Biodefensive Based on Piper nigrum Essential Oil for Controlling of Anopheles aquasalis Larvae: Influence of Temperature (35 °C) and Preservatives
Abstract
:1. Introduction
2. Materials and Methods
2.1. Essential Oil Extraction
2.2. Gelatin/PCL-Based Particles and Encapsulation Efficiency
2.3. Stability Evaluation
- (i).
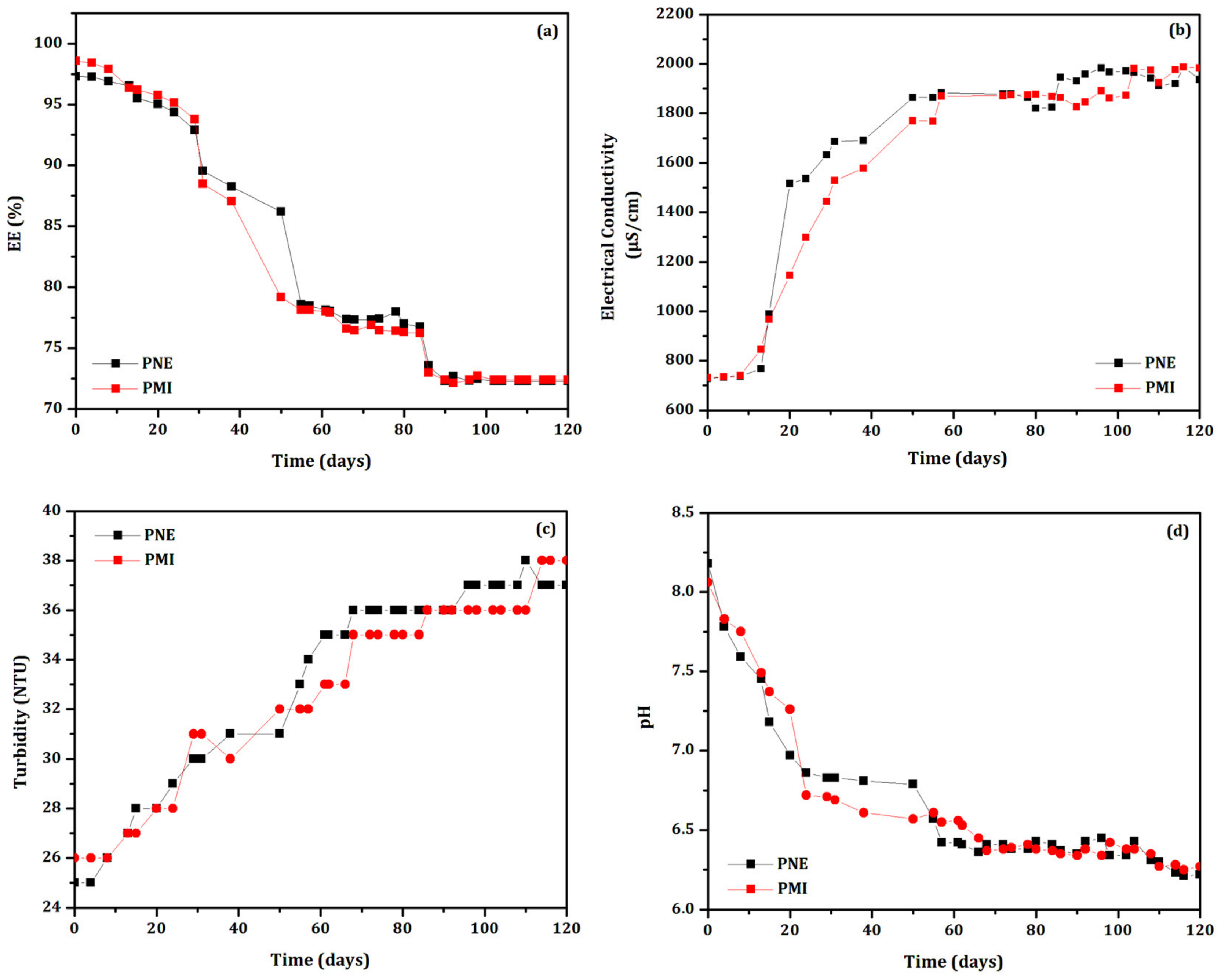
- Stability under constant handling: Biodefensives containing essential oil and preservatives (PCA, PNE, PMI, PTI, PED and PBS) were stored in transparent vials, and maintained at (35 ± 2) °C. Vials were opened (and consequently exposed to the laboratory environmental conditions such as air contact, light, and temperature variation) at pre-established time intervals (1–3 days) for 30 days. Physical parameters (pH, electrical conductivity, EE% and turbidity) were measured each time the vials were opened. Then, the more stable biodefensives were evaluated again for 120 days. All measurements were performed in triplicate.
- (ii).
