Epigenetic Regulation in Exposome-Induced Tumorigenesis: Emerging Roles of ncRNAs
Abstract
:1. Introduction
2. ncRNA Subtypes, Biogenesis, and Turnover
2.1. miRNAs
2.2. lncRNAs
2.3. Other ncRNA Biotypes (siRNA, piRNAs, snoRNAs)
3. Epigenetic Alterations Induced by Toxicants
3.1. Toxics in the Air
3.1.1. Ozone
3.1.2. Carbon Monoxide
3.1.3. Lead
3.1.4. Sulfur Dioxide
3.1.5. Nitrogen Dioxide
3.1.6. Particulate Matter
Organic and Elemental Carbon
Sulfates
Polycyclic Aromatic Hydrocarbons
3.1.7. Indoor Air Pollutants
Formaldehyde
Asbestos
Toluene
Radon
Mercury
Cigarette Smoking
3.2. Toxics in Water
3.2.1. Arsenic
3.2.2. Cadmium
3.2.3. Chromium
3.2.4. Nickel
3.2.5. Copper
3.2.6. Trihalomethanes
3.3. Toxicants in Food
3.3.1. Glyphosate
3.3.2. Dioxin
3.3.3. Bisphenol A
3.3.4. Phthalate
3.4. Toxics in Consumer Products
3.4.1. Nanotechnology
Titanium Dioxide
Gold Nanoparticles
Silica
The Balance between the Beneficial and Detrimental Effects of Nanomaterials and Nanoparticles
3.4.2. Pharmaceutically Active Compounds
Diethylstilbestrol
3.4.3. Parabens
3.4.4. Aluminum Hydrochloride
4. Effects of Epigenetic Toxicants in Phenotypic Transformation
Epimutations in Pollution-Associated Diseases
5. ncRNA Dysregulation in Pollution-Related Cancer
5.1. ncRNA Dysregulation in Liver Cancer
5.2. ncRNA Dysregulation in Ovarian Cancer
5.3. ncRNA Dysregulation in Breast Cancer
5.4. ncRNA Dysregulation in Pancreatic Cancer
5.5. ncRNA Dysregulation in Colorectal Cancer
5.6. ncRNA Dysregulation in Lung Cancer
5.7. ncRNA Dysregulation in Kidney Cancer
5.8. ncRNA Dysregulation in Acute Leukemia
5.9. ncRNA Dysregulation in Testis Cancer
5.10. Potential Role of ncRNAs as Therapeutic Agents in Exposome-Associated Diseases
6. Transgenerational Inheritance after Epi-Toxicants Exposure
7. Future Perspective: Biomonitoring and Novel 3D Epigenomic Technologies
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, L.; Lu, Q.; Chang, C. Epigenetics in Health and Disease. Adv. Exp. Med. Biol. 2020, 1253, 3–55. [Google Scholar] [CrossRef] [PubMed]
- Vrijheid, M. The exposome: A new paradigm to study the impact of environment on health. Thorax 2014, 69, 876–878. [Google Scholar] [CrossRef] [Green Version]
- Vermeulen, R.; Schymanski, E.L.; Barabási, A.-L.; Miller, G.W. The exposome and health: Where chemistry meets biology. Science 2020, 367, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Boardman, J.D.; Daw, J.; Freese, J. Defining the Environment in Gene–Environment Research: Lessons From Social Epidemiology. Am. J. Public Health 2013, 103, S64–S72. [Google Scholar] [CrossRef] [PubMed]
- Wild, C.P. Complementing the Genome with an “Exposome”: The Outstanding Challenge of Environmental Exposure Measurement in Molecular Epidemiology. Cancer Epidemiol. Biomark. Prev. 2005, 14, 1847–1850. [Google Scholar] [CrossRef] [Green Version]
- Debord, D.G.; Carreón, T.; Lentz, T.J.; Middendorf, P.J.; Hoover, M.D.; Schulte, P.A. Use of the “Exposome” in the Practice of Epidemiology: A Primer on -Omic Technologies. Am. J. Epidemiol. 2016, 184, 302–314. [Google Scholar] [CrossRef] [Green Version]
- Zhubi, A.; Cook, E.H.; Guidotti, A.; Grayson, D.R. Epigenetic Mechanisms in Autism Spectrum Disorder. Int. Rev. Neurobiol. 2014, 115, 203–244. [Google Scholar] [CrossRef]
- Fabrizio, P.; Garvis, S.; Palladino, F. Histone Methylation and Memory of Environmental Stress. Cells 2019, 8, 339. [Google Scholar] [CrossRef] [Green Version]
- Skinner, M.K.; Manikkam, M.; Guerrero-Bosagna, C. Epigenetic transgenerational actions of environmental factors in disease etiology. Trends Endocrinol. Metab. 2010, 21, 214–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Loh, X.J.; Luo, Y.; Ge, S.; Fan, X.; Ruan, J. Insights into the epigenetic effects of nanomaterials on cells. Biomater. Sci. 2019, 8, 763–775. [Google Scholar] [CrossRef]
- Bollati, V.; Baccarelli, A. Environmental epigenetics. Heredity 2010, 105, 105–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karlsson, O.; Baccarelli, A.A. Environmental Health and Long Non-coding RNAs. Curr. Environ. Health Rep. 2016, 3, 178–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Javierre, B.M.; Fernandez, A.F.; Richter, J.; Al-Shahrour, F.; Martin-Subero, J.I.; Rodriguez-Ubreva, J.; Berdasco, M.; Fraga, M.F.; O’Hanlon, T.P.; Rider, L.G.; et al. Changes in the pattern of DNA methylation associate with twin discordance in systemic lupus erythematosus. Genome Res. 2010, 20, 170–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubota, T. Epigenetic alterations induced by environmental stress associated with metabolic and neurodevelopmental disorders. Environ. Epigenetics 2016, 2, dvw017. [Google Scholar] [CrossRef] [Green Version]
- Fragou, D.; Fragou, A.; Kouidou, S.; Njau, S.; Kovatsi, L. Epigenetic mechanisms in metal toxicity. Toxicol. Mech. Methods 2011, 21, 343–352. [Google Scholar] [CrossRef]
- Treiber, T.; Treiber, N.; Meister, G. Regulation of microRNA biogenesis and its crosstalk with other cellular pathways. Nat. Rev. Mol. Cell Biol. 2019, 20, 5–20, Erratum in Nat. Rev. Mol. Cell Biol. 2019, 20, 321. [Google Scholar] [CrossRef]
- Miguel, V.; Lamas, S.; Espinosa-Diez, C. Role of non-coding-RNAs in response to environmental stressors and consequences on human health. Redox Biol. 2020, 37, 101580. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef] [Green Version]
- Truesdell, S.S.; Mortensen, R.D.; Seo, M.; Schroeder, J.C.; Lee, J.H.; LeTonqueze, O.; Vasudevan, S. MicroRNA-mediated mRNA Translation Activation in Quiescent Cells and Oocytes Involves Recruitment of a Nuclear microRNP. Sci. Rep. 2012, 2, srep00842. [Google Scholar] [CrossRef] [Green Version]
- Vasudevan, S.; Steitz, J.A. AU-Rich-Element-Mediated Upregulation of Translation by FXR1 and Argonaute 2. Cell 2007, 128, 1105–1118. [Google Scholar] [CrossRef] [Green Version]
- Singh, I.; Contreras, A.; Cordero, J.; Rubio, K.; Dobersch, S.; Günther, S.; Jeratsch, S.; Mehta, A.; Krüger, M.; Graumann, J.; et al. MiCEE is a ncRNA-protein complex that mediates epigenetic silencing and nucleolar organization. Nat. Genet. 2018, 50, 990–1001. [Google Scholar] [CrossRef] [PubMed]
- Rubio, K.; Singh, I.; Dobersch, S.; Sarvari, P.; Günther, S.; Cordero, J.; Mehta, A.; Wujak, L.; Cabrera-Fuentes, H.; Chao, C.-M.; et al. Inactivation of nuclear histone deacetylases by EP300 disrupts the MiCEE complex in idiopathic pulmonary fibrosis. Nat. Commun. 2019, 10, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bose, M.; Bhattacharyya, S.N. Target-dependent biogenesis of cognate microRNAs in human cells. Nat. Commun. 2016, 7, 12200. [Google Scholar] [CrossRef] [PubMed]
- Cheloufi, S.; Dos Santos, C.O.; Chong, M.M.W.; Hannon, G.J. A dicer-independent miRNA biogenesis pathway that requires Ago catalysis. Nature 2010, 465, 584–589. [Google Scholar] [CrossRef] [Green Version]
- Cifuentes, D.; Xue, H.; Taylor, D.W.; Patnode, H.; Mishima, Y.; Cheloufi, S.; Ma, E.; Mane, S.; Hannon, G.J.; Lawson, N.D.; et al. A Novel miRNA Processing Pathway Independent of Dicer Requires Argonaute2 Catalytic Activity. Science 2010, 328, 1694–1698. [Google Scholar] [CrossRef] [Green Version]
- Statello, L.; Guo, C.-J.; Chen, L.-L.; Huarte, M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 2021, 22, 96–118, Correction in 2021, 22, 159, doi:10.1038/s41580-021-00330-4. [Google Scholar] [CrossRef]
- Ruiz-Orera, J.; Messeguer, X.; Subirana, J.; Alba, M.M. Long non-coding RNAs as a source of new peptides. eLife 2014, 3, e03523. [Google Scholar] [CrossRef] [Green Version]
- Guttman, M.; Amit, I.; Garber, M.; French, C.; Lin, M.F.; Feldser, D.; Huarte, M.; Zuk, O.; Carey, B.W.; Cassady, J.P.; et al. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature 2009, 458, 223–227. [Google Scholar] [CrossRef]
- Schlackow, M.; Nojima, T.; Gomes, T.; Dhir, A.; Carmo-Fonseca, M.; Proudfoot, N.J. Distinctive Patterns of Transcription and RNA Processing for Human lincRNAs. Mol. Cell 2016, 65, 25–38. [Google Scholar] [CrossRef] [Green Version]
- Marques, A.A.; Hughes, J.; Graham, B.; Kowalczyk, M.S.; Higgs, D.R.; Ponting, C.P. Chromatin signatures at transcriptional start sites separate two equally populated yet distinct classes of intergenic long noncoding RNAs. Genome Biol. 2013, 14, R131. [Google Scholar] [CrossRef]
- Marchese, F.P.; Raimondi, I.; Huarte, M. The multidimensional mechanisms of long noncoding RNA function. Genome Biol. 2017, 18, 206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.H.; Chen, Y. Targeting long non-coding RNAs in cancers: Progress and prospects. Int. J. Biochem. Cell Biol. 2013, 45, 1895–1910. [Google Scholar] [CrossRef]
- Elbashir, S.M.; Harborth, J.; Lendeckel, W.; Yalcin, A.; Weber, K.; Tuschl, T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 2001, 411, 494–498. [Google Scholar] [CrossRef]
- Ketting, R.F.; Fischer, S.E.; Bernstein, E.; Sijen, T.; Hannon, G.J.; Plasterk, R.H. Dicer functions in RNA interference and in synthesis of small RNA involved in developmental timing in C. elegans. Genes Dev. 2001, 15, 2654–2659. [Google Scholar] [CrossRef] [Green Version]
- Okamura, K.; Ishizuka, A.; Siomi, H.; Siomi, M.C. Distinct roles for Argonaute proteins in small RNA-directed RNA cleavage pathways. Genes Dev. 2004, 18, 1655–1666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carthew, R.W.; Sontheimer, E.J. Origins and Mechanisms of miRNAs and siRNAs. Cell 2009, 136, 642–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarkar, A.; Maji, R.K.; Saha, S.; Ghosh, Z. piRNAQuest: Searching the piRNAome for silencers. BMC Genom. 2014, 15, 555. [Google Scholar] [CrossRef] [Green Version]
- Rayford, K.; Cooley, A.; Rumph, J.; Arun, A.; Rachakonda, G.; Villalta, F.; Lima, M.; Pratap, S.; Misra, S.; Nde, P. piRNAs as Modulators of Disease Pathogenesis. Int. J. Mol. Sci. 2021, 22, 2373. [Google Scholar] [CrossRef]
- Guo, B.; Li, D.; Du, L.; Zhu, X. piRNAs: Biogenesis and their potential roles in cancer. Cancer Metastasis Rev. 2020, 39, 567–575. [Google Scholar] [CrossRef]
- Ojha, S.; Malla, S.; Lyons, S.M. snoRNPs: Functions in Ribosome Biogenesis. Biomolecules 2020, 10, 783. [Google Scholar] [CrossRef]
- Hirose, T.; Shu, M.-D.; Steitz, J.A. Splicing-Dependent and -Independent Modes of Assembly for Intron-Encoded Box C/D snoRNPs in Mammalian Cells. Mol. Cell 2003, 12, 113–123. [Google Scholar] [CrossRef]
- Ooi, S.L.; Samarsky, D.A.; Fournier, M.J.; Boeke, J.D. Intronic snoRNA biosynthesis in Saccharomyces cerevisiae depends on the lariat-debranching enzyme: Intron length effects and activity of a precursor snoRNA. RNA 1998, 4, 1096–1110. [Google Scholar] [CrossRef] [Green Version]
- Mei, Y.-P.; Liao, J.-P.; Shen, J.; Yu, L.; Liu, B.L.; Liu, L.; Li, R.-Y.; Ji, L.; Dorsey, S.G.; Jiang, Z.-R.; et al. Small nucleolar RNA 42 acts as an oncogene in lung tumorigenesis. Oncogene 2012, 31, 2794–2804. [Google Scholar] [CrossRef] [PubMed]
- Patterson, D.; Roberts, J.T.; King, V.M.; Houserova, D.; Barnhill, E.C.; Crucello, A.; Polska, C.J.; Brantley, L.W.; Kaufman, G.C.; Nguyen, M.; et al. Human snoRNA-93 is processed into a microRNA-like RNA that promotes breast cancer cell invasion. NPJ Breast Cancer 2017, 3, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Marsit, C.J. Influence of environmental exposure on human epigenetic regulation. J. Exp. Biol. 2015, 218, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Tohyama, C. Towards comprehensive health risk assessments of chemicals for occupational and environmental health. Ind. Health 2017, 55, 199–200. [Google Scholar] [CrossRef] [Green Version]
- Farhadi, Z.; Gorgi, H.A.; Shabaninejad, H.; Delavar, M.A.; Torani, S. Association between PM2.5 and risk of hospitalization for myocardial infarction: A systematic review and a meta-analysis. BMC Public Health 2020, 20, 1–12. [Google Scholar] [CrossRef]
- Peng, F.; Tsuji, G.; Zhang, J.-Z.; Chen, Z.; Furue, M. Potential role of PM2.5 in melanogenesis. Environ. Int. 2019, 132, 105063. [Google Scholar] [CrossRef]
- Rider, C.F.; Carlsten, C. Air pollution and DNA methylation: Effects of exposure in humans. Clin. Epigenetics 2019, 11, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Xu, J.; Chen, Y.; Guo, X.; Zheng, Y.; Wang, Q.; Chen, Y.; Ni, Y.; Zhu, Y.; Joyce, B.T.; et al. Characterization of genome-wide H3K27ac profiles reveals a distinct PM2.5-associated histone modification signature. Environ. Health 2015, 14, 65. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Ma, J.; Li, X.; Chan, M.T.V.; Wu, W.K.K.; Wu, Z.; Shen, J. Aberrantly expressed long non-coding RNAs in air pollution-induced congenital defects. J. Cell. Mol. Med. 2019, 23, 7717–7725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasahara, D.I.; Shore, S.A. IL-33, diet-induced obesity, and pulmonary responses to ozone. Respir. Res. 2020, 21, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Tashiro, H.; Cho, Y.; Kasahara, D.I.; Brand, J.D.; Bry, L.; Yeliseyev, V.; Abu-Ali, G.; Huttenhower, C.; Shore, S.A. Microbiota Contribute to Obesity-related Increases in the Pulmonary Response to Ozone. Am. J. Respir. Cell Mol. Biol. 2019, 61, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Shore, S.A. Mechanistic Basis for Obesity-related Increases in Ozone-induced Airway Hyperresponsiveness in Mice. Ann. Am. Thorac. Soc. 2017, 14, S357–S362. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.J.; Tovar, A.; Kanke, M.; Wang, Y.; Deshane, J.S.; Sethupathy, P.; Kelada, S.N.P. Ozone-induced changes in the murine lung extracellular vesicle small RNA landscape. Physiol. Rep. 2021, 9, e15054. [Google Scholar] [CrossRef] [PubMed]
