Switching Roles: Beneficial Effects of Adipose Tissue-Derived Mesenchymal Stem Cells on Microglia and Their Implication in Neurodegenerative Diseases
Abstract
:1. Introduction
2. Role of Microglia in Health and Disease
3. Origin and Characteristics of Adipose Tissue-Derived Mesenchymal Stem Cells (ASC)
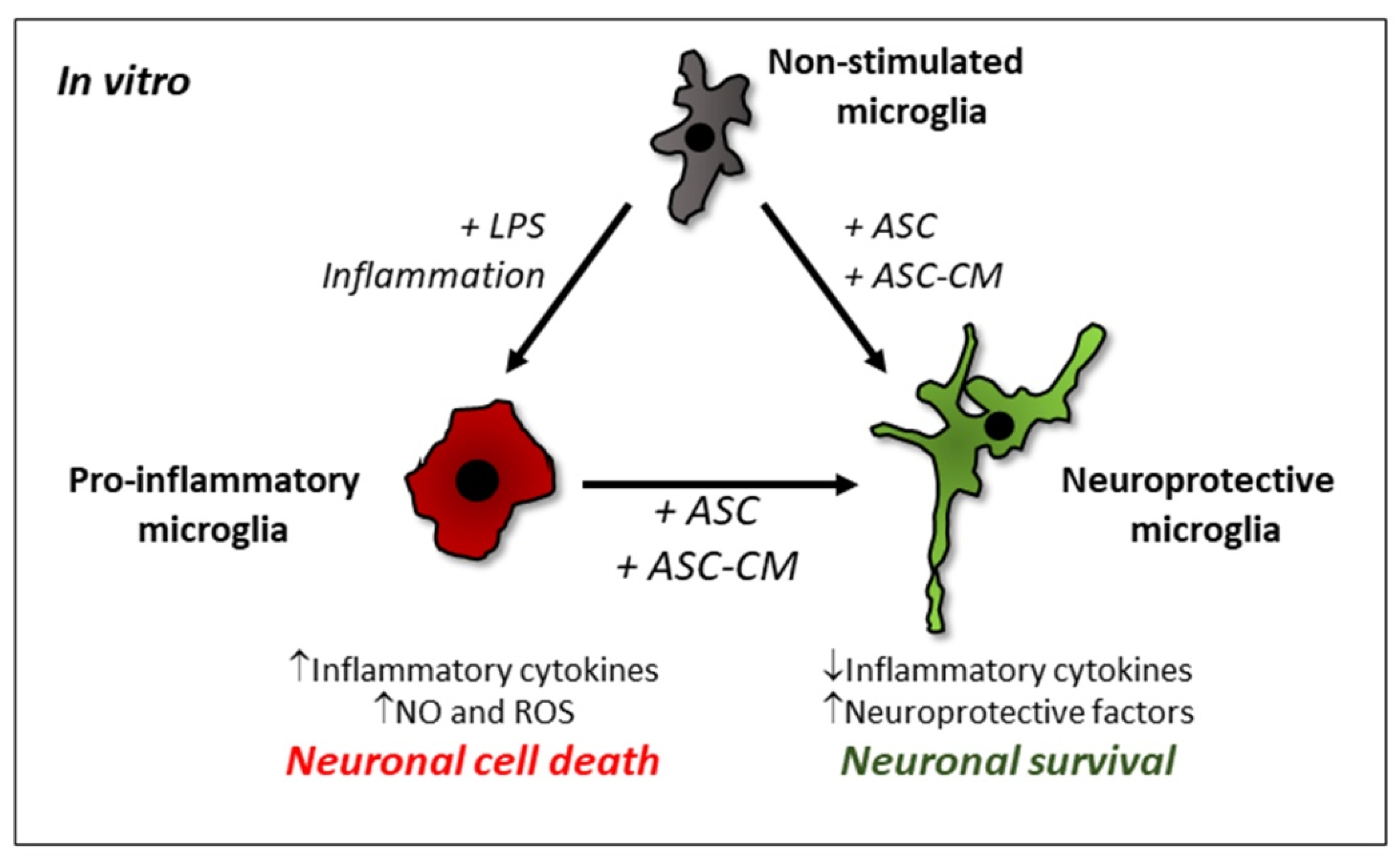
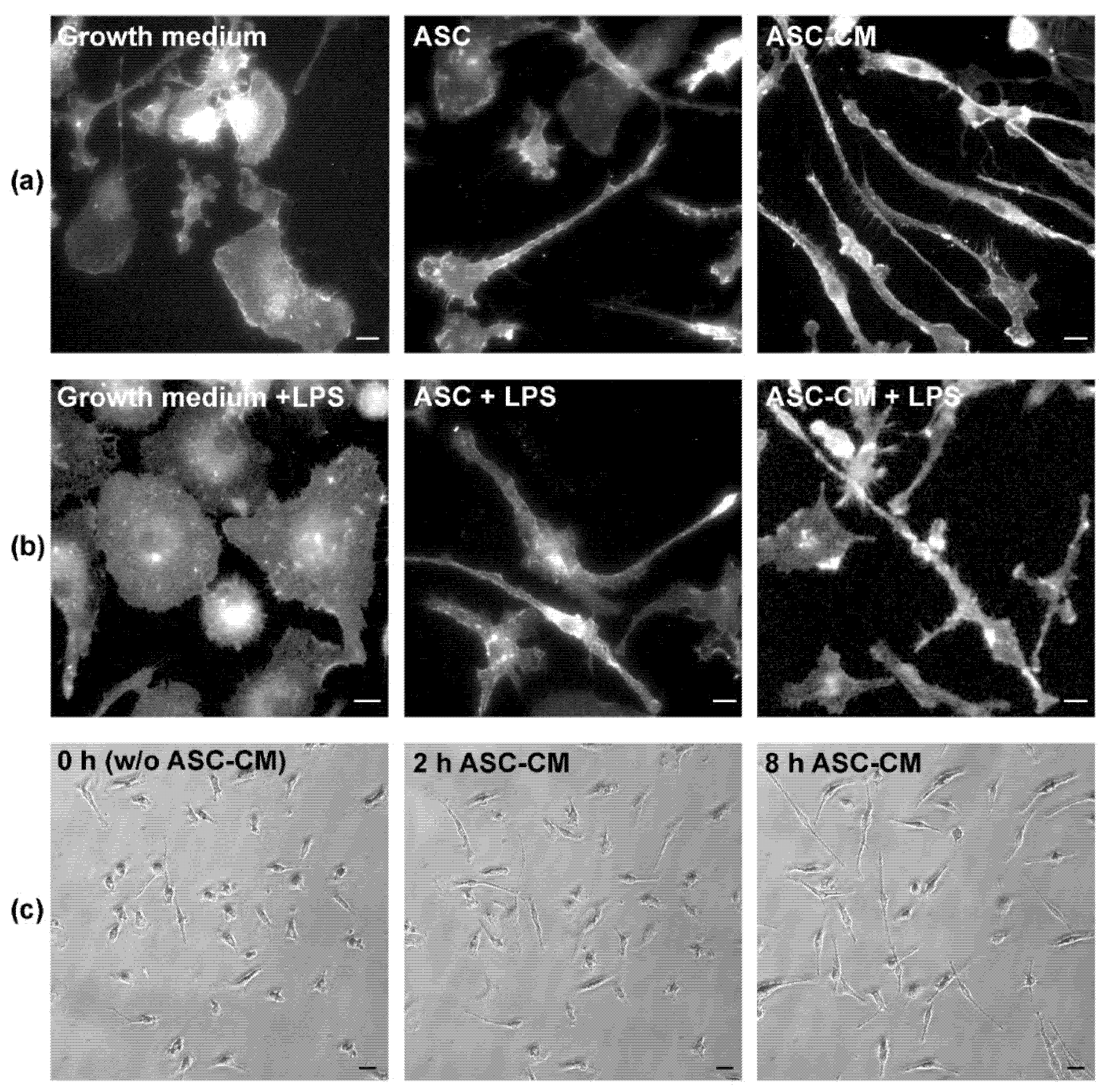
4. Effect of ASC on Microglia Phenotype In Vitro
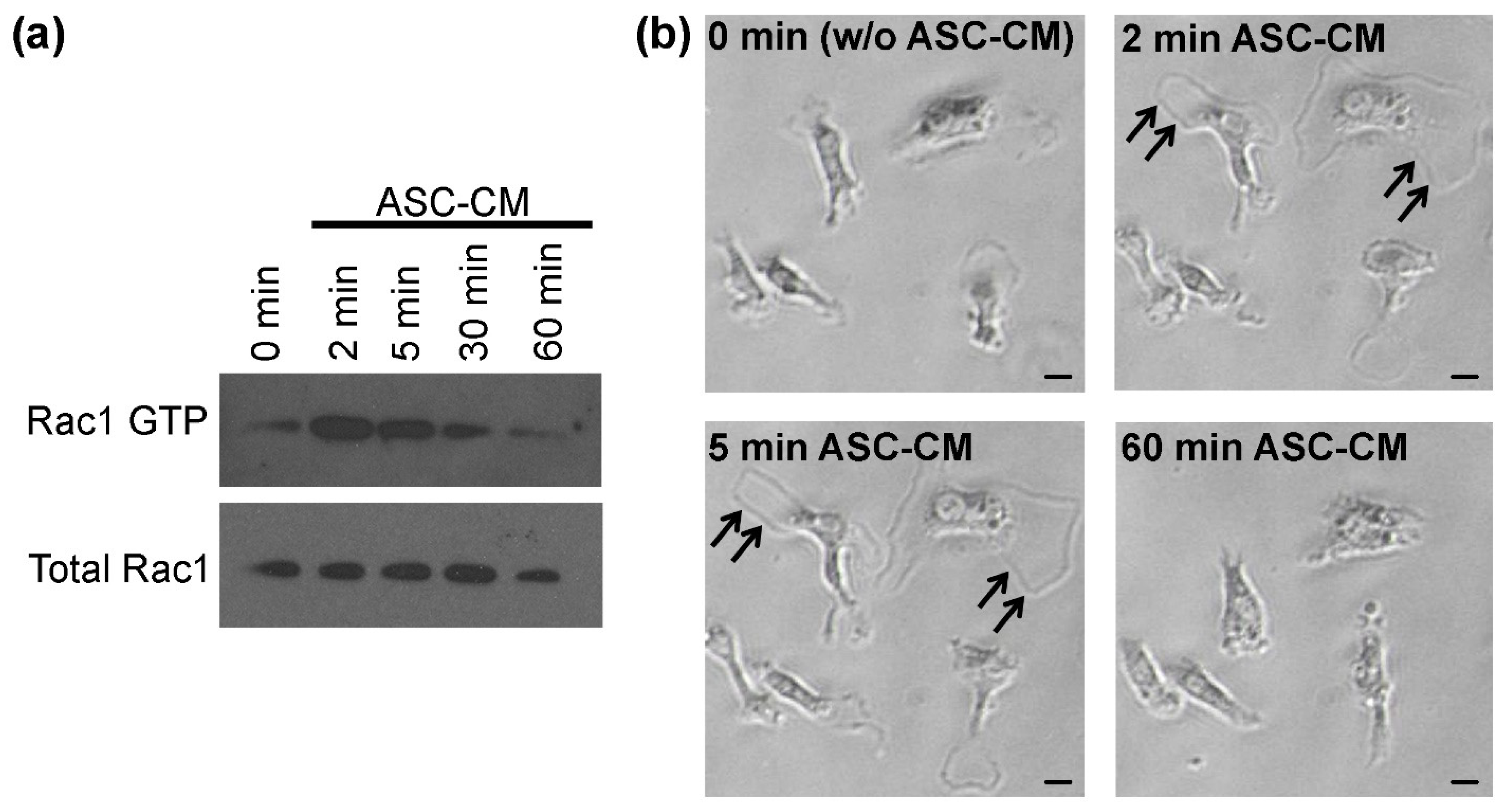
5. ASC-Induced Activation of the PI3K/Akt/RhoGTPase Signaling Pathway in Microglia
6. Application of ASC In Vivo
6.1. Application of ASC In Vivo by Cell Transplantation and ASC-CM Injection
6.2. Application of ASC-Generated Extracellular Vesicles (EV) In Vivo
7. Conclusions and Outlook
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADNP | activity-dependent neurotrophic protein |
| Aβ | β-amyloid peptide |
| AD | Alzheimer´s disease |
| AIS | acute ischemic stroke |
| APP | Alzheimer Aβ precursor protein |
| ASC | adipose tissue-derived mesenchymal stem cells |
| ASC-CCM | concentrated conditioned medium from ASC |
| ASC-CM | conditioned medium from ASC |
| ASC-EV | extracellular vesicles from ASC |
| BDNF | brain-derived neurotrophic factor |
| BM-MSC | bone marrow-derived MSC |
| CM | conditioned medium |
| CMS | chronic mild stress |
| CNS | central nervous system |
| EAE | experimental autoimmune encephalomyelitis |
| EMA | European Medicines Agency |
| EV | extracellular vesicles |
| FGF-2 | fibroblast-growth factor-2 |
| GCI | global cerebral ischemia |
| GMP | good manufacturing practices |
| HLA | human leucocyte antigen |
| HO-1 | heme oxygenase-1 |
| IFN-γ | interferon-γ |
| IL | Interleukin |
| iNOS | inducible nitric oxide synthase |
| LPS | lipopolysaccharide |
| MS | multiple sclerosis |
| MSC | mesenchymal stem cells |
| mTBI | mild traumatic brain injury |
| NK | natural killer cells |
| NO | nitric oxide |
| PD | Parkinson´s disease |
| PS1 | Presilin1 |
| PI3K | phosphoinositol-3-kinase |
| PKB | protein kinase B/Akt |
| PIP3 | phophoinositol-3-phosphate |
| SCI | spinal cord injury |
| SN | substantia nigra |
| TBI | traumatic brain injury |
| TH | tyrosine hydroxylase |
| TMEV | MS model of Theiler’s murine encephalomyelitis virus |
| TNF-α | tumor necrosis factor-α |
| RhoGEF | Rho guanine nucleotide exchange factor |
| VIP | vasoactive intestinal peptide |
References
- Heneka, M.T.; Golenbock, D.T.; Latz, E. Innate immunity in Alzheimer’s disease. Nat. Immunol. 2015, 16, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.E.; Lang, A.E. Parkinson disease. Nat. Rev. Dis. Primers 2017, 3, 17013. [Google Scholar] [CrossRef] [PubMed]
- Block, M.L.; Hong, J.S. Microglia and inflammation-mediated neurodegeneration: Multiple triggers with a common mechanism. Prog. Neurobiol. 2005, 76, 77–98. [Google Scholar] [CrossRef] [PubMed]
- Block, M.L.; Zecca, L.; Hong, J.S. Microglia-mediated neurotoxicity: Uncovering the molecular mechanisms. Nat. Rev. Neurosci. 2007, 8, 57–69. [Google Scholar] [CrossRef]
- Whitney, N.P.; Eidem, T.M.; Peng, H.; Huang, Y.; Zheng, J.C. Inflammation mediates varying effects in neurogenesis: Relevance to the pathogenesis of brain injury and neurodegenerative disorders. J. Neurochem. 2009, 108, 1343–1359. [Google Scholar] [CrossRef]
- Anderson, P.; Gonzalez-Rey, E.; O’Valle, F.; Martin, F.; Oliver, F.J.; Delgado, M. Allogeneic Adipose-Derived Mesenchymal Stromal Cells Ameliorate Experimental Autoimmune Encephalomyelitis by Regulating Self-Reactive T Cell Responses and Dendritic Cell Function. Stem Cells Int. 2017, 2017, 2389753. [Google Scholar] [CrossRef]
- Huang, X.; Fei, G.Q.; Liu, W.J.; Ding, J.; Wang, Y.; Wang, H.; Ji, J.L.; Wang, X. Adipose-derived mesenchymal stem cells protect against CMS-induced depression-like behaviors in mice via regulating the Nrf2/HO-1 and TLR4/NF-kappaB signaling pathways. Acta Pharmacol. Sin. 2020, 41, 612–619. [Google Scholar] [CrossRef]
- Jha, K.A.; Pentecost, M.; Lenin, R.; Klaic, L.; Elshaer, S.L.; Gentry, J.; Russell, J.M.; Beland, A.; Reiner, A.; Jotterand, V.; et al. Concentrated Conditioned Media from Adipose Tissue Derived Mesenchymal Stem Cells Mitigates Visual Deficits and Retinal Inflammation Following Mild Traumatic Brain Injury. Int. J. Mol. Sci. 2018, 19, 2016. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.J.; Park, H.J.; Lee, G.; Bang, O.Y.; Ahn, Y.H.; Joe, E.; Kim, H.O.; Lee, P.H. Neuroprotective effects of human mesenchymal stem cells on dopaminergic neurons through anti-inflammatory action. Glia 2009, 57, 13–23. [Google Scholar] [CrossRef]