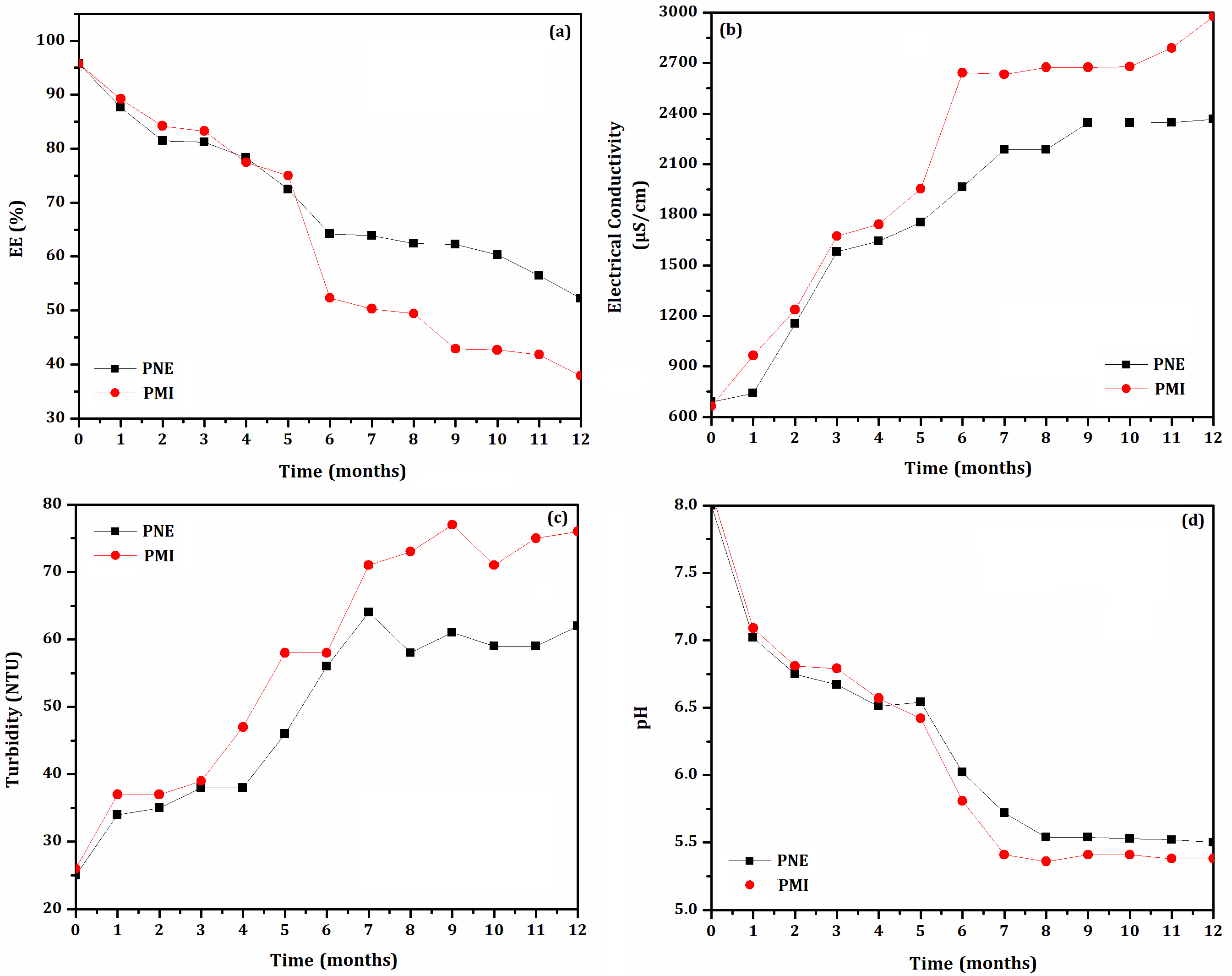
- Shelf-life test: The biodefensives selected in the stability test under constant handling at (35 ± 2) °C were submitted to the shelf-life test at (35 ± 2) °C. Biodefensives were stored in sealed vials until reach EE% equal to or less than 70%. Vials were opened every 30 days. Physical parameters (pH, electrical conductivity, EE% and turbidity) were measured each time the vials were opened. All measurements were performed in triplicate.
2.4. Essential Oil Release
2.5. Zeta Potential
2.6. Nanoparticle Tracking Analysis (NTA)
2.7. Atomic Force Microscopy (AFM)
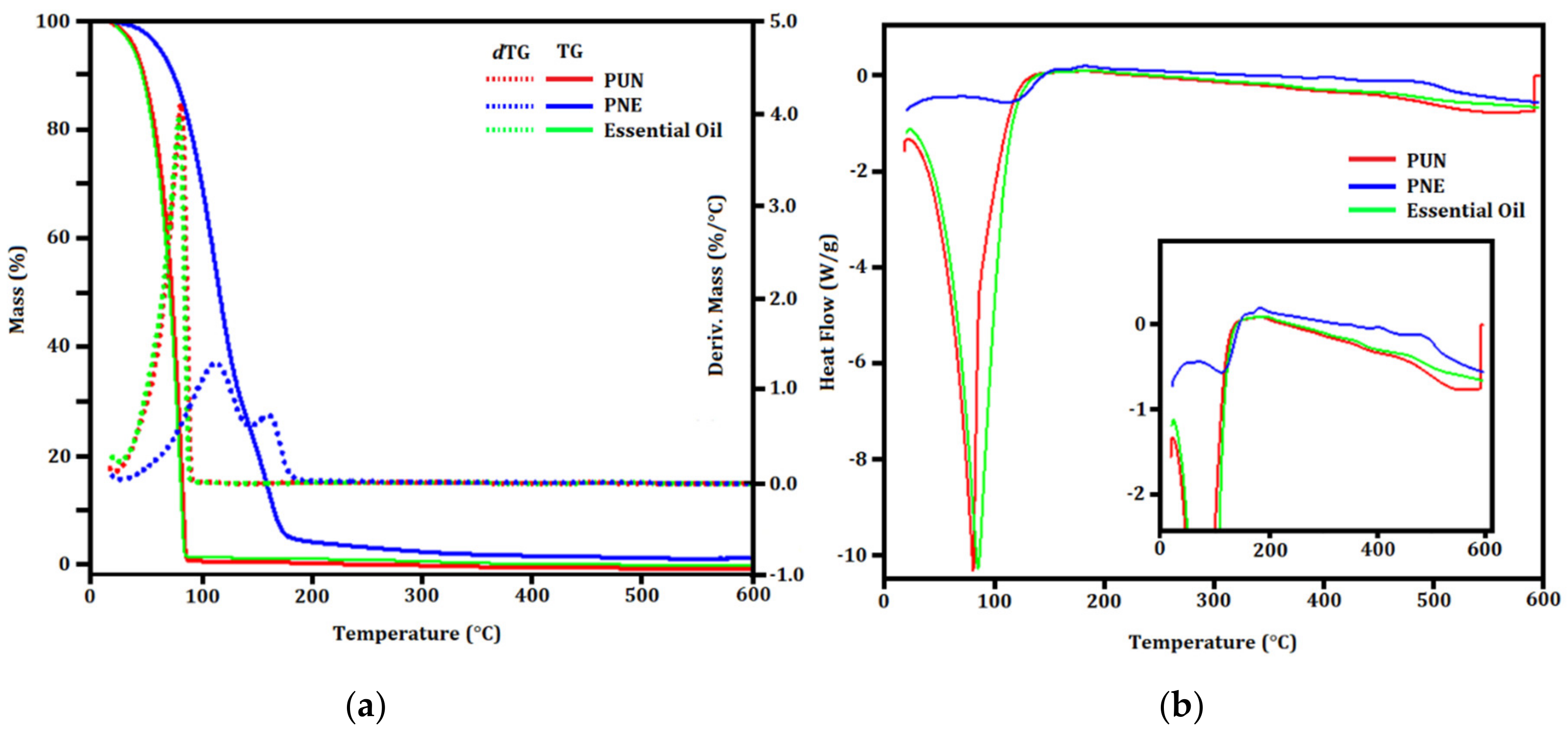
2.8. Thermogravimetry/Derivative Thermogravimetry (TG/dTG) and Differential Scanning Calorimetry (DSC)
2.9. Larvicidal Bioassays
2.10. Statistical Analysis
3. Results and Discussion
3.1. Stability under Constant Handling at (35 ± 2) °C
3.2. Shelf-Life Storage at (35 ± 2) °C
3.3. Zeta Potential and Particle Size Distribution
3.4. TG/dTG and DSC Evaluation
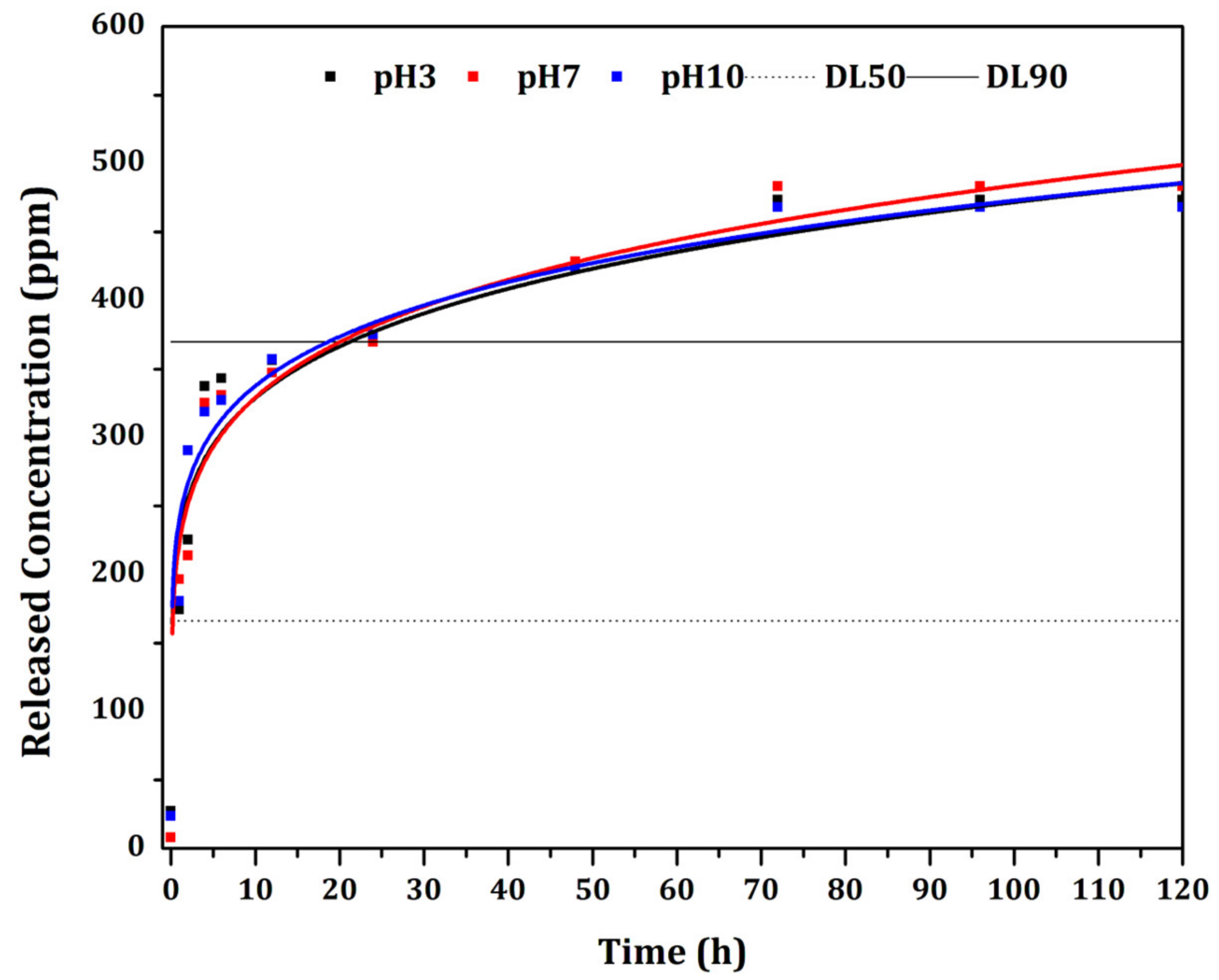
3.5. Controlled Release
3.6. Larvicidal Bioassays
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, W.; Wang, S.; Jacobs-Lorena, M. Use of Microbiota to Fight Mosquito-Borne Disease. Front. Genet. 2020, 11, 196. [Google Scholar] [CrossRef] [PubMed]
- Osanloo, M.; Amani, A.; Sereshti, H.; Abai, M.R.; Esmaeili, F.; Sedaghat, M.M. Preparation and Optimization Nanoemulsion of Tarragon (Artemisia dracunculus) Essential Oil as Effective Herbal Larvicide against Anopheles stephensi. Ind. Crops Prod. 2017, 109, 214–219. [Google Scholar] [CrossRef]
- He, Q.; Wang, W.; Zhu, L. Larvicidal Activity of Zanthoxylum acanthopodium Essential Oil against the Malaria Mosquitoes, Anopheles anthropophagus and Anopheles sinensis. Malar. J. 2018, 17, 194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, A.; Mazigo, H.D.; Manjurano, A.; Morona, D.; Kweka, E.J. Evaluation of Active Ingredients and Larvicidal Activity of Clove and Cinnamon Essential Oils against Anopheles gambiae (Sensu Lato). Parasit. Vectors 2017, 10, 411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Recht, J.; Siqueira, A.M.; Monteiro, W.M.; Herrera, S.M.; Herrera, S.; Lacerda, M.V.G. Malaria in Brazil, Colombia, Peru and Venezuela: Current Challenges in Malaria Control and Elimination. Malar. J. 2017, 16, 273. [Google Scholar] [CrossRef]
