Adrenomedullin: Not Just Another Gastrointestinal Peptide
Abstract
:1. Introduction
2. Adrenomedullin and Proadrenomedullin N-Terminal 20 Peptide
2.1. Adrenomedullin and PAMP Biosynthesis and Structure
2.2. Adrenomedullin and PAMP Receptors
2.3. Main Physiological Effects of Adrenomedullin and PAMP
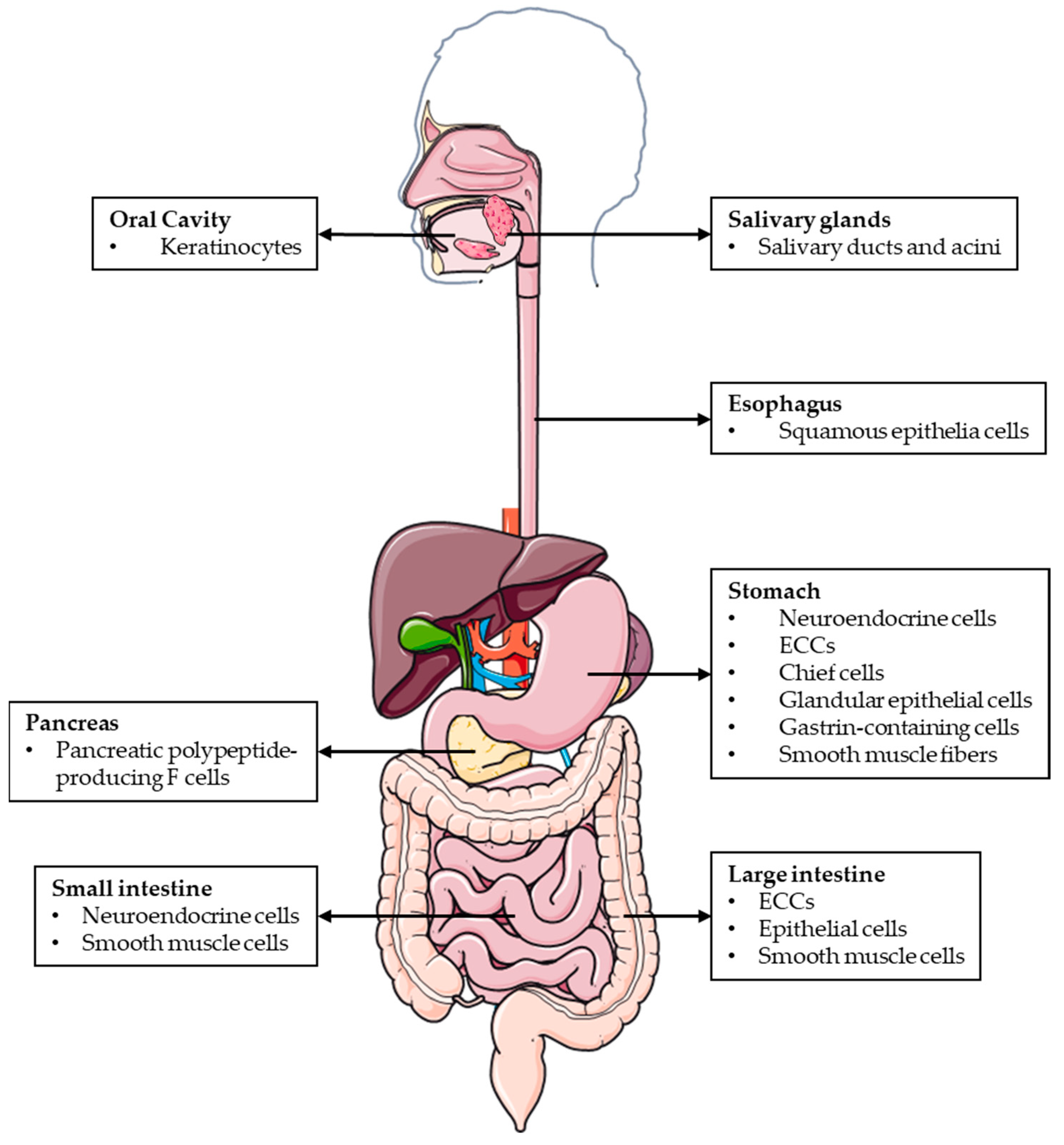
3. Adrenomedullin/PAMP’s Roles in the Digestive System under Physiological Conditions
3.1. Adrenomedullin Actions in the Oral Cavity
3.2. Role of Adrenomedullin in Stomach Physiology
3.3. Adrenomedullin and PAMP Actions in the Intestine
3.4. Role of Adrenomedullin in Pancreatic Physiology
3.5. Adrenomedullin/PAMP’s Impact on Microbiota Composition
4. Pathophysiological Involvement of Adrenomedullin/PAMP in the Most Relevant Diseases of the Digestive System
4.1. Adrenomedullin and Peptic Ulcer Disease
4.2. Adrenomedullin and Intestinal Ischemia
4.3. Adrenomedullin and Diabetes
4.4. Adrenomedullin and Cancer in the Digestive System
4.5. Adrenomedullin/PAMP and Inflammatory Bowel Disease
4.5.1. AM as an Anti-Inflammatory Factor
4.5.2. AM’s Role in Maintaining Intestinal Epithelial Barrier Integrity
4.5.3. AM Improves Mucosal Healing and Re-Epithelialization
4.5.4. AM Actions on Enteric Vasculature
4.5.5. AM and PAMP Modulation of Intestinal Microbiome
5. From Animal Models to Patients: New Pharmacological Treatments for Gastrointestinal Disorders Based on Adrenomedullin
5.1. Adrenomedullin-Based Therapies for IBD
5.2. Positive Effects of AM in PUD
5.3. AM as a CRC Treatment
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hajishafiee, M.; Bitarafan, V.; Feinle-Bisset, C. Gastrointestinal Sensing of Meal-Related Signals in Humans, and Dysregulations in Eating-Related Disorders. Nutrients 2019, 11, 1298. [Google Scholar] [CrossRef] [Green Version]
- Monteiro, M.P.; Batterham, R.L. The Importance of the Gastrointestinal Tract in Controlling Food Intake and Regulating Energy Balance. Gastroenterology 2017, 152, 1707–1717. [Google Scholar] [CrossRef]
- Milani, C.; Duranti, S.; Bottacini, F.; Casey, E.; Turroni, F.; Mahony, J.; Belzer, C.; Palacio, S.D.; Montes, S.A.; Mancabelli, L.; et al. The First Microbial Colonizers of the Human Gut: Composition, Activities, and Health Implications of the Infant Gut Microbiota. Microbiol. Mol. Biol. Rev. 2017, 81, e00036-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dockray, G.J. Gastrointestinal hormones and the dialogue between gut and brain. J. Physiol. 2014, 30, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Lv, J.; Xi, R. The specification and function of enteroendocrine cells in Drosophila and mammals: A comparative review. FEBS J. 2021. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Dockray, G.J. Enteroendocrine cell signalling via the vagus nerve. Curr. Opin. Pharmacol. 2013, 13, 954–958. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Chen, R.; Zhan, G.; Wang, D.; Tan, X.; Xu, H. Enterochromaffin Cells: Sentinels to Gut Microbiota in Hyperalgesia? Front. Cell Infect. Microbiol. 2021, 11, 760076. [Google Scholar] [CrossRef]
- Cussotto, S.; Sandhu, K.V.; Dinan, T.G.; Cryan, J.F. The Neuroendocrinology of the Microbiota-Gut-Brain Axis: A Behavioural Perspective. Front. Neuroendocrinol. 2018, 51, 80–101. [Google Scholar] [CrossRef]
- Hinson, J.P.; Kapas, S.; Smith, D.M. Adrenomedullin, a multifunctional regulatory peptide. Endocr. Rev. 2000, 21, 138–167. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Herrero, S.; Martinez, A. Adrenomedullin regulates intestinal physiology and pathophysiology. Domest. Anim. Endocrinol. 2016, 56 (Suppl. S66–S83). [Google Scholar] [CrossRef]
- Marutsuka, K.; Hatakeyama, K.; Sato, Y.; Yamashita, A.; Sumiyoshi, A.; Asada, Y. Immunohistological localization and possible functions of adrenomedullin. Hypertens Res. 2003, 26 (Suppl. S33–S40), S33–S40). [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez, J.; Martinez, A. Cell and molecular biology of the multifunctional peptide, adrenomedullin. Int. Rev. Cytol. 2002, 221, 1–92. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Herrero, S.; Pérez-Matute, P.; Villanueva-Millán, M.; Oteo, J.; Martínez, A. Changes in Gut Microbiota Induced by Lack of Adrenomedullin. Microbiology ASF. In Proceedings of the 54th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC®), Washington, DC, USA, 6 September 2014. [Google Scholar]
- Martinez-Herrero, S.; Larrayoz, I.M.; Narro-Iniguez, J.; Villanueva-Millan, M.J.; Recio-Fernandez, E.; Perez-Matute, P.; Oteo, J.A.; Martínez, A. Lack of Adrenomedullin Results in Microbiota Changes and Aggravates Azoxymethane and Dextran Sulfate Sodium-Induced Colitis in Mice. Front. Physiol. 2016, 7, 595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Herrero, S.; Larrayoz, I.M.; Narro-Iniguez, J.; Rubio-Mediavilla, S.; Martinez, A. Lack of Adrenomedullin Aggravates Acute TNBS-Induced Colitis Symptoms in Mice, Especially in Females. Front. Physiol. 2017, 8, 1058. [Google Scholar] [CrossRef] [Green Version]
- Clementi, G.; Caruso, A.; Cutuli, V.M.; de Bernardis, E.; Prato, A.; Mangano, N.G.; Amico-Roxas, M. Effects of centrally of peripherally injected adrenomedullin on reserpine-induced gastric lesions. Eur. J. Pharmacol. 1998, 36, 51–54. [Google Scholar] [CrossRef]
- Ashizuka, S.; Inatsu, H.; Kita, T.; Kitamura, K. Adrenomedullin Therapy in Patients with Refractory Ulcerative Colitis: A Case Series. Dig. Dis. Sci. 2016, 61, 872–880. [Google Scholar] [CrossRef] [Green Version]
