Milk-Derived Proteins and Peptides in Head and Neck Carcinoma Treatment
Abstract
:1. Introduction
- 1.
- How effective is the use of milk-derived proteins as an anticancer treatment for HNSCC?
- 2.
- What are the anticancer mechanisms of action behind these milk-derived proteins?
- 3.
- How are the milk-derived proteins extracted, obtained, and synthesised?
- 4.
- What is the specificity of milk-derived proteins in targeting HNSCC vs. normal host cells?
- 5.
- Are the effects of milk-derived proteins cell line, dose, and time dependent?
2. Materials and Methods
2.1. Study Selection
2.1.1. Inclusion Criteria
- P = Patients/population/case: HNSCC.
- I = Intervention: Milk-derived proteins as the sole treatment or cotreatment.
- C = Control: Specified control in each study.
- O = Outcome: Prognosis of head and neck carcinoma.
- S = Study design: All original studies including in vitro and/or in vivo and/or in silico.
2.1.2. Exclusion Criteria
- 1.
- Published only in non-English languages or if the full text was not available.
- 2.
- Irrelevant to the association of milk-derived proteins and HNSCC.
- 3.
- Involving cancers that occur in head and neck regions but are not usually classified as head and neck cancers, including cancer of the scalp, skin, muscles, bones, eye, brain, ear, oesophageal, thyroid, and parathyroid.
- 4.
- Focusing on prevention of cancer rather than on the treatment of cancer.
- 5.
- Exploring the role of milk-derived proteins as a drug delivery vehicle.
2.1.3. Screening Process
- Step 1.
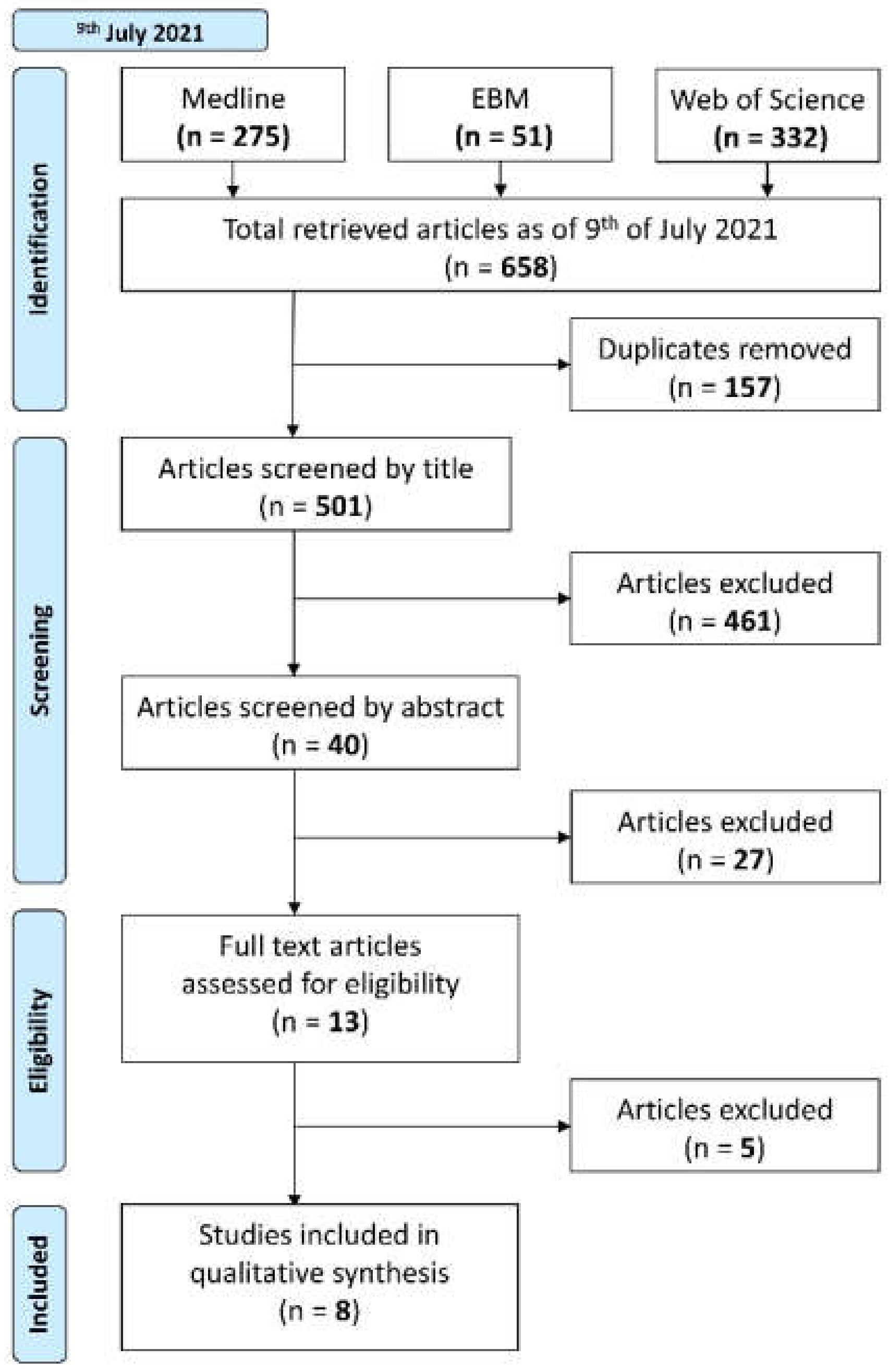
