3.1. Transcriptomic Analyses Reveal Differential Root/Shoot DEGs of Oxidative Stress-Related Activity in Plants Exposed to NNMF
A. thaliana seedlings vertically grown in Petri dishes and exposed to NNMF conditions from 10 min to 96 h were separately sampled by collecting roots and shoots. Controls plants were grown in the same experimental conditions (i.e., temperature, gravity, atmospheric pressure, and photosynthetic phlux density—PPD) but under GMF. Samples were assayed by microarray gene expression.
Supplementary Dataset S1 collects all information on the gene expression fold change volcano plots for all evaluated time points. Gene microarray data were then filtered by considering all genes coding for enzymes involved in oxidative reactions. A total of 194 DEGs were selected. A consistent percentage of these DEGs explained a fold change value >2 at almost all time points (
Supplementary Table S2). In the following text, the term “not regulated” is referred to genes that are DEGs with fold change values below the established threshold limits of >2 and <0.5.
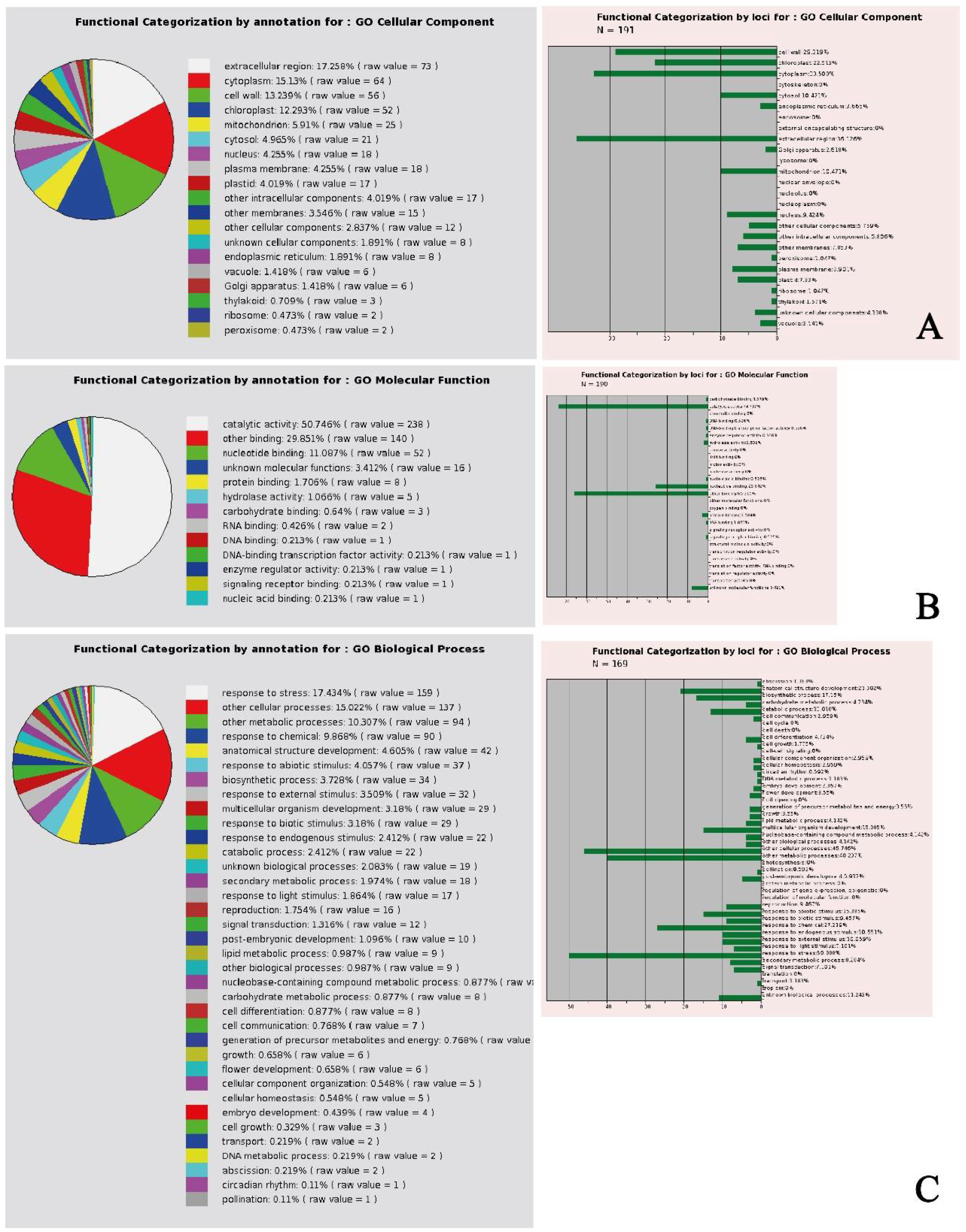
In general, the gene ontology (GO) analysis of the selected genes showed that the cellular component DEGs were mainly expressed in the extracellular region, followed by cytoplasm, cell wall, and chloroplast-related DEGs (
Figure 1A), with a molecular function mainly identified in catalytic activity and binding (
Figure 1B). The biological process was associated to DEGs responding to stress, and other cellular and metabolic processes (
Figure 1C). The data matrix of
Supplementary Table S2 was then subjected to heatmapping in order to identify specific root/shoot patterns of DEGs.