- Zanier, E.R.; Pischiutta, F.; Riganti, L.; Marchesi, F.; Turola, E.; Fumagalli, S.; Perego, C.; Parotto, E.; Vinci, P.; Veglianese, P.; et al. Bone marrow mesenchymal stromal cells drive protective M2 microglia polarization after brain trauma. Neurotherapeutics 2014, 11, 679–695. [Google Scholar] [CrossRef] [Green Version]
- Shariati, A.; Nemati, R.; Sadeghipour, Y.; Yaghoubi, Y.; Baghbani, R.; Javidi, K.; Zamani, M.; Hassanzadeh, A. Mesenchymal stromal cells (MSCs) for neurodegenerative disease: A promising frontier. Eur. J. Cell Biol. 2020, 99, 151097. [Google Scholar] [CrossRef] [PubMed]
- Abumaree, M.; Al Jumah, M.; Pace, R.A.; Kalionis, B. Immunosuppressive properties of mesenchymal stem cells. Stem Cell Rev. Rep. 2012, 8, 375–392. [Google Scholar] [CrossRef] [PubMed]
- Friedenstein, A.J.; Chailakhjan, R.K.; Lalykina, K.S. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970, 3, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Nimmerjahn, A.; Kirchhoff, F.; Helmchen, F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 2005, 308, 1314–1318. [Google Scholar] [CrossRef] [Green Version]
- Kettenmann, H.; Hanisch, U.K.; Noda, M.; Verkhratsky, A. Physiology of microglia. Physiol. Rev. 2011, 91, 461–553. [Google Scholar] [CrossRef]
- Wolf, S.A.; Boddeke, H.W.; Kettenmann, H. Microglia in Physiology and Disease. Annu. Rev. Physiol. 2017, 79, 619–643. [Google Scholar] [CrossRef]
- Hanisch, U.K.; Kettenmann, H. Microglia: Active sensor and versatile effector cells in the normal and pathologic brain. Nat. Neurosci. 2007, 10, 1387–1394. [Google Scholar] [CrossRef]
- Perry, V.H.; Nicoll, J.A.; Holmes, C. Microglia in neurodegenerative disease. Nat. Rev. Neurol. 2010, 6, 193–201. [Google Scholar] [CrossRef]
- Holtman, I.R.; Noback, M.; Bijlsma, M.; Duong, K.N.; van der Geest, M.A.; Ketelaars, P.T.; Brouwer, N.; Vainchtein, I.D.; Eggen, B.J.; Boddeke, H.W. Glia Open Access Database (GOAD): A comprehensive gene expression encyclopedia of glia cells in health and disease. Glia 2015, 63, 1495–1506. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, H.; Sun, G.; Zhang, J.; Edwards, N.J.; Aronowski, J. Neuronal Interleukin-4 as a Modulator of Microglial Pathways and Ischemic Brain Damage. J. Neurosci. 2015, 35, 11281–11291. [Google Scholar] [CrossRef] [Green Version]
- Giunti, D.; Parodi, B.; Usai, C.; Vergani, L.; Casazza, S.; Bruzzone, S.; Mancardi, G.; Uccelli, A. Mesenchymal stem cells shape microglia effector functions through the release of CX3CL1. Stem Cells 2012, 30, 2044–2053. [Google Scholar] [CrossRef] [PubMed]
- Neubrand, V.E.; Pedreno, M.; Caro, M.; Forte-Lago, I.; Delgado, M.; Gonzalez-Rey, E. Mesenchymal stem cells induce the ramification of microglia via the small RhoGTPases Cdc42 and Rac1. Glia 2014, 62, 1932–1942. [Google Scholar] [CrossRef]
- Munder, M. Arginase: An emerging key player in the mammalian immune system. Br. J. Pharmacol. 2009, 158, 638–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cherry, J.D.; Olschowka, J.A.; O’Banion, M.K. Neuroinflammation and M2 microglia: The good, the bad, and the inflamed. J. Neuroinflammation 2014, 11, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Galipeau, J.; Sensebe, L. Mesenchymal Stromal Cells: Clinical Challenges and Therapeutic Opportunities. Cell Stem Cell 2018, 22, 824–833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saldanha-Araujo, F.; Melgaco Garcez, E.; Silva-Carvalho, A.E.; Carvalho, J.L. Mesenchymal Stem Cells: A New Piece in the Puzzle of COVID-19 Treatment. Front. Immunol. 2020, 11, 1563. [Google Scholar] [CrossRef]
- Gonzalez-Rey, E.; Anderson, P.; Gonzalez, M.A.; Rico, L.; Buscher, D.; Delgado, M. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut 2009, 58, 929–939. [Google Scholar] [CrossRef]
- Gonzalez, M.A.; Gonzalez-Rey, E.; Rico, L.; Buscher, D.; Delgado, M. Treatment of experimental arthritis by inducing immune tolerance with human adipose-derived mesenchymal stem cells. Arthritis Rheum. 2009, 60, 1006–1019. [Google Scholar] [CrossRef]
- Gonzalez-Rey, E.; Gonzalez, M.A.; Varela, N.; O’Valle, F.; Hernandez-Cortes, P.; Rico, L.; Buscher, D.; Delgado, M. Human adipose-derived mesenchymal stem cells reduce inflammatory and T cell responses and induce regulatory T cells in vitro in rheumatoid arthritis. Ann. Rheum. Dis. 2010, 69, 241–248. [Google Scholar] [CrossRef] [Green Version]
- Anderson, P.; Souza-Moreira, L.; Morell, M.; Caro, M.; O’Valle, F.; Gonzalez-Rey, E.; Delgado, M. Adipose-derived mesenchymal stromal cells induce immunomodulatory macrophages which protect from experimental colitis and sepsis. Gut 2013, 62, 1131–1141. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Francos, S.; Eiro, N.; Gonzalez-Galiano, N.; Vizoso, F.J. Mesenchymal Stem Cell-Based Therapy as an Alternative to the Treatment of Acute Respiratory Distress Syndrome: Current Evidence and Future Perspectives. Int. J. Mol. Sci. 2021, 22, 7850. [Google Scholar] [CrossRef] [PubMed]