- Romito, B.T.; McBroom, M.M.; Bryant, D.; Gamez, J.; Merchant, A.; E Hill, S. The effect of SANGUINATE® (PEGylated carboxyhemoglobin bovine) on cardiopulmonary bypass functionality using a bovine whole blood model of normovolemic hemodilution. Perfusion 2019, 35, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, S.; Salt, O.; Durukan, P.; Sen, A.; Bulbul, E.; Duman, A.; Kavalci, C. The relationship among plasma copeptin, carboxyhemoglobin, and lactate levels in carbon monoxide poisoning. Hum. Exp. Toxicol. 2019, 39, 311–318. [Google Scholar] [CrossRef]
- Terzikhan, N.; Xu, H.; Edris, A.; Bracke, K.R.; Verhamme, F.M.; Stricker, B.H.C.; Dupuis, J.; Lahousse, L.; O’Connor, G.T.; Brusselle, G.G. Epigenome-wide association study on diffusing capacity of the lung. ERJ Open Res. 2020, 7. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, J.; Xu, Y. Epigenetic Basis of Lead-Induced Neurological Disorders. Int. J. Environ. Res. Public Health 2020, 17, 4878. [Google Scholar] [CrossRef]
- Kovatsi, L.; Georgiou, E.; Ioannou, A.; Haitoglou, C.; Tzimagiorgis, G.; Tsoukali, H.; Kouidou, S. p16promoter methylation in Pb2+-exposed individuals. Clin. Toxicol. 2010, 48, 124–128. [Google Scholar] [CrossRef]
- Wu, M.-F.; Chen, Y.-H.; Chen, H.-C.; Huang, W.-C. Interactions among Obstructive Sleep Apnea Syndrome Severity, Sex, and Obesity on Circulatory Inflammatory Biomarkers in Patients with Suspected Obstructive Sleep Apnea Syndrome: A Retrospective, Cross-Sectional Study. Int. J. Environ. Res. Public Health 2020, 17, 4701. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Li, J.; Zhang, W.; Wang, Z.; Wu, J.; Ge, X.; Wu, J.; Cao, Y.; Xie, Y.; Ying, D.; et al. Emission of sulfur dioxide from polyurethane foam and respiratory health effects. Environ. Pollut. 2018, 242, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Ku, T.; Chen, M.; Li, B.; Yun, Y.; Li, G.; Sang, N. Synergistic effects of particulate matter (PM2.5) and sulfur dioxide (SO2) on neurodegeneration via the microRNA-mediated regulation of tau phosphorylation. Toxicol. Res. 2017, 6, 7–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fenech, S.; Aquilina, N.J. Trends in ambient ozone, nitrogen dioxide, and particulate matter concentrations over the Maltese Islands and the corresponding health impacts. Sci. Total Environ. 2019, 700, 134527. [Google Scholar] [CrossRef]
- Ritz, B.; Hoffmann, B.; Peters, A. The Effects of Fine Dust, Ozone, and Nitrogen Dioxide on Health. Dtsch Arztebl Int 2019, 51–52, 881–886. [Google Scholar] [CrossRef]
- Jiang, Y.; Niu, Y.; Xia, Y.; Liu, C.; Lin, Z.; Wang, W.; Ge, Y.; Lei, X.; Wang, C.; Cai, J.; et al. Effects of personal nitrogen dioxide exposure on airway inflammation and lung function. Environ. Res. 2019, 177, 108620. [Google Scholar] [CrossRef]
- Seo, M.Y.; Kim, S.-H.; Park, M.J. Air pollution and childhood obesity. Clin. Exp. Pediatr. 2020, 63, 382–388. [Google Scholar] [CrossRef] [Green Version]
- Sahay, D.; Terry, M.B.; Miller, R. Is breast cancer a result of epigenetic responses to traffic-related air pollution? A review of the latest evidence. Epigenomics 2019, 11, 701–714. [Google Scholar] [CrossRef]
- Brown, J.S.; Gordon, T.; Price, O.; Asgharian, B. Thoracic and respirable particle definitions for human health risk assessment. Part. Fibre Toxicol. 2013, 10, 12. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Lv, Y.; Hao, J.; Sun, H.; Gao, N.; Zhang, C.; Lu, R.; Wang, S.; Yin, L.; Pu, Y.; et al. Role of microRNA-4516 involved autophagy associated with exposure to fine particulate matter. Oncotarget 2016, 7, 45385–45397. [Google Scholar] [CrossRef]
- Yang, D.; Ma, M.; Zhou, W.; Yang, B.; Xiao, C. Inhibition of miR-32 activity promoted EMT induced by PM2.5 exposure through the modulation of the Smad1-mediated signaling pathways in lung cancer cells. Chemosphere 2017, 184, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Zhong, Y.; Hu, Y.; Sun, C.; Wang, Y.; Wang, G. PM2.5 downregulates miR-194-3p and accelerates apoptosis in cigarette-inflamed bronchial epithelium by targeting death-associated protein kinase 1. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 2339–2349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Li, Z. microRNA-16 via Twist1 inhibits EMT induced by PM2.5 exposure in human hepatocellular carcinoma. Open Med. 2019, 14, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zheng, M.; Pu, J.; Zhou, Y.; Hong, W.; Fu, X.; Peng, Y.; Zhou, W.; Pan, H.; Li, B.; et al. Identification of abnormally expressed lncRNAs induced by PM2.5 in human bronchial epithelial cells. Biosci. Rep. 2018, 38, BSR20171577. [Google Scholar] [CrossRef] [Green Version]
- Song, B.; Ye, L.; Wu, S.; Jing, Z. Long non-coding RNA MEG3 regulates CSE-induced apoptosis and inflammation via regulating miR-218 in 16HBE cells. Biochem. Biophys. Res. Commun. 2019, 521, 368–374. [Google Scholar] [CrossRef]
- Tan, Y.; Wang, Y.; Zou, Y.; Zhou, C.; Yi, Y.; Ling, Y.; Liao, F.; Jiang, Y.; Peng, X. LncRNA LOC101927514 regulates PM2.5-driven inflammation in human bronchial epithelial cells through binding p-STAT3 protein. Toxicol. Lett. 2019, 319, 119–128. [Google Scholar] [CrossRef]
- Khuzestani, R.B.; Schauer, J.J.; Shang, J.; Cai, T.; Fang, D.; Wei, Y.; Zhang, L.; Zhang, Y. Source apportionments of PM2.5 organic carbon during the elevated pollution episodes in the Ordos region, Inner Mongolia, China. Environ. Sci. Pollut. Res. 2018, 25, 13159–13172. [Google Scholar] [CrossRef]
- Peng, X.-L.; Hao, Q.-J.; Wen, T.-X.; Ji, D.-S.; Liu, Z.-R.; Wang, Y.-S.; Chen, J.-B.; Jiang, C.-S. [Pollution Characteristics of Organic Carbon and Elemental Carbon in Atmospheric Aerosols in Beibei District, Chongqing]. Huan jing ke xue Huanjing kexue 2018, 39, 3502–3510. [Google Scholar] [CrossRef]
- Xing, L.; Li, G.; Pongpiachan, S.; Wang, Q.; Han, Y.; Cao, J.; Tipmanee, D.; Palakun, J.; Aukkaravittayapun, S.; Surapipith, V.; et al. Quantifying the contributions of local emissions and regional transport to elemental carbon in Thailand. Environ. Pollut. 2020, 262, 114272. [Google Scholar] [CrossRef]
- Plato, N.; Lewné, M.; Gustavsson, P. A historical job-exposure matrix for occupational exposure to diesel exhaust using elemental carbon as an indicator of exposure. Arch. Environ. Occup. Health 2019, 75, 321–332. [Google Scholar] [CrossRef] [Green Version]
- Sagai, M. Toxic Components of PM2.5 and Their Toxicity Mechanisms—On the Toxicity of Sulfate and Carbon Components. Nippon Eiseigaku Zasshi 2019, 74, 19004. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Feng, N.; Zheng, M.; Ye, X.; Lin, H.; Yu, X.; Gan, Z.; Fang, Z.; Zhang, H.; Gao, M.; et al. PM2.5 exposure-induced autophagy is mediated by lncRNA loc146880 which also promotes the migration and invasion of lung cancer cells. Biochim. et Biophys. Acta (BBA)-Gen. Subj. 2016, 1861, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Feng, N.; Ching, T.; Wang, Y.; Liu, B.; Lin, H.; Shi, O.; Zhang, X.; Zheng, M.; Zheng, X.; Gao, M.; et al. Analysis of Microarray Data on Gene Expression and Methylation to Identify Long Non-coding RNAs in Non-small Cell Lung Cancer. Sci. Rep. 2016, 6, 37233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yenjerla, M.; Panopoulos, A.; Reynaud, C.; Fotedar, R.; Margolis, R.L. TD-60 is required for interphase cell cycle progression. Cell Cycle 2013, 12, 837–841. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.; Zhang, X.; Feng, N.; Wang, R.; Zhang, W.; Deng, X.; Wang, Y.; Yu, X.; Ye, X.; Li, L.; et al. LncRNA LCPAT1 Mediates Smoking/Particulate Matter 2.5-Induced Cell Autophagy and Epithelial-Mesenchymal Transition in Lung Cancer Cells via RCC2. Cell. Physiol. Biochem. 2018, 47, 1244–1258. [Google Scholar] [CrossRef]
- Zheng, L.; Chen, X.; Dong, X.; Wei, X.; Jiang, C.; Tang, Q. Using δ34S–SO4 and δ18O–SO4 to trace the sources of sulfate in different types of surface water from the Linhuan coal-mining subsidence area of Huaibei, China. Ecotoxicol. Environ. Saf. 2019, 181, 231–240. [Google Scholar] [CrossRef]
- Madrigano, J.; Baccarelli, A.; Mittleman, M.; Wright, R.; Sparrow, D.; Vokonas, P.S.; Tarantini, L.; Schwartz, J. Prolonged Exposure to Particulate Pollution, Genes Associated with Glutathione Pathways, and DNA Methylation in a Cohort of Older Men. Environ. Health Perspect. 2011, 119, 977–982. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Liu, M.; Yu, Y.; Li, Y. Characterization and source apportionment of PM2.5-bound polycyclic aromatic hydrocarbons from Shanghai city, China. Environ. Pollut. 2016, 218, 118–128. [Google Scholar] [CrossRef]
- Duca, R.-C.; Grova, N.; Ghosh, M.; Do, J.-M.; Hoet, P.H.M.; Vanoirbeek, J.A.J.; Appenzeller, B.M.R.; Godderis, L. Exposure to Polycyclic Aromatic Hydrocarbons Leads to Non-monotonic Modulation of DNA and RNA (hydroxy)methylation in a Rat Model. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.; Feng, Y.; Yu, H.; Xie, Y.; Luo, F.; Wang, Y. A novel lncRNA, loc107985872, promotes lung adenocarcinoma progression via the notch1 signaling pathway with exposure to traffic-originated PM2.5 organic extract. Environ. Pollut. 2020, 266, 115307. [Google Scholar] [CrossRef]
- Wang, Y.; Zhong, Y.; Hou, T.; Liao, J.; Zhang, C.; Sun, C.; Wang, G. PM2.5 induces EMT and promotes CSC properties by activating Notch pathway in vivo and vitro. Ecotoxicol. Environ. Saf. 2019, 178, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Wei, H.; Guo, H.; Li, Y.; Feng, Y.; Bian, Q.; Wang, Y. LncRNA MALAT1, an lncRNA acting via the miR-204/ZEB1 pathway, mediates the EMT induced by organic extract of PM2.5 in lung bronchial epithelial cells. Am. J. Physiol. Cell. Mol. Physiol. 2019, 317, L87–L98. [Google Scholar] [CrossRef]
- Zhao, G.; Zou, J.; Zhang, T.; Li, C.; Zhou, S.; Jiao, F. Recent progress on removal of indoor air pollutants by catalytic oxidation. Rev. Environ. Health 2020, 35, 311–321. [Google Scholar] [CrossRef]
- Bhargava, B.; Malhotra, S.; Chandel, A.; Rakwal, A.; Kashwap, R.R.; Kumar, S. Mitigation of indoor air pollutants using Areca palm potted plants in real-life settings. Environ. Sci. Pollut. Res. 2020, 28, 8898–8906. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Zhang, X.; Gao, J.; Cao, G.; Zhou, X.; Su, X. Indoor air pollutants, ventilation rate determinants and potential control strategies in Chinese dwellings: A literature review. Sci. Total Environ. 2017, 586, 696–729. [Google Scholar] [CrossRef]
- Siza, C.; Morrison, M.; Harris, S.; Hatch, T.; Tyler, M. Assessment of Community Awareness and Practices Concerning Indoor Air Pollutants—Madison County, Alabama, June 2017. MMWR. Morb. Mortal. Wkly. Rep. 2018, 67, 447–450. [Google Scholar] [CrossRef] [Green Version]
- Vincent, M.; Chemarin, C. Impact sanitaire de la pollution particulaire minérale à l’intérieur des locaux. Rev. des Mal. Respir. 2011, 28, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Yadav, I.C.; Devi, N.L.; Li, J.; Zhang, G. Polychlorinated biphenyls and organochlorines pesticides in indoor dust: An exploration of sources and health exposure risk in a rural area (Kopawa) of Nepal. Ecotoxicol. Environ. Saf. 2020, 195, 110376. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Y.; He, X.; Yang, P.; Zong, T.; Sun, P.; Sun, R.; Yu, T.; Jiang, Z. The cellular function and molecular mechanism of formaldehyde in cardiovascular disease and heart development. J. Cell. Mol. Med. 2021, 25, 5358–5371. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Y.; Ju, H.; He, X.; Sun, P.; Tian, Y.; Yang, P.; Song, X.-X.; Yu, T.; Jiang, Z. Comprehensive profile of circRNAs in formaldehyde induced heart development. Food Chem. Toxicol. 2022, 162, 112899. [Google Scholar] [CrossRef]
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and cancer. Ann. Afr. Med. 2019, 18, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.Y.; Rath, E.M.; Linton, A.; Yuen, M.L.; Takahashi, K.; Lee, K. The Current Understanding Of Asbestos-Induced Epigenetic Changes Associated With Lung Cancer. Lung Cancer Targets Ther. 2020, 11, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilk, E.; Krówczyńska, M. Malignant mesothelioma and asbestos exposure in Europe: Evidence of spatial clustering. Geospat. Health 2021, 16, 1. [Google Scholar] [CrossRef] [PubMed]
- Rozitis, E.; Johnson, B.; Cheng, Y.Y.; Lee, K. The Use of Immunohistochemistry, Fluorescence in situ Hybridization, and Emerging Epigenetic Markers in the Diagnosis of Malignant Pleural Mesothelioma (MPM): A Review. Front. Oncol. 2020, 10, 1742. [Google Scholar] [CrossRef]
- Matboli, M.; Shafei, A.E.; Ali, M.A.; Gaber, A.I.; Galal, A.; Tarek, O.; Marei, M.; Khairy, E.; El-Khazragy, N.; Anber, N.; et al. Clinical significance of serum DRAM1 mRNA, ARSA mRNA, hsa-miR-2053 and lncRNA-RP1-86D1.3 axis expression in malignant pleural mesothelioma. J. Cell. Biochem. 2018, 120, 3203–3211. [Google Scholar] [CrossRef]
- Mozzoni, P.; Ampollini, L.; Goldoni, M.; Alinovi, R.; Tiseo, M.; Gnetti, L.; Carbognani, P.; Rusca, M.; Mutti, A.; Percesepe, A.; et al. MicroRNA Expression in Malignant Pleural Mesothelioma and Asbestosis: A Pilot Study. Dis. Markers 2017, 2017, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Weber, D.G.; Casjens, S.; Brik, A.; Raiko, I.; Lehnert, M.; Taeger, D.; Gleichenhagen, J.; Kollmeier, J.; Bauer, T.T.; Brüning, T.; et al. Circulating long non-coding RNA GAS5 (growth arrest-specific transcript 5) as a complement marker for the detection of malignant mesothelioma using liquid biopsies. Biomark. Res. 2020, 8, 1–11. [Google Scholar] [CrossRef]
- Jiménez-Garza, O.; Baccarelli, A.; Byun, H.-M.; Márquez-Gamiño, S.; Barrón-Vivanco, B.S.; Albores, A. CYP2E1 epigenetic regulation in chronic, low-level toluene exposure: Relationship with oxidative stress and smoking habit. Toxicol. Appl. Pharmacol. 2015, 286, 207–215. [Google Scholar] [CrossRef]
