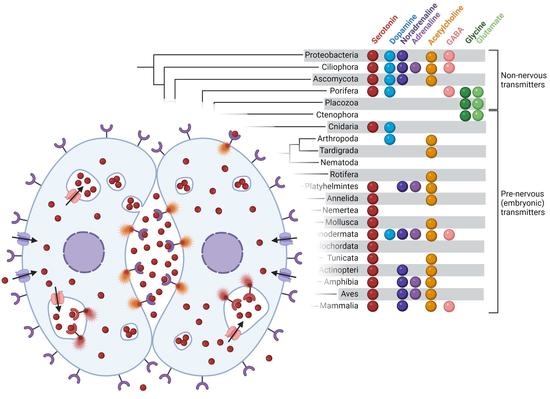
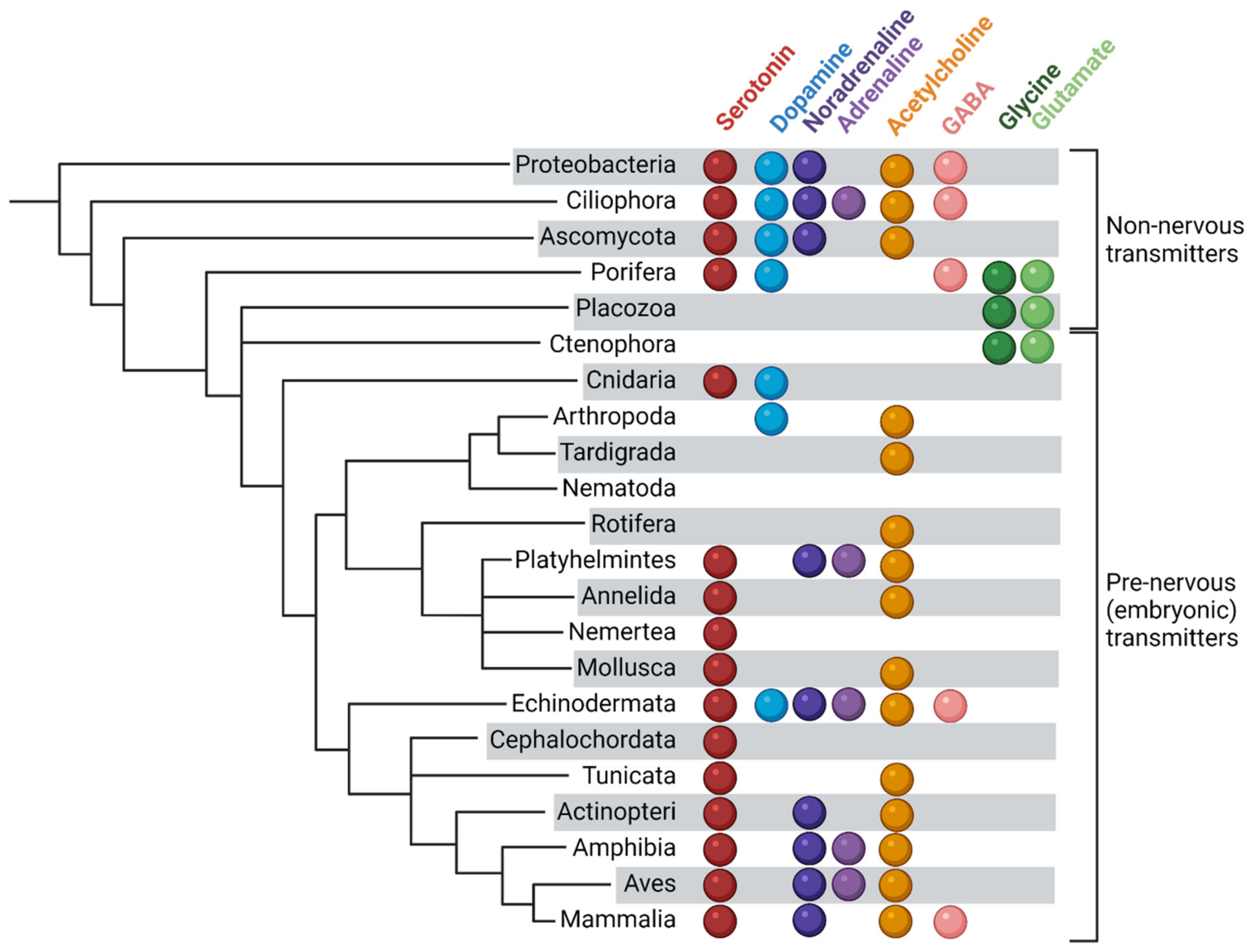
Non-Neuronal Transmitter Systems in Bacteria, Non-Nervous Eukaryotes, and Invertebrate Embryos
Abstract
:1. Introduction
2. Transmitter Systems in Bacteria
3. Transmitter Systems in Unicellular Organisms
4. Transmitter Systems in Non-Neural Multicellular Invertebrates
5. Transmitter Systems in Invertebrate Prenervous Embryogenesis
5.1. Molecular Biology of Embryonic Transmitter Signalization
5.2. Physiological Effects of the Transmitters
5.3. Intracellular Transmitter Activity
5.4. Intercellular Transmitter Signaling
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Loewi, O. Über humorale übertragbarkeit der Herznervenwirkund. I: Mittellung. Pflügers Arch. 1921, 189, 239–242. [Google Scholar] [CrossRef]
- Rowatt, E. The relation of pantothenic acid to acetylcholine formation by a strain of Lactobacillus plantarum. J. Gen. Microbiol. 1948, 2, 25–30. [Google Scholar] [CrossRef] [Green Version]
- Numanoi, H. Studies on the fertilization substances. IV. Presence of acetylcholine-like substance and cholinesterase in echinoderm-germ cells during fertilization. Scient. Papers Coll. Gen. Educ. Univ. Tokyo 1953, 3, 193–200. [Google Scholar]
- Buznikov, G.A.; Manukhin, B.N. 5-HT influence on the embryonic motility of nudibranch molluscs. Zh. Obshch. Boil. 1960, 21, 347–352. (in Russian). [Google Scholar]
- Koshtoyants, K.S.; Buznikov, G.A.; Manukhin, B.N. The possible role of 5-hydroxytryptamine in the motor activity of embryos of some marine gastropods. Comp. Biochem. Physiol. 1961, 3, 20–26. [Google Scholar] [CrossRef]
- Buznikov, G.A.; Chudakova, I.V.; Zvezdina, N.D. The role of neurohumors in early embryogenesis. I. 5-HT content of developing embryos of sea urchin and loach. J. Embryol. Exp. Morph. 1964, 12, 563–573. [Google Scholar]
- Buznikov, G.A.; Sakharova, A.V.; Manukhin, B.N.; Markova, L.N. The role of neurohumors in early embryogenesis. IV. Fluorometric and histochemical study of 5-HT in cleaving eggs and larvae of sea urchins. J. Embryol. Exp. Morph. 1972, 27, 339–351. [Google Scholar]
- Shmukler, Y.B.; Buznikov, G.A. Functional coupling of neurotransmitters with second messengers during cleavage divisions: Facts and hypotheses. Perspect. Dev. Neurobiol. 1998, 5, 469–480. [Google Scholar]
- Buznikov, G.A. Transmitters in early embryogenesis (new data). Ontogenez 1989, 20, 637–646. (in Russian). [Google Scholar]
- Shmukler, Y.; Nikishin, D. Transmitters in Blastomere Interactions. In Cell Interactions; Gowder, S., Ed.; InTech: Rijeka, Croatia, 2012; Ch. 2; pp. 31–65. [Google Scholar]
- Moroz, L.L.; Romanova, D.Y.; Kohn, A.B. Neural versus alternative integrative systems: Molecular insights into origins of neurotransmitters. Phil. Trans. R. Soc. B 2021, 376, 20190762. [Google Scholar] [CrossRef]
- Girvin, G.T.; Stevenson, J.W. Cell free “choline acetylase” from Lactobacillus plantarum. Can. J. Biochem. Physiol. 1954, 32, 131–146. [Google Scholar] [CrossRef]
- Wall, R.; Cryan, J.F.; Ross, R.P.; Fitzgerald, G.F.; Dinan, T.G.; Stanton, C. Bacterial neuroactive compounds produced by probiotics. In Advanced Experimental Medical Biology; Microbial Endocrinology: The Microbiota-Gut-Brain Axis in Health and Disease; Lyte, M., Cryan, J.F., Eds.; Springer Nature: New York, NY, USA, 2014; Volume 817, pp. 221–239. [Google Scholar] [CrossRef]
- Horiuchi, Y.; Kimura, R.; Kato, N.; Fujii, T.; Seki, M.; Endo, T.; Kato, T.; Kawashima, K. Evolutional study on acetylcholine expression. Life Sci. 2003, 72, 1745–1756. [Google Scholar] [CrossRef]
- Hsu, S.C.; Johansson, K.R.; Donahue, M.J. The bacterial flora of the intestine of Ascaris suum and 5-hydroxytryptamine production. J. Parasitol. 1986, 72, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Shahkolahi, A.M.; Donahue, M.J. Bacterial flora, a possible source of serotonin in the intestine of adult female Ascaris suum. J. Parasitol. 1993, 79, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Özogul, F. Effects of specific lactic acid bacteria species on biogenic amine production by foodborne pathogens. Int. J. Food Sci. Technol. 2011, 46, 478–484. [Google Scholar] [CrossRef]
- Tsavkelova, E.A.; Botvinko, I.B.; Kudrin, V.S.; Oleskin, A.V. Detection of neuromediator amines in microorganisms by high-performance liquid chromatography. Dokl. Akad. Nauk 2000, 372, 840–842. [Google Scholar]
- Shishov, V.A.; Kirovskaya, T.A.; Kudrin, V.S.; Oleskin, A.V. Amine Neuromediators, Their Precursors, and Oxidation Products in the Culture of Escherichia coli. Appl. Biochem. Microbiol. 2009, 45, 494–497. [Google Scholar] [CrossRef]
- Lyte, M.; Ernst, S. Catecholamine induced growth of gram negative bacteria. Life Sci. 1992, 50, 203–212. [Google Scholar] [CrossRef]
- Anuchin, A.M.; Chuvelev, D.I.; Kirovskaya, T.A.; Oleskin, A.V. Effect of neuromediator monoamines on the growth-related variables of Escherichia coli K-12. Microbiology 2008, 77, 758–765. [Google Scholar] [CrossRef]
- Lyte, M.; Ernst, S. Alpha and beta adrenergic receptor involvement in catecholamine-induced growth of gram-negative bacteria. Biochem. Biophys. Res. Commun. 1993, 190, 447–452. [Google Scholar] [CrossRef]