- Miceli, V.; Bulati, M.; Iannolo, G.; Zito, G.; Gallo, A.; Conaldi, P.G. Therapeutic Properties of Mesenchymal Stromal/Stem Cells: The Need of Cell Priming for Cell-Free Therapies in Regenerative Medicine. Int. J. Mol. Sci. 2021, 22, 763. [Google Scholar] [CrossRef]
- Zriek, F.; Di Battista, J.A.; Alaaeddine, N. Mesenchymal Stromal Cell Secretome: Immunomodulation, Tissue Repair and Effects on Neurodegenerative Conditions. Curr. Stem Cell Res. Ther. 2021, 16, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Dabrowska, S.; Andrzejewska, A.; Janowski, M.; Lukomska, B. Immunomodulatory and Regenerative Effects of Mesenchymal Stem Cells and Extracellular Vesicles: Therapeutic Outlook for Inflammatory and Degenerative Diseases. Front. Immunol. 2020, 11, 591065. [Google Scholar] [CrossRef] [PubMed]
- Uccelli, A.; Prockop, D.J. Why should mesenchymal stem cells (MSCs) cure autoimmune diseases? Curr. Opin. Immunol. 2010, 22, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, R.; Yan, K.; Chen, F.; Huang, W.; Lv, B.; Sun, C.; Xu, L.; Li, F.; Jiang, X. Mesenchymal stem cells inhibit lipopolysaccharide-induced inflammatory responses of BV2 microglial cells through TSG-6. J. Neuroinflammation 2014, 11, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ooi, Y.Y.; Ramasamy, R.; Rahmat, Z.; Subramaiam, H.; Tan, S.W.; Abdullah, M.; Israf, D.A.; Vidyadaran, S. Bone marrow-derived mesenchymal stem cells modulate BV2 microglia responses to lipopolysaccharide. Int. Immunopharmacol. 2010, 10, 1532–1540. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, C.; Chi, S.; Xu, Y.; Teng, J.; Wang, H.; Song, Y.; Zhao, R. Effects of human marrow stromal cells on activation of microglial cells and production of inflammatory factors induced by lipopolysaccharide. Brain Res. 2009, 1269, 23–30. [Google Scholar] [CrossRef]
- Raffaele, S.; Gelosa, P.; Bonfanti, E.; Lombardi, M.; Castiglioni, L.; Cimino, M.; Sironi, L.; Abbracchio, M.P.; Verderio, C.; Fumagalli, M. Microglial vesicles improve post-stroke recovery by preventing immune cell senescence and favoring oligodendrogenesis. Mol. Ther. 2021, 29, 1439–1458. [Google Scholar] [CrossRef]
- Krabbe, G.; Halle, A.; Matyash, V.; Rinnenthal, J.L.; Eom, G.D.; Bernhardt, U.; Miller, K.R.; Prokop, S.; Kettenmann, H.; Heppner, F.L. Functional impairment of microglia coincides with Beta-amyloid deposition in mice with Alzheimer-like pathology. PLoS ONE 2013, 8, e60921. [Google Scholar] [CrossRef] [PubMed]
- Jha, K.A.; Pentecost, M.; Lenin, R.; Gentry, J.; Klaic, L.; Del Mar, N.; Reiner, A.; Yang, C.H.; Pfeffer, L.M.; Sohl, N.; et al. TSG-6 in conditioned media from adipose mesenchymal stem cells protects against visual deficits in mild traumatic brain injury model through neurovascular modulation. Stem Cell Res. Ther. 2019, 10, 318. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Chen, B.Y.; Sun, X.L.; Luo, Z.J.; Rao, Z.R.; Wang, J.J.; Chen, L.W. LPS-induced proNGF synthesis and release in the N9 and BV2 microglial cells: A new pathway underling microglial toxicity in neuroinflammation. PLoS ONE 2013, 8, e73768. [Google Scholar] [CrossRef] [PubMed]
- Consoli, V.; Sorrenti, V.; Grosso, S.; Vanella, L. Heme Oxygenase-1 Signaling and Redox Homeostasis in Physiopathological Conditions. Biomolecules 2021, 11, 589. [Google Scholar] [CrossRef] [PubMed]
- Ginhoux, F.; Greter, M.; Leboeuf, M.; Nandi, S.; See, P.; Gokhan, S.; Mehler, M.F.; Conway, S.J.; Ng, L.G.; Stanley, E.R.; et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 2010, 330, 841–845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Szretter, K.J.; Vermi, W.; Gilfillan, S.; Rossini, C.; Cella, M.; Barrow, A.D.; Diamond, M.S.; Colonna, M. IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nat. Immunol. 2012, 13, 753–760. [Google Scholar] [CrossRef] [Green Version]
- Bourette, R.P.; Rohrschneider, L.R. Early events in M-CSF receptor signaling. Growth Factors 2000, 17, 155–166. [Google Scholar] [CrossRef]
- Pixley, F.J.; Stanley, E.R. CSF-1 regulation of the wandering macrophage: Complexity in action. Trends Cell Biol. 2004, 14, 628–638. [Google Scholar] [CrossRef]
- Ferguson, K.M.; Kavran, J.M.; Sankaran, V.G.; Fournier, E.; Isakoff, S.J.; Skolnik, E.Y.; Lemmon, M.A. Structural basis for discrimination of 3-phosphoinositides by pleckstrin homology domains. Mol. Cell 2000, 6, 373–384. [Google Scholar] [CrossRef]
- Rossman, K.L.; Der, C.J.; Sondek, J. GEF means go: Turning on RHO GTPases with guanine nucleotide-exchange factors. Nat. Rev. Mol. Cell Biol. 2005, 6, 167–180. [Google Scholar] [CrossRef]
