A New Approach to Inhibiting Triple-Negative Breast Cancer: In Vitro, Ex Vivo and In Vivo Antiangiogenic Effect of BthTx-II, a PLA2-Asp-49 from Bothrops jararacussu Venom
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Venom and Purification of Asp-49 PLA2 BthTx-II
2.3. Cell Culture
2.4. Cell Viability by MTT Assay
2.5. Proliferation Assay
2.6. Cell Adhesion Assay
2.7. Cell Cycle Analysis
2.8. Cell Migration Assay
2.9. In Vitro Angiogenesis (Tubulogenesis Assay)
2.10. Dosing of VEGF in the Supernatant of HUVEC
2.11. Ex Vivo Angiogenesis Assay (Mouse Aortic Ring Assay)
2.12. Characterization of Co-Culture HUVEC and MDA-MB-231 Cells and Angiogenic Sprouting
2.13. Co-Culture Invasion Assay
2.14. Chick Chorioallantoic Membrane Angiogenesis Assay
2.15. Statistical Analysis
3. Results
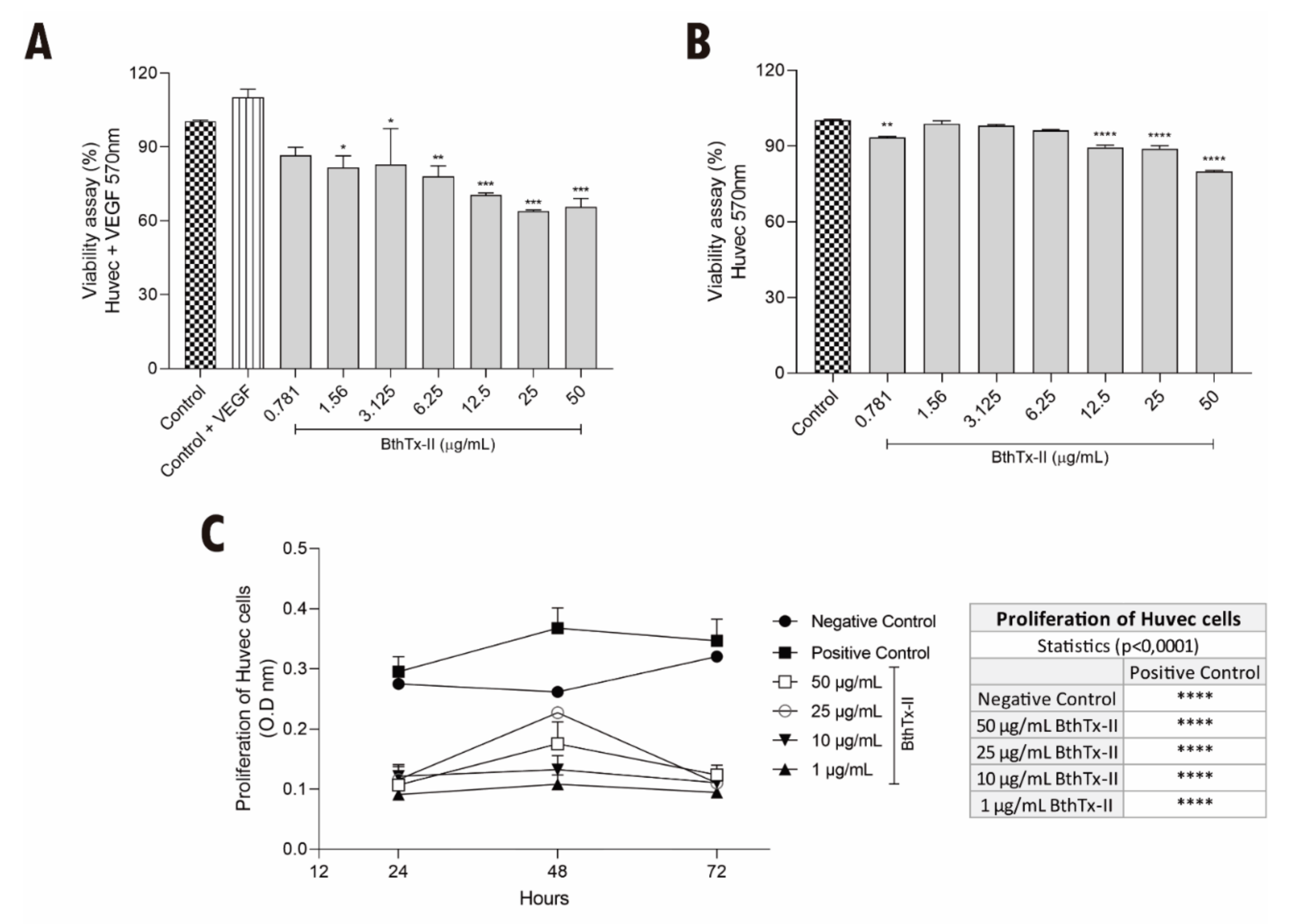
3.1. BthTx-II Induces Cytotoxicity and Decreases the Proliferation of HUVEC
3.2. BthTx-II Promotes G0-G1 Phase Cell Cycle Arrest in HUVEC
3.3. BthTx-II Interferes with the Adhesion and Migration of HUVEC
3.4. BthTx-II Inhibits Vessel Formation and Decreases the Production of Endothelial Growth Factor (VEGF) (In Vitro Model)