- Oleskin, A.V.; Kirovskaya, T.A.; Botvinko, I.V.; Lysak, L.V. Effect of 5-HT (5-hydroxytryptamine) on the growth and differentiation of microorganisms. Microbiology 1998, 67, 306–311. [Google Scholar]
- De Sousa, A.K.; Rocha, J.E.; de Souza, T.G.; de Freitas, T.S.; Ribeiro-Filho, J.; Coutinho, H.D.M. New roles of fluoxetine in pharmacology: Antibacterial effect and modulation of antibiotic activity. Microb. Pathog. 2018, 123, 368–371. [Google Scholar] [CrossRef] [PubMed]
- Oleskin, A.V.; Sorokina, E.V.; Zarubina, A.P.; Parkhomenko, I.M. Testing neurotransmitters for toxicity with a luminescent biosensor: Implications for microbial endocrinology. J. Pharm. Nutr. Sci. 2017, 7, 88–94. [Google Scholar] [CrossRef] [Green Version]
- Sorokina, E.V.; Vodolazov, I.R.; Oleskin, A.V. Stimulatory and Toxic Effects of Neurotransmitters on the lux Operon-Dependent Bioluminescence of Escherichia coli K12 TGI. J. Pharm. Nutr. Sci. 2019, 9, 136–143. [Google Scholar]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef] [Green Version]
- Johnson, K.V.A.; Foster, K.R. Why does the microbiome affect behavior? Nat. Rev. Microbiol. 2018, 16, 647–655. [Google Scholar] [CrossRef] [Green Version]
- LeRoith, D.; Delahunty, G.; Wilson, G.L.; Roberts, C.T., Jr.; Shemer, J.; Hart, C.; Lesniak, M.A.; Shiloach, J.; Roth, J. Evolutionary aspects of the endocrine and nervous systems. Recent Prog. Horm. Res. 1986, 42, 549–587. [Google Scholar] [CrossRef]
- Lenard, J. Mammalian hormones in microbial cells. Trends Biochem. Sci. 1992, 17, 147–150. [Google Scholar] [CrossRef]
- Knecht, L.D.; O’Connor, G.; Mittal, R.; Liu, X.Z.; Daftarian, P.; Deo, S.K.; Daunert, S. Serotonin Activates Bacterial Quorum Sensing and Enhances the Virulence of Pseudomonas aeruginosa in the Host. EBioMedicine 2016, 9, 161–169. [Google Scholar] [CrossRef] [Green Version]
- Ishii, E.; Eguchi, Y. Diversity in Sensing and Signaling of Bacterial Sensor Histidine Kinases. Biomolecules 2021, 11, 1524. [Google Scholar] [CrossRef]
- Thomassin, J.-L.; Leclerc, J.-M.; Giannakopoulou, N.; Zhu, L.; Salmon, K.; Portt, A.; Gruenheid, S. Systematic Analysis of Two-Component Systems in Citrobacter rodentium Reveals Positive and Negative Roles in Virulence. Infect. Immun. 2017, 85, e00654-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zschiedrich, C.P.; Keidel, V.; Szurmant, H. Molecular Mechanisms of Two-Component Signal Transduction. J. Mol. Biol. 2016, 428, 3752–3775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clarke, M.B.; Hughes, D.T.; Zhu, C.; Boedeker, E.C.; Sperandio, V. The QseC sensor kinase: A bacterial adrenergic receptor. Proc. Natl. Acad. Sci. USA 2006, 103, 10420–10425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reading, N.C.; Torres, A.G.; Kendall, M.M.; Hughes, D.T.; Yamamoto, K.; Sperandio, V. A Novel Two-Component Signaling System That Activates Transcription of an Enterohemorrhagic Escherichia coli Effector Involved in Remodeling of Host Actin. J. Bacteriol. 2007, 189, 2468–2476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hughes, D.T.; Clarke, M.B.; Yamamoto, K.; Rasko, D.A.; Sperandio, V. The QseC adrenergic signaling cascade in Enterohemorrhagic, E. coli (EHEC). PLoS Pathog. 2009, 5, e1000553. [Google Scholar] [CrossRef] [Green Version]
- Moreira, C.G.; Russell, R.; Mishra, A.A.; Narayanan, S.; Ritchie, J.M.; Waldor, M.K.; Curtis, M.M.; Winter, S.E.; Weinshenker, D.; Sperandio, V. Bacterial Adrenergic Sensors Regulate Virulence of Enteric Pathogens in the Gut. mBio 2016, 7, e00826-16. [Google Scholar] [CrossRef] [Green Version]
- Kendall, M.M.; Sperandio, V. What a Dinner Party! Mechanisms and Functions of Interkingdom Signaling in Host-Pathogen Associations. mBio 2016, 7, e01748-15. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Russell, R.M.; Pifer, R.; Menezes-Garcia, Z.; Cuesta, S.; Narayanan, S.; MacMillan, J.B.; Sperandio, V. The Serotonin Neurotransmitter Modulates Virulence of Enteric Pathogens. Cell Host Microbe 2020, 28, 41–53. [Google Scholar] [CrossRef]
- Kumar, A.; Sperandio, V. Indole Signaling at the Host-Microbiota-Pathogen Interface. mBio 2019, 10, e01031-19. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Ding, X.; Rather, P.N. Indole can act as an extracellular signal in Escherichia coli. J. Bacteriol. 2001, 183, 4210–4216. [Google Scholar] [CrossRef] [Green Version]
- Stock, A.M.; Robinson, V.L.; Goudreau, P.N. Two-Component Signal Transduction. Annu. Rev. Biochem. 2000, 69, 183–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galperin, M.Y. Bacterial signal transduction network in a genomic perspective. Environ. Microbiol. 2004, 6, 552–567. [Google Scholar] [CrossRef] [PubMed]
- Galperin, M.Y.; Makarova, K.S.; Wolf, Y.I.; Koonin, E.V. Phyletic Distribution and Lineage-Specific Domain Architectures of Archaeal Two-Component Signal Transduction Systems. J. Bacteriol. 2018, 200, e00681-17. [Google Scholar] [CrossRef] [Green Version]
- Stephenson, K.; Hoch, J.A. Two-Component and Phosphorelay Signal-Transduction Systems as Therapeutic Targets. Curr. Opin. Pharmacol. 2002, 2, 507–512. [Google Scholar] [CrossRef]
