Unconventional Functions of Amino Acid Transporters: Role in Macropinocytosis (SLC38A5/SLC38A3) and Diet-Induced Obesity/Metabolic Syndrome (SLC6A19/SLC6A14/SLC6A6)
Abstract
:1. Introduction
2. Unconventional Functions of Amino Acid Transporters
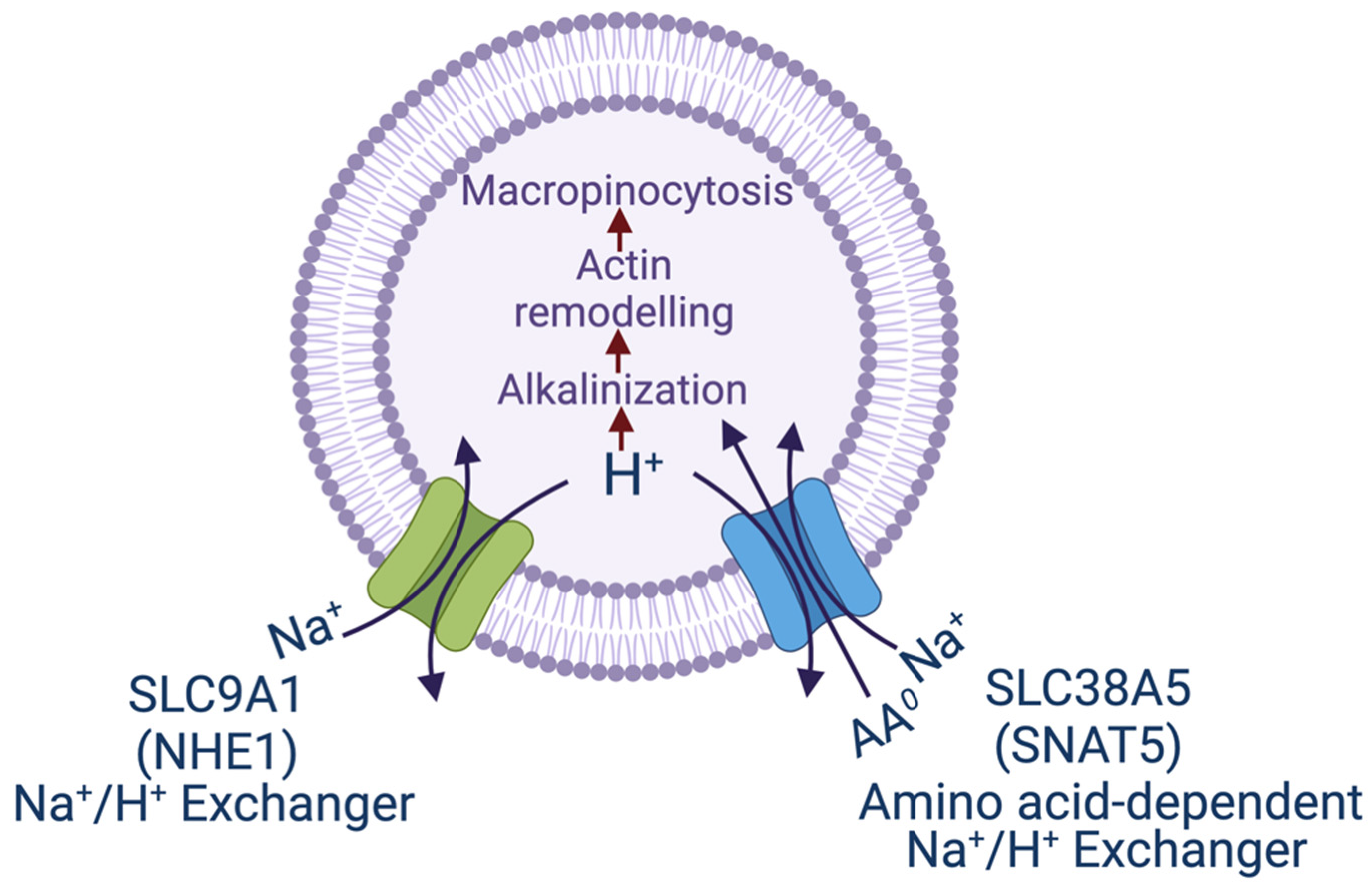
3. Macropinocytosis and SLC38A5/SLC38A3
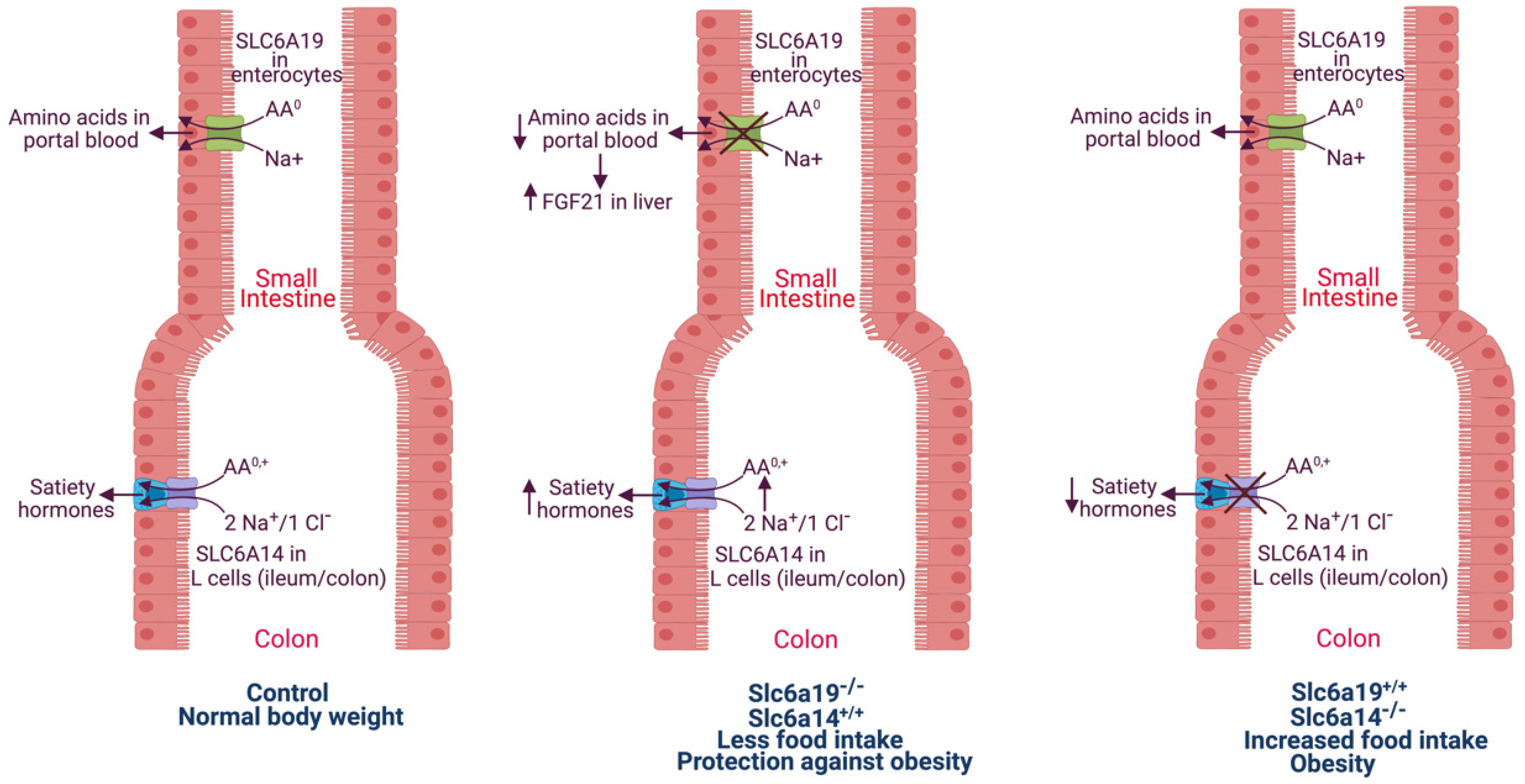
4. Obesity and SLC6A19: Deficiency of SLC6A19 Protects against Diet-Induced Obesity/Metabolic Syndrome in Mice via Increased Secretion of FGF21 and GLP-1
5. Deficiency of SLC6A14 Promotes Diet-Induced Obesity and Metabolic Syndrome in Humans and Mice: SLC6A14 Differs from SLC6A19 with Regard to Obesity
6. Significance of SLC6A19 and SLC6A14 to Obesity in Humans
7. Taurine and Its Transporter in Connection with Obesity
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Broer, S. Amino acid transport across mammalian intestinal and renal epithelia. Physiol. Rev. 2008, 88, 249–286. [Google Scholar] [CrossRef] [PubMed]
- Thwaites, D.T.; Anderson, C.M.H. The SLC36 family of proton-coupled amino acid transporters and their potential role in drug transport. Br. J. Pharmacol. 2011, 164, 1802–1816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pochini, L.; Scalise, M.; Galluccio, M.; Indiveri, C. Membrane transporters for the special amino acid glutamine: Structure/function relationships and relevance to human health. Front. Chem. 2014, 2, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papalazarou, V.; Maddocks, O.D.K. Supply and demand: Cellular nutrient uptake and exchange in cancer. Mol. Cell 2021, 81, 3731–3748. [Google Scholar] [CrossRef] [PubMed]
- Wyant, G.A.; Abu-Remaileh, M.; Wolfson, R.L.; Chen, W.W.; Freinkman, E.; Danai, L.V.; Vander Heiden, M.G.; Sabatini, D.M. mTORC1 activator SLC38A9 is required to efflux essential amino acids from lysosomes and use protein as a nutrient. Cell 2017, 171, 642–654.e12. [Google Scholar] [CrossRef] [PubMed]
- Huizing, M.; Gahl, W.A. Inherited disorders of lysosomal membrane transporters. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183336. [Google Scholar] [CrossRef]
- Kunji, E.R.S.; King, M.S.; Ruprecht, J.J.; Thangaratnarajah, C. The SLC25 carrier family: Important transport proteins in mitochondrial physiology and pathology. Physiology 2020, 35, 302–327. [Google Scholar] [CrossRef] [PubMed]
- Broer, A.; Cavanaugh, J.A.; Rasko, J.E.J.; Broer, S. The molecular basis of neutral aminoacidurias. Pflug. Arch. 2006, 451, 511–517. [Google Scholar] [CrossRef]
- Broer, S. Diseases associated with general amino acid transporters of the solute carrier 6 family (SLC6). Curr. Mol. Pharmacol. 2013, 6, 74–87. [Google Scholar] [CrossRef]
- Broer, S.; Fairweather, S.J. Amino acid transport across the mammalian intestine. Compr. Physiol. 2018, 9, 343–373. [Google Scholar] [PubMed]
