Phenotyping of Drosophila Melanogaster—A Nutritional Perspective
Abstract
1. Introduction
2. Phenotyping of D. melanogaster
2.1. Body Weight
2.2. Body Composition: Lipids, Proteins, Carbohydrates
2.2.1. Lipid Determination
2.2.2. Protein Determination
2.2.3. Carbohydrate Determination
2.3. Metabolic Rate
2.4. Food Intake
2.5. Lifespan
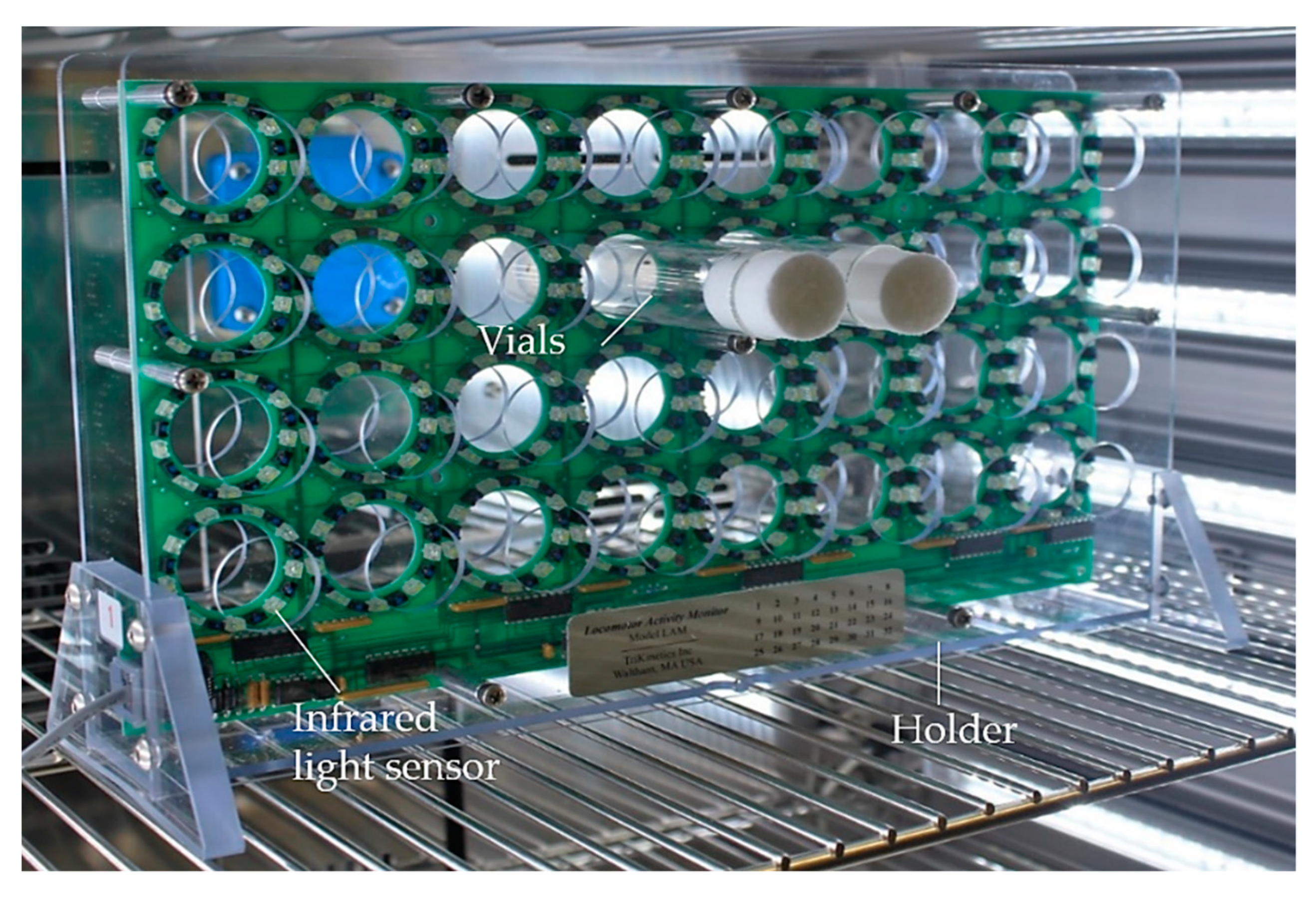
2.6. Spontaneous and Induced Locomotor Activity
2.7. Heart Rate Measurement
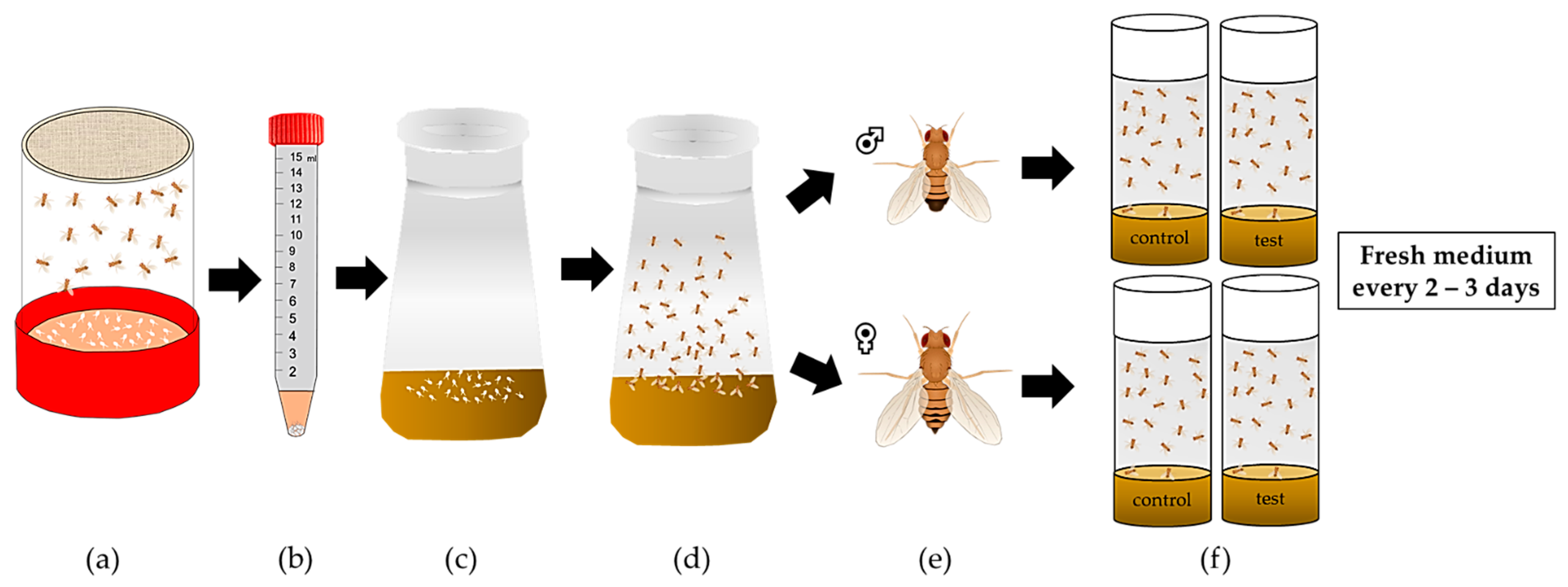
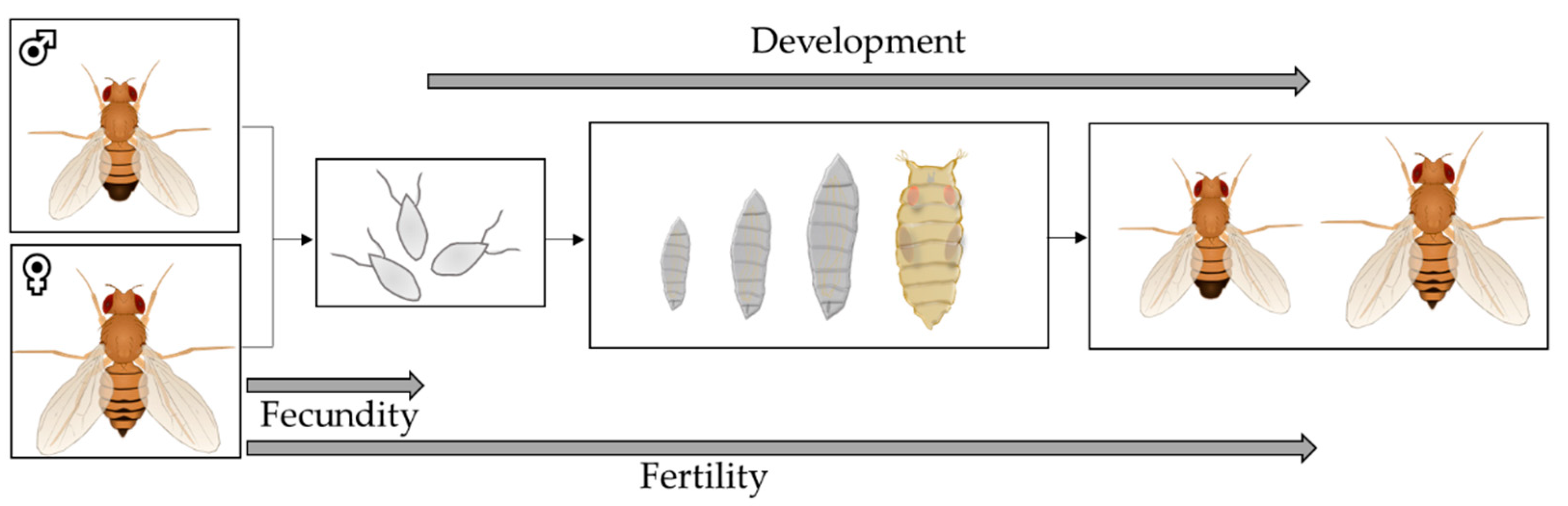
2.8. Fecundity, Fertility and Development as Parameters of Reproduction
2.9. Stress Assays
3. Obesity Phenotype of D. melanogaster Following a High-Sugar or High-Fat Diet
3.1. High-Sugar Diet
3.2. High-Fat Diet
4. Molecular Changes Following a High-Sugar Diet and High-Fat Diet
4.1. Changes in Transcriptome
4.1.1. Transcriptional Changes Induced by HSD
4.1.2. Transcriptional Changes Induced by HFD
4.2. Changes in Metabolome
4.2.1. HSD Metabolome
4.2.2. HFD Metabolome
5. Relevance of Findings in D. melanogaster for Other Species
5.1. Lipid Metabolism
5.2. Obesity and Related Comorbidities
5.3. Lessons Learned from D. Melanogaster
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 7-ADD | 7-Aminoactinomycin D |
| Acetyl-CoA | Acetyl-coenzyme A |
| 4EBP | eIF4E-binding protein |
| AKHR | Adipokinetic hormone receptor |
| AKT | Protein kinase B |
| AMPK | Adenosine monophosphate-activated protein kinase |
| ATGL | Adipose triglyceride lipase |
| BCA | Bicinchoninic acid |
| CAFE | CApillary FEeder |
| CCA | Coupled colorimetric assay |
| Con-Ex | Consumption-excretion |
| DAG | Diacylglycerol |
| DAM | Drosophila Activity Monitoring |
| dFOXO | Forkhead Box O |
| DHE | Dihydroethidium |
| Dilp | Insulin-like peptide |
| EB, EC, EE | Enteroblast, enterocytes, enteroendocrine |
| ERK | Extracellular signal-regulated kinase |
| EX-Q | Excreta quantification |
| FA | Fatty acid |
| FAS | Fatty acid synthase |
| FLIC | Fly Liquid-Food Interaction Counter |
| flyPAD | Fly Proboscis and Activity Detector |
| GABA | Gamma-amino-butyric acid |
| GFP | Green fluorescent protein |
| GO | Glucose oxidase |
| HBP | Hexosamine biosynthetic pathway |
| HFD | High-fat diet |
| HK | Hexokinase |
| HSD | High-sugar diet |
| Hsl | Hormone-sensitive lipase |
| ISC | Intestinal stem cell |
| JAK | Janus kinase |
| JNK | c-Jun N-terminal kinase |
| LC/MS; GC/MS | Liquid chromatography or gas chromatography/mass spectrometry |
| LSD | Low-sugar diet |
| MAG | Monoacylglycerol |
| MAFE | Manual feeding |
| MARK | Mitogen-activated protein kinase |
| NADH | Nicotinamide-adenine dinucleotide |
| NADPH | Nicotinamide-adenine dinucleotide phosphate |
| O-GlcNAc | O-linked N-acetylglucosamine |
| OGT | O-GlcNAc transferase |
| PAT | Perilipin, ADRP, TIP47 |
| PBT | Dulbecco’s phosphate-buffered saline with Triton X-100 |
| PER | Proboscis extension response |
| PI | Preference index |
| PKA | Protein kinase A |
| qRT–PCR | Real-time quantitative PCR |
| RING | Rapid iterative negative geotaxis |
| SAM (ELISA) | S-Adenosylmethionine ELISA |
| SIK3 | Serine/threonine-protein kinase 3 |
| STAT | Signal transducer and activator of transcription |
| TAG | Triacylglyceride |
| TCA | Tricarboxylic acid |
| TLC | Thin-layer chromatography |
| TOR | Target of rapamycin |
| TUNEL | Terminal deoxynucleotidyl transferase dUTP nick end labeling |
| VLDL | Very-low-density lipoprotein |
| WDTC | WD40/tetratricopeptide-repeat-domain protein |
References
- Fernández-Moreno, M.A.; Farr, C.L.; Kaguni, L.S.; Garesse, R. Drosophila melanogaster as a Model System to Study Mitochondrial Biology. Methods Mol. Biol. 2007, 372, 33–49. [Google Scholar] [PubMed]
- Adams, M.D. The Genome Sequence of Drosophila melanogaster. Science 2000, 287, 2185–2195. [Google Scholar] [CrossRef] [PubMed]
- Bier, E. Drosophila, the golden bug, emerges as a tool for human genetics. Nat. Rev. Genet. 2005, 6, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Lüersen, K.; Röder, T.; Rimbach, G. Drosophila melanogaster in nutrition research—The importance of standardizing experimental diets. Genes Nutr. 2019, 14, 3737. [Google Scholar] [CrossRef] [PubMed]
- Apidianakis, Y.; Rahme, L.G. Drosophila melanogaster as a model for human intestinal infection and pathology. Dis. Model. Mech. 2010, 4, 21–30. [Google Scholar] [CrossRef]
- Graham, P.; Pick, L. Drosophila as a Model for Diabetes and Diseases of Insulin Resistance. In Fly Models of Human Diseases, 1st ed.; Pick, L., Ed.; Elsevier/Academic Press: Cambridge, MA, USA, 2017; pp. 397–419. [Google Scholar]
- Kreipke, R.E.; Kwon, Y.V.; Shcherbata, H.R.; Ruohola-Baker, H. Drosophila melanogaster as a Model of Muscle Degeneration Disorders. In Fly Models of Human Diseases, 1st ed.; Pick, L., Ed.; Elsevier/Academic Press: Cambridge, MA, USA, 2017; pp. 83–109. [Google Scholar]
- Kayashima, Y.; Murata, S.; Sato, M.; Matsuura, K.; Asanuma, T.; Chimoto, J.; Ishii, T.; Mochizuki, K.; Kumazawa, S.; Nakayama, T.; et al. Tea polyphenols ameliorate fat storage induced by high-fat diet in Drosophila melanogaster. Biochem. Biophys. Rep. 2015, 4, 417–424. [Google Scholar] [CrossRef]
- Wagner, A.E.; Piegholdt, S.; Rabe, D.; Baenas, N.; Schloesser, A.; Eggersdorfer, M.; Stocker, A.; Rimbach, G. Epigallocatechin gallate affects glucose metabolism and increases fitness and lifespan in Drosophila melanogaster. Oncotarget 2015, 6, 30568–30578. [Google Scholar] [CrossRef]
- Piegholdt, S.; Rimbach, G.; Wagner, A.E. The phytoestrogen prunetin affects body composition and improves fitness and lifespan in male Drosophila melanogaster. FASEB J. 2016, 30, 948–958. [Google Scholar] [CrossRef]
- Staats, S.; Wagner, A.E.; Lüersen, K.; Künstner, A.; Meyer, T.; Kahns, A.K.; Derer, S.; Graspeuntner, S.; Rupp, J.; Busch, H.; et al. Dietary ursolic acid improves health span and life span in male Drosophila melanogaster. BioFactors 2018, 45, 169–186. [Google Scholar] [CrossRef]
- Staats, S.; Wagner, A.; Kowalewski, B.; Rieck, F.; Soukup, S.; Kulling, S.; Rimbach, G. Dietary Resveratrol Does Not Affect Life Span, Body Composition, Stress Response, and Longevity-Related Gene Expression in Drosophila melanogaster. Int. J. Mol. Sci. 2018, 19, 223. [Google Scholar] [CrossRef]
- Piper, M.D.W.; Partridge, L. Protocols to Study Aging in Drosophila. Methods Mol. Biol. 2016, 1478, 291–302. [Google Scholar] [PubMed]
- Staats, S.; Lüersen, K.; Wagner, A.E.; Rimbach, G. Drosophila melanogaster as a Versatile Model Organism in Food and Nutrition Research. J. Agric. Food Chem. 2018, 66, 3737–3753. [Google Scholar] [CrossRef] [PubMed]
- Jumbo-Lucioni, P.; Ayroles, J.F.; Chambers, M.M.; Jordan, K.W.; Leips, J.; Mackay, T.F.C.; de Luca, M. Systems genetics analysis of body weight and energy metabolism traits in Drosophila melanogaster. BMC Genom. 2010, 11, S12. [Google Scholar] [CrossRef]
- Burggren, W.; Souder, B.M.; Ho, D.H. Metabolic rate and hypoxia tolerance are affected by group interactions and sex in the fruit fly (Drosophila melanogaster): New data and a literature survey. Biol. Open 2017, 23, 59. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, A.; Bickmeyer, I.; Kühnlein, R.P. Reliable Drosophila body fat quantification by a coupled colorimetric assay. PLoS ONE 2011, 6, e23796. [Google Scholar] [CrossRef]
- Wei, C.; Yan, Y.; Miao, X.; Jiao, R. Dissection and Lipid Droplet Staining of Oenocytes in Drosophila Larvae. J. Vis. Exp. 2019, e60606. [Google Scholar] [CrossRef]
- Häder, T.; Müller, S.; Aguilera, M.; Eulenberg, K.G.; Steuernagel, A.; Ciossek, T.; Kühnlein, R.P.; Lemaire, L.; Fritsch, R.; Dohrmann, C.; et al. Control of triglyceride storage by a WD40/TPR-domain protein. EMBO Rep. 2003, 4, 511–516. [Google Scholar] [CrossRef]
- Ugrankar, R.; Liu, Y.; Provaznik, J.; Schmitt, S.; Lehmann, M. Lipin is a central regulator of adipose tissue development and function in Drosophila melanogaster. Mol. Cell Biol. 2011, 31, 1646–1656. [Google Scholar] [CrossRef]
- Grönke, S.; Beller, M.; Fellert, S.; Ramakrishnan, H.; Jäckle, H.; Kühnlein, R.P. Control of Fat Storage by a Drosophila PAT Domain Protein. Curr. Biol. 2003, 13, 603–606. [Google Scholar] [CrossRef]
- Birse, R.T.; Choi, J.; Reardon, K.; Rodriguez, J.; Graham, S.; Diop, S.; Ocorr, K.; Bodmer, R.; Oldham, S. High-Fat-Diet-Induced Obesity and Heart Dysfunction Are Regulated by the TOR Pathway in Drosophila. Cell Metab. 2010, 12, 533–544. [Google Scholar] [CrossRef]
- Diop, S.B.; Bisharat-Kernizan, J.; Birse, R.T.; Oldham, S.; Ocorr, K.; Bodmer, R. PGC-1/Spargel Counteracts High-Fat-Diet-Induced Obesity and Cardiac Lipotoxicity Downstream of TOR and Brummer ATGL Lipase. Cell Rep. 2015, 10, 1572–1584. [Google Scholar] [CrossRef]
- Diop, S.; Birse, R.; Bodmer, R. High Fat Diet Feeding and High Throughput Triacylglyceride Assay in Drosophila Melanogaster. J. Vis. Exp. 2017, 127, 56029. [Google Scholar] [CrossRef] [PubMed]
- McGowan, M.W.; Artiss, J.D.; Strandbergh, D.R.; Zak, B. A peroxidase-coupled method for the colorimetric determination of serum triglycerides. Clin. Chem. 1983, 29, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Fossati, P.; Prencipe, L. Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin. Chem. 1982, 28, 2077–2080. [Google Scholar] [CrossRef] [PubMed]
