Secondary Metabolites Diversity of Aspergillus unguis and Their Bioactivities: A Potential Target to Be Explored
Abstract
:1. Introduction
2. Biology of A. unguis
3. Secondary Metabolism
3.1. Depsidones
3.2. Depsides
3.3. Phthalides
3.4. Cyclopeptides
3.5. Pyrone
3.6. Diarylethers
3.7. Orcinol/Orsenillate Derivatives and Benzoic Acid Derivatives
3.8. Indanones
3.9. Anthraquinones
3.10. Terpenoids and Sterols
4. Biological Potential of Natural Products Isolated from A. unguis
4.1. Antifungal, Antibacterial, Antimalarial and Larvicidal Activities
4.2. Anti-Osteoclastogenic Activity
4.3. Cytotoxic Activity against Cancer Cell Lines
4.4. Anti-Inflammatory Activity
4.5. Enzyme Inhibitors
5. Summary and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Newman, D.J.; Cragg, G.M.; Snader, K.M. The influence of natural products upon drug discovery. Nat. Prod. Rep. 2000, 17, 215–234. [Google Scholar] [CrossRef] [Green Version]
- Brakhage, A.A. Regulation of fungal secondary metabolism. Nat. Rev. Microbiol. 2013, 11, 21–32. [Google Scholar] [CrossRef]
- Bills, G.F.; Gloer, J.B. Biologically Active Secondary Metabolites from the Fungi. Microbiol. Spectr. 2016, 4, 1087–1119. [Google Scholar]
- Brakhage, A.A.; Schroeckh, V. Fungal secondary metabolites—Strategies to activate silent gene clusters. Fungal Genet. Biol. 2011, 48, 15–22. [Google Scholar] [CrossRef]
- Blackwell, M. The fungi: 1, 2, 3 …5.1 million species? Am. J. Bot. 2011, 98, 426–438. [Google Scholar] [CrossRef]
- Richards, T.A.; Leonard, G.; Wideman, J.G. What Defines the “Kingdom” Fungi? Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M.; Newman, D.J. Natural products: A continuing source of novel drug leads. Biochim. Biophys. Acta 2013, 1830, 3670–3695. [Google Scholar]
- Pham, J.V.; Yilma, M.A.; Feliz, A.; Majid, M.T.; Maffetone, N.; Walker, J.R.; Kim, E.; Cho, H.J.; Reynolds, J.M.; Song, M.C.; et al. A Review of the Microbial Production of Bioactive Natural Products and Biologics. Front. Microbiol. 2019, 10, 1404. [Google Scholar]
- Keller, N.P. Fungal secondary metabolism: Regulation, function and drug discovery. Nat. Rev. Microbiol. 2019, 17, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Berdy, J. Thoughts and facts about antibiotics: Where we are now and where we are heading. J. Antibiot. 2012, 65, 385–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inglis, D.O.; Binkley, J.; Skrzypek, M.S.; Arnaud, M.B.; Cerqueira, G.C.; Shah, P.; Wymore, F.; Wortman, J.R.; Sherlock, G. Comprehensive annotation of secondary metabolite biosynthetic genes and gene clusters of Aspergillus nidulans, A. fumigatus, A. niger and A. oryzae. BMC Microbiol. 2013, 13, 91. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, J.F.; Somoza, A.D.; Keller, N.P.; Wang, C.C. Advances in Aspergillus secondary metabolite research in the post-genomic era. Nat. Prod. Rep. 2012, 29, 351–371. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.L.; Hamayun, M.; Kim, Y.-H.; Kang, S.-M.; Lee, J.-H.; Lee, I.-J. Gibberellins producing endophytic Aspergillus fumigatus sp. LH02 influenced endogenous phytohormonal levels, isoflavonoids production and plant growth in salinity stress. Process Biochem. 2011, 46, 440–447. [Google Scholar] [CrossRef]
- Frisvad, J.C.; Larsen, T.O. Chemodiversity in the genus Aspergillus. Appl. Microbiol. Biotechnol. 2015, 99, 7859–7877. [Google Scholar] [CrossRef]
- Sharaf, E.F.; Alharbi, E. Removal of heavy metals from waste water of tanning leather industry by fungal species isolated from polluted soil. Afr. J. Biotechnol. 2013, 12, 4351–4355. [Google Scholar]
- Madhavan, A.; Pandey, A.; Sukumaran, R.K. Expression system for heterologous protein expression in the filamentous fungus Aspergillus unguis. Bioresour. Technol. 2017, 245, 1334–1342. [Google Scholar] [CrossRef]
- Shruthi, K.; Yadav, P.S.; Prasad, B.V.S.; Chandra, M.S. Cellulase production by Aspergillus unguis in solid state fermentation. J. For. Res. 2019, 30, 205–212. [Google Scholar] [CrossRef]
- Kooloth Valappil, P.; Rajasree, K.P.; Abraham, A.; Christopher, M.; Sukumaran, R.K. Characterization of a glucose tolerant beta-glucosidase from Aspergillus unguis with high potential as a blend-in for biomass hydrolyzing enzyme cocktails. Biotechnol. Lett. 2019, 41, 1201–1211. [Google Scholar] [CrossRef]