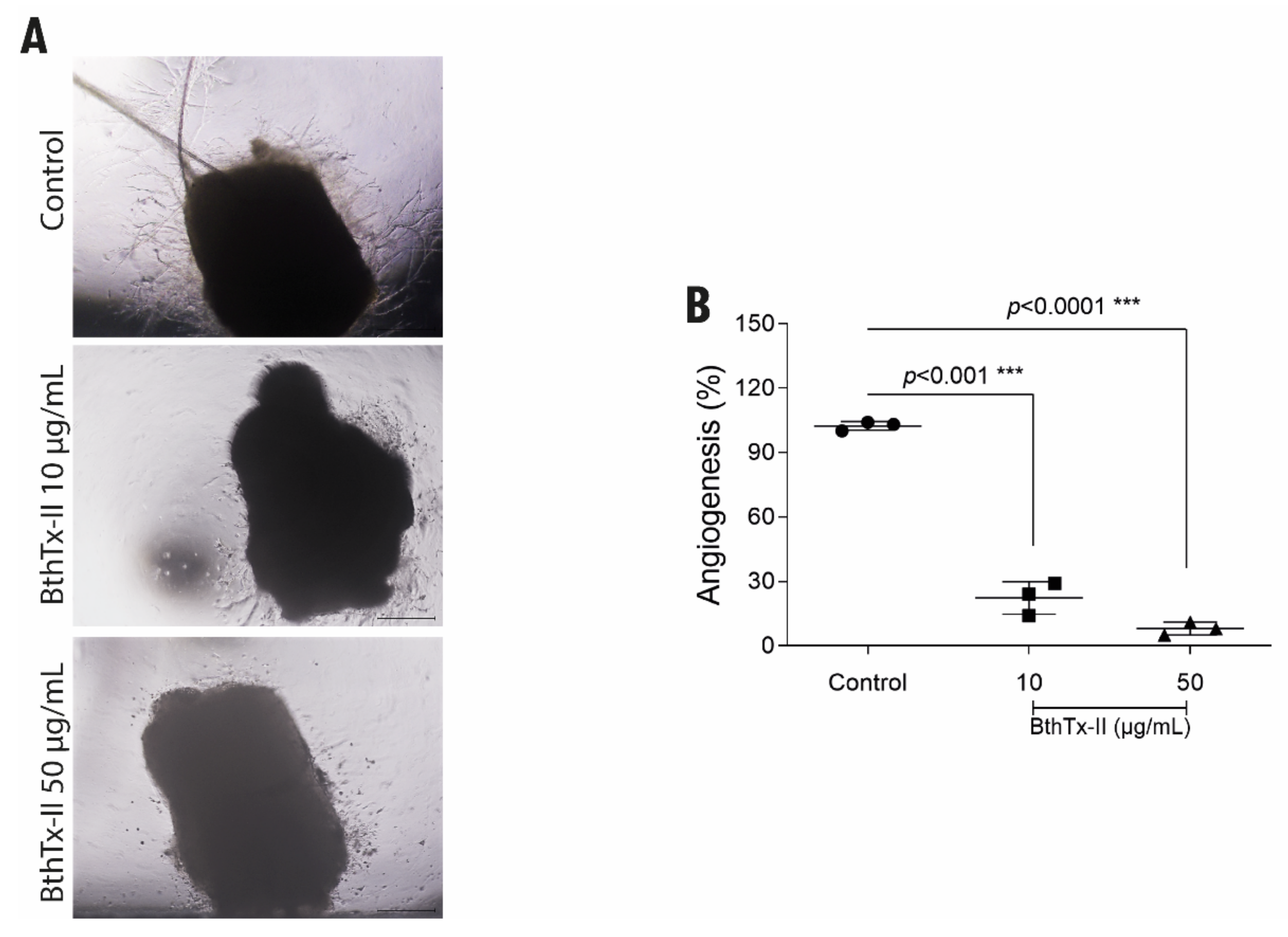
3.5. BthTx-II Inhibits the Formation of Vessels through the Aortic Ring of Mice
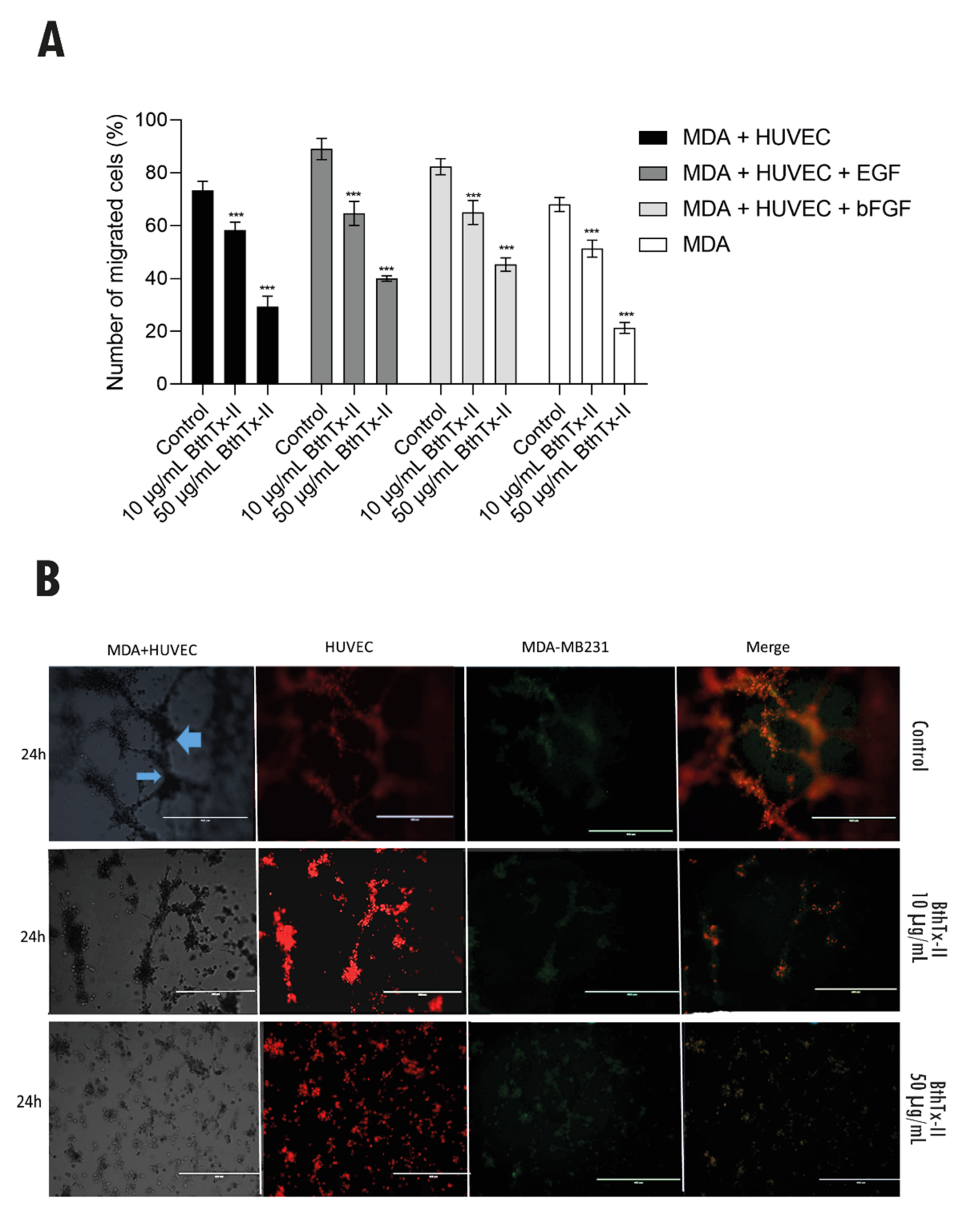
3.6. BthTx-II Inhibits Transwell/Matrigel Invasion of MDA-MB-231 Cells: HUVEC and Tumor Angiogenesis Assessed by Co-Culture Assay
3.7. BthTx-II Inhibits Tumor Angiogenesis In Vivo through the Chick Chorioallantoic Membrane (CAM)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Globol Cancer Observatory. New Global Cancer Data: GLOBOCAN 2020. Available online: http://gco.iarc.fr/ (accessed on 2 February 2021).