- Yahyaoui, R.; Perez-Frias, J. Amino acid transport defects in human inherited metabolic disorders. Int. J. Mol. Sci. 2019, 21, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, P.M. Role of amino acid transporters in amino acid sensing. Am. J. Clin. Nutr. 2014, 99, 223S–230S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinilla, J.; Aledo, J.C.; Cwiklinski, E.; Hyde, R.; Taylor, P.M.; Hundal, H.S. SNAT2 transceptor signalling via mTOR: A role in cell growth and proliferation? Front. Biosci. 2011, 3, 1289–1299. [Google Scholar] [CrossRef]
- Broer, S. The SLC38 family of sodium-amino acid co-transporters. Pflug. Arch. 2014, 466, 155–172. [Google Scholar] [CrossRef] [PubMed]
- Varoqui, H.; Zhu, H.; Yao, D.; Ming, H.; Erickson, J.D. Cloning and functional identification of a neuronal glutamine transporter. J. Biol. Chem. 2000, 275, 4049–4054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Huang, W.; Sugawara, M.; Devoe, L.D.; Leibach, F.H.; Prasad, P.D.; Ganapathy, V. Cloning and functional expression of ATA1, a subtype of amino acid transporter A, from human placenta. Biochem. Biophys. Res. Commun. 2000, 273, 1175–1179. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, M.; Nakanishi, T.; Fei, Y.J.; Huang, W.; Ganapathy, M.E.; Leibach, F.H.; Ganapathy, V. Cloning of an amino acid transporter with functional characteristics and tissue expression pattern identical to that of system A. J. Biol. Chem. 2000, 275, 16473–16477. [Google Scholar] [CrossRef] [Green Version]
- Sugawara, M.; Nakanishi, T.; Fei, Y.J.; Martindale, R.G.; Ganapathy, M.E.; Leibach, F.H.; Ganapathy, V. Structure and function of ATA3, a new subtype of amino acid transport system A, primarily expressed in the liver and skeletal muscle. Biochim. Biophys. Acta 2000, 1509, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Hyde, R.; Cwiklinski, E.L.; MacAulay, K.; Taylor, P.M.; Hundal, H.S. Distinct sensor pathways in the hierarchical control of SNAT2, a putative amino acid transporter, by amino acid availability. J. Biol. Chem. 2007, 282, 19788–19798. [Google Scholar] [CrossRef] [Green Version]
- Ling, R.; Bridges, C.C.; Sugawara, M.; Fujita, T.; Leibach, F.H.; Prasad, P.D.; Ganapathy, V. Involvement of transporter recruitment as well as gene expression in the substrate-induced adaptive regulation of amino acid transport system A. Biochim. Biophys. Acta 2001, 1512, 15–21. [Google Scholar] [CrossRef] [Green Version]
- Rebsamen, M.; Pochini, L.; Stasyk, T.; de Araujo, M.E.; Galluccio, M.; Kandasamy, R.K.; Snijder, B.; Fauster, A.; Rudashevskaya, E.L.; Bruckner, M.; et al. SLC38A9 is a component of the lysosomal amino acid sensing machinery that controls mTORC1. Nature 2015, 519, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Tsun, Z.Y.; Wolfson, R.L.; Shen, K.; Wyant, G.A.; Plovanich, M.E.; Yuan, E.D.; Jones, T.D.; Chantranupong, L.; Comb, W.; et al. Lysosomal amino acid transporter SLC38A9 signals arginine sufficiency to mTORC1. Science 2015, 347, 188–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heublein, S.; Kazi, S.; Ogmundsdottir, M.H.; Attwood, E.V.; Kala, S.; Boyd, C.A.; Wilson, C.; Goberdhan, D.C. Proton-assisted amino-acid transporters are conserved regulators of proliferation and amino-acid-dependent mTORC1 activation. Oncogene 2010, 29, 4068–4079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goberdhan, D.C.; Wilson, C.; Harris, A.L. Amino acid sensing by mTORC1: Intracellular transporters mark the spot. Cell Metab. 