- Faccio, R.; Takeshita, S.; Colaianni, G.; Chappel, J.; Zallone, A.; Teitelbaum, S.L.; Ross, F.P. M-CSF regulates the cytoskeleton via recruitment of a multimeric signaling complex to c-Fms Tyr-559/697/721. J. Biol. Chem. 2007, 282, 18991–18999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nobes, C.D.; Hall, A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell 1995, 81, 53–62. [Google Scholar] [CrossRef] [Green Version]
- Pixley, F.J. Macrophage Migration and Its Regulation by CSF-1. Int J. Cell Biol. 2012, 2012, 501962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neubrand, V.E.; Forte-Lago, I.; Caro, M.; Delgado, M. The atypical RhoGTPase RhoE/Rnd3 is a key molecule to acquire a neuroprotective phenotype in microglia. J. Neuroinflammation 2018, 15, 343. [Google Scholar] [CrossRef]
- Mukai, T.; Di Martino, E.; Tsuji, S.; Blomgren, K.; Nagamura-Inoue, T.; Aden, U. Umbilical cord-derived mesenchymal stromal cells immunomodulate and restore actin dynamics and phagocytosis of LPS-activated microglia via PI3K/Akt/Rho GTPase pathway. Cell Death Discov. 2021, 7, 46. [Google Scholar] [CrossRef]
- Constantin, G.; Marconi, S.; Rossi, B.; Angiari, S.; Calderan, L.; Anghileri, E.; Gini, B.; Bach, S.D.; Martinello, M.; Bifari, F.; et al. Adipose-derived mesenchymal stem cells ameliorate chronic experimental autoimmune encephalomyelitis. Stem Cells 2009, 27, 2624–2635. [Google Scholar] [CrossRef]
- Xu, C.; Diao, Y.F.; Wang, J.; Liang, J.; Xu, H.H.; Zhao, M.L.; Zheng, B.; Luan, Z.; Wang, J.J.; Yang, X.P.; et al. Intravenously Infusing the Secretome of Adipose-Derived Mesenchymal Stem Cells Ameliorates Neuroinflammation and Neurological Functioning After Traumatic Brain Injury. Stem Cells Dev. 2020, 29, 222–234. [Google Scholar] [CrossRef] [Green Version]
- Kosloski, L.M.; Kosmacek, E.A.; Olson, K.E.; Mosley, R.L.; Gendelman, H.E. GM-CSF induces neuroprotective and anti-inflammatory responses in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine intoxicated mice. J. Neuroimmunol. 2013, 265, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Boissier, P.; Huynh-Do, U. The guanine nucleotide exchange factor Tiam1: A Janus-faced molecule in cellular signaling. Cell Signal. 2014, 26, 483–491. [Google Scholar] [CrossRef]
- Mukai, T.; Sei, K.; Nagamura-Inoue, T. Mesenchymal Stromal Cells Perspective: New Potential Therapeutic for the Treatment of Neurological Diseases. Pharmaceutics 2021, 13, 1159. [Google Scholar] [CrossRef]
- McCoy, M.K.; Martinez, T.N.; Ruhn, K.A.; Wrage, P.C.; Keefer, E.W.; Botterman, B.R.; Tansey, K.E.; Tansey, M.G. Autologous transplants of Adipose-Derived Adult Stromal (ADAS) cells afford dopaminergic neuroprotection in a model of Parkinson’s disease. Exp. Neurol. 2008, 210, 14–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, T.; Gong, K.; Ao, Q.; Yan, Y.; Song, B.; Huang, H.; Zhang, X.; Gong, Y. Intracerebral transplantation of adipose-derived mesenchymal stem cells alternatively activates microglia and ameliorates neuropathological deficits in Alzheimer’s disease mice. Cell Transplant. 2013, 22 Suppl 1, S113–S126. [Google Scholar] [CrossRef] [Green Version]
- Gordon, S. Alternative activation of macrophages. Nat. Rev. Immunol. 2003, 3, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Cherry, J.D.; Olschowka, J.A.; O’Banion, M.K. Are “resting” microglia more “m2”? Front. Immunol. 2014, 5, 594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munoz, M.F.; Arguelles, S.; Medina, R.; Cano, M.; Ayala, A. Adipose-derived stem cells decreased microglia activation and protected dopaminergic loss in rat lipopolysaccharide model. J. Cell Physiol. 2019, 234, 13762–13772. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Huang, M.; Zheng, M.; Dai, C.; Song, Q.; Zhang, Q.; Li, Q.; Gu, X.; Chen, H.; Jiang, G.; et al. ADSCs-derived extracellular vesicles alleviate neuronal damage, promote neurogenesis and rescue memory loss in mice with Alzheimer’s disease. J. Control. Release 2020, 327, 688–702. [Google Scholar] [CrossRef] [PubMed]
- Mehrabadi, S.; Motevaseli, E.; Sadr, S.S.; Moradbeygi, K. Hypoxic-conditioned medium from adipose tissue mesenchymal stem cells improved neuroinflammation through alternation of toll like receptor (TLR) 2 and TLR4 expression in model of Alzheimer’s disease rats. Behav. Brain Res. 2020, 379, 112362. [Google Scholar] [CrossRef]
- Chen, K.H.; Chen, C.H.; Wallace, C.G.; Yuen, C.M.; Kao, G.S.; Chen, Y.L.; Shao, P.L.; Chen, Y.L.; Chai, H.T.; Lin, K.C.; et al. Intravenous administration of xenogenic adipose-derived mesenchymal stem cells (ADMSC) and ADMSC-derived exosomes markedly reduced brain infarct volume and preserved neurological function in rat after acute ischemic stroke. Oncotarget 2016, 7, 74537–74556. [Google Scholar] [CrossRef] [Green Version]