- Electronic literature searches using the workgroup-defined search strategies were conducted by two blind reviewers (DZ, WX) on 9 July 2021 in Medline (Ovid), Evidence-based Medicine (EBM), and Web of Science databases. The syntaxes for these searches are shown in Appendix B. The results were imported into Endnote X9 (Clarivate Analytics, Philadelphia, PA, USA); automated deduplication was completed by Endnote. The subsequent library was exported to Covidence (Veritas Health Innovation, Melbourne, Australia) for screening, where another automated deduplication was performed.
- Step 2.
- Papers were then screened by title only.
- Step 3.
- Papers were then screened by abstract.
- Step 4.
- Papers were lastly screened by full text.
2.2. Statistical Analysis
2.3. Risk of Bias
- Was the administered dose or exposure level adequately randomized?
- Was the allocation to study groups adequately concealed?
- Were the experimental conditions identical across study groups?
- Were the research personnel and human subjects blinded to the study group in the study?
- Was the outcome data complete without attrition or exclusion from the analysis?
- Can we be confident in the exposure characterization?
- Can we be confident in the outcome assessment?
- Were all the measured outcomes reported?
- Statistics: Were the statistical methods appropriate?
- Unintended coexposures for experimental studies: Did the study design or analysis account for important confounding and modifying variables (including unintended coexposures) in experimental studies?