- Al-Anzi, B.; Zinn, K. Colorimetric measurement of triglycerides cannot provide an accurate measure of stored fat content in Drosophila. PLoS ONE 2010, 5, e12353. [Google Scholar] [CrossRef]
- Matsuda, H.; Yamada, T.; Yoshida, M.; Nishimura, T. Flies without trehalose. J. Biol. Chem. 2015, 290, 1244–1255. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Al-Anzi, B.; Sapin, V.; Waters, C.; Zinn, K.; Wyman, R.J.; Benzer, S. Obesity-blocking neurons in Drosophila. Neuron 2009, 63, 329–341. [Google Scholar] [CrossRef]
- Rietveld, A.; Neutz, S.; Simons, K.; Eaton, S. Association of sterol- and glycosylphosphatidylinositol-linked proteins with Drosophila raft lipid microdomains. J. Biol. Chem. 1999, 274, 12049–12054. [Google Scholar] [CrossRef]
- Hammad, L.A.; Cooper, B.S.; Fisher, N.P.; Montooth, K.L.; Karty, J.A. Profiling and quantification of Drosophila melanogaster lipids using liquid chromatography/mass spectrometry. Rapid Commun. Mass Spectrom. 2011, 25, 2959–2968. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Lam, S.M.; Xin, J.; Yang, X.; Liu, Z.; Liu, Y.; Wang, Y.; Shui, G.; Huang, X. Drosophila TRF2 and TAF9 regulate lipid droplet size and phospholipid fatty acid composition. PLoS Genet. 2017, 13, e1006664. [Google Scholar] [CrossRef] [PubMed]
- Köhler, K.; Brunner, E.; Guan, X.L.; Boucke, K.; Greber, U.F.; Mohanty, S.; Barth, J.M.I.; Wenk, M.R.; Hafen, E. A combined proteomic and genetic analysis identifies a role for the lipid desaturase Desat1 in starvation-induced autophagy in Drosophila. Autophagy 2009, 5, 980–990. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Krohn, R.I. The colorimetric detection and quantitation of total protein. Curr. Protoc. Cell Biol. 2011, 23, A-31. [Google Scholar]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Tennessen, J.M.; Barry, W.E.; Cox, J.; Thummel, C.S. Methods for studying metabolism in Drosophila. Methods 2014, 68, 105–115. [Google Scholar] [CrossRef]
- Haselton, A.T.; Fridell, Y.-W.C. Insulin injection and hemolymph extraction to measure insulin sensitivity in adult Drosophila melanogaster. J. Vis. Exp. 2011, e2722. [Google Scholar] [CrossRef]
- Trinder, P. Determination of blood glucose using an oxidase-peroxidase system with a non-carcinogenic chromogen. J. Clin. Pathol. 1969, 22, 158–161. [Google Scholar] [CrossRef]
- Galant, A.L.; Kaufman, R.C.; Wilson, J.D. Glucose: Detection and analysis. Food Chem. 2015, 188, 149–160. [Google Scholar] [CrossRef]
- Yatsenko, A.S.; Marrone, A.K.; Kucherenko, M.M.; Shcherbata, H.R. Measurement of Metabolic Rate in Drosophila using Respirometry. J. Vis. Exp. 2014, e51681. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, M.; Rundle, H.D.; Videlier, M.; Careau, V.; Herberstein, M. Territoriality in Drosophila: Indirect effects and covariance with body mass and metabolic rate. Behav. Ecol. 2021, 102, 591. [Google Scholar] [CrossRef]
- Fiorino, A.; Thompson, D.; Yadlapalli, S.; Jiang, C.; Shafer, O.T.; Reddy, P.; Meyhofer, E. Parallelized, real-time, metabolic-rate measurements from individual Drosophila. Sci. Rep. 2018, 8, 14452. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, T.S.; Gendron, C.M.; Lyu, Y.; Munneke, A.S.; DeMarco, M.N.; Hoisington, Z.W.; Pletcher, S.D. Sensory perception of dead conspecifics induces aversive cues and modulates lifespan through serotonin in Drosophila. Nat Commun. 2019, 10, 804. [Google Scholar] [CrossRef] [PubMed]
- Walsberg, G.E.; Hoffman, T.C.M. Direct calorimetry reveals large errors in respirometric estimates of energy expenditure. J. Exp. Biol. 2005, 208, 1035–1043. [Google Scholar] [CrossRef]
- Van Voorhies, W.A.; Khazaeli, A.A.; Curtsinger, J.W. Selected contribution: Long-lived Drosophila melanogaster lines exhibit normal metabolic rates. J. Appl. Physiol. 2003, 95, 2605–2613, discussion 2604. [Google Scholar] [CrossRef]
- Levine, J.A. Measurement of energy expenditure. Public Health Nutr. 2005, 8, 1123–1132. [Google Scholar] [CrossRef]
- Orgad, S.; Nelson, H.; Segal, D.; Nelson, N. Metal ions suppress the abnormal taste behavior of the Drosophila mutant malvolio. J. Exp. Biol. 1998, 201, 115–120. [Google Scholar] [CrossRef]
- Bahadorani, S.; Bahadorani, P.; Phillips, J.P.; Hilliker, A.J. The effects of vitamin supplementation on Drosophila life span under normoxia and under oxidative stress. J. Gerontol. A Biol. Sci. Med. Sci. 2008, 63, 35–42. [Google Scholar] [CrossRef]
- Bahadorani, S.; Hilliker, A.J. Cocoa confers life span extension in Drosophila melanogaster. Nutr. Res. 2008, 28, 377–382. [Google Scholar] [CrossRef]
- Peng, C.; Chan, H.Y.E.; Huang, Y.; Yu, H.; Chen, Z.-Y. Apple polyphenols extend the mean lifespan of Drosophila melanogaster. J. Agric. Food Chem. 2011, 59, 2097–2106. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.; Piper, M.D.W.; Blanc, E.; Partridge, L. Pitfalls of measuring feeding rate in the fruit fly Drosophila melanogaster. Nat. Methods 2008, 5, 214–215. [Google Scholar] [CrossRef] [PubMed]
- Shell, B.C.; Schmitt, R.E.; Lee, K.M.; Johnson, J.C.; Chung, B.Y.; Pletcher, S.D.; Grotewiel, M. Measurement of solid food intake in Drosophila via consumption-excretion of a dye tracer. Sci. Rep. 2018, 8, 11536. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Yu, G.; Park, S.J.; Gao, Y.; Ja, W.W.; Yang, M. Excreta Quantification (EX-Q) for Longitudinal Measurements of Food Intake in Drosophila. iScience 2020, 23, 100776. [Google Scholar] [CrossRef]
- Geer, B.W.; Olander, R.M.; Sharp, P.L. Quantification of dietary choline utilization in adult Drosophila melanogaster by radioisotope methods. J. Insect Physiol. 1970, 16, 33–43. [Google Scholar] [CrossRef]
- Ayaki, T.; Ohshima, K.; Okumura, Y.; Yoshikawa, I.; Shiomi, T. The relationship between lethal mutation yield and intake of ethylnitrosourea (ENU) in Drosophila melanogaster. Environ. Mol. Mutagen. 1984, 6, 483–488. [Google Scholar] [CrossRef]
- Thompson, E.D.; Reeder, B.A. Method for selecting exposure levels for the Drosophila sex-linked recessive lethal assay. Environ. Mol. Mutagen. 1987, 10, 357–365. [Google Scholar] [CrossRef]
- Thompson, E.D.; Reeder, B.A.; Bruce, R.D. Characterization of a method for quantitating food consumption for mutation assays in Drosophila. Environ. Mol. Mutagen. 1991, 18, 14–21. [Google Scholar] [CrossRef]
- Brummel, T.; Ching, A.; Seroude, L.; Simon, A.F.; Benzer, S. Drosophila lifespan enhancement by exogenous bacteria. Proc. Natl. Acad. Sci. USA 2004, 101, 12974–12979. [Google Scholar] [CrossRef]
- Carvalho, G.B.; Kapahi, P.; Benzer, S. Compensatory ingestion upon dietary restriction in Drosophila melanogaster. Nat. Methods 2005, 2, 813–815. [Google Scholar] [CrossRef]
- Ja, W.W.; Carvalho, G.B.; Zid, B.M.; Mak, E.M.; Brummel, T.; Benzer, S. Water- and nutrient-dependent effects of dietary restriction on Drosophila lifespan. Proc. Natl. Acad. Sci. USA 2009, 106, 18633–18637. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, S.A.; Carvalho, G.B.; Amador, A.; Phillips, A.M.; Hoxha, S.; Lizotte, K.J.; Ja, W.W. Quantifying Drosophila food intake: Comparative analysis of current methodology. Nat. Methods 2014, 11, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Ja, W.W.; Carvalho, G.B.; Mak, E.M.; Noelle, N.; Fang, A.Y.; Liong, J.C.; Brummel, T.; Benzer, S. Prandiology of Drosophila and the CAFE assay. Proc. Natl. Acad. Sci. USA 2007, 104, 8253–8256. [Google Scholar] [CrossRef] [PubMed]
- Diegelmann, S.; Jansen, A.; Jois, S.; Kastenholz, K.; Velo Escarcena, L.; Strudthoff, N.; Scholz, H. The CApillary FEeder Assay Measures Food Intake in Drosophila melanogaster. J. Vis. Exp. 2017, 121, 55024. [Google Scholar] [CrossRef] [PubMed]
- Garlapow, M.E.; Huang, W.; Yarboro, M.T.; Peterson, K.R.; Mackay, T.F.C.; Ko, D.C. Quantitative Genetics of Food Intake in Drosophila melanogaster. PLoS ONE 2015, 10, e0138129. [Google Scholar] [CrossRef]
- Qi, W.; Yang, Z.; Lin, Z.; Park, J.-Y.; Suh, G.S.B.; Wang, L. A quantitative feeding assay in adult Drosophila reveals rapid modulation of food ingestion by its nutritional value. Mol. Brain 2015, 8, 87. [Google Scholar] [CrossRef]
- Shiraiwa, T.; Carlson, J.R. Proboscis extension response (PER) assay in Drosophila. J. Vis. Exp. 2007, 193. [Google Scholar] [CrossRef]
- Itskov, P.M.; Moreira, J.-M.; Vinnik, E.; Lopes, G.; Safarik, S.; Dickinson, M.H.; Ribeiro, C. Automated monitoring and quantitative analysis of feeding behaviour in Drosophila. Nat. Commun. 2014, 5, 4560. [Google Scholar] [CrossRef]
- Ro, J.; Harvanek, Z.M.; Pletcher, S.D. FLIC: High-throughput, continuous analysis of feeding behaviors in Drosophila. PLoS ONE 2014, 9, e101107. [Google Scholar] [CrossRef]
- Linford, N.J.; Bilgir, C.; Ro, J.; Pletcher, S.D. Measurement of lifespan in Drosophila melanogaster. J. Vis. Exp. 2013, e50068. [Google Scholar] [CrossRef]
- Sun, Y.; Yolitz, J.; Wang, C.; Spangler, E.; Zhan, M.; Zou, S. Aging Studies in Drosophila melanogaster. Methods Mol. Biol. 2013, 1048, 77–93. [Google Scholar] [PubMed]