- Ashizuka, S.; Kuroishi, N.; Nakashima, K.; Inatsu, H.; Kita, T.; Kitamura, K. Adrenomedullin: A Novel Therapy for Intractable Crohn’s Disease with a Loss of Response to Infliximab. Intern. Med. 2019, 58, 1573–1576. [Google Scholar] [CrossRef] [Green Version]
- Ashizuka, S.; Kita, T.; Inatsu, H.; Kitamura, K. Adrenomedullin: A Novel Therapeutic for the Treatment of Inflammatory Bowel Disease. Biomedicines 2021, 9, 1068. [Google Scholar] [CrossRef]
- Kitamura, K.; Kangawa, K.; Kawamoto, M.; Ichiki, Y.; Nakamura, S.; Matsuo, H.; Eto, T. Adrenomedullin: A novel hypotensive peptide isolated from human pheochromocytoma. Biochem. Biophys. Res. Commun. 1993, 192, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Ishimitsu, T.; Kojima, M.; Kangawa, K.; Hino, J.; Matsuoka, H.; Kitamura, K.; Eto, T.; Matsuo, H. Genomic structure of human adrenomedullin gene. Biochem. Biophys. Res. Commun. 1994, 203, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Beltowski, J.; Jamroz, A. Adrenomedullin—What do we know 10 years since its discovery? Pol. J. Pharmacol. 2004, 56, 5–27. [Google Scholar] [PubMed]
- Martínez, A.; Hodge, D.L.; Garayoa, M.; Young, H.A.; Cuttitta, F. Alternative splicing of the proadrenomedullin gene results in differential expression of gene products. J. Mol. Endocrinol. 2001, 27, 31–41. [Google Scholar] [CrossRef] [Green Version]
- Takei, Y.; Inoue, K.; Ogoshi, M.; Kawahara, T.; Bannai, H.; Miyano, S. Identification of novel adrenomedullin in mammals: A potent cardiovascular and renal regulator. FEBS Lett. 2004, 556, 53–58. [Google Scholar] [CrossRef]
- Roh, J.; Chang, C.L.; Bhalla, A.; Klein, C.; Hsu, S.Y. Intermedin is a calcitonin/calcitonin gene-related peptide family peptide acting through the calcitonin receptor-like receptor/receptor activity-modifying protein receptor complexes. J. Biol. Chem. 2004, 279, 7264–7274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez-Castells, J.; Martin-Santamaria, S.; Nieto, L.; Ramos, A.; Martinez, A.; de Pascual-Teresa, B.; Jimenez-Barbero, J. Structure of Micelle-Bound Adrenomedullin: A First Step Toward the Analysis of Its Interactions with Receptors and Small Molecules. Biopolymers 2012, 97, 45–53. [Google Scholar] [CrossRef]
- Lucyk, S.; Taha, H.; Yamamoto, H.; Miskolzie, M.; Kotovych, G. NMR conformational analysis of proadrenomedullin N-terminal 20 peptide, a proangiogenic factor involved in tumor growth. Biopolymers 2006, 81, 295–308. [Google Scholar] [CrossRef]
- Martinez, A.; Bengoechea, J.A.; Cuttitta, F. Molecular evolution of proadrenomedullin N-terminal 20 peptide (PAMP): Evidence for gene co-option. Endocrinology 2006, 147, 3457–3461. [Google Scholar] [CrossRef] [Green Version]
- Garayoa, M.; Martínez, A.; Lee, S.; Pío, R.; An, W.G.; Neckers, L.; Trepel, J.; Montuenga, L.M.; Ryan, H.; Johnson, R.; et al. Hypoxia-inducible factor-1 (HIF-1) up-regulates adrenomedullin expression in human tumor cell lines during oxygen deprivation: A possible promotion mechanism of carcinogenesis. Mol. Endocrinol. 2000, 148, 48–62. [Google Scholar] [CrossRef] [PubMed]
- Poyner, D.R.; Sexton, P.M.; Marshall, I.; Smith, D.M.; Quirion, R.; Born, W.; Muff, R.; Fischer, J.A.; Foord, S.M. International Union of Pharmacology. XXXII. The mammalian calcitonin gene-related peptides, adrenomedullin, amylin, and calcitonin receptors. Pharmacol. Rev. 2002, 54, 233–246. [Google Scholar] [CrossRef] [Green Version]
- Qi, T.; Christopoulos, G.; Bailey, R.J.; Christopoulos, A.; Sexton, P.M.; Hay, D.L. Identification of N-terminal receptor activity-modifying protein residues important for calcitonin gene-related peptide, adrenomedullin, and amylin receptor function. Mol. Pharmacol. 2008, 74, 1059–1071. [Google Scholar] [CrossRef] [Green Version]
- Gibbons, C.; Dackor, R.; Dunworth, W.; Fritz-Six, K.; Caron, K.M. Receptor activity-modifying proteins: RAMPing up adrenomedullin signaling. Mol. Endocrinol. 2007, 21, 783–796. [Google Scholar] [CrossRef] [Green Version]
- Kamohara, M.; Matsuo, A.; Takasaki, J.; Kohda, M.; Matsumoto, M.; Matsumoto, S.; Soga, T.; Hiyama, H.; Katou, M. Identification of MrgX2 as a human G-protein-coupled receptor for proadrenomedullin N-terminal peptides. Biochem. Biophys. Res. Commun. 2005, 330, 1146–1152. [Google Scholar] [CrossRef]
- Larrayoz, I.M.; Martinez-Herrero, S.; Ochoa-Callejero, L.; Garcia-Sanmartin, J.; Martinez, A. Is the Cytoskeleton an Intracellular Receptor for Adrenomedullin and PAMP? Curr. Protein Pept. Sci. 2013, 14, 429–443. [Google Scholar] [CrossRef] [PubMed]
- Meyrath, M.; Palmer, C.B.; Reynders, N.; Vanderplasschen, A.; Ollert, M.; Bouvier, M.; Szpakowska, M.; Chevigné, A. Proadrenomedullin N-Terminal 20 Peptides (PAMPs) Are Agonists of the Chemokine Scavenger Receptor ACKR3/CXCR7. ACS Pharmacol. Transl. Sci. 2021, 48, 13–23. [Google Scholar] [CrossRef]
- Bachelerie, F.; Ben-Baruch, A.; Burkhardt, A.M.; Combadiere, C.; Farber, J.M.; Graham, G.J.; Horuk, R.; Sparre-Ulrich, A.H.; Locati, M.; Luster, A.D.; et al. International Union of Basic and Clinical Pharmacology. [corrected]. LXXXIX. Update on the extended family of chemokine receptors and introducing a new nomenclature for atypical chemokine receptors. Pharmacol. Rev. 2014, 66, 1–79. [Google Scholar] [CrossRef] [Green Version]