3. Results
3.1. Study Selection
Study Characteristics
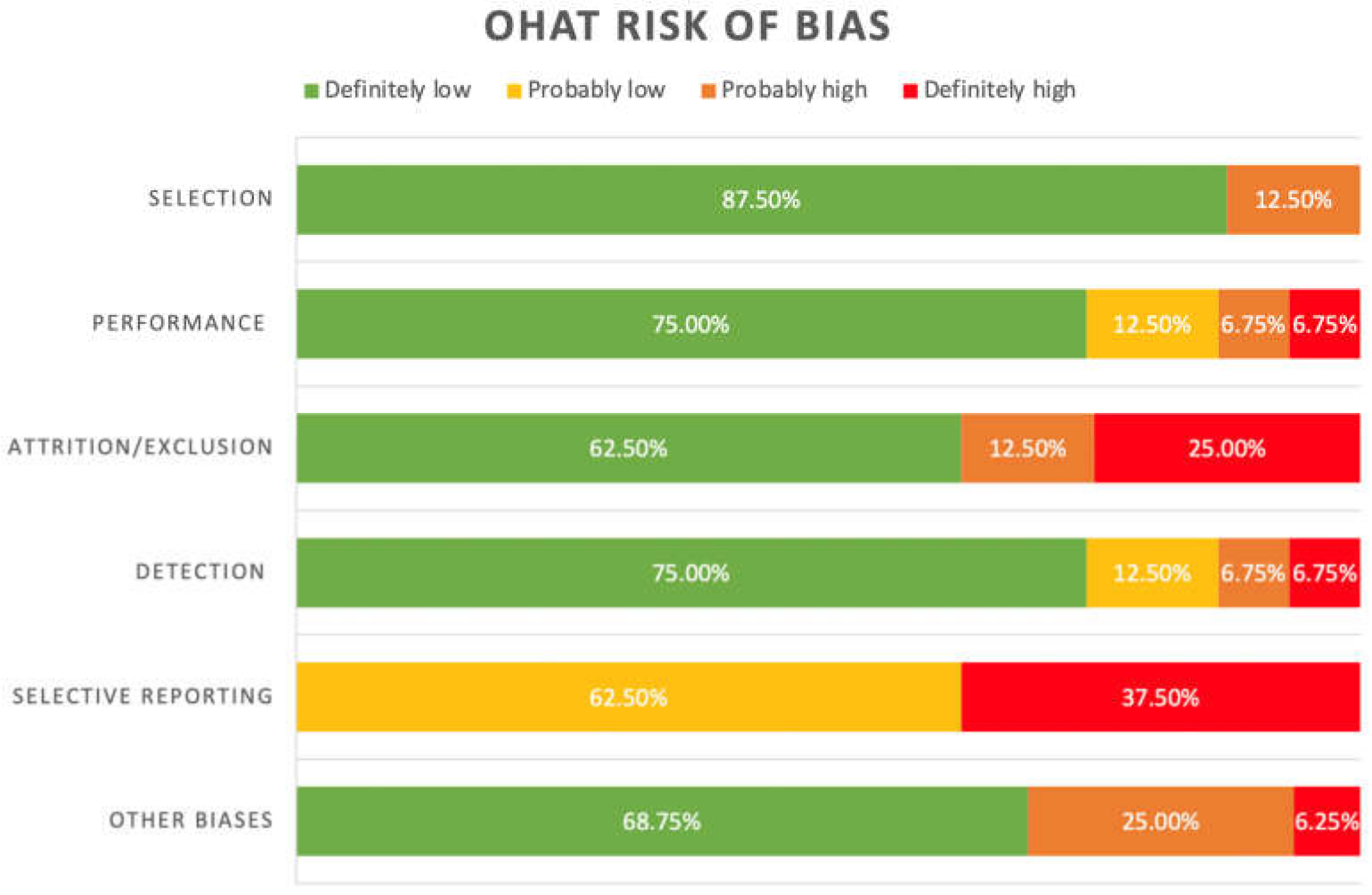
3.2. Risk of Bias
3.3. Specimen Types
3.4. Source of Milk-Derived Proteins and Peptides
3.5. Milk-Derived Proteins and Peptides Examined
3.5.1. Whey and Casein
3.5.2. Lactoferrin (LF)
3.5.3. Pepsin-Digested Lactoferrin (pLF)
3.5.4. Lactoperoxidase (LP)
3.5.5. Alpha-Lactalbumin (α-LA)
3.5.6. BAMLET/HAMLET
3.5.7. Alpha Lactalbumin-Oleic Acid (LA-OA) Complexes
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Potential Biases | First Author Surname, Year | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sinevicci, 2020 | Wolf, 2003 | Panihipour, 2020 | Mohan, 2007 | Sakai, 2005 | Permyakov, 2011 | Panahipour, 2021 | Knyazeva, 2008 | ||
| Selection | Was administered dose or exposure level adequately randomised? | ↓↓ | ↑ | ↓↓ | ↓↓ | ↓↓ | ↓↓ | ↓↓ | ↓↓ |
| Was allocation to study groups adequately concealed? | ↓↓ | ↑ | ↓↓ | ↓↓ | ↓↓ | ↓↓ | ↓↓ | ↓↓ | |
| Performance | Were experimental conditions identical across study groups? | ↓↓ | ↑↑ | ↓↓ | ↓↓ | ↓↓ | ↓ | ↓↓ | ↓ |
| Were the study personnel and human subjects blinded to the study groups during the study? | ↓↓ | ↑ | ↓↓ | ↓↓ | ↓↓ | ↓↓ | ↓↓ | ↓↓ | |
| Attrition/ Exclusion | Were outcome data complete without attrition or exclusion from the analysis? | ↓↓ | ↑↑ | ↓↓ | ↓↓ | ↓↓ | ↑↑ | ↓↓ | ↑ |
| Detection | Can we be confident in the exposure characterisation? | ↓↓ | ↑↑ | ↓↓ | ↓↓ | ↓↓ | ↓ | ↓↓ | ↓ |
| Can we be confident in the outcome assessment? | ↓↓ | ↑ | ↓↓ | ↓↓ | ↓↓ | ↓↓ | ↓↓ | ↓↓ | |
| Selective Reporting | Were all measured outcomes reported? | ↓ | ↑↑ | ↓ | ↓ | ↓ | ↑↑ | ↓ | ↑↑ |
| Other Biases | Statistics: were statistical methods appropriate | ↓↓ | ↓↓ | ↓↓ | ↓↓ | ↑ | ↑↑ | ↓↓ | ↓↓ |
| Unintended co-exposures for experimental studies: did the study design or analysis account for important confounding and modifying variables (including unintended co-exposures) in experimental studies? | ↓↓ | ↑ | ↓↓ | ↓↓ | ↑ | ↑ | ↓↓ | ↓↓ | |
Appendix B
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, N.W.; Jayasekara, P.; Amarasinghe, A.A. Squamous cell carcinoma and precursor lesions of the oral cavity: Epidemiology and aetiology. Periodontol. 2000 2011, 57, 19–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cancer Research UK. Head and Neck Cancers Survival Statistics. 2013. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/head-and-neck-cancers/survival (accessed on 22 June 2021).