- Baia-da-Silva, D.C.; Brito-Sousa, J.D.; Rodovalho, S.R.; Peterka, C.; Moresco, G.; Lapouble, O.M.M.; Melo, G.C.d.; Sampaio, V.d.S.; das Graças Costa Alecrim, M.; Pimenta, P.; et al. Current Vector Control Challenges in the Fight against Malaria in Brazil. Rev. Soc. Bras. Med. Trop. 2019, 52, 1–10. [Google Scholar] [CrossRef]
- Taban, A.; Saharkhiz, M.J.; Khorram, M. Formulation and Assessment of Nano-Encapsulated Bioherbicides Based on Biopolymers and Essential Oil. Ind. Crops Prod. 2020, 149, 112348. [Google Scholar] [CrossRef]
- Mar, J.M.; Silva, L.S.; Azevedo, S.G.; França, L.P.; Goes, A.F.F.; dos Santos, A.L.; Bezerra, J.d.A.; de Cássia, S.; Nunomura, R.; Machado, M.B.; et al. Lippia origanoides Essential Oil: An Efficient Alternative to Control Aedes aegypti, Tetranychus urticae and Cerataphis lataniae. Ind. Crops Prod. 2018, 111, 292–297. [Google Scholar] [CrossRef]
- Silva, L.S.; Mar, J.M.; Azevedo, S.G.; Rabelo, M.S.; Bezerra, J.A.; Campelo, P.H.; Machado, M.B.; Trovati, G.; Santos, L.; Fonseca, D.; et al. Encapsulation of Piper aduncum and Piper hispidinervum Essential Oils in Gelatin Nanoparticles: A Possible Sustainable Control Tool of Aedes aegypti, Tetranychus urticae and Cerataphis lataniae. J. Sci. Food Agric. 2018, 99, 685–695. [Google Scholar] [CrossRef]
- Azevedo, S.G.; Mar, J.M.; da Silva, L.S.; França, L.P.; Machado, M.B.; Tadei, W.P.; Bezerra, J.d.A.; dos Santos, A.L.; Sanches, E.A. Bioactivity of Licaria puchury-major Essential Oil Against Aedes aegypti, Tetranychus urticae and Cerataphis lataniae. Rec. Nat. Prod. 2018, 12, 229–238. [Google Scholar] [CrossRef]
- Oliveira, L.M.D.; Lima, S.X.; Silva, L.S.; Mar, J.M.; Azevedo, S.G.; Rabelo, M.S.; Henrique, D.; Filho, F.; Campelo, P.H.; Sanches, E.A. Controlled Release of Licaria puchury-major Essential Oil Encapsulated in PCL/Gelatin-Based Colloidal Systems and Membranes. Am. J. Essent. Oils Nat. Prod. 2019, 7, 23–29. [Google Scholar]
- Rocha, A.L.F.; de Aguiar Nunes, R.Z.; Matos, R.S.; da Fonseca Filho, H.D.; de Araújo Bezerra, J.; Lima, A.R.; Guimarães, F.E.G.; Pamplona, A.M.S.R.; Majolo, C.; de Souza, M.G.; et al. Alternative Controlling Agent of Theobroma grandiflorum Pests: Nanoscale Surface and Fractal Analysis of Gelatin/PCL Loaded Particles Containing Lippia origanoides Essential Oil. Nanomaterials 2022, 12, 2712. [Google Scholar] [CrossRef] [PubMed]