- Khaled, N.; Bidet, Y. New Insights into the Implication of Epigenetic Alterations in the EMT of Triple Negative Breast Cancer. Cancers 2019, 11, 559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bielenberg, D.R.; Zetter, B.R. The Contribution of Angiogenesis to the Process of Metastasis. Cancer J. 2015, 21, 267–273. [Google Scholar] [CrossRef] [Green Version]
- Rajabi, M.; Mousa, S.A. The Role of Angiogenesis in Cancer Treatment. Biomedicines 2017, 5, 34. [Google Scholar] [CrossRef] [Green Version]
- Shojaei, F. Anti-angiogenesis therapy in cancer: Current challenges and future perspectives. Cancer Lett. 2012, 320, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Stankic, M.; Pavlovic, S.; Chin, Y.; Brogi, E.; Padua, D.; Norton, L.; Massagué, J.; Benezra, R. TGF-β-Id1 Signaling Opposes Twist1 and Promotes Metastatic Colonization via a Mesenchymal-to-Epithelial Transition. Cell Rep. 2013, 5, 1228–1242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Risau, W. Mechanisms of angiogenesis. Nature 1997, 386, 671–674. [Google Scholar] [CrossRef]
- Correa de Sampaio, P.; Auslaender, D.; Krubasik, D.; Failla, A.V.; Skepper, J.N.; Murphy, G.; English, W.R. A Heterogeneous In Vitro Three Dimensional Model of Tumour-Stroma Interactions Regulating Sprouting Angiogenesis. PLoS ONE 2012, 7, e30753. [Google Scholar] [CrossRef] [Green Version]
- Hirschhaeuser, F.; Menne, H.; Dittfeld, C.; West, J.; Mueller-Klieser, W.; Kunz-Schughart, L.A. Multicellular tumor spheroids: An underestimated tool is catching up again. J. Biotechnol. 2010, 148, 3–15. [Google Scholar] [CrossRef]
- Pepper, M.S.; Mandriota, S.J.; Jeltsch, M.; Kumar, V.; Alitalo, K. Vascular endothelial growth factor (VEGF)-C synergizes with basic fibroblast growth factor and VEGF in the induction of angiogenesis in vitro and alters endothelial cell extracellular proteolytic activity. J. Cell. Physiol. 1998, 177, 439–452. [Google Scholar] [CrossRef]
- Sant, S.; Johnston, P.A. The production of 3D tumor spheroids for cancer drug discovery. Drug Discov. Today Technol. 2017, 23, 27–36. [Google Scholar] [CrossRef] [Green Version]
- Shoval, H.; Karsch-Bluman, A.; Brill-Karniely, Y.; Stern, T.; Zamir, G.; Hubert, A.; Benny, O. Tumor cells and their crosstalk with endothelial cells in 3D spheroids. Sci. Rep. 2017, 7, 10428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polloni, L.; Azevedo, F.V.P.V.; Teixeira, S.C.; Moura, E.; Costa, T.R.; Gimenes, S.N.C.; Correia, L.I.V.; Freitas, V.; Yoneyama, K.A.G.; Rodrigues, R.S.; et al. Antiangiogenic effects of phospholipase A2 Lys49 BnSP-7 from Bothrops pauloensis snake venom on endothelial cells: An in vitro and ex vivo approach. Toxicol. In Vitro 2021, 72, 105099. [Google Scholar] [CrossRef]
- Calderon, L.A.; Sobrinho, J.C.; Zaqueo, K.D.; de Moura, A.A.; Grabner, A.N.; Mazzi, M.V.; Marcussi, S.; Nomizo, A.; Fernandes, C.F.; Zuliani, J.P.; et al. Antitumoral activity of snake venom proteins: New trends in cancer therapy. BioMed Res. Int. 2014, 2014, 203639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gebrim, L.C.; Marcussi, S.; Menaldo, D.L.; de Menezes, C.S.; Nomizo, A.; Hamaguchi, A.; Silveira-Lacerda, E.P.; Homsi-Brandeburgo, M.I.; Sampaio, S.V.; Soares, A.M.; et al. Antitumor effects of snake venom chemically modified Lys49 phospholipase A2-like BthTX-I and a synthetic peptide derived from its C-terminal region. Biol. J. Int. Assoc. Biol. Stand. 2009, 37, 222–229. [Google Scholar] [CrossRef]