- Chung, T.N.; Kim, J.H.; Choi, B.Y.; Chung, S.P.; Kwon, S.W.; Suh, S.W. Adipose-derived mesenchymal stem cells reduce neuronal death after transient global cerebral ischemia through prevention of blood-brain barrier disruption and endothelial damage. Stem Cells Transl. Med. 2015, 4, 178–185. [Google Scholar] [CrossRef]
- Chung, T.N.; Kim, J.H.; Choi, B.Y.; Jeong, J.Y.; Chung, S.P.; Kwon, S.W.; Suh, S.W. Effect of Adipose-Derived Mesenchymal Stem Cell Administration and Mild Hypothermia Induction on Delayed Neuronal Death After Transient Global Cerebral Ischemia. Crit. Care Med. 2017, 45, e508–e515. [Google Scholar] [CrossRef]
- Otero-Ortega, L.; Laso-Garcia, F.; Gomez-de Frutos, M.D.; Rodriguez-Frutos, B.; Pascual-Guerra, J.; Fuentes, B.; Diez-Tejedor, E.; Gutierrez-Fernandez, M. White Matter Repair After Extracellular Vesicles Administration in an Experimental Animal Model of Subcortical Stroke. Sci. Rep. 2017, 7, 44433. [Google Scholar] [PubMed] [Green Version]
- Yu, Z.; Wenyan, T.; Xuewen, S.; Baixiang, D.; Qian, W.; Zhaoyan, W.; Yinxiang, Y.; Suqing, Q.; Zuo, L. Immunological effects of the intraparenchymal administration of allogeneic and autologous adipose-derived mesenchymal stem cells after the acute phase of middle cerebral artery occlusion in rats. J. Transl. Med. 2018, 16, 339. [Google Scholar] [CrossRef]
- Payne, N.L.; Dantanarayana, A.; Sun, G.; Moussa, L.; Caine, S.; McDonald, C.; Herszfeld, D.; Bernard, C.C.; Siatskas, C. Early intervention with gene-modified mesenchymal stem cells overexpressing interleukin-4 enhances anti-inflammatory responses and functional recovery in experimental autoimmune demyelination. Cell Adh. Migr. 2012, 6, 179–189. [Google Scholar] [CrossRef] [Green Version]
- Cobo, M.; Anderson, P.; Benabdellah, K.; Toscano, M.G.; Munoz, P.; Garcia-Perez, A.; Gutierrez, I.; Delgado, M.; Martin, F. Mesenchymal stem cells expressing vasoactive intestinal peptide ameliorate symptoms in a model of chronic multiple sclerosis. Cell Transplant. 2013, 22, 839–854. [Google Scholar] [CrossRef] [Green Version]
- Laso-Garcia, F.; Ramos-Cejudo, J.; Carrillo-Salinas, F.J.; Otero-Ortega, L.; Feliu, A.; Gomez-de Frutos, M.; Mecha, M.; Diez-Tejedor, E.; Guaza, C.; Gutierrez-Fernandez, M. Therapeutic potential of extracellular vesicles derived from human mesenchymal stem cells in a model of progressive multiple sclerosis. PLoS ONE 2018, 13, e0202590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bae, J.S.; Carter, J.E.; Jin, H.K. Adipose tissue-derived stem cells rescue Purkinje neurons and alleviate inflammatory responses in Niemann-Pick disease type C mice. Cell Tissue Res. 2010, 340, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Berg, J.; Roch, M.; Altschuler, J.; Winter, C.; Schwerk, A.; Kurtz, A.; Steiner, B. Human adipose-derived mesenchymal stem cells improve motor functions and are neuroprotective in the 6-hydroxydopamine-rat model for Parkinson’s disease when cultured in monolayer cultures but suppress hippocampal neurogenesis and hippocampal memory function when cultured in spheroids. Stem Cell Rev. Rep. 2015, 11, 133–149. [Google Scholar] [PubMed]
- Schwerk, A.; Altschuler, J.; Roch, M.; Gossen, M.; Winter, C.; Berg, J.; Kurtz, A.; Steiner, B. Human adipose-derived mesenchymal stromal cells increase endogenous neurogenesis in the rat subventricular zone acutely after 6-hydroxydopamine lesioning. Cytotherapy 2015, 17, 199–214. [Google Scholar] [CrossRef]
- Shao, M.; Jin, M.; Xu, S.; Zheng, C.; Zhu, W.; Ma, X.; Lv, F. Exosomes from Long Noncoding RNA-Gm37494-ADSCs Repair Spinal Cord Injury via Shifting Microglial M1/M2 Polarization. Inflammation 2020, 43, 1536–1547. [Google Scholar] [CrossRef]
- Zhou, Z.; Tian, X.; Mo, B.; Xu, H.; Zhang, L.; Huang, L.; Yao, S.; Huang, Z.; Wang, Y.; Xie, H.; et al. Adipose mesenchymal stem cell transplantation alleviates spinal cord injury-induced neuroinflammation partly by suppressing the Jagged1/Notch pathway. Stem Cell Res. Ther. 2020, 11, 212. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Ma, B.; Li, N.; Wang, S.; Sun, Z.; Xue, C.; Han, Q.; Wei, J.; Zhao, R.C. MSC-derived exosomes promote recovery from traumatic brain injury via microglia/macrophages in rat. Aging 2020, 12, 18274–18296. [Google Scholar] [CrossRef]
- Baglio, S.R.; Rooijers, K.; Koppers-Lalic, D.; Verweij, F.J.; Perez Lanzon, M.; Zini, N.; Naaijkens, B.; Perut, F.; Niessen, H.W.; Baldini, N.; et al. Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res. Ther. 2015, 6, 127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valadi, H.; Ekstrom, K.; Bossios, A.; Sjostrand, M.; Lee, J.J.; Lotvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez, M.; Avila, J.; Hernandez, F. Propagation of Tau via Extracellular Vesicles. Front. Neurosci. 2019, 13, 698. [Google Scholar] [CrossRef] [PubMed]