- Regard, J.B.; Sato, I.T.; Coughlin, S.R. Anatomical profiling of G protein-coupled receptor expression. Cell 2008, 135, 561–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garayoa, M.; Bodegas, E.; Cuttitta, F.; Montuenga, L.M. Adrenomedullin in mammalian embryogenesis. Microsc. Res. Tech. 2002, 57, 40–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, K.G.; Hosoe, M.; Sakumoto, R.; Takahashi, T. Temporo-spatial expression of adrenomedullin and its receptors in the bovine placenta. Reprod. Biol. Endocrinol. 2013, 11, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shindo, T.; Kurihara, Y.; Nishimatsu, H.; Moriyama, N.; Kakoki, M.; Wang, Y.; Imai, Y.; Ebihara, A.; Kuwaki, T.; Ju, K.-H.; et al. Vascular abnormalities and elevated blood pressure in mice lacking adrenomedullin gene. Circulation 2001, 104, 1964–1971. [Google Scholar] [CrossRef] [Green Version]
- Shimosawa, T.; Shibagaki, Y.; Ishibashi, K.; Kitamura, K.; Kangawa, K.; Kato, S.; Ando, K.; Fujita, T. Adrenomedullin, an endogenous peptide, counteracts cardiovascular damage. Circulation 2002, 105, 106–111. [Google Scholar] [CrossRef] [Green Version]
- Dackor, R.T.; Fritz-Six, K.; Dunworth, W.P.; Gibbons, C.L.; Smithies, O.; Caron, K.M. Hydrops fetalis, cardiovascular defects, and embryonic lethality in mice lacking the calcitonin receptor-like receptor gene. Mol. Cell Biol. 2006, 26, 2511–2518. [Google Scholar] [CrossRef] [Green Version]
- Ichikawa-Shindo, Y.; Sakurai, T.; Kamiyoshi, A.; Kawate, H.; Iinuma, N.; Yoshizawa, T.; Koyama, T.; Fukuchi, J.; Iimuro, S.; Moriyama, N.; et al. The GPCR modulator protein RAMP2 is essential for angiogenesis and vascular integrity. J. Clin. Investig. 2008, 118, 29–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dackor, R.; Fritz-Six, K.; Smithies, O.; Caron, K. Receptor activity-modifying proteins 2 and 3 have distinct physiological functions from embryogenesis to old age. J. Biol. Chem. 2007, 282, 18094–18099. [Google Scholar] [CrossRef] [Green Version]
- Plück, A. Conditional mutagenesis in mice: The Cre/loxP recombination system. Int. J. Exp. Pathol. 1996, 77, 269–278. [Google Scholar] [PubMed]
- Nicholls, M.G. Hemodynamic and hormonal actions of adrenomedullin. Braz. J. Med. Biol. Res. 2004, 37, 1247–1253. [Google Scholar] [CrossRef]
- Samson, W.K.; Murphy, T.C.; Resch, Z.T. Central mechanisms for the hypertensive effects of preproadrenomedullin-derived peptides in conscious rats. Am. J. Physiol. 1998, 274, R1505–R1509. [Google Scholar] [CrossRef]
- Martinez, A. A new family of angiogenic factors. Cancer Lett. 2006, 236, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Koyama, T.; Ochoa-Callejero, L.; Sakurai, T.; Kamiyoshi, A.; Ichikawa-Shindo, Y.; Iinuma, N.; Arai, T.; Yoshizawa, T.; Iesato, Y.; Lei, Y.; et al. Vascular Endothelial Adrenomedullin-RAMP2 System Is Essential for Vascular Integrity and Organ Homeostasis. Circulation 2013, 127, 842–853. [Google Scholar] [CrossRef]
- Garcia-Honduvilla, N.; Cifuentes, A.; Bellon, J.M.; Bujan, J.; Martinez, A. The angiogenesis promoter, proadrenomedullin N-terminal 20 peptide (PAMP), improves healing in both normoxic and ischemic wounds either alone or in combination with autologous stem/progenitor cells. Histol. Histopathol. 2013, 28, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.; Yamahara, K.; Ohnishi, S.; Otani, K.; Kanoh, H.; Ishibashi-Ueda, H.; Minamino, N.; Kangawa, K.; Nagaya, N.; Ikeda, T. Sustained-release adrenomedullin ointment accelerates wound healing of pressure ulcers. Regul. Pept. 2011, 168, 21–26. [Google Scholar] [CrossRef]
- Nishikimi, T. Adrenomedullin in the kidney-renal physiological and pathophysiological roles. Curr. Med. Chem. 2007, 14, 1689–1699. [Google Scholar] [CrossRef] [PubMed]
- Jougasaki, M.; Wei, C.M.; Aarhus, L.L.; Heublein, D.M.; Sandberg, S.M.; Burnett, J.C. Renal localization and actions of adrenomedullin: A natriuretic peptide. Am. J. Physiol. 1995, 268, 657–663. [Google Scholar] [CrossRef] [PubMed]
- López, J.; Cuesta, N.; Martínez, A.; Montuenga, L.; Cuttitta, F. Proadrenomedullin N-terminal 20 peptide (PAMP) immunoreactivity in vertebrate juxtaglomerular granular cells identified by both light and electron microscopy. Gen. Comp. Endocrinol. 1999, 116, 192–203. [Google Scholar] [CrossRef]
- Zudaire, E.; Cuttitta, F.; Martínez, A. Regulation of pancreatic physiology by adrenomedullin and its binding protein. Regul. Pept. 2003, 112, 121–130. [Google Scholar] [CrossRef]
- Martinez-Herrero, S.; Larrayoz, I.M.; Ochoa-Callejero, L.; Fernandez, L.J.; Allueva, A.; Ochoa, I.; Martínez, A. Prevention of Bone Loss in a Model of Postmenopausal Osteoporosis through Adrenomedullin Inhibition. Front. Physiol. 2016, 7, 280. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.L.; Zhao, T.J.; Li, R.L.; Sherbet, D.P.; Liang, G.; Brown, M.S. Surviving starvation: Essential role of the ghrelin-growth hormone axis. Cold Spring Harb. Symp. Quant. Biol. 2011, 76, 121–127. [Google Scholar] [CrossRef] [Green Version]
- Serrano, J.; Alonso, D.; Fernández, A.P.; Encinas, J.M.; López, J.C.; Castro-Blanco, S.; Fernández-Vizarra, P.; Richart, A.; Santacana, M.; Uttenthal, L.O.; et al. Adrenomedullin in the central nervous system. Microsc. Res. Tech. 2002, 57, 76–90. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, K.; Itoh, H.; Arai, H.; Suganami, T.; Sawada, N.; Fukunaga, Y.; Sone, M.; Yamahara, K.; Yurugi-Kobayashi, T.; Park, K.; et al. The neuroprotective and vasculo-neuro-regenerative roles of adrenomedullin in ischemic brain and its therapeutic potential. Endocrinology 2006, 147, 1642–1653. [Google Scholar] [CrossRef] [Green Version]