- Azevedo, F.V.; Lopes, D.S.; Cirilo Gimenes, S.N.; Ache, D.C.; Vecchi, L.; Alves, P.T.; Guimaraes Dde, O.; Rodrigues, R.S.; Goulart, L.R.; Rodrigues Vde, M.; et al. Antitumor and antimetastatic effects of PLA2-BthTX-II from Bothrops jararacussu venom on human breast cancer cells. Int. J. Biol. Macromol. 2019, 82, 671–677. [Google Scholar] [CrossRef]
- Silva, M.A.; Lopes, D.S.; Teixeira, S.C.; Gimenes, S.N.C.; Azevedo, F.V.P.V.; Polloni, L.; Borges, B.C.; da Silva, M.S.; Barbosa, M.J.; Oliveira Júnior, R.J.d.; et al. Genotoxic effects of BnSP-6, a Lys-49 phospholipase A2 (PLA2) homologue from Bothrops pauloensis snake venom, on MDA-MB-231 breast cancer cells. Int. J. Biol. Macromol. 2018, 118, 311–319. [Google Scholar] [CrossRef]
- Kessentini-Zouari, R.; Jebali, J.; Taboubi, S.; Srairi-Abid, N.; Morjen, M.; Kallech-Ziri, O.; Bezzine, S.; Marvaldi, J.; Ayeb, M.E.L.; Marrakchi, N.; et al. CC-PLA2-1 and CC-PLA2-2, two Cerastes cerastes venom-derived phospholipases A2, inhibit angiogenesis both in vitro and in vivo. Lab. Investig. 2010, 90, 510–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bazaa, A.; Luis, J.; Srairi-Abid, N.; Kallech-Ziri, O.; Kessentini-Zouari, R.; Defilles, C.; Lissitzky, J.C.; El Ayeb, M.; Marrakchi, N. MVL-PLA2, a phospholipase A2 from Macrovipera lebetina transmediterranea venom, inhibits tumor cells adhesion and migration. Matrix Biol. J. Int. Soc. Matrix Biol. 2009, 28, 188–193. [Google Scholar] [CrossRef]
- Homsi-Brandeburgo, M.I.; Queiroz, L.S.; Santo-Neto, H.; Rodrigues-Simioni, L.; Giglio, J.R. Fractionation of Bothrops jararacussu snake venom: Partial chemical characterization and biological activity of bothropstoxin. Toxicon 1988, 26, 615–627. [Google Scholar] [CrossRef]
- Kini, R.M. Excitement ahead: Structure, function and mechanism of snake venom phospholipase A2 enzymes. Toxicon 2003, 42, 827–840. [Google Scholar] [CrossRef]
- Rodrigues, R.S.; Izidoro, L.F.M.; De Oliveira, J.R.; Robson, J.; Sampaio, S.V.; Soares, A.M.; Rodrigues, V.M. Snake venom phospholipases A2: A new class of antitumor agents. Protein Pept. Lett. 2009, 16, 894–898. [Google Scholar] [CrossRef]
- Fuly, A.L.; Soares, A.M.; Marcussi, S.; Giglio, J.R.; Guimarães, J.A. Signal transduction pathways involved in the platelet aggregation induced by a D-49 phospholipase A2 isolated from Bothrops jararacussu snake venom. Biochimie 2004, 86, 731–739. [Google Scholar] [CrossRef]
- Rodrigues, V.; Lopes, D.; Castanheira, L.; Gimenes, S.; Souza, D.; Achê, D.; Borges, I.; Yoneyama, K.; Rodrigues, R. Bothrops pauloensis Snake Venom Toxins: The Search for New Therapeutic Models. Curr. Top. Med. Chem. 2015, 15, 670–684. [Google Scholar] [CrossRef] [PubMed]
- Akalu, A.; Cretu, A.; Brooks, P.C. Targeting integrins for the control of tumour angiogenesis. Expert Opin. Investig. Drugs 2005, 14, 1475–1486. [Google Scholar] [CrossRef]
- Nicolas, J.P.; Lambeau, G.; Lazdunski, M. Identification of the binding domain for secretory phospholipases A2 on their M-type 180-kDa membrane receptor. J. Biol. Chem. 1995, 270, 28869–28873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lomonte, B.; Angulo, Y.; Moreno, E. Synthetic peptides derived from the C-terminal region of Lys49 phospholipase A2 homologues from viperidae snake venoms: Biomimetic activities and potential applications. Curr. Pharm. Des. 2010, 16, 3224–3230. [Google Scholar] [CrossRef] [PubMed]
