LPS Administration Impacts Glial Immune Programs by Alternative Splicing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. LPS Administration
2.3. Collecting Hippocampal Samples
2.4. RNA-Sequencing and Data Analysis
2.5. Statistical Methods
3. Results
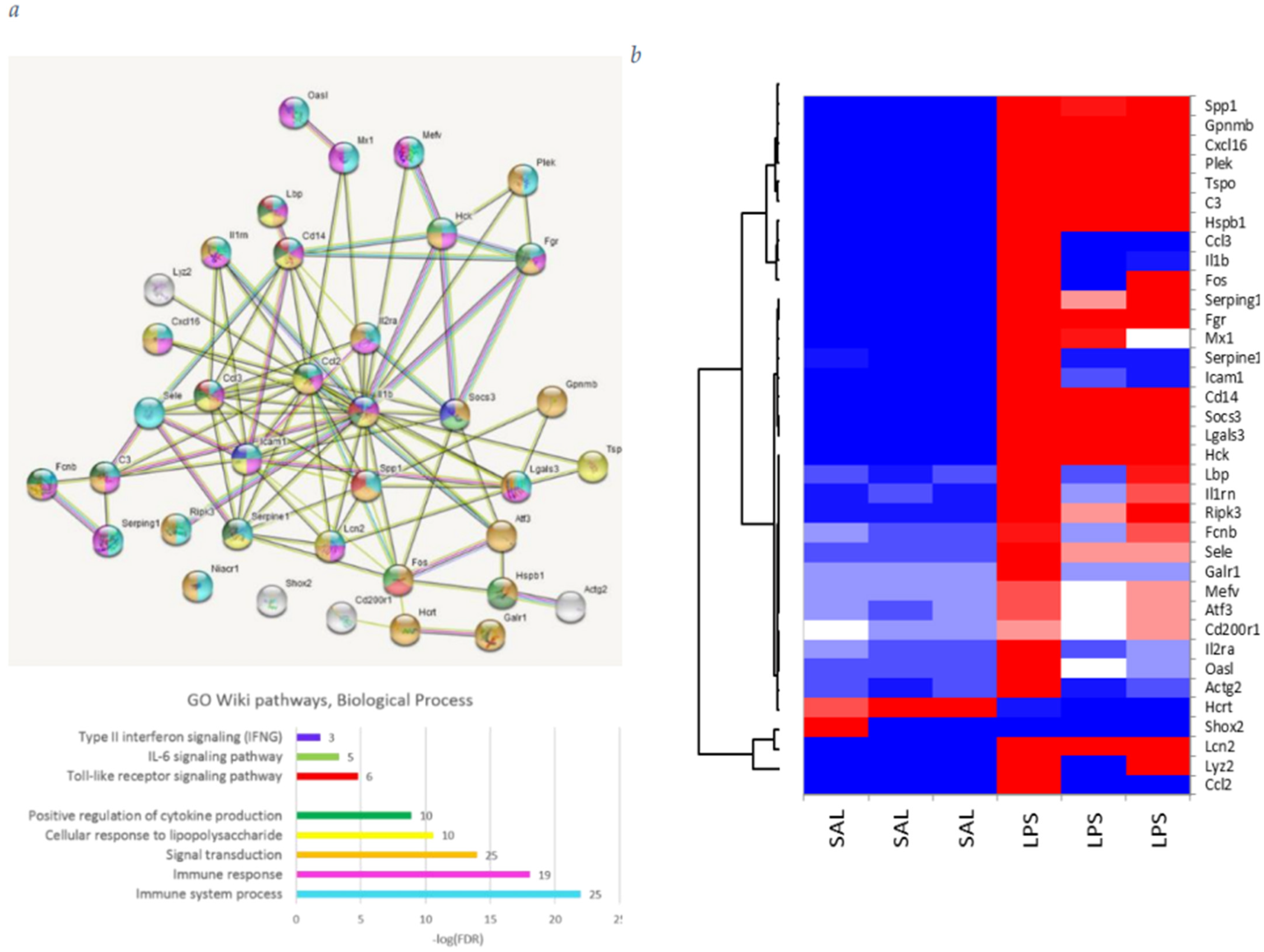
3.1. GO DEGs Analysis
3.2. DAS Genes Analysis
3.2.1. Cell-Specific Expression of DAS Genes
3.2.2. Sampled DAS Genes
3.3. Microglial Genes
3.3.1. Rhog Gene
3.3.2. Ptbp1 Exon 8 Skipping Preference
3.4. Endothelial Specific DAS Genes
3.4.1. Cast Gene
3.4.2. Phactr4 Gene
3.4.3. Pkn2 Gene
3.4.4. Ndufb3 vs. AABR07019088 Genes
3.4.5. Pan3 and Nebl Genes
3.5. Astrocyte Specific DAS Genes
Fnbp1 Gene
3.6. Splicing Factors (SF) in Brain
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Radenovic, L.; Nenadic, M.; Ułamek-Kozioł, M.; Januszewski, S.; Czuczwar, S.J.; Andjus, P.R.; Pluta, R. Heterogeneity in brain distribution of activated microglia and astrocytes in a rat ischemic model of Alzheimer’s disease after 2 years of survival. Aging 2020, 12, 12251–12267. [Google Scholar] [CrossRef] [PubMed]
- Batista, C.R.A.; Gomes, G.F.; Candelario-Jalil, E.; Fiebich, B.L.; de Oliveira, A.C.P. Lipopolysaccharide-Induced Neuroinflammation as a Bridge to Understand Neurodegeneration. Int. J. Mol. Sci. 2019, 20, 2293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonow, R.H.; Aid, S.; Zhang, Y.; Becker, K.G.; Bosetti, F. The brain expression of genes involved in inflammatory response, the ribosome, and learning and memory is altered by centrally injected lipopolysaccharide in mice. Pharm. J. 2009, 9, 116–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazin, P.V.; Jiang, X.; Fu, N.; Han, D.; Guo, M.; Gelfand, M.S.; Khaitovich, P. Conservation, evolution, and regulation of splicing during prefrontal cortex development in humans, chimpanzees, and macaques. RNA 2018, 24, 585–596. [Google Scholar] [CrossRef]
- Mazin, P.V.; Khaitovich, P.; Cardoso-Moreira, M.; Kaessmann, H. Alternative splicing during mammalian organ development. Nat. Genet. 2021, 53, 925–934. [Google Scholar] [CrossRef]
- Pinner, E.; Gruper, Y.; Ben Zimra, M.; Kristt, D.; Laudon, M.; Naor, D.; Zisapel, N. CD44 Splice Variants as Potential Players in Alzheimer’s Disease Pathology. J. Alzheimers Dis. 2017, 58, 1137–1149. [Google Scholar] [CrossRef] [PubMed]