- Pletcher, S.D.; Curtsinger, J.W. The influence of environmentally induced heterogeneity on age-specific genetic variance for mortality rates. Genet. Res. 2000, 75, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Skorupa, D.A.; Dervisefendic, A.; Zwiener, J.; Pletcher, S.D. Dietary composition specifies consumption, obesity, and lifespan in Drosophila melanogaster. Aging Cell 2008, 7, 478–490. [Google Scholar] [CrossRef] [PubMed]
- Pletcher, S.D.; Stumpf, M.P.H. Population Genomics: Ageing by Association. Curr. Biol. 2002, 12, R328–R330. [Google Scholar] [CrossRef]
- Nicolai, S.; Rossi, A.; Di Daniele, N.; Melino, G.; Annicchiarico-Petruzzelli, M.; Raschellà, G. DNA repair and aging: The impact of the p53 family. Aging 2015, 7, 1050–1065. [Google Scholar] [CrossRef]
- Rogina, B.; Helfand, S.L. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc. Natl. Acad. Sci. USA 2004, 101, 15998–16003. [Google Scholar] [CrossRef]
- Benzer, S. Behavioral mutants of Drosophila isolated by countercurrent distribution. Proc. Natl. Acad. Sci. USA 1967, 58, 1112–1119. [Google Scholar] [CrossRef]
- Konopka, R.J.; Benzer, S. Clock Mutants of Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 1971, 68, 2112–2116. [Google Scholar] [CrossRef]
- Nichols, C.D.; Becnel, J.; Pandey, U.B. Methods to assay Drosophila behavior. J. Vis. Exp. 2012, e3795. [Google Scholar] [CrossRef]
- Gargano, J.W.; Martin, I.; Bhandari, P.; Grotewiel, M.S. Rapid iterative negative geotaxis (RING): A new method for assessing age-related locomotor decline in Drosophila. Exp. Gerontol. 2005, 40, 386–395. [Google Scholar] [CrossRef]
- Madabattula, S.; Strautman, J.; Bysice, A.; O’Sullivan, J.; Androschuk, A.; Rosenfelt, C.; Doucet, K. Quantitative Analysis of Climbing Defects in a Drosophila Model of Neurodegenerative Disorders. J. Vis. Exp. 2015, 52741. [Google Scholar] [CrossRef] [PubMed]
- Scaplen, K.M.; Mei, N.J.; Bounds, H.A.; Song, S.L.; Azanchi, R.; Kaun, K.R. Automated real-time quantification of group locomotor activity in Drosophila melanogaster. Sci. Rep. 2019, 9, 2112. [Google Scholar] [CrossRef] [PubMed]
- Maia Chagas, A.; Prieto-Godino, L.L.; Arrenberg, A.B.; Baden, T. The €100 lab: A 3D-printable open-source platform for fluorescence microscopy, optogenetics, and accurate temperature control during behaviour of zebrafish, Drosophila, and Caenorhabditis elegans. PLoS Biol. 2017, 15, e2002702. [Google Scholar] [CrossRef] [PubMed]
- Wolf, F.W.; Rodan, A.R.; Tsai, L.T.-Y.; Heberlein, U. High-Resolution Analysis of Ethanol-Induced Locomotor Stimulation in Drosophila. J. Neurosci. 2002, 22, 11035–11044. [Google Scholar] [CrossRef]
- Seong, K.-H.; Matsumura, T.; Shimada-Niwa, Y.; Niwa, R.; Kang, S. The Drosophila Individual Activity Monitoring and Detection System (DIAMonDS). elife 2020, 9, 1321. [Google Scholar] [CrossRef]
- Donelson, N.; Kim, E.Z.; Slawson, J.B.; Vecsey, C.G.; Huber, R.; Griffith, L.C.; van Swinderen, B. High-Resolution Positional Tracking for Long-Term Analysis of Drosophila Sleep and Locomotion Using the “Tracker” Program. PLoS ONE 2012, 7, e37250. [Google Scholar] [CrossRef]
- Pfeiffenberger, C.; Lear, B.C.; Keegan, K.P.; Allada, R. Locomotor Activity Level Monitoring Using the Drosophila Activity Monitoring (DAM) System. Cold Spring Harb. Protoc. 2010, 2010, 1238–1241. [Google Scholar] [CrossRef]
- Vogler, G.; Ocorr, K. Visualizing the Beating Heart in Drosophila. J. Vis. Exp. 2009, e1425. [Google Scholar] [CrossRef]
- Pitnick, S.; Markow, T.A.; Spicer, G.S. Delayed male maturity is a cost of producing large sperm in Drosophila. Proc. Natl. Acad. Sci. USA 1995, 92, 10614–10618. [Google Scholar] [CrossRef]
- Klepsatel, P.; Gáliková, M.; Xu, Y.; Kühnlein, R.P. Thermal stress depletes energy reserves in Drosophila. Sci. Rep. 2016, 6, 229. [Google Scholar] [CrossRef]
- Miller, P.B.; Obrik-Uloho, O.T.; Phan, M.H.; Medrano, C.L.; Renier, J.S.; Thayer, J.L.; Wiessner, G.; Bloch Qazi, M.C. The song of the old mother: Reproductive senescence in female drosophila. Fly (Austin) 2014, 8, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Buck Louis, G.M. Fecundity and Fertility: Overview of human fecundity and fertility. In Reproductive and Perinatal Epidemiology, 1st ed.; Buck Louis, G.M., Platt, R.W., Eds.; Oxford University Press Inc.: New York, NY, USA, 2011; pp. 6–8. [Google Scholar]
- Gayathri, M.V.; Krishnamurthy, N.B. Studies on the toxicity of the mercurial fungicide Agallol 3 in Drosophila melanogaster. Environ. Res. 1981, 24, 89–95. [Google Scholar] [CrossRef]
- Menon, A.; Varma, V.; Sharma, V.K. Rhythmic egg-laying behaviour in virgin females of fruit flies Drosophila melanogaster. Chronobiol. Int. 2014, 31, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Kochar, E.; Prasad, N.G.; Schausberger, P. Egg Viability, Mating Frequency and Male Mating Ability Evolve in Populations of Drosophila melanogaster Selected for Resistance to Cold Shock. PLoS ONE 2015, 10, e0129992. [Google Scholar] [CrossRef][Green Version]
- Burke, M.K.; Barter, T.T.; Cabral, L.G.; Kezos, J.N.; Phillips, M.A.; Rutledge, G.A.; Phung, K.H.; Chen, R.H.; Nguyen, H.D.; Mueller, L.D.; et al. Rapid divergence and convergence of life-history in experimentally evolved Drosophila melanogaster. Evolution 2016, 70, 2085–2098. [Google Scholar] [CrossRef]
- Ng’oma, E.; King, E.G.; Middleton, K.M. A model-based high throughput method for fecundity estimation in fruit fly studies. Fly (Austin) 2018, 12, 183–190. [Google Scholar] [CrossRef]
- Novoseltsev, V.N.; Novoseltseva, J.A.; Yashin, A.I. What does a fly’s individual fecundity pattern look like?: The dynamics of resource allocation in reproduction and ageing. Mech. Ageing Dev. 2003, 124, 605–617. [Google Scholar] [CrossRef]
- Aruna, S.; Flores, H.A.; Barbash, D.A. Reduced Fertility of Drosophila melanogaster Hybrid male rescue (Hmr) Mutant Females Is Partially Complemented by Hmr Orthologs from Sibling Species. Genetics 2009, 181, 1437–1450. [Google Scholar] [CrossRef]
- Fricke, C.; Bretman, A.; Chapman, T. Adult male nutrition and reproductive success in Drosophila melanogaster. Evolution 2008, 62, 3170–3177. [Google Scholar] [CrossRef]
- Tan, C.K.W.; Pizzari, T.; Wigby, S. Parental age, gametic age, and inbreeding interact to modulate offspring viability in Drosophila melanogaster. Evolution 2013, 99, 3043–3051. [Google Scholar]
- Flaven-Pouchon, J.; Garcia, T.; Abed-Vieillard, D.; Farine, J.-P.; Ferveur, J.-F.; Everaerts, C. Transient and permanent experience with fatty acids changes Drosophila melanogaster preference and fitness. PLoS ONE 2014, 9, e92352. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.B.; Kim, J.H.; Kim, D.; Clark, J.M.; Park, Y. Conjugated Linoleic Acid Regulates Body Composition and Locomotor Activity in a Sex-Dependent Manner in Drosophila melanogaster. Lipids 2018, 53, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Zandawala, M.; Nguyen, T.; Balanyà Segura, M.; Johard, H.A.D.; Amcoff, M.; Wegener, C.; Paluzzi, J.-P.; Nässel, D.R.; Schoofs, L. A neuroendocrine pathway modulating osmotic stress in Drosophila. PLoS Genet. 2021, 17, e1009425. [Google Scholar] [CrossRef] [PubMed]
- Dillon, M.E.; Wang, G.; Garrity, P.A.; Huey, R.B. Thermal preference in Drosophila. J. Therm. Biol. 2009, 34, 109–119. [Google Scholar] [CrossRef]
- Hercus, M.J. Lifespan extension of Drosophila melanogaster through hormesis by repeated mild heat stress. Biogerontology 2003, 4, 149–156. [Google Scholar] [CrossRef]
- Bettencourt, B.R.; Hogan, C.C.; Nimali, M.; Drohan, B.W. Inducible and constitutive heat shock gene expression responds to modification of Hsp70 copy number in Drosophila melanogaster but does not compensate for loss of thermotolerance in Hsp70null flies. BMC Biol. 2008, 6, 155. [Google Scholar] [CrossRef]
- Heinrichsen, E.T.; Haddad, G.G.; Missirlis, F. Role of High-Fat Diet in Stress Response of Drosophila. PLoS ONE 2012, 7, e42587. [Google Scholar] [CrossRef]
- Andersen, J.L.; Manenti, T.; Sørensen, J.G.; MacMillan, H.A.; Loeschcke, V.; Overgaard, J.; Woods, A. How to assess Drosophila cold tolerance: Chill coma temperature and lower lethal temperature are the best predictors of cold distribution limits. Funct. Ecol. 2014, 29, 55–65. [Google Scholar] [CrossRef]
- Livingston, D.B.H.; Patel, H.; Donini, A.; MacMillan, H.A. Active transport of brilliant blue FCF across the Drosophila midgut and Malpighian tubule epithelia. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2020, 239, 110588. [Google Scholar] [CrossRef]
- Al-Momani, F.A.; Massadeh, A.M. Effect of Different Heavy-Metal Concentrations on Drosophila melanogaster Larval Growth and Development. Biol. Trace Elem. Res. 2005, 108, 271–278. [Google Scholar] [CrossRef]
- Bahadorani, S.; Hilliker, A.J. Biological and Behavioral Effects of Heavy Metals in Drosophila melanogaster Adults and Larvae. J. Insect Behav. 2009, 22, 399–411. [Google Scholar] [CrossRef]
- Tettweiler, G. Starvation and oxidative stress resistance in Drosophila are mediated through the eIF4E-binding protein, d4E-BP. Genes Dev. 2005, 19, 1840–1843. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Chaudhary, S.U.; Afzal, A.J.; Tariq, M. Starvation-Induced Dietary Behaviour in Drosophila melanogaster Larvae and Adults. Sci. Rep. 2015, 5, 956. [Google Scholar] [CrossRef] [PubMed]