- Yu, S.Y.; Koh, E.J.; Kim, S.H.; Song, B.; Lee, J.S.; Son, S.W.; Seo, H.; Hwang, S.Y. Analysis of multi-omics data on the relationship between epigenetic changes and nervous system disorders caused by exposure to environmentally harmful substances. Environ. Toxicol. 2021, 37, 802–813. [Google Scholar] [CrossRef]
- Yu, S.Y.; Koh, E.J.; Kim, S.H.; Lee, S.Y.; Lee, J.S.; Son, S.W.; Hwang, S.Y. Integrated analysis of multi-omics data on epigenetic changes caused by combined exposure to environmental hazards. Environ. Toxicol. 2021, 36, 1001–1010. [Google Scholar] [CrossRef]
- Sun, L.; Pan, Y.; Wang, X.; Gao, G.; Wu, L.; Piao, C.; Ruan, J.; Liu, J. Screening for Potential Biomarkers in Peripheral Blood From Miners Exposed to Radon Radiation. Dose-Response 2020, 18, 1559325820904600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, E.; Guo, J.; Bai, Y.; Zhang, H.; Liu, X.; Cai, W.; Zhong, L.; Zhu, B. MiR-92a and miR-486 are potential diagnostic biomarkers for mercury poisoning and jointly sustain NF-κB activity in mercury toxicity. Sci. Rep. 2017, 7, 15980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guida, N.; Laudati, G.; Mascolo, L.; Valsecchi, V.; Sirabella, R.; Selleri, C.; Di Renzo, G.; Canzoniero, L.M.T.; Formisano, L. p38/Sp1/Sp4/HDAC4/BDNF Axis Is a Novel Molecular Pathway of the Neurotoxic Effect of the Methylmercury. Front. Neurosci. 2017, 11, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zong, D.; Liu, X.; Li, J.; Ouyang, R.; Chen, P. The role of cigarette smoke-induced epigenetic alterations in inflammation. Epigenetics Chromatin 2019, 12, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Sundar, I.K.; Rahman, I. Gene expression profiling of epigenetic chromatin modification enzymes and histone marks by cigarette smoke: Implications for COPD and lung cancer. Am. J. Physiol. Cell. Mol. Physiol. 2016, 311, L1245–L1258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, G.; Begum, R.; Thota, S.; Batra, S. A systematic review of smoking-related epigenetic alterations. Arch. Toxicol. 2019, 93, 2715–2740. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, F.; Xu, Y.; Wang, B.; Zhao, Y.; Xu, W.; Shi, L.; Lu, X.; Liu, Q. Epithelial-mesenchymal transition and cancer stem cells, mediated by a long non-coding RNA, HOTAIR, are involved in cell malignant transformation induced by cigarette smoke extract. Toxicol. Appl. Pharmacol. 2015, 282, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Rubio, K.; Castillo-Negrete, R.; Barreto, G. Non-coding RNAs and nuclear architecture during epithelial-mesenchymal transition in lung cancer and idiopathic pulmonary fibrosis. Cell. Signal. 2020, 70, 109593. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Luo, F.; Liu, Y.; Liu, X.; Shi, L.; Lu, X.; Liu, Q. Posttranscriptional silencing of the lncRNA MALAT1 by miR-217 inhibits the epithelial–mesenchymal transition via enhancer of zeste homolog 2 in the malignant transformation of HBE cells induced by cigarette smoke extract. Toxicol. Appl. Pharmacol. 2015, 289, 276–285. [Google Scholar] [CrossRef]
- Hasan, K.; Shahriar, A.; Jim, K.U. Water pollution in Bangladesh and its impact on public health. Heliyon 2019, 5, e02145. [Google Scholar] [CrossRef] [Green Version]
- Kurwadkar, S. Occurrence and distribution of organic and inorganic pollutants in groundwater. Water Environ. Res. 2019, 91, 1001–1008. [Google Scholar] [CrossRef] [Green Version]
- Tracy, J.W.; Guo, A.; Liang, K.; Bartram, J.; Fisher, M. Sources of and Solutions to Toxic Metal and Metalloid Contamination in Small Rural Drinking Water Systems: A Rapid Review. Int. J. Environ. Res. Public Health 2020, 17, 7076. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.B.; Guo, A.Z.; Tracy, J.W.; Prasad, S.K.; Cronk, R.D.; Browning, E.G.; Liang, K.R.; Kelly, E.R.; Bartram, J.K. Occurrence of Lead and Other Toxic Metals Derived from Drinking-Water Systems in Three West African Countries. Environ. Health Perspect. 2021, 129, 47012. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Huang, Z.; Wang, L.; Lin, Z.; Cao, Z.; Li, X.; Zhang, F.; Wang, H.; Li, Y.; Ma, X. Malignant Transformation of Human Bronchial Epithelial Cells Induced by Arsenic through STAT3/miR-301a/SMAD4 Loop. Sci. Rep. 2018, 8, 13291. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wu, D.; Zhao, L.; Yang, Y.; Ding, J.; Dong, L.; Hu, L.; Wang, F.; Zhao, X.; Cai, Y.; et al. Arsenic Trioxide Reduces Global Histone H4 Acetylation at Lysine 16 through Direct Binding to Histone Acetyltransferase hMOF in Human Cells. PLoS ONE 2015, 10, e0141014. [Google Scholar] [CrossRef]
- Pournara, A.; Kippler, M.; Holmlund, T.; Ceder, R.; Grafström, R.; Vahter, M.; Broberg, K.; Wallberg, A.E. Arsenic alters global histone modifications in lymphocytes in vitro and in vivo. Cell Biol. Toxicol. 2016, 32, 275–284. [Google Scholar] [CrossRef]
- Yuan, D.; Ye, S.; Pan, Y.; Bao, Y.; Chen, H.; Shao, C. Long-term cadmium exposure leads to the enhancement of lymphocyte proliferation via down-regulating p16 by DNA hypermethylation. Mutat. Res. Toxicol. Environ. Mutagen. 2013, 757, 125–131. [Google Scholar] [CrossRef]
- Gao, M.; Chen, M.; Li, C.; Xu, M.; Liu, Y.; Cong, M.; Sang, N.; Liu, S. Long non-coding RNA MT1DP shunts the cellular defense to cytotoxicity through crosstalk with MT1H and RhoC in cadmium stress. Cell Discov. 2018, 4, 1–19. [Google Scholar] [CrossRef]
- Zhou, Z.-H.; Lei, Y.-X.; Wang, C.-X. Analysis of Aberrant Methylation in DNA Repair Genes During Malignant Transformation of Human Bronchial Epithelial Cells Induced by Cadmium. Toxicol. Sci. 2011, 125, 412–417. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Kluz, T.; Fang, L.; Zhang, X.; Sun, H.; Jin, C.; Costa, M. Hexavalent Chromium (Cr(VI)) Down-Regulates Acetylation of Histone H4 at Lysine 16 through Induction of Stressor Protein Nupr1. PLoS ONE 2016, 11, e0157317. [Google Scholar] [CrossRef] [Green Version]
- Tsuboi, M.; Kondo, K.; Soejima, S.; Kajiura, K.; Kawakita, N.; Toba, H.; Kawakami, Y.; Yoshida, M.; Takizawa, H.; Tangoku, A. Chromate exposure induces DNA hypermethylation of the mismatch repair gene MLH1 in lung cancer. Mol. Carcinog. 2019, 59, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Zamudio, R.; Ha, H.C. Environmental epigenetics in metal exposure. Epigenetics 2011, 6, 820–827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jose, C.C.; Wang, Z.; Tanwar, V.S.; Zhang, X.; Zang, C.; Cuddapah, S. Nickel-induced transcriptional changes persist post exposure through epigenetic reprogramming. Epigenetics Chromatin 2019, 12, 1–15. [Google Scholar] [CrossRef]
- Long, P.; Wang, Q.; Zhang, Y.; Zhu, X.; Yu, K.; Jiang, H.; Liu, X.; Zhou, M.; Yuan, Y.; Liu, K.; et al. Profile of copper-associated DNA methylation and its association with incident acute coronary syndrome. Clin. Epigenetics 2021, 13, 1–12. [Google Scholar] [CrossRef]
- A Salas, L.; Bustamante, M.; Gonzalez, J.R.; Gracia-Lavedan, E.; Moreno, V.; Kogevinas, M.; Villanueva, C.M. DNA methylation levels and long-term trihalomethane exposure in drinking water: An epigenome-wide association study. Epigenetics 2015, 10, 650–661. [Google Scholar] [CrossRef] [Green Version]
- Villanueva, C.M.; Cordier, S.; Font-Ribera, L.; Salas, L.A.; Levallois, P. Overview of Disinfection By-products and Associated Health Effects. Curr. Environ. Health Rep. 2015, 2, 107–115. [Google Scholar] [CrossRef] [Green Version]
- Tiffon, C. The Impact of Nutrition and Environmental Epigenetics on Human Health and Disease. Int. J. Mol. Sci. 2018, 19, 3425. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Fang, M. Nutritional and Environmental Contaminant Exposure: A Tale of Two Co-Existing Factors for Disease Risks. Environ. Sci. Technol. 2020, 54, 14793–14796. [Google Scholar] [CrossRef]
- Hodjat, M.; Rahmani, S.; Khan, F.; Niaz, K.; Navaei-Nigjeh, M.; Nejad, S.M.; Abdollahi, M. Environmental toxicants, incidence of degenerative diseases, and therapies from the epigenetic point of view. Arch. Toxicol. 2017, 91, 2577–2597. [Google Scholar] [CrossRef]
- Saigenji, K. [Tests required for the diagnosis of gastric and duodenal ulcer and their significance]. Nursing Tech. 1988, 34, 858–859. [Google Scholar]
- Devaux, C.A.; Raoult, D. The Microbiological Memory, an Epigenetic Regulator Governing the Balance Between Good Health and Metabolic Disorders. Front. Microbiol. 2018, 9, 1379. [Google Scholar] [CrossRef] [PubMed]
- Woo, V.; Alenghat, T. Host–microbiota interactions: Epigenomic regulation. Curr. Opin. Immunol. 2017, 44, 52–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woźniak, E.; Reszka, E.; Jablonska, E.; Balcerczyk, A.; Broncel, M.; Bukowska, B. Glyphosate affects methylation in the promoter regions of selected tumor suppressors as well as expression of major cell cycle and apoptosis drivers in PBMCs (in vitro study). Toxicol. Vitr. 2019, 63, 104736. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, E.; Reszka, E.; Jabłońska, E.; Michałowicz, J.; Huras, B.; Bukowska, B. Glyphosate and AMPA Induce Alterations in Expression of Genes Involved in Chromatin Architecture in Human Peripheral Blood Mononuclear Cells (In Vitro). Int. J. Mol. Sci. 2021, 22, 2966. [Google Scholar] [CrossRef] [PubMed]
- Sharavanan, V.J.; Sivaramakrishnan, M.; Sivarajasekar, N.; Senthilrani, N.; Kothandan, R.; Dhakal, N.; Sivamani, S.; Show, P.L.; Awual, R.; Naushad, M. Pollutants inducing epigenetic changes and diseases. Environ. Chem. Lett. 2019, 18, 325–343. [Google Scholar] [CrossRef]
- De Gannes, M.; Ko, C.-I.; Zhang, X.; Biesiada, J.; Niu, L.; E Koch, S.; Medvedovic, M.; Rubinstein, J.; Puga, A. Dioxin Disrupts Dynamic DNA Methylation Patterns in Genes That Govern Cardiomyocyte Maturation. Toxicol. Sci. 2020, 178, 325–337. [Google Scholar] [CrossRef]
- Cimmino, I.; Fiory, F.; Perruolo, G.; Miele, C.; Beguinot, F.; Formisano, P.; Oriente, F. Potential Mechanisms of Bisphenol A (BPA) Contributing to Human Disease. Int. J. Mol. Sci. 2020, 21, 5761. [Google Scholar] [CrossRef]
- Greathouse, K.L.; Bredfeldt, T.; I Everitt, J.; Lin, K.; Berry, T.; Kannan, K.; Mittelstadt, M.L.; Ho, S.-M.; Walker, C.L. Environmental Estrogens Differentially Engage the Histone Methyltransferase EZH2 to Increase Risk of Uterine Tumorigenesis. Mol. Cancer Res. 2012, 10, 546–557. [Google Scholar] [CrossRef] [Green Version]
- Chou, C.; Huang, H.; Yang, C.; Dahms, H.; Liang, S.; Wang, T.; Kuo, P.; Hsi, E.; Tsai, E.; Chiu, C. Reduced camptothecin sensitivity of estrogen receptor-positive human breast cancer cells following exposure to di(2-ethylhexyl)phthalate (DEHP) is associated with DNA methylation changes. Environ. Toxicol. 2019, 34, 401–414. [Google Scholar] [CrossRef]
- Shahidehnia, M. Epigenetic Effects of Endocrine Disrupting Chemicals. J. Environ. Anal. Toxicol. 2016, 6, 4. [Google Scholar] [CrossRef]
- Sonkar, R.; Powell, C.A.; Choudhury, M. Benzyl butyl phthalate induces epigenetic stress to enhance adipogenesis in mesenchymal stem cells. Mol. Cell. Endocrinol. 2016, 431, 109–122. [Google Scholar] [CrossRef]
- Li, D.; Suh, S. Health risks of chemicals in consumer products: A review. Environ. Int. 2019, 123, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Xie, H. Nanoparticles in Daily Life: Applications, Toxicity and Regulations. J. Environ. Pathol. Toxicol. Oncol. 2018, 37, 209–230. [Google Scholar] [CrossRef] [PubMed]
- Bleeker, E.A.; de Jong, W.H.; Geertsma, R.; Groenewold, M.; Heugens, E.H.; Koers-Jacquemijns, M.; van de Meent, D.; Popma, J.R.; Rietveld, A.G.; Wijnhoven, S.W.; et al. Considerations on the EU definition of a nanomaterial: Science to support policy making. Regul. Toxicol. Pharmacol. 2013, 65, 119–125. [Google Scholar] [CrossRef]
- Pogribna, M.; Hammons, G. Epigenetic Effects of Nanomaterials and Nanoparticles. J. Nanobiotechnol. 2021, 19, 1–18. [Google Scholar] [CrossRef]
- Gedda, M.R.; Babele, P.K.; Zahra, K.; Madhukar, P. Epigenetic Aspects of Engineered Nanomaterials: Is the Collateral Damage Inevitable? Front. Bioeng. Biotechnol. 2019, 7, 228. [Google Scholar] [CrossRef]
- Shyamasundar, S.; Ng, C.T.; Yung, L.Y.L.; Dheen, S.T.; Bay, B.H. Epigenetic mechanisms in nanomaterial-induced toxicity. Epigenomics 2015, 7, 395–411. [Google Scholar] [CrossRef]
- Sierra, M.I.; Valdés, A.; Fernández, A.; Torrecillas, R.; Fraga, M.F. The effect of exposure to nanoparticles and nanomaterials on the mammalian epigenome. Int. J. Nanomed. 2016, 11, 6297–6306. [Google Scholar] [CrossRef] [Green Version]
- Patil, N.A.; Gade, W.N.; Deobagkar, D.D. Epigenetic modulation upon exposure of lung fibroblasts to TiO2 and ZnO nanoparticles: Alterations in DNA methylation. Int. J. Nanomed. 2016, 11, 4509–4519. [Google Scholar] [CrossRef] [Green Version]
- Black, J.C.; Van Rechem, C.; Whetstine, J.R. Histone Lysine Methylation Dynamics: Establishment, Regulation, and Biological Impact. Mol. Cell 2012, 48, 491–507. [Google Scholar] [CrossRef] [Green Version]
- Valdiglesias, V.; Giunta, S.; Fenech, M.; Neri, M.; Bonassi, S. γH2AX as a marker of DNA double strand breaks and genomic instability in human population studies. Mutat. Res. Mutat. Res. 2013, 753, 24–40. [Google Scholar] [CrossRef] [PubMed]
- Kopp, B.; Dario, M.; Zalko, D.; Audebert, M. Assessment of a panel of cellular biomarkers and the kinetics of their induction in comparing genotoxic modes of action in HepG2 cells. Environ. Mol. Mutagen. 2018, 59, 516–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dobersch, S.; Rubio, K.; Singh, I.; Günther, S.; Graumann, J.; Cordero, J.; Castillo-Negrete, R.; Huynh, M.B.; Mehta, A.; Braubach, P.; et al. Positioning of nucleosomes containing γ-H2AX precedes active DNA demethylation and transcription initiation. Nat. Commun. 2021, 12, 1–20. [Google Scholar] [CrossRef]