- Marchese, E.; Valentini, M.; Di Sante, G.; Cesari, E.; Adinolfi, A.; Corvino, V.; Ria, F.; Sette, C.; Geloso, M.C. Alternative splicing of neurexins 1–3 is modulated by neuroinflammation in the prefrontal cortex of a murine model of multiple sclerosis. Exp. Neurol. 2021, 335, 113497. [Google Scholar] [CrossRef]
- Makeyev, E.V.; Zhang, J.; Carrasco, M.A.; Maniatis, T. The MicroRNA miR-124 promotes neuronal differentiation by triggering brain-specific alternative pre-mRNA splicing. Mol Cell. 2007, 27, 435–448. [Google Scholar] [CrossRef] [Green Version]
- Linares, A.J.; Lin, C.H.; Damianov, A.; Adams, K.L.; Novitch, B.G.; Black, D.L. The splicing regulator PTBP1 controls the activity of the transcription factor Pbx1 during neuronal differentiation. eLife 2015, 4, e09268. [Google Scholar] [CrossRef]
- Vuong, J.K.; Lin, C.H.; Zhang, M.; Chen, L.; Black, D.L.; Zheng, S. PTBP1 and PTBP2 Serve Both Specific and Redundant Functions in Neuronal Pre-mRNA Splicing. Cell Rep. 2016, 17, 2766–2775. [Google Scholar] [CrossRef] [Green Version]
- Georgilis, A.; Klotz, S.; Hanley, C.J.; Herranz, N.; Weirich, B.; Morancho, B.; Leote, A.C.; D’Artista, L.; Gallage, S.; Seehawer, M.; et al. PTBP1-Mediated Alternative Splicing Regulates the Inflammatory Secretome and the Pro-tumorigenic Effects of Senescent Cells. Cancer Cell. 2018, 34, 85.e9–102.e9. [Google Scholar] [CrossRef] [Green Version]
- Pan, J.; Ma, N.; Yu, B.; Zhang, W.; Wan, J. Transcriptomic profiling of microglia and astrocytes throughout aging. J. Neuroinflamm. 2020, 17, 97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geng, G.; Xu, C.; Peng, N.; Li, Y.; Liu, J.; Wu, J.; Liang, J.; Zhu, Y.; Shi, L. PTBP1 is necessary for dendritic cells to regulate T-cell homeostasis and antitumour immunity. Immunology 2021, 163, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Jeong, K.; Li, J.; Murphy, J.M.; Vukadin, L.; Stone, J.K.; Richard, A.; Tran, J.; Gillespie, G.Y.; Flemington, E.K.; et al. SON drives oncogenic RNA splicing in glioblastoma by regulating PTBP1/PTBP2 switching and RBFOX2 activity. Nat. Commun. 2021, 12, 5551. [Google Scholar] [CrossRef] [PubMed]
- Shishkina, G.T.; Gulyaeva, N.V.; Lanshakov, D.A.; Kalinina, T.S.; Onufriev, M.V.; Moiseeva, Y.V.; Sukhareva, E.V.; Babenko, V.N.; Dygalo, N.N. Identifying the Involvement of Pro-Inflammatory Signal in Hippocampal Gene Expression Changes after Experimental Ischemia: Transcriptome-Wide Analysis. Biomedicines 2021, 9, 1840. [Google Scholar] [CrossRef] [PubMed]