- Saita, M.; Shimokawa, A.; Kunitake, T.; Kato, K.; Hanamori, T.; Kitamura, K.; Eto, T.; Kannan, H. Central actions of adrenomedullin on cardiovascular parameters and sympathetic outflow in conscious rats. Am. J. Physiol. 1998, 274, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Kis, B.; Abrahám, C.S.; Deli, M.A.; Kobayashi, H.; Niwa, M.; Yamashita, H.; Busija, D.W.; Ueta, Y. Adrenomedullin, an autocrine mediator of blood-brain barrier function. Hypertens Res. 2003, 26, S61–S70. [Google Scholar] [CrossRef] [Green Version]
- Fernandez, A.P.; Serrano, J.; Tessarollo, L.; Cuttitta, F.; Martinez, A. Lack of adrenomedullin in the mouse brain results in behavioral changes, anxiety, and lower survival under stress conditions. Proc. Natl. Acad. Sci. USA 2008, 105, 12581–12586. [Google Scholar] [CrossRef] [Green Version]
- Fernandez, A.P.; Serrano, J.; Martinez-Murillo, R.; Martinez, A. Lack of Adrenomedullin in the Central Nervous System Results in Apparently Paradoxical Alterations on Pain Sensitivity. Endocrinology 2010, 151, 4908–4915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gröschl, M.; Wendler, O.; Topf, H.G.; Bohlender, J.; Köhler, H. Significance of salivary adrenomedullin in the maintenance of oral health: Stimulation of oral cell proliferation and antibacterial properties. Regul. Pept. 2009, 154, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Hiroshima, Y.; Bando, M.; Kataoka, M.; Inagaki, Y.; Herzberg, M.C.; Ross, K.F.; Hosoi, K.; Nagata, T.; Kido, J.-I. Regulation of antimicrobial peptide expression in human gingival keratinocytes by interleukin-1α. Arch. Oral. Biol. 2011, 56, 761–767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLachlan, J.L.; Smith, A.J.; Bujalska, I.J.; Cooper, P.R. Gene expression profiling of pulpal tissue reveals the molecular complexity of dental caries. Biochim. Biophys. Acta 2005, 1741, 271–281. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Tomikawa, M.; Jones, M.K.; Sarfeh, I.J.; Tarnawski, A.S. Ethanol injury triggers activation of adrenomedullin and its receptor genes in gastric mucosa. Dig. Dis. Sci. 1999, 44, 1390–1400. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K.; Tsukada, H.; Oya, M.; Onomura, M.; Kodama, M.; Nakamura, H.; Hosokawa, M.; Seino, Y. Adrenomedullin promotes epithelial restitution of rat and human gastric mucosa in vitro. Peptides 1999, 20, 127–132. [Google Scholar] [CrossRef]
- Sakata, J.; Asada, Y.; Shimokubo, T.; Kitani, M.; Inatsu, H.; Kitamura, K.; Kangawa, K.; Matsuo, H.; Sumiyoshi, A.; Eto, T. Adrenomedullin in the gastrointestinal tract. Distribution and gene expression in rat and augmented gastric adrenomedullin after fasting. J. Gastroenterol. 1998, 33, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Rossowski, W.J.; Jiang, N.Y.; Coy, D.H. Adrenomedullin, amylin, calcitonin gene-related peptide and their fragments are potent inhibitors of gastric acid secretion in rats. Eur. J. Pharmacol. 1997, 336, 51–63. [Google Scholar] [CrossRef]
- Rossowski, W.J.; Cheng, B.L.; Jiang, N.Y.; Coy, D.H. Examination of somatostatin involvement in the inhibitory action of GIP, GLP-1, amylin and adrenomedullin on gastric acid release using a new SRIF antagonist analogue. Br. J. Pharmacol. 1998, 125, 1081–1087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirsch, A.B.; McCuen, R.W.; Arimura, A.; Schubert, M.L. Adrenomedullin stimulates somatostatin and thus inhibits histamine and acid secretion in the fundus of the stomach. Regul. Pept. 2003, 110, 189–195. [Google Scholar] [CrossRef]
- Clementi, G.; Caruso, A.; Cutuli, V.M.; Mangano, N.G.; Salomone, S.; Lempereur, L.; Prato, A.; Matera, M.; Amico-Roxas, M. Gastroprotective effect of adrenomedullin administered subcutaneously in the rat. Peptides 2002, 23, 1149–1153. [Google Scholar] [CrossRef]
- Salomone, S.; Caruso, A.; Cutuli, V.M.; Mangano, N.G.; Prato, A.; Amico-Roxas, M.; Bianchi, A.; Clementi, G. Effects of adrenomedullin on the contraction of gastric arteries during reserpine-induced gastric ulcer. Peptides 2003, 24, 117–122. [Google Scholar] [CrossRef]
- Martínez, V.; Cuttitta, F.; Taché, Y. Central action of adrenomedullin to inhibit gastric emptying in rats. Endocrinology 1997, 138, 3749–3755. [Google Scholar] [CrossRef] [PubMed]
- Holzer-Petsche, U.; Seitz, H.; Lembeck, F. Effect of capsaicin on gastric corpus smooth muscle of the rat in vitro. Eur. J. Pharmacol. 1989, 162, 29–36. [Google Scholar] [CrossRef]
- Ebert, E.C. The thyroid and the gut. J. Clin. Gastroenterol. 2010, 44, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Mulder, H.; Ahren, B.; Karlsson, S.; Sundler, F. Adrenomedullin: Localization in the gastrointestinal tract and effects on insulin secretion. Regul. Pept. 1996, 62, 107–112. [Google Scholar] [CrossRef]
- Hussain, S.; Miyazawa, R.; Tomomasa, T.; Kaneko, H.; Takahashi, A.; Watanabe, T.; Arakawa, H.; Morikawa, A. Possible involvement of adrenomedullin in lipopolysaccharide-induced small-intestinal motility changes in conscious rats. J. Gastroenterol. 2005, 40, 1123–1129. [Google Scholar] [CrossRef]