- Cohen, E.E.W.; LaMonte, S.J.; Erb, N.L.; Beckman, K.L.; Sadeghi, N.; Hutcheson, K.A.; Stubblefield, M.D.; Abbott, D.M.; Fisher, P.S.; Stein, K.D.; et al. American Cancer Society Head and Neck Cancer Survivorship Care Guideline. CA Cancer J. Clin. 2016, 66, 203–239. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, D.P.; Mohapatra, S.; Misra, S.; Sahu, P.S. Milk derived bioactive peptides and their impact on human health—A review. Saudi J. Biol. Sci. 2016, 23, 577–583. [Google Scholar] [CrossRef] [Green Version]
- Mossberg, A.K.; Hou, Y.; Svensson, M.; Holmqvist, B.; Svanborg, C. HAMLET treatment delays bladder cancer development. J. Urol. 2010, 183, 1590–1597. [Google Scholar] [CrossRef]
- Fischer, W.; Gustafsson, L.; Mossberg, A.K.; Gronli, J.; Mork, S.; Bjerkvig, R.; Svanborg, C. Human alpha-lactalbumin made lethal to tumor cells (HAMLET) kills human glioblastoma cells in brain xenografts by an apoptosis-like mechanism and prolongs survival. Cancer Res. 2004, 64, 2105–2112. [Google Scholar] [CrossRef] [Green Version]
- Puthia, M.; Storm, P.; Nadeem, A.; Hsiung, S.; Svanborg, C. Prevention and treatment of colon cancer by peroral administration of HAMLET (human α-lactalbumin made lethal to tumour cells). Gut 2014, 63, 131–142. [Google Scholar] [CrossRef] [Green Version]
- Gustafsson, L.; Leijonhufvud, I.; Aronsson, A.; Mossberg, A.K.; Svanborg, C. Treatment of skin papillomas with topical alpha-lactalbumin-oleic acid. New Engl. J. Med. 2004, 350, 2663–2672. [Google Scholar] [CrossRef] [Green Version]
- Mossberg, A.K.; Wullt, B.; Gustafsson, L.; Månsson, W.; Ljunggren, E.; Svanborg, C. Bladder cancers respond to intravesical instillation of HAMLET (human alpha-lactalbumin made lethal to tumor cells). Int. J. Cancer 2007, 121, 1352–1359. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar]
- Wolf, J.S.; Li, D.; Taylor, R.J.; O’Malley, B.W., Jr. Lactoferrin inhibits growth of malignant tumors of the head and neck. ORL J. Oto-Rhino-Laryngol. Its Relat. Spec. 2003, 65, 245–249. [Google Scholar] [CrossRef]
- Zhao, M.; Sano, D.; Pickering, C.R.; Jasser, S.A.; Henderson, Y.C.; Clayman, G.L.; Sturgis, E.M.; Ow, T.J.; Lotan, R.; Carey, T.; et al. Assembly and initial characterization of a panel of 85 genomically validated cell lines from diverse head and neck tumor sites. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2011, 17, 7248–7264. [Google Scholar] [CrossRef] [Green Version]
- Swiss Institute of Bioinformatics. Expasy. 2022. Available online: https://www.expasy.org/ (accessed on 12 December 2021).
- Página Inicial. APABCAM. 2022. Available online: http://bcrj.org.br/ (accessed on 12 December 2021).