2016, 23, 580–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenwood, A.D.; Ishida, Y.; O’Brien, S.P.; Roca, A.L.; Eiden, M.V. Transmission, evolution, and endogenization: Lessons learned from recent retroviral invasions. Microbiol. Mol. Biol. Rev. 2018, 82, e00044-17. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.W.; Closs, E.I.; Albritton, L.M.; Cunningham, J.M. Transport of cationic amino acids by the mouse ecotropic retrovirus receptor. Nature 1991, 352, 725–728. [Google Scholar] [CrossRef]
- Wang, H.; Kavanaugh, M.P.; North, R.A.; Kabat, D. Cell-surface receptor for murine retroviruses is a basic amino-acid transporter. Nature 1991, 352, 729–731. [Google Scholar] [CrossRef]
- Marin, M.; Tailor, C.S.; Nouri, A.; Kabat, D. Sodium-dependent neutral amino acid transporter type 1 is an auxiliary receptor for baboon endogenous retrovirus. J. Virol. 2000, 74, 8085–8093. [Google Scholar] [CrossRef] [Green Version]
- Tailor, C.S.; Nouri, A.; Zhao, Y.; Takeuchi, Y.; Kabat, D. A sodium-dependent neutral amino-acid transporter mediates infections of feline and baboon endogenous retroviruses and simian type D retroviruses. J. Virol. 1999, 73, 4470–4474. [Google Scholar] [CrossRef] [Green Version]
- Rasco, J.E.; Battini, J.L.; Gottschalk, R.J.; Mazo, I.; Miller, A.D. The RD114/simian type D retrovirus receptor is a neutral amino acid transporter. Proc. Natl. Acad. Sci. USA 1999, 96, 2129–2134. [Google Scholar] [CrossRef] [Green Version]
- Singer, D.; Camargo, S.M. Collectrin and ACE2 in renal and intestinal amino acid transport. Channels 2011, 5, 410–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Moore, M.J.; Vasilieva, N.; Sui, J.; Wong, S.K.; Berne, M.A.; Somasundaran, M.; Sullivan, J.L.; Luzuriaga, K.; Greenough, T.C.; et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003, 426, 450–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scialo, F.; Daniele, A.; Amato, F.; Pastore, L.; Matera, M.G.; Cazzola, M.; Castaldo, G.; Bianco, A. ACE2: The major cell entry receptor for SARS-CoV-2. Lung 2020, 198, 867–877. [Google Scholar] [CrossRef] [PubMed]
- Singer, D.; Camargo, S.M.; Ramadan, T.; Schafer, M.; Mariotta, L.; Herzog, B.; Huggel, K.; Wolfer, D.; Werner, S.; Penninger, J.M.; et al. Defective intestinal amino acid absorption in Ace2 null mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G686–G695. [Google Scholar] [CrossRef]
- Lin, X.P.; Mintern, J.D.; Gleeson, P.A. Macropinocytosis in different cell types: Similarities and differences. Membranes 2020, 10, 177. [Google Scholar] [CrossRef]
- Puccini, J.; Badgley, M.A.; Bar-Sagi, D. Exploiting cancer’s drinking problem: Regulation and therapeutic potential of macropinocytosis. Trends Cancer 2021, 8, 54–64. [Google Scholar] [CrossRef]
- Csanyi, G.; Feck, D.M.; Ghoshal, P.; Singla, B.; Lin, H.; Nagarajan, S.; Meijles, D.N.; Ghouleh, I.A.; Cantu-Medellin, N.; Kelley, E.E.; et al. CD47 and Nox1 mediate dynamic fluid-phase macropinocytosis of native LDL. Antioxid. Redox Signal. 2017, 26, 886–901. [Google Scholar] [CrossRef]