Figure 2 shows the heatmap depicting the time-course DEGs of roots and shoots in plants grown under NNMF with respect to GMF (this heatmap includes all DEGs). Based on the expression matrix, we selected five groups characterized by specific expression patterns (groups A–E), which were then organized in tables by selecting DEGs showing a fold change ≥2 or ≤0.5 in at least one time point (
Table 1,
Table 2,
Table 3,
Table 4 and
Table 5). We identified three main timing of magnetic induction: (i) early (10 min to 2 h), (ii) intermediate (4 and 24 h), and (iii) late (48 and 96 h).
Table 1 lists the DEGs of group A (
Figure 2) showing a fold change ≥2 or ≤0.5 in at least one time point that are not regulated or downregulated in roots and upregulated in shoots. Most of the DEGs in this group have a subcellular prediction in the extracellular space and belong to the peroxidase family proteins, followed by laccases and dioxygenases (
Supplementary Table S2). In particular, several shoot peroxidases (
At1g34510,
At2g43480,
At5g24070,
At1g05240,
At4g26010,
At5g67400) were upregulated at early (10 min and 1 h) times, whereas the peroxidases
At1g30870,
At1g49570, and
At1g05250 were upregulated in intermediate (4 h) and late (48–96 h) times. In shoots,
At1g48700, a 2-oxoglutarate (2OG) and Fe(II)-dependent oxygenase, was upregulated at almost all times and downregulated in roots at 48 h, whereas three other 2OG-Fe(II) oxygenases were upregulated at early (
At5g51930), middle and late times (
At1g55290) and at early and late times (
At4g25310). In shoots, a gene that encodes a protein that is similar to laccase-like polyphenol oxidases (
At5g48100) was upregulated at very early times, whereas a putative laccase (
At5g01050) was upregulated at intermediate and late times. A gene coding for a multicopper oxidase type I family protein (
At1g21860) was downregulated at early times in roots and upregulated at almost all times in shoots, whereas a gene with a similar function (
At1g21860) was only upregulated in shoots at intermediate times (
Table 1).
Table 2 lists the DEGs of group B (
Figure 2) that are upregulated in shoots mostly at all times and upregulated in roots mainly at earlier and intermediate times. Even in this group, some DEGs had an extracellular prediction but many were cytosolic. The gene function was dominated by copper ion binding oxidoreductases, dioxygenases, FAD-binding oxidases, and a few peroxidases (
Supplementary Table S2). Four DEGs coding for 2OG-Fe(II) oxygenases (
At1g28030,
At1g52790,
At2g44800, and
At3g28490), two for gibberellin dioxygenases (
At1g60980 and
At2g34555), and four DEGs coding for oxidoreductases with copper ion binding activity (
At4g28090,
At1g55570,
At1g75790, and
At1g21850) were upregulated in shoots at all times and upregulated in roots at early/intermediate times. Five DEGs coding for oxidoreductases with a FAD-binding domain (
At1g11770,
At4g20800,
At2g38960,
At1g32300, and
At5g45180) were always upregulated in shoots, whereas in roots upregulation was absent at late times and the same pattern was observed for three peroxidases (
At1g65990,
At1g24110, and
At3g42570.1) (
Supplementary Table S2).