- Samuel, M.; Oliver, S.V.; Coetzee, M.; Brooke, B.D. The Larvicidal Effects of Black Pepper (Piper nigrum L.) and Piperine against Insecticide Resistant and Susceptible Strains of Anopheles Malaria Vector Mosquitoes. Parasit. Vectors 2016, 9, 238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Detsi, A.; Kavetsou, E.; Kostopoulou, I.; Pitterou, I.; Pontillo, A.R.N.; Tzani, A.; Christodoulou, P.; Siliachli, A.; Zoumpoulakis, P. Nanosystems for the Encapsulation of Natural Products: The Case of Chitosan Biopolymer as a Matrix. Pharmaceutics 2020, 12, 669. [Google Scholar] [CrossRef]
- Ghaderi-Ghahfarokhi, M.; Barzegar, M.; Sahari, M.A.; Ahmadi Gavlighi, H.; Gardini, F. Chitosan-Cinnamon Essential Oil Nano-Formulation: Application as a Novel Additive for Controlled Release and Shelf Life Extension of Beef Patties. Int. J. Biol. Macromol. 2017, 102, 19–28. [Google Scholar] [CrossRef]
- Chenni, M.; El Abed, D.; Neggaz, S.; Rakotomanomana, N.; Fernandez, X.; Chemat, F. Solvent Free Microwave Extraction Followed by Encapsulation of O. basilicum L. Essential Oil for Insecticide Purpose. J. Stored Prod. Res. 2020, 86, 101575. [Google Scholar] [CrossRef]
- Camara, M.C.; Monteiro, R.A.; Carvalho, L.B.; Oliveira, J.L.; Fraceto, L.F. Enzyme Stimuli–Responsive Nanoparticles for Bioinsecticides: An Emerging Approach for Uses in Crop Protection. ACS Sustain. Chem. Eng. 2021, 9, 106–112. [Google Scholar] [CrossRef]
- Silva, J.S.M.; Rabelo, M.S.; Lima, S.X.; Rocha, A.N.A.L.F.; Tadei, W.P.; Chaves, F.C.M.; Bezerra, J.D.E.A.; Biondo, M.M.; Campelo, P.H.; Sanches, E.A. Biodegradable Nanoparticles Loaded with Lippia alba Essential Oil: A Sustainable Alternative for Aedes aegypti Larvae Control. Eur. Acad. Res. 2020, 7, 6237–6258. [Google Scholar]
- de Oliveira, L.M.; Matos, R.S.; Campelo, P.H.; Sanches, E.A.; da Fonseca Filho, H.D. Evaluation of the Nanoscale Surface Applied to Biodegradable Nanoparticles Containing Allium sativum Essential Oil. Mater. Lett. 2020, 275, 128111. [Google Scholar] [CrossRef]
- De Oliveira, L.M.; Silva, L.S.; Mar, J.M.; Azevedo, S.G.; Rabelo, M.S.; Da Fonseca Filho, H.D.; Lima, S.X.; Bezerra, J.D.A.; Machado, M.B.; Campelo, P.H.; et al. Alternative Biodefensive Based on the Essential Oil from Allium sativum Encapsulated in PCL/Gelatin Nanoparticles. J. Food Eng. Technol. 2019, 8, 65–74. [Google Scholar] [CrossRef] [Green Version]