- Doodnauth, S.A.; Grinstein, S.; Maxson, M.E. Constitutive and stimulated macropinocytosis in macrophages: Roles in immunity and in the pathogenesis of atherosclerosis. Philos. Trans. R. Soc. B 2019, 374, 20180147. [Google Scholar] [CrossRef] [Green Version]
- Commisso, C. The pervasiveness of macropinocytosis in oncological malignancies. Philos. Trans. R. Soc. B 2019, 374, 20180153. [Google Scholar] [CrossRef] [Green Version]
- Stow, J.L.; Hung, Y.; Wall, A.A. Macropinocytosis: Insights from immunology and cancer. Curr. Opin. Cell Biol. 2020, 65, 131–140. [Google Scholar] [CrossRef]
- Encarnacion-Rosado, J.; Kimmelman, A.C. Harnessing metabolic dependencies in pancreatic cancers. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 482–492. [Google Scholar] [CrossRef] [PubMed]
- Ganapathy, V.; Thangaraju, M.; Prasad, P.D. Nutrient transporters in cancer: Relevance to Warburg hypothesis and beyond. Pharmacol. Ther. 2009, 121, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Bhutia, Y.D.; Babu, E.; Ramachandran, S.; Ganapathy, V. Amino acid transporters in cancer and their relevance to “glutamine addiction”: Novel targets for the design of a new class of anticancer drugs. Cancer Res. 2015, 75, 1782–1788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhutia, Y.D.; Ganapathy, V. Glutamine transporters in mammalian cells and their functions in physiology and cancer. Biochim. Biophys. Acta 2016, 1863, 2531–2539. [Google Scholar] [CrossRef] [PubMed]
- Broer, S. Amino acid transporters as targets for cancer therapy: Why, where, when and how? Int. J. Mol. Sci. 2020, 21, 6156. [Google Scholar] [CrossRef] [PubMed]
- Schniers, B.K.; Rajasekaran, D.; Korac, K.; Sniegowski, T.; Ganapathy, V.; Bhutia, Y.D. PEPT1 is essential for the growth of pancreatic cancer cells: A viable drug target. Biochem. J. 2021, 478, 3757–3774. [Google Scholar] [CrossRef] [PubMed]
- Weerasekara, V.K.; Patra, K.C.; Bardeesy, N. EGFR pathway links amino acid levels and induction of macropinocytosis. Dev. Cell 2019, 50, 261–263. [Google Scholar] [CrossRef]
- Yoshida, S.; Pacitto, R.; Inoki, K.; Swanson, J. Macropinocytosis, mTORC1 and cellular growth control. Cell. Mol. Life Sci. 2018, 75, 1227–1239. [Google Scholar] [CrossRef] [Green Version]
- Kay, R.R.; Williams, T.D.; Manton, J.D.; Traynor, D.; Paschke, P. Living on soup: Macropinocytic feeding in amoebae. Int. J. Dev. Biol. 2019, 63, 473–483. [Google Scholar] [CrossRef] [Green Version]
- Sasabe, E.; Tomomura, A.; Liu, H.; Sento, S.; Kitamura, N.; Yamamoto, T. EGF/EGFR signaling blockage inhibits tumor cell-derived exosome uptake by oral squamous cell carcinoma through macropinocytosis. Cancer Sci. 2021, in press. [Google Scholar]