- Chen, A.J.; Frisvad, J.C.; Sun, B.D.; Varga, J.; Kocsube, S.; Dijksterhuis, J.; Kim, D.H.; Hong, S.B.; Houbraken, J.; Samson, R.A. Aspergillus section Nidulantes (formerly Emericella): Polyphasic taxonomy, chemistry and biology. Stud. Mycol. 2016, 84, 1–118. [Google Scholar]
- Hubka, V.; Nováková, A.; Peterson, S.W.; Frisvad, J.C.; Sklenář, F.; Matsuzawa, T.; Kubátová, A.; Kolařík, M. A reappraisal of Aspergillus section Nidulantes with descriptions of two new sterigmatocystin-producing species. Plant. Syst. Evol. 2016, 302, 1267–1299. [Google Scholar] [CrossRef]
- Sklenar, F.; Jurjevic, Z.; Peterson, S.W.; Kolarik, M.; Novakova, A.; Flieger, M.; Stodulkova, E.; Kubatova, A.; Hubka, V. Increasing the species diversity in the Aspergillus section Nidulantes: Six novel species mainly from the indoor environment. Mycologia 2020, 112, 342–370. [Google Scholar] [CrossRef]
- Houbraken, J.; Kocsube, S.; Visagie, C.M.; Yilmaz, N.; Wang, X.C.; Meijer, M.; Kraak, B.; Hubka, V.; Bensch, K.; Samson, R.A.; et al. Classification of Aspergillus, Penicillium, Talaromyces and related genera (Eurotiales): An overview of families, genera, subgenera, sections, series and species. Stud. Mycol. 2020, 95, 5–169. [Google Scholar]
- Batista, L.R.; Souza, S.C.; Evangelista, S.R.; Azevedo, N.L. Identificação de fungos: Gêneros Aspergillus, Penicillium e Talaromyces. In Conhecimento, Conservação e Uso de Fungos; Oliveira, L.A., Jesus, M.A., Matsuura, A.B.J., Oliveira, J.G.S., Gasparotto, L., Lima-Neto, R.G., Rocha, L.C., Eds.; Editora INPA: Manaus, Brazil, 2019; pp. 1–10. [Google Scholar]
- Samson, R.A.; Visagie, C.M.; Houbraken, J.; Hong, S.B.; Hubka, V.; Klaassen, C.H.; Perrone, G.; Seifert, K.A.; Susca, A.; Tanney, J.B.; et al. Phylogeny, identification and nomenclature of the genus Aspergillus. Stud. Mycol. 2014, 78, 141–173. [Google Scholar] [CrossRef] [Green Version]
- Phainuphong, P.; Rukachaisirikul, V.; Phongpaichit, S.; Sakayaroj, J.; Kanjanasirirat, P.; Borwornpinyo, S.; Akrimajirachoote, N.; Yimnual, C.; Muanprasat, C. Depsides and depsidones from the soil-derived fungus Aspergillus unguis PSU-RSPG204. Tetrahedron 2018, 74, 5691–5699. [Google Scholar] [CrossRef]
- Li, Y.L.; Gao, Y.; Liu, C.Y.; Sun, C.J.; Zhao, Z.T.; Lou, H.X. Asperunguisins A-F, Cytotoxic Asperane Sesterterpenoids from the Endolichenic Fungus Aspergillus unguis. J. Nat. Prod. 2019, 82, 1527–1534. [Google Scholar] [CrossRef]
- Nielsen, J.; Nielsen, P.H.; Frisvad, J.C. Fungal depside, guisinol, from a marine derived strain of Emericella unguis. Phytochemistry 1999, 50, 263–265. [Google Scholar] [CrossRef]
- Yang, W.C.; Bao, H.Y.; Liu, Y.Y.; Nie, Y.Y.; Yang, J.M.; Hong, P.Z.; Zhang, Y. Depsidone Derivatives and a Cyclopeptide Produced by Marine Fungus Aspergillus unguis under Chemical Induction and by Its Plasma Induced Mutant. Molecules 2018, 23, 2245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sureram, S.; Wiyakrutta, S.; Ngamrojanavanich, N.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P. Depsidones, aromatase inhibitors and radical scavenging agents from the marine-derived fungus Aspergillus unguis CRI282-03. Planta Med. 2012, 78, 582–588. [Google Scholar] [CrossRef]
- Morshed, M.T.; Vuong, D.; Crombie, A.; Lacey, A.E.; Karuso, P.; Lacey, E.; Piggott, A.M. Expanding antibiotic chemical space around the nidulin pharmacophore. Org. Biomol. Chem. 2018, 16, 3038–3051. [Google Scholar] [CrossRef]
- Phainuphong, P.; Rukachaisirikul, V.; Phongpaichit, S.; Preedanon, S.; Sakayaroj, J. Diphenyl ethers and indanones from the soil-derived fungus Aspergillus unguis PSU-RSPG204. Tetrahedron 2017, 73, 5920–5925. [Google Scholar] [CrossRef]
- Cao, V.A.; Kwon, J.H.; Kang, J.S.; Lee, H.S.; Heo, C.S.; Shin, H.J.; Aspersterols, A.-D. Ergostane-Type Sterols with an Unusual Unsaturated Side Chain from the Deep-Sea-Derived Fungus Aspergillus unguis. J. Nat. Prod. 2022, 85, 2177–2183. [Google Scholar] [CrossRef]
- Kamal, A.; Haider, Y.; Khan, Y.A.; Qureshi, I.H.; Qureshi, A.A. Studies in the biochemistry of microorganisms. Part X. Isolation, structure and stereochemistry of yasminin and other metabolic products of Aspergillus unguis Emile-Weil and Gaudin. Pakistan J. Sci. Ind. Res. 1970, 13, 244–250. [Google Scholar]
- Zhang, Y.; Mu, J.; Feng, Y.; Wen, L.; Han, J. Four chlorinated depsidones from a seaweed-derived strain of Aspergillus unguis and their new biological activities. Nat. Prod. Res. 2014, 28, 503–506. [Google Scholar] [CrossRef]