- Azevedo, S.G.; Rocha, A.L.F.; de Aguiar Nunes, R.Z.; da Costa Pinto, C.; Ţălu, Ş.; da Fonseca Filho, H.D.; de Araújo Bezerra, J.; Lima, A.R.; Guimarães, F.E.G.; Campelo, P.H.; et al. Pulsatile Controlled Release and Stability Evaluation of Polymeric Particles Containing Piper nigrum Essential Oil and Preservatives. Materials 2022, 15, 5415. [Google Scholar] [CrossRef] [PubMed]
- ANVISA. Guia de Estabilidade de Produtos Cosméticos, 1st ed.; Agência Nacional de Vigilância Sanitária: Brasília, Brazil, 2004; Volume 1, ISBN 8588233150.
- Schaffazick, S.R.; Pohlmann, A.R. Caracterização e Estudo de Estabilidade de Suspensões de Nanocápsulas e de Nanoesferas Poliméricas Contendo Diclofenaco. Acta Farm. Bonaer. 2002, 21, 99–106. [Google Scholar]
- Boyapally, H.; Nukala, R.K.; Bhujbal, P.; Douroumis, D. Controlled Release from Directly Compressible Theophylline Buccal Tablets. Colloids Surf. B Biointerfaces 2010, 77, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Korsmeyer, R.; Peppas, N. Macromolecular and Modeling Aspects of Swelling Controlled Systems. In Controlled Release Delivery Systems; Marcel Dekker: New York, NY, USA, 1983; pp. 77–90. [Google Scholar]
- Agência Nacional de Vigilância Sanitária. Farmacopeia Brasileira, 5a; Fundação Oswaldo Cruz: Brasília, Brazil, 2010.
- Perry, R.H.; Green, D.W.; Maloney, J.O. Perry’s Chemical Engineers’ Handbook; McGraw-Hill: New York, NY, USA, 2008. [Google Scholar]
- Ghasemishahrestani, Z.; Mehta, M.; Darne, P.; Yadav, A.; Ranade, S. Tunable Synthesis of Gelatin Nanoparticles Employing Sophorolipid and Plant Extract, a Promising Drug Carrier. World J. Pharm. Pharm. Sci. 2015, 4, 1365–1381. [Google Scholar]
- Naidu, B.V.K.; Paulson, A.T. A New Method for the Preparation of Gelatin Nanoparticles: Encapsulation and Drug Release Characteristics. J. Appl. Polym. Sci. 2011, 121, 3495–3500. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Laboratory and Field Testing of Mosquito Larvicides; World Health Organization Communicable Disease Control, Prevention and Eradication Who Pesticide Evaluation Scheme; World Health Organization: Geneva, Switzerland, 2005; pp. 1–41. [Google Scholar]
- Robertson, J.L.; Russell, R.M.; Savin, N.E. POLO: A User’s Guide to Probit or Logit Analysis; Pacific Southwest Forest and Range Experiment Station: Berkeley, CA, USA, 1980; Volume 20.