- Lee, S.W.; Zhang, Y.; Jung, M.; Cruz, N.; Alas, B.; Commisso, C. EGFR-Pak signaling selectively regulates glutamine deprivation-induced macropinocytosis. Dev. Cell 2019, 50, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, C.; Hauser, A.D.; Vucic, E.A.; Bar-Sagi, D. Plasma membrane v-ATPase controls oncogenic Ras-induced macropinocytosis. Nature 2019, 576, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, S.; Sennoune, S.R.; Sharma, M.; Thangaraju, M.; Suresh, V.V.; Sniegowski, T.; Bhutia, Y.D.; Pruitt, K.; Ganapathy, V. Expression and function of SLC38A5, an amino acid-coupled Na+/H+ exchanger, in triple-negative breast cancer and its relevance to macropinocytosis. Biochem. J. 2021, 478, 3857–3976. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, T.; Sugawara, M.; Huang, W.; Martindale, R.G.; Leibach, F.H.; Ganapathy, M.E.; Prasad, P.D.; Ganapathy, V. Structure, function, and tissue expression pattern of human SN2, a subtype of the amino acid transport system N. Biochem. Biophys. Res. Commun. 2001, 281, 1343–1348. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, T.; Kekuda, R.; Fei, Y.J.; Hatanaka, T.; Sugawara, M.; Martindale, R.G.; Leibach, F.H.; Prasad, P.D.; Ganapathy, V. Cloning and functional characterization of a new subtype of the amino acid transport system N. Am. J. Physiol. Cell Physiol. 2001, 281, C1757–C1768. [Google Scholar] [CrossRef] [PubMed]
- Sniegowski, T.; Korac, K.; Bhutia, Y.D.; Ganapathy, V. SLC6A14 and SLC38A5 drive the glutaminolysis and serine-glycine-one-carbon pathways in cancer. Pharmaceuticals 2021, 14, 216. [Google Scholar] [CrossRef]
- Brown, T.P.; Ganapathy, V. Lactate/GPR81 signaling and proton motive fore in cancer: Role in angiogenesis, immune escape, nutrition, and Warburg phenomenon. Pharmacol. Ther. 2020, 206, 107451. [Google Scholar] [CrossRef]
- Busque, S.M.; Wagner, C.A. Potassium restriction, high protein intake, and metabolic acidosis increase expression of the glutamine transporter SNAT3 (Slc38a3) in mouse kidney. Am. J. Physiol. Ren. Physiol. 2009, 297, F440–F450. [Google Scholar] [CrossRef]
- Chan, K.; Busque, S.M.; Sailer, M.; Stoeger, C.; Broer, S.; Daniel, H.; Rubio-Aliaga, I.; Wagner, C.A. Loss of function mutation of the Slc38a3 glutamine transporter reveals its critical role for amino acid metabolism in the liver, brain, and kidney. Pflug. Arch. 2016, 468, 213–227. [Google Scholar] [CrossRef]
- Rubio-Aliaga, R.; Wagner, C.A. Regulation and function of the SLC38A3 (SNAT3) glutamine transporter. Channels 2016, 10, 440–452. [Google Scholar] [CrossRef]
- Umapathy, N.S.; Li, W.; Mysona, B.A.; Smith, S.B.; Ganapathy, V. Expression and function of glutamine transporters SN1 (SNAT3) and SN2 (SNAT5) in retinal Muller cells. Investig. Ophthalmol. Vis. Sci. 2005, 46, 3980–3987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daneman, R.; Zhou, L.; Agalliu, D.; Cahoy, J.D.; Kaushal, A.; Barres, B.A. The mouse blood-brain barrier transcriptome: A new resource for understanding the development and function of brain endothelial cells. PLoS ONE 2010, 5, e13741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broer, S. Epithelial neutral amino acid transporters: Lessons from mouse models. Curr. Opin. Nephrol. Hypertens. 2013, 22, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Broer, S. The role of the neutral amino acid transporter B0AT1 (SLC6A19) in Hartnup disorder and protein nutrition. IUBMB Life 2009, 61, 691–699. [Google Scholar] [CrossRef]