- Gotoh, Y.; Eguchi, Y.; Watanabe, T.; Okamoto, S.; Doi, A.; Utsumi, R. Two-Component Signal Transduction as Potential Drug Targets in Pathogenic Bacteria. Curr. Opin. Microbiol. 2010, 13, 232–239. [Google Scholar] [CrossRef]
- Bem, A.E.; Velikova, N.; Pellicer, M.T.; van Baarlen, P.; Marina, A.; Wells, J.M. Bacterial histidine kinases as novel antibacterial drug targets. ACS Chem. Biol. 2015, 10, 213–224. [Google Scholar] [CrossRef]
- Ponting, C.P.; Schultz, J.; Copley, R.R.; Andrade, M.A.; Bork, P. Evolution of Domain Families. Adv. Protein Chem. 2000, 54, 185–244. [Google Scholar]
- Abriata, L.A.; Albanesi, D.; Dal Peraro, M.; de Mendoza, D. Signal Sensing and Transduction by Histidine Kinases as Unveiled through Studies on a Temperature Sensor. Acc. Chem. Res. 2017, 50, 1359–1366. [Google Scholar] [CrossRef]
- Zhulin, I.B.; Nikolskaya, A.N.; Galperin, M.Y. Common extracellular sensory domains in transmembrane receptors for diverse signal transduction pathways in bacteria and archaea. J. Bacteriol. 2003, 185, 285–294. [Google Scholar] [CrossRef] [Green Version]
- Galperin, M.Y.; Nikolskaya, A.N.; Koonin, E.V. Novel domains of the prokaryotic two-component signal transduction systems. FEMS Microbiol. Lett. 2001, 203, 11–21. [Google Scholar] [CrossRef]
- Kennelly, P.J. Protein kinases and protein phosphatases in prokaryotes: A genomic perspective. FEMS Microbiol. Lett. 2002, 206, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, W.D.; Sullivan, C.F. The acetylcholine content and the effect of hexamethonium bromide in this compound at the various phases of division in Tetrahymena pyriformis Gl. Brôteria. Ciénc. natur. 1964, 33, 17–33. [Google Scholar]
- Janakidevi, K.; Dewey, V.C.; Kidder, G.W. 5-HT in Protozoa. Arch. Biochem. Biophys. 1966, 113, 758–759. [Google Scholar] [CrossRef]
- Blum, J.J. Biogenic amines and metabilic control in Tetrahymena. In Biogenic Amines as Physiological Regulators; Blum, J.J., Ed.; Prentice-Hall, Inc.: Upper Saddle River, NJ, USA, 1970; pp. 95–118. [Google Scholar]
- Corrado Delmonte, M.U.; Ognibene, M.; Trielli, F.; Politi, H.; Passalacqua, M.; Falugi, C. Detection of molecules related to the GABAergic system in a single-cell eukaryote, Paramecium primaurelia. Neurosci. Lett. 2002, 329, 65–68. [Google Scholar] [CrossRef]
- Csaba, G. Biogenic amines at a low level of evolution: Production, functions and regulation in the unicellular Tetrahymena. Acta Microbiol. Immunol. Hung. 2015, 62, 93–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Csaba, G. Hormonal Imprinting: The First Cellular-level Evidence of Epigenetic Inheritance and its Present State. Curr. Genomics 2019, 20, 409–418. [Google Scholar] [CrossRef]
- Baig, A.M.; Rana, Z.; Tariq, S.; Lalani, S.; Ahmad, H.R. Traced on the Timeline: Discovery of Acetylcholine and the Components of the Human Cholinergic System in a Primitive Unicellular Eukaryote Acanthamoeba spp. ACS Chem. Neurosci. 2017, 9, 494–504. [Google Scholar] [CrossRef]
- Ramoino, P.; Fronte, P.; Beltrame, F.; Diaspro, A.; Fato, M.; Raiteri, L.; Stigliani, S.; Usai, C. Swimming behavior regulation by GABAB receptors in Paramecium. Exp. Cell Res. 2003, 291, 398–405. [Google Scholar] [CrossRef]
- Ud-Daula, A.; Pfister, G.; Schramm, K.W. Growth inhibition and biodegradation of catecholamines in the ciliated protozoan Tetrahymena pyriformis. J. Environ. Sci. Health Part A 2008, 43, 1610–1617. [Google Scholar] [CrossRef]
- Wyroba, E. β-adrenergic stimulation of phagocytosis in the unicellular eukariote Paramecium aurelia. Cell Biol. Int. Rep. 1989, 13, 667–678. [Google Scholar] [CrossRef]
- Csaba, G. Is there a hormonal regulation of phagocytosis at unicellular and multicellular levels? A critical review. Acta Microbiol. Immunol. Hung. 2017, 64, 357–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corrado Delmonte, M.U.; Politi, H.; Trielli, F.; Angelini, C.; Falugi, C. Evidence for the presence of a mammalian-like cholinesterase in Paramecium primaurelia (Protista, Ciliophora) developmental cycle. J. Exp. Zool. 1999, 283, 102–105. [Google Scholar] [CrossRef]
- Rodríguez, N.; Renaud, F.L. On the possible role of 5-HT in the regulation of regeneration of cilia. J. Cell Biol. 1980, 85, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Ud-Daula, A.; Pfister, G.; Schramm, K.-W. Identification of Dopamine Receptor in Tetrahymena thermophile by Fluorescent Ligands. Pak. J. Biol. Sci. 2012, 15, 1133–1138. [Google Scholar] [CrossRef] [PubMed]
- Bucci, G.; Ramoino, P.; Diaspro, A.; Usai, C. A role for GABAA receptors in the modulation of Paramecium swimming behavior. Neurosci. Lett. 2005, 386, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Baig, A.M.; Ahmad, H.R. Evidence of a M1-muscarininc GPCR homolog in unicellular eukaryotes: Featuring Acanthamoeba spp bioinformatics 3D-modelling and experimentations. J. Rec. Signal Transduct. 2016, 37, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Ramoino, P.; Scaglione, S.; Diaspro, A.; Beltrame, F.; Fato, M.; Usai, C. GABAA receptor subunits identified in Paramecium by immunofluorescence confocal microscopy. FEMS Microbiol. Lett. 2004, 238, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Connolly, C.M.; Wooltorton, J.R.; Smart, T.G.; Moss, S.J. Subcellular localization of γ-aminobutyric acid type A receptors is determined by receptor β-subunits. Proc. Natl. Acad. Sci. USA 1996, 93, 9899–9904. [Google Scholar] [CrossRef] [Green Version]