- Krupp, J.J.; Nayal, K.; Wong, A.; Millar, J.G.; Levine, J.D. Desiccation resistance is an adaptive life-history trait dependent upon cuticular hydrocarbons, and influenced by mating status and temperature in D. melanogaster. J. Insect Physiol. 2020, 121, 103990. [Google Scholar] [CrossRef] [PubMed]
- Kaneuchi, T. Efficient measurement of H2O2 resistance in Drosophila using an activity monitor. Biogerontology 2003, 4, 157–165. [Google Scholar] [CrossRef]
- Jünger, M.A.; Rintelen, F.; Stocker, H.; Wasserman, J.D.; Végh, M.; Radimerski, T.; Greenberg, M.E.; Hafen, E. The Drosophila Forkhead Transcription Factor FOXO Mediates the Reduction in Cell Number Associated with Reduced Insulin Signaling. J. Biol. 2003, 2, 20. [Google Scholar] [CrossRef] [PubMed]
- Rzezniczak, T.Z.; Douglas, L.A.; Watterson, J.H.; Merritt, T.J.S. Paraquat administration in Drosophila for use in metabolic studies of oxidative stress. Anal. Biochem. 2011, 419, 345–347. [Google Scholar] [CrossRef]
- Lemaitre, B.; Reichhart, J.-M.; Hoffmann, J.A. Drosophila host defense: Differential induction of antimicrobial peptide genes after infection by various classes of microorganisms. Proc. Natl. Acad. Sci. USA 1997, 94, 14614–14619. [Google Scholar] [CrossRef]
- Ambrose, R.L.; Lander, G.C.; Maaty, W.S.; Bothner, B.; Johnson, J.E.; Johnson, K.N. Drosophila A virus is an unusual RNA virus with a T=3 icosahedral core and permuted RNA-dependent RNA polymerase. J. Gen. Virol. 2009, 90, 2191–2200. [Google Scholar] [CrossRef]
- Siva-Jothy, J.A.; Prakash, A.; Vasanthakrishnan, R.B.; Monteith, K.M.; Vale, P.F. Oral Bacterial Infection and Shedding in Drosophila melanogaster. J. Vis. Exp. 2018, 57676. [Google Scholar] [CrossRef]
- Woodcock, K.J.; Kierdorf, K.; Pouchelon, C.A.; Vivancos, V.; Dionne, M.S.; Geissmann, F. Macrophage-derived upd3 cytokine causes impaired glucose homeostasis and reduced lifespan in Drosophila fed a lipid-rich diet. Immunity 2015, 42, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Hariri, N.; Thibault, L. High-fat diet-induced obesity in animal models. Nutr. Res. Rev. 2010, 23, 270–299. [Google Scholar] [CrossRef]
- Gáliková, M.; Klepsatel, P. Obesity and Aging in the Drosophila Model. Int. J. Mol. Sci. 2018, 19, 1896. [Google Scholar] [CrossRef] [PubMed]
- Raubenheimer, D.; Machovsky-Capuska, G.E.; Chapman, C.A.; Rothman, J.M. Geometry of nutrition in field studies: An illustration using wild primates. Oecologia 2015, 177, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Buescher, J.L.; Musselman, L.P.; Wilson, C.A.; Lang, T.; Keleher, M.; Baranski, T.J.; Duncan, J.G. Evidence for transgenerational metabolic programming in Drosophila. Dis. Model. Mech. 2013, 6, 1123–1132. [Google Scholar] [CrossRef] [PubMed]
- Palanker Musselman, L.; Fink, J.L.; Narzinski, K.; Ramachandran, P.V.; Sukumar Hathiramani, S.; Cagan, R.L.; Baranski, T.J. A high-sugar diet produces obesity and insulin resistance in wild-type Drosophila. Dis. Model. Mech. 2011, 4, 842–849. [Google Scholar] [CrossRef]
- Colinet, H.; Larvor, V.; Bical, R.; Renault, D. Dietary sugars affect cold tolerance of Drosophila melanogaster. Metabolomics 2013, 9, 608–622. [Google Scholar] [CrossRef]
- May, C.E.; Vaziri, A.; Lin, Y.Q.; Grushko, O.; Khabiri, M.; Wang, Q.-P.; Holme, K.J.; Pletcher, S.D.; Freddolino, P.L.; Neely, G.G.; et al. High Dietary Sugar Reshapes Sweet Taste to Promote Feeding Behavior in Drosophila melanogaster. Cell Rep. 2019, 27, 1675–1685.e7. [Google Scholar] [CrossRef]
- Wilinski, D.; Winzeler, J.; Duren, W.; Persons, J.L.; Holme, K.J.; Mosquera, J.; Khabiri, M.; Kinchen, J.M.; Freddolino, P.L.; Karnovsky, A.; et al. Rapid metabolic shifts occur during the transition between hunger and satiety in Drosophila melanogaster. Nat. Commun. 2019, 10, 4052. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, G.; Jin, L.H. A high-sugar diet affects cellular and humoral immune responses in Drosophila. Exp. Cell Res. 2018, 368, 215–224. [Google Scholar] [CrossRef]
- Villanueva, J.E.; Livelo, C.; Trujillo, A.S.; Chandran, S.; Woodworth, B.; Andrade, L.; Le, H.D.; Manor, U.; Panda, S.; Melkani, G.C. Time-restricted feeding restores muscle function in Drosophila models of obesity and circadian-rhythm disruption. Nat. Commun. 2019, 10, 2700. [Google Scholar] [CrossRef] [PubMed]
- Na, J.; Musselman, L.P.; Pendse, J.; Baranski, T.J.; Bodmer, R.; Ocorr, K.; Cagan, R.; Rulifson, E. A Drosophila Model of High Sugar Diet-Induced Cardiomyopathy. PLoS Genet. 2013, 9, e1003175. [Google Scholar] [CrossRef] [PubMed]
- Dobson, A.J.; Ezcurra, M.; Flanagan, C.E.; Summerfield, A.C.; Piper, M.D.W.; Gems, D.; Alic, N. Nutritional Programming of Lifespan by FOXO Inhibition on Sugar-Rich Diets. Cell Rep. 2017, 18, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Van Dam, E.; van Leeuwen, L.A.G.; dos Santos, E.; James, J.; Best, L.; Lennicke, C.; Vincent, A.J.; Marinos, G.; Foley, A.; Buricova, M.; et al. Sugar-Induced Obesity and Insulin Resistance Are Uncoupled from Shortened Survival in Drosophila. Cell Metab. 2020, 31, 710–725.e7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jin, Q.; Jin, L.H. High sugar diet disrupts gut homeostasis though JNK and STAT pathways in Drosophila. Biochem. Biophys. Res. Commun. 2017, 487, 910–916. [Google Scholar] [CrossRef]
- Pereira, M.T.; Malik, M.; Nostro, J.A.; Mahler, G.J.; Musselman, L.P. Effect of dietary additives on intestinal permeability in both Drosophila and a human cell co-culture. Dis. Model Mech. 2018, 11, dmm034520. [Google Scholar] [CrossRef] [PubMed]
- Rani, L.; Saini, S.; Shukla, N.; Chowdhuri, D.K.; Gautam, N.K. High sucrose diet induces morphological, structural and functional impairments in the renal tubules of Drosophila melanogaster: A model for studying type-2 diabetes mediated renal tubular dysfunction. Insect Biochem. Mol. Biol. 2020, 125, 103441. [Google Scholar] [CrossRef]
- Catalani, E.; Silvestri, F.; Bongiorni, S.; Taddei, A.R.; Fanelli, G.; Rinalducci, S.; de Palma, C.; Perrotta, C.; Prantera, G.; Cervia, D. Retinal damage in a new model of hyperglycemia induced by high-sucrose diets. Pharmacol. Res. 2021, 166, 105488. [Google Scholar] [CrossRef]
- Brookheart, R.T.; Swearingen, A.R.; Collins, C.A.; Cline, L.M.; Duncan, J.G. High-sucrose-induced maternal obesity disrupts ovarian function and decreases fertility in Drosophila melanogaster. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1255–1263. [Google Scholar] [CrossRef]
- Öst, A.; Lempradl, A.; Casas, E.; Weigert, M.; Tiko, T.; Deniz, M.; Pantano, L.; Boenisch, U.; Itskov, P.M.; Stoeckius, M.; et al. Paternal Diet Defines Offspring Chromatin State and Intergenerational Obesity. Cell 2014, 159, 1352–1364. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Amcoff, M.; Nässel, D.R. Impact of high-fat diet on lifespan, metabolism, fecundity and behavioral senescence in Drosophila. Insect Biochem. Mol. Biol. 2020, 133, 103495. [Google Scholar] [CrossRef] [PubMed]
- Oldham, S. Obesity and nutrient sensing TOR pathway in flies and vertebrates: Functional conservation of genetic mechanisms. Trends Endocrinol. Metab. 2011, 22, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Kim, D.-I.; Han, G.-Y.; Kwon, H. The Effects of High Fat Diet-Induced Stress on Olfactory Sensitivity, Behaviors, and Transcriptional Profiling in Drosophila melanogaster. Int. J. Mol. Sci. 2018, 19, 2855. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Song, T.; Su, H.; Lai, Z.; Qin, W.; Tian, Y.; Dong, X.; Wang, L. High-fat diet enhances starvation-induced hyperactivity via sensitizing hunger-sensing neurons in Drosophila. elife 2020, 9, e53103. [Google Scholar] [CrossRef]
- Wen, D.-T.; Zheng, L.; Yang, F.; Li, H.-Z.; Hou, W.-Q. Endurance exercise prevents high-fat-diet induced heart and mobility premature aging and dsir2 expression decline in aging Drosophila. Oncotarget 2018, 9, 7298. [Google Scholar] [CrossRef]
- Schultzhaus, J.N.; Bennett, C.J.; Iftikhar, H.; Yew, J.Y.; Mallett, J.; Carney, G.E. High fat diet alters Drosophila melanogaster sexual behavior and traits: Decreased attractiveness and changes in pheromone profiles. Sci. Rep. 2018, 8, 647. [Google Scholar] [CrossRef]
- Nazario-Yepiz, N.O.; Loustalot-Laclette, M.R.; Carpinteyro-Ponce, J.; Abreu-Goodger, C.; Markow, T.A.; Missirlis, F. Transcriptional responses of ecologically diverse Drosophila species to larval diets differing in relative sugar and protein ratios. PLoS ONE 2017, 12, e0183007. [Google Scholar] [CrossRef]
- Loreto, J.S.; Ferreira, S.A.; Ardisson-Araújo, D.M.P.; Barbosa, N.V. Human type 2 diabetes mellitus-associated transcriptional disturbances in a high-sugar diet long-term exposed Drosophila melanogaster. Comp. Biochem. Physiol. Part D Genomics Proteomics 2021, 39, 100866. [Google Scholar] [CrossRef]
- Hemphill, W.; Rivera, O.; Talbert, M. RNA-Sequencing of Drosophila melanogaster Head Tissue on High-Sugar and High-Fat Diets. G3 (Bethesda) 2018, 8, 279–290. [Google Scholar] [CrossRef]
- Ng’oma, E.; Williams-Simon, P.A.; Rahman, A.; King, E.G. Diverse biological processes coordinate the transcriptional response to nutritional changes in a Drosophila melanogaster multiparent population. BMC Genom. 2020, 21, 686. [Google Scholar] [CrossRef]