- Ory, D.; Planas, A.; Dresselaers, T.; Gsell, W.; Postnov, A.; Celen, S.; Casteels, C.; Himmelreich, U.; Debyser, Z.; Van Laere, K.; et al. PET imaging of TSPO in a rat model of local neuroinflammation induced by intracerebral injection of lipopolysaccharide. Nucl. Med. Biol. 2015, 42, 753–761. [Google Scholar] [CrossRef] [Green Version]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dobin, A.; Gingeras, T.R. Mapping RNA-seq Reads with STAR. Curr. Protoc. Bioinform. 2015, 51, 11.14.11–11.14.19. [Google Scholar] [CrossRef] [Green Version]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, S.; Park, J.W.; Lu, Z.X.; Lin, L.; Henry, M.D.; Wu, Y.N.; Zhou, Q.; Xing, Y. rMATS: Robust and flexible detection of differential alternative splicing from replicate RNA-Seq data. Proc. Natl. Acad. Sci. USA 2014, 111, E5593–E5601. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Oh, D.H.; Dassanayake, M. GOMCL: A toolkit to cluster, evaluate, and extract non-redundant associations of Gene Ontology-based functions. BMC Bioinform. 2020, 21, 139. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Chen, K.; Sloan, S.A.; Bennett, M.L.; Scholze, A.R.; O’Keeffe, S.; Phatnani, H.P.; Guarnieri, P.; Caneda, C.; Ruderisch, N.; et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. 2014, 34, 11929–11947. [Google Scholar] [CrossRef]
- Gueroussov, S.; Gonatopoulos-Pournatzis, T.; Irimia, M.; Raj, B.; Lin, Z.Y.; Gingras, A.C.; Blencowe, B.J. An alternative splicing event amplifies evolutionary differences between vertebrates. Science 2015, 349, 68–873. [Google Scholar] [CrossRef] [Green Version]
- Ule, J.; Ule, A.; Spencer, J.; Williams, A.; Hu, J.S.; Cline, M.; Wang, H.; Clark, T.; Fraser, C.; Ruggiu, M.; et al. Nova regulates brain-specific splicing to shape the synapse. Nat. Genet. 2005, 37, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Ule, J.; Stefani, G.; Mele, A.; Ruggiu, M.; Wang, X.; Taneri, B.; Gaasterland, T.; Blencowe, B.J.; Darnell, R.B. An RNA map predicting Nova-dependent splicing regulation. Nature 2006, 444, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Porter, R.S.; Jaamour, F.; Iwase, S. Neuron-specific alternative splicing of transcriptional machineries: Implications for neurodevelopmental disorders. Mol. Cell Neurosci. 2018, 87, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Rawish, E.; Nording, H.; Münte, T.; Langer, H.F. Platelets as Mediators of Neuroinflammation and Thrombosis. Front. Immunol. 2020, 11, 548631. [Google Scholar] [CrossRef]