- Kaplanas, R.I.; Antanavichyus, A.I. Isolation and purification of alpha -lactalbumin-A component of lactose synthetase. Biochemistry 1975, 40, 584–587. [Google Scholar]
- Pettersson, J.; Mossberg, A.K.; Svanborg, C. Alpha-Lactalbumin species variation, HAMLET formation, and tumor cell death. Biochem. Biophys. Res. Commun. 2006, 345, 260–270. [Google Scholar] [CrossRef] [Green Version]
- Svensson, M.; Håkansson, A.; Mossberg, A.K.; Linse, S.; Svanborg, C. Conversion of α-lactalbumin to a protein. Proc. Natl. Acad. Sci. USA 2000, 97, 4221–4226. [Google Scholar] [CrossRef] [Green Version]
- Protein Data Bank. RCSB PDB. 2022. Available online: https://www.rcsb.org/ (accessed on 12 December 2021).
- Panahipour, L.; Husejnovic, S.; Nasirzade, J.; Semelmayer, S.; Gruber, R. Micellar Casein and Whey Powder Hold a TGF-β Activity and Regulate ID Genes In Vitro. Molecules 2021, 26, 507. [Google Scholar] [CrossRef]
- Mohan, K.; Gunasekaran, P.; Varalakshmi, E.; Hara, Y.; Nagini, S. In vitro evaluation of the anticancer effect of lactoferrin and tea polyphenol combination on oral carcinoma cells. Cell Biol Int. 2007, 31, 599–608. [Google Scholar] [CrossRef]
- Sakai, T.; Banno, Y.; Kato, Y.; Nozawa, Y.; Kawaguchi, M. Pepsin-digested bovine lactoferrin induces apoptotic cell death with JNK/SAPK activation in oral cancer cells. J. Pharmacol. Sci. 2005, 98, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Panahipour, L.; De Biasi, M.; Bokor, T.; Thajer, A.; Haiden, N.; Guber, R. Milk lactoperoxidase decreases ID1 and ID3 expression in human oral squamous cell carcinoma cell lines. Sci. Rep. 2020, 10, 5836. [Google Scholar] [CrossRef] [Green Version]
- Sinevici, N.; Harte, N.; O’Grady, I.; Xie, Y.; Min, S.; Mok, K.; O’Sullivan, J. The novel therapeutic potential of bovine α-lactalbumin made lethal to tumour cells (BALMET) and oleic acid in oral squamous cell carcinoma (OSCC). Eur. J. Cancer Prev. 2021, 30, 178–187. [Google Scholar] [CrossRef]
- Knyazeva, E.; Grishchenko, V.; Fadeev, R.; Akatov, V.; Permyakov, S.; Permyakov, E. Who is Mr. HAMLET? Interaction of human alpha-lactalbumin with monomeric oleic acid. Biochemistry 2008, 47, 13127–13137. [Google Scholar]
- Permyakov, S.; Knyazeva, E.; Leonteva, M.; Fadeev, R.; Chekanov, A.; Håkansson, A.P.; Akatov, V.S.; Permyakov, E.A. A novel method for preparation of HAMLET-like protein complexes. Biochimie 2011, 93, 1495–1501. [Google Scholar] [CrossRef]
- Håkansson, A.; Zhivotovsky, B.; Orrenius, S.; Sabharwal, H.; Svanborg, C. Apoptosis induced by a human milk protein. Proc. Natl. Acad. Sci. USA 1995, 92, 8064–8068. [Google Scholar] [CrossRef] [Green Version]