- Ye, J.; Cui, X.; Loraine, A.; Bynum, K.; Kim, N.C.; White, G.; De Luca, M.; Garfinkel, M.G.; Lu, X.; Ruden, D.M. Methods for nutrigenomics and longevity studies in Drosophila: Effects of diets high in sucrose, palmitic acid, soy, or beef. Methods Mol. Biol. 2007, 371, 111–141. [Google Scholar] [PubMed]
- Ruden, D.M.; de Luca, M.; Garfinkel, M.D.; Bynum, K.L.; Lu, X. DROSOPHILA NUTRIGENOMICS CAN PROVIDE CLUES TO HUMAN GENE-NUTRIENT INTERACTIONS. Annu. Rev. Nutr. 2005, 25, 499–522. [Google Scholar] [CrossRef] [PubMed]
- Heinrichsen, E.T.; Zhang, H.; Robinson, J.E.; Ngo, J.; Diop, S.; Bodmer, R.; Joiner, W.J.; Metallo, C.M.; Haddad, G.G. Metabolic and transcriptional response to a high-fat diet in Drosophila melanogaster. Mol. Metab. 2014, 3, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Rivera, O.; McHan, L.; Konadu, B.; Patel, S.; Sint Jago, S.; Talbert, M.E. A high-fat diet impacts memory and gene expression of the head in mated female Drosophila melanogaster. J. Comp. Physiol. B 2019, 189, 179–198. [Google Scholar] [CrossRef] [PubMed]
- Grönke, S.M. Identification and characterization of genes controlling energy homeostasis in Drosophila melanogaster. Ph.D. Dissertation, Technischen Universität Carolo-Wilhelmina, Braunschweig, Germany, 2005. [Google Scholar]
- Stobdan, T.; Sahoo, D.; Azad, P.; Hartley, I.; Heinrichsen, E.; Zhou, D.; Haddad, G.G.; Skoulakis, E.M.C. High fat diet induces sex-specific differential gene expression in Drosophila melanogaster. PLoS ONE 2019, 14, e0213474. [Google Scholar] [CrossRef]
- Fabian, L.; Brill, J.A. Drosophila spermiogenesis. Spermatogenesis 2014, 2, 197–212. [Google Scholar] [CrossRef]
- Ekengren, S.; Hultmark, D. A Family of Turandot-Related Genes in the Humoral Stress Response of Drosophila. Biochem. Biophys. Res. Commun. 2001, 284, 998–1003. [Google Scholar] [CrossRef]
- Azuma, M.; Fat Le, T.; Yoshimoto, Y.; Hiraki, N.; Yamanaka, M.; Omura, F.; Inoue, Y.H. RNA-seq analysis of diet-driven obesity and anti-obesity effects of quercetin glucoside or epigallocatechin gallate in Drosophila adults. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 857–876. [Google Scholar]
- Reed, L.K.; Lee, K.; Zhang, Z.; Rashid, L.; Poe, A.; Hsieh, B.; Deighton, N.; Glassbrook, N.; Bodmer, R.; Gibson, G. Systems Genomics of Metabolic Phenotypes in Wild-Type Drosophila melanogaster. Genetics 2014, 197, 781–793. [Google Scholar] [CrossRef]
- Williams, S.; Dew-Budd, K.; Davis, K.; Anderson, J.; Bishop, R.; Freeman, K.; Davis, D.; Bray, K.; Perkins, L.; Hubickey, J.; et al. Metabolomic and Gene Expression Profiles Exhibit Modular Genetic and Dietary Structure Linking Metabolic Syndrome Phenotypes in Drosophila. G3 (Bethesda) 2015, 5, 2817–2829. [Google Scholar] [CrossRef]
- Enell, L.E.; Kapan, N.; Söderberg, J.A.E.; Kahsai, L.; Nässel, D.R.; Bergmann, A. Insulin Signaling, Lifespan and Stress Resistance Are Modulated by Metabotropic GABA Receptors on Insulin Producing Cells in the Brain of Drosophila. PLoS ONE 2010, 5, e15780. [Google Scholar] [CrossRef] [PubMed]
- Simard, C.J.; Touaibia, M.; Allain, E.P.; Hebert-Chatelain, E.; Pichaud, N. Role of the Mitochondrial Pyruvate Carrier in the Occurrence of Metabolic Inflexibility in Drosophila melanogaster Exposed to Dietary Sucrose. Metabolites 2020, 10, 411. [Google Scholar] [CrossRef] [PubMed]
- Bricker, D.K.; Taylor, E.B.; Schell, J.C.; Orsak, T.; Boutron, A.; Chen, Y.-C.; Cox, J.E.; Cardon, C.M.; van Vranken, J.G.; Dephoure, N.; et al. A Mitochondrial Pyruvate Carrier Required for Pyruvate Uptake in Yeast, Drosophila, and Humans. Science 2012, 337, 96–100. [Google Scholar] [CrossRef]
- Gillette, C.M.; Hazegh, K.E.; Nemkov, T.; Stefanoni, D.; D’Alessandro, A.; Taliaferro, J.M.; Reis, T. Gene–Diet Interactions: Dietary Rescue of Metabolic Defects in spen-Depleted Drosophila melanogaster. Genetics 2020, 214, 961–975. [Google Scholar] [CrossRef]
- Tuthill, B.F.; Searcy, L.A.; Yost, R.A.; Musselman, L.P. Tissue-specific analysis of lipid species in Drosophila during overnutrition by UHPLC-MS/MS and MALDI-MSI. J. Lipid Res. 2020, 61, 275–290. [Google Scholar] [CrossRef]
- Hardy, C.M.; Birse, R.T.; Wolf, M.J.; Yu, L.; Bodmer, R.; Gibbs, A.G. Obesity-associated cardiac dysfunction in starvation-selected Drosophila melanogaster. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 309, R658–R667. [Google Scholar] [CrossRef] [PubMed]
- Oza, V.; Aicher, J.; Reed, L. Random Forest Analysis of Untargeted Metabolomics Data Suggests Increased Use of Omega Fatty Acid Oxidation Pathway in Drosophila Melanogaster Larvae Fed a Medium Chain Fatty Acid Rich High-Fat Diet. Metabolites 2019, 9, 5. [Google Scholar] [CrossRef]
- Cormier, R.J.; Strang, R.; Menail, H.; Touaibia, M.; Pichaud, N. Systemic and mitochondrial effects of metabolic inflexibility induced by high fat diet in Drosophila melanogaster. Insect Biochem. Mol. Biol. 2021, 133, 103556. [Google Scholar] [CrossRef]
- Miguel-Aliaga, I.; Jasper, H.; Lemaitre, B. Anatomy and Physiology of the Digestive Tract of Drosophila melanogaster. Genetics 2018, 210, 357–396. [Google Scholar]
- Horne, I.; Haritos, V.S.; Oakeshott, J.G. Comparative and functional genomics of lipases in holometabolous insects. Insect Biochem. Mol. Biol. 2009, 39, 547–567. [Google Scholar] [CrossRef]
- Sieber, M.H.; Thummel, C.S. Coordination of Triacylglycerol and Cholesterol Homeostasis by DHR96 and the Drosophila LipA Homolog magro. Cell Metab. 2012, 15, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Grönke, S.; Mildner, A.; Fellert, S.; Tennagels, N.; Petry, S.; Müller, G.; Jäckle, H.; Kühnlein, R.P. Brummer lipase is an evolutionary conserved fat storage regulator in Drosophila. Cell Metab. 2005, 1, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Chapman, R.F. The Insects: Structure and Function, 5th ed.; Chapman, R.F., Simpson, S.J., Douglas, A.E., Eds.; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Demignot, S.; Beilstein, F.; Morel, E. Triglyceride-rich lipoproteins and cytosolic lipid droplets in enterocytes: Key players in intestinal physiology and metabolic disorders. Biochimie 2014, 96, 48–55. [Google Scholar] [CrossRef]
- Heier, C.; Kühnlein, R.P. Triacylglycerol Metabolism in Drosophila melanogaster. Genetics 2018, 210, 1163–1184. [Google Scholar] [CrossRef]
- Voght, S.P.; Fluegel, M.L.; Andrews, L.A.; Pallanck, L.J. Drosophila NPC1b Promotes an Early Step in Sterol Absorption from the Midgut Epithelium. Cell Metab. 2007, 5, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Majerowicz, D.; Gondim, K. Insect Lipid Metabolism: Insights into Gene Expression Regulation: Recent Trends in Gene Expression. In Recent Trends in Gene Expression, 1st ed; Mandal, S.S., Ed.; Nova Science Publishers: New York, NY, USA, 2013; pp. 147–190. [Google Scholar]
- Palm, W.; Sampaio, J.L.; Brankatschk, M.; Carvalho, M.; Mahmoud, A.; Shevchenko, A.; Eaton, S. Lipoproteins in Drosophila melanogaster—Assembly, Function, and Influence on Tissue Lipid Composition. PLoS Genet 2012, 8, e1002828. [Google Scholar] [CrossRef]
- Gesta, S.; Tseng, Y.-H.; Kahn, C.R. Developmental Origin of Fat: Tracking Obesity to Its Source. Cell 2007, 131, 242–256. [Google Scholar] [CrossRef]
- Ameer, F.; Scandiuzzi, L.; Hasnain, S.; Kalbacher, H.; Zaidi, N. De novo lipogenesis in health and disease. Metabolism 2014, 63, 895–902. [Google Scholar] [CrossRef]
- Biesalski, H.K.; Grimm, P.; Nowitzki-Grimm, S. Taschenatlas Ernährung, 8th ed.; Georg Thieme Verlag: Stuttgart, Germany, 2020. [Google Scholar]
- Coleman, R.A.; Mashek, D.G. Mammalian Triacylglycerol Metabolism: Synthesis, Lipolysis, and Signaling. Chem. Rev. 2011, 111, 6359–6386. [Google Scholar] [CrossRef]
- Bi, J.; Xiang, Y.; Chen, H.; Liu, Z.; Grönke, S.; Kühnlein, R.P.; Huang, X. Opposite and redundant roles of the two Drosophila perilipins in lipid mobilization. J. Cell Sci. 2012, 125, 3568–3577. [Google Scholar] [CrossRef]
- Duncan, R.E.; Ahmadian, M.; Jaworski, K.; Sarkadi-Nagy, E.; Sul, H.S. Regulation of Lipolysis in Adipocytes. Annu. Rev. Nutr. 2007, 27, 79–101. [Google Scholar] [CrossRef] [PubMed]
- Thaker, V.V. Genetic and epigenetic causes of obesity. Adolesc. Med. State Art Rev. 2017, 28, 379–405. [Google Scholar] [PubMed]
- Grönke, S.; Müller, G.; Hirsch, J.; Fellert, S.; Andreou, A.; Haase, T.; Jäckle, H.; Kühnlein, R.P.; O’Rahilly, S. Dual Lipolytic Control of Body Fat Storage and Mobilization in Drosophila. PLoS Biol. 2007, 5, e137. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, R.; Xie, H.; Schweiger, M. Of mice and men: The physiological role of adipose triglyceride lipase (ATGL). Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 880–899. [Google Scholar] [CrossRef]
- Guilleminault, L.; Williams, E.; Scott, H.; Berthon, B.; Jensen, M.; Wood, L. Diet and Asthma: Is It Time to Adapt Our Message? Nutrients 2017, 9, 1227. [Google Scholar] [CrossRef]
- Hu, F.B. Dietary pattern analysis: A new direction in nutritional epidemiology. Curr. Opin. Lipidol. 2002, 13, 3–9. [Google Scholar] [CrossRef]
- Roser, M.; Ritchie, H. Food Supply. Available online: https://ourworldindata.org/food-supply (accessed on 1 November 2021).