- Göhde, R.; Naumann, B.; Laundon, D.; Imig, C.; McDonald, K.; Cooper, B.H.; Varoqueaux, F.; Fasshauer, D.; Burkhardt, P. Choanoflagellates and the ancestry of neurosecretory vesicles. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2021, 376, 20190759. [Google Scholar] [CrossRef]
- Malikina, K.D.; Shishov, V.A.; Chuvelev, D.I.; Kudrin, V.S.; Oleskin, A.V. Regulatory Role of Monoamine Neurotransmitters in Saccharomyces cerevisiae Cells. Appl. Biochem. Microbiol. 2010, 46, 620–625. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Ye, D.Q.; Zhu, B.Q.; Wu, G.F.; Duan, C.Q. Rapid HPLC analysis of amino acids and biogenic amines in wines during fermentation and evaluation of matrix effect. Food Chem. 2014, 163, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Cordente, A.G.; Schmidt, S.; Beltran, G.; Torija, M.J.; Curtin, C.D. Harnessing yeast metabolism of aromatic amino acids for fermented beverage bioflavouring and bioproduction. Appl. Microbiol. Biotechnol. 2019, 103, 4325–4336. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Cruz, E.; Álvarez-Fernández, M.A.; Valero, E.; Troncoso, A.M.; GarcíaParrilla, M.C. Validation of an analytical method to determine melatonin and compounds related to L-tryptophan metabolism using UHPLC/HRMS. Food Anal. Methods 2016, 9, 3327–3336. [Google Scholar] [CrossRef]
- Fernández-Cruz, E.; González, B.; Muñiz-Calvo, S.; Morcillo-Parra, M.A.; Bisquert, R.; Troncoso, A.M.; García-Parrilla, M.C.; Torija, M.J.; Guillamón, J.M. Intracellular biosynthesis of melatonin and other indolic compounds in Saccharomyces and non-Saccharomyces wine yeasts. Eur. Food Res. Technol. 2019, 245, 1553–1560. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Cruz, E.; Carrasco-Galán, F.; Cerezo-López, A.B.; Valero, E.; Morcillo-Parra, M.Á.; Beltran, G.; Torija, M.-J.; Troncoso, A.M.; García-Parrilla, M.C. Occurrence of melatonin and indolic compounds derived from l-tryptophan yeast metabolism in fermented wort and commercial beers. Food Chem. 2020, 331, 127192. [Google Scholar] [CrossRef] [PubMed]
- Strakhovskaya, M.G.; Ivanova, E.V.; Fraikin, G.Y. Stimulatory influence of 5-HT onto Candida guilliermondii yeast and Streptococcus faecalis bacteria growth. Mikrobiologiya 1993, 62, 46–49. [Google Scholar]
- Romanova, D.Y.; Heyland, A.; Sohn, D.; Kohn, A.B.; Fasshauer, D.; Varoqueaux, F.; Moroz, L.L. Glycine as a signaling molecule and chemoattractant in Trichoplax (Placozoa): Insights into the early evolution of neurotransmitters. Neuroreport 2020, 31, 490–497. [Google Scholar] [CrossRef]
- Srivastava, M.; Begovic, E.; Chapman, J.; Putnam, N.H.; Hellsten, U.; Kawashima, T.; Kuo, A.; Mitros, T.; Salamov, A.; Carpenter, M.L.; et al. The Trichoplax genome and the nature of placozoans. Nature 2008, 454, 955–960. [Google Scholar] [CrossRef] [Green Version]
- Varoqueaux, F.; Fasshauer, D. Getting Nervous: An Evolutionary Overhaul for Communication. Annu. Rev. Genet. 2017, 51, 455–476. [Google Scholar] [CrossRef]
- Romanova, D.Y.; Smirnov, I.V.; Nikitin, M.A.; Kohn, A.B.; Borman, A.I.; Malyshev, A.Y.; Balaban, P.M.; Moroz, L.L. Sodium action potentials in placozoa: Insights into behavioral integration and evolution of nerveless animals. Biochem. Biophys. Res. Commun. 2020, 532, 120–126. [Google Scholar] [CrossRef]
- Senatore, A.; Raiss, H.; Le, P. Physiology and Evolution of Voltage-Gated Calcium Channels in Early Diverging Animal Phyla: Cnidaria, Placozoa, Porifera and Ctenophora. Front. Physiol. 2016, 7, 481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senatore, A.; Reese, T.S.; Smith, C.L. Neuropeptidergic integration of behavior in Trichoplax adhaerens, an animal without synapses. J. Exp. Biol. 2017, 220, 3381–3390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moroz, L.L.; Nikitin, M.A.; Poličar, P.G.; Kohn, A.B.; Romanova, D.Y. Evolution of glutamatergic signaling and synapses. Neuropharmacology 2021, 199, 108740. [Google Scholar] [CrossRef] [PubMed]
- Musser, J.M.; Schippers, K.J.; Nickel, M.; Mizzon, G.; Kohn, A.B.; Pape, C.; Ronchi, P.; Papadopoulos, N.; Tarashansky, A.J.; Hammel, J.U.; et al. Profiling cellular diversity in sponges informs animal cell type and nervous system evolution. Science 2021, 374, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Lentz, T.L. Histochemical localisation of neurohumors in a sponge. J. Exp. Zool. 1966, 162, 171–180. [Google Scholar] [CrossRef]
- Weyrer, S.; Rutzler, K.; Rieger, R. 5-HT in Porifera? Evidence from developing Tedania ignis, the Caribbean fire sponge (Demospongiae). Mem. Queensl. Mus. 1999, 44, 659–665. [Google Scholar]
- Sokolova, A.M.; Voronezhskaya, E.E. Morphological background for non-canonical action of monoamines in Porifera. In Proceedings of the Society for Integrative and Comparative Biology (SICB) Virtual Annual Meeting, Online, 3 January–28 February 2021. [Google Scholar]