- Hallgren, O.; Gustafsson, L.; Irjala, H.; Selivanova, G.; Orrenius, S.; Svanborg, C. HAMLET triggers apoptosis but tumor cell death is independent of caspases, Bcl-2 and p53. Apoptosis 2006, 11, 221–233. [Google Scholar] [CrossRef]
- Ho, J.C.S.; Nadeem, A.; Svanborg, C. HAMLET-A protein-lipid complex with broad tumoricidal activity. Biochem. Biophys. Res. Commun. 2017, 482, 454–458. [Google Scholar] [CrossRef]
- Roschger, C.; Cabrele, C. The Id-protein family in developmental and cancer-associated pathways. Cell Commun. Signal. 2017, 15, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Viqar, S. TGF-β Signaling in Cancer. J. Cell. Biochem. 2016, 117, 1279–1287. [Google Scholar]
- Blank, U.; Karlsson, S. The role of Smad signaling in hematopoiesis and translational hematology. Leukemia 2011, 25, 1379–1388. [Google Scholar] [CrossRef] [Green Version]
- Van Slyke, L.L.; Baker, J.C. Handbook of Food Proteins; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing: Cambridge, UK, 2011. [Google Scholar]
- Neyestani, T.R.; Djalali, M.; Pezeshkic, M. Isolation of α-lactalbumin, β-lactoglobulin, and bovine serum albumin from cow’s milk using gel filtration and anion-exchange chromatography including evaluation of their antigenicity. Protein Expr. Purif. 2003, 29, 202–208. [Google Scholar] [CrossRef]
| Author, Year, Country | Major Proteins and Peptides | Model | Experimental Method | Source of Proteins and Peptides | Major Findings |
|---|---|---|---|---|---|
| Sinevici et al., 2020, Ireland |
|
|
| BAMLET: Ca2+ depleted α-LA added into DEAE column matrix preconditioned with OA then eluted with NaCl buffer, desalted overnight and lyophilized. |
|
| Wolf et al., 2003, USA |
|
|
| NR |
|
|
|
| NR | ||
| Panahipour et al., 2020, Austria |
|
|
|
|
|
| Mohan et al., 2007, India |
|
|
| bLF: Morinaga Milk Industry Co. Ltd., Tokyo Japan. |
|
| Sakai et al., 2005, Japan |
|
|
|
|
|
| Permyakov et al., 2011, Russia |
|
|
|
|
|
| Panahipour et al., 2021, Switzerland |
|
|
|
|
|
| Knyazeva et al., 2008, Russia |
|
|
|
|
|
| Cell Lines | Tissue of Origin | Genetic Characteristics and Mutations [14] | Notable Characteristics [14,15] |
|---|---|---|---|
| DOK | Tongue | TP53 mutation | Mild to moderate dysplastic Stratification in confluent cultures and contain a keratin profile similar to the original dysplasia Nontumourigenic in nu/nu mice |
| TR146 | Buccal mucosa | p53 wild type | Well-differentiated Polygonal Tumourigenic in female (nu/nu) mice Metastatic |