- Caballero, B. Humans against Obesity: Who Will Win? Adv. Nutr. 2019, 10, S4–S9. [Google Scholar] [CrossRef]
- Kopp, W. How Western Diet And Lifestyle Drive The Pandemic Of Obesity And Civilization Diseases. Diabetes Metab. Syndr. Obes. 2019, 12, 2221–2236. [Google Scholar] [CrossRef]
- Swinburn, B.A.; Caterson, I.; Seidell, J.C.; James, W.P. Diet, nutrition and the prevention of excess weight gain and obesity. Public Health Nutr. 2004, 7, 123–146. [Google Scholar]
- NCD-RisC. Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet 2016, 387, 1377–1396. [Google Scholar] [CrossRef]
- de Moura e Dias, M.; dos Reis, S.A.; da Conceição, L.L.; de Oliveira Sediyama, C.M.N.; Pereira, S.S.; de Oliveira, L.L.; do Carmo Gouveia Peluzio, M.; Martinez, J.A.; Milagro, F.I. Diet-induced obesity in animal models: Points to consider and influence on metabolic markers. Diabetol. Metab. Syndr. 2021, 13, 47. [Google Scholar] [CrossRef] [PubMed]
- Bortolin, R.C.; Vargas, A.R.; Gasparotto, J.; Chaves, P.R.; Schnorr, C.E.; Martinello, K.B.; Silveira, A.K.; Rabelo, T.K.; Gelain, D.P.; Moreira, J.C.F. A new animal diet based on human Western diet is a robust diet-induced obesity model: Comparison to high-fat and cafeteria diets in term of metabolic and gut microbiota disruption. Int. J. Obes. 2018, 42, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, N.J.; Stock, M.J. The development of obesity in animals: The role of dietary factors. Clin. Endocrinol. Metab. 1984, 13, 437–449. [Google Scholar] [CrossRef]
- Murashov, A.K.; Pak, E.S.; Lin, C.-T.; Boykov, I.N.; Buddo, K.A.; Mar, J.; Bhat, K.M.; Neufer, P.D. Preference and detrimental effects of high fat, sugar, and salt diet in wild-caught Drosophila simulans are reversed by flight exercise. FASEB Bioadv. 2021, 3, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Drewnowski, A.; Kurth, C.; Holden-Wiltse, J.; Saari, J. Food preferences in human obesity: Carbohydrates versus fats. Appetite 1992, 18, 207–221. [Google Scholar] [CrossRef]
- Lampuré, A.; Schlich, P.; Deglaire, A.; Castetbon, K.; Péneau, S.; Hercberg, S.; Méjean, C. Sociodemographic, Psychological, and Lifestyle Characteristics Are Associated with a Liking for Salty and Sweet Tastes in French Adults. Br. J. Nutr. 2014, 112, 1353–1363. [Google Scholar] [CrossRef] [PubMed]
- Bartoshuk, L.M.; Duffy, V.B.; Hayes, J.E.; Moskowitz, H.R.; Snyder, D.J. Psychophysics of sweet and fat perception in obesity: Problems, solutions and new perspectives. Phil. Trans. R. Soc. B 2006, 361, 1137–1148. [Google Scholar] [CrossRef]
- Proserpio, C.; Laureati, M.; Bertoli, S.; Battezzati, A.; Pagliarini, E. Determinants of Obesity in Italian Adults: The Role of Taste Sensitivity, Food Liking, and Food Neophobia. Chem. Senses 2015, 4, bjv072. [Google Scholar] [CrossRef]
- Berthoud, H.-R.; Zheng, H. Modulation of taste responsiveness and food preference by obesity and weight loss. Physiol. Behav. 2012, 107, 527–532. [Google Scholar] [CrossRef]
- Spinelli, S.; Monteleone, E. Food Preferences and Obesity. Endocrinol. Metab. 2021, 36, 209–219. [Google Scholar] [CrossRef]
- Deutsche Gesellschaft für Ernährung; Österreichische Gesellschaft für Ernährung; Schweizerische Gesellschaft für Ernährungsforschung. D-A-CH-Referenzwerte für die Nährstoffzufuhr, 2nd ed.; Neuer Umschau Buchverl.: Neustadt an der Weinstraße, Germany, 2015. [Google Scholar]
- HHS; USDA. Dietary Guidelines for Americans, 2020–2025: Make Every Bite Count with the Dietary Guidelines, 9th ed.; U.S. Department of Agriculture: Washington, DC, USA, 2020. [Google Scholar]
- Wolfram, G.; Bechthold, A.; Boeing, H.; Ellinger, S.; Hauner, H.; Kroke, A.; Leschik-Bonnet, E.; Linseisen, J.; Lorkowski, S.; Schulze, M.; et al. Evidence-Based Guideline of the German Nutrition Society: Fat Intake and Prevention of Selected Nutrition-Related Diseases. Ann. Nutr. Metab. 2015, 67, 141–204. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Han, T.; Chu, X.; Lu, H.; Yang, X.; Zi, T.; Zhao, Y.; Wang, X.; Liu, Z.; Ruan, J.; et al. An isocaloric moderately high-fat diet extends lifespan in male rats and Drosophila. Cell Metab. 2021, 33, 581–597.e9. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, Z.; Syed, S.; Lyu, C.; Lincoln, S.; O’Nell, J.; Nguyen, A.D.; Feng, I.; Young, M.W. Chronic social isolation signals starvation and reduces sleep in Drosophila. Nature 2021, 597, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Lebedev, A.A.; Moskalev, A.R.; Abrosimov, M.E.; Vetlugin, E.A.; Pshenichnaya, A.G.; Lebedev, V.A.; Evdokimova, N.R.; Bychkov, E.R.; Shabanov, P.D. Effect of neuropeptide Y antagonist BMS193885 on overeating and emotional responses induced by social isolation in rats. Rev. Clin. Pharm. Drug Ther. 2021, 19, 189–202. [Google Scholar] [CrossRef]
- Anversa, R.G.; Campbell, E.J.; Ch’ng, S.S.; Gogos, A.; Lawrence, A.J.; Brown, R.M. A model of emotional stress-induced binge eating in female mice with no history of food restriction. Genes Brain Behav. 2019, 19, e12613. [Google Scholar] [CrossRef]
- Dos Santos Quaresma, M.V.L.; Marques, C.G.; Magalhães, A.C.O.; dos Santos, R.V.T. Emotional eating, binge eating, physical inactivity, and vespertine chronotype are negative predictors of dietary practices during COVID-19 social isolation: A cross-sectional study. Nutrition 2021, 90, 111223. [Google Scholar] [CrossRef]
- Kalkan Uğurlu, Y.; Mataracı Değirmenci, D.; Durgun, H.; Gök Uğur, H. The examination of the relationship between nursing students’ depression, anxiety and stress levels and restrictive, emotional, and external eating behaviors in COVID-19 social isolation process. Perspect. Psychiatr. Care 2021, 57, 507–516. [Google Scholar] [CrossRef]
- Zhang, Q. Characterization of Circadian Feeding Rhythms in Drosophila Using the Fly Liquid-Food Interaction Counter (FLIC) Assay. Ph.D. Dissertation, University of Michigan, Ann Arbor, MI, USA, 2016. [Google Scholar]
- Harbison, S.T.; Sehgal, A.; Louis, M. Energy Stores Are Not Altered by Long-Term Partial Sleep Deprivation in Drosophila melanogaster. PLoS ONE 2009, 4, e6211. [Google Scholar] [CrossRef]
- Froy, O. Circadian Rhythms and Obesity in Mammals. ISRN Obes. 2012, 2012, 437198. [Google Scholar] [CrossRef][Green Version]
- Sherman, H.; Genzer, Y.; Cohen, R.; Chapnik, N.; Madar, Z.; Froy, O. Timed high-fat diet resets circadian metabolism and prevents obesity. FASEB J. 2012, 26, 3493–3502. [Google Scholar] [CrossRef]
- Marcheva, B.; Ramsey, K.M.; Buhr, E.D.; Kobayashi, Y.; Su, H.; Ko, C.H.; Ivanova, G.; Omura, C.; Mo, S.; Vitaterna, M.H.; et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature 2010, 466, 627–631. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; He, L.; Perrimon, N. Regulation of insulin and adipokinetic hormone/glucagon production in flies. WIREs Dev Biol 2020, 9, 65. [Google Scholar] [CrossRef] [PubMed]
- Nässel, D.R.; Kubrak, O.I.; Liu, Y.; Luo, J.; Lushchak, O.V. Factors that regulate insulin producing cells and their output in Drosophila. Front. Physiol. 2013, 4, 252. [Google Scholar] [CrossRef]
- Claeys, I.; Simonet, G.; Poels, J.; van Loy, T.; Vercammen, L.; de Loof, A.; Vanden Broeck, J. Insulin-related peptides and their conserved signal transduction pathway. Peptides 2002, 23, 807–816. [Google Scholar] [CrossRef]
- Blenis, J. Signal transduction via the MAP kinases: Proceed at your own RSK. Proc. Natl. Acad. Sci. USA 1993, 90, 5889–5892. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, P.R.; Withers, D.J.; Siddle, K. Phosphoinositide 3-kinase: The key switch mechanism in insulin signalling. Biochem. J. 1998, 333, 471–490. [Google Scholar] [CrossRef]
- Rintelen, F.; Stocker, H.; Thomas, G.; Hafen, E. PDK1 regulates growth through Akt and S6K in Drosophila. Proc. Natl. Acad. Sci. USA 2001, 98, 15020–15025. [Google Scholar] [CrossRef]
- Wessells, R.; Fitzgerald, E.; Piazza, N.; Ocorr, K.; Morley, S.; Davies, C.; Lim, H.-Y.; Elmén, L.; Hayes, M.; Oldham, S.; et al. d4eBP acts downstream of both dTOR and dFoxo to modulate cardiac functional aging in Drosophila. Aging Cell 2009, 8, 542–552. [Google Scholar] [CrossRef]
- Morris, S.N.S.; Coogan, C.; Chamseddin, K.; Fernandez-Kim, S.O.; Kolli, S.; Keller, J.N.; Bauer, J.H. Development of diet-induced insulin resistance in adult Drosophila melanogaster. Biochim. Biophys. Acta 2012, 1822, 1230–1237. [Google Scholar] [CrossRef]
- Reaven, G.M. Pathophysiology of insulin resistance in human disease. Physiol. Rev. 1995, 75, 473–486. [Google Scholar] [CrossRef]
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006, 444, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Ugur, B.; Chen, K.; Bellen, H.J. Drosophila tools and assays for the study of human diseases. Dis. Model. Mech. 2016, 9, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Choma, M.A.; Suter, M.J.; Vakoc, B.J.; Bouma, B.E.; Tearney, G.J. Physiological homology between Drosophila melanogaster and vertebrate cardiovascular systems. Dis. Model. Mech. 2011, 4, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Csige, I.; Ujvárosy, D.; Szabó, Z.; Lőrincz, I.; Paragh, G.; Harangi, M.; Somodi, S. The Impact of Obesity on the Cardiovascular System. J. Diabetes Res. 2018, 2018, 3407306. [Google Scholar] [CrossRef] [PubMed]
- Bailey, A.P.; Koster, G.; Guillermier, C.; Hirst, E.M.A.; MacRae, J.I.; Lechene, C.P.; Postle, A.D.; Gould, A.P. Antioxidant Role for Lipid Droplets in a Stem Cell Niche of Drosophila. Cell 2015, 163, 340–353. [Google Scholar] [CrossRef]
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of liver diseases in the world. J. Hepatol. 2019, 70, 151–171. [Google Scholar] [CrossRef]
- Gutierrez, E.; Wiggins, D.; Fielding, B.; Gould, A.P. Specialized hepatocyte-like cells regulate Drosophila lipid metabolism. Nature 2007, 445, 275–280. [Google Scholar] [CrossRef]
- Moraes, K.C.M.; Montagne, J. Drosophila melanogaster: A Powerful Tiny Animal Model for the Study of Metabolic Hepatic Diseases. Front. Physiol. 2021, 12, 390. [Google Scholar] [CrossRef]