- Zhou, M.; Chaudry, I.H.; Wang, P. The small intestine is an important source of adrenomedullin release during polymicrobial sepsis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 281, 654–660. [Google Scholar] [CrossRef] [Green Version]
- Fukuda, K.; Tsukada, H.; Onomura, M.; Saito, T.; Kodama, M.; Nakamura, H.; Taniguchi, T.; Tominaga, M.; Hosokawa, M.; Seino, Y. Effect of adrenomedullin on ion transport and muscle contraction in rat distal colon. Peptides 1998, 19, 1043–1047. [Google Scholar] [CrossRef]
- Kiyomizu, A.; Kitamura, K.; Kawamoto, M.; Eto, T. Distribution and molecular forms of adrenomedullin and proadrenomedullin N-terminal 20 peptide in the porcine gastrointestinal tract. J. Gastroenterol. 2001, 36, 18–23. [Google Scholar] [CrossRef]
- Kravtsov, G.M.; Hwang, I.S.; Tang, F. The inhibitory effect of adrenomedullin in the rat ileum: Cross-talk with beta3-adrenoceptor in the serotonin-induced muscle contraction. J. Pharmacol. Exp. Ther. 2004, 308, 241–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, T. Interdigestive migrating motor complex -its mechanism and clinical importance. J. Smooth Muscle Res. 2013, 49, 99–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández de Arcaya, I.; Lostao, M.P.; Martínez, A.; Berjón, A.; Barber, A. Effect of adrenomedullin and proadrenomedullin N-terminal 20 peptide on sugar transport in the rat intestine. Regul. Pept. 2005, 129, 147–154. [Google Scholar] [CrossRef]
- Martínez, A.; Cuttitta, F.; Teitelman, G. Expression pattern for adrenomedullin during pancreatic development in the rat reveals a common precursor with other endocrine cell types. Cell Tissue Res. 1998, 293, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Martínez, A.; Weaver, C.; López, J.; Bhathena, S.J.; Elsasser, T.H.; Miller, M.J.; Moody, T.W.; Unsworth, E.J.; Cuttitta, F. Regulation of insulin secretion and blood glucose metabolism by adrenomedullin. Endocrinology 1996, 137, 2626–2632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sekine, N.; Takano, K.; Kimata-Hayashi, N.; Kadowaki, T.; Fujita, T. Adrenomedullin inhibits insulin exocytosis via pertussis toxin-sensitive G protein-coupled mechanism. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E9–E14. [Google Scholar] [CrossRef]
- Martínez, A.; Pío, R.; López, J.; Cuttitta, F. Expression of the adrenomedullin binding protein, complement factor H, in the pancreas and its physiological impact on insulin secretion. J. Endocrinol. 2001, 170, 503–511. [Google Scholar] [CrossRef] [Green Version]
- Tsuchida, T.; Ohnishi, H.; Tanaka, Y.; Mine, T.; Fujita, T. Inhibition of stimulated amylase secretion by adrenomedullin in rat pancreatic acini. Endocrinology 1999, 140, 865–870. [Google Scholar] [CrossRef]
- Wan, X.; Song, M.; Wang, A.; Zhao, Y.; Wei, Z.; Lu, Y. Microbiome Crosstalk in Immunotherapy and Antiangiogenesis Therapy. Front. Immunol. 2021, 12, 747914. [Google Scholar] [CrossRef] [PubMed]
- Trakman, G.L.; Fehily, S.; Basnayake, C.; Hamilton, A.L.; Russell, E.; Wilson-O’Brien, A.; Kamm, M.A. Diet and gut microbiome in gastrointestinal disease. J. Gastroenterol. Hepatol. 2021. online ahead of print. [Google Scholar] [CrossRef]
- Martínez, A.; Elsasser, T.H.; Muro-Cacho, C.; Moody, T.W.; Miller, M.J.; Macri, C.J.; Cuttitta, F. Expression of adrenomedullin and its receptor in normal and malignant human skin: A potential pluripotent role in the integument. Endocrinology 1997, 138, 5597–5604. [Google Scholar] [CrossRef]
- Allaker, R.P.; Kapas, S. Adrenomedullin and mucosal defence: Interaction between host and microorganism. Regul. Pept. 2003, 112, 147–152. [Google Scholar] [CrossRef]
- Kishikawa, H.; Nishida, J.; Ichikawa, H.; Kaida, S.; Morishita, T.; Miura, S.; Hibi, T. Lipopolysaccharides stimulate adrenomedullin synthesis in intestinal epithelial cells: Release kinetics and secretion polarity. Peptides 2009, 30, 906–912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matheson, P.J.; Mays, M.P.; Hurt, R.T.; Harris, P.D.; Garrison, R.N. Adrenornedullin is increased in the portal circulation during chronic sepsis in rats. Am. J. Surg. 2003, 186, 519–525. [Google Scholar] [CrossRef]
- Walsh, T.J.; Martinez, A.; Peter, J.; Unsworth, E.; Cuttitta, F. Antimicrobial activity of adrenomedullin and its gene-related peptides. Clin. Infect. Dis. 1996, 23, 96. [Google Scholar]
- Allaker, R.P.; Zihni, C.; Kapas, S. An investigation into the antimicrobial effects of adrenomedullin on members of the skin, oral, respiratory tract and gut microflora. FEMS Immunol. Med. Microbiol. 1999, 23, 289–293. [Google Scholar] [CrossRef]
- Kapas, S.; Bansal, A.; Bhargava, V.; Maher, R.; Malli, D.; Hagi-Pavli, E.; Allaker, R.P. Adrenomedullin expression in pathogen-challenged oral epithelial cells. Peptides 2001, 22, 1485–1489. [Google Scholar] [CrossRef]
- Marutsuka, K.; Nawa, Y.; Asada, Y.; Hara, S.; Kitamura, K.; Eto, T.; Sumiyoshi, A. Adrenomedullin and proadrenomudullin N-terminal 20 peptide (PAMP) are present in human colonic epithelia and exert an antimicrobial effect. Exp. Physiol. 2001, 86, 543–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allaker, R.P.; Grosvenor, P.W.; McAnerney, D.C.; Sheehan, B.E.; Srikanta, B.H.; Pell, K.; Kapas, S. Mechanisms of adrenomedullin antimicrobial action. Peptides 2006, 27, 661–666. [Google Scholar] [CrossRef]