- Sadorn, K.; Saepua, S.; Bunbamrung, N.; Boonyuen, N.; Komwijit, S.; Rachtawee, P.; Pittayakhajonwut, P. Diphenyl ethers and depsidones from the endophytic fungus Aspergillus unguis BCC54176. Tetrahedron 2022, 105, 132612. [Google Scholar] [CrossRef]
- Anh, C.V.; Kwon, J.H.; Kang, J.S.; Lee, H.S.; Heo, C.S.; Shin, H.J. Antibacterial and Cytotoxic Phenolic Polyketides from Two Marine-Derived Fungal Strains of Aspergillus unguis. Pharmaceuticals 2022, 15, 74. [Google Scholar] [CrossRef] [PubMed]
- Handayani, D.; Rendowati, A.; Aminah, I.; Ariantari, N.P.; Proksch, P. Bioactive compounds from marine sponge derived fungus Aspergillus unguis WR8. Rasayan. J. Chem. 2020, 13, 2633–2638. [Google Scholar] [CrossRef]
- Kawahara, N.; Nakajima, S.; Satoh, Y.; Yamazaki, M.; Kawai, K.-c. Studies on fungal products. XVIII.: Isolation and structures of a new fungal depsidone related to nidulin and a new phthalide from Emericella unguis. Chem. Pharm. Bull. 1988, 36, 1970–1975. [Google Scholar] [CrossRef]
- Kawahara, N.; Nozawa, K.; Nakajima, S.; Kawai, K. Isolation and structures of novel fungal depsidones, emeguisins, A, B, and C, from Emericella unguis. J. Chem. Soc. Perkin Trans. 1988, 1, 2611–2614. [Google Scholar] [CrossRef]
- Klaiklay, S.; Rukachaisirikul, V.; Aungphao, W.; Phongpaichit, S.; Sakayaroj, J. Depsidone and phthalide derivatives from the soil-derived fungus Aspergillus unguis PSU-RSPG199. Tetrahedron. Lett. 2016, 57, 4348–4351. [Google Scholar] [CrossRef]
- Saetang, P.; Rukachaisirikul, V.; Phongpaichit, S.; Preedanon, S.; Sakayaroj, J.; Hadsadee, S.; Jungsuttiwong, S. Antibacterial and Antifungal Polyketides from the Fungus Aspergillus unguis PSU-MF16. J. Nat. Prod. 2021, 84, 1498–1506. [Google Scholar] [CrossRef]
- Sureram, S.; Kesornpun, C.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P. Directed biosynthesis through biohalogenation of secondary metabolites of the marine-derived fungus Aspergillus unguis. RSC Adv. 2013, 3, 1781–1788. [Google Scholar] [CrossRef]
- Turner, W.B.; Aldridge, D.C. Fungal Metabolites II; Academic Press: London, UK, 1983. [Google Scholar]
- Yang, J.; Zhou, L.; Zhou, Z.; Song, Y.; Ju, J. Anti-pathogenic depsidones and its derivatives from a coral-derived fungus Aspergillus sp. SCSIO SX7S7. Biochem. Syst. Ecol. 2022, 102, 104415. [Google Scholar] [CrossRef]
- Dean, F.M.; Roberts, J.C.; Robertson, A. The chemistry of fungi. Part XXII. Nidulin and nornidulin (“ustin”): Chlorine-containing metabolic products of Aspergillus nidulans. J. Chem. Soc. 1954, 1432–1439. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Huang, B.; Liu, K.; Peng, S.; Liu, X.; Gao, C.; Liu, Y.; Tan, Y.; Luo, X. Anti-Osteoclastogenic and Antibacterial Effects of Chlorinated Polyketides from the Beibu Gulf Coral-Derived Fungus Aspergillus unguis GXIMD 02505. Mar. Drugs 2022, 20, 178. [Google Scholar] [CrossRef] [PubMed]
- Uchida, R.; Nakajyo, K.; Kobayashi, K.; Ohshiro, T.; Terahara, T.; Imada, C.; Tomoda, H. 7-Chlorofolipastatin, an inhibitor of sterol O-acyltransferase, produced by marine-derived Aspergillus ungui NKH-007. J. Antibiot. 2016, 69, 647–651. [Google Scholar] [CrossRef] [PubMed]
- Feighner, S.D.; Salituro, G.M.; Smith, J.L.; Tsou, N.N. Unguinol and Analogs as Animal Growth Promoters. U.S. Patent 5350763A, 1994. [Google Scholar]
- Motti, C.A.; Bourne, D.G.; Burnell, J.N.; Doyle, J.R.; Haines, D.S.; Liptrot, C.H.; Llewellyn, L.E.; Ludke, S.; Muirhead, A.; Tapiolas, D.M. Screening marine fungi for inhibitors of the C4 plant enzyme pyruvate phosphate dikinase: Unguinol as a potential novel herbicide candidate. Appl. Environ. Microbiol. 2007, 73, 1921–1927. [Google Scholar] [CrossRef] [Green Version]
- Chottanapund, S.; Van Duursen, M.B.M.; Zwartsen, A.; Timtavorn, S.; Navasumrit, P.; Kittakoop, P.; Sureram, S.; Ruchirawat, M.; Van den Berg, M. Depsidones inhibit aromatase activity and tumor cell proliferation in a co-culture of human primary breast adipose fibroblasts and T47D breast tumor cells. Toxicol. Rep. 2017, 4, 165–171. [Google Scholar] [CrossRef]
- Zwartsen, A.; Chottanapund, S.; Kittakoop, P.; Navasumrit, P.; Ruchirawat, M.; Van Duursen, M.B.M.; Van den Berg, M. Evaluation of anti-tumour properties of two depsidones—Unguinol and Aspergillusidone D—In triple-negative MDA-MB-231 breast tumour cells. Toxicol. Rep. 2019, 6, 1216–1222. [Google Scholar] [CrossRef]