DEGs of group C (
Figure 2) with a fold change ≥2 or ≤0.5 are listed in
Table 3. Group C contains DEGs that are highly upregulated in shoots at 24 h (
Figure 1). Two cytoplasmic DEGs encoding for oxidoreductases with a FAD-binding domain were both upregulated in shoots at 24 h, but one of them (
At1g26410.1) was downregulated in roots at earlier time stages, whereas the other (
At1g26390.1) was upregulated at earlier times both in roots and shoots. A gene coding for a peroxidase (
At5g05340.1) with extracellular localization and a chloroplastic methionine sulfoxide reductase (
At4g21830.1), which respond to singlet oxygen, were only upregulated in shoots at 24 h (
Supplementary Table S2).
Group D contains DEGs that are highly upregulated in shoots at 48 h (
Figure 2). DEGs of this group with a fold change ≥2 or ≤0.5 are listed in
Table 4. A gene localized in the cytoplasm coding for a jasmonate-induced dioxygenase (
At2g38240.1) was only upregulated in shoots at 48 h whereas another cytosolic gene coding for a galactose-oxidase (
At3g57620.1) showed a high upregulation at 48 h also in roots. A gene coding for a plasma membrane-located ferric-chelate reductase (
At5g23990.1) was upregulated in shoots at almost all times and upregulated in roots at 48 h, whereas a gene coding for an extracellular peroxidase (
At3g50990.1) was highly upregulated in shoots at 48 h and was also upregulated in roots at intermediate and late times (
Supplementary Table S2).
The last group, E, is made by DEGs that are downregulated in shoots at 24 h (
Figure 2). The only gene with a fold change ≥2 or ≤0.5 (
Table 5) is coding for a putative 2-aminoethanethiol dioxygenase (
At1g18490.1) (
Supplementary Table S2).
3.3. GMF Induces a Significant ROS Production
In order to evaluate the ROS production in NNMF conditions, we measured the H2O2 content during the middle and late stages of plant growth (24 h, 48 h, and 96 h). For technical reasons, measurements at early times were not possible due to the tiny amount of material. Analyses were performed on both roots and shoots of plants grown in GMF and NNMF.
In general, the content of H
2O
2 was reduced 5-fold at 24 h, 2-fold at 48 h, and 0.2-fold at 24 h in plants grown under NNMF in comparison to GMF condition, independently of the analyzed plant tissue (
Table 7).
Under GMF, the shoot H
2O
2 content decreased with time, whereas under NNMF, the H
2O
2 concentration was practically unaffected at 24 and 48 h and significantly decreased only at 96 h (
Table 7). By calculating the NNMF/GMF ratio, a general increasing trend was observed for both shoots and roots. In shoots, NNMF always showed highly significantly (
p < 0.01) lower H
2O
2 production with respect to GMF (
Table 7).
Under GMF, root H
2O
2 production was almost constant with time, whereas a significant (
p < 0.05) increase in H
2O
2 was observed with time in roots exposed to NNMF (
Table 7). However, as for shoots, the NNMF/GMF ratio indicated a general reduction of root H
2O
2 in NNMF exposed plants (
Table 7).
Because the observed trend could be related to different DEGs involved in H
2O
2 metabolism, DEGs involved in H
2O
2 metabolic processes were extracted from
Supplementary Table S2 (see Supplementary Table S4). These genes were then used to generate a heatmap calculated using a Pearson distance and average linkage method (
Figure 3).
DEGs (NNMF/GMF fold change ratio) were considered by analyzing data from 4 h to 48 h in order to evaluate the possible regulation before the first (24 h) and last (96 h) time considered for ROS scavenging/production (
Table 7). In general, the heatmap confirms the DEGs regulation of roots and shoots ROS-scavenging enzymes. In particular, roots showed a marked downregulation of most of DEGs responsible for the transcription of ROS-scavenging enzymes. The only exception was for
RBOHH and
RBOHG at 4 h and some peroxidases at 4 h (
PRX71,
At1g77100,
At5g64110,
At4g33870,
At5g58400), 24 h (
PRX71,
At1g77100,
At5g24070) and 48 h (
At1g77100,
At5g19880,
At4g17690,
At2g18150), which were upregulated (
Figure 3). These data are in agreement with the general time-course increasing H
2O
2 levels in roots exposed to NNMF (
Table 7). On the other hand, shoot upregulation of most of ROS-scavenging DEGs was evident (
Figure 3), with stronger upregulation present at 4 h for
CAT1 (
At1g20630),
APX2 (
At3g09640),
PRX2 (
At1g05250), and
PER57 (
At5g17820); at 24 h for
APX1 (
At1g07890),
CCS (
At1g12520),
SOD1 (
At1g08830), and
SOD2 (
At2g28190); and at 48 h for
RBOHJ (
At3g45810),
RBOHG (
At4g25090), and
PER36 (
At3g50990). Again, DEGs data obtained by microarray were in agreement with the decreasing shoot H
2O
2 production with time in NNMF exposed plants (
Table 7).