- Aires, I.D.; Ribeiro-Rodrigues, T.; Boia, R.; Ferreira-Rodrigues, M.; Girao, H.; Ambrosio, A.F.; Santiago, A.R. Microglial Extracellular Vesicles as Vehicles for Neurodegeneration Spreading. Biomolecules 2021, 11, 770. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, A.; Serpe, C.; Chece, G.; Nigro, V.; Sarra, A.; Ruzicka, B.; Relucenti, M.; Familiari, G.; Ruocco, G.; Pascucci, G.R.; et al. Microglia-Derived Microvesicles Affect Microglia Phenotype in Glioma. Front. Cell Neurosci. 2019, 13, 41. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Li, Z.; He, T.; Qu, M.; Jiang, L.; Li, W.; Shi, X.; Pan, J.; Zhang, L.; Wang, Y.; et al. M2 microglia-derived exosomes protect the mouse brain from ischemia-reperfusion injury via exosomal miR-124. Theranostics 2019, 9, 2910–2923. [Google Scholar] [CrossRef]
- Guo, M.; Hao, Y.; Feng, Y.; Li, H.; Mao, Y.; Dong, Q.; Cui, M. Microglial Exosomes in Neurodegenerative Disease. Front. Mol. Neurosci. 2021, 14, 630808. [Google Scholar] [CrossRef]
- Lombardi, M.; Parolisi, R.; Scaroni, F.; Bonfanti, E.; Gualerzi, A.; Gabrielli, M.; Kerlero de Rosbo, N.; Uccelli, A.; Giussani, P.; Viani, P.; et al. Detrimental and protective action of microglial extracellular vesicles on myelin lesions: Astrocyte involvement in remyelination failure. Acta Neuropathol. 2019, 138, 987–1012. [Google Scholar] [CrossRef] [Green Version]
- Casella, G.; Colombo, F.; Finardi, A.; Descamps, H.; Ill-Raga, G.; Spinelli, A.; Podini, P.; Bastoni, M.; Martino, G.; Muzio, L.; et al. Extracellular Vesicles Containing IL-4 Modulate Neuroinflammation in a Mouse Model of Multiple Sclerosis. Mol. Ther. 2018, 26, 2107–2118. [Google Scholar]
- Wiklander, O.P.; Nordin, J.Z.; O’Loughlin, A.; Gustafsson, Y.; Corso, G.; Mager, I.; Vader, P.; Lee, Y.; Sork, H.; Seow, Y.; et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J. Extracell. Vesicles 2015, 4, 26316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, R.C.; Tan, S.S.; Teh, B.J.; Sze, S.K.; Arslan, F.; de Kleijn, D.P.; Choo, A.; Lim, S.K. Proteolytic Potential of the MSC Exosome Proteome: Implications for an Exosome-Mediated Delivery of Therapeutic Proteasome. Int. J. Proteom. 2012, 2012, 971907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lecuyer, M.; Pathipati, P.; Faustino, J.; Vexler, Z.S. Neonatal stroke enhances interaction of microglia-derived extracellular vesicles with microglial cells. Neurobiol. Dis. 2021, 157, 105431. [Google Scholar] [CrossRef] [PubMed]
- Pathipati, P.; Lecuyer, M.; Faustino, J.; Strivelli, J.; Phinney, D.G.; Vexler, Z.S. Mesenchymal Stem Cell (MSC)-Derived Extracellular Vesicles Protect from Neonatal Stroke by Interacting with Microglial Cells. Neurotherapeutics 2021, 18, 1939–1952. [Google Scholar] [CrossRef] [PubMed]
- Sepulveda, J.C.; Tome, M.; Fernandez, M.E.; Delgado, M.; Campisi, J.; Bernad, A.; Gonzalez, M.A. Cell senescence abrogates the therapeutic potential of human mesenchymal stem cells in the lethal endotoxemia model. Stem Cells 2014, 32, 1865–1877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, N.; Boyette, L.B.; Tuan, R.S. Characterization of bone marrow-derived mesenchymal stem cells in aging. Bone 2015, 70, 37–47. [Google Scholar] [CrossRef]
- DelaRosa, O.; Sanchez-Correa, B.; Morgado, S.; Ramirez, C.; del Rio, B.; Menta, R.; Lombardo, E.; Tarazona, R.; Casado, J.G. Human adipose-derived stem cells impair natural killer cell function and exhibit low susceptibility to natural killer-mediated lysis. Stem Cells Dev. 2012, 21, 1333–1343. [Google Scholar] [CrossRef] [Green Version]
- Melief, S.M.; Zwaginga, J.J.; Fibbe, W.E.; Roelofs, H. Adipose tissue-derived multipotent stromal cells have a higher immunomodulatory capacity than their bone marrow-derived counterparts. Stem Cells Transl. Med. 2013, 2, 455–463. [Google Scholar] [CrossRef]
- Li, C.Y.; Wu, X.Y.; Tong, J.B.; Yang, X.X.; Zhao, J.L.; Zheng, Q.F.; Zhao, G.B.; Ma, Z.J. Comparative analysis of human mesenchymal stem cells from bone marrow and adipose tissue under xeno-free conditions for cell therapy. Stem Cell Res. Ther. 2015, 6, 55. [Google Scholar] [CrossRef] [Green Version]
- Damasceno, P.K.F.; de Santana, T.A.; Santos, G.C.; Orge, I.D.; Silva, D.N.; Albuquerque, J.F.; Golinelli, G.; Grisendi, G.; Pinelli, M.; Ribeiro Dos Santos, R.; et al. Genetic Engineering as a Strategy to Improve the Therapeutic Efficacy of Mesenchymal Stem/Stromal Cells in Regenerative Medicine. Front. Cell Dev. Biol. 2020, 8, 737. [Google Scholar] [CrossRef]
| Disease | Experimental Model | ASC Administration | Publication Title | References |
|---|---|---|---|---|
| AD | APP/PS1 transgenic mouse | Intracerebral transplantation (hippocampus) of ASC | Intracerebral transplantation of adipose-derived mesenchymal stem cells alternatively activates microglia and ameliorates neuropathological deficits in Alzheimer’s disease mice | [62] |