- Ndika, J.; Karisola, P.; Kinaret, P.; Ilves, M.; Alenius, H. Profiling Non-Coding RNA Changes Associated with 16 Different Engineered Nanomaterials in a Mouse Airway Exposure Model. Cells 2021, 10, 1085. [Google Scholar] [CrossRef] [PubMed]
- Pogribna, M.; Koonce, N.A.; Mathew, A.; Word, B.; Patri, A.K.; Lyn-Cook, B.; Hammons, G. Effect of titanium dioxide nanoparticles on DNA methylation in multiple human cell lines. Nanotoxicology 2020, 14, 534–553. [Google Scholar] [CrossRef] [PubMed]
- Enea, M.; Pereira, E.; Costa, J.; Soares, M.E.; da Silva, D.D.; Bastos, M.D.L.; Carmo, H.F. Cellular uptake and toxicity of gold nanoparticles on two distinct hepatic cell models. Toxicol. Vitr. 2020, 70, 105046. [Google Scholar] [CrossRef]
- Ng, C.-T.; Dheen, S.T.; Yip, W.-C.G.; Ong, C.N.; Bay, B.-H.; Yung, L.-Y.L. The induction of epigenetic regulation of PROS1 gene in lung fibroblasts by gold nanoparticles and implications for potential lung injury. Biomaterials 2011, 32, 7609–7615. [Google Scholar] [CrossRef] [PubMed]
- Brzóska, K.; Grądzka, I.; Kruszewski, M. Silver, Gold, and Iron Oxide Nanoparticles Alter miRNA Expression but Do Not Affect DNA Methylation in HepG2 Cells. Materials 2019, 12, 1038. [Google Scholar] [CrossRef] [Green Version]
- Abdullah, A.S.; Sayed, I.E.T.E.; El-Torgoman, A.M.A.; Alghamdi, N.A.; Ullah, S.; Wageh, S.; Kamel, M.A. Preparation and Characterization of Silymarin-Conjugated Gold Nanoparticles with Enhanced Anti-Fibrotic Therapeutic Effects against Hepatic Fibrosis in Rats: Role of MicroRNAs as Molecular Targets. Biomedicines 2021, 9, 1767. [Google Scholar] [CrossRef]
- Chen, L.; Liu, J.; Zhang, Y.; Zhang, G.; Kang, Y.; Chen, A.; Feng, X.; Shao, L. The toxicity of silica nanoparticles to the immune system. Nanomedicine 2018, 13, 1939–1962. [Google Scholar] [CrossRef] [Green Version]
- Seidel, C.; Kirsch, A.; Fontana, C.; Visvikis, A.; Remy, A.; Gaté, L.; Darne, C.; Guichard, Y. Epigenetic changes in the early stage of silica-induced cell transformation. Nanotoxicology 2017, 11, 923–935. [Google Scholar] [CrossRef] [Green Version]
- Dusinska, M.; Tulinska, J.; El Yamani, N.; Kuricova, M.; Liskova, A.; Rollerova, E.; Rundén-Pran, E.; Smolkova, B. Immunotoxicity, genotoxicity and epigenetic toxicity of nanomaterials: New strategies for toxicity testing? Food Chem. Toxicol. 2017, 109, 797–811. [Google Scholar] [CrossRef] [PubMed]
- Koedrith, P.; Rahman, M.; Jang, Y.J.; Shin, D.Y.; Seo, Y.R. Nanoparticles: Weighing the Pros and Cons from an Eco-genotoxicological Perspective. J. Cancer Prev. 2021, 26, 83–97. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Fan, C.-Q.; Dong, H.; Wang, S.-M.; Yang, X.-C.; Yang, S.-M. Current applications and future prospects of nanomaterials in tumor therapy. Int. J. Nanomed. 2017, 12, 1815–1825. [Google Scholar] [CrossRef] [Green Version]
- Han, J.; Zhao, D.; Li, D.; Wang, X.; Jin, Z.; Zhao, K. Polymer-Based Nanomaterials and Applications for Vaccines and Drugs. Polymers 2018, 10, 31. [Google Scholar] [CrossRef] [Green Version]
- Hueso, M.; Mallén, A.; Suñé-Pou, M.; Aran, J.M.; Suñé-Negre, J.M.; Navarro, E. ncRNAs in Therapeutics: Challenges and Limitations in Nucleic Acid-Based Drug Delivery. Int. J. Mol. Sci. 2021, 22, 11596. [Google Scholar] [CrossRef]
- Winkle, M.; El-Daly, S.M.; Fabbri, M.; Calin, G.A. Noncoding RNA therapeutics — challenges and potential solutions. Nat. Rev. Drug Discov. 2021, 20, 629–651. [Google Scholar] [CrossRef]
- Ghadimi, M.; Zangenehtabar, S.; Homaeigohar, S. An Overview of the Water Remediation Potential of Nanomaterials and Their Ecotoxicological Impacts. Water 2020, 12, 1150. [Google Scholar] [CrossRef] [Green Version]
- Sun, T.Y.; Bornhöft, N.A.; Hungerbuehler, K.; Nowack, B. Dynamic Probabilistic Modeling of Environmental Emissions of Engineered Nanomaterials. Environ. Sci. Technol. 2016, 50, 4701–4711. [Google Scholar] [CrossRef]
- Majumder, A.; Gupta, B.; Gupta, A.K.; Majumder, A.; Gupta, B.; Gupta, A.K. Pharmaceutically active compounds in aqueous environment: A status, toxicity and insights of remediation. Environ. Res. 2019, 176, 108542. [Google Scholar] [CrossRef]
- Song, X.; Wang, X.; Bhandari, R.K. Developmental abnormalities and epigenetic alterations in medaka (Oryzias latipes) embryos induced by triclosan exposure. Chemosphere 2020, 261, 127613. [Google Scholar] [CrossRef] [PubMed]
- Guyon, A.; Smith, K.F.; Charry, M.P.; Champeau, O.; Tremblay, L.A. Effects of Chronic Exposure to Benzophenone and Diclofenac on DNA Methylation Levels and Reproductive Success in a Marine Copepod. J. Xenobiotics 2018, 8, 1–3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guillette, L.J.; Parrott, B.B.; Nilsson, E.; Haque, M.; Skinner, M.K. Epigenetic programming alterations in alligators from environmentally contaminated lakes. Gen. Comp. Endocrinol. 2016, 238, 4–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manikkam, M.; Tracey, R.; Guerrero-Bosagna, C.; Skinner, M.K. Plastics Derived Endocrine Disruptors (BPA, DEHP and DBP) Induce Epigenetic Transgenerational Inheritance of Obesity, Reproductive Disease and Sperm Epimutations. PLoS ONE 2013, 8, e55387. [Google Scholar] [CrossRef] [PubMed]
- Vilahur, N.; Bustamante, M.; Byun, H.-M.; Fernandez, M.F.; Marina, L.S.; Basterrechea, M.; Ballester, F.; Murcia, M.; Tardón, A.; Fernández-Somoano, A.; et al. Prenatal exposure to mixtures of xenoestrogens and repetitive element DNA methylation changes in human placenta. Environ. Int. 2014, 71, 81–87. [Google Scholar] [CrossRef] [Green Version]
- Warita, K.; Mitsuhashi, T.; Sugawara, T.; Tabuchi, Y.; Tanida, T.; Wang, Z.-Y.; Matsumoto, Y.; Yokoyama, T.; Kitagawa, H.; Miki, T.; et al. Direct effects of diethylstilbestrol on the gene expression of the cholesterol side-chain cleavage enzyme (P450scc) in testicular Leydig cells. Life Sci. 2010, 87, 281–285. [Google Scholar] [CrossRef]
- Meng, F.; Jiao, X.; Chen, F.; Zhang, X.; Duan, Z.; Ding, Z.; Wu, D.; Wang, Y.; Zhang, S.; Miao, Y.; et al. Isobutylparaben Negatively Affects Porcine Oocyte Maturation Through Increasing Oxidative Stress and Cytoskeletal Abnormalities. Environ. Mol. Mutagen. 2020, 61, 433–444. [Google Scholar] [CrossRef]
- Leppert, B.; Strunz, S.; Seiwert, B.; Schlittenbauer, L.; Schlichting, R.; Pfeiffer, C.; Röder, S.; Bauer, M.; Borte, M.; Stangl, G.I.; et al. Maternal paraben exposure triggers childhood overweight development. Nat. Commun. 2020, 11, 1–12. [Google Scholar] [CrossRef]
- Silvestre, A.L.P.; Milani, M.I.; Rossini, E.L.; Pezza, L.; Pezza, H.R. A paper platform for colorimetric determination of aluminum hydrochloride in antiperspirant samples. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 204, 432–435. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Zhang, D.-D.; Wang, C.-Q.; Shi, M.; Wang, L.-L. Protective effects of low-intensity pulsed ultrasound on aluminum overload-induced cerebral damage through epigenetic regulation of brain-derived neurotrophic factor expression. Biosci. Rep. 2019, 39, BSR20181185. [Google Scholar] [CrossRef] [Green Version]
- Matozzo, V.; Fabrello, J.; Masiero, L.; Ferraccioli, F.; Finos, L.; Pastore, P.; Di Gangi, I.M.; Bogialli, S. Ecotoxicological risk assessment for the herbicide glyphosate to non-target aquatic species: A case study with the mussel Mytilus galloprovincialis. Environ. Pollut. 2018, 233, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Espinoza-Montero, P.J.; Vega-Verduga, C.; Alulema-Pullupaxi, P.; Fernández, L.; Paz, J.L. Technologies Employed in the Treatment of Water Contaminated with Glyphosate: A Review. Molecules 2020, 25, 5550. [Google Scholar] [CrossRef] [PubMed]
- Zouaoui, K.; Dulaurent, S.; Gaulier, J.-M.; Moesch, C.; Lachâtre, G. Determination of glyphosate and AMPA in blood and urine from humans: About 13 cases of acute intoxication. Forensic Sci. Int. 2013, 226, e20–e25. [Google Scholar] [CrossRef] [PubMed]
- Soukup, S.T.; Merz, B.; Bub, A.; Hoffmann, I.; Watzl, B.; Steinberg, P.; Kulling, S.E. Glyphosate and AMPA levels in human urine samples and their correlation with food consumption: Results of the cross-sectional KarMeN study in Germany. Arch. Toxicol. 2020, 94, 1575–1584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, Y.; Zhang, Y.; Ni, H.; Gao, J.; Yang, Y.; Xu, W.; Tao, L. Evaluation of the cytotoxic effects of glyphosate herbicides in human liver, lung, and nerve. J. Environ. Sci. Health Part B 2019, 54, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Duforestel, M.; Nadaradjane, A.; Bougras-Cartron, G.; Briand, J.; Olivier, C.; Frenel, J.-S.; Vallette, F.M.; Lelièvre, S.A.; Cartron, P.-F. Glyphosate Primes Mammary Cells for Tumorigenesis by Reprogramming the Epigenome in a TET3-Dependent Manner. Front. Genet. 2019, 10, 885. [Google Scholar] [CrossRef] [Green Version]
- Bechtel, W.; McGoohan, S.; Zeisberg, E.M.; Müller, G.A.; Kalbacher, H.; Salant, D.J.; Müller, C.A.; Kalluri, R.; Zeisberg, M. Methylation determines fibroblast activation and fibrogenesis in the kidney. Nat. Med. 2010, 16, 544–550. [Google Scholar] [CrossRef] [Green Version]
- Negreros, M.; Hagood, J.S.; Espinoza, C.R.; Balderas-Martinez, Y.I.; Selman, M.; Pardo, A. Transforming growth factor beta 1 induces methylation changes in lung fibroblasts. PLoS ONE 2019, 14, e0223512. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Zhu, J.; Wang, W.; Ruan, P.; Rajeshkumar, S.; Li, X. Biochemical and molecular impacts of glyphosate-based herbicide on the gills of common carp. Environ. Pollut. 2019, 252, 1288–1300. [Google Scholar] [CrossRef]
- Zhu, Y.; Costa, M. Metals and molecular carcinogenesis. Carcinogenesis 2020, 41, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Li, Q.; Arita, A.; Sun, H.; Costa, M. Effects of nickel, chromate, and arsenite on histone 3 lysine methylation. Toxicol. Appl. Pharmacol. 2009, 236, 78–84. [Google Scholar] [CrossRef] [Green Version]
- Zoroddu, M.A.; Peana, M.; Medici, S.; Casella, L.; Monzani, E.; Costa, M. Nickel binding to histone H4. Dalton Trans. 2009, 39, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Mo, Y.; Zhang, Y.; Wan, R.; Jiang, M.; Xu, Y.; Zhang, Q. miR-21 mediates nickel nanoparticle-induced pulmonary injury and fibrosis. Nanotoxicology 2020, 14, 1175–1197. [Google Scholar] [CrossRef] [PubMed]
- Mo, Y.; Zhang, Y.; Mo, L.; Wan, R.; Jiang, M.; Zhang, Q. The role of miR-21 in nickel nanoparticle-induced MMP-2 and MMP-9 production in mouse primary monocytes: In vitro and in vivo studies. Environ. Pollut. 2020, 267, 115597. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Kluz, T.; Zhang, R.; Costa, M. Hypoxia and nickel inhibit histone demethylase JMJD1A and repress Spry2 expression in human bronchial epithelial BEAS-2B cells. Carcinogenesis 2010, 31, 2136–2144. [Google Scholar] [CrossRef] [PubMed]
- Sutterlüty, H.; Mayer, C.-E.; Setinek, U.; Attems, J.; Ovtcharov, S.; Mikula, M.; Mikulits, W.; Micksche, M.; Berger, W. Down-Regulation of Sprouty2 in Non–Small Cell Lung Cancer Contributes to Tumor Malignancy via Extracellular Signal-Regulated Kinase Pathway-Dependent and -Independent Mechanisms. Mol. Cancer Res. 2007, 5, 509–520. [Google Scholar] [CrossRef] [Green Version]
- Jose, C.C.; Jagannathan, L.; Tanwar, V.S.; Zhang, X.; Zang, C.; Cuddapah, S. Nickel exposure induces persistent mesenchymal phenotype in human lung epithelial cells through epigenetic activation of ZEB1. Mol. Carcinog. 2018, 57, 794–806. [Google Scholar] [CrossRef]
- Zhan, H.; Chang, X.; Wang, X.; Yang, M.; Gao, Q.; Liu, H.; Li, C.; Li, S.; Sun, Y. LncRNA MEG3 mediates nickel oxide nanoparticles-induced pulmonary fibrosis via suppressing TGF-β1 expression and epithelial-mesenchymal transition process. Environ. Toxicol. 2021, 36, 1099–1110. [Google Scholar] [CrossRef]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The Effects of Cadmium Toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef]
- Gu, S.; Dai, J.; Qu, T.; He, Z. Emerging Roles of MicroRNAs and Long Noncoding RNAs in Cadmium Toxicity. Biol. Trace Element Res. 2019, 195, 481–490. [Google Scholar] [CrossRef]
- Ngalame, N.N.O.; Waalkes, M.P.; Tokar, E.J. Silencing KRAS Overexpression in Cadmium-Transformed Prostate Epithelial Cells Mitigates Malignant Phenotype. Chem. Res. Toxicol. 2016, 29, 1458–1467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fay, M.J.; Alt, L.A.C.; Ryba, D.; Salamah, R.; Peach, R.; Papaeliou, A.; Zawadzka, S.; Weiss, A.; Patel, N.; Rahman, A.; et al. Cadmium Nephrotoxicity Is Associated with Altered MicroRNA Expression in the Rat Renal Cortex. Toxics 2018, 6, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.; Zheng, C.; Shen, H.; Zhou, Z.; Lei, Y. MicroRNAs-mRNAs Expression Profile and Their Potential Role in Malignant Transformation of Human Bronchial Epithelial Cells Induced by Cadmium. BioMed Res. Int. 2015, 2015, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hassan, F.; Nuovo, G.J.; Crawford, M.; Boyaka, P.; Kirkby, S.; Nana-Sinkam, S.P.; Cormet-Boyaka, E. MiR-101 and miR-144 Regulate the Expression of the CFTR Chloride Channel in the Lung. PLoS ONE 2012, 7, e50837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Z.; Liu, H.; Wang, C.; Lu, Q.; Huang, Q.; Zheng, C.; Lei, Y. Long non-coding RNAs as novel expression signatures modulate DNA damage and repair in cadmium toxicology. Sci. Rep. 2015, 5, srep15293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Q.; Lu, Q.; Chen, B.; Shen, H.; Liu, Q.; Zhou, Z.; Lei, Y. LncRNA-MALAT1 as a novel biomarker of cadmium toxicity regulates cell proliferation and apoptosis. Toxicol. Res. 2017, 6, 361–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fabbri, M. Whole genome analysis and microRNAs regulation in HepG2 cells exposed to cadmium. ALTEX 2012, 29, 173–182. [Google Scholar] [CrossRef]