- Javed, K.; Broer, S. Mice lacking the intestinal and renal neutral amino acid transporter SLC6A19 demonstrate the relationship between dietary protein intake and amino acid malabsorption. Nutrients 2019, 11, 2024. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Rose, A.J.; Sijmonsma, T.P.; Broer, A.; Pfenninger, A.; Herzig, S.; Schmoll, D.; Broer, S. Mice lacking neutral amino acid transporter B0AT1 (SLC6A19) have elevated levels of FGF21 and GLP-1 and improved glycaemic control. Mol. Metab. 2015, 4, 406–417. [Google Scholar] [CrossRef]
- Cheng, Q.; Shah, N.; Broer, A.; Fairweather, S.; Jiang, Y.; Schmoll, D.; Corry, B.; Broer, S. Identification of novel inhibitors of the amino acid transporter B0AT1 (SLC6A19), a potential target to induce protein restriction and to treat type 2 diabetes. Br. J. Pharmacol. 2017, 174, 468–482. [Google Scholar] [CrossRef] [Green Version]
- Javed, K.; Cheng, Q.; Carroll, A.J.; Truong, T.T.; Broer, S. Development of biomarkers for inhibition of SLC6A19 (B0AT1)—A potential target to treat metabolic disorders. Int. J. Mol. Sci. 2018, 19, 3597. [Google Scholar] [CrossRef] [Green Version]
- Sonoda, J.; Chen, M.Z.; Baruch, A. FGF21-receptor agonists: An emerging therapeutic class for obesity-related diseases. Horm. Mol. Biol. Clin. Investig. 2017, 30. [Google Scholar] [CrossRef]
- Geng, L.; Lam, K.S.L.; Xu, A. The therapeutic potential of FGF21 in metabolic diseases: From bench to clinic. Nat. Rev. Endocrinol. 2020, 16, 654–667. [Google Scholar] [CrossRef]
- Kuhre, R.E.; Deacon, C.F.; Holst, J.J.; Petersen, N. What is an L-cell and how do we study the secretory mechanisms of the L-cell? Front. Endocrinol. 2021, 12, 694284. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.V.; Efanov, A.M.; Fang, X.; Beavers, L.S.; Wang, X.; Wang, J.; Gonzalez Valcarcel, I.C.; Ma, T. GPR142 controls tryptophan-induced insulin and incretin hormone secretion to improve glucose metabolism. PLoS ONE 2016, 11, e0157298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudenko, O.; Shang, J.; Munk, A.; Ekberg, J.P.; Petersen, N.; Engelstoft, M.S.; Hjorth, S.A.; Wu, M.; Feng, Y.; Zhou, Y.P.; et al. The aromatic amino acid sensor GPR142 controls metabolism through balanced regulation of pancreatic and gut hormones. Mol. Metab. 2019, 19, 49–64. [Google Scholar] [CrossRef]
- Suviolahti, E.; Oksanen, L.J.; Ohman, M.; Cantor, R.M.; Ridderstrale, M.; Tuomi, T.; Kaprio, J.; Rissanen, A.; Mustajoki, P.; Jousilahti, P.; et al. The SLC6A14 gene shows evidence of association with obesity. J. Clin. Investig. 2003, 112, 1762–1772. [Google Scholar] [CrossRef] [PubMed]
- Durand, E.; Boutin, P.; Meyre, D.; Charles, M.A.; Clement, K.; Dina, C.; Froguel, P. Polymorphisms in the amino acid transporter solute carrier family 6 (neurotransmitter transporter) member 14 gene contribute to polygenic obesity in French Caucasians. Diabetes 2004, 53, 2483–2486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corpeleijn, E.; Petersen, L.; Holst, C.; Saris, W.H.; Astrup, A.; Langin, D.; MacDonald, I.; Martinez, J.A.; Oppert, J.M.; Polak, J.; et al. Obesity-related polymorphisms and their associations with the ability to regulate fat oxidation in obese Europeans: The NUGENOB study. Obesity 2010, 18, 1369–1377. [Google Scholar] [CrossRef] [PubMed]