- Ambrosio, A.L.B.; Nonato, M.C.; de Araújo, H.S.S.; Arni, R.; Ward, R.J.; Ownby, C.L.; de Souza, D.H.F.; Garratt, R.C. A Molecular Mechanism For Lys49-Phospholipase A2 Activity Based on Ligand-Induced Conformational Change *. J. Biol. Chem. 2005, 280, 7326–7335. [Google Scholar] [CrossRef] [Green Version]
- Arnaoutova, I.; Kleinman, H.K. In vitro angiogenesis: Endothelial cell tube formation on gelled basement membrane extract. Nat. Protoc. 2010, 5, 628–635. [Google Scholar] [CrossRef]
- Baker, M.; Robinson, S.D.; Lechertier, T.; Barber, P.R.; Tavora, B.; D’amico, G.; Jones, D.T.; Vojnovic, B.; Hodivala-Dilke, K. Use of the Mouse Aortic Ring Assay to Study Angiogenesis. Nat. Protoc. 2011, 7, 89–104. [Google Scholar] [CrossRef]
- Nowak-Sliwinska, P.; Alitalo, K.; Allen, E.; Anisimov, A.; Aplin, A.C.; Auerbach, R.; Augustin, H.G.; Bates, D.O.; van Beijnum, J.R.; Bender, R.H.F.; et al. Consensus guidelines for the use and interpretation of angiogenesis assays. Angiogenesis 2018, 21, 425–532. [Google Scholar] [CrossRef] [Green Version]
- Gimenes, S.N.C.; Lopes, D.S.; Alves, P.T.; Azevedo, F.; Vecchi, L.; Goulart, L.R.; Rodrigues, T.C.S.; Santos, A.L.Q.; Brites, V.L.C.; Teixeira, T.L.; et al. Antitumoral effects of gammaCdcPLI, a PLA2 inhibitor from Crotalus durissus collilineatus via PI3K/Akt pathway on MDA-MB-231 breast cancer cell. Sci. Rep. 2017, 7, 7077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swaminathan, S.; Ngo, O.; Basehore, S.; Clyne, A.M. Vascular Endothelial–Breast Epithelial Cell Coculture Model Created from 3D Cell Structures. ACS Biomater. Sci. Eng. 2017, 3, 2999–3006. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Gerber, H.P. The Role of Vascular Endothelial Growth Factor in Angiogenesis. Acta Haematol. 2001, 106, 148–156. [Google Scholar] [CrossRef]
- Jiménez–Charris, E.; Lopes, D.S.; Gimenes, S.N.C.; Teixeira, S.C.; Montealegre–Sánchez, L.; Solano–Redondo, L.; Fierro–Pérez, L.; Rodrigues Ávila, V.d.M. Antitumor potential of Pllans–II, an acidic Asp49–PLA2 from Porthidium lansbergii lansbergii snake venom on human cervical carcinoma HeLa cells. Int. J. Biol. Macromol. 2019, 122, 1053–1061. [Google Scholar] [CrossRef]
- Han, R.; Liang, H.; Qin, Z.H.; Liu, C.Y. Crotoxin induces apoptosis and autophagy in human lung carcinoma cells in vitro via activation of the p38MAPK signaling pathway. Acta Pharmacol. Sin. 2014, 35, 1323–1332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Lv, P.; Liu, J.; Xu, K. Apoptosis of human hepatocellular carcinoma cell (HepG2) induced by cardiotoxin III through S-phase arrest. Exp. Toxicol. Pathol. 2009, 61, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Hartwell, L.H.; Weinert, T.A. Checkpoints: Controls that Ensure the Order of Cell Cycle Events. Science 1989, 246, 629–634. [Google Scholar] [CrossRef] [Green Version]
- Behzadian, M.A.; Windsor, L.J.; Ghaly, N.; Liou, G.; Tsai, N.-T.; Caldwell, R.B. VEGF-induced paracellular permeability in cultured endothelial cells involves urokinase and its receptor. FASEB J. 2003, 17, 752–754. [Google Scholar] [CrossRef]
- Bazaa, A.; Pasquier, E.; Defilles, C.; Limam, I.; Kessentini-Zouari, R.; Kallech-Ziri, O.; Battari, A.E.; Braguer, D.; Ayeb, M.E.; Marrakchi, N.; et al. MVL-PLA2, a snake venom phospholipase A2, inhibits angiogenesis through an increase in microtubule dynamics and disorganization of focal adhesions. PLoS ONE 2010, 5, e10124. [Google Scholar] [CrossRef] [Green Version]
- Xie, Q.; Tang, N.; Wan, R.; Qi, Y.; Lin, X.; Lin, J. Recombinant snake venom cystatin inhibits tumor angiogenesis in vitro and in vivo associated with downregulation of VEGF-A165, Flt-1 and bFGF. Anti-Cancer Agents Med. Chem. 2013, 13, 663–671. [Google Scholar] [CrossRef]