- Gao, M.; Li, C.; Xu, M.; Liu, Y.; Cong, M.; Liu, S. LncRNA MT1DP Aggravates Cadmium-Induced Oxidative Stress by Repressing the Function of Nrf2 and is Dependent on Interaction with miR-365. Adv. Sci. 2018, 5, 1800087. [Google Scholar] [CrossRef]
- Sun, H.; Brocato, J.; Costa, M. Oral Chromium Exposure and Toxicity. Curr. Environ. Health Rep. 2015, 2, 295–303. [Google Scholar] [CrossRef] [Green Version]
- Stout, M.D.; Herbert, R.A.; Kissling, G.E.; Collins, B.J.; Travlos, G.S.; Witt, K.L.; Melnick, R.L.; Abdo, K.M.; Malarkey, D.E.; Hooth, M.J. Hexavalent Chromium Is Carcinogenic to F344/N Rats and B6C3F1 Mice after Chronic Oral Exposure. Environ. Health Perspect. 2009, 117, 716–722. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.Y.; Murphy, A.; Sun, H.; Costa, M. Molecular and epigenetic mechanisms of Cr(VI)-induced carcinogenesis. Toxicol. Appl. Pharmacol. 2019, 377, 114636. [Google Scholar] [CrossRef] [PubMed]
- Amrani, I.; Haddam, N.; Garat, A.; Allorge, D.; Zerimech, F.; Schraen, S.; Taleb, A.; Merzouk, H.; Edme, J.-L.; Lo-Guidice, J.-M. Exposure to metal fumes and circulating miRNAs in Algerian welders. Int. Arch. Occup. Environ. Health 2019, 93, 553–561. [Google Scholar] [CrossRef]
- Jia, J.; Li, T.; Yao, C.; Chen, J.; Feng, L.; Jiang, Z.; Shi, L.; Liu, J.; Chen, J.; Lou, J. Circulating differential miRNAs profiling and expression in hexavalent chromium exposed electroplating workers. Chemosphere 2020, 260, 127546. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, P.; Yu, S.; Zhang, J.; Wang, T.; Jia, G. miR-3940-5p associated with genetic damage in workers exposed to hexavalent chromium. Toxicol. Lett. 2014, 229, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Qiu, J.-G.; He, J.; Liu, W.-J.; Ge, X.; Zhou, F.-M.; Huang, Y.-X.; Jiang, B.-H.; Liu, L.-Z. Suppression of miR-143 contributes to overexpression of IL-6, HIF-1α and NF-κB p65 in Cr(VI)-induced human exposure and tumor growth. Toxicol. Appl. Pharmacol. 2019, 378, 114603. [Google Scholar] [CrossRef] [PubMed]
- Pratheeshkumar, P.; Son, Y.-O.; Divya, S.P.; Turcios, L.; Roy, R.V.; Hitron, J.A.; Wang, L.; Kim, D.; Dai, J.; Asha, P.; et al. Hexavalent chromium induces malignant transformation of human lung bronchial epithelial cells via ROS-dependent activation of miR-21-PDCD4 signaling. Oncotarget 2016, 7, 51193–51210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, J.; Qian, X.; Carpenter, R.; Xu, Q.; Wang, L.; Qi, Y.; Wang, Z.-X.; Liu, L.-Z.; Jiang, B.-H. Repression of miR-143 Mediates Cr (VI)–Induced Tumor Angiogenesis via IGF-IR/IRS1/ERK/IL-8 Pathway. Toxicol. Sci. 2013, 134, 26–38. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Lin, H.-P.; Li, Y.; Tao, H.; Yang, P.; Xie, J.; Maddy, D.; Kondo, K.; Yang, C. Chronic Hexavalent Chromium Exposure Induces Cancer Stem Cell-Like Property and Tumorigenesis by Increasing c-Myc Expression. Toxicol. Sci. 2019, 172, 252–264. [Google Scholar] [CrossRef]
- Wang, J.; Song, Y.-X.; Ma, B.; Wang, J.-J.; Sun, J.-X.; Chen, X.-W.; Zhao, J.-H.; Yang, Y.-C.; Wang, Z.-N. Regulatory Roles of Non-Coding RNAs in Colorectal Cancer. Int. J. Mol. Sci. 2015, 16, 19886–19919. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.; Gregory, R.I. MicroRNA biogenesis pathways in cancer. Nat. Cancer 2015, 15, 321–333. [Google Scholar] [CrossRef]
- Zhang, B.; Pan, X. RDX Induces Aberrant Expression of MicroRNAs in Mouse Brain and Liver. Environ. Health Perspect. 2009, 117, 231–240. [Google Scholar] [CrossRef]
- Hui, P.; Gysler, S.M.; Uduman, M.; Togun, T.A.; Prado, D.E.; Brambs, C.E.; Nallur, S.; Schwartz, P.E.; Rutherford, T.J.; Santin, A.D.; et al. MicroRNA signatures discriminate between uterine and ovarian serous carcinomas. Hum. Pathol. 2018, 76, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Iorio, M.V.; Visone, R.; Di Leva, G.; Donati, V.; Petrocca, F.; Casalini, P.; Taccioli, C.; Volinia, S.; Liu, C.-G.; Alder, H.; et al. MicroRNA Signatures in Human Ovarian Cancer. Cancer Res. 2007, 67, 8699–8707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, M.; Hu, Y.; Gou, R.; Nie, X.; Li, X.; Liu, J.; Lin, B. Identification three LncRNA prognostic signature of ovarian cancer based on genome-wide copy number variation. Biomed. Pharmacother. 2020, 124, 109810. [Google Scholar] [CrossRef] [PubMed]
- Martini, P.; Paracchini, L.; Caratti, G.; Mello-Grand, M.; Fruscio, R.; Beltrame, L.; Calura, E.; Sales, G.; Ravaggi, A.; Bignotti, E.; et al. lncRNAs as Novel Indicators of Patients’ Prognosis in Stage I Epithelial Ovarian Cancer: A Retrospective and Multicentric Study. Clin. Cancer Res. 2016, 23, 2356–2366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, C.; Zhou, J.-Q.; Liao, Y.-S. Autophagy-related long non-coding RNA signature for ovarian cancer. J. Int. Med Res. 2020, 48, 300060520970761. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, H.; Zhang, M.; Xing, L.; Yang, C.; Xia, B.; Lou, G. Integrated analysis of a competing endogenous RNA network reveals an 11-lncRNA prognostic signature in ovarian cancer. Aging 2020, 12, 25153–25171. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wu, Y.; Che, X.; Zhao, J.; Wang, F.; Wang, P.; Qu, X.; Liu, Y.; Li, Z. Cox-LASSO Analysis Reveals a Ten-lncRNA Signature to Predict Outcomes in Patients with High-Grade Serous Ovarian Cancer. DNA Cell Biol. 2019, 38, 1519–1528. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, Q.; He, J.; Chen, P.; Wan, J.; Li, J.; Yang, Y.; Li, X. Recurrence-Associated Multi-RNA Signature to Predict Disease-Free Survival for Ovarian Cancer Patients. BioMed Res. Int. 2020, 2020, 1618527-19. [Google Scholar] [CrossRef]
- Hong, H.-C.; Chuang, C.-H.; Huang, W.-C.; Weng, S.-L.; Chen, C.-H.; Chang, K.-H.; Liao, K.-W.; Huang, H.-D. A panel of eight microRNAs is a good predictive parameter for triple-negative breast cancer relapse. Theranostics 2020, 10, 8771–8789. [Google Scholar] [CrossRef]
- Yu, X.; Liang, J.; Xu, J.; Li, X.; Xing, S.; Li, H.; Liu, W.; Liu, D.; Xu, J.; Huang, L.; et al. Identification and Validation of Circulating MicroRNA Signatures for Breast Cancer Early Detection Based on Large Scale Tissue-Derived Data. J. Breast Cancer 2018, 21, 363–370. [Google Scholar] [CrossRef]
- Sathipati, S.Y.; Ho, S.-Y. Identifying a miRNA signature for predicting the stage of breast cancer. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Lai, J.; Wang, H.; Pan, Z.; Su, F. A novel six-microRNA-based model to improve prognosis prediction of breast cancer. Aging 2019, 11, 649–662. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Ma, W.; Zeng, Q.; Tan, J.; Cao, K.; Luo, L. Identification of miRNA-Based Signature as a Novel Potential Prognostic Biomarker in Patients with Breast Cancer. Dis. Markers 2019, 2019, 3815952-17. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Zhao, F.; Yu, X.; Guan, S.; Suo, H.; Tao, Z.; Qiu, Y.; Wu, Y.; Cao, Y.; Jin, F. Immune-related lncRNAs as predictors of survival in breast cancer: A prognostic signature. J. Transl. Med. 2020, 18, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, Y.; Wang, X.; Yang, Q. Identification of a Six-Immune-Related Long Non-coding RNA Signature for Predicting Survival and Immune Infiltrating Status in Breast Cancer. Front. Genet. 2020, 11, 680. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Y.; Tian, G.; Jiang, T. Long non-coding RNA-based signatures to improve prognostic prediction of breast cancer. Medicine 2020, 99, e22203. [Google Scholar] [CrossRef]
- Liu, Z.; Li, M.; Hua, Q.; Li, Y.; Wang, G. Identification of an eight-lncRNA prognostic model for breast cancer using WGCNA network analysis and a Cox-proportional hazards model based on L1-penalized estimation. Int. J. Mol. Med. 2019, 44, 1333–1343. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Chen, X.; Liang, Y.; Wang, W.; Fang, Y.; Shen, K. Long Noncoding RNA Signature and Disease Outcome in Estrogen Receptor-Positive Breast Cancer Patients Treated with Tamoxifen. J. Breast Cancer 2018, 21, 277–287. [Google Scholar] [CrossRef]
- Huang, P.; Li, F.; Mo, Z.; Geng, C.; Wen, F.; Zhang, C.; Guo, J.; Wu, S.; Li, L.; Brünner, N.; et al. A Comprehensive RNA Study to Identify circRNA and miRNA Biomarkers for Docetaxel Resistance in Breast Cancer. Front. Oncol. 2021, 11, 669270. [Google Scholar] [CrossRef]
- Zhou, X.; Huang, Z.; Xu, L.; Zhu, M.; Zhang, L.; Zhang, H.; Wang, X.; Li, H.; Zhu, W.; Shu, Y.; et al. A panel of 13-miRNA signature as a potential biomarker for predicting survival in pancreatic cancer. Oncotarget 2016, 7, 69616–69624. [Google Scholar] [CrossRef] [Green Version]
- Liang, L.; Wei, D.; Li, J.; Luo, D.; Chen, G.; Dang, Y.; Cai, X. Prognostic microRNAs and their potential molecular mechanism in pancreatic cancer: A study based on The Cancer Genome Atlas and bioinformatics investigation. Mol. Med. Rep. 2017, 17, 939–951. [Google Scholar] [CrossRef] [Green Version]
- Duell, E.J.; Lujan-Barroso, L.; Sala, N.; McElyea, S.D.; Overvad, K.; Tjonneland, A.; Olsen, A.; Weiderpass, E.; Busund, L.-T.; Moi, L.; et al. Plasma microRNAs as biomarkers of pancreatic cancer risk in a prospective cohort study. Int. J. Cancer 2017, 141, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Vila-Navarro, E.; Duran-Sanchon, S.; Vila-Casadesús, M.; Moreira, L.; Ginès, À.; Cuatrecasas, M.; Lozano, J.J.; Bujanda, L.; Castells, A.; Gironella, M. Novel Circulating miRNA Signatures for Early Detection of Pancreatic Neoplasia. Clin. Transl. Gastroenterol. 2019, 10, e00029. [Google Scholar] [CrossRef] [PubMed]
- Shams, R.; Saberi, S.; Zali, M.; Sadeghi, A.; Ghafouri-Fard, S.; Aghdaei, H.A. Identification of potential microRNA panels for pancreatic cancer diagnosis using microarray datasets and bioinformatics methods. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Wang, S.; Zhou, Q.; Zhao, J.; Xia, X.; Chen, W.; Zheng, Y.; Xue, M.; Yang, F.; Fu, D.; et al. A Long Non-coding RNA Signature to Improve Prognostic Prediction of Pancreatic Ductal Adenocarcinoma. Front. Oncol. 2019, 9, 1160. [Google Scholar] [CrossRef] [Green Version]
- Shi, X.; Zhao, Y.; He, R.; Zhou, M.; Pan, S.; Yu, S.; Xie, Y.; Li, X.; Wang, M.; Guo, X.; et al. Three-lncRNA signature is a potential prognostic biomarker for pancreatic adenocarcinoma. Oncotarget 2018, 9, 24248–24259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.; Pan, X.; Chen, Z.; Lin, S.; Chen, S. Identification of an Immune-Related Nine-lncRNA Signature Predictive of Overall Survival in Colon Cancer. Front. Genet. 2020, 11, 318. [Google Scholar] [CrossRef]
- Pichler, M.; Stiegelbauer, V.; Vychytilova-Faltejskova, P.; Ivan, C.; Ling, H.; Winter, E.; Zhang, X.; Goblirsch, M.; Wulf-Goldenberg, A.; Ohtsuka, M.; et al. Genome-Wide miRNA Analysis Identifies miR-188-3p as a Novel Prognostic Marker and Molecular Factor Involved in Colorectal Carcinogenesis. Clin. Cancer Res. 2016, 23, 1323–1333. [Google Scholar] [CrossRef] [Green Version]
- Shen, L.; Chen, L.; Wang, Y.; Jiang, X.; Xia, H.; Zhuang, Z. Long noncoding RNA MALAT1 promotes brain metastasis by inducing epithelial-mesenchymal transition in lung cancer. J. Neuro-Oncol. 2014, 121, 101–108. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Zhang, G.; Lu, C.; Chu, H.; Yang, R.; Zhao, G. MALAT1/miR-101-3p/MCL1 axis mediates cisplatin resistance in lung cancer. Oncotarget 2017, 9, 7501–7512. [Google Scholar] [CrossRef] [Green Version]
- Ghafouri-Fard, S.; Shoorei, H.; Anamag, F.T.; Taheri, M. The Role of Non-Coding RNAs in Controlling Cell Cycle Related Proteins in Cancer Cells. Front. Oncol. 2020, 10, 608975. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Yang, Y.-E.; Jin, J.; Zhang, M.-Y.; Liu, X.; Yin, Y.-H.; Qu, Y.-Q. Identification of lncRNA biomarkers in lung squamous cell carcinoma using comprehensive analysis of lncRNA mediated ceRNA network. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3246–3258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, W.; Yang, L.; Wang, Y.; Xian, J.; Qiu, F.; Liu, L.; Lin, M.; Feng, Y.; Zhou, Y.; Lu, J. Analysis of Survival-Related lncRNA Landscape Identifies A Role for LINC01537 in Energy Metabolism and Lung Cancer Progression. Int. J. Mol. Sci. 2019, 20, 3713. [Google Scholar] [CrossRef] [Green Version]
- Acha-Sagredo, A.; Uko, B.; Pantazi, P.; Bediaga, N.G.; Moschandrea, C.; Rainbow, L.; Marcus, M.W.; Davies, M.P.A.; Field, J.K.; Liloglou, T. Long non-coding RNA dysregulation is a frequent event in non-small cell lung carcinoma pathogenesis. Br. J. Cancer 2020, 122, 1050–1058. [Google Scholar] [CrossRef]
- Liu, H.; Ye, T.; Yang, X.; Lv, P.; Wu, X.; Zhou, H.; Zeng, J.; Tang, K.; Ye, Z. A Panel of Four-lncRNA Signature as a Potential Biomarker for Predicting Survival in Clear Cell Renal Cell Carcinoma. J. Cancer 2020, 11, 4274–4283. [Google Scholar] [CrossRef] [PubMed]
- Ghafouri-Fard, S.; Shirvani-Farsani, Z.; Branicki, W.; Taheri, M. MicroRNA Signature in Renal Cell Carcinoma. Front. Oncol. 2020, 10, 596359. [Google Scholar] [CrossRef]
- Youssef, Y.M.; White, N.M.; Grigull, J.; Krizova, A.; Samy, C.; Mejia-Guerrero, S.; Evans, A.; Yousef, G.M. Accurate Molecular Classification of Kidney Cancer Subtypes Using MicroRNA Signature. Eur. Urol. 2011, 59, 721–730. [Google Scholar] [CrossRef]
- Rodriguez, P.; Paculova, H.; Kogut, S.; Heath, J.; Schjerven, H.; Frietze, S. Non-Coding RNA Signatures of B-Cell Acute Lymphoblastic Leukemia. Int. J. Mol. Sci. 2021, 22, 2683. [Google Scholar] [CrossRef]
- Fernando, T.R.; Rodriguez-Malave, N.I.; Waters, E.V.; Yan, W.; Casero, D.; Basso, G.; Pigazzi, M.; Rao, D.S. LncRNA Expression Discriminates Karyotype and Predicts Survival in B-Lymphoblastic Leukemia. Mol. Cancer Res. 2015, 13, 839–851. [Google Scholar] [CrossRef] [Green Version]