3.4. Reduction of GMF to NNMF Modulates the Production of Antioxidant Polyphenols
HPLC-DAD-MS/MS analyses were performed on the same extracts used for H
2O
2 detection. The considered time points were then 24 h, 48 h, and 96 h for both shoots and roots. In general, 44 compounds belonging to different polyphenol classes were identified in both NNMF and GMF samples, including 8 flavanols (
Figure 4 compounds
7,
11,
16,
19,
26,
34,
41, and
45), 13 flavonols (compounds
10,
13,
14,
15,
18,
20,
22,
24,
29,
33,
39,
42, and
43), 10 isoflavanones (compounds
3,
5,
6,
9,
21,
28,
31,
35,
36, and
40), and 6
O-methylated flavonols (compounds
12,
17,
30,
32,
44, and
23) (see
Supplementary Table S5 for quantitative variation and
Figure 4 for chemical structures).
Table 8 shows the content of the total identified polyphenols expressed as µg g
−1 FW, along with the NNMF/GMF ratio. In general, at 96 h, both roots and shoots showed a total polyphenol content that was always lower under NNMF than GMF. From 24 h to 48 h, the shoots polyphenol content showed a decreasing trend with time, whereas in roots the highest value was found at 48 h (
Table 8).
Figure 4.
Chemical structure of polyphenolic compounds identified in
Arabidopsis thaliana roots and shoots grown under GMF and NNMF conditions. Formulae numbers refer to the compounds cited in the main text and in
Table 9.
Figure 4.
Chemical structure of polyphenolic compounds identified in
Arabidopsis thaliana roots and shoots grown under GMF and NNMF conditions. Formulae numbers refer to the compounds cited in the main text and in
Table 9.
In order to evaluate whether NNMF could affect the content of specific polyphenols, raw data obtained from HPLC-DAD-MS/MS analyses (see
Supplementary Table S5) were used to calculate the NNMF/GMF ratio (
Table 9). Data indicate that NNMF differently modulates the content of polyphenols, not only within the two organs, but also with time.
In roots, plants grown for 24 h under NNMF conditions showed a general increase in polyphenols, when compared to GMF, with the sole exception for isorhamnetin glucoside (
32) and quercetin glucoside (
33) (
Table 9). At 48 h, the production of polyphenols was even higher in NNMF, with the exception for dihydroquercetin-rutinoside (
11) and isorhamnetin (
44), which were reduced. At 96 h, root polyphenols showed a reversed trend and only daidzein-galactoside (
1), dihydrogenistein-glucuronide (
6), isorhamnetin-sambubioside (
23), dihydroquercetin-glucoside (
26), dihydromyricetin-glucoside (
35) and genistein (
37) remained upregulated; interestingly, dihydroquercetin-rutinoside (
11), which was reduced at 24 h, increased at 96 h (
Table 9).
At all times, shoots contained 14 polyphenols that were not found in roots: genistein-galactoside (
2), dihydrogenistein-galactoside (
3), genistein-glucoside (
4), dihydrogenistein-glucoside (
5), dihydrokaempferol-glucoside (
7), dihydrohesperetin-neohesperidoside (
12), kaempferol-sambubioside (
20), dihydromyricetin-sambubioside (
28), myricetin-diglucoside (
22), dihydromyricetin-rutinoside (
31), dihydroquercetin-diglucoside (
34), kaempferol (
39), dihydrokaempferol (
41) and dihydroquercetin (
45). However, shoots lacked isorhamnetin-galactoside (
30) and isorhamnetin-glucoside (
32) at all times and kaempferol-rutinoside (
13) at 24 h, with respect to roots. At 24 h, most of the polyphenols were upregulated in NNMF, with particular reference to dihydrogenistein-galactoside (
3); however, a significant reduction was found for genistein-galactoside (
2), dihydrogenistein-glucuronide (
6), dihydroquercetin-rutinoside (
11), daidzein-glucuronide (
25), dihydromyricetin-galactoside (
28), quercetin-glucoside (
33), and an unknown polyphenol (
27). Upregulation was also found at 48 h NNMF treated plants for most of the identified compounds, with a strong increase of dihydrokaempferol (
41); whereas dihydrogenistein-galactoside (
3) and dihydroquercetin-glucoside (
26) were severely reduced. Finally, at 96 h a general reduction of most of the shoot polyphenols was observed in NNMF plants, with the sole exception for myricetin-diglucoside (
22), dihydrokaempferol (
41), and an unknown polyphenol (
27) that showed a higher induction with respect to GMF plants (
Table 9).