- Allocca, M.; Zola, S.; Bellosta, P. The Fruit Fly, Drosophila melanogaster: Modeling of Human Diseases (Part II). In Drosophila melanogaster - Model for Recent Advances in Genetics and Therapeutics, 1st ed.; Perveen, F.K., Ed.; InTech: Rijeka, Croatia, 2018; pp. 138–139. [Google Scholar]
- Na, J.; Sweetwyne, M.T.; Park, A.S.D.; Susztak, K.; Cagan, R.L. Diet-Induced Podocyte Dysfunction in Drosophila and Mammals. Cell Rep. 2015, 12, 636–647. [Google Scholar] [CrossRef]
- Zhuang, S.; Shao, H.; Guo, F.; Trimble, R.; Pearce, E.; Abmayr, S.M. Sns and Kirre, the Drosophila orthologs of Nephrin and Neph1, direct adhesion, fusion and formation of a slit diaphragm-like structure in insect nephrocytes. Development 2009, 136, 2335–2344. [Google Scholar] [CrossRef]
- Kovesdy, C.P.; Furth, S.; Zoccali, C.; on Behalf of the World Kidney Day Steering Comittee. Obesity and kidney disease: Hidden consequences of the epidemic. Indian J. Nephrol. 2017, 27, 85. [Google Scholar] [CrossRef] [PubMed]
- Wiederkehr, M.R.; Moe, O.W. Uric Acid Nephrolithiasis: A Systemic Metabolic Disorder. Clinic Rev. Bone Miner. Metab. 2011, 9, 207–217. [Google Scholar] [CrossRef]
- Poore, W.; Boyd, C.J.; Singh, N.P.; Wood, K.; Gower, B.; Assimos, D.G. Obesity and Its Impact on Kidney Stone Formation. Rev. Urol. 2020, 22, 17–23. [Google Scholar] [PubMed]
- Chen, Y.-H.; Liu, H.-P.; Chen, H.-Y.; Tsai, F.-J.; Chang, C.-H.; Lee, Y.-J.; Lin, W.-Y.; Chen, W.-C. Ethylene glycol induces calcium oxalate crystal deposition in Malpighian tubules: A Drosophila model for nephrolithiasis/urolithiasis. Kidney Int. 2011, 80, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, E.; Xu, E.Y. Identification and characterization of novel mammalian spermatogenic genes conserved from fly to human. Mol. Hum. Reprod. 2008, 14, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, J.; Wigby, S. Differential effects of male nutrient balance on pre- and post-copulatory traits, and consequences for female reproduction in Drosophila melanogaster. Sci. Rep. 2016, 6, 1415. [Google Scholar] [CrossRef]
- Campos-Silva, P.; Furriel, A.; Costa, W.S.; Sampaio, F.J.B.; Gregório, B.M. Metabolic and Testicular Effects of the Long-Term Administration of Different High-Fat Diets in Adult Rats. Int. Braz. J. Urol. 2015, 41, 569–575. [Google Scholar] [CrossRef]
- Leisegang, K.; Sengupta, P.; Agarwal, A.; Henkel, R. Obesity and male infertility: Mechanisms and management. Andrologia 2021, 53, 2380. [Google Scholar] [CrossRef]
- Armstrong, A.R. Drosophila melanogaster as a model for nutrient regulation of ovarian function. Reproduction 2020, 159, R69–R82. [Google Scholar] [CrossRef]
- Depalo, R.; Garruti, G.; Totaro, I.; Panzarino, M.; Vacca, M.P.; Giorgino, F.; Selvaggi, L.E. Oocyte morphological abnormalities in overweight women undergoing in vitro fertilization cycles. Gynecol. Endocrinol. 2011, 27, 880–884. [Google Scholar] [CrossRef]
- Leary, C.; Leese, H.J.; Sturmey, R.G. Human embryos from overweight and obese women display phenotypic and metabolic abnormalities. Hum. Reprod. 2014, 30, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Practice Committee of the American Society for Reproductive Medicine. Obesity and reproduction: A committee opinion. Fertil. Steril. 2015, 104, 1116–1126. [Google Scholar] [CrossRef] [PubMed]
- Snider, A.P.; Wood, J.R. Obesity induces ovarian inflammation and reduces oocyte quality. Reproduction 2019, 158, R79–R90. [Google Scholar] [CrossRef] [PubMed]
- Groh, B.S.; Yan, F.; Smith, M.D.; Yu, Y.; Chen, X.; Xiong, Y. The antiobesity factor WDTC 1 suppresses adipogenesis via the CRL 4 WDTC1 E3 ligase. EMBO Rep. 2016, 17, 638–647. [Google Scholar] [CrossRef]
- Suh, J.M.; Zeve, D.; McKay, R.; Seo, J.; Salo, Z.; Li, R.; Wang, M.; Graff, J.M. Adipose Is a Conserved Dosage-Sensitive Antiobesity Gene. Cell Metab. 2007, 6, 195–207. [Google Scholar] [CrossRef]
- Lai, C.-Q.; Parnell, L.D.; Arnett, D.K.; García-Bailo, B.; Tsai, M.Y.; Kabagambe, E.K.; Straka, R.J.; Province, M.A.; An, P.; Borecki, I.B.; et al. WDTC1, the Ortholog of Drosophila Adipose Gene, Associates With Human Obesity, Modulated by MUFA Intake. Obesity 2009, 17, 593–600. [Google Scholar] [CrossRef]
- Baumbach, J.; Hummel, P.; Bickmeyer, I.; Kowalczyk, K.M.; Frank, M.; Knorr, K.; Hildebrandt, A.; Riedel, D.; Jäckle, H.; Kühnlein, R.P. A Drosophila In Vivo Screen Identifies Store-Operated Calcium Entry as a Key Regulator of Adiposity. Cell Metab. 2014, 19, 331–343. [Google Scholar] [CrossRef]
- Pospisilik, J.A.; Schramek, D.; Schnidar, H.; Cronin, S.J.F.; Nehme, N.T.; Zhang, X.; Knauf, C.; Cani, P.D.; Aumayr, K.; Todoric, J.; et al. Drosophila genome-wide obesity screen reveals hedgehog as a determinant of brown versus white adipose cell fate. Cell 2010, 140, 148–160. [Google Scholar] [CrossRef]
- Arruda, A.P.; Pers, B.M.; Parlakgul, G.; Güney, E.; Goh, T.; Cagampan, E.; Lee, G.Y.; Goncalves, R.L.; Hotamisligil, G.S. Defective STIM-mediated store operated Ca2+ entry in hepatocytes leads to metabolic dysfunction in obesity. elife 2017, 6, 14485. [Google Scholar] [CrossRef]
- Baranski, T.J.; Kraja, A.T.; Fink, J.L.; Feitosa, M.; Lenzini, P.A.; Borecki, I.B.; Liu, C.-T.; Cupples, L.A.; North, K.E.; Province, M.A.; et al. A high throughput, functional screen of human Body Mass Index GWAS loci using tissue-specific RNAi Drosophila melanogaster crosses. PLoS Genet. 2018, 14, e1007222. [Google Scholar] [CrossRef]
- Agrawal, N.; Lawler, K.; Davidson, C.M.; Keogh, J.M.; Legg, R.; Barroso, I.; Farooqi, I.S.; Brand, A.H.; Tapon, N. Predicting novel candidate human obesity genes and their site of action by systematic functional screening in Drosophila. PLoS Biol. 2021, 19, e3001255. [Google Scholar] [CrossRef] [PubMed]
- Men, T.T.; van Thanh, D.N.; Yamaguchi, M.; Suzuki, T.; Hattori, G.; Arii, M.; Huy, N.T.; Kamei, K. A Drosophila Model for Screening Antiobesity Agents. Biomed Res. Int. 2016, 2016, 6293163. [Google Scholar] [CrossRef] [PubMed]
- Sanhueza, S.; Tobar, N.; Cifuentes, M.; Quenti, D.; Varì, R.; Scazzocchio, B.; Masella, R.; Herrera, K.; Paredes, A.; Morales, G.; et al. Lampaya Medicinalis Phil. decreases lipid-induced triglyceride accumulation and proinflammatory markers in human hepatocytes and fat body of Drosophila melanogaster. Int. J. Obes. 2021, 45, 1464–1475. [Google Scholar] [CrossRef] [PubMed]
- Günther, I.; Rimbach, G.; Nevermann, S.; Neuhauser, C.; Stadlbauer, V.; Schwarzinger, B.; Schwarzinger, C.; Ipharraguerre, I.R.; Weghuber, J.; Lüersen, K. Avens Root (Geum urbanum L.) Extract Discovered by Target-Based Screening Exhibits Antidiabetic Activity in the Hen’s Egg Test Model and Drosophila melanogaster. Front. Pharmacol. 2021, 12, 55. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Romey-Glüsing, R.; Tahan Zadeh, N.; von Frieling, J.; Hoffmann, J.; Huebbe, P.; Bruchhaus, I.; Rimbach, G.; Fink, C.; Roeder, T. Furbellow (Brown Algae) Extract Increases Lifespan in Drosophila by Interfering with TOR-Signaling. Nutrients 2020, 12, 1172. [Google Scholar] [CrossRef]
- Huebbe, P.; Nikolai, S.; Schloesser, A.; Herebian, D.; Campbell, G.; Glüer, C.-C.; Zeyner, A.; Demetrowitsch, T.; Schwarz, K.; Metges, C.C.; et al. An extract from the Atlantic brown algae Saccorhiza polyschides counteracts diet-induced obesity in mice via a gut related multi-factorial mechanisms. Oncotarget 2017, 8, 73501–73515. [Google Scholar] [CrossRef]

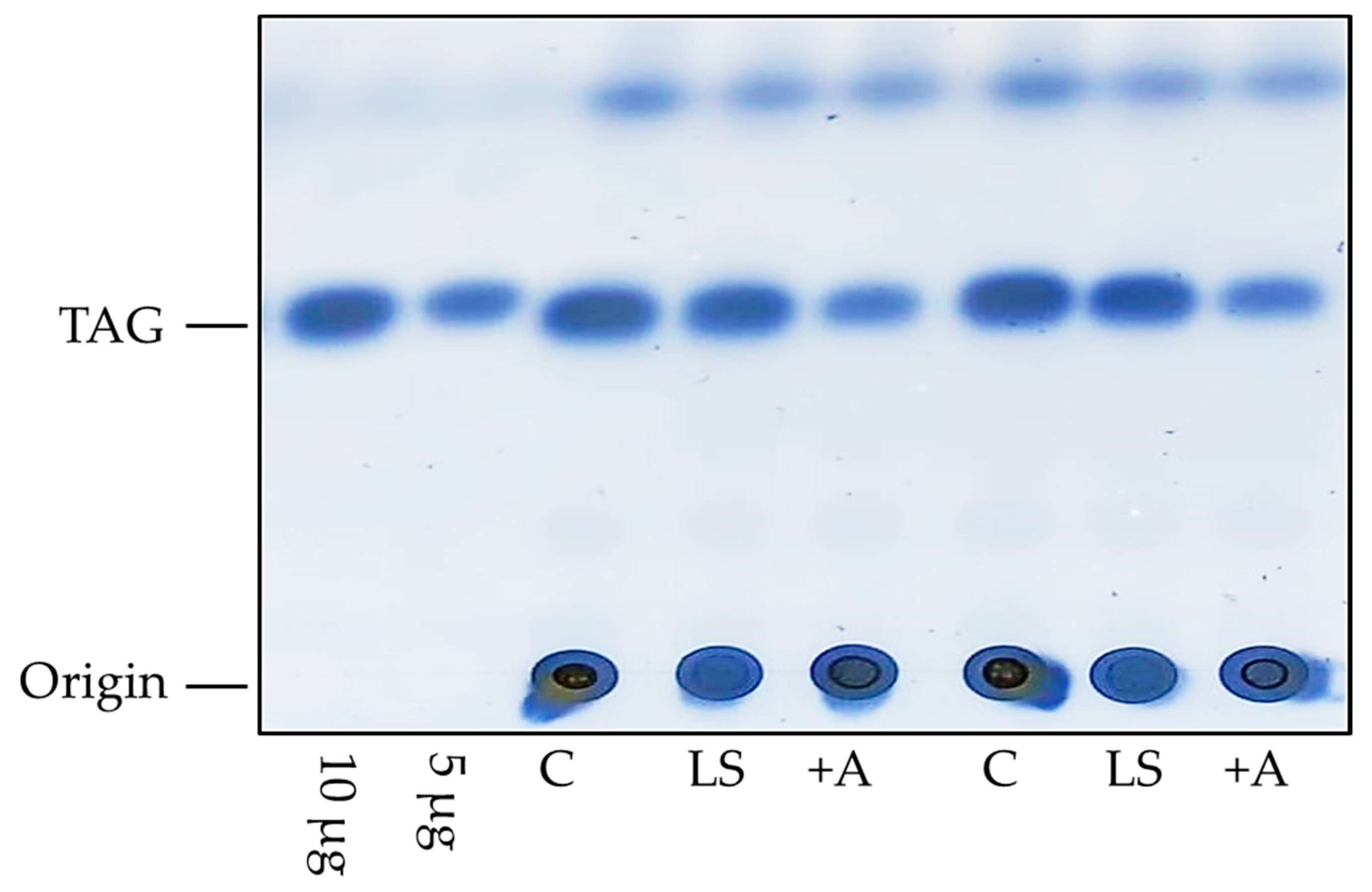











| Method | Advantages | Disadvantages |
|---|---|---|
| Dye staining (Nile red, BODIPY, Harris hematoxylin) | Cellular level: Determination of the number and size of lipid droplets | No quantification of total TAG content |
| Coupled colorimetric assays | Determination of the whole-body TAG content | No identification of the proportions of different glycerides |
| Rapid determination | Removal of heads (due to colored eye pigment) recommended | |
| Large sample size | ||
| Thin-layer chromatography | Proportions of TAG, diglycerides, fatty acids measurable | Extraction performed with organic solvents like chloroform or methanol |
| Quantification of lipid classes available | Not compatible with the protocol shown in Figure 1 | |
| Liquid chromatography or gas chromatography/mass spectrometry | Specific and highly sensitive | High cost per measurement |
| Method | Advantages | Disadvantages |
|---|---|---|
| Dye labeling of the food |
|
|
| Consumption-excretion (Con-Ex); Excreta quantification (EX-Q) |
|
|
| Radioactive labeling of the food |
|
|
| CApillary FEeder (CAFE) assay |
|
|
| Proboscis extension |
|
|
| MAnual FEeding (MAFE) assay |
|
|
| Automated detection of food intake |
|
|
| Fly Liquid-Food Interaction Counter (FLIC) |
|
|
| fly Proboscis and Activity Detector(flyPAD) |
|
|
| Diet | Strain, Sex, Age | Time Period of Intervention | Assays (Number of Larvae or Flies per Treatment or n = Number of Treatments) | Outcomes | Publication |
|---|---|---|---|---|---|
| HSD | |||||
| Combination of different sugar and yeast ratios HSD 2.5–40% sugar 2.5–40% yeast 1.5% agar 1.8% fungicide | yw; w1118; Canton-S Males, females 1 d | Prefeeding 3 d 10% sugar/yeast 13 d, 26–40 d, 52–56 d |
|
| Skorupa et al. [75] |
| Control diet 5% sugar 10% yeast 2% peptone 1% agar HSD 34% sugar | Canton-S L3 larvae | Shortterm: 12 h or Longterm: Egg to L3 Wandering L3 stage |
|
| Palanker Musselman et al. [129] |
|
|
|
| Results for sucrose:
Results for fructose:
Results for glucose:
Results for trehalose:
| Colinet et al. [130] |
| Control diet 5% sucrose 10% yeast 2% peptone 1% agar HSD 34% sucrose | w1118 L3 larvae Females | Virgin females 7 d |
| Females:
| Buescher et al. [128] |
| Control diet 5% sucrose 10% yeast 2% peptone 1% agar magnesium sulfate calcium chloride HSD 34% sucrose | w1118 Males After eclosion | 3 weeks |
|
| Na et al. [135] |
| Paternal diet: Control diet 3% sucrose 1% yeast 3% soy flour 1.2% agar HSD 10 or 30% sucrose F1 flies: 1.8% yeast 1% soy flour 8% yellow corn meal 2.2% molasses 8% malt extract 1.2% agar | w1118 Males 4–5 d | Parental flies 2 d on HSD Investigation of F1 flies 7 d post eclosion |