- Ellwanger, K.; Nickel, M. Neuroactive substances specifically modulate rhythmic body contractions in the nerveless metazoon Tethya wilhelma (Demospongiae, Porifera). Front. Zool. 2006, 3, 7. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, M.; Simakov, O.; Chapman, J.; Fahey, B.; Gauthier, M.E.A.; Mitros, T.; Richards, G.S.; Conaco, C.; Dacre, M.; Hellsten, U.; et al. The Amphimedon queenslandica genome and the evolution of animal complexity. Nature 2010, 466, 720–726. [Google Scholar] [CrossRef]
- Small, D.H.; Wurtman, R.J. Association of 5-HT, dopamine, or norepinephrinee with an actin-like component in pheochromocytoma (PC12) cells. J. Neurochem. 1985, 45, 825–831. [Google Scholar] [CrossRef]
- Small, D.H.; Wurtman, R.J. Binding of 3H-5-HT to skeletal muscle actin. J. Neurochem. 1985, 45, 819–824. [Google Scholar] [CrossRef]
- Walther, D.J.; Peter, J.U.; Winter, S.; Holtje, M.; Paulmann, N.; Grohmann, M.; Vowinckel, J.; Alamo-Bethencourt, V.; Wilhelm, C.S.; Ahnert-Hilger, G.; et al. Serotonylation of small GTPases is a signal transduction pathway that triggers platelet alpha-granule release. Cell 2003, 115, 851–862. [Google Scholar] [CrossRef] [Green Version]
- Buznikov, G.A. Low-molecular Weight Regulators of Embryonic Development; Nauka Publishers: Moscow, Russia, 1967; 265p. (in Russian) [Google Scholar]
- Brown, K.M.; Anitole, K.G. 5-HT in early embryogenesis. Trends Comp. Biochem. Physiol. 1993, 1, 281–288. [Google Scholar]
- Buznikov, G.A. Neurotransmitters in Embryogenesis; Academic Press: Chur, Switzerland, 1990; 526p. [Google Scholar]
- Turlejski, K. Evolutionary anncient roles of 5-HT: Long-lasting regulation of activity and development. Acta Neurobiol. Exp. 1996, 56, 619–636. [Google Scholar]
- Candiani, S.; Augello, A.; Oliveri, D.; Passalacqua, M.; Pennati, R.; De Bernardi, F.; Pestarino, M. Immunocytochemical localization of 5-HT in embryos, larvae and adults of the lancelet, Branchiostoma floridae. Histochem. J. 2001, 33, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Il’kova, G.; Rehak, P.; Vesela, J.; Čikoš, Š.; Fabian, D.; Czikkova, S.; Koppel, J. 5-HT localization and its functional significance during mouse preimplantation embryo development. Zygote 2004, 12, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Amireault, P.; Dubé, F. 5-HT and its antidepressantsensitive transport in mouse cumulus–oocyte complexes and early embryos. Biol. Reprod. 2005, 73, 358–365. [Google Scholar] [CrossRef]
- Dubé, F.; Amireault, P. Local serotonergic signaling in mammalian follicles, oocytes and early embryos. Life Sci. 2007, 81, 1627–1637. [Google Scholar] [CrossRef]
- Mayorova, T.D.; Kosevich, I.A.; Melekhova, O.P. On Some Features of Embryonic Development and Metamorphosis of Aurelia aurita (Cnidaria, Scyphozoa). Russ. J. Developm. Biol. 2012, 43, 271–285. [Google Scholar] [CrossRef]
- Kandel, E.R. Cellular insights into behavior and learning. In The Harvey Lectures; Ser. 73; Academic Press: New York, NY, USA, 1979; pp. 19–92. [Google Scholar]
- Kusano, K.; Miledi, R.; Stinnakre, J. Acetylcholine receptors in the oocyte membrane. Nature 1977, 270, 739–741. [Google Scholar] [CrossRef]
- Kusano, K. Comparative electrophysiological study on the oocyte membrane ‘neuro-receptors’. Biol. Bull. 1978, 155, 450. [Google Scholar]
- Eusebi, F.; Mangia, F.; Alfei, L. Acetylcholine-elicited responses in primary and secondary mammalian oocytes disappear after fertilisation. Nature 1979, 277, 651–653. [Google Scholar] [CrossRef]
- Piomboni, P.; Baccetti, B.; Moretti, E.; Gambera, L.; Angelini, C.; Falugi, C. Localization of molecules related to cholinergic signaling in eggs and zygotes of the sea urchin, Paracentrotus lividus. J. Submicrosc. Cytol. Pathol. 2001, 33, 187–193. [Google Scholar]
- Falugi, C.; Borgiani, L.; Faraldi, G.; Tafliafierro, G.; Toso, F.; Drews, U. Localization and possible functions of neurotransmitter systems in sperms of different animal species. In Comparative Spermatology, 20 Years After; Baccetti, B., Ed.; Raven Press: New York, NY, USA, 1991; Volume 75, pp. 475–478. [Google Scholar]
- Falugi, C. Localization and possible role of molecules associated with the cholinergic system during “non-nervous” developmental events. Eur. J. Histochem. 1993, 37, 287–294. [Google Scholar] [PubMed]
- Angelini, C.; Baccetti, B.; Piomboni, P.; Trombino, S.; Aluigi, M.G.; Stringara, S.; Gallus, L.; Falugi, C. Acetylcholine synthesis and possible functions during sea urchin development. Eur. J. Histochem. 2004, 48, 235–243. [Google Scholar] [PubMed]
- Colas, J.F.; Launay, J.M.; Kellermann, O.; Rosay, P.; Maroteaux, L. Drosophila 5-HT2 5-HT receptor: Coexpression with fushi-tarazu during segmentation. Proc. Natl. Acad. Sci. USA 1995, 92, 5441–5445. [Google Scholar] [CrossRef] [Green Version]