- Guimaraes, D.O.; Lopes, D.S.; Azevedo, F.V.; Gimenes, S.N.; Silva, M.A.; Ache, D.C.; Gomes, M.S.; Vecchi, L.; Goulart, L.R.; Yoneyama, K.A.; et al. In vitro antitumor and antiangiogenic effects of Bothropoidin, a metalloproteinase from Bothrops pauloensis snake venom. Int. J. Biol. Macromol. 2017, 97, 770–777. [Google Scholar] [CrossRef] [PubMed]
- Kini, R.M.; Evans, H.J. A model to explain the pharmacological effects of snake venom phospholipases A2. Toxicon 1989, 27, 613–635. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Matsunaga, Y.; Nakano, Y.; Morita, T. Identification of vascular endothelial growth factor receptor-binding protein in the venom of eastern cottonmouth. A new role of snake venom myotoxic Lys49-phospholipase A2. J. Biol. Chem. 2005, 280, 29989–29992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujisawa, D.; Yamazaki, Y.; Lomonte, B.; Morita, T. Catalytically inactive phospholipase A2 homologue binds to vascular endothelial growth factor receptor-2 via a C-terminal loop region. Biochem. J. 2008, 411, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Lambeau, G.; Ancian, P.; Nicolas, J.-P.; Beiboer, S.H.W.; Moinier, D.; Verheij, H.; Lazdunski, M. Structural Elements of Secretory Phospholipases A2 Involved in the Binding to M-type Receptors (*). J. Biol. Chem. 1995, 270, 5534–5540. [Google Scholar] [CrossRef] [Green Version]
- Massimino, M.L.; Simonato, M.; Spolaore, B.; Franchin, C.; Arrigoni, G.; Marin, O.; Monturiol-Gross, L.; Fernández, J.; Lomonte, B.; Tonello, F. Cell surface nucleolin interacts with and internalizes Bothrops asper Lys49 phospholipase A2 and mediates its toxic activity. Sci. Rep. 2018, 8, 10619. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, T.; Hiroki, K.; Yamashita, Y. The role of epidermal growth factor receptor in cancer metastasis and microenvironment. BioMed Res. Int. 2013, 2013, 546318. [Google Scholar] [CrossRef] [Green Version]
- Melincovici, C.S.; Bosca, A.B.; Susman, S.; Marginean, M.; Mihu, C.; Istrate, M.; Moldovan, I.M.; Roman, A.L.; Mihu, C.M. Vascular endothelial growth factor (VEGF)—Key factor in normal and pathological angiogenesis. Rom. J. Morphol. Embryol. 2018, 59, 455–467. [Google Scholar]
- Pinto, M.P.; Dye, W.W.; Jacobsen, B.M.; Horwitz, K.B. Malignant stroma increases luminal breast cancer cell proliferation and angiogenesis through platelet-derived growth factor signaling. BMC Cancer 2014, 14, 735. [Google Scholar] [CrossRef] [Green Version]
- Eiro, N.; Gonzalez, L.; Martinez-Ordonez, A.; Fernandez-Garcia, B.; Gonzalez, L.O.; Cid, S.; Dominguez, F.; Perez-Fernandez, R.; Vizoso, F.J. Cancer-associated fibroblasts affect breast cancer cell gene expression, invasion and angiogenesis. Cell. Oncol. 2018, 41, 369–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, R.; D’Amico, G.; Hodivala-Dilke, K.M.; Reynolds, L.E. Integrins: The keys to unlocking angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1703–1713. [Google Scholar] [CrossRef]
- Machado, A.R.T.; Aissa, A.F.; Ribeiro, D.L.; Costa, T.R.; Ferreira, R.S., Jr.; Sampaio, S.V.; Antunes, L.M.G. Cytotoxic, genotoxic, and oxidative stress-inducing effect of an l-amino acid oxidase isolated from Bothrops jararacussu venom in a co-culture model of HepG2 and HUVEC cells. Int. J. Biol. Macromol. 2019, 127, 425–432. [Google Scholar] [CrossRef]