|
| Öst et al. [143] |
| Control diet 5% sucrose 10% yeast 2% peptone 1% agar HSD 34% sucrose | w1118 Females 1 d | Virgin females 7 d on HSD F1 adult males 14 d on HSD |
|
| Brookheart et al. [142] |
| Control diet 5% sucrose 10% yeast 1.5% agar HSD 40% sucrose | w1118 Females 2 d | 1–3 weeks, followed by 1 week on control diet |
|
| Dobson et al. [136] |
| Control diet (cornmeal/yeast fly medium) HSD +1 M sucrose | w1118 Females 5–7 d | 5–7 d |
|
| Zhang et al. [138] |
| Control diet 5% sucrose 8% yeast 2% yeast extract 2% peptone 1% agar HSD 34% sucrose (1 M) | w1118 Females 3 d | 3 weeks |
|
| Pereira et al. [139] |
| Control diet sucrose (0.15 M) cornmeal/yeast medium HSD sucrose (1 M) | w1118 Post-eclosion L3 larvae | 3–5 d |
|
| Yu et al. [133] |
| Control diet 5% sucrose 7.5% white corn syrup 2% yeast 1% soy flour 7% cornmeal 1% agar HSD: 20 or 30% sucrose | w1118 Males 2–5 d; 6–8 d (RNAi lines) | 2 and 7 d |
|
| May et al. [131] |
| Control diet 8% sucrose 0.5% yeast 2% agar 0.16% calcium chloride dihydrate 0.16% ferrous sulfate heptahydrate 0.8% sodium potassium tartrate tetrahydrate 0.05% sodium chloride 0.05% manganese chloride tetrahydrate 0.53% nipagin HSD +300 mM sucrose | Canton-S; w1118 Males, females 1, 3, 5 weeks | 4 d |
|
| Villanueva et al. [134] |
| Control diet 7.5% white corn syrup 1.7% yeast 1% soy flour 7% cornmeal 0.7% agar 0.9% propionic acid 0.4% tegosept HSD +30% sugar | w1118 Males 2–4 d | 18–24 h fasting or HSD feeding (refeeding) |
|
| Wilinski et al. [132] |
| Control diet 15 g sugar (0.15 M) 6 g yeast 17 g maize 1.5 g agar 1 mL propionic acid 1 g methyl-paraben HSD 1 M sucrose | Oregon-R Male 1 d | 5, 10, 15, 20 and 25 d |
|
| Rani et al. [140] |
| Control diet 5% sucrose 10% Brewer’s yeast 1.5% agar 0.3% nipagin 0.3% propionic acid HSD 20% sucrose (partly 30%, 40%) | wDah; w1118 Males, females 2 d | 7 and 28 d Adjustment of setup: water source |
|
| van Dam et al. [137] |
| Control diet 5% sucrose 6.7% yeast 6.7% cornmeal 0.5% agar HSD 20 or 35% sucrose | Oregon-R Males, females 5–6 d | 10 d |
|
| Catalani et al. [141] |
| HFD | |||||
| Control diet Yeast Corn starch Molasses HFD +3, 7, 15 or 30% (w/v) coconut oil | w1118 Females 10–15 d | Control diet prefeeding 5 d 2, 5 and 10 d (mainly 5 d) |
|
| Birse et al. [22] |
| Control diet 5% sugar 10% yeast 2% peptone 1% agar HFD: 14.1% “Crisco fat” (soybean and palm oil) | Canton-S L3 larvae | Shortterm: 12 h or Longterm: Egg to L3 Wandering L3 stage |
|
| Palanker Musselman et al. [129] |
| Control diet yeast corn starch molasses HFD +5, 10 or 20% (w/v) coconut oil (mainly 20%) | w1118; Canton; Oregon-R Females 3 d | 7 d |
|
| Heinrichsen et al. [110] |
| Control dietyeast corn starch molasses HFD +30% (w/v) coconut oil | w1118 Females 5–10 d | Prefeeding 5 d 2, 5 and 10 d (mainly 5 d) |
|
| Diop et al. [23] |
| Control diet 10% glucose 7% corn meal 4% yeast 0.6% agar HFD +5% or 15% (w/v) lard | w1118 Males, females L3 larvae 1 d | L3 larvae 60 h post-hatching 10 d |
|
| Kayashima et al. [8] |
| Control diet 8% fructose 10% yeast 2% polenta 0.8% agar HFD +6.3 or 15% (w/v) lard | w1118 Males | 10, 20, 30, 40 d |
|
| Woodcock et al. [124] |
| Control diet 10% sugar 10% yeast 1.5% agar 1% Tween 80 (w/v) HFD +2% (w/v) palmitic acid | Canton-S Males 3 d | Prefeeding 3 d 7 and 14 d |
|
| Jung et al. [146] |
| Control diet 0.75% sucrose 1.3% yeast 6.5% cornmeal 0.7% agar HFD +3, 7, 15 or 30% (w/v) coconut oil (mainly 15%) | Canton-S Females Post eclosion | During development or only in adults |
|
| Schultzhaus et al. [149] |
| Control diet 10% sugar 10% yeast 2% agar HFD +30% (w/v) coconut oil | w1118 Females 2 d | 5 weeks |
|
| Wen et al. [148] |
| Control diet 8% sucrose 0.5% yeast 2% agar 0.16% calcium chloride dihydrate 0.16% ferrous sulfate heptahydrate 0.8% sodium potassium tartrate tetrahydrate 0.05% sodium chloride 0.05% manganese chloride tetrahydrate 0.53% nipagin HFD +5% (w/v) coconut oil | Canton-S; w1118 Males, females 1, 3, 5 weeks | 4 d |
|
| Villanueva et al. [134] |
| Control diet yeast corn agar HFD +20% (w/v) coconut oil Partly: +5, 10 or 20% (w/v) coconut oil Partly: +coconut oil, soybean oil or lard | Oregon-R; Orgon-S; 2 control stains Virgin female flies | 5–6 d Starvation 36 h |
|
| Huang et al. [147] |
| Control diet 10% sugar 5% yeast 1.2% agar HFD +10 or 30% (w/v) coconut oil | w1118 Females 1 d post-eclosion (virgins) 4–5 d (mated) | 1–3 weeks |
|
| Liao et al. [144] |
| Diet | Strain, Sex, Age | Time Period of Intervention | Method(Tissue) | Outcomes(Process, e.g., Genes) | Publication |
|---|---|---|---|---|---|
| HSD | |||||
| Control diet 5% sugar 10% yeast 2% peptone 1% agar HSD 34% sugar | Canton-S Males L3 larvae | Shortterm: 12 h or Longterm: Egg to L3 Wandering L3 stage | Microarray (whole body) | Results short-term intervention:
| Palanker Musselman et al. [129] |
| Control diet 5% sucrose 10% yeast 1.5% agar HSD 40% sucrose | w1118 Females 2 d | 1–3 weeks, followed by 1 week on control diet | RNA sequencing (whole body) |
| Dobson et al. [136] |
| Control diet 3% sucrose 4% inactive yeast 2.6% cornmea l0.8% agar 1.5% tegosept 0.3% propionic acid HSD 20% sucrose | Oregon R-C Females 2 d | 7 d | RNA sequencing (heads) |
| Hemphill et al. [152] |
| Control diet 2% sucrose 22% cornmea l3% wheat germ 0.1% powdered milk 0.1% salt 0.1% soybean flour 0.1% rye flour Nipagin Lyophilized yeast HSD 30% sucrose | Oregon-R Males, females Eggs on control diet/HSD | 7 d | RNA sequencing (whole body) |
| Loreto et al. [151] |
| HFD | |||||
| Control diet yeast corn starch molasses HFD 20% (w/v) coconut oil | w1118 Females 3–5 d | 7 d | Microarray (whole body) |
| Heinrichsen et al. [156] |
| Control diet 3% sucrose 4% inactive yeast 2.6% cornmea l0.8% agar 1.5% tegosept 0.3% propionic acid HFD 20% (w/v) coconut oil | Oregon R-C Females 2 d | 7 d | RNA sequencing (heads) |
| Hemphill et al. [152] |
| Control diet 10% sucrose 10% yeast 5% cornmea l1% agar 3% tegosept 0.3% propionic acid HFD 20% (w/v) coconut oil | w1118 Females 2–7 d | 7 d | Microarray (heads) |
| Rivera et al. [157] |
| Control diet Food mix (New Horizon Foods) HFD 20% (w/v) coconut oil | w1118 Males, females 3 d | 7 d | RNA sequencing (heads, whole body) | Results for males:
| Stobdan et al. [159] |
| Control diet 4% sucrose 2% yeast 4% cornmea l1% agarose 1% parahydroxybenzonate 1% propionic acid HFD 20% (w/v) coconut oil | Canton-S Males, females 5–10 d | 7 d | RNA sequencing (whole body) |
| Azuma et al. [162] |
| Diet | Strain, Sex, Age | Time Period of Intervention | Method | Outcomes | Publication |
|---|---|---|---|---|---|
| HSD | |||||
| Control diet 10,400 mM sucrose, fructose, glucose or trehalose 8% yeast 1.5% agar HSD 1000 mM sucrose, fructose, glucose or trehalose | Mix of two wild populations Females 5 d | Egg to 5 d adult females | Gas chromatography/mass spectrometry | Results for sucrose:
| Colinet et al. [130] |
| Control diet 1% corn syrup 4% yeast 4% malt extract 7% cornmeal 1% soy flour 0.5% agar 0.4% propionic acid 0.8% tegosept HSD 18% corn syrup | w1118, split ends (Spen)-depleted D. melanogaster Males, females Larvae 22–24 h | First to late third instar larvae | Liquid chromatography/mass spectrometry | Results for w1118: Metabolic change: 4% of the analyzed metabolites were changed Results for Spen-depleted flies: Metabolic change: 7% of the analyzed metabolites were changed
| Gillette et al. [168] |
| Control diet 0.5% sugar 3% yeast 0.6% agar 5% cornmeal nipagin propionic acid HSD 18% and 30% sucrose | w1118; D. melanogaster with mild mitochondrial pyruvate carrier (MPC) 1 deficiency Males 15 d | 15–25 d | 1H Nuclear magnetic resonance spectroscopy | Results for w1118:
| Simard et al. [166] |
| Control diet 5% sugar 10% yeast 2% peptone 1% agar HSD 34% sucrose | w1118 Males, females 1 d | 3–5 weeks | Ultra-high performance liquid chromatography/mass spectrometry/mass spectrometry | Results for fat body:
Altered: 44 lipids
| Tuthill et al. [169] |
| HFD | |||||
| Control diet yeast corn starch molasses HFD 20% (w/v) coconut oil | w1118 Females 3–5 d | 7 d | Gas chromatography/mass spectrometry |
| Heinrichsen et al. [156] |
| Control diet Cornmeal-molasses food HFD 3% (w/v) coconut oil | 16 different cultured genotypes Larvae First instar | First to late third instar larvae | Liquid chromatography/mass spectrometry Gas chromatography/mass spectrometry |
| Oza et al. [171] |
| Control diet 1% light corn syrup 4% yeast 4% light malt extract 7% yellow cornmeal 1% soy flour 0.5% agar 0.4% propionic acid 0.8% tegosept HFD 15% (w/v) coconut oil | w1118; split ends (Spen)-depleted D. melanogaster Males, females Larvae 22–24 h | First to late third instar larvae | Liquid chromatography/mass spectrometry | Results for w1118
| Gillette et al. [168] |
| Control diet 0.5% agar 0.6% sugar 3% yeast 5% cornmeal nipagin propionic acid HFD 20% (w/v) coconut oil | w1118 Males 10 d | 10 d | Nuclear magnetic resonance spectroscopy |
| Cormier et al. [172] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eickelberg, V.; Lüersen, K.; Staats, S.; Rimbach, G. Phenotyping of Drosophila Melanogaster—A Nutritional Perspective. Biomolecules 2022, 12, 221. https://doi.org/10.3390/biom12020221
Eickelberg V, Lüersen K, Staats S, Rimbach G. Phenotyping of Drosophila Melanogaster—A Nutritional Perspective. Biomolecules. 2022; 12(2):221. https://doi.org/10.3390/biom12020221
Chicago/Turabian StyleEickelberg, Virginia, Kai Lüersen, Stefanie Staats, and Gerald Rimbach. 2022. "Phenotyping of Drosophila Melanogaster—A Nutritional Perspective" Biomolecules 12, no. 2: 221. https://doi.org/10.3390/biom12020221
APA StyleEickelberg, V., Lüersen, K., Staats, S., & Rimbach, G. (2022). Phenotyping of Drosophila Melanogaster—A Nutritional Perspective. Biomolecules, 12(2), 221. https://doi.org/10.3390/biom12020221