| AD | APP/PS1 transgenic mouse | Intranasal administration of ASC-EV | ADSCs-derived extracellular vesicles alleviate neuronal damage, promote neurogenesis and rescue memory loss in mice with Alzheimer’s disease | [66] |
| AD | Intra-hippocampal injections of Aβ in rats | Intraperitoneal injections of ASC-CM | Hypoxic-conditioned medium from adipose tissue mesenchymal stem cells T improved neuroinflammation through alternation of toll-like receptor (TLR) 2 and TLR4 expression in a model of Alzheimer’s disease rats | [67] |
| CMS | CMS induction in mice | Intravenous injection of murine ASC 3 weeks after CMS induction | Adipose-derived mesenchymal stem cells protect against CMS-induced depression-like behaviors in mice via regulatingthe Nrf2/HO-1 and TLR4/NF-κB signaling pathways | [7] |
| Ischemic stroke | Acute ischemic stroke (AIS) model in rats | Intravenous injection of pig ASC and exosomes 3 h after AIS induction | Intravenous administration of xenogenic adipose-derived mesenchymal stem cells (ADMSC) and ADMSC-derived exosomes markedly reduced brain infarct volume and preserved neurological function in rats after acute ischemic stroke | [68] |
| Ischemic stroke | Transient global cerebral ischemia (GCI) model in rats | Intravenous injection of human ASC directly after induction of GCI | Adipose-derived mesenchymal stem cells reduce neuronal death after transient global cerebral ischemia through prevention of blood-brain barrier disruption and endothelial damage 1 | [69] |
| Ischemic stroke | Transient GCI model in rats | Intravenous injection of human ASC | Effect of adipose-derived mesenchymal stem cell administration and mild hypothermia induction on delayed neuronal death after transient global cerebral ischemia | [70] |
| Ischemic stroke | Intracerebral injection of endothelin-1 to induce subcortical ischemic stroke | Intravenous injection of rat EV 24 h after stroke induction | White matter repair after extracellular vesicles administration in an experimental animal model of subcortical stroke 1 | [71] |
| Ischemic stroke | Middle cerebral artery occlusion in rats | Intracerebral transplantation of rat ASC 8 days after ischemia induction | Immunological effects of the intraparenchymal administration of allogeneic and autologous adipose-derived mesenchymal stem cells after the acute phase of middle cerebral artery occlusion in rats 1 | [72] |
| MS | EAE mouse model | Intraperitoneal injection of human allogenic ASC | Allogeneic adipose-derived mesenchymal stromal cells ameliorate experimental autoimmune encephalomyelitis by regulating self-reactive T cell responses and dendritic cell function | [6] |
| MS | EAE mouse model | Intraperitoneal administration of human allogenic ASC expressing IL-4 at disease onset | Early intervention with gene-modified mesenchymal stem cells overexpressing interleukin-4 enhances anti-inflammatory responses and functional recovery in experimental autoimmune demyelination | [73] |
| MS | EAE mouse model | Intraperitoneal administration of mouse allogenic ASC expressing VIP at the peak of disease | Mesenchymal stem cells expressing vasoactive intestinal peptides ameliorate symptoms in a model of chronic multiple sclerosis. | [74] |
| MS | EAE mouse model | Intravenous administration of ASC before and after disease onset | Adipose-derived mesenchymal stem cells ameliorate chronic experimental autoimmune encephalomyelitis 1 | [56] |
| MS | MS model of Theiler’s murine encephalomyelitis virus (TMEV) | Intravenous injection of ASC-EV on day 60 postinfection | Therapeutic potential of extracellular vesicles derived from human mesenchymal stem cells in a model of progressive multiple sclerosis | [75] |
| Neuro-inflammation | BV2 cells | ASC-BV2 cells co-culture | Adipose-derived mesenchymal stem cells protect against CMS-induced depression-like behaviors in mice via regulating the Nrf2/HO-1 and TLR4/NF-κB signaling pathways | [7] |
| Neuro-inflammation | BV2 cells | Microglia incubated with ASC-CCM | Concentrated conditioned media from adipose tissue-derived mesenchymal stem cells mitigates visual deficits and retinal inflammation following mild traumatic brain injury | [8] |
| Neuro-inflammation | BV2 cells | Microglia incubated with ASC-CCM | TSG-6 in conditioned media from adipose mesenchymal stem cells protects against visual deficits in mild traumatic brain injury model through neurovascular modulation | [42] |
| Neuro-inflammation | Primary mouse microglia | Microglia with ASC plated in transwells, microglia incubated with ASC-CM | Mesenchymal stem cells induce the ramification of microglia via the small RhoGTPases Cdc42 and Rac1 | [22] |