- Barci, L.A.G.; de Almeida, J.E.M.; de Campos Nogueira, A.H.; do Prado, A.P. Determinação da CL90 e TL90 do Isolado IBCB66 de Beauveria bassiana (Ascomycetes: Clavicipitaceae) para o Controle de Rhipicephalus (Boophilus) Microplus (Acari: Ixodidae). Rev. Bras. Parasitol. Vet. 2009, 18, 34–39. [Google Scholar] [CrossRef]
- Snima, K.S.; Arunkumar, P.; Jayakumar, R.; Lakshmanan, V.K. Silymarin. Encapsulated Poly(D, L-Lactic-Co-Glycolic Acid) Nanoparticles: A Prospective Candidate for Prostate Cancer Therapy. J. Biomed. Nanotechnol. 2014, 10, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Turek, C.; Stintzing, F.C. Stability of Essential Oils: A Review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]
- Coradi, P.C.; Melo, E.d.C.; Rocha, R.P. Evaluation of Electrical Conductivity as a Quality Parameter of Lemongrass Leaves (Cymbopogon citratus Stapf) Submitted to Drying Process. Dry. Technol. 2014, 32, 969–980. [Google Scholar] [CrossRef]
- Gharanjig, H.; Gharanjig, K.; Hosseinnezhad, M.; Jafari, S.M. Development and Optimization of Complex Coacervates Based on Zedo Gum, Cress Seed Gum and Gelatin. Int. J. Biol. Macromol. 2020, 148, 31–40. [Google Scholar] [CrossRef]
- Bastos, L.P.H.; Vicente, J.; Santos, C.H.C.d.; Carvalho, M.G.d.; Garcia-Rojas, E.E. Encapsulation of Black Pepper (Piper nigrum L.) Essential Oil with Gelatin and Sodium Alginate by Complex Coacervation. Food Hydrocoll. 2020, 102, 105605. [Google Scholar] [CrossRef]
- González-reza, R.M.; Quintanar-guerrero, D.; Real-lópez, A.D.; Piñon-segundo, E. Effect of Sucrose Concentration and PH onto the Physical Stability of β-Carotene Nanocapsules. LWT Food Sci. Technol. 2018, 90, 354–361. [Google Scholar] [CrossRef]
- Guterres, S.S.; Alves, M.P.; Pohlmann, A.R. Polymeric Nanoparticles, Nanospheres and Nanocapsules for Cutaneous Applications. Drug Target Insights 2007, 147–157. [Google Scholar] [CrossRef] [Green Version]
- Elzoghby, A.O. Gelatin-Based Nanoparticles as Drug and Gene Delivery Systems: Reviewing Three Decades of Research. J. Control. Release 2013, 172, 1075–1091. [Google Scholar] [CrossRef]
- Schöler, N.; Zimmermann, E.; Katzfey, U.; Hahn, H.; Müller, R.H.; Liesenfeld, O. Preserved Solid Lipid Nanoparticles (SLN) at Low Concentrations Do Cause Neither Direct nor Indirect Cytotoxic Effects in Peritoneal Macrophages. Int. J. Pharm. 2000, 196, 235–239. [Google Scholar] [CrossRef]
- Agrawal, A.; Pandwar, U.; Pandya, N. Are Phenoxyethanol Products Safe for Babies?—A Review of Current Evidences. Indian J. Child Health 2022, 9, 63–67. [Google Scholar] [CrossRef]
- Dréno, B.; Zuberbier, T.; Gelmetti, C.; Gontijo, G.; Marinovich, M. Safety Review of Phenoxyethanol When Used as a Preservative in Cosmetics. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 15–24. [Google Scholar] [CrossRef] [Green Version]
- Lundov, M.D.; Krongaard, T.; Menné, T.L.; Johansen, J.D. Methylisothiazolinone Contact Allergy: A Review. Br. J. Dermatol. 2011, 165, 1178–1182. [Google Scholar] [CrossRef]
- Castanedo-Tardana, M.P.; Zug, K.A. Methylisothiazolinone. Dermatitis 2013, 24, 2–6. [Google Scholar] [CrossRef]
- Schaffazick, S.R.; Guterres, S.S.; De Lucca Freitas, L.; Pohlmann, A.R. Physicochemical Characterization and Stability of the Polymeric Nanoparticle Systems for Drug Administration. Quim. Nova 2003, 26, 726–737. [Google Scholar] [CrossRef]
- Honary, S.; Zahir, F. Effect of Zeta Potential on the Properties of Nano-Drug Delivery Systems—A Review (Part 2). Trop. J. Pharm. Res. 2013, 12, 265–273. [Google Scholar] [CrossRef]
- Jiménez, M.; Domínguez, J.A.; Pascual-Pineda, L.A.; Azuara, E.; Beristain, C.I. Elaboration and Characterization of O/W Cinnamon (Cinnamomum zeylanicum) and Black Pepper (Piper nigrum) Emulsions. Food Hydrocoll. 2018, 77, 902–910. [Google Scholar] [CrossRef]
- Seibert, J.B.; Viegas, J.S.R.; Almeida, T.C.; Amparo, T.R.; Rodrigues, I.V.; Lanza, J.S.; Frézard, F.J.G.; Soares, R.D.O.A.; Teixeira, L.F.M.; de Souza, G.H.B.; et al. Nanostructured Systems Improve the Antimicrobial Potential of the Essential Oil from Cymbopogon densiflorus Leaves. J. Nat. Prod. 2019, 82, 3208–3220. [Google Scholar] [CrossRef] [PubMed]