- Miranda, R.C.; Vetter, S.B.; Genro, J.P.; Campagnolo, P.D.; Mattevi, V.S.; Vitolo, M.R.; Almeida, S. SLC6A14 and 5-HTR2C polymorphisms are associated with food intake and nutritional status in children. Clin. Biochem. 2015, 48, 1277–1282. [Google Scholar] [CrossRef] [PubMed]
- Sloan, J.L.; Mager, S. Cloning and functional expression of a human Na+ and Cl−-dependent neutral and cationic amino acid transporter B0,+. J. Biol. Chem. 1999, 274, 23740–23745. [Google Scholar] [CrossRef] [Green Version]
- Sivaprakasam, S.; Sikder, M.O.F.; Ramalingam, L.; Kaur, G.; Dufour, J.M.; Moustaid-Moussa, N.; Wachtel, M.S.; Ganapathy, V. SLC6A14 deficiency is linked to obesity, fatty liver, and metabolic syndrome but only under conditions of a high-fat diet. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 166087. [Google Scholar] [CrossRef]
- Sikder, M.O.F.; Yang, S.; Ganapathy, V.; Bhutia, Y.D. The Na+/Cl−-coupled, broad-specific amino acid transporter SLC6A14 (ATB0,+): Emerging role in multiple diseases and therapeutic potential for treatment and diagnosis. AAPS J. 2017, 20, 12. [Google Scholar] [CrossRef]
- Lambert, I.H.; Kristensen, D.M.; Holm, J.B.; Mortensen, O.H. Physiological role of taurine–from organism to organelle. Acta Physiol. 2015, 213, 191–212. [Google Scholar] [CrossRef] [PubMed]
- Sturman, J. Review: Taurine deficiency and the cat. Adv. Exp. Med. Biol. 1992, 315, 1–5. [Google Scholar] [PubMed]
- Smith, K.E.; Borden, L.A.; Wang, C.H.; Hartig, P.R.; Branchek, T.A.; Weinshank, R.L. Cloning and expression of a high affinity taurine transporter from rat brain. Mol. Pharmacol. 1992, 42, 563–569. [Google Scholar] [PubMed]
- Ramamoorthy, S.; Leibach, F.H.; Mahesh, V.B.; Han, H.; Yang-Feng, T.; Blakely, R.D.; Ganapathy, V. Functional characterization and chromosomal localization of a clone taurine transporter from human placenta. Biochem. J. 1994, 300, 893–900. [Google Scholar] [CrossRef] [Green Version]
- Sattari, M.R.; Mashayekhi, S. Obesity and taurine. Adv. Obes. Weight Manag. Control 2015, 2, 70–71. [Google Scholar] [CrossRef] [Green Version]
- Irving, B.A.; Wood, G.C.; Bennotti, P.N.; Babu, E.; Deshpande, A.; Lent, M.R.; Petrick, A.; Gabrielsen, J.; Strodel, W.; Gerhard, G.S.; et al. Nutrient transporter expression in the jejunum in relation to body mass index in patients undergoing bariatric surgery. Nutrients 2016, 8, 683. [Google Scholar] [CrossRef] [Green Version]
- Miyamoto, Y.; Balkovetz, D.F.; Leibach, F.H.; Mahesh, V.B.; Ganapathy, V. Na+ + Cl−-gradient-driven, high-affinity, uphill transport of taurine in human placental brush-border membrane vesicles. FEBS Lett. 1988, 231, 263–267. [Google Scholar] [CrossRef] [Green Version]