| Neuro-inflammation | Primary mouse microglia | Microglia incubated with ASC-CM | The atypical RhoGTPase RhoE/Rnd3 is a key molecule to acquire a neuroprotective phenotype in microglia | [54] |
| Niemann-Pick disease type C | Niemann–Pick disease type C model mice | Transplantation of ASC in mouse cerebellum | Adipose tissue-derived stem cells rescue Purkinje neurons and alleviate inflammatory responses in Niemann-Pick disease type C mice 1 | [76] |
| PD | Intrastriatal 6-hydroxydopamine injections of rats | Intracerebral transplantation (SN) of human ASC | Human adipose-derived mesenchymal stem cells improve motor functions and are neuroprotective in the 6-hydroxydopamine-rat model for Parkinson’s disease when cultured in monolayer cultures but suppress hippocampal neurogenesis and hippocampal memory function when cultured in spheroids 1 | [77] |
| PD | Intrastriatal 6-hydroxydopamine injections of mice | Intracerebral transplantation (SN) of ASC one week after the 6-hydroxydopamine injections | Autologous transplants of adipose-derived adult stromal (ADAS) afford dopaminergic neuroprotection in a model of Parkinson’s disease 1 | [61] |
| PD | LPS-injection into SN | Intracerebral transplantation (SN) of ASC at the same time as LPS injection | Adipose-derived stem cells decreased microglial activation and protected dopaminergic loss in a rat lipopolysaccharide model | [65] |
| PD | Intrastriatal 6-hydroxydopamine injections of rats | Intracerebral transplantation (SN) of human ASC | Human adipose-derived mesenchymal stromal cells increase endogenous neurogenesis in the rat subventricular zone acutely after 6-hydroxydopamine lesioning 1 | [78] |
| Retinal inflammation following mTBI | mTBI mouse model | Intravitreal injections of ASC-CCM | Concentrated conditioned media from adipose tissue-derived mesenchymal stem cells mitigates visual deficits and retinal inflammation following mild traumatic brain injury | [8] |
| Retinal inflammation following mTBI | mTBI mouse model | Intravitreal injections of ASC-CCM | TSG-6 in conditioned media from adipose mesenchymal stem cells protects against visual deficits in mild traumatic brain injury model through neurovascular modulation | [42] |
| SCI | SCI model in mice | Intravenous injection of ASC-EV immediately after SCI induction | Exosomes from long noncoding RNA-Gm37494-ADSCs repair spinal cord injury via shifting microglial M1/M2 polarization | [79] |
| SCI | Moderate contusion injury of the spinal cord in mice | Injection of ASC into SCI epicenter directly after SCI induction | Adipose mesenchymal stem cell transplantation alleviates spinal cord injury-induced neuroinflammation partly by suppressing the Jagged1/Notch pathway 1 | [80] |
| TBI | TBI rat model | Intra-cerebroventricular injection of human ASC-EV | MSC-derived exosomes promote recovery from traumatic brain injury via microglia/macrophages in rat | [81] |
| TBI | TBI rat model | Intravenous injection of CM from human ASC after TBI | Intravenously infusing the secretome of adipose-derived mesenchymal stem cells ameliorates neuroinflammation and neurological functioning after traumatic brain injury | [57] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Castillo, A.I.; Sepúlveda, M.R.; Marín-Teva, J.L.; Cuadros, M.A.; Martín-Oliva, D.; González-Rey, E.; Delgado, M.; Neubrand, V.E. Switching Roles: Beneficial Effects of Adipose Tissue-Derived Mesenchymal Stem Cells on Microglia and Their Implication in Neurodegenerative Diseases. Biomolecules 2022, 12, 219. https://doi.org/10.3390/biom12020219
Sánchez-Castillo AI, Sepúlveda MR, Marín-Teva JL, Cuadros MA, Martín-Oliva D, González-Rey E, Delgado M, Neubrand VE. Switching Roles: Beneficial Effects of Adipose Tissue-Derived Mesenchymal Stem Cells on Microglia and Their Implication in Neurodegenerative Diseases. Biomolecules. 2022; 12(2):219. https://doi.org/10.3390/biom12020219
Chicago/Turabian StyleSánchez-Castillo, Ana Isabel, M. Rosario Sepúlveda, José Luis Marín-Teva, Miguel A. Cuadros, David Martín-Oliva, Elena González-Rey, Mario Delgado, and Veronika E. Neubrand. 2022. "Switching Roles: Beneficial Effects of Adipose Tissue-Derived Mesenchymal Stem Cells on Microglia and Their Implication in Neurodegenerative Diseases" Biomolecules 12, no. 2: 219. https://doi.org/10.3390/biom12020219
APA StyleSánchez-Castillo, A. I., Sepúlveda, M. R., Marín-Teva, J. L., Cuadros, M. A., Martín-Oliva, D., González-Rey, E., Delgado, M., & Neubrand, V. E. (2022). Switching Roles: Beneficial Effects of Adipose Tissue-Derived Mesenchymal Stem Cells on Microglia and Their Implication in Neurodegenerative Diseases. Biomolecules, 12(2), 219. https://doi.org/10.3390/biom12020219