- Sugamori, K.S.; Demchyshyn, L.L.; McConkey, F.; Forte, M.A.; Niznik, H.B. A primordial dopamine D1-like adenylyl cyclase-linked receptor from Drosophila melanogaster displaying poor affinity for benzazepines. FEBS Lett. 1995, 362, 131–138. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.C.; Lee, H.G.; Seong, C.S.; Han, K.A. Expression of a D1 dopamine receptor dDA1/DmDOP1 in the central nervous system of Drosophila melanogaster. Gene Expr. Patterns 2003, 3, 237–245. [Google Scholar] [CrossRef]
- Mustard, J.A.; Beggs, K.T.; Mercer, A.R. Molecular biology of the invertebrate dopamine receptors. Arch. Insect. Biochem Physiol. 2005, 59, 103–117. [Google Scholar] [CrossRef]
- Cazzamali, G.; Klaerke, D.A.; Grimmelikhuijzen, C.J.P. A new family of insect tyramine receptors. Biochem. Biophys. Res. Commun. 2005, 338, 1189–1196. [Google Scholar] [CrossRef]
- Wu, S.F.; Yao, Y.; Huang, J.; Ye, G.Y. Characterization of a β-adrenergic-like octopamine receptor from the rice stem borer (Chilo suppressalis). J. Exp. Biol. 2012, 215, 2646–2652. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.F.; Jv, X.M.; Li, J.; Xu, G.J.; Cai, X.Y.; Gao, C.F. Pharmacological characterisation and functional roles for egg-laying of a β-adrenergic-like octopamine receptor in the brown planthopper Nilaparvata lugens. Insect Biochem. Mol. Biol. 2017, 87, 55–64. [Google Scholar] [CrossRef]
- Huang, Q.T.; Ma, H.H.; Deng, X.L.; Zhu, H.; Liu, J.; Zhou, Y.; Zhou, X.M. Pharmacological characterization of a beta-adrenergic-like octopamine receptor in Plutella xylostella. Arch. Insect Biochem. Physiol. 2018, 98, e21466. [Google Scholar] [CrossRef]
- Yang, S.; Xi, G.; Wang, G. Molecular Cloning and Expression Analysis of 5-hydroxytryptamine Receptor 7 in Ant Polyrhachis vicina Roger (Hymenoptera: Formicidae). J. Insect Sci. 2019, 19, 2. [Google Scholar] [CrossRef]
- Qi, Y.-X.; Wang, J.-L.; Xu, G.; Song, Q.-S.; Stanley, D.; Fang, Q.; Ye, G.-Y. Biogenic amine biosynthetic and transduction genes in the endoparasitoid wasp Pteromalus puparum (Hymenoptera: Pteromalidae). Arch. Insect Biochem. Physiol. 2020, 103, e21632. [Google Scholar] [CrossRef]
- Buznikov, G.A.; Bezuglov, V.V. Polyenic acid 5-hydroxytryptamides and 3-hydroxytyramides as tools for studying of the pre-nervous biogenic monoamine functions. Ross. Fiziol. Zh. Im. IM Sechenova 2000, 86, 1093–1098. (in Russian). [Google Scholar] [PubMed]
- Boyd, C.A.R. Chemical neurotransmission: A hypothesis concerning the evolution of neurotransmitters substances. J. Theoret. Biol. 1979, 76, 413–417. [Google Scholar] [CrossRef]
- Buznikov, G.A.; Nikitina, L.A.; Voronezhskaya, E.E.; Bezuglov, V.V.; Willows, A.O.D.; Nezlin, L.P. Localization of 5-HT and its possible role in early embryos of Tritonia diomedea (Mollusca: Nudibranchia). Cell Tissue Res. 2003, 311, 259–266. [Google Scholar] [CrossRef]
- Emanuelsson, H. Localization of 5-HT in cleavage embryos of Ophryotrocha labronica La Greca and Bacci. Wilh. Roux’ Arch. 1974, 175, 253–271. [Google Scholar] [CrossRef] [PubMed]
- Manukhin, B.N.; Buznikov, G.A. 5-HT in the embryogenesis of marine invertebrates. Zh. Obshch. Biol. 1963, 24, 23–29. [Google Scholar]
- Renaud, F.; Parisi, E.; Capasso, A.; De Prisco, E.P. On the role of 5-HT and 5-methoxytryptamine in the regulation of cell division in sea urchin eggs. Dev. Biol. 1983, 98, 37–47. [Google Scholar] [CrossRef]
- Capasso, A.; Cretì, P.; De Petrocellis, B.; De Prisco, P.P.; Parisi, E. Role of dopamine and indolamine derivatives in the regulation of the sea urchin adenylate cyclase. Biochem. Biophys. Res. Commun. 1988, 154, 758–764. [Google Scholar] [CrossRef]
- Nikishin, D.A.; Milošević, I.; Gojković, M.; Rakić, Lj.; Bezuglov, V.V.; Shmukler, Y.B. Expression and functional activity of neurotransmitter system components in sea urchins’ early development. Zygote 2016, 24, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Nikishin, D.A.; Malchenko, L.A.; Milošević, I.; Rakić, L.; Shmukler, Y.B. Effects of Haloperidol and Cyproheptadine on the Cytoskeleton of the Sea Urchin Embryos. Biochem. (Mosc.) Suppl. Ser. A Membr. Cell Biol. 2020, 14, 249–254. [Google Scholar] [CrossRef]
- Buznikov, G.A.; Shmukler, Y.B.; Lauder, J.M. From oocyte to neuron: Do neurotransmitters function in the same way throughout development? Cell. Molec. Neurobiol. 1996, 16, 532–559. [Google Scholar] [CrossRef]
- Hamdan, F.F.; Ungrin, M.D.; Abramovitz, M.; Ribeiro, P. Characterization of a Novel 5-HT Receptor from Caenorhabditis elegans: Cloning and Expression of Two Splice Variants. J. Neurochem. 1999, 72, 1372–1383. [Google Scholar] [CrossRef]
- Nikishin, D.A.; Semenova, M.N.; Shmukler, Y.B. Expression of transmitter receptor genes in early development of sea urchin Paracentrotus lividus. Russ. J. Dev.Biol. 2012, 43, 3–181. [Google Scholar] [CrossRef]
- Shi, L.; Javitch, J.A. The binding site of aminergic G protein-coupled receptors: The transmembrane segments and second extracellular loop. Annu. Rev. Pharmacol. Toxicol. 2002, 42, 437–467. [Google Scholar] [CrossRef]
- Coffman, J.A.; Robertson, A.J.; Clifton, S.; Pape, D.; Hillier, L.; Martin, J.; Wylie, T.; Dante, M.; Meyer, R.; Theising, B.; et al. WashU Sea Urchin EST Project. GenBank: CX685095.1. 2010. Available online: http://www.seaurchinproject.org/ (accessed on 13 January 2022).