- Jaffrey, S.R.; Wilkinson, M.F. Nonsense-mediated RNA decay in the brain: Emerging modulator of neural development and disease. Nat. Rev. Neurosci. 2018, 19, 715–728. [Google Scholar] [CrossRef] [PubMed]
- Mironov, A.; Denisov, S.; Gress, A.; Kalinina, O.V.; Pervouchine, D.D. An extended catalogue of tandem alternative splice sites in human tissue transcriptomes. PLoS Comput. Biol. 2021, 7, e1008329. [Google Scholar] [CrossRef]
- La Manno, G.; Soldatov, R.; Zeisel, A.; Braun, E.; Hochgerner, H.; Petukhov, V.; Lidschreiber, K.; Kastriti, M.E.; Lönnerberg, P.; Furlan, A.; et al. RNA velocity of single cells. Nature 2018, 560, 494–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Xue, Y.; Ouyang, K.; Huang, J.; Zhou, Y.; Ouyang, H.; Li, H.; Wang, G.; Wu, Q.; Wei, C.; Bi, Y.; et al. Direct conversion of fibroblasts to neurons by reprogramming PTB-regulated microRNA circuits. Cell 2013, 152, 82–96. [Google Scholar] [CrossRef] [Green Version]
- Qian, H.; Kang, X.; Hu, J.; Zhang, D.; Liang, Z.; Meng, F.; Zhang, X.; Xue, Y.; Maimon, R.; Dowdy, S.F.; et al. Reversing a model of Parkinson’s disease with in situ converted nigral neurons. Nature 2020, 582, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Qian, H.; Hu, J.; Zhou, B.; Zhou, Y.; Hu, X.; Karakhanyan, A.; Pang, Z.; Fu, X.D. Sequential regulatory loops as key gatekeepers for neuronal reprogramming in human cells. Nat. Neurosci. 2016, 19, 807–815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Li, Y.; Fan, Y.; Yu, X.; Mao, X.; Jin, F. PTBP1 promotes the growth of breast cancer cells through the PTEN/Akt pathway and autophagy. J. Cell Physiol. 2018, 233, 8930–8939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reshetnikov, V.V.; Kisaretova, P.E.; Ershov, N.I.; Shulyupova, A.S.; Oshchepkov, D.Y.; Klimova, N.V.; Ivanchihina, A.V.; Merkulova, T.I.; Bondar, N.P. Genes associated with cognitive performance in the Morris water maze: An RNA-seq study. Sci Rep. 2020, 10, 22078. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Z.; Sun, Y.; Zhu, R.; Jiao, N.; Tang, K.; Cao, Z.; Ma, C. Functional Cross-Talking between Differentially Expressed and Alternatively Spliced Genes in Human Liver Cancer Cells Treated with Berberine. PLoS ONE 2015, 10, e0143742. [Google Scholar] [CrossRef] [Green Version]