In order to evaluate whether the observed differences in the root and shoot polyphenols could match the DEGs, we extracted from the general gene microarray analysis those genes that were involved in flavonoid biosynthesis (see
Supplementary Table S7). Data were then used, along with those reported in
Table 9, to generate heatmaps coupled with hierarchical cluster analysis (
Figure 5).
A direct comparison between polyphenol quantity (
Figure 5A) and DEGs expressing polyphenol metabolism (
Figure 5B) shows a gene upregulation of
chalcone synthase (
At5g13930),
chalcone isomerase (
At5g05270) and
UDP-glucosyl transferase (
At5g17040) in roots at 4 h, that preceded the reduced biosynthesis of isorhamnetin-galactoside (
30), daidzein (
38), quercetin-dirhamnopyranoside (
15), kaempferol-rutinoside (
13), dihydrogenistein (
40), and isorhamnetin-sambubioside (
23) that were detected at 24 h (
Figure 5 green arrows). At 24 h, root upregulation of
phenylcoumaran benzylic ether reductase 1 (
At4g39230),
pinoresinol reductase 1 (
At1g32100),
pinoresinol reductase 2 (
At4g13660), and
polyketide synthase A (
At1g02050) preceded the biosynthesis of several polyphenols at 48 h, with particular reference to quercetin-glucoside (
33), myricetin (
43), quercetin-galactoside (
29), and dihydrogenistein (
40) (
Figure 5 orange arrows). Finally, the root DEGs at 48 h were characterized by general downregulation, which matched with the low amount of polyphenols detected at 96 h (
Figure 4A), with the exception for
DFR (
At5g42800),
EPSPS (
At1g48860),
CYP75B1 (
At5g07990), and a putative
isoflavone reductase (
At1g75290), which upregulation-matched with the higher levels of dihydrogenistein-glucuronide (
6), dihydroquercetin-glucoside (
26), and dihydromyricetin-glucoside (
35) (
Figure 5 blue arrows).
With regards shoots, the gene upregulation of chalcone synthase (At4g00040 and At1g02050), chalcone isomerase (At3g55120), flavonol synthase (At5g63580), and UDP-glucosyl transferase (At5g17040 and At2g18560) preceded the synthesis of the most polyphenols detected at 24 h, including genistein (
37), quercetin-sambubioside (
14), quercetin-glucoside (
33), quercetin-galactoside (
29), kaempferol (
39), and kaempferol-rutinoside (
13) (
Figure 5 purple arrows). The shoot DEGs at 24 h were characterized by a downregulation of several flavonol synthases including FLS3 (At5g63590), FLS4 (At5g63595), and FLS5 (At5g63600) that matched with the reduced production of dihydrogenistein-galactoside (
3), daidzein-glucuronide (
25), and quercetin-galactoside (
29) at 48 h (
Figure 3 black arrows). The upregulation of UDP-glucosyl transferase (At5g17030), FLS6 (At5g43935), and a putative isoflavone reductase (At1g75290) at 24 h matched with the increased amount, among others, of dihydrogenistein-glucuronide (
6), dihydroquercetin-rutinoside (
11), quercetin-dirhamnopyranoside (
15), dihydromyricetin-sambubioside (
21), and dihydrokaempferol (
41) (
Figure 3 maroon arrows). Finally, the shoot DEGs at 48 h were characterized by the upregulation of an isoflavone reductase (At1g75290), FLS6 (At5g43935), chalcone and stilbene synthase (At5g66220), UDP-glucosyl transferase (At5g17030), DFR (At5g42800), and BAN (At1g61720), that matched the higher production of myricetin-diglucoside (
22), an unknown polyphenol (
27), and dihydrokaempferol (
41) at 96 h (
Figure 5 light blue arrow).