- Rawoof, A.; Swaminathan, G.; Tiwari, S.; A Nair, R.; Kumar, L.D. LeukmiR: A database for miRNAs and their targets in acute lymphoblastic leukemia. Database 2020, 2020, baz151. [Google Scholar] [CrossRef] [PubMed]
- Mi, S.; Lu, J.; Sun, M.; Li, Z.; Zhang, H.; Neilly, M.B.; Wang, Y.; Qian, Z.; Jin, J.; Zhang, Y.; et al. MicroRNA expression signatures accurately discriminate acute lymphoblastic leukemia from acute myeloid leukemia. Proc. Natl. Acad. Sci. USA 2007, 104, 19971–19976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Luo, X.-Q.; Zhang, P.; Huang, L.-B.; Zheng, Y.-S.; Wu, J.; Zhou, H.; Qu, L.-H.; Xu, L.; Chen, Y.-Q. MicroRNA Patterns Associated with Clinical Prognostic Parameters and CNS Relapse Prediction in Pediatric Acute Leukemia. PLoS ONE 2009, 4, e7826. [Google Scholar] [CrossRef] [PubMed]
- Han, B.-W.; Feng, D.-D.; Li, Z.-G.; Luo, X.-Q.; Zhang, H.; Li, X.-J.; Zhang, X.-J.; Zheng, L.-L.; Zeng, C.-W.; Lin, K.-Y.; et al. A set of miRNAs that involve in the pathways of drug resistance and leukemic stem-cell differentiation is associated with the risk of relapse and glucocorticoid response in childhood ALL. Hum. Mol. Genet. 2011, 20, 4903–4915. [Google Scholar] [CrossRef] [PubMed]
- Avigad, S.; Verly, I.R.; Lebel, A.; Kordi, O.; Shichrur, K.; Ohali, A.; Hameiri-Grossman, M.; Kaspers, G.J.; Cloos, J.; Fronkova, E.; et al. miR expression profiling at diagnosis predicts relapse in pediatric precursor B-cell acute lymphoblastic leukemia. Genes Chromosom. Cancer 2015, 55, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Gaffo, E.; Boldrin, E.; Molin, A.D.; Bresolin, S.; Bonizzato, A.; Trentin, L.; Frasson, C.; Debatin, K.-M.; Meyer, L.H.; Kronnie, G.T.; et al. Circular RNA differential expression in blood cell populations and exploration of circRNA deregulation in pediatric acute lymphoblastic leukemia. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bresesti, C.; Vezzoli, V.; Cangiano, B.; Bonomi, M. Long Non-Coding RNAs: Role in Testicular Cancers. Front. Oncol. 2021, 11, 605606. [Google Scholar] [CrossRef] [PubMed]
- Lobo, J.; Nunes, S.P.; Gillis, A.J.M.; Barros-Silva, D.; Miranda-Gonçalves, V.; Berg, A.V.D.; Cantante, M.; Guimarães, R.; Henrique, R.; Jerónimo, C.; et al. XIST-Promoter Demethylation as Tissue Biomarker for Testicular Germ Cell Tumors and Spermatogenesis Quality. Cancers 2019, 11, 1385. [Google Scholar] [CrossRef] [Green Version]
- Regouc, M.; Belge, G.; Lorch, A.; Dieckmann, K.-P.; Pichler, M. Non-Coding microRNAs as Novel Potential Tumor Markers in Testicular Cancer. Cancers 2020, 12, 749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wentzensen, N.; Wacholder, S. From Differences in Means between Cases and Controls to Risk Stratification: A Business Plan for Biomarker Development. Cancer Discov. 2013, 3, 148–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nava, V.E.; Perera, P.-Y.; Kumar, N.; Jain, M. Noncoding-RNA-Based Therapeutics with an Emphasis on Prostatic Carcinoma—Progress and Challenges. Vaccines 2022, 10, 276. [Google Scholar] [CrossRef]
- Juliano, R.L. The delivery of therapeutic oligonucleotides. Nucleic Acids Res. 2016, 44, 6518–6548. [Google Scholar] [CrossRef] [PubMed]
- Battistelli, C.; Garbo, S.; Riccioni, V.; Montaldo, C.; Santangelo, L.; Vandelli, A.; Strippoli, R.; Tartaglia, G.G.; Tripodi, M.; Cicchini, C. Design and functional validation of a mutant variant of the lncRNA HOTAIR to counteract Snail function in Epithelial-to-Mesenchymal Transition. Cancer Res. 2020, 81, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Egli, M.; Manoharan, M. Re-Engineering RNA Molecules into Therapeutic Agents. Accounts Chem. Res. 2019, 52, 1036–1047. [Google Scholar] [CrossRef]
- Das, S.K.; Menezes, M.E.; Bhatia, S.; Wang, X.-Y.; Emdad, L.; Sarkar, D.; Fisher, P.B. Gene Therapies for Cancer: Strategies, Challenges and Successes. J. Cell. Physiol. 2014, 230, 259–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toden, S.; Zumwalt, T.J.; Goel, A. Non-coding RNAs and potential therapeutic targeting in cancer. Biochim. et Biophys. Acta 2020, 1875, 188491. [Google Scholar] [CrossRef]
- Skinner, M.K. What is an epigenetic transgenerational phenotype?: F3 or F2. Reprod. Toxicol. 2008, 25, 2–6. [Google Scholar] [CrossRef] [Green Version]
- Morris, I.D. Sperm DNA damage and cancer treatment1. Int. J. Androl. 2002, 25, 255–261. [Google Scholar] [CrossRef]
- Hajkova, P.; Erhardt, S.; Lane, N.; Haaf, T.; El-Maarri, O.; Reik, W.; Walter, J.; Surani, M. Epigenetic reprogramming in mouse primordial germ cells. Mech. Dev. 2002, 117, 15–23. [Google Scholar] [CrossRef] [Green Version]
- Reik, W.; Walter, J. Genomic imprinting: Parental influence on the genome. Nat. Rev. Genet. 2001, 2, 21–32. [Google Scholar] [CrossRef]
- Ptashne, M. Epigenetics: Core misconcept. Proc. Natl. Acad. Sci. USA 2013, 110, 7101–7103. [Google Scholar] [CrossRef] [Green Version]
- Waddington, C.H. Canalization of Development and Genetic Assimilation of Acquired Characters. Nature 1959, 183, 1654–1655. [Google Scholar] [CrossRef] [PubMed]
- Anway, M.D.; Cupp, A.S.; Uzumcu, M.; Skinner, M.K. Epigenetic Transgenerational Actions of Endocrine Disruptors and Male Fertility. Science 2005, 308, 1466–1469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, H.D.; Sutherland, H.; Martin, D.I.; Whitelaw, E. Epigenetic inheritance at the agouti locus in the mouse. Nat. Genet. 1999, 23, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Raval, A.; Tanner, S.M.; Byrd, J.C.; Angerman, E.B.; Perko, J.D.; Chen, S.-S.; Hackanson, B.; Grever, M.R.; Lucas, D.M.; Matkovic, J.J.; et al. Downregulation of Death-Associated Protein Kinase 1 (DAPK1) in Chronic Lymphocytic Leukemia. Cell 2007, 129, 879–890. [Google Scholar] [CrossRef] [Green Version]
- Hitchins, M.P.; Rapkins, R.W.; Kwok, C.-T.; Srivastava, S.; Wong, J.; Khachigian, L.; Polly, P.; Goldblatt, J.; Ward, R. Dominantly Inherited Constitutional Epigenetic Silencing of MLH1 in a Cancer-Affected Family Is Linked to a Single Nucleotide Variant within the 5′UTR. Cancer Cell 2011, 20, 200–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daxinger, L.; Whitelaw, E. Understanding transgenerational epigenetic inheritance via the gametes in mammals. Nat. Rev. Genet. 2012, 13, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.P.; Brunet, A. Bridging the transgenerational gap with epigenetic memory. Trends Genet. 2013, 29, 176–186. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, E.E.; Sadler-Riggleman, I.; Skinner, M.K. Environmentally induced epigenetic transgenerational inheritance of disease. Environ. Epigenetics 2018, 4, dvy016. [Google Scholar] [CrossRef] [Green Version]
- Belleannee, C.; Calvo, É.; Caballero, J.; Sullivan, R. Epididymosomes Convey Different Repertoires of MicroRNAs throughout the Bovine Epididymis1. Biol. Reprod. 2013, 89, 30. [Google Scholar] [CrossRef]
- Chamorro-García, R.; Sahu, M.; Abbey, R.J.; Laude, J.; Pham, N.; Blumberg, B. Transgenerational Inheritance of Increased Fat Depot Size, Stem Cell Reprogramming, and Hepatic Steatosis Elicited by Prenatal Exposure to the Obesogen Tributyltin in Mice. Environ. Health Perspect. 2013, 121, 359–366. [Google Scholar] [CrossRef] [Green Version]
- Diaz-Castillo, C.; Chamorro-Garcia, R.; Shioda, T.; Blumberg, B. Transgenerational Self-Reconstruction of Disrupted Chromatin Organization After Exposure To An Environmental Stressor in Mice. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, T.; Tomizawa, S.-I.; Mitsuya, K.; Totoki, Y.; Yamamoto, Y.; Kuramochi-Miyagawa, S.; Iida, N.; Hoki, Y.; Murphy, P.J.; Toyoda, A.; et al. Role for piRNAs and Noncoding RNA in de Novo DNA Methylation of the Imprinted Mouse Rasgrf1 Locus. Science 2011, 332, 848–852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gapp, K.; Jawaid, A.; Sarkies, P.; Bohacek, J.; Pelczar, P.; Prados, J.; Farinelli, L.; Miska, E.; Mansuy, I.M. Implication of sperm RNAs in transgenerational inheritance of the effects of early trauma in mice. Nat. Neurosci. 2014, 17, 667–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grandjean, V.; Fourré, S.; De Abreu, D.A.F.; Derieppe, M.-A.; Remy, J.-J.; Rassoulzadegan, M. RNA-mediated paternal heredity of diet-induced obesity and metabolic disorders. Sci. Rep. 2015, 5, 18193. [Google Scholar] [CrossRef] [Green Version]
- Barbosa, T.D.C.; Ingerslev, L.R.; Alm, P.S.; Versteyhe, S.; Massart, J.; Rasmussen, M.; Donkin, I.; Sjögren, R.; Mudry, J.M.; Vetterli, L.; et al. High-fat diet reprograms the epigenome of rat spermatozoa and transgenerationally affects metabolism of the offspring. Mol. Metab. 2016, 5, 184–197. [Google Scholar] [CrossRef]
- Kawano, M.; Kawaji, H.; Grandjean, V.; Kiani, J.; Rassoulzadegan, M. Novel Small Noncoding RNAs in Mouse Spermatozoa, Zygotes and Early Embryos. PLoS ONE 2012, 7, e44542. [Google Scholar] [CrossRef]
- Rassoulzadegan, M.; Grandjean, V.; Gounon, P.; Vincent, S.; Gillot, I.; Cuzin, F. RNA-mediated non-mendelian inheritance of an epigenetic change in the mouse. Nature 2006, 441, 469–474. [Google Scholar] [CrossRef]
- Rodgers, A.B.; Morgan, C.P.; Leu, N.A.; Bale, T.L. Transgenerational epigenetic programming via sperm microRNA recapitulates effects of paternal stress. Proc. Natl. Acad. Sci. USA 2015, 112, 13699–13704. [Google Scholar] [CrossRef] [Green Version]
- Schuster, A.; Skinner, M.K.; Yan, W. Ancestral vinclozolin exposure alters the epigenetic transgenerational inheritance of sperm small noncoding RNAs. Environ. Epigenetics 2016, 2, dvw001. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Babatola, S.S. Global burden of diseases attributable to air pollution. J. Public Health Afr. 2018, 9, 813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.-M.; Chen, Y.-Q. Principles and innovative technologies for decrypting noncoding RNAs: From discovery and functional prediction to clinical application. J. Hematol. Oncol. 2020, 13, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhou, B.; Chen, L.; Gou, L.-T.; Li, H.; Fu, X.-D. GRID-seq reveals the global RNA–chromatin interactome. Nat. Biotechnol. 2017, 35, 940–950. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, S.; Davis, R.W.; Saha, A.K. Microfluidic Point-of-Care Testing: Commercial Landscape and Future Directions. Front. Bioeng. Biotechnol. 2021, 8, 602659. [Google Scholar] [CrossRef]
- Wang, Y.-M.; Trinh, M.P.; Zheng, Y.; Guo, K.; Jimenez, L.A.; Zhong, W. Analysis of circulating non-coding RNAs in a non-invasive and cost-effective manner. TrAC Trends Anal. Chem. 2019, 117, 242–262. [Google Scholar] [CrossRef]
- Mishra, K.; Kanduri, C. Understanding Long Noncoding RNA and Chromatin Interactions: What We Know So Far. Non-Coding RNA 2019, 5, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agency for Toxic Substances and Disease Registry. ToxFAQsTM for DDT, DDE, and DDD. 2020. Available online: https://wwwn.cdc.gov/TSP/ToxFAQs/ToxFAQsDetails.aspx?faqid=80&toxid=20 (accessed on 10 February 2022).
- Agency for Toxic Substances and Disease Registry. Polychlorinated Biphenyls (PCBs) Toxicity. 2014. Available online: https://www.atsdr.cdc.gov/csem/polychlorinated-biphenyls/adverse_health.html (accessed on 10 February 2022).
- American Cancer Society. Perfluorooctanoic Acid (PFOA), Teflon, and Related Chemicals. 2022. Available online: https://www.cancer.org/cancer/cancer-causes/teflon-and-perfluorooctanoic-acid-pfoa.html (accessed on 10 February 2022).
- Alli, L.A. Blood level of cadmium and lead in occupationally exposed persons in Gwagwalada, Abuja, Nigeria. Interdiscip. Toxicol. 2015, 8, 146–150. [Google Scholar] [CrossRef] [Green Version]
- Alvarado, A.; Blanco, R.; Mora, E. El cromo como elemento esencial en los humanos. Rev. Costarric. De Cienc. Médicas 2002, 23, 55–68. [Google Scholar]
- Anyanwu, B.O.; Orisakwe, O.E. Current mechanistic perspectives on male reproductive toxicity induced by heavy metals. J. Environ. Sci. Health Part C 2020, 38, 204–244. [Google Scholar] [CrossRef]
- Avissar-Whiting, M.; Veiga, K.R.; Uhl, K.M.; Maccani, M.A.; Gagne, L.A.; Moen, E.L.; Marsit, C.J. Bisphenol A exposure leads to specific microRNA alterations in placental cells. Reprod. Toxicol. 2010, 29, 401–406. [Google Scholar] [CrossRef] [Green Version]
- Awadalla, A.; Mortada, W.I.; Abol-Enein, H.; Shokeir, A.A. Correlation between blood levels of cadmium and lead and the expression of microRNA-21 in Egyptian bladder cancer patients. Heliyon 2020, 6, e05642. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.-S.; Ryu, D.-Y.; Choi, B.-S.; Park, J.-D. Urinary Arsenic Concentrations and their Associated Factors in Korean Adults. Toxicol. Res. 2013, 29, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Baker, B.A.; Cassano, V.A.; Murray, C. Arsenic Exposure, Assessment, Toxicity, Diagnosis, and Management. J. Occup. Environ. Med. 2018, 60, e634–e639. [Google Scholar] [CrossRef] [PubMed]
- Beck, R.; Bommarito, P.; Douillet, C.; Kanke, M.; Del Razo, L.M.; García-Vargas, G.; Fry, R.C.; Sethupathy, P.; Stýblo, M. Circulating miRNAs Associated with Arsenic Exposure. Environ. Sci. Technol. 2018, 52, 14487–14495. [Google Scholar] [CrossRef]
- Blockhuys, S.; Wittung-Stafshede, P. Roles of Copper-Binding Proteins in Breast Cancer. Int. J. Mol. Sci. 2017, 18, 871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boffetta, P.; Jourenkova, N.; Gustavsson, P. Riesgo de cáncer por exposición ocupacional y ambiental a hidrocarbu-ros aromáticos policíclicos. Cancer Causes Control. 1997, 8, 444–472. [Google Scholar] [CrossRef]
- Born, S.C. Chapter 62. dioxins. In Poisoning & Drug Overdose, 6th ed.; Olson, K.R., Ed.; McGraw Hill: New York, NY, USA, 2012; Available online: https://accessmedicine.mhmedical.com/content.aspx?bookid=391§ionid=42069876 (accessed on 10 February 2022).