- Hamano, K.; Kinoshita-Okami, M.; Hemmi, A.; Sato, A.; Hisamoto, M.; Matsuda, K.; Yoda, K.; Haruyama, H.; Hosoya, T.; Tanzawa, K. Folipastatin, a new depsidone compound from Aspergillus unguis as an inhibitor of phospholipase A2. Taxonomy, fermentation, isolation, structure determination and biological properties. J. Antibiot. 1992, 45, 1195–1201. [Google Scholar] [CrossRef] [Green Version]
- Kamal, A.; Haider, Y.; Qureshi, A.A.; Khan, Y.A. Studies in the biochemistry of microorganisms XII. Isolation and structures of haiderin, rubinin, shirin and nasrin metabolic products of Aspergillus unguis Emile-Weil and Gaudin. Pak. J. Sci. Ind. Res. 1970, 13, 364–372. [Google Scholar]
- Kamal, A.; Haider, Y.; Qureshi, A.A.; Wahid, M.A.; Khan, Y.A. Studies in the biochemistry of microorganisms. Part XIII. Biosynthesis of yasimin, a metabolic product of Aspergillus unguis. Pak. J. Sci. Ind. Res. 1970, 13, 373–377. [Google Scholar]
- Malmstrom, J. Unguisins A and B: New cyclic peptides from the marine-derived fungus Emericella unguis. J. Nat. Prod. 1999, 62, 787–789. [Google Scholar] [CrossRef] [PubMed]
- Stodola, F.H.; Vesonder, R.F.; Fennell, D.I.; Weisleder, D. A new depsidone from Aspergillus unguis. Phytochemistry 1972, 11, 2107–2108. [Google Scholar] [CrossRef]
- Sierankiewicz, J.; Gatenbeck, S. A new depsidone from Aspergillus nidulans. Acta Chem. Scand. 1972, 26, 455–458. [Google Scholar] [CrossRef] [Green Version]
- Maha, A.; Phainuphong, P.; Rukachaisirikul, V.; Saithong, S.; Phongpaichit, S.; Hadsadee, S.; Jungsuttiwong, S.; Preedanon, S.; Sakayaroj, J. Blennolide derivatives from the soil-derived fungus Trichoderma asperellum PSU-PSF14. Tetrahedron 2018, 74, 5659–5664. [Google Scholar] [CrossRef]
- Hosoe, T.; Gloer, J.B.; Wicklow, D.T.; Raja, H.A.; Shearer, C.A. New nonadride analogues from a freshwater isolate of an undescribed fungus belonging to the order Pleosporales. Heterocycles 2010, 81, 2123–2130. [Google Scholar] [CrossRef]
- Okoye, F.B.C.; Nworu, C.S.; Akah, P.A.; Esimone, C.O.; Debbab, A.; Proksch, P. Inhibition of inflammatory mediators and reactive oxygen and nitrogen species by some depsidones and diaryl ether derivatives isolated from Corynespora cassiicola, an endophytic fungus of Gongronema latifolium leaves. Immunopharmacol Immunotoxicol. 2013, 35, 662–668. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, D.; Kuang, M.; Peng, W.; Chen, Y.; Tan, J.; Kang, F.; Xu, K.; Zou, Z. Rhytidhylides A and B, Two New Phthalide Derivatives from the Endophytic Fungus Rhytidhysteron sp. BZM-9. Molecules 2021, 26, 6092. [Google Scholar] [CrossRef]
- Anh, C.V.; Kang, J.S.; Choi, B.K.; Lee, H.S.; Heo, C.S.; Shin, H.J. Polyketides and Meroterpenes from the Marine-Derived Fungi Aspergillus unguis 158SC-067 and A. flocculosus 01NT-1.1.5 and Their Cytotoxic and Antioxidant Activities. Mar. Drugs 2021, 19, 415. [Google Scholar] [CrossRef]
- Malmstrom, J.; Ryager, A.; Anthoni, U.; Nielsen, P.H. Unguisin C, a GABA-containing cyclic peptide from the fungus Emericella unguis. Phytochemistry 2002, 60, 869–872. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Shen, Y. A new cyclic peptide from the marine fungal strain Aspergillus sp. AF119. Chem. Nat. Compd. 2011, 47, 786–788. [Google Scholar] [CrossRef]
- Akone, S.H.; Daletos, G.; Lin, W.; Proksch, P. Unguisin F, a new cyclic peptide from the endophytic fungus Mucor irregularis. Z. Naturforsch. C J. Biosci. 2016, 71, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Rajachan, O.A.; Kanokmedhakul, S.; Kanokmedhakul, K.; Soytong, K. Bioactive depsidones from the fungus Pilobolus heterosporus. Planta Med. 2014, 80, 1635–1640. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.S.; Seshadri, T.R. Chemical examination of Indian lichens. V. Occurrence of active montagnetol in Roccella montagnei. Proceedings 1942, 15, 429–431. [Google Scholar]
- Anh, C.V.; Yoon, Y.D.; Kang, J.S.; Lee, H.S.; Heo, C.S.; Shin, H.J. Nitrogen-Containing Secondary Metabolites from a Deep-Sea Fungus Aspergillus unguis and Their Anti-Inflammatory Activity. Mar. Drugs 2022, 20, 217. [Google Scholar] [CrossRef]