- Paredes, A.J.; Asencio, C.M.; Manuel, L.J.; Allemandi, D.A. Nanoencapsulation in the Food Industry: Manufacture, Applications and Characterization. J. Food Bioeng. Nanoprocessing 2016, 1, 56–79. [Google Scholar]
- Reddy, L.H.; Arias, J.L.; Nicolas, J.; Couvreur, P. Magnetic Nanoparticles: Design and Characterization, Toxicity and Biocompatibility, Pharmaceutical and Biomedical Applications. Chem. Rev. 2012, 112, 5818–5878. [Google Scholar] [CrossRef]
- Rangelov, A.; Arnaudov, L.; Stoyanov, S.; Spassov, T. Gelatinization of Industrial Starches Studied by DSC and TG. Bulg. Chem. Commun. 2017, 49, 422–429. [Google Scholar]
- Delancey, J.M.; Cavazza, M.D.; Rendos, M.G.; Ulisse, C.J.; Palumbo, S.G.; Mathers, R.T. Controlling Crosslinking in Thermosets via Chain Transfer with Monoterpenes. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 3719–3727. [Google Scholar] [CrossRef]
- Han, Z.; Zhuang, D.; Yan, H.; Zhao, H.; Sun, B.; Li, D.; Sun, Y.; Hu, W.; Xuan, Q.; Chen, J.; et al. Thermogravimetric and Kinetic Analysis of Thermal Decomposition Characteristics of Microbial Calcites Induced by Cyanobacteria Synechocystis Sp. PCC6803. J. Therm. Anal. Calorim. 2017, 127, 1371–1379. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of Solute Release from Porous Hydrophilic Polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Maderuelo, C.; Zarzuelo, A.; Lanao, J.M. Critical Factors in the Release of Drugs from Sustained Release Hydrophilic Matrices. J. Control. Release 2011, 154, 2–19. [Google Scholar] [CrossRef]
- Siepmann, J.; Siepmann, F. Mathematical Modeling of Drug Dissolution. Int. J. Pharm. 2013, 453, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Vinceković, M.; Jurić, S.; Đermić, E.; Topolovec-Pintarić, S. Kinetics and Mechanisms of Chemical and Biological Agents Release from Biopolymeric Microcapsules. J. Agric. Food Chem. 2017, 65, 9608–9617. [Google Scholar] [CrossRef] [PubMed]
- Dahham, S.; Tabana, Y.; Iqbal, M.; Ahamed, M.; Ezzat, M.; Majid, A.; Majid, A. The Anticancer, Antioxidant and Antimicrobial Properties of the Sesquiterpene β-Caryophyllene from the Essential Oil of Aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef] [PubMed]
- Ghelardini, C.; Galeotti, N.; Di Cesare Mannelli, L.; Mazzanti, G.; Bartolini, A. Local Anaesthetic Activity of β-Caryophyllene. Il Farmaco 2001, 56, 387–389. [Google Scholar] [CrossRef]
- Dahham, S.S.; Tabana, Y.M.; Ahamed, M.B.K.; Majid, A.M.S.A. In Vivo Anti-Inflammatory Activity of β-Caryophyllene, Evaluated by Molecular Imaging. Mol. Med. Chem. 2016, 1, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Govindarajan, M.; Rajeswary, M.; Hoti, S.L.; Bhattacharyya, A.; Benelli, G. Eugenol, α-Pinene and β-Caryophyllene from Plectranthus barbatus Essential Oil as Eco-Friendly Larvicides against Malaria, Dengue and Japanese Encephalitis Mosquito Vectors. Parasitol. Res. 2016, 115, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Dória, G.A.A.; Silva, W.J.; Carvalho, G.A.; Alves, P.B.; Cavalcanti, S.C.H. A Study of the Larvicidal Activity of Two Croton Species from Northeastern Brazil against Aedes aegypti. Pharm. Biol. 2010, 48, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.-X.; Wong, C.-L.; Ahmad, R.; Jantan, I. Mosquitocidal and Oviposition Repellent Activities of the Extracts of Seaweed Bryopsis pennata on Aedes aegypti and Aedes albopictus. Molecules 2015, 20, 14082–14102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nararak, J.; Sathantriphop, S.; Kongmee, M.; Mahiou-Leddet, V.; Ollivier, E.; Manguin, S.; Chareonviriyaphap, T. Excito-Repellent Activity of β-Caryophyllene Oxide against Aedes aegypti and Anopheles minimus. Acta Trop. 2019, 197, 105030. [Google Scholar] [CrossRef]
- Houndété, T.A.; Fournier, D.; Ketoh, G.K.; Glitho, I.A.; Nauen, R.; Martin, T. Biochemical Determination of Acetylcholinesterase Genotypes Conferring Resistance to the Organophosphate Insecticide Chlorpyriphos in Field Populations of Bemisia tabaci from Benin, West Africa. Pestic. Biochem. Physiol. 2010, 98, 115–120. [Google Scholar] [CrossRef]