- Azhari, H.; Underwood, F.; King, J.; Coward, S.; Shah, S.; Ng, S.; Ho, G.; Chan, C.; Tang, W.; Kaplan, G.G. The Global Incidence of Peptic Ulcer Disease and Its Complications at the Turn of the 21st Century: A Systematic Review. Am. J. Gastroenterol. 2018, 1 (Suppl. S2), 61–62. [Google Scholar] [CrossRef] [Green Version]
- Kaneko, H.; Mitsuma, T.; Nagai, H.; Mori, S.; Iyo, T.; Kusugami, K.; Tache, Y. Central action of adrenomedullin to prevent ethanol-induced gastric injury through vagal pathways in rats. Am. J. Physiol. 1998, 274, 1783–1788. [Google Scholar] [CrossRef]
- Egerod, K.L.; Engelstoft, M.S.; Lund, M.L.; Grunddal, K.V.; Zhao, M.; Barir-Jensen, D.; Nygaard, E.B.; Petersen, N.; Holst, J.J.; Schwartz, T.W. Transcriptional and Functional Characterization of the G Protein-Coupled Receptor Repertoire of Gastric Somatostatin Cells. Endocrinology 2015, 156, 3909–3923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khadzhiev, O.C.; Lupal’tsov, V.I.; Simonenkov, A.P.; Klimenko, N.A.; Tatarko, S.V. Microcirculatory disturbances in gastric mucosa during ulcer disease and effects of serotonin on their dynamics. Bull. Exp. Biol. Med. 2000, 130, 843–845. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, H.; Akimoto, M.; Maeda, A.; Shigemoto, M.; Yamashita, K.; Yokoyama, I. Changes in vasoactive substances during gastric ulcer healing. J. Cardiovasc. Pharmacol. 2000, 36 (Suppl. S1), 278–281. [Google Scholar] [CrossRef]
- Cudnik, M.T.; Darbha, S.; Jones, J.; Macedo, J.; Stockton, S.W.; Hiestand, B.C. The diagnosis of acute mesenteric ischemia: A systematic review and meta-analysis. Acad. Emerg. Med. 2013, 20, 1087–1100. [Google Scholar] [CrossRef]
- Coelho, A.; Logo, M.; Gouveia, R.; Campos, J.; Augusto, R.; Canedo, A. Acute Mesenteric Ischemia: Epidemiology, Risk Ractors and Determinants of Mortality. Rev. Port. Cir. Cardiotorac. Vasc. 2016, 23, 137–143. [Google Scholar]
- Oldenburg, W.A.; Lau, L.L.; Rodenberg, T.J.; Edmonds, H.J.; Burger, C.D. Acute mesenteric ischemia: A clinical review. Arch. Intern. Med. 2004, 164, 1054–1062. [Google Scholar] [CrossRef]
- Higuchi, S.; Wu, R.; Zhou, M.; Marini, C.P.; Ravikumar, T.S.; Wang, P. Gut Hyperpermiability after Ischemia and Reperfusion: Attenuation with Adrenomedullin and its Binding Protein Treatment. Int. J. Clin. Exp. Pathol. 2008, 1, 409–418. [Google Scholar]
- Zhang, F.; Wu, R.; Zhou, M.; Blau, S.A.; Wang, P. Human adrenomedullin combined with human adrenomedullin binding protein-1 is protective in gut ischemia and reperfusion injury in the rat. Regul. Pept. 2009, 152, 82–87. [Google Scholar] [CrossRef] [Green Version]
- Looi, Y.H.; Kane, K.A.; McPhaden, A.R.; Wainwright, C.L. Adrenomedullin acts via nitric oxide and peroxynitrite to protect against myocardial ischaemia-induced arrhythmias in anaesthetized rats. Br. J. Pharmacol. 2006, 148, 599–609. [Google Scholar] [CrossRef] [Green Version]
- Okumura, H.; Nagaya, N.; Itoh, T.; Okano, I.; Hino, J.; Mori, K.; Tsukamoto, Y.; Ishibashi-Ueda, H.; Miwa, S.; Tambara, K.; et al. Adrenomedullin infusion attenuates myocardial ischemia/reperfusion injury through the phosphatidylinositol 3-kinase/Akt-dependent pathway. Circulation 2004, 109, 242–248. [Google Scholar] [CrossRef] [Green Version]
- Nishimatsu, H.; Hirata, Y.; Shindo, T.; Kurihara, H.; Kakoki, M.; Nagata, D.; Hayakawa, H.; Satonaka, H.; Sata, M.; Tojo, A.; et al. Role of endogenous adrenomedullin in the regulation of vascular tone and ischemic renal injury: Studies on transgenic/knockout mice of adrenomedullin gene. Circ. Res. 2002, 90, 657–663. [Google Scholar] [CrossRef] [Green Version]
- Wong, H.K.; Tang, F.; Cheung, T.T.; Cheung, B.M. Adrenomedullin and diabetes. World J. Diabetes 2014, 5, 364–371. [Google Scholar] [CrossRef]
- Rulle, S.; Ah Kioon, M.D.; Asensio, C.; Mussard, J.; Ea, H.K.; Boissier, M.C.; Lioté, F.; Falgarone, G. Adrenomedullin, a neuropeptide with immunoregulatory properties induces semi-mature tolerogenic dendritic cells. Immunology 2012, 136, 252–264. [Google Scholar] [CrossRef] [PubMed]
- García-Unzueta, M.T.; Montalbán, C.; Pesquera, C.; Berrazueta, J.R.; Amado, J.A. Plasma adrenomedullin levels in type 1 diabetes. Relationship with clinical parameters. Diabetes Care 1998, 21, 999–1003. [Google Scholar] [CrossRef] [PubMed]
- Martínez, A.; Elsasser, T.H.; Bhathena, S.J.; Pío, R.; Buchanan, T.A.; Macri, C.J.; Cuttitta, F. Is adrenomedullin a causal agent in some cases of type 2 diabetes? Peptides 1999, 20, 1471–1478. [Google Scholar] [CrossRef]
- Larrayoz, I.M.; Martinez-Herrero, S.; Garcia-Sanmartin, J.; Ochoa-Callejero, L.; Martinez, A. Adrenomedullin and tumour microenvironment. J. Transl. Med. 2014, 12, 339. [Google Scholar] [CrossRef] [Green Version]
- Sion-Vardy, N.; Tzikinovsky, A.; Bolotyn, A.; Segal, S.; Fishman, D. Augmented expression of chromogranin A and serotonin in peri-malignant benign prostate epithelium as compared to adenocarcinoma. Pathol. Res. Pract. 2004, 200, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Uemura, M.; Yamamoto, H.; Takemasa, I.; Mimori, K.; Mizushima, T.; Ikeda, M.; Sekimoto, M.; Doki, Y.; Mori, M. Hypoxia-inducible adrenomedullin in colorectal cancer. Anticancer Res. 2011, 31, 507–514. [Google Scholar]
- Benyahia, Z.; Dussault, N.; Cayol, M.; Sigaud, R.; Berenguer-Daizé, C.; Delfino, C.; Tounsi, A.; Garcia, S.; Martin, P.M.; Mabrouk, K.; et al. Stromal fibroblasts present in breast carcinomas promote tumor growth and angiogenesis through adrenomedullin secretion. Oncotarget 2017, 8, 15744–15762. [Google Scholar] [CrossRef] [Green Version]