- Lee, Y.M.; Li, H.; Hong, J.; Cho, H.Y.; Bae, K.S.; Kim, M.A.; Kim, D.K.; Jung, J.H. Bioactive metabolites from the sponge-derived fungus Aspergillus versicolor. Arch. Pharm. Res. 2010, 33, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Abdou, R.; Scherlach, K.; Dahse, H.M.; Sattler, I.; Hertweck, C. Botryorhodines A-D, antifungal and cytotoxic depsidones from Botryosphaeria rhodina, an endophyte of the medicinal plant Bidens pilosa. Phytochemistry 2010, 71, 110–116. [Google Scholar] [CrossRef]
- Cane, D.E.; Kudo, F.; Kinoshita, K.; Khosla, C. Precursor-directed biosynthesis: Biochemical basis of the remarkable selectivity of the erythromycin polyketide synthase toward unsaturated triketides. Chem. Biol. 2002, 9, 131–142. [Google Scholar] [CrossRef] [Green Version]
- Duong, T.H.; Huynh, B.L.C.; Chavasiri, W.; Chollet-Krugler, M.; Nguyen, V.K.; Nguyen, T.H.T.; Hansen, P.E.; Le Pogam, P.; Thus, H.; Boustie, J.; et al. New erythritol derivatives from the fertile form of Roccella montagnei. Phytochemistry 2017, 137, 156–164. [Google Scholar] [CrossRef]
- Cueto, M.; Jensen, P.R.; Fenical, W. Aspergilloxide, a novel sesterterpene epoxide from a marine-derived fungus of the genus Aspergillus. Org. Lett. 2002, 4, 1583–1585. [Google Scholar] [CrossRef] [PubMed]
- Isaka, M.; Yangchum, A.; Supothina, S.; Veeranondha, S.; Komwijit, S.; Phongpaichit, S. Semisynthesis and antibacterial activities of nidulin derivatives. J. Antibiot. 2019, 72, 181–184. [Google Scholar] [CrossRef]
- Wei, Q.; Bai, J.; Yan, D.; Bao, X.; Li, W.; Liu, B.; Zhang, D.; Qi, X.; Yu, D.; Hu, Y. Genome mining combined metabolic shunting and OSMAC strategy of an endophytic fungus leads to the production of diverse natural products. Acta Pharm. Sin. B 2021, 11, 572–587. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.P.; Huang, Y.M.; Liu, S.W.; Wu, G.; Yang, Q.; Liu, L.F.; Zhang, H.T.; Qi, Y.; Wang, T.; Jiang, Z.K.; et al. Metabolomics Tools Assisting Classic Screening Methods in Discovering New Antibiotics from Mangrove Actinomycetia in Leizhou Peninsula. Mar. Drugs 2021, 19, 688. [Google Scholar] [CrossRef] [PubMed]
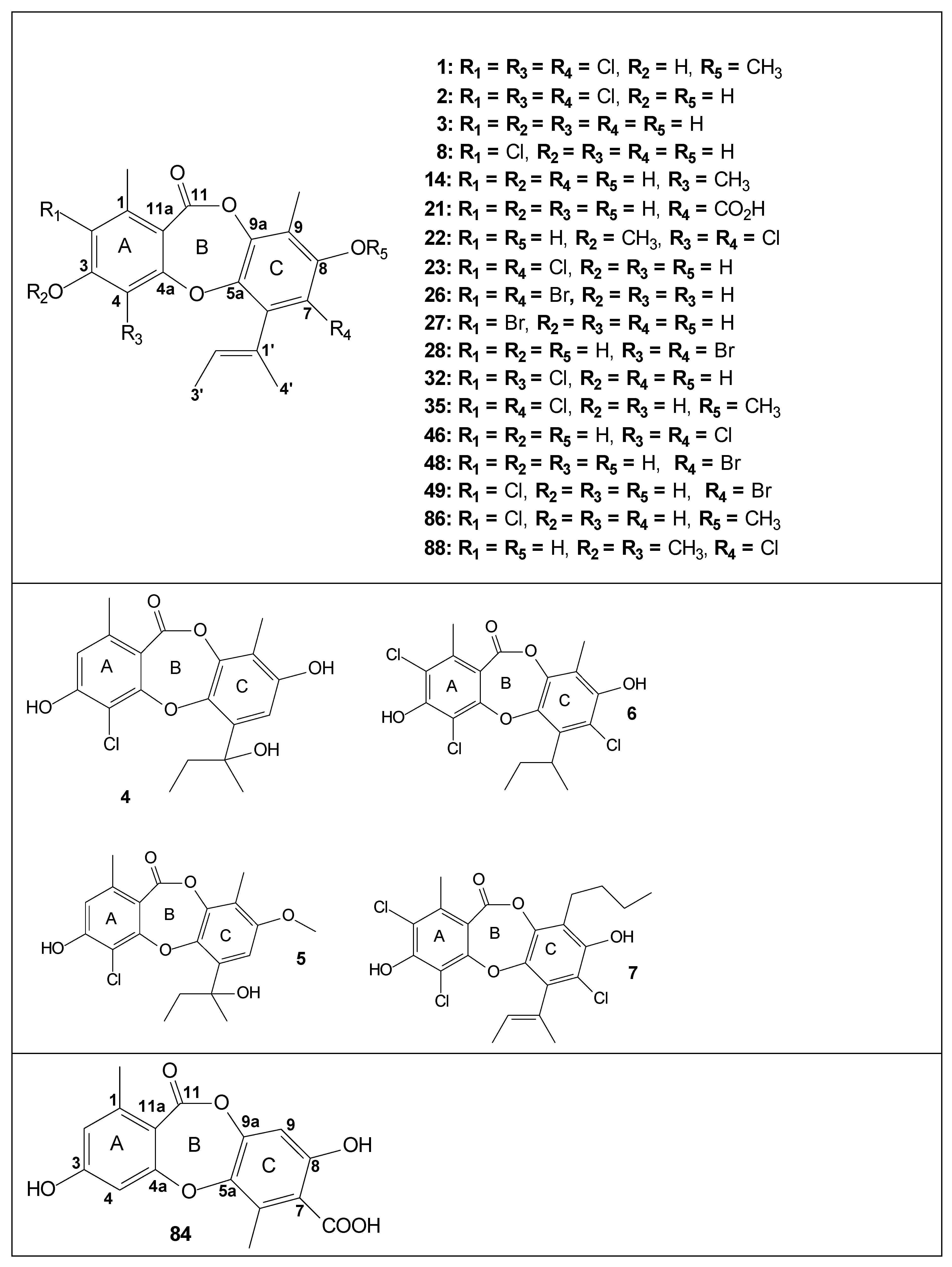
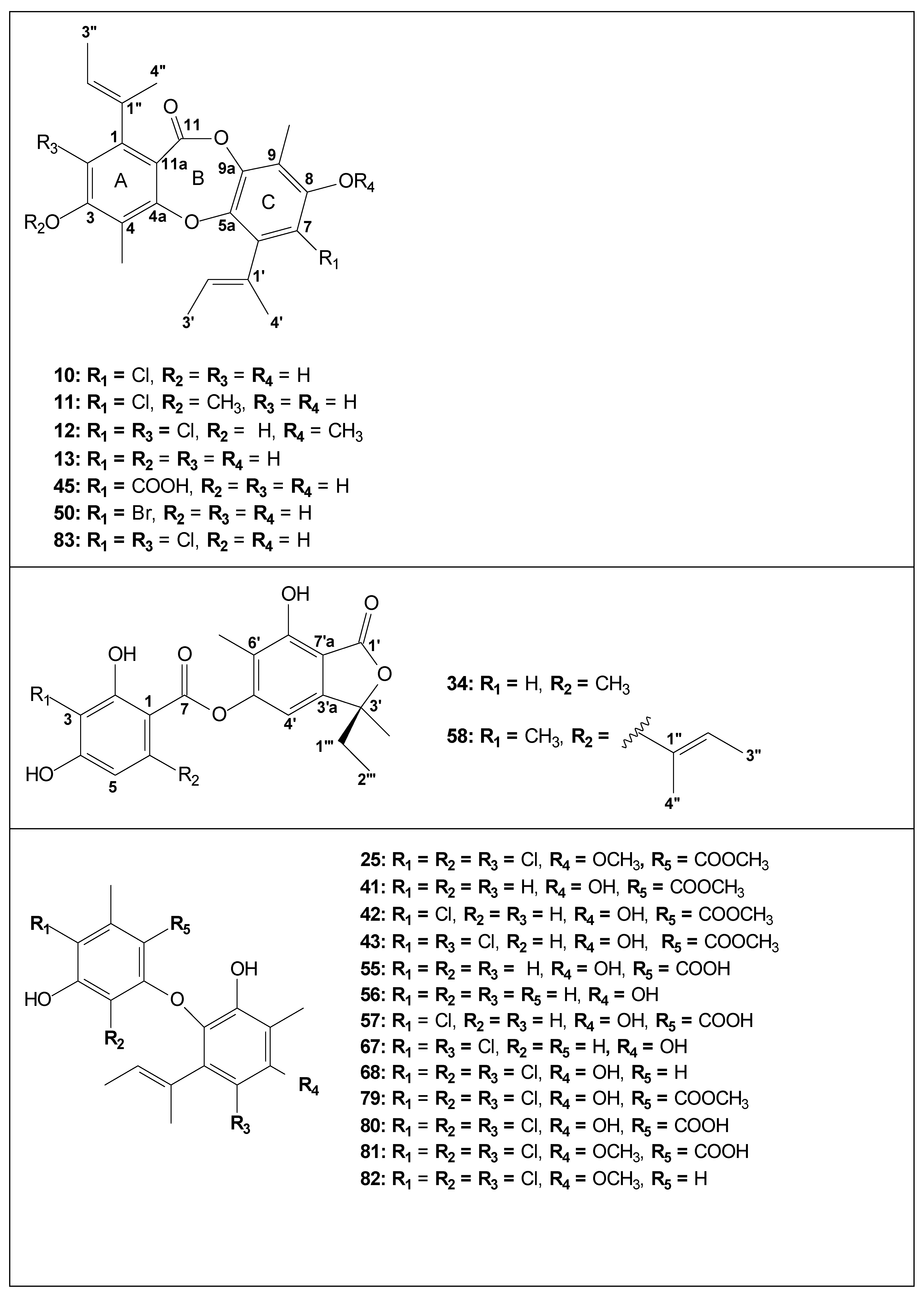
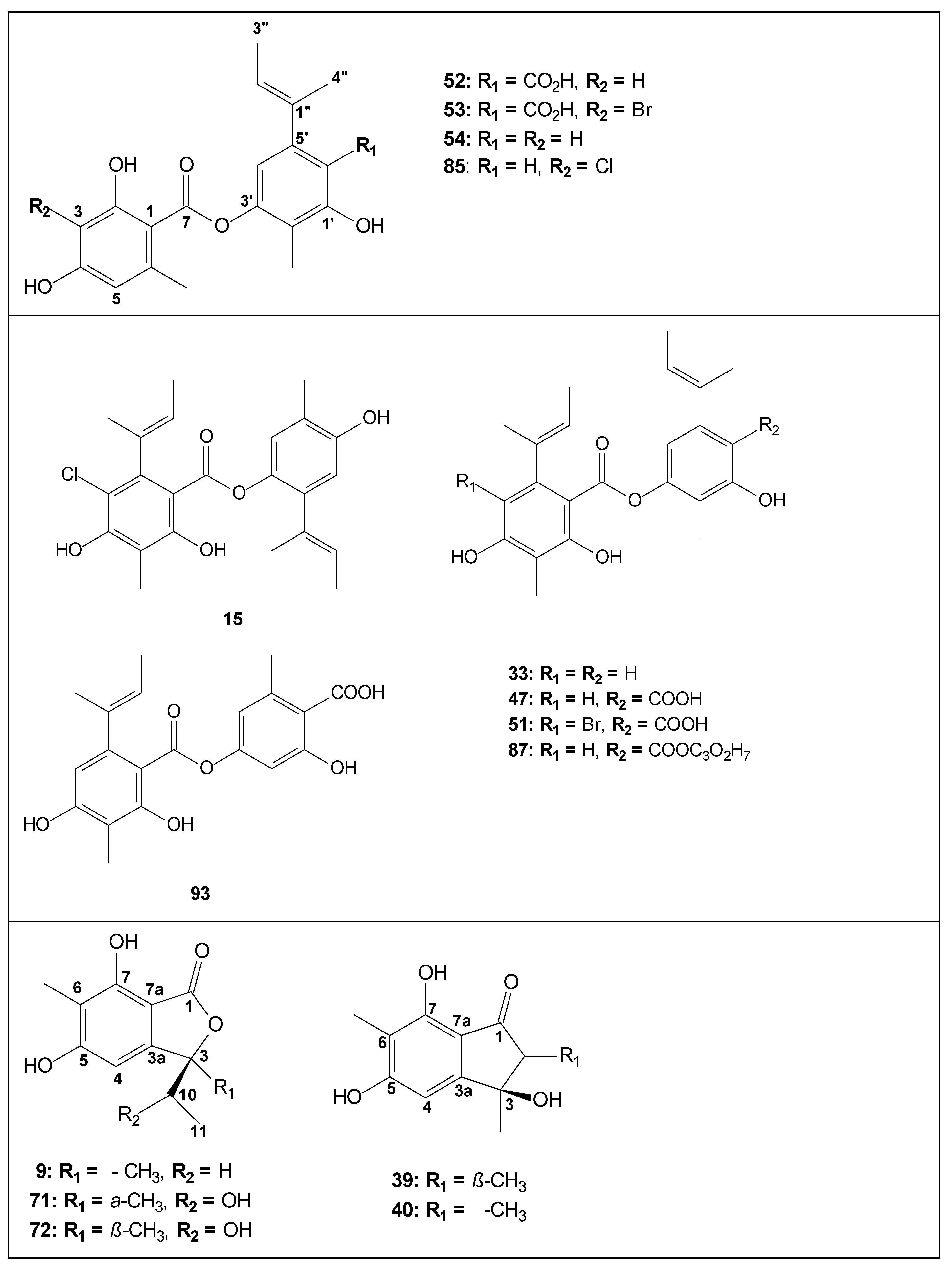
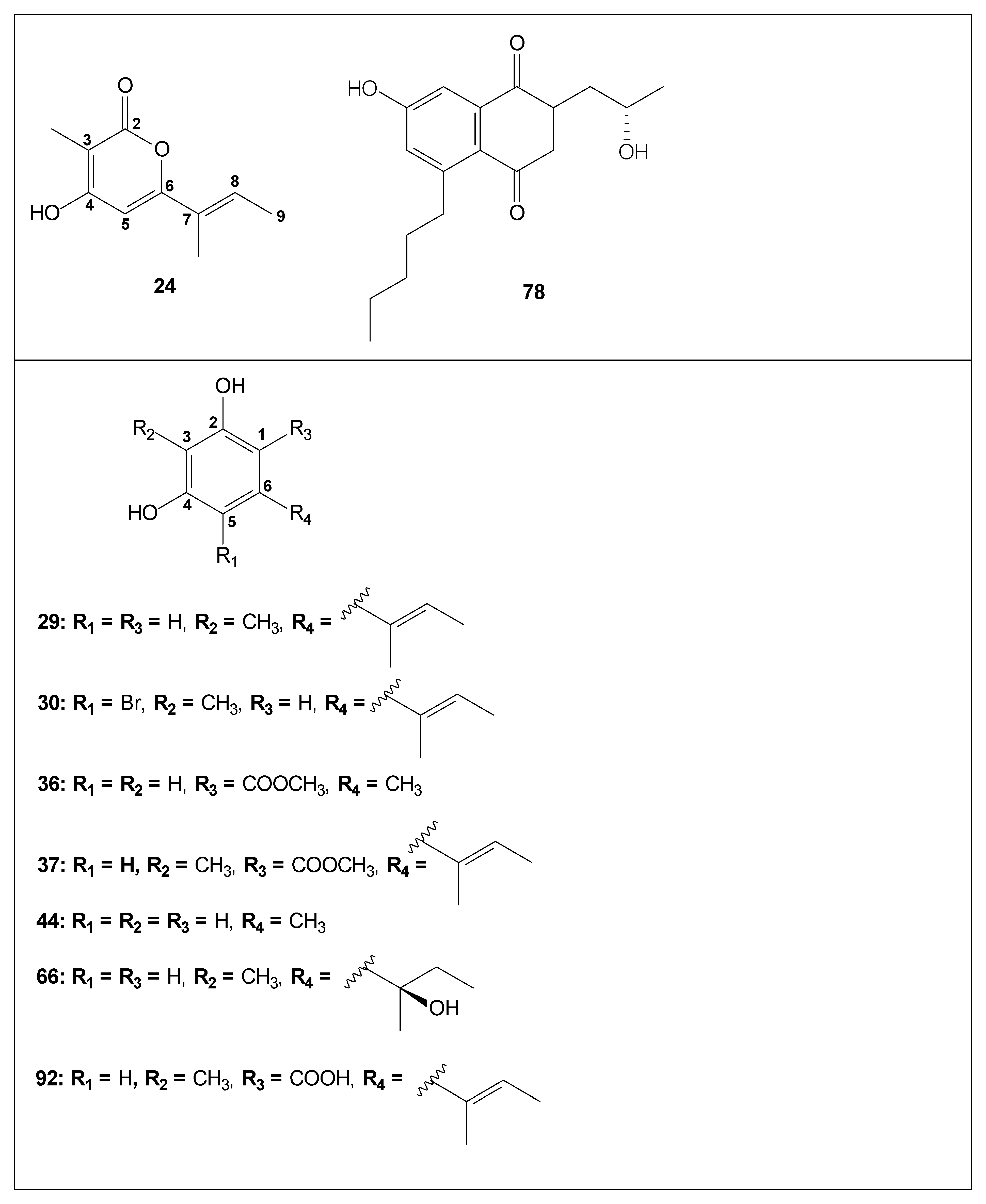

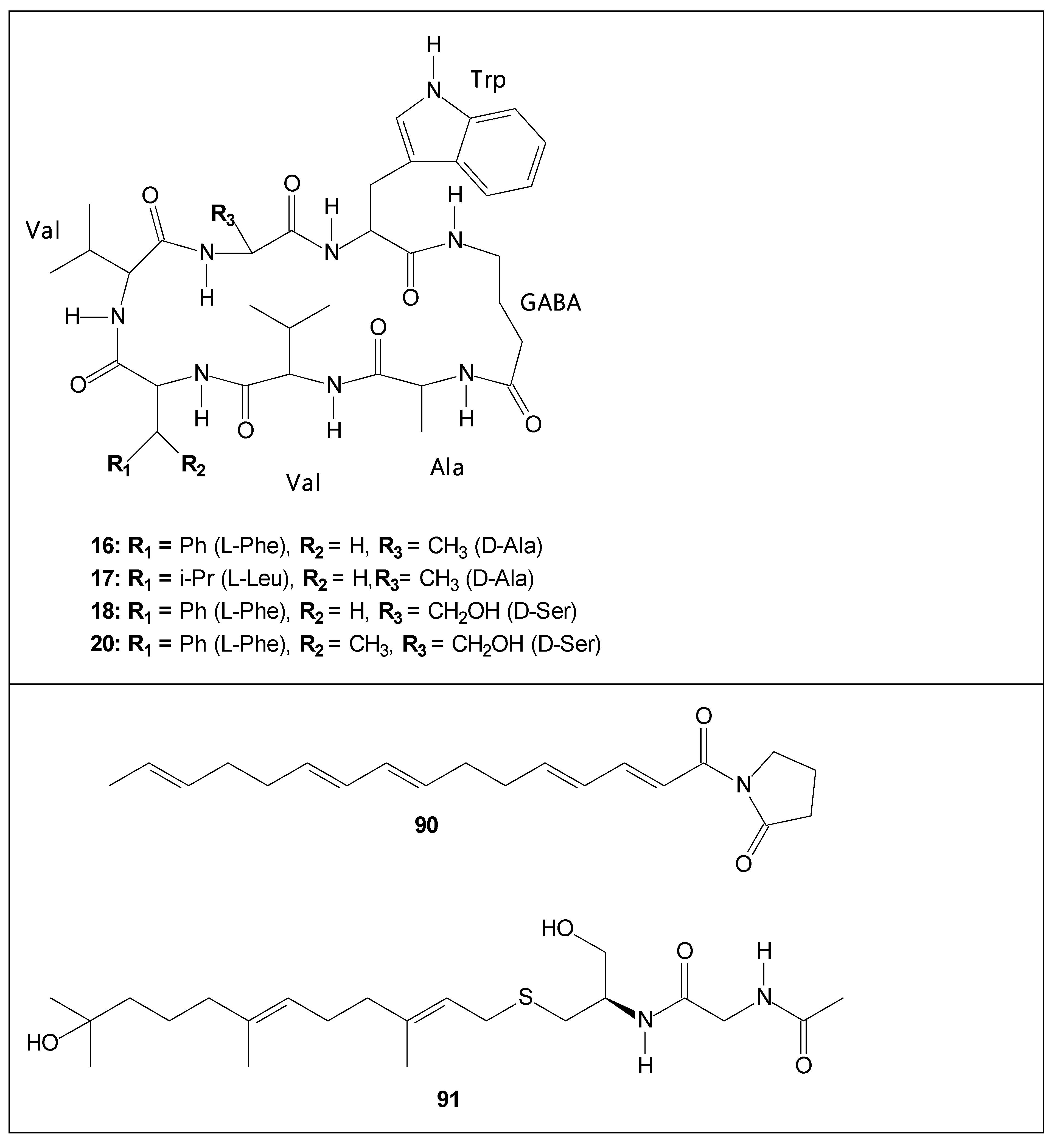
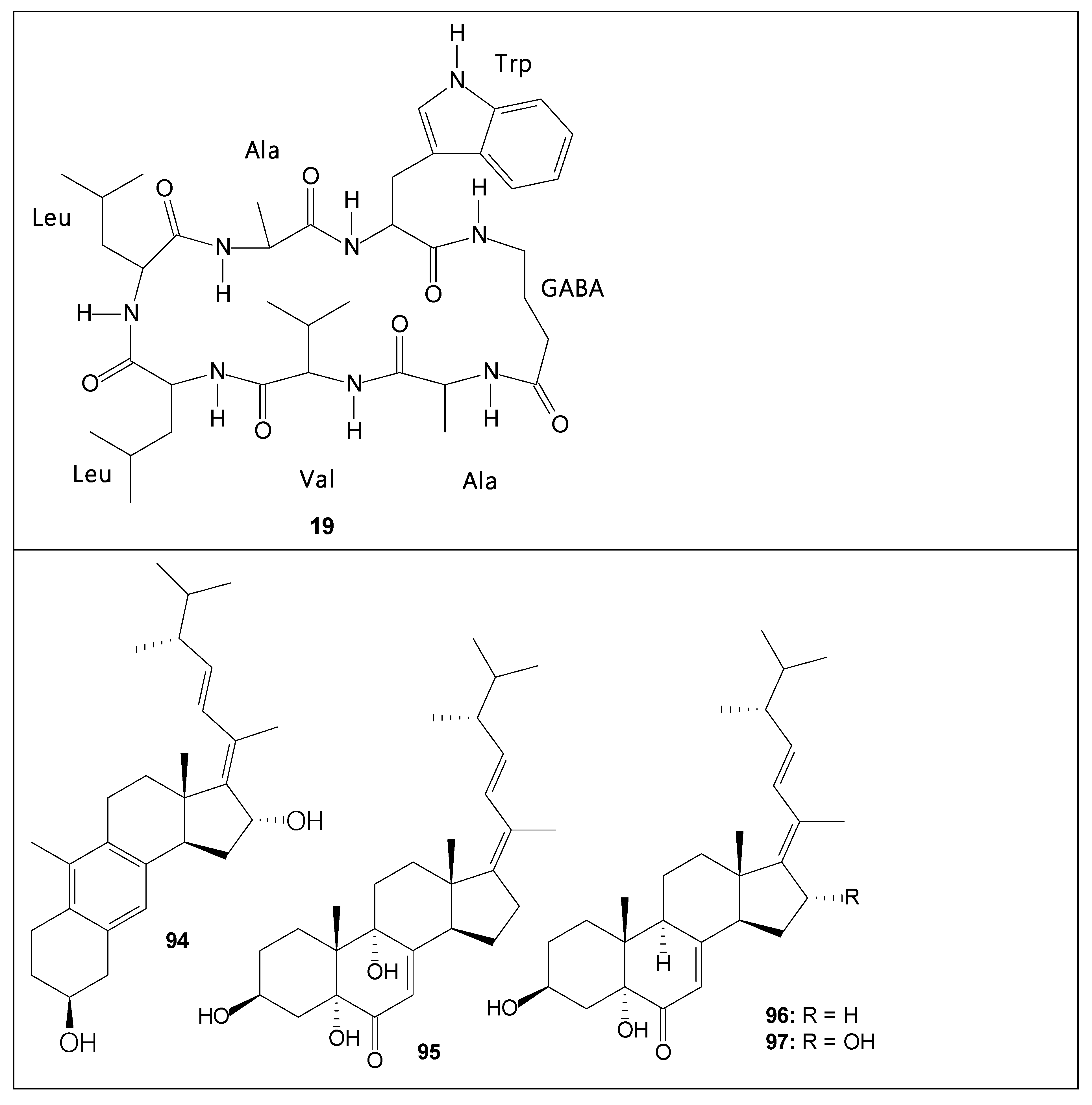
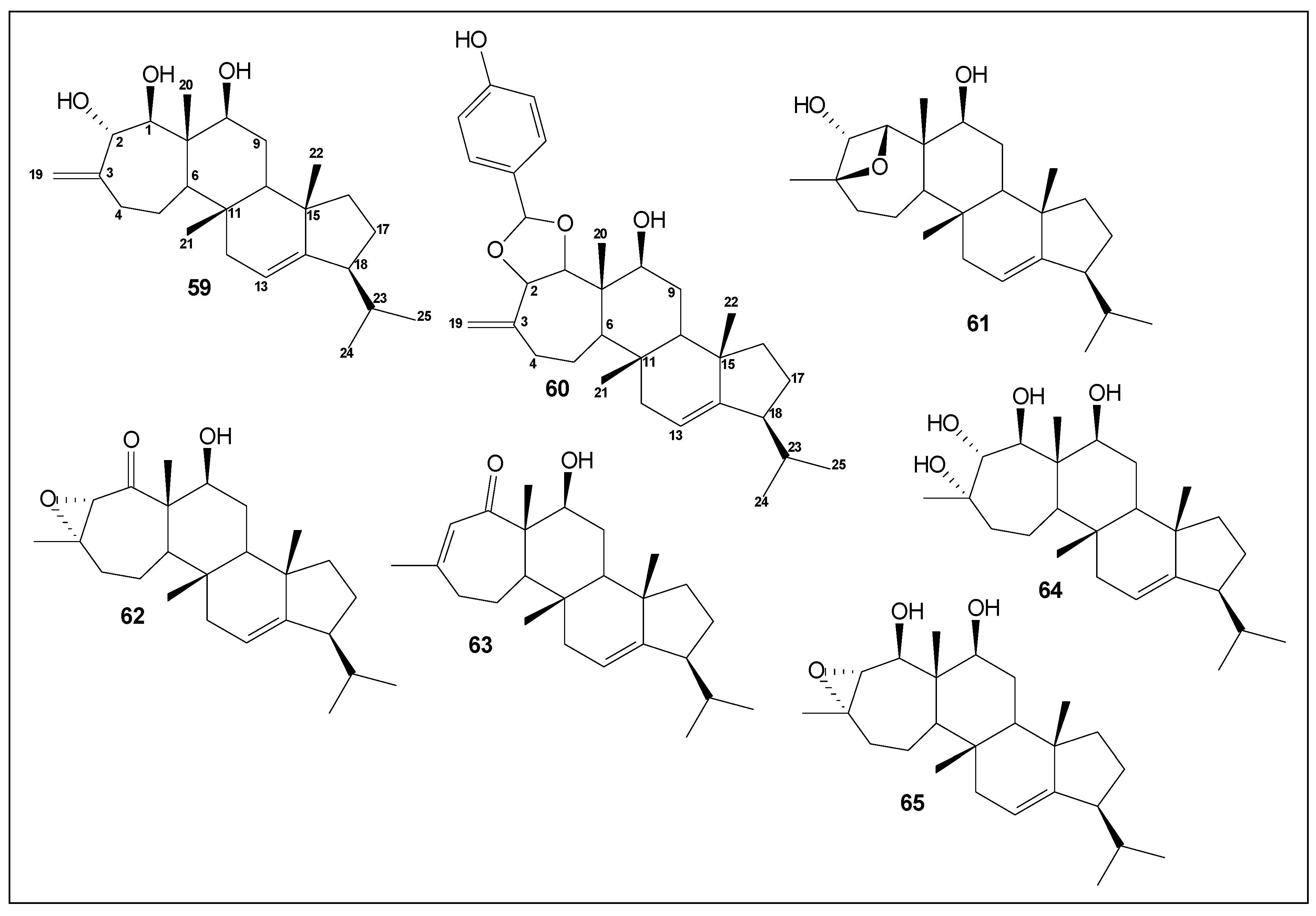
| Code | Compound | Biological Activities | Isolation and Identification |
|---|---|---|---|
| Depsidones | |||
| 1 | nidulin | Inhibition of xanthine oxidase [29]; aromatase inhibitory activity [29]; HuCCA-1, HepG2, A549, MOLT-3 cancer cell line (weak cytotoxicity) [29]; anti-MRSA and brine shrimp larva lethality test strong bioactivity [34]; DNA-damage repair test (anti-AB3027) [34]; antimicrobial activity against BS and SA [30]; antimicrobial activity against SA and MRSA [25]; larvicidal activity using brine shrimp model [28]; antimicrobial activity against BC [35]; anti-phytopathogenic activity against CA [35]; growth inhibition against human cancer cell lines [36] | [25,28,29,30,33,34,35,36,37,38,39,40,41,42,43,44] also isolated from A. nidulans [45] |
| 2 | nornidulin | Inhibition of xanthine oxidase [29]; aromatase inhibitory activity [29]; HuCCA-1, HepG2, A549, MOLT-3 cancer cell line (weak cytotoxicity) [29]; anti-MRSA and brine shrimp larva lethality test [34]; DNA-damage repair test (anti-AB3027) [34]; antimicrobial in SA, MRSA, and CN [42]; antimicrobial activity against BS and SA [30]; antimicrobial activity against SA, MRSA and CN [25]; larvicidal activity/brine shrimp model [28]; antimicrobial activity against BC [35]. anti-phytopathogenic activity against AB and CA [35]; growth inhibition against human cancer cell lines [36]; inhibition effect on LPS-induced NF-kβ activation [46]. Antimicrobial activity against MRSA, MV, MJ and VP [46]; antimicrobial activity against Gram-positive and Gram-negative bacteria [41] | [25,27,28,29,30,33,34,35,36,40,41,42,43,44,46,47] also isolated from A. nidulans [45] |