- Amann, A.; Zwierzina, M.; Koeck, S.; Gamerith, G.; Pechriggl, E.; Huber, J.M.; Lorenz, E.; Kelm, J.M.; Hilbe, W.; Zwierzina, H.; et al. Development of a 3D angiogenesis model to study tumour—Endothelial cell interactions and the effects of anti-angiogenic drugs. Sci. Rep. 2017, 7, 2963. [Google Scholar] [CrossRef] [Green Version]
- Richardson, M.; Singh, G. Observations on the Use of the Avian Chorioallantoic Membrane (CAM) Model in Investigations into Angiogenesis. Curr. Drug Targets—Cardiovasc. Hematol. Disord. 2003, 3, 155–185. [Google Scholar] [CrossRef]
- Strojnik, T.; Kavalar, R.; Barone, T.A.; Plunkett, R.J. Experimental model and immunohistochemical comparison of U87 human glioblastoma cell xenografts on the chicken chorioallantoic membrane and in rat brains. Anticancer Res. 2010, 30, 4851–4860. [Google Scholar]
- Lokman, N.A.; Elder, A.S.; Ricciardelli, C.; Oehler, M.K. Chick chorioallantoic membrane (CAM) assay as an in vivo model to study the effect of newly identified molecules on ovarian cancer invasion and metastasis. Int. J. Mol. Sci. 2012, 13, 9959–9970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Z.; Lin, W.; Huang, Z.; Chen, X.; Zhao, J.; Zheng, L.; Ye, H.; Liu, Z.; Liao, L.; Du, J. Jiedu Xiaozheng Yin, a Chinese herbal formula, inhibits tumor angiogenesis via downregulation of VEGF-A and VEGFR-2 expression in vivo and in vitro. Oncol. Rep. 2013, 29, 1080–1086. [Google Scholar] [CrossRef]
- Lin, J.; Chen, Y.; Wei, L.; Hong, Z.; Sferra, T.J.; Peng, J. Ursolic acid inhibits colorectal cancer angiogenesis through suppression of multiple signaling pathways. Int. J. Oncol. 2013, 43, 1666–1674. [Google Scholar] [CrossRef] [PubMed] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Petten de Vasconcelos Azevedo, F.; Lopes, D.S.; Zóia, M.A.P.; Correia, L.I.V.; Saito, N.; Fonseca, B.B.; Polloni, L.; Teixeira, S.C.; Goulart, L.R.; de Melo Rodrigues Ávila, V. A New Approach to Inhibiting Triple-Negative Breast Cancer: In Vitro, Ex Vivo and In Vivo Antiangiogenic Effect of BthTx-II, a PLA2-Asp-49 from Bothrops jararacussu Venom. Biomolecules 2022, 12, 258. https://doi.org/10.3390/biom12020258
Van Petten de Vasconcelos Azevedo F, Lopes DS, Zóia MAP, Correia LIV, Saito N, Fonseca BB, Polloni L, Teixeira SC, Goulart LR, de Melo Rodrigues Ávila V. A New Approach to Inhibiting Triple-Negative Breast Cancer: In Vitro, Ex Vivo and In Vivo Antiangiogenic Effect of BthTx-II, a PLA2-Asp-49 from Bothrops jararacussu Venom. Biomolecules. 2022; 12(2):258. https://doi.org/10.3390/biom12020258
Chicago/Turabian StyleVan Petten de Vasconcelos Azevedo, Fernanda, Daiana Silva Lopes, Mariana Alves Pereira Zóia, Lucas Ian Veloso Correia, Natieli Saito, Belchiolina Beatriz Fonseca, Lorena Polloni, Samuel Cota Teixeira, Luiz Ricardo Goulart, and Veridiana de Melo Rodrigues Ávila. 2022. "A New Approach to Inhibiting Triple-Negative Breast Cancer: In Vitro, Ex Vivo and In Vivo Antiangiogenic Effect of BthTx-II, a PLA2-Asp-49 from Bothrops jararacussu Venom" Biomolecules 12, no. 2: 258. https://doi.org/10.3390/biom12020258
APA StyleVan Petten de Vasconcelos Azevedo, F., Lopes, D. S., Zóia, M. A. P., Correia, L. I. V., Saito, N., Fonseca, B. B., Polloni, L., Teixeira, S. C., Goulart, L. R., & de Melo Rodrigues Ávila, V. (2022). A New Approach to Inhibiting Triple-Negative Breast Cancer: In Vitro, Ex Vivo and In Vivo Antiangiogenic Effect of BthTx-II, a PLA2-Asp-49 from Bothrops jararacussu Venom. Biomolecules, 12(2), 258. https://doi.org/10.3390/biom12020258






