth | 5 (31.3) | 2 (10.0) |
| Premolars | 4 (25.0) | 5 (25.0) |
| Molars | 7 (43.7) | 9 (65.0) |
| Morphology [n (%)] | ||
| 1-wall | 3 (18.8) | 2 (12.5) |
| 2-wall | 4 (25.0) | 5 (31.3) |
| 3-wall | 4 (25.0) | 4 (25.0) |
| combination | 5 (31.2) | 5 (31.3) |
| Depth (mm; mean ± SD) | 4.72 ± 1.88 (range, 3.0–11.0) | 4.53 ± 1.09 (range, 3.0–6.5) |
| Width (mm; mean ± SD) | 2.88 ± 0.82 (range, 2.0–5.0) | 3.59 ± 1.87 (range, 2.0–10.0) |
| Variable/Group | Baseline (Post-IP) | 6 Months | 1 Year | 2 Years | 4 Years |
|---|---|---|---|---|---|
| CAL (mm) | |||||
| rhFGF-2 | 7.28 ± 1.73 (6.5; 6.36–8.20) | 4.56 ± 1.44 ** (4; 3.80–5.33) | 4.25 ± 1.53 *** (4; 3.44–5.06) | 4.18 ± 1.35 *** (4; 3.47–4.90) | 4.56 ± 1.25 ** (4.25; 3.90–5.23) |
| rhFGF-2 + DBBM | 7.31 ± 1.62 (7; 6.45–8.18) | 4.22 ± 1.17 *** (4; 3.60–4.84) | 4.22 ± 0.97 ** (4.25; 3.70–4.73) | 3.93 ± 0.89 *** (4; 3.46–4.41) | 3.81 ± 0.91 *** (4; 3.33–4.30) |
| Difference between groups | N.S. | N.S. | N.S. | N.S. | N.S. |
| PPD (mm) | |||||
| rhFGF-2 | 6.28 ± 1.46 (5; 5.50–7.01) | 2.94 ± 0.87 *** (3; 2.47–3.40) | 2.75 ± 0.86 *** (3; 2.29–2.99) | 2.68 ± 0.89 *** (2.25; 2.21–3.16) | 3.03 ± 0.97 ** (2.75; 2.51–3.55) |
| rhFGF-2 + DBBM | 6.00 ± 1.27 (6.5; 5.33–6.67) | 2.66 ± 0.72 *** (2.50; 2.27–3.04) | 2.72 ± 0.52 *** (3; 2.44–2.99) | 2.59 ± 0.49 *** (3; 2.33–2.86) | 2.63 ± 0.62 *** (2.50; 2.30–2.96) |
| Difference between groups | N.S. | N.S. | N.S. | N.S. | N.S. |
| GR (mm) | |||||
| rhFGF-2 | 0.93 ± 1.24 (0.50; 0.27–1.60) | 1.37 ± 1.53 (1; 0.56–2.19) | 1.50 ± 1.27 (1.5; 0.83–2.17) | 1.50 ± 1.30 (1.25; 0.78–2.22) | 1.53 ± 1.30 (1.25; 0.84–2.22) |
| rhFGF-2 + DBBM | 1.31 ± 1.35 (1; 0.59–2.03) | 1.56 ± 1.22 (1.25; 0.91–2.21) | 1.44 ± 0.98 (1.25; 0.91–1,96) | 1.25 ± 0.93 (1.25; 0.75–1.75) | 1.22 ± 0.88 (1; 0.75–1.69) |
| Difference between groups | N.S. | N.S. | N.S. | N.S. | N.S. |
| BOP positive (%) | |||||
| rhFGF-2 | 62.5 | 12.5 *** | 6.3 *** | 0.0 *** | 0.0 *** |
| rhFGF-2 + DBBM | 62.5 | 6.3 *** | 0.0 *** | 0.0 *** | 0.0 *** |
| Difference between groups a | N.S. | N.S. | N.S. | N.S. | N.S. |
| TM | |||||
| rhFGF-2 | 0.11 ± 0.32 (0; −0.05–0.27) | 0.06 ± 0.24 (0; −0.06–0.17) | 0.06 ± 0.24 (0; −0.06–0.17) | 0.11 ± 0.32 (0; −0.05–0.27) | 0.11 ± 0.32 (0; −0.05–0.27) |
| rhFGF-2 + DBBM | 0.20 ± 0.41 (0; 0.01–0.39) | 0.05 ± 0.22 (0; −0.05–0.15) | 0.05 ± 0.22 (0;−0.05–0.15) | 0.05 ± 0.22 (0; −0.05–0.15) | 0.05 ± 0.22 (0; −0.05–0.15) |
| Differencebetween groups | N.S. | N.S. | N.S. | N.S. | N.S. |