| 3 | unguinol (yasimin) | Animal growth permittant [48]; pyruvate phosphate dikinase (PPDK) inhibitor (a potential herbicide target) [49]; aromatase inhibitory activity [37]; HuCCA-1, HepG2, A549, MOLT-3 cancer cell line (weak cytotoxicity) [37]; KB, MCF-7 and Vero cancer cell line (cytotoxicity) [42]; T47D tumor cells most likely via inhibition of aromatase (CYP19) activity [50]; SOAT1 and SOAT2 isozymes inhibition [47]; antimicrobial activity against BS [30]; apoptosis induction and cell cycle arrest in MDA-MB-231 cells induction [51]; antimicrobial activity against MT [35]; anti-phytopathogenic activity against AB [35]; growth inhibition against human cancer cell lines [36]; inhibition effect on LPS-induced NF-kβ activation [46] | [25,28,30,33,35,36,38,39,40,41,42,43,46,47,48,49,50,52,53,54,55,56] also isolated from Aspergillus nidulans and Trichoderma asperellum [57,58] |
| 4 | haiderin | - | [53] |
| 5 | rubinin | - | [53] |
| 6 | (-)-shirin | - | [53] |
| 7 | nasrin | - | [53] |
| 8 | 2-chlorounguinol | Inhibition of xanthine oxidase [29]; aromatase inhibitory activity [29]; antimicrobial in SA, MRSA, CA, CN and MG [42]; SOAT1 and SOAT2 isozymes inhibition [47]; antimicrobial activity against SA, MRSA, CA, and MG [25]; antimicrobial activity against PA and MRSA [28]; larvicidal activity/brine shrimp model [28]; antimicrobial activity against MT, BC [35]. Anti-phytopathogenic activity against AB and CA [35]; growth inhibition against human cancer cell lines [36] | [25,28,29,30,35,36,38,39,40,41,42,44,47,48] also isolated from Trichoderma asperellum [58] |
| 10 | emeguisin A/7-chlorofolipastatin | Antimicrobial in SA, MRSA and CN [42]; antimalarial activity against PF [35,42]; antimicrobial activity against BS, SA and SC [30]; antimicrobial activity against SA, MRSA, CA and CN [25]; cytotoxic activity against KB and Vero cells [25]; SOAT1 and SOAT2 isozymes inhibition [47]; antimicrobial activity against MT, BC [35]; anti-phytopathogenic activity against CA [35]; growth inhibition against human cancer cell lines [36]; antimicrobial activity against Gram-positive and Gram-negative bacteria [41] | [25,30,35,36,39,40,41,44] |
| 11 | emeguisin B | Antimalarial activity against PF [35] | [35,39,41,44] |
| 12 | emeguisin C | Antimalarial activity against PF [35]; Antimicrobial activity against MT, BC [35] | [35,39] |
| 13 | folipastatin | Phospholipase A inhibitor [55]; antimicrobial in SA, MRSA, and CN [42]; antimalarial activity against PF [35,42]; SOAT1 and SOAT2 isozymes inhibition [47]; antimicrobial activity against BS and SA [30]; cytotoxity against NS-1 [30]; antimicrobial activity against SA, MRSA and CN [25]; cytotoxic activity against Vero cells [25]; growth inhibition against human cancer cell lines [36]; antimicrobial activity against Gram-positive and Gram-negative bacteria [41] | [25,30,35,36,40,41,44,47,48,52] also isolated from Wicklowia aquatica [59] |
| 14 | 4-methylunguinol | - | [48] |
| 21 | aspergillusidone A | Inhibition of superoxide anion radical formation by xanthine/xanthine oxidase [29]; aromatase inhibitory activity [29]; HuCCA-1, HepG2, A549, MOLT-3 cancer cell line (only weak cytotoxicity) [29]; T47D tumor cells most likely via inhibition of aromatase (CYP19) activity [50]; antimicrobial activity against MRSA and CA [28]. AChE inhibitory activity [28]. Larvicidal activity using brine shrimp model [28]; anti-phytopathogenic activity against CA [35] | [25,28,29,35,41,44,50] |
| 22 | aspergillusidone B | Inhibition of superoxide anion radical formation by xanthine/xanthine oxidase [29]; aromatase inhibitory activity [29]; antimalarial activity against PF [35]; antimicrobial activity against BC [35]; inhibition effect on LPS-induced NF-kβ activation [46] | [25,29,35,44,46] |
| 23 | aspergillusidone C(2,7-dichlorounginol) | Aromatase inhibitory activity [29]; inhibition of xanthine oxidase [29]; HuCCA-1, HepG2, A549, MOLT-3 cancer cell line (only weak cytotoxicity) [29]; anti-A549 tumor cell line [34]; anti-MRSA and brine shrimp larva lethality test strong bioactivity [34]; DNA-damage repair test (anti-AB3027) [34]; antimicrobial in SA, MRSA, CA, CN and MG [42]; antimalarial [42]; VERO cell line (strong cytotoxicity) [42]; antimicrobial activity against BS, SA and SC [30]; antimicrobial activity against SA, MRSA, and MG [25]. Cytotoxic activity against Vero cells [25]; antimicrobial activity against PA [28]. Larvicidal activity using brine shrimp model [28]; antimicrobial activity against MT, BC [35]; anti-phytopathogenic activity against AB and CA [35]; growth inhibition against human cancer cell lines [36]; antimicrobial activity against MRSA, MV, MJ and VP [46]; | [25,28,29,30,34,35,36,40,41,44,46,47] also isolated from Trichoderma asperellum [58] |