- Carvalho, S.C.G.d.; Martins Junior, A.d.J.; Lima, J.B.P.; Valle, D. Temperature Influence on Embryonic Development of Anopheles albitarsis and Anopheles aquasalis. Mem. Inst. Oswaldo Cruz 2002, 97, 1117–1120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisenberg, J.; Washburn, J.; Schreiber, S. Generalist Feeding Behaviors of Aedes sierrensis Larvae and Their Effects on Protozoan Populations. Ecol. Soc. Am. 2000, 81, 921–935. [Google Scholar] [CrossRef]



| Parameters | Biodefensive PNE |
|---|---|
| Mean (nm) | 236 ± 34 |
| Mode (nm) | 136 ± 10 |
| SD (nm) | 157 ± 12 |
| D10 (nm) | 127 ± 10 |
| D50 (nm) | 178 ± 35 |
| D90 (nm) | 472 ± 78 |
| Concentration (particles.mL−1) | (7.0 ± 0.5) × 1010 |
| Model | Coefficient | pH = 3 | pH = 7 | pH = 10 |
|---|---|---|---|---|
| Korsmeyer-Peppas | k | 92.7 a | 92.7 a | 89.8 b |
| n | 0.64 a | 0.65 a | 0.64 a | |
| R2 | 0.93 | 0.96 | 0.94 |
| Time (h) | Concentration (μg.mL−1) | Mortality (%) | LD50 ± SD (μg.mL−1) LCL–UCL | LD90 ± SD (μg.mL−1) LCL–UCL | Equation |
|---|---|---|---|---|---|
| 125 | 20 | 230 ± 2 a (211.5–263.9) | 628 ± 5 a (617.5–643.9) | Y = (−1.058 + 1.273) + 0.38X | |
| 250 | 70 | ||||
| 24 | 375 | 60 | |||
| 454 | 70 | ||||
| 625 | 100 | ||||
| NC | 0 | ||||
| 125 | 70 | 167 ± 2 b (152.6–181.2) | 370 ± 3 b (366.8–378.2) | Y = (−0.826 + 1.610) + 0.44X | |
| 250 | 90 | ||||
| 375 | 90 | ||||
| 48 | 454 | 90 | |||
| 625 | 100 | ||||
| NC | 0 | ||||
| 125 | 80 | 74.3 ± 0.7 c (68.4–85.3) | 356 ± 4 b (349.3–361.9) | Y = (0.243 + 0.818) + 0.41X | |
| 250 | 90 | ||||
| 72 | 375 | 90 | |||
| 454 | 90 | ||||
| 625 | 100 | ||||
| NC | 0 |
| Time (h) | Concentration (μg.mL−1) | Mortality ± SD in vitro (%) | Concentration (μg.mL−1) | Mortality ± SD in loco (%) |
|---|---|---|---|---|
| 100 | 6.7 ± 0.8 | 100 | 8.3 ± 0.8 | |
| 200 | 37 ± 4 | 200 | 58 ± 4 | |
| 24 | 400 | 88 ± 3 | 300 | 83 ± 6 |
| 500 | 88 ± 1 | 500 | 92 ± 0 | |
| NC | 0 | NC | 0 | |
| 100 | 70 ± 2 | 100 | 10 ± 5 | |
| 200 | 82 ± 8 | 200 | 67 ± 2 | |
| 48 | 400 | 92 ± 8 | 300 | 93 ± 6 |
| 500 | 95 ± 5 | 500 | 100 ± 0 | |
| NC | 0 | NC | 0 | |
| 100 | 98 ± 3 | 100 | 25 ± 2 | |
| 200 | 97 ± 3 | 200 | 80 ± 5 | |
| 72 | 400 | 97 ± 3 | 300 | 97 ± 6 |
| 500 | 100 ± 0 | 500 | 100 ± 0 | |
| NC | 0 | NC | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcião Vieira, A.C.; Azevedo, S.G.; Linhares, R.A.; Brandão Justiniano, S.C.; Pontes, G.O.; Lima, A.R.; Campelo, P.H.; Bezerra, J.d.A.; da Costa Pinto, C.; Fonseca Filho, H.D.d.; et al. Biodefensive Based on Piper nigrum Essential Oil for Controlling of Anopheles aquasalis Larvae: Influence of Temperature (35 °C) and Preservatives. Biomolecules 2022, 12, 1711. https://doi.org/10.3390/biom12111711
Marcião Vieira AC, Azevedo SG, Linhares RA, Brandão Justiniano SC, Pontes GO, Lima AR, Campelo PH, Bezerra JdA, da Costa Pinto C, Fonseca Filho HDd, et al. Biodefensive Based on Piper nigrum Essential Oil for Controlling of Anopheles aquasalis Larvae: Influence of Temperature (35 °C) and Preservatives. Biomolecules. 2022; 12(11):1711. https://doi.org/10.3390/biom12111711
Chicago/Turabian StyleMarcião Vieira, Ayná Caroline, Sidney Gomes Azevedo, Ramon Andrade Linhares, Silvia Cássia Brandão Justiniano, Grafe Oliveira Pontes, Alessandra Ramos Lima, Pedro Henrique Campelo, Jaqueline de Araújo Bezerra, Camila da Costa Pinto, Henrique Duarte da Fonseca Filho, and et al. 2022. "Biodefensive Based on Piper nigrum Essential Oil for Controlling of Anopheles aquasalis Larvae: Influence of Temperature (35 °C) and Preservatives" Biomolecules 12, no. 11: 1711. https://doi.org/10.3390/biom12111711
APA StyleMarcião Vieira, A. C., Azevedo, S. G., Linhares, R. A., Brandão Justiniano, S. C., Pontes, G. O., Lima, A. R., Campelo, P. H., Bezerra, J. d. A., da Costa Pinto, C., Fonseca Filho, H. D. d., Matos, R. S., Ţălu, Ş., Bagnato, V. S., Inada, N. M., & Sanches, E. A. (2022). Biodefensive Based on Piper nigrum Essential Oil for Controlling of Anopheles aquasalis Larvae: Influence of Temperature (35 °C) and Preservatives. Biomolecules, 12(11), 1711. https://doi.org/10.3390/biom12111711