| RBF (%) | |||||
| rhFGF-2 | – | 31.2 ± 14.1 (28.7; 23.6–38.7) | 36.7 ± 16.1 (34.9; 28.1–45.3) | 39.6 ± 18.0 (34.9; 30.1–49.2) | 41.5 ± 21.5 †† (36.2; 29.6–53.4) |
| rhFGF-2 + DBBM | – | 50.6 ± 16.7 (52.8; 41.7–59.5) | 58.2 ± 15.7 (63.6; 49.9–66.6) | 60.1 ± 15.8 (63.6; 51.7–68.5) | 61.8 ± 16.0 ††† (65.7; 53.2–70.3) |
| Difference between groups | p = 0.003 | p = 0.001 | p = 0.003 | p = 0.006 |
| Outcome | Defect | rhFGF-2 (Control) | Difference | rhFGF-2 + DBBM (Test) | Difference |
|---|---|---|---|---|---|
| CAL gain (mm) | 3-wall | 3.19 ± 1.36 (3.50; 2.05–4.33) | N.S. | 3.72 ± 1.52 (4.00; 2.55–4.89) | N.S. |
| 1–2-wall | 2.25 ± 1.41 (2.00; 1.07–3.43) | 3.21 ± 1.32 (3.00; 2.00–4.43) | |||
| RBF (%) | 3-wall | 47.6 ± 14.4 (48.6; 36.6–58.7) | p = 0.03 | 61.4 ± 13.3 (65.2; 51.2–73.1) | N.S. |
| 1–2-wall | 32.3 ± 28.1 (25.5; 2.7–61.8) | 61.1 ± 19.6 * (67.3; 43.1–79.2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seshima, F.; Bizenjima, T.; Aoki, H.; Imamura, K.; Kita, D.; Irokawa, D.; Matsugami, D.; Kitamura, Y.; Yamashita, K.; Sugito, H.; et al. Periodontal Regenerative Therapy Using rhFGF-2 and Deproteinized Bovine Bone Mineral versus rhFGF-2 Alone: 4-Year Extended Follow-Up of a Randomized Controlled Trial. Biomolecules 2022, 12, 1682. https://doi.org/10.3390/biom12111682
Seshima F, Bizenjima T, Aoki H, Imamura K, Kita D, Irokawa D, Matsugami D, Kitamura Y, Yamashita K, Sugito H, et al. Periodontal Regenerative Therapy Using rhFGF-2 and Deproteinized Bovine Bone Mineral versus rhFGF-2 Alone: 4-Year Extended Follow-Up of a Randomized Controlled Trial. Biomolecules. 2022; 12(11):1682. https://doi.org/10.3390/biom12111682
Chicago/Turabian StyleSeshima, Fumi, Takahiro Bizenjima, Hideto Aoki, Kentaro Imamura, Daichi Kita, Daisuke Irokawa, Daisuke Matsugami, Yurie Kitamura, Keiko Yamashita, Hiroki Sugito, and et al. 2022. "Periodontal Regenerative Therapy Using rhFGF-2 and Deproteinized Bovine Bone Mineral versus rhFGF-2 Alone: 4-Year Extended Follow-Up of a Randomized Controlled Trial" Biomolecules 12, no. 11: 1682. https://doi.org/10.3390/biom12111682
APA StyleSeshima, F., Bizenjima, T., Aoki, H., Imamura, K., Kita, D., Irokawa, D., Matsugami, D., Kitamura, Y., Yamashita, K., Sugito, H., Tomita, S., & Saito, A. (2022). Periodontal Regenerative Therapy Using rhFGF-2 and Deproteinized Bovine Bone Mineral versus rhFGF-2 Alone: 4-Year Extended Follow-Up of a Randomized Controlled Trial. Biomolecules, 12(11), 1682. https://doi.org/10.3390/biom12111682