| 26 | aspergillusidone D | Aromatase inhibitory activity [37]; HuCCA-1, HepG2, A549, MOLT-3 cancer cell line (only weak cytotoxicity) [37]; antimicrobial activity against BS, SA and SC [30]; apoptosis induction and cell cycle arrest in MDA-MB-231 cells induction [51] | [30,42] |
| 27 | aspergillusidone E | Aromatase inhibitory activity [37]; HuCCA-1, HepG2, A549, MOLT-3 cancer cell line (only weak cytotoxicity) [37]; antimicrobial activity against BS, SA and SC [30] | [30,42] |
| 28 | aspergillusidone F | Aromatase inhibitory activity [37]; HuCCA-1, HepG2, A549, MOLT-3 cancer cell line (only weak cytotoxicity) [37]; antimicrobial activity against BS, SA and SC [30]; antimicrobial activity against PA and MRSA [28]. Larvicidal activity using brine shrimp model [28]. | [28,30,42] |
| 32 | 2,4-dichlorounguinol | Antimicrobial activity against SA and MRSA [25] | [25,42] |
| 35 | aspersidone | Antimicrobial activity against SA and MRSA [25]. Cytotoxic activity against Vero cells [25]; antimicrobial activity against BC [35]; anti-phytopathogenic activity against AB and CA [35]; growth inhibition against human cancer cell lines [36] | [25,35,36,40,41] |
| 45 | 7-carboxifolipastatin | - | [30,44] |
| 46 | 4,7-dichlorounguinol | Antimicrobial activity against BS, SA and SC [30] | [30] |
| 48 | 7-bromounguinol | Antimicrobial activity against BS, SA and SC [30] | [30] |
| 49 | 2-chloro-7-bromounguinol | Antimicrobial activity against BS, SA and SC [30] | [30] |
| 50 | 7-bromofolipastatin | Antimicrobial activity against BS and SA [30] | [30] |
| 69 | asperunguissidone A | Antimicrobial activity against SA and MRSA [38] | [41] |
| 70 | asperunguissidone B | - | [41] |
| 83 | emeguisin D | Antimalarial activity against PF [35]; antimicrobial activity against MT, BC and SA [35]; | [35] |
| 84 | corynesidone D | Antimicrobial activity against BC [35]; | [35] also isolated from Corynespora cassiicola [60] |
| 86 | aspersidone B | Antimicrobial activity against BS, ML and SA [36]; growth inhibition against human cancer cell lines [36] | [36] |
| 88 | aspergillusidone H | Inhibition effect on LPS-induced NF-kβ activation [46] | [46] |
| Depsides | |||
| 15 | guisinol | Growth inhibition against human cancer cell lines [36]; antimicrobial activity against MRSA, MV, MJ and VP [46] | [27,36,46] |
| 33 | agonodepside A | Growth inhibition against human cancer cell lines [36]; | [28,36,41,42,44] |
| 47 | agonodepside B | Antimicrobial activity against BS [30]; growth inhibition against human cancer cell lines [36] | [30,36] |
| 51 | 5-bromoagonodepside B | Antimicrobial activity against BS [30] | [30] |
| 52 | aspergiside B/unguidepside A/aspergillusidone G | - | [25,30,41,44] |
| 53 | 3-bromounguidepside A | - | [30] |
| 54 | aspergiside A/decarboxyunguidepside A | Antimicrobial activity against BS [30];antimicrobial activity against SA and MRSA [25]; growth inhibition against human cancer cell lines [36] | [25,30,36,41] |
| 85 | unguidepside C | Antimicrobial activity against BS, ML and SA [36]; growth inhibition against human cancer cell lines [36] | [36] |
| 87 | agonodepside C | Antimicrobial activity against BS, ML and SA [36] | [36] |
| 93 | asperdepside A | - | [44] |
| Phthalides | |||
| 9* | (3S)-3-ethyl-5,7-dihydroxy-3,6-dimethylphthalide | Antimicrobial in SA, MRSA, CA, CN and MG [42]; antimalarial [42]; HB carcinoma cell line (strong cytotoxicity) [42]; antimicrobial activity against SA, MRSA, and MG [25] | [25,38,40,41,42] also isolated from Rhytidhysteron sp. [61] |
| 34 | asperlide | - | [40,41] |
| 58 | aspergiside C | - | [25,41] |
| 71 | asperunguislide A | - | [41] |
| 72 | asperunguislide B | - | [41] |
| Cyclopeptides | |||
| 16 | unguisin A | - | [28,55,62] |
| 17 | unguisin B | - | [55] |
| 18 | unguisin C | - | [63] |
| 19 | unguisin D | - | [63] |
| 20 | unguisin E | - | [55] also isolated from Mucor irregularis and Aspergillus candidus [64,65] |
| Nitrogen-containing Compounds | |||
| 90 | variotin B | Anti-inflamatory activity [62] | [62] |
| 91 | coniosulfide E | - | [62] |
| Indanones | |||
| 39 | asperunguisone A | - | [31,41] |
| 40 | asperunguisone B | - | [31,41] |
| Diarylethers | |||
| 25 | aspergillusether A | HuCCA-1, HepG2, A549, MOLT-3 cancer cell line (weak cytotoxicity) [29] | [29,34] |
| 41 | aspergillusether B | - | [31] |