- Udy, D.B.; Bradley, R.K. Nonsense-mediated mRNA decay uses complementary mechanisms to suppress mRNA and protein accumulation. Life Sci. Alliance 2021, 5, e202101217. [Google Scholar] [CrossRef]





















| Endothelial Cells | Microglia | Astrocytes |
|---|---|---|
| Ptbp1 | Ptbp1 | Ank2 |
| Cast | Rhog | Med12l |
| Phactr4 | Atp13a2 | Tead1 |
| Nebl | Afmid | Pan3 |
| Lrp8 | Milt10 | |
| Pkn2 | Ptn2 | |
| Ndufb3 | Fnbp1 | |
| Zfp1 | ||
| Wrd78 | ||
| Eogt | ||
| Dctd | ||
| Phc3 | ||
| Mok |
| GO_id | Definition | Obs/Exp | FDR |
|---|---|---|---|
| GO: 0002366 | Immune system process | 25 of 945 | 9.70 × 10−23 |
| GO: 0006955 | Immune response | 19 of 506 | 8.32 × 10−19 |
| GO: 0007165 | Signal transduction | 25 of 2142 | 1.03 × 10−14 |
| GO: 0071222 | Cellular response to lipopolysaccharide | 10 of 153 | 2.39 × 10−11 |
| GO: 0001819 | Positive regulation of cytokine production | 10 of 239 | 1.18 × 10−09 |
| GO: 0001817 | Regulation of cytokine production | 15 of 390 | 1.84 × 10−08 |
| GO: 0032496 | Response to lipopolysaccharide | 14 of 306 | 1.26 × 10−08 |
| (a) Pancreas | Liver | Whole Blood | Heart—Left Ventricle | Brain—Frontal Cortex (BA9) |
|---|---|---|---|---|
| 9.4 (1.01) | 8.42 (1.7) | 7.39 (3.1) | 6.28 (1.6) | 6.72 (4.5) |
| (b) | ||||
| Adipose—Subcutaneous | Lung | Cells—Transformed Fibroblasts | Cells—EBV-Transformed Lymphocytes | Cervix—Ectocervix |
| 82.4 (3.1) | 85.5 (2.6) | 80.5 (2.4) | 101.4 (4.5) | 57.3 (1.9) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Babenko, V.N.; Shishkina, G.T.; Lanshakov, D.A.; Sukhareva, E.V.; Dygalo, N.N. LPS Administration Impacts Glial Immune Programs by Alternative Splicing. Biomolecules 2022, 12, 277. https://doi.org/10.3390/biom12020277
Babenko VN, Shishkina GT, Lanshakov DA, Sukhareva EV, Dygalo NN. LPS Administration Impacts Glial Immune Programs by Alternative Splicing. Biomolecules. 2022; 12(2):277. https://doi.org/10.3390/biom12020277
Chicago/Turabian StyleBabenko, Vladimir N., Galina T. Shishkina, Dmitriy A. Lanshakov, Ekaterina V. Sukhareva, and Nikolay N. Dygalo. 2022. "LPS Administration Impacts Glial Immune Programs by Alternative Splicing" Biomolecules 12, no. 2: 277. https://doi.org/10.3390/biom12020277
APA StyleBabenko, V. N., Shishkina, G. T., Lanshakov, D. A., Sukhareva, E. V., & Dygalo, N. N. (2022). LPS Administration Impacts Glial Immune Programs by Alternative Splicing. Biomolecules, 12(2), 277. https://doi.org/10.3390/biom12020277