- Baranello, C.; Mariani, M.; Andreoli, M.; Fanelli, M.; Martinelli, E.; Ferrandina, G.; Scambia, G.; Shahabi, S.; Ferlini, C. Adrenomedullin in ovarian cancer: Foe in vitro and friend in vivo? PLoS ONE 2012, 7, e40678. [Google Scholar] [CrossRef] [PubMed]
- Keleg, S.; Kayed, H.; Jiang, X.; Penzel, R.; Giese, T.; Büchler, M.W.; Friess, H.; Kleeff, J. Adrenomedullin is induced by hypoxia and enhances pancreatic cancer cell invasion. Int. J. Cancer 2007, 121, 21–32. [Google Scholar] [CrossRef]
- Letizia, C.; Tamburrano, G.; Alo, P.; Paoloni, A.; Caliumi, C.; Marinoni, E.; di Iorio, R.; d’Erasmo, E. Adrenomedullin, a new peptide, in patients with insulinoma. Eur. J. Endocrinol. 2001, 144, 517–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aggarwal, G.; Ramachandran, V.; Javeed, N.; Arumugam, T.; Dutta, S.; Klee, G.G.; Smyrk, T.C.; Bamlet, W.; Han, J.J.; Vittar, N.B.R.; et al. Adrenomedullin is up-regulated in patients with pancreatic cancer and causes insulin resistance in β cells and mice. Gastroenterology 2012, 143, 1510–1517.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, M.; Qi, F.; Zhang, S.; Ma, X.; Wang, S.; Wang, C.; Fu, Y.; Luo, Y. Adrenomedullin promotes the growth of pancreatic ductal adenocarcinoma through recruitment of myelomonocytic cells. Oncotarget 2016, 7, 55043–55056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiao, F.; Fang, J.; Xu, J.; Zhao, W.; Ni, Y.; Akuo, B.A.; Zhang, W.; Liu, Y.; Ding, F.; Li, G.; et al. The role of adrenomedullin in the pathogenesis of gastric cancer. Oncotarget 2017, 8, 88464–88474. [Google Scholar] [CrossRef] [Green Version]
- Nouguerede, E.; Berenguer, C.; Garcia, S.; Bennani, B.; Delfino, C.; Nanni, I.; Dahan, L.; Gasmi, M.; Seitz, J.-F.; Martin, P.-M.; et al. Expression of adrenomedullin in human colorectal tumors and its role in cell growth and invasion in vitro and in xenograft growth in vivo. Cancer Med. 2013, 2, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Callejero, L.; Garcia-Sanmartin, J.; Martinez-Herrero, S.; Rubio-Mediavilla, S.; Narro-Iniguez, J.; Martinez, A. Small molecules related to adrenomedullin reduce tumor burden in a mouse model of colitis-associated colon cancer. Sci. Rep. 2017, 7, 17488. [Google Scholar] [CrossRef] [Green Version]
- Leone, V.; Chang, E.B.; Devkota, S. Diet, microbes, and host genetics: The perfect storm in inflammatory bowel diseases. J. Gastroenterol. 2013, 48, 315–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ford, A.C.; Moayyedi, P.; Hanauer, S.B. Ulcerative colitis. BMJ 2013, 346, f432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jergens, A.E.; Simpson, K.W. Inflammatory bowel disease in veterinary medicine. Front. Biosci. 2012, 4, 1404–1419. [Google Scholar] [CrossRef]
- Yesudhas, D.; Gosu, V.; Anwar, M.A.; Choi, S. Multiple roles of toll-like receptor 4 in colorectal cancer. Front. Immunol. 2014, 5, 334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ha, C.W.; Lam, Y.Y.; Holmes, A.J. Mechanistic links between gut microbial community dynamics, microbial functions and metabolic health. World J. Gastroenterol. 2014, 20, 16498–16517. [Google Scholar] [CrossRef] [PubMed]
- Pedreño, M.; Morell, M.; Robledo, G.; Souza-Moreira, L.; Forte-Lago, I.; Caro, M.; O’Valle, F.; Ganea, D.; Gonzalez-Rey, E. Adrenomedullin protects from experimental autoimmune encephalomyelitis at multiple levels. Brain Behav. Immun. 2014, 37, 152–163. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Rey, E.; Delgado-Maroto, V.; Souza Moreira, L.; Delgado, M. Neuropeptides as therapeutic approach to autoimmune diseases. Curr. Pharm. Des. 2010, 16, 3158–3172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashizuka, S.; Ishikawa, N.; Kato, J.; Yamaga, J.; Inatsu, H.; Eto, T.; Kitamura, K. Effect of adrenomedullin administration on acetic acid-induced colitis in rats. Peptides 2005, 26, 2610–2615. [Google Scholar] [CrossRef]
- Ashizuka, S.; Inagaki-Ohara, K.; Kuwasako, K.; Kato, J.; Inatsu, H.; Kitamura, K. Adrenomedullin treatment reduces intestinal inflammation and maintains epithelial barrier function in mice administered dextran sulphate sodium. Microbiol. Immunol. 2009, 53, 573–581. [Google Scholar] [CrossRef]
- Talero, E.; Sanchez-Fidalgo, S.; de la Lastra, C.A.; Illanes, M.; Calvo, J.R.; Motilva, V. Acute and chronic responses associated with adrenomedullin administration in experimental colitis. Peptides 2008, 29, 2001–2012. [Google Scholar] [CrossRef] [PubMed]
- MacManus, C.F.; Campbell, E.L.; Keely, S.; Burgess, A.; Kominsky, D.J.; Colgan, S.P. Anti-inflammatory actions of adrenomedullin through fine tuning of HIF stabilization. FASEB J. 2011, 25, 1856–1864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinoshita, Y.; Arita, S.; Murazoe, H.; Kitamura, K.; Ashizuka, S.; Inagaki-Ohara, K. Subcutaneously administered adrenomedullin exerts a potent therapeutic effect in a murine model of ulcerative colitis. Hum. Cell. 2019, 32, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.; Fan, H.; Liu, X.; Tang, Q.; Zuo, D.; Yang, J. Adrenomedullin improves intestinal epithelial barrier function by downregulating myosin light chain phosphorylation in ulcerative colitis rats. Mol. Med. Rep. 2015, 12, 3615–3620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, Y.; Narumi, K.; Tsuji, S.; Tsubokawa, T.; Nakaya, M.A.; Wakayama, T.; Zuka, M.; Ohshima, T.; Yamagishi, M.; Okada, T. Impact of adrenomedullin on dextran sulfate sodium-induced inflammatory colitis in mice: Insights from in vitro and in vivo experimental studies. Int. J. Colorectal. Dis. 2011, 26, 1453–1462. [Google Scholar] [CrossRef]