- Katow, H.; Yaguchi, S.; Kiyomoto, M.; Washio, M. 5-HT receptor cell is a new member of secondary mesenchyme descendants and forms major blastocoelar network in sea urchin larvae. Mech. Dev. 2004, 121, 325–337. [Google Scholar] [CrossRef]
- Katow, H.; Suyemitsu, T.; Ooka, S.; Yaguchi, J.; Jin-Nai, T.; Kuwahara, I.; Katow, T.; Yaguchi, S.; Abe, H. Development of a dopaminergic system in sea urchin embryos and larvae. J. Exp. Biol. 2010, 213, 2808–2819. [Google Scholar] [CrossRef] [Green Version]
- Katow, H.; Abe, K.; Katow, T.; Zamani, A.; Abe, H. Development of the GABA-ergic signaling system and its role in larval swimming in the sea urchin. J. Exp. Biol. 2013, 216, 1704–1716. [Google Scholar]
- Falugi, C.; Prestipino, G. Localization of putative nicotinic cholinoreceptors in the early development of Paracentrotus lividus. Cell. Mol. Biol. 1989, 35, 147–161. [Google Scholar] [PubMed]
- Baccetti, B.; Burrini, A.G.; Collodel, G.; Falugi, C.; Morett, E.; Piomboni, P. Localisation of two classes of acetylcholine receptor-like molecules. in sperms of different animal species. Zygote 1995, 3, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Angelini, C.; Aluigi, M.G.; Sgro, M.; Trombino, S.; Thielecke, H.; Falugi, C. Cell signalling during sea urchin development: A model for assessing toxicity of environmental contaminants. Prog. Mol. Subcell. Biol. 2005, 39, 45–70. [Google Scholar] [CrossRef] [PubMed]
- Aluigi, M.G.; Diaspro, A.; Ramoino, P.; Russo, P.; Falugi, C. The sea urchin, Paracentrotus lividus, as a model to investigate the onset of molecules immunologically related to the α-7 subunit of nicotinic receptors during embryonic and larval development. Curr. Drug Targets 2012, 13, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Conner, S.; Leaf, D.; Wessel, G. Members of the SNARE hypothesis are associated with cortical granule exocytosis in the sea urchin egg. Mol. Reprod. Dev. 1997, 48, 106–118. [Google Scholar] [CrossRef]
- Conner, S.; Wessel, G.M. Rab3 mediates cortical granule exocytosis in the sea urchin egg. Dev. Biol. 1998, 203, 334–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conner, S.D.; Wessel, G.M. Syntaxin is required for cell division. Mol. Biol. Cell 1999, 10, 2735–2743. [Google Scholar] [CrossRef] [Green Version]
- Conner, S.D.; Wessel, G.M. A rab3 homolog in sea urchin functions in cell division. FASEB J. 2000, 14, 1559–1566. [Google Scholar] [CrossRef]
- Conner, S.D.; Wessel, G.M. Syntaxin, VAMP, and Rab3 are SelectivelyExpressed During Sea Urchin Embryogenesis. Mol. Reprod. Dev. 2001, 58, 22–29. [Google Scholar] [CrossRef]
- Ciccone, M.A.; Timmons, M.; Phillips, A.; Quick, M.W. Calcium/calmodulin-dependent kinase II regulates the interaction between the 5-HT transporter and syntaxin 1A. Neuropharmacology 2008, 55, 763–770. [Google Scholar] [CrossRef] [Green Version]
- Leguia, M.; Wessel, G.M. Selective expression of a sec1/munc18 member in sea urchin eggs and embryos. Gene Expr. Patterns 2004, 4, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Falugi, C.; Pieroni, M.; Moretti, E. Cholinergic molecules and sperm functions. J. Submicrosc. Anat. Pathol. 1993, 25, 63–69. [Google Scholar]
- Limatola, N.; Vasilev, F.; Santella, L.; Chun, J.T. Nicotine Induces Polyspermy in Sea Urchin Eggs through a Non-Cholinergic Pathway Modulating Actin Dynamics. Cells 2019, 9, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrison, P.K.; Falugi, C.; Angelini, C.; Whitaker, M.J. Muscarinic signalling affects intracellular calcium concentration during the first cell cycle of sea urchin embryos. Cell Calcium 2002, 31, 289–297. [Google Scholar] [CrossRef]
- Leguia, M.; Wessel, G.M. The histamine H1 receptor activates the nitric oxide pathway at fertilization. Mol. Reprod. Dev. 2006, 73, 1550–1563. [Google Scholar] [CrossRef]
- Shmukler, Y.B.; Nikishin, D.A. On the Intracellular Transmitter Reception. Neurochem. J. 2018, 12, 295–298. [Google Scholar] [CrossRef]
- Buznikov, G.A.; Lambert, H.W.; Lauder, J.M. 5-HT and 5-HT-like substances as regulators of early embryogenesis and morphogenesis. Cell Tissue Res. 2001, 305, 177–186. [Google Scholar] [CrossRef]
- Buznikov, G.A.; Peterson, R.E.; Nikitina, L.A.; Bezuglov, V.V.; Lauder, J.M. The pre-nervous serotonergic system of developing sea urchin embryos and larvae: Pharmacologic and immunocytochemical evidence. Neurochem. Res. 2005, 30, 825–837. [Google Scholar] [CrossRef]
- Falugi, C.; Moretti, E.; Lammerding-Köppel, M.; Drews, U. A cholinergic system is involved in gamete interaction. In Cellular Communication in Reproduction; Facchinetti, F., Henderson, H.W., Pierantoni, R., Polzonetti Magni, A., Eds.; Society for Endocrinology: Bristol, UK, 1993; pp. 101–104. [Google Scholar]
- Grigor’ev, N.G. The cortical layer of the cytoplasm—A possible site of the action of prenervous transmitters. Zh. Evol. Biokhim. Fiziol. 1988, 24, 5–625. (in Russian). [Google Scholar]
- Cornea-Hebert, V.; Watkins, K.C.; Roth, B.L.; Kroeze, W.K.; Gaudreau, P.; Leclerc, N.; Descarries, L. Similar ultrastructural distribution of the 5-HT(2A) 5-HT receptor and microtubule-associated protein MAP1A in cortical dendrites of adult rat. Neuroscience 2002, 113, 23–35. [Google Scholar] [CrossRef]
- Shmukler, Y.B.; Buznikov, G.A.; Whitaker, M.J. Action of 5-HT antagonists on cytoplasmic calcium level in early embryos of sea urchin Lytechinus pictus. Int. J. Dev. Biol. 1999, 42, 3–179. [Google Scholar]
- Capasso, A.; Parisi, E.; De Prisco, P.; De Petrocellis, B. Catecholamine secretion and adenylate cyclase activation in sea urchin eggs. Cell Biol. Int. Rep. 1987, 11, 457–463. [Google Scholar] [CrossRef]
- Carginale, V.; Capasso, A.; Madonna, L.; Borrelli, L.; Parisi, E. Adenylate cyclase from sea urchin eggs is positively and negatively regulated by D-1 and D-2 dopamine receptors. Exp. Cell Res. 1992, 203, 491–494. [Google Scholar] [CrossRef]
- Buznikov, G.A.; Kabankin, A.C.; Kolbanov, V.M.; Landau, M.A.; Aroyan, A.A.; Ovsepyan, T.R.; Teplitz, N.A. On the correlation between alkoxibenzylalkylamines’ embryotoxic activity and lipophility. Khim.-Farm. Zh. 1976, 10, 23–27. (in Russian). [Google Scholar]
- Landau, M.A.; Buznikov, G.A.; Kabankin, A.S.; Kolbanov, V.M.; Suvorov, N.N.; Teplitz, N.A. Embryotoxic activity of indole derivatives. Khim.-Farm. Zh. 1977, 11, 57–60. (in Russian). [Google Scholar] [CrossRef]