| Ca9.22 | Gingiva | p53 mutation | Tumourigenic Epithelial-like Expressing remarkable EGF receptors |
| O22 | Larynx | TP53 mutation | Metastatic |
| O12 | Larynx | TP53 mutation | Metastatic |
| FaDu | Hypopharynx | CDKN2A, FAT1, or TP53 mutation | Tumourigenic in nu/nu mice Epithelial morphology Contain bundles of tonofilaments in the cell cytoplasm and desmosomal regions were prominent at cell boundaries. |
| HSC2 | Oral | TP53 mutation or PIK3CA mutation | Epithelial-like |
| CAL-27 | Tongue | TRET or TP53 mutation | Epithelial, polygonal with highly granular cytoplasm Tumourigenic in nu/nu mice Aneuploid Tumourigenic; solid tumours developed within 6 weeks in nude mice inoculated with 2 × 106 cells subcutaneously Immunocytochemical studies show strong positive staining with antikeratin antibodies |
| SAS | Tongue | TP53 mutation | Tumourigenic Epithelial-like General cell growth properties |
| HEp-2 | Larynx | Contaminated with HeLa marker chromosome Contains HPV DNA sequence | Epithelial morphology |
| Protein/Peptide | Structure (Obtained from [19]) | Weight (kDa) | Solubility |
|---|---|---|---|
| Lactoferrin | 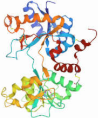 | ~80 kDa | Soluble |
| Lactoperoxidase | 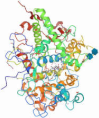 | ~78 kDa | Soluble |
| Alpha-lactalbumin | 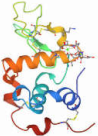 | ~14 kDa | Soluble |
| BAMLET/HAMLET | 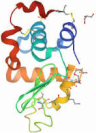 | ~110 kDa | Soluble |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Liu, X.; Ng, Y.Y.; Tarleton, K.; Tran, A.; Tran, T.; Xue, W.Y.; Youssef, P.; Yuan, P.; Zhang, D.; et al. Milk-Derived Proteins and Peptides in Head and Neck Carcinoma Treatment. Biomolecules 2022, 12, 290. https://doi.org/10.3390/biom12020290
Wang T, Liu X, Ng YY, Tarleton K, Tran A, Tran T, Xue WY, Youssef P, Yuan P, Zhang D, et al. Milk-Derived Proteins and Peptides in Head and Neck Carcinoma Treatment. Biomolecules. 2022; 12(2):290. https://doi.org/10.3390/biom12020290
Chicago/Turabian StyleWang, Theresa, Xinyi Liu, Yah Ying Ng, Kiera Tarleton, Amy Tran, Thomas Tran, Wen Yue Xue, Paul Youssef, Peiyu Yuan, Daniel Zhang, and et al. 2022. "Milk-Derived Proteins and Peptides in Head and Neck Carcinoma Treatment" Biomolecules 12, no. 2: 290. https://doi.org/10.3390/biom12020290
APA StyleWang, T., Liu, X., Ng, Y. Y., Tarleton, K., Tran, A., Tran, T., Xue, W. Y., Youssef, P., Yuan, P., Zhang, D., Paolini, R., & Celentano, A. (2022). Milk-Derived Proteins and Peptides in Head and Neck Carcinoma Treatment. Biomolecules, 12(2), 290. https://doi.org/10.3390/biom12020290