| 42 | aspergillusether C | - | [31,41] |
| 43 | aspergillusether D | Antimicrobial activity against CA, CN and PM [31] | [31,35,41] |
| 55 | unguinolic acid | - | [30] |
| 56 | decarboxyunguinolic acid | - | [30] |
| 57 | 5-chlorounguinolic acid | [30] | |
| 67 | aspergillusether E | Antimicrobial activity against SA, MRSA, CA, CN and MG [38]; cytotoxic activity against Vero cells [38] | [41] |
| 68 | aspergillusether F | Antimicrobial activity against BC [35]; anti-phytopathogenic activity against CA [35]; inhibition effect on LPS-induced NF-kβ activation [46]. Antimicrobial activity against MRSA, MV, MJ and VP [46] | [35,41,46] |
| 79 | aspergillusether G | - | [35] |
| 80 | aspergillusether H | Antimicrobial activity against MT, BC and SA [35]; | [35] |
| 81 | aspergillusether I | Antimicrobial activity against BC and SA [35]; Anti-phytopathogenic activity against CA [35]; | [35] |
| 82 | aspergillusether J | Antimicrobial activity against MT, BC and SA [35]; Anti-phytopathogenic activity against CA [35]; Inhibition effect on LPS-induced NF-kβ activation [46]. Antimicrobial activity against MRSA, MV, MJ and VP [46]; | [35,46] |
| Pyrone | |||
| 24 | 3-methyl-4-hydroxy-6-(1-trans-methyl-1-propenyl)-2-pyrone | Antimicrobial activity against MT [35]; | [29,35,41] |
| Orcinol/Orsenillate Derivatives/Phloroglucinol | |||
| 29 | aspergillusphenol A | Antimicrobial activity against SA and MRSA [31] | [29,31,40,41,44] |
| 30 | aspergillusphenol B | - | [42] |
| 36 | methyl orsellinate | - | [31,40,41] |
| 37 | pilobolusate | Antimicrobial in SA, MRSA, CA, CN and MG [42]; antimalarial [42]; KB cell line (cytotoxicity) [31] | [31,40,41] also isolated from Pilobolus heterosporus [66] |
| 38 | (+)-montagnetol | - | [31,40,41] also isolated from Roccella montagnei [67] |
| 44 | orcinol | - | [31,41] |
| 66 | aspergillusphenol C | - | [41] |
| 73 | grifolin A | - | [68] |
| 74 | grifolin B | DPPH radical scavenging activity [68] | [68] |
| 89 | 1-(2,6-dihydroxy-4-methoxy-3,5-dimethylphenyl)-methylbutan-1-one | Inhibition effect on LPS-induced NF-kβ activation [46]; antimicrobial activity against MV, MJ and VP [46] | [46] |
| 92 | aspergillusphenol A carboxylic acid | - | [44] |
| Benzoic Acid Derivatives | |||
| 31 | 3,5-dibromo-2,4-dihydroxy-6-methyl-benzoic acid methyl ester | - | [42] |
| Anthraquinones | |||
| 75* | averantin | - | [68] also isolated from Aspergillus versicolor [69] |
| 76 | 7-chloroaveratin | - | [68] |
| 77 | 1′-O-methylaveration | - | [68] |
| Chromone | |||
| 78 | 7-hydroxy-2-(2-hydroxypropyl-5-pentyl-chromone | - | [68] |
| Sesterterpenoids | |||
| 59 | asperunguisin A | Cytotoxicity against A549 and HepG2 human cancer cells [26] | [26] |
| 60 | asperunguisin B | Cytotoxicity against SMMC-7721 human cancer cells [26] | [26] |
| 61 | asperunguisin C | Cytotoxicity against HT-29, A549, U251, U87, SMMC-7721 and HepG2 human cancer cells [26] | [26] |
| 62 | asperunguisin D | - | [26] |
| 63 | asperunguisin E | - | [26] |
| 64 | asperunguisin F | - | [26] |
| 65 | aspergilloxide | - | [26] |
| Sterols | |||
| 94 | aspersterol A | Cytotoxicity against cancer cell lines [32] | [32] |
| 95 | aspersterol B | Anti-inflammatory activity [32] | [32] |
| 96 | aspersterol C | Anti-inflammatory activity [32] | [32] |
| 97 | aspersterol D | Anti-inflammatory activity [32] | [32] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domingos, L.T.S.; Martins, R.d.S.; Lima, L.M.d.; Ghizelini, A.M.; Ferreira-Pereira, A.; Cotinguiba, F. Secondary Metabolites Diversity of Aspergillus unguis and Their Bioactivities: A Potential Target to Be Explored. Biomolecules 2022, 12, 1820. https://doi.org/10.3390/biom12121820
Domingos LTS, Martins RdS, Lima LMd, Ghizelini AM, Ferreira-Pereira A, Cotinguiba F. Secondary Metabolites Diversity of Aspergillus unguis and Their Bioactivities: A Potential Target to Be Explored. Biomolecules. 2022; 12(12):1820. https://doi.org/10.3390/biom12121820
Chicago/Turabian StyleDomingos, Levy Tenório Sousa, Raquel dos Santos Martins, Leonardo Melo de Lima, Angela Michelato Ghizelini, Antonio Ferreira-Pereira, and Fernando Cotinguiba. 2022. "Secondary Metabolites Diversity of Aspergillus unguis and Their Bioactivities: A Potential Target to Be Explored" Biomolecules 12, no. 12: 1820. https://doi.org/10.3390/biom12121820