- Jayanthi, J.D.; Ramamoorthy, S.; Mahesh, V.B.; Leibach, F.H.; Ganapathy, V. Substrate-specific regulation of the taurine transporter in human placental choriocarcinoma cells (JAR). Biochim. Biophys. Acta 1995, 1235, 351–360. [Google Scholar] [CrossRef] [Green Version]
- Ditchfield, A.M.; Desforges, M.; Mills, T.A.; Glazier, J.D.; Wareing, M.; Mynett, K.; Sibley, C.P.; Greenwood, S.L. Maternal obesity is associated with a reduction in placental taurine transporter activity. Int. J. Obes. 2015, 39, 557–564. [Google Scholar] [CrossRef] [Green Version]
- Heller-Stilb, B.; van Roeyen, C.; Rascher, K.; Hartwig, H.G.; Huth, A.; Seeliger, M.W.; Warskulat, U.; Haussinger, D. Disruption of the taurine transporter gene (taut) leads to retinal degeneration in mice. FASEB J. 2002, 16, 231–233. [Google Scholar] [CrossRef]
- Warskulat, U.; Heller-Stilb, B.; Oermann, E.; Zilles, K.; Haas, H.; Lang, F.; Haussinger, D. Phenotype of the taurine transporter knockout mouse. Methods Enzymol. 2007, 428, 439–458. [Google Scholar] [PubMed]
- Preising, M.N.; Gorg, B.; Friedburg, C.; Qvartskhava, N.; Budde, B.S.; Bonus, M.; Toliat, M.R.; Pfleger, C.; Altmuller, J.; Herebian, D.; et al. Biallelic mutation of human SLC6A6 encoding the taurine transporter TAUT is linked to early retinal degeneration. FASEB J. 2019, 33, 11507–11527. [Google Scholar] [CrossRef] [PubMed]
| Transporter | Species | Virus |
|---|---|---|
| Cat 1 (Slc7a1) | Mouse | Ecotropic murine leukemia virus; Bovine leukemia virus |
| ASCT 1 (SLC1A4) | Human | Feline endogenous retrovirus RD-114; Baboon endogenous retrovirus |
| ASCT 2 (SLC1A5) | Human | Baboon endogenous retrovirus; Human endogenous retrovirus HERV-W |
| ACE 2 (chaperone for intestinal amino acid transporters SLC6A19 and SLC6A20) | Human | COVID-19 virus SARS-CoV-2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhutia, Y.D.; Mathew, M.; Sivaprakasam, S.; Ramachandran, S.; Ganapathy, V. Unconventional Functions of Amino Acid Transporters: Role in Macropinocytosis (SLC38A5/SLC38A3) and Diet-Induced Obesity/Metabolic Syndrome (SLC6A19/SLC6A14/SLC6A6). Biomolecules 2022, 12, 235. https://doi.org/10.3390/biom12020235
Bhutia YD, Mathew M, Sivaprakasam S, Ramachandran S, Ganapathy V. Unconventional Functions of Amino Acid Transporters: Role in Macropinocytosis (SLC38A5/SLC38A3) and Diet-Induced Obesity/Metabolic Syndrome (SLC6A19/SLC6A14/SLC6A6). Biomolecules. 2022; 12(2):235. https://doi.org/10.3390/biom12020235
Chicago/Turabian StyleBhutia, Yangzom D., Marilyn Mathew, Sathish Sivaprakasam, Sabarish Ramachandran, and Vadivel Ganapathy. 2022. "Unconventional Functions of Amino Acid Transporters: Role in Macropinocytosis (SLC38A5/SLC38A3) and Diet-Induced Obesity/Metabolic Syndrome (SLC6A19/SLC6A14/SLC6A6)" Biomolecules 12, no. 2: 235. https://doi.org/10.3390/biom12020235
APA StyleBhutia, Y. D., Mathew, M., Sivaprakasam, S., Ramachandran, S., & Ganapathy, V. (2022). Unconventional Functions of Amino Acid Transporters: Role in Macropinocytosis (SLC38A5/SLC38A3) and Diet-Induced Obesity/Metabolic Syndrome (SLC6A19/SLC6A14/SLC6A6). Biomolecules, 12(2), 235. https://doi.org/10.3390/biom12020235