- Bouwman, H.; Becker, P.J.; Schutte, C.H. Malaria control and longitudinal changes in levels of DDT and its me-tabolites in human serum from KwaZulu. Bull. World Health Organ. 1994, 72, 921–930. [Google Scholar]
- Brucker, N.; Charão, M.F.; Moro, A.M.; Ferrari, P.; Bubols, G.; Sauer, E.; Fracasso, R.; Durgante, J.; Thiesen, F.; Duarte, M.M.; et al. Atherosclerotic process in taxi drivers occupationally exposed to air pollution and co-morbidities. Environ. Res. 2014, 131, 31–38. [Google Scholar] [CrossRef]
- Burstyn, I.; Kromhout, H.; Partanen, T.; Svane, O.; Langård, S.; Ahrens, W.; Kauppinen, T.; Stücker, I.; Shaham, J.; Heederik, D.; et al. Polycyclic Aromatic Hydrocarbons and Fatal Ischemic Heart Disease. Epidemiology 2005, 16, 744–750. [Google Scholar] [CrossRef]
- CDC. Polycyclic Aromatic Hydrocarbons (PAHs). Centers for Disease Control and Prevention. 2009. Available online: https://www.epa.gov/sites/default/files/2014-03/documents/pahs_factsheet_cdc_2013.pdf (accessed on 10 February 2022).
- CDC. Dichlorodiphenyltrichloroethane (DDT). Centers for Disease Control and Prevention. 2009. Available online: https://www.cdc.gov/biomonitoring/pdf/ddt_factsheet.pdf (accessed on 10 February 2022).
- CDC. Lead Compounds (as Pb). Centers for Disease Control and Prevention. 2014. Available online: https://www.cdc.gov/niosh/idlh/7439921.html (accessed on 10 February 2022).
- CDC. Smoking and Cardiovascular Disease. Centers for Disease Control and Prevention. 2014. Available online: https://www.cdc.gov/tobacco/data_statistics/sgr/50th-anniversary/pdfs/fs_smoking_CVD_508.pdf (accessed on 10 February 2022).
- CDC. ToxFAQs™—DDT, DDE y DDD. Centers for Disease Control and Prevention. 2016. Available online: https://www.atsdr.cdc.gov/es/toxfaqs/es_tfacts35.html (accessed on 10 February 2022).
- CDC. Bisphenol A (BPA) Factsheet. Centers for Disease Control and Prevention. 2017. Available online: https://www.cdc.gov/biomonitoring/BisphenolA_FactSheet.html (accessed on 10 February 2022).
- CDC. Sources of Lead Exposure. Centers for Disease Control and Prevention. 2020. Available online: https://www.cdc.gov/nceh/lead/prevention/sources.htm (accessed on 10 February 2022).
- CDC. Health Effects of Cigarette Smoking. Centers for Disease Control and Prevention. 2021. Available online: https://www.cdc.gov/tobacco/data_statistics/fact_sheets/health_effects/effects_cig_smoking/index.htm (accessed on 10 February 2022).
- Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/biomonitoring/Phthalates_FactSheet.html (accessed on 25 June 2020).
- Chuang, S.-C.; Chen, H.-C.; Sun, C.-W.; Chen, Y.-A.; Wang, Y.-H.; Chiang, C.-J.; Chen, C.-C.; Wang, S.-L.; Chen, C.-J.; Hsiung, C.A. Phthalate exposure and prostate cancer in a population-based nested case-control study. Environ. Res. 2019, 181, 108902. [Google Scholar] [CrossRef]
- Cobellis, L.; Colacurci, N.; Trabucco, E.; Carpentiero, C.; Grumetto, L. Measurement of bisphenol A and bisphenol B levels in human blood sera from healthy and endometriotic women. Biomed. Chromatogr. 2009, 23, 1186–1190. [Google Scholar] [CrossRef] [PubMed]
- Cohn, B.A.; Wolff, M.S.; Cirillo, P.M.; Sholtz, R.I. DDT and Breast Cancer in Young Women: New Data on the Significance of Age at Exposure. Environ. Health Perspect. 2007, 115, 1406–1414. [Google Scholar] [CrossRef]
- Collaborative on Health and the Environment. Arsenic. 2019. Available online: https://www.healthandenvironment.org/environmental-health/environmental-risks/chemical-environment-overview/arsenic (accessed on 10 February 2022).
- Craig, S. Chapter 78. Glyphosate. In Poisoning & Drug Overdose, 6th ed.; Olson, K.R., Ed.; McGraw Hill: New York, NY, USA, 2012; Available online: https://accessmedicine.mhmedical.com/content.aspx?bookid=391§ionid=42069892 (accessed on 10 February 2022).
- Dann, A.B.; Hontela, A. Triclosan: Environmental exposure, toxicity and mechanisms of action. J. Appl. Toxicol. 2010, 31, 285–311. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Huang, S.; Zhang, X.; Zhang, W.; Feng, J.; Wang, T.; Hu, D.; Guan, L.; Li, J.; Dai, X.; et al. Plasma microRNA Expression and Micronuclei Frequency in Workers Exposed to Polycyclic Aromatic Hydrocarbons. Environ. Health Perspect. 2014, 122, 719–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinwiddie, M.T.; Terry, P.D.; Chen, J. Recent Evidence Regarding Triclosan and Cancer Risk. Int. J. Environ. Res. Public Health 2014, 11, 2209–2217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dioni, L.; Sucato, S.; Motta, V.; Iodice, S.; Angelici, L.; Favero, C.; Cavalleri, T.; Vigna, L.; Albetti, B.; Fustinoni, S.; et al. Urinary chromium is associated with changes in leukocyte miRNA expression in obese subjects. Eur. J. Clin. Nutr. 2016, 71, 142–148. [Google Scholar] [CrossRef]
- Du, Z.; Chai, X.; Li, X.; Ren, G.; Yang, X.; Yang, Z. Nano-CuO causes cell damage through activation of dose-dependent autophagy and mitochondrial lncCyt b-AS/ND5-AS/ND6-AS in SH-SY5Y cells. Toxicol. Mech. Methods 2021, 32, 37–48. [Google Scholar] [CrossRef]
- Falagan-Lotsch, P.; Murphy, C.J. Network-based analysis implies critical roles of microRNAs in the long-term cellular responses to gold nanoparticles. Nanoscale 2020, 12, 21172–21187. [Google Scholar] [CrossRef]
- Gao, H.; Yang, B.-J.; Li, N.; Feng, L.-M.; Shi, X.-Y.; Zhao, W.-H.; Liu, S.-J. Bisphenol A and Hormone-Associated Cancers: Current progress and perspectives. Medicine 2015, 94, e211. [Google Scholar] [CrossRef]
- Garshick, E.; Laden, F.; Hart, J.E.; Davis, M.E.; Eisen, E.A.; Smith, T.J. Lung Cancer and Elemental Carbon Exposure in Trucking Industry Workers. Environ. Health Perspect. 2012, 120, 1301–1306. [Google Scholar] [CrossRef] [PubMed]
- Gaum, P.M.; Gube, M.; Schettgen, T.; Putschögl, F.M.; Kraus, T.; Fimm, B.; Lang, J. Polychlorinated biphenyls and depression: Cross-sectional and longitudinal investigation of a dopamine-related Neurochemical path in the German HELPcB surveillance program. Environ. Health 2017, 16, 106. [Google Scholar] [CrossRef] [Green Version]
- Gerona, R.R.; Pan, J.; Zota, A.R.; Schwartz, J.M.; Friesen, M.; Taylor, J.A.; Hunt, P.A.; Woodruff, T.J. Direct measurement of Bisphenol A (BPA), BPA glucuronide and BPA sulfate in a diverse and low-income population of pregnant women reveals high exposure, with potential implications for previous exposure estimates: A cross-sectional study. Environ. Health 2016, 15, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonskikh, Y.; Gerstl, M.; Kos, M.; Borth, N.; Schosserer, M.; Grillari, J.; Polacek, N. Modulation of mammalian translation by a ribosome-associated tRNA half. RNA Biol. 2020, 17, 1125–1136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Government of Spain. DDT. 2020. Available online: https://prtr-es.es/DDT,15620,11,2007.html (accessed on 10 February 2022).
- Harari, F.; Barregard, L.; Östling, G.; Sallsten, G.; Hedblad, B.; Forsgard, N.; Borné, Y.; Fagerberg, B.; Engström, G. Blood Lead Levels and Risk of Atherosclerosis in the Carotid Artery: Results from a Swedish Cohort. Environ. Health Perspect. 2019, 127, 127002. [Google Scholar] [CrossRef] [PubMed]
- Hollensteiner, J.; Schneider, D.; Poehlein, A.; Daniel, R. Complete Genome of Roseobacter ponti DSM 106830T. Genome Biol. Evol. 2020, 12, 1013–1018. [Google Scholar] [CrossRef]
- Hong, Y.-S.; Song, K.-H.; Chung, J.-Y. Health Effects of Chronic Arsenic Exposure. J. Prev. Med. Public Health 2014, 47, 245–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iida, T.; Todaka, T. Measurement of Dioxins in Human Blood: Improvement of Analytical Method. Ind. Health 2003, 41, 197–204. [Google Scholar] [CrossRef]
- Kalinina, T.S.; Kononchuk, V.V.; Ovchinnikov, V.Y.; Chanyshev, M.D.; Gulyaeva, L.F. Expression of the miR-190 family is increased under DDT exposure in vivo and in vitro. Mol. Biol. Rep. 2018, 45, 1937–1945. [Google Scholar] [CrossRef]
- Kim, J.H.; Cho, Y.H.; Hong, Y.-C. MicroRNA expression in response to bisphenol A is associated with high blood pressure. Environ. Int. 2020, 141, 105791. [Google Scholar] [CrossRef]
- Konieczna, A.; Rutkowska, A.; Rachoń, D. Health risk of exposure to Bisphenol A (BPA). Rocz. Panstw. Zakl. Hig. 2015, 66, 5–11. [Google Scholar] [PubMed]
- Lee, H.-W.; Jose, C.C.; Cuddapah, S. Epithelial-mesenchymal transition: Insights into nickel-induced lung diseases. Semin. Cancer Biol. 2021, 76, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.-W.; Kim, D.H.; Ryu, J.Y. Association between urinary polycyclic aromatic hydrocarbons and hypertension in the Korean population: Data from the Second Korean National Environmental Health Survey (2012–2014). Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Lim, J.-H.; Song, M.-K.; Cho, Y.; Kim, W.; Han, S.O.; Ryu, J.-C. Comparative analysis of microRNA and mRNA expression profiles in cells and exosomes under toluene exposure. Toxicol. Vitr. 2017, 41, 92–101. [Google Scholar] [CrossRef] [PubMed]
- López-Carrillo, L.; Hernández-Ramírez, R.U.; Calafat, A.M.; Torres-Sánchez, L.; Galván-Portillo, M.; Needham, L.L.; Ruiz-Ramos, R.; Cebrián, M.E. Exposure to Phthalates and Breast Cancer Risk in Northern Mexico. Environ. Health Perspect. 2010, 118, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Luz, A.; DeLeo, P.; Pechacek, N.; Freemantle, M. Human health hazard assessment of quaternary ammonium compounds: Didecyl dimethyl ammonium chloride and alkyl (C12–C16) dimethyl benzyl ammonium chloride. Regul. Toxicol. Pharmacol. 2020, 116, 104717. [Google Scholar] [CrossRef]
- Messerlian, C.; Wylie, B.J.; Mínguez-Alarcón, L.; Williams, P.L.; Ford, J.B.; Souter, I.C.; Calafat, A.M.; Hauser, R. Urinary Concentrations of Phthalate Metabolites and Pregnancy Loss Among Women Conceiving with Medically Assisted Reproduction. Epidemiology 2016, 27, 879–888. [Google Scholar] [CrossRef]
- Mohamed, F.; Gawarammana, I.; Robertson, T.; Roberts, M.; Palangasinghe, C.; Zawahir, S.; Jayamanne, S.; Kandasamy, J.; Eddleston, M.; Buckley, N.A.; et al. Acute Human Self-Poisoning with Imidacloprid Compound: A Neonicotinoid Insecticide. PLoS ONE 2009, 4, e5127. [Google Scholar] [CrossRef]
- Mundhe, S.A.; Birajdar, S.V.; Chavan, S.S.; Pawar, N.R. Imidacloprid poisoning: An emerging cause of potentially fatal poisoning. Indian J. Crit. Care Med. 2017, 21, 786–788. [Google Scholar] [CrossRef]
- Muzembo, B.A.; Iwai-Shimada, M.; Isobe, T.; Arisawa, K.; Shima, M.; Fukushima, T.; Nakayama, S.F. Dioxins levels in human blood after implementation of measures against dioxin exposure in Japan. Environ. Health Prev. Med. 2019, 24, 6. [Google Scholar] [CrossRef] [Green Version]
- Nassan, F.L.; Mínguez-Alarcón, L.; Williams, P.L.; Dadd, R.; Petrozza, J.C.; Ford, J.B.; Calafat, A.M.; Hauser, R. Urinary triclosan concentrations and semen quality among men from a fertility clinic. Environ. Res. 2019, 177, 108633. [Google Scholar] [CrossRef] [PubMed]
- National Academy of Sciences. 2 Phthalate Exposure Assessment in Humans. In Phthalates and Cumulative Risk Assessment: The Tasks Ahead; 2008. Available online: https://www.ncbi.nlm.nih.gov/books/NBK215044/ (accessed on 10 February 2022).
- National Institute of Environmental Health Science. Bisphenol A (BPA). 2021. Available online: https://www.niehs.nih.gov/health/topics/agents/sya-bpa/index.cfm (accessed on 10 February 2022).
- National Research Council (US) Committee on Measuring Lead in Critical Populations. 2. Adverse Health Ef-fects of Exposure to Lead. In Measuring Lead Exposure in Infants, Children, and Other Sensitive Populations; National Academies Press: Washington, DC, USA, 1993. Available online: https://www.ncbi.nlm.nih.gov/books/NBK236465 (accessed on 10 February 2022).
- New Jersey Departament of Health. Hazardous Substance Fact Sheet. 2010. Available online: https://nj.gov/health/eoh/rtkweb/documents/fs/0709.pdf (accessed on 10 February 2022).
- Oregon State University. Imidacloprid. 2010. Available online: http://npic.orst.edu/factsheets/imidagen.html#exposed (accessed on 10 February 2022).
- Papoutsopoulou, S.; Satsangi, J.; Campbell, B.J.; Probert, C.S. Review article: Impact of cigarette smoking on intestinal inflammation-direct and indirect mechanisms. Aliment. Pharmacol. Ther. 2020, 51, 1268–1285. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Hu, J.; Xia, W.; Zhang, B.; Liu, W.; Zhang, C.; Yang, J.; Hu, C.; Zhou, A.; Chen, Z.; et al. Prenatal chromium exposure and risk of preterm birth: A cohort study in Hubei, China. Sci. Rep. 2017, 7, 3048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan American Health Organization. (w.d.). Arsenic. Available online: https://www3.paho.org/hq/index.php?option=com_content&view=article&id=9201:2013-arsenic&Itemid=40132&lang=es (accessed on 10 February 2022).