- Buznikov, G.A.; Shmukler, I.B. Effect of antimediator preparations on the intercellular relations in early embryos of the sea urchin. Ontogenez 1978, 9, 173–178. (in Russian). [Google Scholar]
- Shmukler, Y.B.; Grigoriev, N.G.; Buznikov, G.A.; Turpaev, T.M. Regulation of cleavage divisions: Participation of “prenervous” neurotransmitters coupled with second messengers. Comp. Biochem. Physiol. 1986, 83C, 423–427. [Google Scholar]
- Basu, B.; Desai, R.; Balaji, J.; Chaerkady, R.; Sriram, V.; Maiti, S.; Panicker, M.M. 5-HT in pre-implantation mouse embryos is localized to the mitochondria and can modulate mitochondrial potential. Reproduction 2008, 135, 5657–5669. [Google Scholar] [CrossRef] [Green Version]
- Rostomyan, M.A.; Abramyan, K.S.; Buznikov, G.A.; Gusareva, E.V. Ultracytochemical detection of adenylate cyclase in early sea urchin embryos. Tsitologiya 1985, 27, 877–881. (in Russian). [Google Scholar]
- Yamamoto, S.; Kawamura, K.; James, T.N. Intracellular distribution of adenylate cyclase in human cardiocytes determined by electron microscopic cytochemistry. Microsc. Res. Tech. 1998, 40, 479–487. [Google Scholar] [CrossRef]
- Brandes, L.J.; Davie, J.P.; Paraskevas, F.; Sukhu, F.; Bogdanovic, R.P.; LaBella, F.S. The antiproliferative potency of histamine antagonists correlates with inhibition of binding of [H3]-histamine to novel intracellular receptors (HIC) in microsomal and nuclear fractions of rat liver. Agents Actions 1991, 33, 325–342. [Google Scholar] [PubMed]
- Brandes, L.J.; LaBella, F.S.; Glavin, G.B.; Paraskevas, F.; Saxena, S.P.; Nicol, A.; Gerrard, J.M. Histamine as an intracellular messenger. Biochem. Pharmacol. 1990, 40, 1677–1681. [Google Scholar] [CrossRef]
- Muneoka, K.T.; Takigawa, M. 5-Hydroxytryptamine7 (5-HT7) receptor immunoreactivity-positive ‘stigmoid body’-like structure in developing rat brains. Int. J. Dev. Neurosci. 2003, 21, 133–143. [Google Scholar] [CrossRef]
- Uwada, J.; Yoshiki, H.; Masuoka, T.; Nishio, M.; Muramatsu, I. Intracellular localization of the M1 muscarinic acetylcholine receptor through clathrin-dependent constitutive internalization is mediated by a C-terminal tryptophan-based motif. J. Cell Sci. 2014, 127, 3131–3140. [Google Scholar] [CrossRef] [Green Version]
- Turpaev, T.M.; Yurchenko, O.P.; Grigoriev, N.G. Alteration of the acetylcholine response by intra- and extracellular 5-HT application in intracellularly perfused neurons of Lymnaea stagnalis. Cell. Mol. Neurobiol. 1987, 7, 381–390. [Google Scholar] [CrossRef]
- Yurchenko, O.P.; Grigoriev, N.G.; Turpaev, T.M.; Konjević, D.; Rakić, L. Intracellular injection of dopamine enhances acetylcholine responses of neuron R2 in the Aplysia abdominal ganglion. Comp. Biochem. Physiol. 1987, 87, 389–391. [Google Scholar] [CrossRef]
- Yurchenko, O.P.; S-Rózsa, K. Modulatory effect of 5-HT on the acetylcholine sensitivity of identified neurons in the brain of Helix pomatia L. Comp. Biochem. Physiol. Comp. Pharmacol. Toxicol. 1984, 77, 127–133. [Google Scholar] [CrossRef]
- Prou, D.; Gu, W.J.; Crom, L.S.; Vincent, J.D.; Salamero, J.; Vernier, P. Intracellular retention of the two isoforms of the D(2) dopamine receptor promotes endoplasmic reticulum disruption. J. Cell Sci. 2001, 114, 3517–3527. [Google Scholar] [CrossRef]
- Takizawa, P.A.; Yucel, J.K.; Veit, B.; Faulkner, D.J.; Deerinck, T.; Soto, G.; Ellisman, M.; Malhotra, V. Complete vesiculation of Golgi membranes and inhibition of protein transport by a novel sea sponge metabolite, ilimaquinone. Cell 1993, 73, 1079–1090. [Google Scholar] [CrossRef]
- Shmukler, Y.B. On the possibility of membrane reception of neurotransmitter in sea urchin early embryos. Comp. Biochem. Physiol. 1993, 106, 269–273. [Google Scholar] [CrossRef]
- Shmukler, Y.B. Cellular interactions in early sea urchin embryos. III. Influences of neuropharmaca on the pattern of cleavage of Scaphechinus mirabilis half-embryos. Ontogenez 1981, 12, 4–404. (in Russian). [Google Scholar]
- Buznikov, G.A.; Shmukler, Y.B. The possible role of “prenervous” neurotransmitters in cellular interactions of early embryogenesis: A hypothesis. Neurochem. Res. 1981, 6, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Shmukler, Y.B.; Silvestre, F.; Tosti, E. 5-НТ-receptive structures are localized in the interblastomere cleft of Paracеntrotus lividus early embryos. Zygote 2008, 16, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Shmukler, Y.B. A “micromere model” of cell-cell interactions in sea urchin early embryos. Biophysics 2010, 55, 399–405. [Google Scholar] [CrossRef]
- Shmukler, Y.B.; Tosti, E. Serotonergic-induced ion currents in cleaving sea urchin embryo. Invertebr. Reprod. Dev. 2002, 42, 43–49. [Google Scholar] [CrossRef]
- Vacquier, V.D.; Mazia, D. Twinning of sand dollar embryos by means of dithiothreitol. The structural basis of blastomere interactions. Exp. Cell Res. 1968, 52, 209–219. [Google Scholar] [CrossRef]
- Katow, H.; Katow, T.; Yoshida, H.; Kiyomoto, M.; Uemura, I. Immunohistochemical and ultrastructural properties of the larval ciliary band associated strand in the sea urchin Hemicentrotus pulcherrimus. Front. Zool. 2016, 13, 27. [Google Scholar] [CrossRef] [Green Version]
- Gustafson, T.; Toneby, M. On the role of 5-HT and acetylcholine in sea urchin morphogenesis. Exp. Cell Res. 1970, 62, 102–117. [Google Scholar] [CrossRef]
- Martynova, L.E.; Beloussov, L.V. Effect of neuropharmacologic preparations and colchicine on morphogenetic processes in embryonal amphibian cells. Ontogenez 1978, 9, 382–389. (in Russian). [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shmukler, Y.B.; Nikishin, D.A. Non-Neuronal Transmitter Systems in Bacteria, Non-Nervous Eukaryotes, and Invertebrate Embryos. Biomolecules 2022, 12, 271. https://doi.org/10.3390/biom12020271
Shmukler YB, Nikishin DA. Non-Neuronal Transmitter Systems in Bacteria, Non-Nervous Eukaryotes, and Invertebrate Embryos. Biomolecules. 2022; 12(2):271. https://doi.org/10.3390/biom12020271
Chicago/Turabian StyleShmukler, Yuri B., and Denis A. Nikishin. 2022. "Non-Neuronal Transmitter Systems in Bacteria, Non-Nervous Eukaryotes, and Invertebrate Embryos" Biomolecules 12, no. 2: 271. https://doi.org/10.3390/biom12020271
APA StyleShmukler, Y. B., & Nikishin, D. A. (2022). Non-Neuronal Transmitter Systems in Bacteria, Non-Nervous Eukaryotes, and Invertebrate Embryos. Biomolecules, 12(2), 271. https://doi.org/10.3390/biom12020271