- Hayakawa, H.; Hirata, Y.; Kakoki, M.; Suzuki, Y.; Nishimatsu, H.; Nagata, D.; Suzuki, E.; Kikuchi, K.; Nagano, T.; Kangawa, K.; et al. Role of nitric oxide-cGMP pathway in adrenomedullin-induced vasodilation in the rat. Hypertension 1999, 33, 689–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talero, E.; Sanchez-Fidalgo, S.; Villegas, I.; de la Lastra, C.A.; Illanes, M.; Motilva, V. Role of different inflammatory and tumor biomarkers in the development of ulcerative colitis-associated carcinogenesis. Inflamm. Bowel Dis. 2011, 17, 696–710. [Google Scholar] [CrossRef]
- Davis, R.B.; Kechele, D.O.; Blakeney, E.S.; Pawlak, J.B.; Caron, K.M. Lymphatic deletion of calcitonin receptor-like receptor exacerbates intestinal inflammation. JCI Insight 2017, 2, e92465. [Google Scholar] [CrossRef] [Green Version]
- Zudaire, E.; Portal-Núñez, S.; Cuttitta, F. The central role of adrenomedullin in host defense. J. Leukoc. Biol. 2006, 80, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Ashizuka, S.; Inatsu, H.; Inagaki-Ohara, K.; Kita, T.; Kitamura, K. Adrenomedullin as a Potential Therapeutic Agent for Inflammatory Bowel Disease. Curr. Protein Pept. Sci. 2013, 14, 246–255. [Google Scholar] [CrossRef]
- Liang, J.; Sha, S.M.; Wu, K.C. Role of the intestinal microbiota and fecal transplantation in inflammatory bowel diseases. J. Dig. Dis. 2014, 15, 641–646. [Google Scholar] [CrossRef]
- Ashizuka, S.; Inatsu, H.; Kita, T.; Kitamura, K. The First Clinical Pilot Study of Adrenomedullin Therapy in Refractory Ulcerative Colitis: The Initial Six Cases. Gastroenterology 2012, 142, S353. [Google Scholar] [CrossRef]
- Ashizuka, S.; Kita, T.; Inatsu, H.; Kitamura, K. Adrenomedullin: A Novel Therapy for Intractable Ulcerative Colitis. Inflamm. Bowel Dis. 2013, 19, 26–27. [Google Scholar] [CrossRef]
- Lainchbury, J.G.; Troughton, R.W.; Lewis, L.K.; Yandle, T.G.; Richards, A.M.; Nicholls, M.G. Hemodynamic, hormonal, and renal effects of short-term adrenomedullin infusion in healthy volunteers. J. Clin. Endocrinol. Metab. 2000, 85, 1016–1020. [Google Scholar] [CrossRef]
- Nagaya, N.; Goto, Y.; Satoh, T.; Sumida, H.; Kojima, S.; Miyatake, K.; Kangawa, K. Intravenous adrenomedullin in myocardial function and energy metabolism in patients after myocardial infarction. J. Cardiovas. Pharmacol. 2002, 39, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Troughton, R.W.; Lewis, L.K.; Yandle, T.G.; Richards, A.M.; Nicholls, M.G. Hemodynamic, hormone, and urinary effects of adrenomedullin infusion in essential hypertension. Hypertension 2000, 36, 588–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kataoka, Y.; Miyazaki, S.; Yasuda, S.; Nagaya, N.; Noguchi, T.; Yamada, N.; Morii, I.; Kawamura, A.; Doi, K.; Miyatake, K.; et al. The First Clinical Pilot Study of Intravenous Adrenomedullin Administration in Patients With Acute Myocardial Infarction. J. Cardiovasc. Pharmacol. 2010, 56, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Nagata, S.; Yamasaki, M.; Kitamura, K. Anti-Inflammatory Effects of PEGylated Human Adrenomedullin in a Mouse DSS-Induced Colitis Model. Drug Dev. Res. 2017, 78, 129–134. [Google Scholar] [CrossRef]
- Martinez, A.; Julian, M.; Bregonzio, C.; Notari, L.; Moody, T.W.; Cuttitta, F. Identification of vasoactive nonpeptidic positive and negative modulators of adrenomedullin using a neutralizing antibody-based screening strategy. Endocrinology 2004, 145, 3858–3865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, H.; You, N.; Chen, H.; Teng, Y.S.; Liu, Y.G.; Lv, Y.P.; Mao, F.Y.; Cheng, P.; Chen, W.; Zhao, Z.; et al. Helicobacter pylori-induced adrenomedullin modulates IFN-γ-producing T-cell responses and contributes to gastritis. Cell Death Dis. 2020, 11, 189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.Y.; Park, W.D.; Lee, S.; Park, J.H. Adrenomedullin is involved in the progression of colonic adenocarcinoma. Mol. Med. Rep. 2012, 6, 1030–1034. [Google Scholar] [CrossRef]
- Kaafarani, I.; Fernandez-Sauze, S.; Berenguer, C.; Chinot, O.; Delfino, C.; Dussert, C.; Metellus, P.; Boudouresque, F.; Mabrouk, K.; Grisoli, F.; et al. Targeting adrenomedullin receptors with systemic delivery of neutralizing antibodies inhibits tumor angiogenesis and suppresses growth of human tumor xenografts in mice. FASEB J. 2009, 23, 3424–3435. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Herrero, S.; Martínez, A. Adrenomedullin: Not Just Another Gastrointestinal Peptide. Biomolecules 2022, 12, 156. https://doi.org/10.3390/biom12020156
Martínez-Herrero S, Martínez A. Adrenomedullin: Not Just Another Gastrointestinal Peptide. Biomolecules. 2022; 12(2):156. https://doi.org/10.3390/biom12020156
Chicago/Turabian StyleMartínez-Herrero, Sonia, and Alfredo Martínez. 2022. "Adrenomedullin: Not Just Another Gastrointestinal Peptide" Biomolecules 12, no. 2: 156. https://doi.org/10.3390/biom12020156
APA StyleMartínez-Herrero, S., & Martínez, A. (2022). Adrenomedullin: Not Just Another Gastrointestinal Peptide. Biomolecules, 12(2), 156. https://doi.org/10.3390/biom12020156