- Parvez, S.; Gerona, R.R.; Proctor, C.; Friesen, M.; Ashby, J.L.; Reiter, J.L.; Lui, Z.; Winchester, P.D. Glyphosate exposure in pregnancy and shortened gestational length: A prospective Indiana birth cohort study. Environ. Health 2018, 17, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Perkins, J.T.; Petriello, M.C.; Newsome, B.J.; Hennig, B. Polychlorinated biphenyls and links to cardiovascular disease. Environ. Sci. Pollut. Res. 2015, 23, 2160–2172. [Google Scholar] [CrossRef] [Green Version]
- Persson, E.C.; Graubard, B.I.; Evans, A.A.; London, W.T.; Weber, J.-P.; Leblanc, A.; Chen, G.; Lin, W.; McGlynn, K.A. Dichlorodiphenyltrichloroethane and risk of hepatocellular carcinoma. Int. J. Cancer 2012, 131, 2078–2084. [Google Scholar] [CrossRef] [Green Version]
- Pesatori, A.C.; Consonni, D.; Rubagotti, M.; Grillo, P.; Bertazzi, P.A. Cancer incidence in the population exposed to dioxin after the “Seveso accident”: Twenty years of follow-up. Environ. Health 2009, 8, 39. [Google Scholar] [CrossRef] [Green Version]
- Poothong, S.; Papadopoulou, E.; Padilla-Sánchez, J.A.; Thomsen, C.; Haug, L.S. Multiple pathways of human exposure to poly- and perfluoroalkyl substances (PFASs): From external exposure to human blood. Environ. Int. 2020, 134, 105244. [Google Scholar] [CrossRef]
- Pozuelos, G.L.; Kagda, M.S.; Schick, S.; Girke, T.; Volz, D.C.; Talbot, P. Experimental Acute Exposure to Thirdhand Smoke and Changes in the Human Nasal Epithelial Transcriptome: A Randomized Clinical Trial. JAMA Netw. Open 2019, 2, e196362. [Google Scholar] [CrossRef] [Green Version]
- Rahimzadeh, M.R.; Rahimzadeh, M.R.; Kazemi, S.; Moghadamnia, A.A. Cadmium toxicity and treatment: An update. Casp. J. Intern. Med. 2017, 8, 135–145. [Google Scholar]
- Rezg, R.; El-Fazaa, S.; Gharbi, N.; Mornagui, B. Bisphenol A and human chronic diseases: Current evidences, possible mechanisms, and future perspectives. Environ. Int. 2014, 64, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Rivers, A.R.; Burns, A.S.; Chan, L.-K.; Moran, M.A. Experimental Identification of Small Non-Coding RNAs in the Model Marine Bacterium Ruegeria pomeroyi DSS-3. Front. Microbiol. 2016, 7, 380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schecter, A.; Stanley, J.; Boggess, K.; Masuda, Y.; Mes, J.; Wolff, M.; Fürst, P.; Furst, C.; Wilson-Yang, K.; Chisholm, B. Polychlorinated biphenyl levels in the tissues of exposed and nonexposed humans. Environ. Health Perspect. 1994, 102, 149–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shankar, A.; Xiao, J.; Ducatman, A. Perfluorooctanoic Acid and Cardiovascular Disease in US Adults. Arch. Intern. Med. 2012, 172, 1397–1403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shanker, A.; Venkateswarlu, B. Chromium: Environmental Pollution, Health Effects and Mode of Action. Encycl. Environ. Health 2011, 650–659. [Google Scholar] [CrossRef]
- Sharma, M.K.; Kumar, M. Sulphate contamination in groundwater and its remediation: An overview. Environ. Monit. Assess. 2020, 192, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Shelnutt, S.; Kind, J.; Allaben, W. Bisphenol A: Update on newly developed data and how they address NTP’s 2008 finding of “Some Concern”. Food Chem. Toxicol. 2013, 57, 284–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shvedova, A.A.; Yanamala, N.; Kisin, E.R.; Khailullin, T.O.; Birch, M.E.; Fatkhutdinova, L. Integrated Analysis of Dysregulated ncRNA and mRNA Expression Profiles in Humans Exposed to Carbon Nanotubes. PLoS ONE 2016, 11, e0150628. [Google Scholar] [CrossRef]
- Skarha, J.; Mínguez-Alarcón, L.; Williams, P.L.; Korevaar, T.I.; De Poortere, R.A.; Broeren, M.A.; Ford, J.B.; Eliot, M.; Hauser, R.; Braun, J.M. Cross-sectional associations between urinary triclosan and serum thyroid function biomarker concentrations in women. Environ. Int. 2018, 122, 256–262. [Google Scholar] [CrossRef]
- Steenland, K.; Boffetta, P. Lead and cancer in humans: Where are we now? Am. J. Ind. Med. 2000, 38, 295–299. [Google Scholar] [CrossRef]
- Steenland, K.; Fletcher, T.; Stein, C.R.; Bartell, S.M.; Darrow, L.; Lopez-Espinosa, M.-J.; Ryan, P.B.; Savitz, D.A. Review: Evolution of evidence on PFOA and health following the assessments of the C8 Science Panel. Environ. Int. 2020, 145, 106125. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Zhao, T.; Long, K.; Wu, M.; Zhang, Z. Triclosan down-regulates fatty acid synthase through microRNAs in HepG2 cells. Eur. J. Pharmacol. 2021, 907, 174261. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Xu, A.; Li, M.; Xia, X.; Li, P.; Han, R.; Fei, G.; Zhou, S.; Wang, R. Effect of Methylation Status of lncRNA-MALAT1 and MicroRNA-146a on Pulmonary Function and Expression Level of COX2 in Patients With Chronic Obstructive Pulmonary Disease. Front. Cell Dev. Biol. 2021, 9, 667624. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Wang, H.; Chen, E.; Bian, E.; Xu, Y.; Ji, X.; Yang, Z.; Hua, X.; Zhang, Y.; Zhao, B. LncRNA-ATB promotes TGF-β-induced glioma cells invasion through NF-κB and P38/MAPK pathway. J. Cell. Physiol. 2019, 234, 23302–23314. [Google Scholar] [CrossRef]
- Tani, H.; Numajiri, A.; Aoki, M.; Umemura, T.; Nakazato, T. Short-lived long noncoding RNAs as surrogate indicators for chemical stress in HepG2 cells and their degradation by nuclear RNases. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- University of Tennessee. Toxicity Profiles. Formal Toxicity Summary for Cadmium. 2020. Available online: https://rais.ornl.gov/tox/profiles/cadmium.html (accessed on 10 February 2022).
- University of Tennessee. Toxicity Profiles. Formal Toxicity Summary for Chromium. 2020. Available online: https://rais.ornl.gov/tox/profiles/chromium.html (accessed on 10 February 2022).
- United States Environmental Protection Agency (EPA). Polychlorinated Biphenyls (PCBs). 2021. Available online: https://www.epa.gov/pcbs/learn-about-polychlorinated-biphenyls-pcbs (accessed on 10 February 2022).
- United States Environmental Protection Agency (EPA). PFOA, PFOS and Other PFAS. 2021. Available online: https://www.epa.gov/pfas/pfas-explained (accessed on 10 February 2022).
- US Department of Health and Human Services. Toxicological Profile for Glyphosate. Agency for toxic Subtances and Disease Registry. 2020. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp214.pdf (accessed on 10 February 2022).
- Wahlang, B.; Petriello, M.C.; Perkins, J.T.; Shen, S.; Hennig, B. Polychlorinated biphenyl exposure alters the expression profile of microRNAs associated with vascular diseases. Toxicol. Vitr. 2016, 35, 180–187. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Shim, Y.K.; Michalek, J.E.; Barber, E.; Saleh, L.M.; Choi, B.Y.; Wang, C.-P.; Ketchum, N.; Costello, R.; Marti, G.E.; et al. Serum microRNA profiles among dioxin exposed veterans with monoclonal gammopathy of undetermined significance. J. Toxicol. Environ. Health Part A 2020, 83, 269–278. [Google Scholar] [CrossRef]
- Wani, A.L.; Ara, A.; Usmani, J.A. Lead toxicity: A review. Interdiscip. Toxicol. 2015, 8, 55–64. [Google Scholar] [CrossRef] [Green Version]
- Weatherly, L.M.; Gosse, J.A. Triclosan exposure, transformation, and human health effects. J. Toxicol. Environ. Health. Part B Crit. Rev. 2017, 20, 447–469. [Google Scholar] [CrossRef]
- Woeller, C.F.; Thatcher, T.H.; Thakar, J.; Cornwell, A.; Smith, M.R.; Jones, D.P.; Hopke, P.K.; Sime, P.J.; Krahl, P.; Mallon, T.M.; et al. Exposure to Heptachlorodibenzo-p-dioxin (HpCDD) Regulates microRNA Expression in Human Lung Fibroblasts. J. Occup. Environ. Med. 2019, 61, S82–S89. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Regional Office for Europe, Copenhagen, Denmark. Chapter 6.4 Chromium. 2000. Available online: https://www.euro.who.int/__data/assets/pdf_file/0017/123074/AQG2ndEd_6_4Chromium.PDF (accessed on 10 February 2022).
- World Health Organization. Preventing Disease through Healthy Environments Exposure to Dioxins and Dioxin-like Substances: A Major Public Health Concern. 2010. Available online: https://www.who.int/ipcs/features/dioxins.pdf (accessed on 10 February 2022).
- World Health Organization. Preventing Disease through Healthy Environments. Exposure to Cadmium: A Major Public Health Concern. 2019. Available online: https://apps.who.int/iris/bitstream/handle/10665/329480/WHO-CED-PHE-EPE-19.4.3-eng.pdf?ua=1 (accessed on 10 February 2022).
- World Health Organization. IARC Monograph on Glyphosate. 2022. Available online: https://www.iarc.who.int/featured-news/media-centre-iarc-news-glyphosate/ (accessed on 10 February 2022).
- World Health Organization, International Agency for Research on Cancer. Triclosan (TCS) (Compound). 2022. Available online: http://exposome-explorer.iarc.fr/compounds/1420 (accessed on 10 February 2022).
- World Health Organization. Regional Office for Europe, Copenhagen, Denmark. Chapter 5.10 Polychlorinated bi-phenyls (PCBs). 2000. Available online: https://www.euro.who.int/__data/assets/pdf_file/0016/123064/AQG2ndEd_5_10PCBs.PDF (accessed on 10 February 2022).
- World Health Organization. Regional Office for Europe, Copenhagen, Denmark. Chapter 8.1 Environmental Tobacco Smoke. 2000. Available online: https://www.euro.who.int/__data/assets/pdf_file/0003/123087/AQG2ndEd_8_1ETS.PDF (accessed on 10 February 2022).
- Xu, M.; Yu, Z.; Hu, F.; Zhang, H.; Zhong, L.; Han, L.; An, Y.; Zhu, B.; Zhang, H. Identification of differential plasma miRNA profiles in Chinese workers with occupational lead exposure. Biosci. Rep. 2017, 37, BSR20171111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Jurkovic-Mlakar, S.; Li, Y.; Wahlberg, K.; Scott, K.; Pineda, D.; Lindh, C.H.; Jakobsson, K.; Engström, K. Association between serum concentrations of perfluoroalkyl substances (PFAS) and expression of serum microRNAs in a cohort highly exposed to PFAS from drinking water. Environ. Int. 2020, 136, 105446. [Google Scholar] [CrossRef]
- Yan, W.; Yue, H.; Ji, X.; Li, G.; Sang, N. Prenatal NO2 exposure and neurodevelopmental disorders in offspring mice: Transcriptomics reveals sex-dependent changes in cerebral gene expression. Environ. Int. 2020, 138, 105659. [Google Scholar] [CrossRef] [PubMed]
- Zefferino, R.; Piccoli, C.; Ricciardi, N.; Scrima, R.; Capitanio, N. Possible Mechanisms of Mercury Toxicity and Cancer Promotion: Involvement of Gap Junction Intercellular Communications and Inflammatory Cytokines. Oxidative Med. Cell. Longev. 2017, 2017, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.-Z.; Hu, G.-L.; Lu, L.-Y.; Hu, S.-F.; Li, Y.-S.; Su, X.; Dong, W.-Y.; Zhen, C.-A.; Liu, R.-Q.; Kong, F.-B.; et al. Identification of differentially expressed microRNAs under imidacloprid exposure in Sitobion miscanthi. Pestic. Biochem. Physiol. 2021, 177, 104885. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; He, L.; Liu, X.-D.; Guan, H.; Li, Y.; Huang, R.-X.; Zhou, P.-K. Integrated Analysis of lncRNA and mRNA Transcriptomes Reveals New Regulators of Ubiquitination and the Immune Response in Silica-Induced Pulmonary Fibrosis. BioMed Res. Int. 2019, 2019, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zota, A.R.; Geller, R.J.; Vannoy, B.N.; Marfori, C.Q.; Tabbara, S.; Hu, L.Y.; A Baccarelli, A.; Moawad, G.N. Phthalate Exposures and MicroRNA Expression in Uterine Fibroids: The FORGE Study. Epigenetics Insights 2020, 13, 2516865720904057. [Google Scholar] [CrossRef] [Green Version]


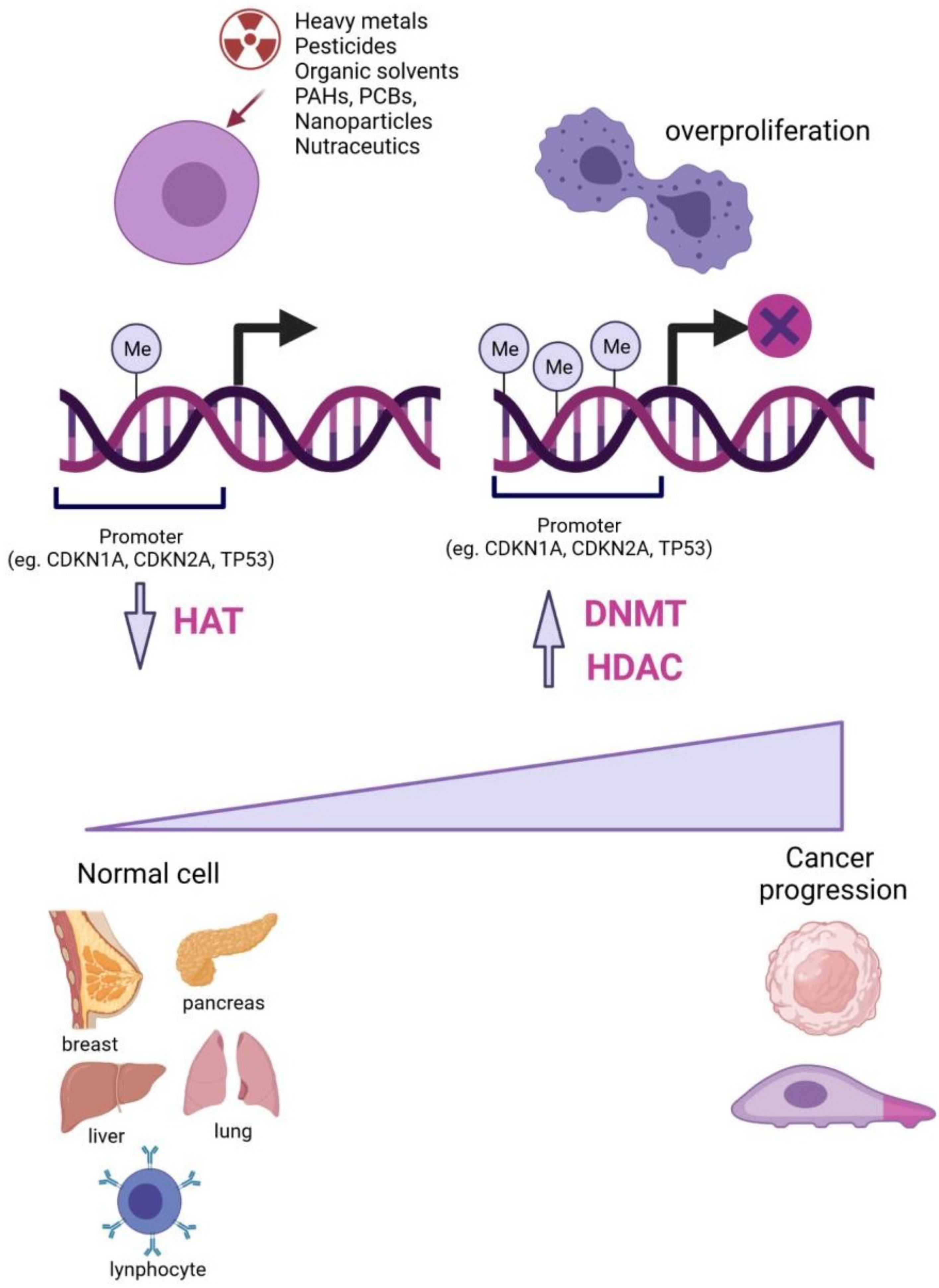
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olmedo-Suárez, M.Á.; Ramírez-Díaz, I.; Pérez-González, A.; Molina-Herrera, A.; Coral-García, M.Á.; Lobato, S.; Sarvari, P.; Barreto, G.; Rubio, K. Epigenetic Regulation in Exposome-Induced Tumorigenesis: Emerging Roles of ncRNAs. Biomolecules 2022, 12, 513. https://doi.org/10.3390/biom12040513
Olmedo-Suárez MÁ, Ramírez-Díaz I, Pérez-González A, Molina-Herrera A, Coral-García MÁ, Lobato S, Sarvari P, Barreto G, Rubio K. Epigenetic Regulation in Exposome-Induced Tumorigenesis: Emerging Roles of ncRNAs. Biomolecules. 2022; 12(4):513. https://doi.org/10.3390/biom12040513
Chicago/Turabian StyleOlmedo-Suárez, Miguel Ángel, Ivonne Ramírez-Díaz, Andrea Pérez-González, Alejandro Molina-Herrera, Miguel Ángel Coral-García, Sagrario Lobato, Pouya Sarvari, Guillermo Barreto, and Karla Rubio. 2022. "Epigenetic Regulation in Exposome-Induced Tumorigenesis: Emerging Roles of ncRNAs" Biomolecules 12, no. 4: 513. https://doi.org/10.3390/biom12040513
APA StyleOlmedo-Suárez, M. Á., Ramírez-Díaz, I., Pérez-González, A., Molina-Herrera, A., Coral-García, M. Á., Lobato, S., Sarvari, P., Barreto, G., & Rubio, K. (2022). Epigenetic Regulation in Exposome-Induced Tumorigenesis: Emerging Roles of ncRNAs. Biomolecules, 12(4), 513. https://doi.org/10.3390/biom12040513






